Summary
Children/adolescents with mature B-cell non-Hodgkin lymphoma (B-NHL) have an excellent prognosis but relapses still occur. While chromosomal aberrations and/or clonal immunoglobulin (Ig) gene rearrangements may indicate risk of failure, a more universal approach was developed to detect minimal disease (MD). Children/adolescents with intermediate-risk B-NHL were treated with French-British-American/Lymphome Malins de Burkitt 96 (FAB/LMB96) B4 modified chemotherapy and rituximab. Specimens from diagnosis, end of induction (EOI), and end of therapy (EOT) were assayed for MD. Initial specimens were screened for IGHV family usage with primer pools followed by individual primers to identify MD. Thirty-two diagnostic/staging specimens screened positive with primer pools and unique IGHV family primers were identified. Two patients relapsed; first relapse (4 months from diagnosis) was MD-positive at EOI, the second (36 months from diagnosis) was MD-positive at EOT. At EOI, recurrent rates were similar between the MRD-positive and MRD-negative patients (p=0.40). At EOT, only 13/32 patients had MRD data available with 1 relapse in the MRD-positive group and no recurrences in the MRD-negative group (p=0.077). The study demonstrated molecular-disseminated disease in which IgIGHV primer pools could be used to assess MD. This feasibility study supports future investigations to assess the validity and significance of MD screening in a larger cohort of patients with intermediate-risk mature B-NHL.
Keywords: non-Hodgkin lymphoma, minimal residual disease, lymphoma, polymerase chain reaction, immunoglobulin rearrangement
Introduction
The recent results of the first international randomized study (French-British-American/Lymphome Malins de Burkitt 96 [FAB/LMB 96]) in children and adolescents with intermediate-risk mature B-cell non-Hodgkin lymphoma (Stages I-IV) (B-NHL) reported a 4-year event-free survival (EFS) and overall survival (OS) of 90.2 and 92.7%, respectively (Gerrard et al 2008, Patte et al 2007). The randomized study demonstrated that intermediate risk patients could receive reduced alkylator exposure and reduced period of therapy without diminishment in EFS. In spite of the excellent results, patients with advanced B-NHL (bone marrow [BM] involvement ≥25% blasts, B cell acute lymphoblastic leukaemia [B-ALL]; ± central nervous system [CNS] involvement) do less well with 4-year EFS and OS of 79±3 and 82±3%, respectively (Cairo et al 2007). Additionally, patients with recurrent or refractory disease (regardless of initial therapy stratification) have poor salvage and survival rates (< 30%). The next major advances hypothesized to improved prognosis in childhood B-NHL, second to newer targeted therapies, may lie in preemptively identifying patients at risk for relapse. From the international FAB/LMB96 adolescent and childhood B-NHL study, a poor radiographic and/or BM response to a 7 day reduction phase was shown to portend significantly inferior EFS, even with escalation of therapy in poor responders (Gerrard et al 2008, Patte et al 2007). In addition, recurrent cytogenetic abnormalities, including R8q24, +7q and del(13q), were significantly associated with an inferior EFS, suggesting that cytogenetic risk-adapted therapy in childhood mature B-NHL might be an important consideration for the future (Poirel et al 2009). Another potentially important strategy to identify children at risk for relapse focuses on detecting minimal disease (MD). However, one challenge in addressing MD is access to original tumour or diagnostic tissue (Sabesan et al 2003, Stark et al 2009). During normal B-cell ontogeny, a distinct IGHV family usage occurs by immunoglobulin (Ig) gene rearrangements through assembling distinct variable (V), diversity (D), and joining (J) gene segments. Therefore, we developed a more universal approach by exploiting the IGHV family usage of each patient's malignant B-cell clone (Cook and Tomlinson 1995). The current study was designed to test the feasibility of assessing MD using IgIGHV primer pools in a pilot Phase II study (Children’s Oncology Group Advanced B-Cell Leukemia/ Lymphoma [COG ANHL] 01P1) which added rituximab to the induction and consolidation phases of FAB Group B4 therapy from CCG5961 to patients with Stage III/IV intermediate-risk mature B-NHL (Cairo et al 2007).
Methods
Patients and Specimens
The study was reviewed and approved by the University of Hawaii Institutional Review Board (IRB) to analyse specimens sent to the reference laboratory as part of the Phase II study for children and adolescents with mature B-NHL, COG ANHL01P1, “A Pilot Study to Determine the Toxicity of the Addition of Rituximab to the Induction and Consolidation Phases and the Addition of Rasburicase to the Reduction Phase in Children with Newly Diagnosed Advanced B-Cell Leukaemia/Lymphoma Treated with LMB/FAB Therapy”. All patients and families signed institution-specific IRB-approved informed consent prior to entry into the study. From ANHL01P1, 45 evaluable children and adolescents ≤21 years of age with newly diagnosed B-NHL were enrolled onto the Group B therapy arm (Cairo et al 2008; Cairo et al 2010; Goldman et al 2008). Staging was performed as described by Murphy (1980), where abdominal tumours cannot be stage I. Risk classification was defined as low risk (Group A), which included resected stage I and abdominal completely resected stage II, high risk (Group C) with BM disease (<25% L3 blasts) and/or central nervous system (CNS) disease, and intermediate risk (Group B), which included all others not included in Groups A or C (Patte et al 2001). Patients diagnosed with mature CD20 + B cell lymphoma, including diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma (BL), or primary mediastinal B-cell lymphoma (PMBL) were eligible for inclusion into the research protocol. All eligible patients had Group B disease (intermediate-risk), however the study, which tested the safety of rituximab, was restricted to St. Jude stages III/IV only (rituximab was generously supplied by Genentech, South San Francisco, CA). Therapy consisted of FAB Group B therapy as previously described (Gerrard et al 2008, Patte et al 2007). Briefly, patients received cyclophosphamide, vincristine and prednisone (COP), followed by two cycles cyclophosphamide, vincristine, prednisone, doxorubicin and high-dose methotrexate (COPADM 1 + 2), as part of the induction phase of chemotherapy. At the end of induction (EOI), patients received 2 cytarabine and methotrexate consolidation cycles as previously reported to complete therapy (end of therapy, EOT) (Gerrard et al 2008; Patte et al 2007). The institutions that enrolled patients were encouraged to send diagnostic tissue and staging specimens (pre-treatment BM; pre-treatment peripheral whole blood [PB]) to the reference laboratory as well as follow-up specimens from a second evaluation time at EOI; and third evaluation time point (EOT) (Cairo et al 2008; Cairo et al 2010; Goldman et al 2008).
Minimal Disease (MD) Assessment
Specimens were requested at diagnosis, EOI and EOT and were sent by express to the central reference laboratory, where they were processed immediately upon arrival and batch-processed for DNA isolation. DNA was extracted from primary diagnostic tumour tissue and staging/follow-up specimens (PB, BM) from paraffin-embedded diagnostic tissue and unstained PB/BM/tissue slides using nucleic acid isolation kits (Qiagen, Valencia, CA) and assessed by ultraviolet spectrophotometry and HBB (5’-GAA GAG CCA AGG ACA GGT AC-3’ and 5’-CAA CTT CAT CCA CGT TCA CC-3’) polymerase chain reaction (PCR) for quality. Diagnostic or staging specimen DNA was screened for IGHV family usage with the following primer pools: VH1/VH2; VH3/VH4; VH5, VH6/VH7 as previously described (Agsalda et al 2009). Briefly, 5’-primers from the FR1 region of the variable IGH regions, IGHV1-IGHV7 (Operon Biotechnologies, Inc, Huntsville, AL) were combined to form three sets of primer pools: IGHV1 & IGHV2; IGHV3 & IGHV4; IGHV5, IGHV6, & IGHV7 (Table I). The two consensus 3’-primers were: IGL@-IGHJ@(5’-VLJH TGA GGA GAC GGT GAC C-3’) and IGLV@-IGHJ@ (5’-GTG ACC AGG GNC CTT GGC CCC AG-3’). Semi-nested real-time PCR was performed in triplicate using a StepOnePlus thermal cycler (Applied Biosystems, Foster City, CA). Reactions were set up with 2× iQ SYBR Green Supermix (Biorad Laboratories, Hercules, CA), 10 pmol 5’-IgIGHV primer pools and 10 pmol 3’-primer (LJH) and parameters: 95°C/3 min; 35 cycles 95°C/10 s, 60°C/30 s; 72°C/3 min; final extension of 72°C/3 min. PCR products were purified using ExoSAP-it (USB Corp, Cleveland, OH) and recovered in 10 µl. An aliquot of 1 µl was used for second-round PCR with 10 pmol 5’-primer pools and 10 pmol 3’-primer (IGLV@-IGHJ@). Following threshold-dependent cycling, melt curves were generated from 60°C to 95°C at either 0.5 °C/s or 0.1 °C/s melt rates (Agsalda et al 2009). As previously described, melt curves were characterized based on height and width: 1) positive MD with peak height ≥ width at half height; 2) negative MD with peak height ≤ width at half height; or 3) equivocal MD with multiple peaks (Agsalda et al 2009). Appropriate positive and negative control DNA was included in every assay.
Table I.
IGHV Primer Pool Sequences
| Primer Pools | Primer | Sequence (5’-3’) |
|---|---|---|
| IGHV1/IGHV2 | IGHV1a | CAG GT(GT) CAG CTG GTG CAG |
| IGHV1b | CAG GTC CAG CTT GTG CAG | |
| IGHV1c | (GC)AG GTC CAG CTG GTA CAG | |
| IGHV1d | CA(AG) ATG CAG CTG GTG CAG | |
| IGHV2a | CAG ATC ACC TTG AAG GAG | |
| IGHV2b | CAG GTC ACC TTG A(AG)G GAG | |
| IGHV3/IGHV4 | IGHV3a | GA(AG) GTG CAG CTG GTG GAG |
| IGHV3b | CAG GTG CAG CTG GTG GAG | |
| IGHV3c | GAG GTG CAG CTG TTG GAG | |
| IGHV4a | CAG (CG)TG CAG CTG CAG GAG | |
| IGHV4b | CAG (CG)TG CAG CTG CAG GAG | |
| IGHV5 | IGHV5a | GA(AG) GTG CAG CTG GTG CAG |
| IGHV6/IGHV7 | IGHV6a | CAG GTA CAG CTG CAG CAG |
| IGHV7a | CAG GT(CG) CAG CTG GTG CAA | |
The algorithm was set up for follow-up analysis if primer pool reactions were positive. For positive results, individual IGHV primers were used to separately reassess the specimen DNA to identify the variable region involved. Individual IGHV primers were then used to assess MD on follow-up specimens from the same patient. As previously noted, the sensitivity of the assay demonstrated the ability to detect 1 tumour cell per 105 normal cells (Agsalda et al 2009). Specimens were analysed blindly without knowledge of the clinical status of the patients.
Statistical Analysis
Fisher's exact test was used to compare MD-positive and MD-negative results at EOI and EOT with remission/relapse status.
Results
Of the 45 eligible children and adolescents treated on the Group B arm, specimens from 32 patients were available for analysis which included diagnostic and at least one follow-up specimen (Cairo et al 2008; Cairo et al 2010; Goldman et al 2008). The clinical research protocol did not require submission of biology study specimens to assess MD assessments. Therefore, institutions that were able to submit specimens to the research laboratory are reflected in the 32 eligible patients (DLBCL, n=8; BL, n=22; PMBL, n=2).
We previously reported the 2-year EFS and OS of the cohort to be 93% and 96%, respectively, details of which are reviewed under separate cover (Cairo et al 2010). From the 32 MD-evaluable patients, 12 had diagnostic tumour tissues (Stage III DLBCL, n=3; Stage III BL, n=6; Stage IV BL, n=2; Stage III PMBL, n=1) available, which were verified to be positive with the primer pools. For those without diagnostic tumour tissue available, 2 patients with <25% BM blasts at diagnosis had successful primers made from PB and BM. An additional 18 patients had histologically-tumour-negative PB or BM at diagnosis but when assayed with the IGHV primer pools, the specimens were MD-positive. These 20 patients with only PB and/or BM at diagnosis were Stage III DLBCL n=4; Stage IV DLBCL n=1; Stage III BL n=13; Stage IV BL n-1; and Stage III PMBL n=1. From all of the 32 patients it was possible to positively-screen diagnostic and staging specimens to identify IGHV family usage from the 12 primary tumours and the 20 BM and/or PB specimens where primary tumour tissues were unavailable. The 12 cases with primary tumour tissues had IGHV family usage identified from the primary tumours and the same IGHV family was identified from histologically-tumour-negative PB or BM specimens from the same patients. Of significance, even though the remaining 20 patients did not have primary tumour tissues available, corresponding staging specimens adequately provided IGHV family usage identification that could be used as tumour markers to assess MD. IGHV family usage was successfully identified from all three mature B-NHL histological subtypes including BL (69%), DLBCL (25%) and PMBL(6%).
The identified unique primary IGHV family primers were used as markers to monitor the follow-up specimens for MD from each respective subject. The number of specimens available for MD assessment at the three time points (diagnosis, EOI, and EOT) varied and was dependent on the institution’s ability to obtain and send the specimens, given that submission of specimens for MD analysis was not required (Fig. 1). There were 32/32 patients who had PB/BM from diagnosis, 32/32 who had EOI specimens, and 13/32 who had EOT specimens (Fig. 1). Thirty patients completed chemotherapy and were in continued remission with a median follow-up of approximately 48 months.
Figure 1.
Minimal Disease (MD) Assay Time Points. At diagnosis, 32 subjects had specimens available for MD analysis, which were all MD-positive. At end of induction (EOI), 7 of 33 specimens were MD-positive; of which 6 became MD-negative at end of therapy (EOT); the other patient with the MD-positive EOI specimen relapsed at 4 months and did not have an EOT specimen available. Only 13 EOT specimens were available for MD assessment, of which 1 was MD-positive and this patient had a clinical relapse at 36 months.
From the 32 EOI specimens, there were 7 patients who were MD-positive at the EOI time point. By EOT, 6 of these 7 patients cleared their disease, had MD-negative specimens, and remained in clinical remission. The other patient who had an MD-positive EOI specimen did not have an EOT specimen available for analysis and relapsed 4 months after diagnosis.
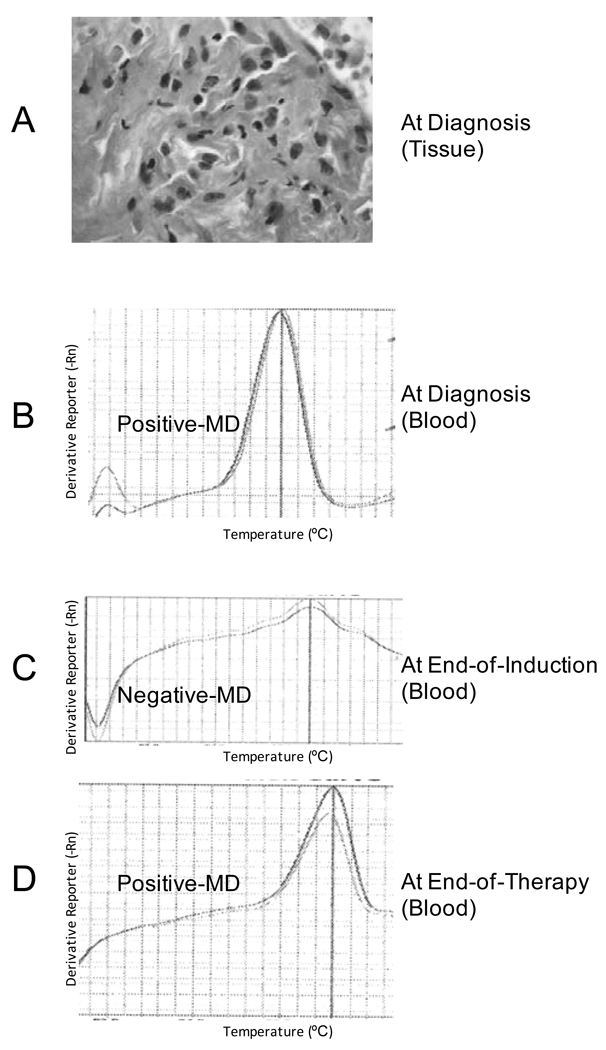
There were only 13 patients from whom EOT specimens were available for MD assessment (Fig. 1). Twelve of these 13 patients had MD-negative EOT specimens (Fig. 1). The other EOT specimen was MD-positive and the patient relapsed 36 months from the time of diagnosis (Fig. 1 and Fig. 2). This patient previously had an MD-negative specimen at EOI, suggesting clearance of disease after induction but a reappearance of disease at EOT. Figure 2 highlights the positive-melt curves for the patient with Stage III/ DLBCL who relapsed at 36 months from the diagnostic blood and EOT blood specimens with the negative-melt curve from the intervening blood specimen at EOI.
Figure 2.
Histology and Minimal Disease (MD) Melt Curves and Associated Amplified Products from a patient with Stage III/ Diffuse Large B-cell Lymphoma (DLBCL). Panel A: Haematoxylin and eosin stain of DLBCL tissue at diagnosis. Original magnification ×400; Panel B: Amplified product and positive-melt curve from diagnostic blood; Panel C: Negative-melt curve of from blood specimen at end of induction (EOI); Panel D: Positive melt curve of amplified product from blood specimen at end of therapy (EOT).
In summary, the two patients who relapsed had MD-positive specimens on their last available specimens. All of the evaluable patients had specimens assayed at EOI but only 13 of the 32 patients had specimens at EOT to analyse for MD. At EOI, the recurrent rates were similar between the MRD-positive and MRD-negative patients. One out of seven recurred in the MRD-positive group and 1 out of 25 recurred in the MRD negative group (p=0.40). At EOT, only 13 patients had MRD data available. 1 out of 1 recurred in the MRD-positive group while 0 out of 12 recurred in the MRD-negative group (p=0.077), which is marginally significant.
Discussion
We found molecular evidence of disseminated disease in PB and/or BM at diagnosis in all studied children and adolescents with intermediate-risk (St. Jude Stage III/IV) B-NHL (DLBCL, BL, and PMBL). The MD assay was feasible in the different B-NHL subtypes that were eligible for treatment on the study. Although only 3/32 of these patients had morphological presence of disease in the BM at diagnosis, each of the 32 patients was found to have MD-positive specimens by PCR at diagnosis. The findings are similar to those reported by Coustan-Smith et al (2009), where more than two thirds of children with T-cell lymphoblastic lymphoma had sub-microscopic disseminated disease at diagnosis by flow cytometry (Coustan-Smith et al 2009). We report that, even in the absence of peripheral blasts and BM involvement, MD was detected by PCR in at least one of the diagnostic staging PB and/or BM specimens. Our ability to capture 100% of one of the staging/diagnostic specimens and identify a marker that can be used to assess MD may bode well as a clinical tool for future prospects (Agsalda et al 2009; Lo Nigro et al 2004; Lovisa et al 2009). As the current study was limited to Group B Stage III/IV patients, similar investigations in children and adolescents with Group A and Group B Stage I/II B-NHL remain to be completed. In the current study, diagnostic tumour tissue and/or staging specimens from 32 of 32 children and adolescents with mature B-cell NHL provided an adequate amount of material to be screened for a unique IGHV family primer. While the total number of cases was low, the data suggest that MD-negative EOT specimens may identify patients who are ultimately cured, as there were no recurrences among patients with MD-negative EOT specimens (n=12) (Fig. 1). Even though all of the patients had specimens available for analysis at the EOI, the total numbers were still low and showed that 1/7 MD-positive patients and 1/25 MD-negative patients relapsed. Thus evaluation of early response to therapy at EOI in this small study appeared to have less significance, in contrast to what is reported in childhood ALL (Bruggemann et al 2010; Cui et al 2010). While we hypothesize that EOT assessment may be an important time point in B-NHL therapy that predicts outcome, the low number of EOT specimens available from the current study precluded any conclusions to be made. Thus a larger study with samples routinely collected at all times points is necessary in order to draw any conclusions about whether EOI and/or EOT positivity is predictive of outcome.
The IGHV primer pool method described in the current study provided a means to assess MD that was potentially more efficient and more manageable than other methods requiring diagnostic tissue (Mussolin et al 2003; Sabesan et al 2003), and similar to the study by Lovisa et al (2009) in which patient-specific IGH@and IGK@ light-chain rearrangements were used as the target sequences. One group reported use of MD assays in patients with B-cell NHL (Mussolin et al 2003; Mussolin et al 2007). In a prospective analysis of MD in paediatric BL cases, Mussolin et al (2003) applied a long-distance PCR (LD-PCR) assay that detected the t(8;14) in BM samples and showed that LD-PCR could be used to study MD in BL (Mussolin et al 2003). In an attempt to identify target Ig gene arrangements in paediatric NHL cases that were t(8;14)-negative, Lovisa et al (2009) evaluated characteristics of patient-specific Ig gene rearrangements as targets for MD analysis. They found that a single sensitive Ig target in the great majority of BL cases could be used as a tool to measure MD, which was similar to the concept reported by Agsalda et al (2009) in measuring MD in paediatric BL cases.
Our approach was particularly useful when diagnostic tumour tissue was not available because the MD assay was able to detect disease in either BM or blood in 100% of the patients at diagnosis, suggesting disseminated disease at diagnosis. The current study provides data in support of larger prospective studies to assess the utility and significance of MD tools in B-NHL. Based on our result we propose testing a larger number of children and adolescents with convenient blood-based MD assessment at diagnosis, EOI, and EOT to further define the positive and negative predictive value of MD at EOI and EOT. As the chemo-immunotherapy in this cohort is relatively short (3–4 months) it may be possible to salvage MD-positive EOT intermediate-risk patients with further intensification, such as high-dose cytarabine with etoposide/rituximab consolidation utilized for Group C patients. Currently, only patients with histologically-proven residual disease at this time point receive this therapy. Finally, MD at EOT may prove to be a useful predictive surrogate in defining the efficacy of immunotherapy in future planned randomized rituximab trials.
Acknowledgements
Bruce Shiramizu designed and performed the research and wrote the paper, Stanton Goldman designed the research study, analysed the data, and wrote the paper, Ian Kusao performed the research and wrote the paper, Melissa Agsalda performed the research and wrote the paper, James Lynch analysed the data, Lynette Smith analysed the data and wrote the paper, Lauren Harrison wrote the paper, Erin Morris wrote the paper, Thomas G. Gross designed the research study, Warren Sanger designed the research study and wrote the paper, Sherrie Perkins designed the research study and wrote the paper, and Mitchell S. Cairo designed the research study, analysed the data, and wrote the paper. The authors would also like to thank Ms. Amber Quilatan, and Erin Morris, RN for their editorial assistance in the development of this manuscript.
The study was supported by NIH CA121955 (BS, IK, MA), P20RR011091 (BS, IK, MA), and grants from Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Department of Health and Human Services (COG Chair’s Grant U10 CA98543-08 and Statistics and Data Center Grant U10 CA98413-08).
Footnotes
Presented in part at the American Society of Clinical Oncology Meeting, June 2010, Chicago, IL
CONFLICT OF INTEREST DISCLOSURE: T.G. is on the scientific advisory board for Roche. All other authors declare no conflict of interest.
REFERENCES
- Agsalda M, Kusao I, Troelstrup D, Shiramizu B. Screening for Residual Disease in Pediatric Burkitt Lymphoma Using Consensus Primer Pools. Adv Hematol. 2009;2009:412163. doi: 10.1155/2009/412163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruggemann M, Schrauder A, Raff T, Pfeifer H, Dworzak M, Ottmann OG, Asnafi V, Baruchel A, Bassan R, Benoit Y, Biondi A, Cave H, Dombret H, Fielding AK, Foa R, Gokbuget N, Goldstone AH, Goulden N, Henze G, Hoelzer D, Janka-Schaub GE, Macintyre EA, Pieters R, Rambaldi A, Ribera JM, Schmiegelow K, Spinelli O, Stary J, von Stackelberg A, Kneba M, Schrappe M, van Dongen JJ. Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18-20 September 2008. Leukemia. 2010;24:521–535. doi: 10.1038/leu.2009.268. [DOI] [PubMed] [Google Scholar]
- Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J, Weston C, Perkins SL, Raphael M, McCarthy K, Patte C. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109:2736–2743. doi: 10.1182/blood-2006-07-036665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairo M, Lynch J, Harrison L, Van de Ven C, Gross T, Shiramizu B, Sanger W, Perkins S, Goldman S. Safety, efficacy and rituximab levels following chemoimmunotherapy (rituximab + FAB chemotherapy) in children and adolescents with mature B-cell non-Hodgkin lymphoma (B-NHL): a Children’s Oncology Group report. Blood (ASH meeting abstracts) 2008;112:838a. [Google Scholar]
- Cairo MS, Lynch JC, Harrison L, Perkins SL, Shiramizu B, Gross TG, Sanger W, Goldman S. Safety, kinetics, and outcome following rituximab (R) in combination with FAB chemotherapy in children and adolescents (C+A) with stage III/IV (Group B) and BM+/CNS+ (Group C) mature B-NHL: A Children's Oncology Group report. J Clin Oncol (Meeting Abstracts) 2010;28 9536-. [Google Scholar]
- Cook GP, Tomlinson IM. The human immunoglobulin VH repertoire. Immunol Today. 1995;16:237–242. doi: 10.1016/0167-5699(95)80166-9. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Sandlund JT, Perkins SL, Chen H, Chang M, Abromowitch M, Campana D. Minimal disseminated disease in childhood T-cell lymphoblastic lymphoma: a report from the children's oncology group. J Clin Oncol. 2009;27:3533–3539. doi: 10.1200/JCO.2008.21.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Li Z, Wu M, Li W, Gao C, Deng G. Combined analysis of minimal residual disease at two time points and its value for risk stratification in childhood B-lineage acute lymphoblastic leukemia. Leuk Res. 2010;34:1314–1319. doi: 10.1016/j.leukres.2009.11.031. [DOI] [PubMed] [Google Scholar]
- Gerrard M, Cairo MS, Weston C, Auperin A, Pinkerton R, Lambilliote A, Sposto R, McCarthy K, Lacombe MJ, Perkins SL, Patte C. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin's lymphoma: results of the FAB/LMB 96 international study. Br J Haematol. 2008;141:840–847. doi: 10.1111/j.1365-2141.2008.07144.x. [DOI] [PubMed] [Google Scholar]
- Goldman S, Lynch J, Davenport V, Perkins S, Shiramizu B, Sanger W, Gross T, Harrison L, Bancroft M, Cairo MS. Preliminary results of a phase II study of chemoimmunotherapy (rituximab + FAB chemotherapy) in children and adolescents with intermediate risk B-cell NHL: a Children’s Oncology Group report. Annals of Oncology. 2008;12:084a. [Google Scholar]
- Lo Nigro L, Sainati L, Mirabile E, Lanciotti M, Poli A, Leszl A, Basso G. Association of cytogenetic abnormalities with detection of BCR-ABL fusion transcripts in children with T-lineage lymphoproliferative diseases (T-ALL and T-NHL) Pediatr Blood Cancer. 2004;42:278–280. doi: 10.1002/pbc.10453. [DOI] [PubMed] [Google Scholar]
- Lovisa F, Mussolin L, Corral L, Pillon M, Cazzaniga G, Biondi A, Rosolen A. IGH and IGK gene rearrangements as PCR targets for pediatric Burkitt's lymphoma and mature B-ALL MRD analysis. Lab Invest. 2009;89:1182–1186. doi: 10.1038/labinvest.2009.81. [DOI] [PubMed] [Google Scholar]
- Murphy SB. Classification, staging and end results of treatment of childhood non-Hodgkin's lymphomas: dissimilarities from lymphomas in adults. Semin Oncol. 1980;7:332–339. [PubMed] [Google Scholar]
- Mussolin L, Basso K, Pillon M, D'Amore ES, Lombardi A, Luzzatto L, Zanesco L, Rosolen A. Prospective analysis of minimal bone marrow infiltration in pediatric Burkitt's lymphomas by long-distance polymerase chain reaction for t(8;14)(q24;q32) Leukemia. 2003;17:585–589. doi: 10.1038/sj.leu.2402828. [DOI] [PubMed] [Google Scholar]
- Mussolin L, Pillon M, Conter V, Piglione M, Lo Nigro L, Pierani P, Micalizzi C, Buffardi S, Basso G, Zanesco L, Rosolen A. Prognostic role of minimal residual disease in mature B-cell acute lymphoblastic leukemia of childhood. J Clin Oncol. 2007;25:5254–5261. doi: 10.1200/JCO.2007.11.3159. [DOI] [PubMed] [Google Scholar]
- Patte C, Auperin A, Michon J, Behrendt H, Leverger G, Frappaz D, Lutz P, Coze C, Perel Y, Raphael M, Terrier-Lacombe MJ. The Societe Francaise d'Oncologie Pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97:3370–3379. doi: 10.1182/blood.v97.11.3370. [DOI] [PubMed] [Google Scholar]
- Patte C, Auperin A, Gerrard M, Michon J, Pinkerton R, Sposto R, Weston C, Raphael M, Perkins SL, McCarthy K, Cairo MS. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109:2773–2780. doi: 10.1182/blood-2006-07-036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirel HA, Cairo MS, Heerema NA, Swansbury J, Auperin A, Launay E, Sanger WG, Talley P, Perkins SL, Raphael M, McCarthy K, Sposto R, Gerrard M, Bernheim A, Patte C. Specific cytogenetic abnormalities are associated with a significantly inferior outcome in children and adolescents with mature B-cell non-Hodgkin's lymphoma: results of the FAB/LMB 96 international study. Leukemia. 2009;23:323–331. doi: 10.1038/leu.2008.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabesan V, Cairo MS, Lones MA, Perkins SL, Morris E, Sposto R, Van De Ven C, Shiramizu B. Assessment of minimal residual disease in childhood non-hodgkin lymphoma by polymerase chain reaction using patient-specific primers. J Pediatr Hematol Oncol. 2003;25:109–113. doi: 10.1097/00043426-200302000-00005. [DOI] [PubMed] [Google Scholar]
- Stark B, Avigad S, Luria D, Manor S, Reshef-Ronen T, Avrahami G, Yaniv I. Bone marrow minimal disseminated disease (MDD) and minimal residual disease (MRD) in childhood T-cell lymphoblastic lymphoma stage III, detected by flow cytometry (FC) and real-time quantitative polymerase chain reaction (RQ-PCR) Pediatr Blood Cancer. 2009;52:20–25. doi: 10.1002/pbc.21823. [DOI] [PubMed] [Google Scholar]