Abstract
Objective
Diabetic ketoacidosis (DKA) may result in both dehydration and cerebral edema but these processes may have opposing effects on blood pressure. We examined the relationship between dehydration and blood pressure in pediatric DKA.
Design
Retrospective review
Setting
Seattle Children's Hospital, Seattle, WA
Participants
Hospitalized children less than 18 years.
Intervention(s) or Main Exposures
DKA (venous pH < 7.3, glucose > 300 mg/dL, HCO3 < 15 meq/l and urinary ketosis).
Outcome Measures
Dehydration was calculated as percent body weight lost at admission compared to discharge. Hypertension (systolic and/or diastolic blood pressure percentile ≥ 95%ile) was defined based on 2004 National Heart, Lung, and Blood Institute nomograms and hypotension was defined as systolic blood pressure < 70 + 2 [age]
Results
Thirty-three patients (median 10.9 years; range 10 months - 17 years) were included. Fifty-eight percent of patients (19/33) had hypertension on admission prior to treatment and 82% had hypertension during the first 6 hours of admission. None had admission hypotension. Hypertension forty-eight hours after treatment and weeks after discharge was common (28% and 19%, respectively). Based on weight gained by discharge, 27% of patients had mild, 61% had moderate, and 12% presented with severe dehydration.
Conclusion
Despite dehydration, most children admitted with severe DKA had hypertension.
Keywords: blood pressure, diabetes, pediatric, hypertension
INTRODUCTION
Dehydration from fluid loss secondary to glycosuria is a central feature of diabetic ketoacidosis (DKA) (1-3). Dehydration can theoretically lead to hypovolemia and systemic hypotension. However, there is a paucity of information on blood pressure in DKA.
Many patients (15-67%) evaluated for new onset type 1 diabetes mellitus present with the constellation of dehydration, hyperglycemia and acidosis consistent with DKA (1-3). Dehydration, coupled with systemic hypotension may result in decreased cerebral perfusion and cerebral ischemia (4). Thus, in DKA, both dehydration and cerebral edema may coexist. Blood pressure may be elevated due to cerebral edema and increased intracranial pressure (ICP). In severe cases, Cushing's triad (hypertension, bradycardia, and irregular respirations) may be present (5). In DKA, therefore, blood pressure may not provide an accurate estimate of dehydration. However, neither the incidence of hypotension nor changes in blood pressure have been well characterized during DKA. The purpose of this study was to characterize blood pressure during DKA treatment and to examine the relationship between severity of dehydration and blood pressure. We hypothesized that as children with DKA commonly present with dehydration, blood pressure would be lower on admission compared to recovery, and that hypotension would be related to severity of dehydration.
METHODS
Study participants
After obtaining Institutional Review Board approval, we retrospectively examined data from the records of children who were admitted to either the pediatric intensive care unit (PICU) or hospital ward at Seattle Children's Hospital between September 2005 and November 2008 with a diagnosis of DKA. These patients had been previously consented and enrolled in an ongoing study investigating changes in cerebral hemodynamics in DKA. Eligibility criteria included: age <18 years, venous pH < 7.3, glucose > 300 mg/dL, HCO3 < 15 meq/l and urinary ketosis. There were no specific blood pressure protocols in the PICU or pediatric ward at the time of this project.
Data abstracted
Admission demographic data, physiological data, discharge information and follow up clinic weights and blood pressure, as well as select laboratory tests (venous blood gas, electrolytes, glucose, blood urea nitrogen (BUN) and creatinine) were abstracted. Laboratory values and blood pressures were divided into specific time periods after initial insulin administration (start of treatment): 0 - 5.9 hours, 6 - 11.9 hours, 12 - 17.9 hours, 18 – 23.9 hours, 24 - 48 hours, and discharge. Fluid administration and urine output was similarly recorded during each time period. Blood pressures and weights from two outpatient endocrine office visits after hospital discharge were also collected. All children had heights measured in the supine position on admission by the admitting nurse and these data are entered in the medical records of all patients per standard procedure. All patients had blood pressures taken using an automated oscillometric device in the supine position with appropriately sized arm cuffs. Four patients had an arterial cannula placed during their PICU stay. When there was a discrepancy in arterial line and blood pressure cuff documentation, we used the lower of the two readings in our analysis in an effort to conservatively estimate hypertension. Transfers from outside hospitals were included and data from first hospital site recorded.
Definitions and statistical analysis
National Heart, Lung, and Blood Institute charts (NHLBI, 2004) were referenced to define hypertension by age, height, and gender and include values between the 50th to 99th percentiles. The blood pressure tables for children and adolescents are based on recently revised child height percentiles and also include the BP data from the 1999-2000 National Health and Nutrition Examination Survey (6). NHLBI defines hypertension when either systolic or diastolic blood pressures equal or exceed the 95th percentile for age, height and gender. Hypotension, while not defined in these charts, is traditionally defined as systolic blood pressure (SBP) less than 70mmHg + (2 x age in years) (7). Using a systolic parameter to define hypertension allowed us to compare the incidence of hypotension and hypertension using the same blood pressure variable. However, recognizing that diastolic blood pressure (DBP) is important to mean arterial pressure (MAP) and cerebral perfusion pressure, the incidence of diastolic hypertension was also recorded and the proportion of diastolic hypertension among patients with systolic hypertension on admission was calculated.
For descriptive purposes, cerebral edema was defined by clinical evaluation and/or radiographic studies as either: 1) alteration in mental status and vital signs that led treating physicians to administer hyperosmolar therapy (mannitol/ hypertonic saline), or 2) radiographically confirmed cerebral edema by head computed tomography (CT).
Severity of dehydration was divided into three categories based on American Academy of Pediatrics guidelines as minimal (less than 3% fluid deficit), mild to moderate (3% to 9% fluid deficit), and severe (greater than 9% fluid deficit) (8). Percent loss of body weight (PLBW) was used to determine severity of dehydration and compare admission (pre-treatment) weight to discharge weight as follows (9-11):
Serum sodium values were adjusted for hyperglycemia using the following formula (12): corrected serum sodium concentration = measured serum sodium concentration + 0.016 × (serum glucose concentration – 100). Serum osmolality was calculated using the following equation (13): (2 × serum sodium) + (serum glucose / 18) + (serum urea nitrogen / 2.8).
Data were entered into an Excel spreadsheet (Microsoft Office 2007). Descriptive statistics were used to examine admission clinical characteristics, including blood pressure. Paired Student's T test was used to examine differences in weight between admission, discharge and outpatient visit. Analysis of variance was used to examine changes in continuous variables such as the differences in SBP through the different time periods (0-6, 6-12, 12-24, etc) from admission. Fisher's Exact test or χ2 test was used for categorical variables such as the relationship between admission hypertension and cerebral edema on head CT and the relationship between previous diagnosis of diabetes and hypertension. Data are presented as range, median and standard error of the mean (SEM) as appropriate. P < 0.05 was considered significant.
RESULTS
Clinical characteristics
The records of the first consecutive 33 children enrolled (median 10.9 years; range 10 months - 17 years) in the ongoing study were reviewed. Seventeen (51%) were female, and nineteen (58%) had new onset diabetes. All children had more than one symptom prior to admission, which included emesis (40%), diarrhea (20%), fatigue (20%), weight loss (17%), polyuria (83%), polydipsia (74%), and fever (9%). Glasgow coma scale (GCS) score was recorded for the 24 patients admitted to the PICU (median GCS 10 ± 3). None of the 33 subjects complained of headache or were crying on admission per nursing records. Seventeen (51%) children were transferred to Seattle Children's Hospital after receiving fluid and/or insulin at an outside facility. Most patients (73%) required admission to the PICU with severe DKA (Table 1). All children were treated initially with intravenous fluids (at least one 10-20 cc/kg isotonic normal saline fluid bolus followed by a maintenance infusion of crystalloid). Of patients started on insulin at outside hospitals, two were given insulin boluses prior to transfer, and eleven were started on an insulin infusion. After admission to our facility, all patients were continued on standard intravenous insulin infusions (0.1units/kg/hr). Nine (27%) patients were treated with mannitol due to clinically suspected or CT confirmed cerebral edema either at the referring facility or after arrival to the study institution. One patient had edema noted on head CT, but did not receive mannitol. None of the children had bradycardia during hospitalization. Admission clinical data are summarized in Table 1, and reveal a study population with severe DKA: median pH 7.07, glucose 587 mg/dL and serum HCO3 6.1 mEq/L.
TABLE 1.
Admission demographics and clinical characteristics of children admitted with DKA. Data as median (± SEM) and range
| Clinical Characteristics | |
| Total # patients enrolled | 33 |
| Age (yrs) | 10.9 yrs (10 mo – 17.5 yrs) |
| Female gender (%) | 17 (51%) |
| Weight (kg) | 41 ± 4 (7.2 – 97.7) |
| Height (cm) | 148 ± 6 (72 – 182) |
| BMI percentile (%) | 29 ± 12 |
| BMI ≥ 85th percentile | 0 |
| New DM diagnosis (%) | 19 (58%) |
| PICU admission Glasgow coma scale score | 10 ± 3 (3 – 15) |
| Cerebral edema (CT or clinical assessment) | 9 (27%) |
| Admission serum laboratory values | |
| Glucose (mg/dL) | 587 ± 48 (346 – 1670) |
| BUN (mg/dL) | 17 ± 2.7 (9 – 77) |
| Creatinine (mg/dL) | 1.1 ± 0.1 (0.4 – 3.0) |
| Na (corrected; mEq/L) | 142 ± 1.5 (131 – 164) |
| Serum osmolality (calculated; mOsm) | 311 ± 5 (282 – 398) |
| pH | 7.07 ± 0.02 (6.82 – 7.29) |
| PCO2 (mmH20) | 20 ± 1.5 (6.8 – 37) |
| Bicarbonate (mEq/L) | 6.1 ± 0.6 (1 – 14) |
Severity of dehydration and fluid balance
All patients received fluid boluses on presentation to a medical facility (range 10-66 ml/kg; median 27 ml/kg), and the American Diabetes Association guidelines were used to dictate maintenance hydration (3). Average fluid administered (intake) was greater than urine output during the first 6 hours after presentation. Beyond 6 hours, the difference between intake and output decreased and stabilized. Despite osmotic diuresis, urine output during the first 6 hours did not exceed 2 ml/kg/hr and urine output remained relatively stable during the first 24 hours after treatment. PLBW ranged from 0.4 to 13%. The estimated severity of dehydration based on change in weight between admission and discharge revealed that 9 patients (27%) had minimal dehydration, 20 patients (61%) had moderate dehydration and 4 (12%) had severe dehydration.
Median serum creatinine level for our study population was 1.1 ± 0.9 mg/dL on admission and decreased to 0.55 ± 0.05mg/dL by 48 hours. Two patients had high creatinine values of 1.6 and 3.0 mg/dL, which normalized to discharge levels of 0.5 and 0.9 mg/dL, respectively. BUN levels were elevated on admission (range 9.0 – 77 mg/dL; median 17.0 ± 2.7 mg/dL), but normalized with hydration by 48 hours.
Blood pressure changes during DKA treatment and after discharge
All 33 patients had both admission (pre-treatment) systolic and diastolic blood pressures recorded, either at the referring facility or at our institution. Nineteen (58%) patients had admission systolic hypertension (SBP ≥ 95th %) and fourteen of those also had diastolic hypertension (DBP ≥ 95th %). Diastolic hypertension occurred only in the presence of systolic hypertension. Sixteen (85%) of 19 patients with systolic hypertension had severe hypertension with SBP ≥ 99th % for age/height/gender. None of the 33 patients had hypotension on admission. Mean arterial pressure values from admission through discharge are given in Table 2 for each subject.
TABLE 2.
Median systolic blood pressure percentile per NHLBI charts for age/height and gender (SBP ≥ 95th % = hypertension).
| Median Blood Pressure and Percentile (SBP = Systolic; DBP = Diastolic; MAP = Mean Arterial BP) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Admit | Discharge | Blood Pressure on Admission | 0-5.9 hrs | 6-11.9 hrs | 12-17.9 hrs | 18-23.9 hrs | 24-24 hrs | clinic 1 | |||||||||||||
| Age (yrs) | Sex | Height (cm) | Weight (kg) | Weight (kg) | # Years with DM | SBP | % | DBP | % | MAP | SBP | % | SBP | % | SBP | % | SBP | % | SBP | % | SBP |
| 99th percentile admission blood pressure | |||||||||||||||||||||
| 17.5 | M | 182 | 95 | 98.3 | 5 | 164 | 99 | 135 | 99 | 144 | 149 | 99 | 137 | 99 | 113 | 50 | 134 | 95 | 132 | 95 | 131 |
| 17 | F | 157 | 68.1 | 70.5 | 4 | 153 | 99 | 86 | 95 | 106 | 145 | 99 | 129 | 97 | 117 | 73 | 129 | 96 | 112 | 58 | 146 |
| 14.5 | F | 157 | 60.7 | 62.5 | 3 | 137 | 99 | 60 | 40 | 83 | 116 | 75 | 116 | 75 | 108 | 50 | 120 | 85 | 118 | 80 | 128 |
| 14.1 | M | 172 | 57.9 | 60 | 0 | 143 | 99 | 93 | 99 | 108 | 139 | 99 | 124 | 80 | 129 | 90 | 115 | 50 | 114 | 50 | 112 |
| ^13.9 | M | 163 | 34.8 | 38 | 2 | 124 | 99 | 81 | 95 | 94 | 121 | 80 | 106 | 30 | 105 | 30 | 103 | 20 | 106 | 30 | 114 |
| 13 | F | 157 | 40.9 | 42.5 | 1 | 115 | 99 | 58 | 40 | 75 | 127 | 95 | 126 | 95 | 113 | 70 | 108 | 50 | 108 | 50 | 115 |
| **12 | M | 161 | 53.4 | 53.4 | 8 | 146 | 99 | 92 | 99 | 108 | 140 | 99 | 150 | 99 | 132 | 95 | 144 | 99 | 143 | 99 | 120 |
| 10.9 | F | 153 | 47 | 48.4 | 0 | 132 | 99 | 96 | 99 | 107 | 118 | 85 | 134 | 99 | 116 | 80 | 102 | 30 | 115 | 80 | 122 |
| 10.9 | F | 137 | 33 | 36.1 | 0 | 130 | 99 | 84 | 99 | 98 | 135 | 99 | 92 | 15 | 86 | 5 | 98 | 30 | 99 | 40 | 127 |
| 10.9 | F | 145 | 34.5 | 37.1 | 0 | 127 | 99 | 91 | 99 | 102 | 122 | 95 | 116 | 85 | 104 | 50 | 105 | 50 | 105 | 50 | 119 |
| **9.8 | M | 130 | 23.2 | 23.9 | 0 | 129 | 99 | 87 | 99 | 100 | 129 | 99 | 134 | 99 | 135 | 99 | 134 | 99 | 117 | 95 | 93 |
| **9.5 | F | 142 | 31 | 33.6 | 6 | 126 | 99 | 70 | 80 | 87 | 117 | 90 | 117 | 90 | 112 | 80 | 112 | 80 | 99 | 35 | 97 |
| ^3.3 | F | 105 | 17.5 | 18.1 | 0 | 123 | 99 | 56 | 90 | 76 | 122 | 99 | 128 | 99 | 122 | 99 | 115 | 95 | 100 | 65 | 97 |
| ^1.3 | F | 72 | 8.4 | 9.2 | 0 | 115 | 99 | 58 | 95 | 75 | 118 | 99 | 119 | 99 | 113 | 99 | 118 | 99 | 104 | 95 | 120 |
| ^1.1 | M | 79 | 9.2 | 9.8 | 0 | 134 | 99 | 76 | 99 | 93 | 113 | 99 | 116 | 99 | 127 | 99 | 120 | 99 | 107 | 95 | 90 |
| 0.9 | M | 72 | 7.9 | 8.8 | 0 | 110 | 99 | 79 | 99 | 88 | 107 | 95 | 99 | 90 | 113 | 99 | 109 | 95 | 111 | 99 | 103 |
| 95th percentile admission blood pressure | |||||||||||||||||||||
| **12.4 | F | 148 | 43 | 45 | 5 | 120 | 95 | 62 | 50 | 79 | 117 | 85 | 111 | 70 | 108 | 50 | 119 | 90 | 121 | 95 | 104 |
| 11.9 | F | 148 | 40.9 | 45.1 | 7 | 122 | 95 | 77 | 90 | 91 | 112 | 75 | 104 | 45 | 107 | 60 | 110 | 70 | 119 | 90 | 114 |
| 11.1 | M | 145 | 34.6 | 36.5 | 3 | 122 | 95 | 60 | 50 | 79 | 130 | 99 | 131 | 99 | 118 | 89 | 116 | 84 | 124 | 96 | 112 |
| 50-94th percentile admission blood pressure | |||||||||||||||||||||
| **14 | F | 162 | 74.7 | 75.4 | 2 | 116 | 75 | 69 | 75 | 83 | 113 | 60 | 119 | 80 | 102 | 80 | 117 | 75 | 110 | 50 | 109 |
| 13.5 | F | 164 | 56 | 58.1 | 2 | 118 | 80 | 65 | 50 | 81 | 114 | 65 | 123 | 90 | 116 | 70 | 123 | 90 | 117 | 75 | 94 |
| **12.5 | M | 152 | 40.1 | 43.4 | 0 | 111 | 60 | 79 | 90 | 89 | 119 | 90 | 122 | 90 | 117 | 80 | 125 | 95 | 115 | 75 | 105 |
| 12.4 | M | 172 | 47 | 47.2 | 0 | 117 | 65 | 81 | 95 | 92 | 103 | 20 | 100 | 10 | 111 | 50 | 108 | 35 | 105 | 25 | 99 |
| 12.3 | M | 156 | 41.8 | 43.4 | 0 | 121 | 90 | 77 | 90 | 90 | 116 | 75 | 123 | 95 | 146 | 99 | 122 | 90 | 120 | 85 | 123 |
| 10.8 | F | 142 | 41 | 42.5 | 0 | 113 | 80 | 75 | 90 | 87 | 123 | 95 | 111 | 75 | 111 | 75 | 109 | 70 | 104 | 50 | 100 |
| **10 | M | 139 | 27.9 | 28.8 | 1 | 110 | 75 | 65 | 60 | 79 | 122 | 95 | 110 | 75 | 106 | 60 | 112 | 80 | 116 | 90 | 104 |
| 9.3 | F | 136 | 21.6 | 23.5 | 0 | 116 | 90 | 46 | 40 | 67 | 129 | 99 | 148 | 99 | 137 | 99 | 130 | 99 | 132 | 99 | 113 |
| ^9 | F | 138 | 42.2 | 43 | 0 | 101 | 50 | 73 | 90 | 81 | 114 | 90 | 95 | 25 | 113 | 85 | 116 | 90 | 104 | 60 | 95 |
| **8.3 | M | 136 | 27 | 28.3 | 0 | 106 | 60 | 74 | 90 | 84 | 115 | 90 | 112 | 80 | 112 | 80 | 108 | 70 | 104 | 50 | 110 |
| ^2.5 | F | 95 | 13.7 | 15.1 | 0 | 107 | 75 | 63 | 95 | 76 | 104 | 80 | 102 | 75 | 100 | 70 | 114 | 95 | 107 | 90 | 104 |
| < 50th percentile admission blood pressure | |||||||||||||||||||||
| 14.5 | M | 168 | 65.8 | 67.1 | 6 | 110 | 40 | 66 | 50 | 79 | 112 | 50 | 110 | 40 | 115 | 60 | ***** | ***** | ***** | ***** | 132 |
| **10.8 | M | 153 | 50 | 50.8 | 0 | 100 | 25 | 63 | 50 | 72 | 124 | 95 | 124 | 95 | 133 | 99 | 133 | 99 | 114 | 75 | 123 |
| *1 | M | 74 | 7.2 | 8.3 | 0 | 77 | 10 | 38 | 50 | 50 | 92 | 75 | 89 | 50 | 89 | 50 | 83 | 50 | 86 | 50 | 108 |
Nine patients were diagnosed with cerebral edema and 4 had hypertension.
Subjects were under 5 years of age.
12 month old infant had lowest SBP percentile with admission (pre-treatment) SBP of 77mmHg (“clamped down”) but all remaining SBP during hospitalization were greater than 80mmHg.
Δ 11/19 patients with hypertension (SBP > 95th percentile) had diastolic blood pressure > 95th percentile and 17/19 patients with hypertension, as defined in this study, had admission diastolic blood pressure percentiles ≥ 90th percentile.
New diagnosis of TIDM is reflected by “0” in column addressing # of years with TIDM.
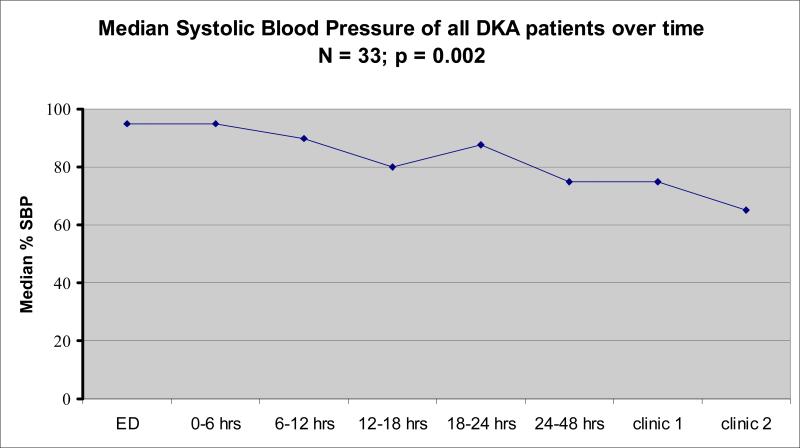
During the first 6 hour period after initiation of insulin therapy, 82% exhibited hypertension on at least one blood pressure reading, and 52% had a median SBP during that time period > 95%ile for age, height and gender. Over time, and after discharge, the number of patients with hypertension decreased. Individual systolic and median blood pressures over the first 6 hours for all subjects are detailed in Table 2, and median SBP percentiles for the entire cohort over all time periods are shown in Figure 1.
FIGURE 1.
Change in median systolic blood pressure percentile in children with DKA. ED represents emergency department.
First outpatient endocrine clinic visits occurred 1 to 8 (median 4 ± 2) weeks after discharge, and second visits were 6 to 24 (median 14 ± 3) weeks after discharge. One patient had their first visit at 12 weeks and second visit at 50 weeks due to noncompliance and repeated admissions. Twenty-seven percent (n=9) of the 33 patients were hypertensive at the first clinic visit, and 21% were hypertensive at the second clinic visit. Seven of the 9 children with admission hypertension had hypertension at clinic 1. Since the development of hypertension is a known complication in patients with diabetes, we examined the association between history of diabetes and hypertension on admission. There was no significant relationship found between pretreatment hypertension in children with DKA by onset of diagnosis (p=0.15).
DISCUSSION
The main findings of this study are: 1) none of the DKA patients had admission (pre-treatment) hypotension despite mostly moderate to severe dehydration, and 2) the majority of patients had pre-treatment hypertension which persisted through hospital stay and discharge (even in newly diagnosed diabetics) despite normalization of renal function. We also observed a positive fluid balance in most patients early in their course of treatment, despite the osmotic diuresis that typically occurs during hyperglycemia. These data suggest that admission SBP may not be a useful indicator of the severity of dehydration in pediatric DKA but rather may be useful in distinguishing patients with dehydration secondary to other causes of hypovolemia. Hypertension despite dehydration in diabetic patients likely reflects systemic pathophysiological processes that may be unique to DKA.
We characterized blood pressure and aimed to determine the incidence of pre-treatment hypotension in pediatric DKA based on the premise that DKA is a hypovolemic state, and hypovolemia may be associated with hypotension. Previous review of clinical factors associated with cerebral edema in DKA revealed that hypocarbia and high serum BUN levels are risk factors for the development of cerebral edema, suggesting a role for dehydration, hypovolemia and cerebral hypoperfusion (4). We sought to detect the presence of hypotension, which has not been previously characterized in DKA patients. Despite dehydration, none of the 33 patients in our study with DKA presented with hypotension and, in contrast, most patients had significant hypertension. Although the median SBP percentile decreased during hospitalization, many patients remained hypertensive through hospitalization and after discharge. Fluid balance after six hours was nearly matched, so overhydration most likely did not occur and did not contribute to hypertension.
We assumed that the degree of hypotension would be associated with the severity of dehydration. We compared admission weight against recovery weight to calculate PLBW and quantify the extent of dehydration. Our data are consistent with previous studies of patients with dehydration due to DKA and to other medical illnesses (8-11,14). While we investigated patients with minimal to severe dehydration, we found no instances of hypotension in our population.
Factors such as pain or anxiety may cause hypertension (15). According to nursing records and physician notes for our study population, most patients did not complain of pain and were often somnolent. Cerebral edema may result in increased ICP and subsequent increase in systemic blood pressure to maintain cerebral perfusion (5). Only five children had cerebral edema documented on head CT and none had bradycardia. However, given the small number of children with available head CTs and the fact that clinical examination may have been more sensitive in detecting cerebral edema than head CT, we cannot draw any definitive conclusions regarding the relationship between hypertension and radiographic cerebral edema.
There are many other factors that we did not study that may contribute to the hypertension observed in our population. Stress response can cause hypertension as even small increases in cerebral edema and ICP may lead to increased catecholamine levels (16,17). Other counter regulatory hormones such as glucagon, cortisol and growth hormone, and proinflammatory cytokines have all been shown to be elevated in DKA which is likely to have an effect on blood pressure acutely (18, 19). As these hormones have also been shown to return to normal levels after the acute event, it may be difficult to attribute long term hypertension to their effects, though this deserves further study. Normalization of cytokine levels after treatment may be due to the anti-inflammatory effect of insulin (19). Hyperosmolality leads to release of antidiuretic hormone (ADH) which increases blood pressure via V2 receptors. Over-activity of the renin-angiotensin system, as well as arginine vasopressin (known to be highly activated in DKA) is also a possible cause of the observed hypertension (17, 20-21).
The presence of hypertension and lack of hypotension in our patients does not support hypovolemia and ischemic injury as the main mechanisms for cerebral edema in DKA. Previously published data in a small series of DKA patients, who underwent transcranial Doppler ultrasonography and cerebral autoregulation testing, show normal to increased cerebral blood flow velocities and impaired cerebral autoregulation early in the course of illness (22). The work by Glaser and colleagues in children with DKA showing elevated apparent diffusion coefficients and shorter mean transit times on MRI study during illness compared to recovery support a predominantly vasogenic mechanism in the development of cerebral edema (23). However, we cannot definitively exclude an ischemic insult prior to hospital arrival. Thus, if cerebral ischemia is involved in the causal pathway leading to cerebral edema, then hypertension may be a physiological response to maintaining cerebral perfusion and would be desirable and protective. Conversely, if cerebral hyperemia is the main pathophysiological process, then treating hypertension may be warranted as a therapy to limit vasogenic cerebral edema. Given the paucity of data on the pathophysiology of DKA, data from our small study provides new information regarding one hemodynamic parameter that appears to be affected in DKA, thereby filling one gap of knowledge in this area. Our data suggest that greater awareness and perhaps more frequent measurement of hypertension is justified throughout hospital admission and during outpatient follow-up visits to further characterize the effect of DKA on blood pressure.
The main limitations of this study are the relatively small sample size, lack of a control group, lack of central venous pressure monitoring (not usual practice at our institution), and lack of continuous arterial blood pressure monitoring in all patients. CT scans were not obtained unless clinically indicated and hence not available for every patient. We used a more severe definition of DKA than the current 2007 criteria for DKA (blood glucose of 200mg/dL). Our study criteria were defined in 2005, prior to the more recent criteria and in accordance with our institutional definition of severe DKA. Finally, weights were not obtained from the same scale at each location, emergency department, hospital ward and clinic, potentially introducing a measurement bias. Pre-illness weights were not available. While there was only one blood pressure reading performed during each outpatient clinic visit, and taking into account the risk of “white coat hypertension”, the true incidence of persistent hypertension in these patients after hospitalization may be overestimated in our sample population. Recent data suggests, however, that single documented episodes of hypertension may, in fact, predispose patients to true hypertension (24). Finally, it is possible that the children who present to our institution are different from those who are treated at other centers and that our findings may not be generalizable to all children with DKA.
In summary, significant hypertension was common in children with severe DKA despite dehydration. Although the etiology of hypertension could not be identified, these data reflect the complex hemodynamic effects of DKA and suggest the need for further studies to decipher the role of hypertension in DKA.
Acknowledgments
The sponsor (NIH) had no involvement in the study design, data collection or any other aspect other than peer review. Drs. Deeter and Vavilala wrote the first draft of the manuscript and no payment was given to anyone to produce the manuscript.
Abbreviations
- ADH
antidiuretic hormone
- CT
computed tomography
- DKA
diabetic ketoacidosis
- GCS
Glasgow Coma Score
- ICP
increased intracranial pressure
- IRB
Institutional Review Board
- SEM
median and standard error of the mean
- SBP
systolic blood pressure
- PICU
pediatric intensive care unit
- NHLBI
National Heart, Lung, and Blood Institute
- PLBW
Percent loss of body weight
References
- 1.Roche EF, Menon A, Gill D, Hoey H. Clinical presentation of type 1 diabetes. Pediatr Diabetes. 2005;6:75–78. doi: 10.1111/j.1399-543X.2005.00110.x. [DOI] [PubMed] [Google Scholar]
- 2.Wolfsdorf J, Craig ME, Daneman D, Dunger D, Edge J, Lee WRW, et al. Diabetic Ketoacidosis. Pediatr Diabetes. 2007;8:28–42. doi: 10.1111/j.1399-5448.2007.00224.x. [DOI] [PubMed] [Google Scholar]
- 3.Wolfsdorf J, Glaser N, Sperling MA. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006;29:1150–1159. doi: 10.2337/diacare.2951150. [DOI] [PubMed] [Google Scholar]
- 4.Glaser N, Barnett P, McCaslin I, et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001;344:264–269. doi: 10.1056/NEJM200101253440404. [DOI] [PubMed] [Google Scholar]
- 5.Brown FK. Cardiovascular effects of acutely raised intracranial pressure. Am J Physiol. 1956;185:510–14. doi: 10.1152/ajplegacy.1956.185.3.510. [DOI] [PubMed] [Google Scholar]
- 6.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl):555–576. 4th Report. [PubMed] [Google Scholar]
- 7.Ralston M, et al., editors. Pediatric Advanced Life Support Provider Manual. American Heart Association, Subcommittee on Pediatric Resuscitation; Dallas: 2006. p. 61. [Google Scholar]
- 8.King CK, Glass R, Bresee JS. Managing acute gastroenteritis among children. Morb Mortal Wkly Rep. 2003;52(RR16):1–16. [PubMed] [Google Scholar]
- 9.Vega RM, Avner JR. A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care. 1997;13(3):179–182. doi: 10.1097/00006565-199706000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Koves IH, Neutze J, Donath S, et al. The accuracy of clinical assessment of dehydration during diabetic ketoacidosis in childhood. Diabetes Care. 2004;27:2485–2487. doi: 10.2337/diacare.27.10.2485. [DOI] [PubMed] [Google Scholar]
- 11.Fagan MJ, Avner J, Khine H. Initial fluid resuscitation for patients with diabetic ketoacidosis: how dry are they? Clin Pediatr (Phila) 2008;47:851–855. doi: 10.1177/0009922808319960. [DOI] [PubMed] [Google Scholar]
- 12.Katz MA. Hyperglycemia-induced hyponatremia--calculation of expected serum sodium depression. N Engl J Med. 1973;289:843–844. doi: 10.1056/NEJM197310182891607. [DOI] [PubMed] [Google Scholar]
- 13.Gennari FJ. Current concepts. Serum osmolality. Uses and limitations. N Engl J Med. 1984;310:102–105. doi: 10.1056/NEJM198401123100207. [DOI] [PubMed] [Google Scholar]
- 14.Steiner MJ, DeWalt DA, Byerley JS. Is This Child Dehydrated? JAMA. 2004;291:2746–2754. doi: 10.1001/jama.291.22.2746. [DOI] [PubMed] [Google Scholar]
- 15.Anand KJS, Phil D, Hickey PR. The neuroanatomy, neurophysiology and neurochemistry of pain, stress and analgesia in newborns and children. Pediatr Clin North Am. 1989;36:795–822. doi: 10.1016/s0031-3955(16)36722-0. [DOI] [PubMed] [Google Scholar]
- 16.Graf CJ, Rossi NP. Catecholamine response to intracranial hypertension. J Neurosurg. 1978;49(6):862–868. doi: 10.3171/jns.1978.49.6.0862. [DOI] [PubMed] [Google Scholar]
- 17.Flynn JT, Tullus K. Severe hypertension in children and adolescents: pathophysiology and treatement. Pediatr Nephrol. 2009;24:1101–1112. doi: 10.1007/s00467-008-1000-1. [DOI] [PubMed] [Google Scholar]
- 18.Umpierrez GE, DiGirolamo M, Tuvlin JA, et al. Differences in metabolic and hormonal milieu in diabetic- and alcohol-induced ketoacidosis. J Crit Care. 2000;15(2):52–59. doi: 10.1053/jcrc.2000.7900. [DOI] [PubMed] [Google Scholar]
- 19.Stentz FB, Umpierrez GE, Cuervo R, Kitabchi AE. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53(8):2079–2086. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 20.Durr JA, Hoffman WH, Hensen J, Sklar AH, el Gammal T, Steinhart CM. Osmoregulation of vasopressin in diabetic ketoacidosis. Am J Physiol. 1990;259(5 Pt 1):E723–8. doi: 10.1152/ajpendo.1990.259.5.E723. [DOI] [PubMed] [Google Scholar]
- 21.Tulassay T, Rascher W, Körner A, Miltényi M. Atrial natriuretic peptide and other vasoactive hormones during treatment of severe diabetic ketoacidosis in children. J Pediatr. 1987;111(3):329–334. doi: 10.1016/s0022-3476(87)80449-3. [DOI] [PubMed] [Google Scholar]
- 22.Roberts JS, Vavilala MS, Schenkman KA, Shaw D, Martin LD, Lam AM. Cerebral hyperemia and impaired cerebral autoregulation associated with diabetic ketoacidosis in critically ill children. Crit Care Med. 2006;34:2217–2223. doi: 10.1097/01.CCM.0000227182.51591.21. [DOI] [PubMed] [Google Scholar]
- 23.Glaser NS, Wootton-Gorges SL, Marcin JP, Buonocore MH, Dicarlo J, Neely EK, et al. Mechanism of cerebral edema in children with diabetic ketoacidosis. J Pediatr. 2004;145:164–71. doi: 10.1016/j.jpeds.2004.03.045. [DOI] [PubMed] [Google Scholar]
- 24.Treiber FA, Musanted L, Kapukua G, Davis C, Litaker M, Davis H. Cardiovascular responsivity (CV) and recovery to acute stress and future CV functioning in youth with family histories of CV disease: a 4-year longitudinal study. Int J Psychophysiol. 2001;41:65–74. doi: 10.1016/s0167-8760(00)00183-5. [DOI] [PubMed] [Google Scholar]