Abstract
Background
Alcohol consumption is influenced by specific genetic risk factors for alcohol use disorders (AUDs), non-specific genetic risk factors for externalizing behaviors and various environmental experiences. We have limited knowledge of how these risk factors inter-relate through development.
Method
Retrospective assessments in 1796 adult male twins using a life history calendar of key environmental exposures and alcohol consumption from early adolescence to mid-adulthood. Analysis by linear mixed models.
Results
The importance of non-specific genetic risk factors on maximal alcohol consumption rose rapidly in early to mid-adolescence, peaked at ages 15–17 years and then declined slowly. Alcohol-specific genetic risk factors increased slowly in influence through mid-adulthood. We detected robust evidence for environmental moderation of genetic effects on alcohol consumption that was more pronounced in early and mid-adolescence than in later periods. Alcohol availability, peer deviance and low prosocial behaviors showing the strongest moderation effects. More interactions with environmental risk factors were seen for the non-specific externalizing disorder risk than for specific genetic risk for AUDs.
Conclusions
The impact of specific and non-specific genetic influences on alcohol consumption have different development trajectories. Genetic effects on alcohol use are more pronounced when social constraints are minimized (e.g. low prosocial behaviors or parental monitoring) or when the environment permits easy access to alcohol and/or encourages its use (e.g. high alcohol availability or peer deviance). Gene–environment interactions influencing alcohol intake may be more robust at younger ages, indicating greater plasticity of genetic influences early in the development of drinking patterns.
Keywords: Adolescence, alcohol, genetics, gene–environment interaction
Introduction
The risk for alcohol use disorders (AUDs) and high alcohol consumption are influenced by genetic factors that are specific to alcohol (Kendler et al. 2003; Hicks et al. 2004; Macgregor et al. 2009) and by a broader genetic susceptibility to externalizing disorders/traits (Kendler et al. 2003; Hicks et al. 2004, 2007). We understand little, however, about the etiologic role of these two classes of genetic risk factors on alcohol-related behaviors across development. The first goal of this study was to examine the impact of specific and non-specific alcohol-related genetic risk factors on alcohol consumption in males from late childhood to mid-adulthood. This is a crucial time in the drinking history, as it is generally during this period that alcohol is initiated and regular patterns of use established.
Environmental exposures can moderate the impact of genetic risk factors on a wide range of psychopathology (Kendler & Eaves, 1986; Moffitt, 2005; Rutter et al. 2006), including alcohol-related traits (Cloninger et al. 1981; Sigvardsson et al. 1996; Dick et al. 2001; Rose et al. 2001; Martens et al. 2007; Viken et al. 2007). However, with a recent exception (Hicks et al. 2009), such studies have typically explored one environmental exposure during one developmental period. Therefore, the secondary goal of this study was to determine whether, across adolescence, the impacts on alcohol consumption of (i) specific alcohol-related genetic risk factors and (ii) non-specific externalizing genetic risk factors are moderated by six relevant environmental exposures: alcohol availability, church attendance, peer group deviance, prosocial behaviors, parental bonding, and parental monitoring. We predicted that, during early adolescence, individuals at elevated genetic risk would be particularly sensitive to environments, which can act either as social constraints on or social triggers of alcohol consumption (Kendler, 2001; Shanahan & Hofer, 2005).
Method
This study used data from the third wave of interviews of adult male–male twin pairs from the Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (Kendler & Prescott, 2006), and originally contained male–male and male–female twin pairs born between 1940 and 1974. Initially, 6814 twins participated in the first wave of interviews (MM1) (participation rate=72.4%). An 82.6% follow-up response rate was obtained for the wave 2 interviews (MM2). The third interview wave (MM3) (1998–2004) was completed solely with members of male–male twin pairs, with interviews completed by 1796 twins, representing 75.1% of the entire cohort and 77.8% of those eligible (excluding those who died and were lost to follow-up). Subjects were aged 24–62 years (mean age=40.3 years, S.D.=9.0). The sample for the present analysis consisted of 469 monozygotic (MZ) and 287 dizygotic (DZ) twin pairs (including two triplet sets subdivided into all possible pairings) and 290 twins whose co-twin did not participate.
Most subjects were interviewed by telephone. After a complete explanation of the research protocol, verbal or signed informed consent was obtained before all interviews. This project was approved by the Committee for the Conduct of Human Research at Virginia Commonwealth University. Interviewers had a Master’s degree in a mental health-related field or a Bachelor’s degree in this area and at least 2 years of clinical experience. Each member of a twin pair was always interviewed by a different interviewer. Zygosity was determined by a combination of self-report measures, photographs and DNA polymorphisms (Kendler et al. 2000).
Assessment
To improve accuracy of retrospective recall, we used a life history calendar interview (Freedman et al. 1988) that assessed a range of relevant constructs for five age periods: 8–11, 12–14, 15–17, 18–21, and 22–25 years. These periods were assessed sequentially after the development of a calendar tracing major developmental events from ages 1–30 years. Interviewers began each new period with specific memory prompts taken from events in the calendar, thereby cuing the respondent into the relevant ‘memory files’. For variables assessed at the MM3 interview, test–retest reliability was assessed from evaluations of 141 subjects interviewed an average of 29 days apart.
Genetic risk for AUDs was indexed from the history of alcohol abuse and dependence in the subject’s parents and co-twin, based on interviews with the co-twin, and family history reports about parents and co-twins obtained from the index twin (Muffler et al. 1991). The contribution of each measure to the total AUD risk was based on a modified ridit score, which is useful for combining scores from binary and ordinal variables with different numbers of levels and different prevalence rates. Each ordinal level is assigned a score that would be the middle score for that level on a uniform distribution of 0–1. For example, an ordinal variable with prevalence rates of 40, 30, 20 and 10% (0–40, 40–70, 70–90 and 90–100%) would be scored 0.20, 0.55, 0.80 and 0.95 respectively. Scores from the MZ co-twins were unmodified from the 0–1 scale whereas scores from the DZ co-twins and parents were adjusted half way back to the mean score of 0.5 (thus based on a uniform distribution on 0.25–0.75) to reflect the fact that MZ twins are twice as similar genetically as DZ twin or parent–offspring pairs. In effect, this results in scores from MZ co-twins being weighted twice as strongly as scores from DZ co-twins or parents.
The genetic risk for externalizing disorders was a composite measure of the co-twin self-report symptoms of DSM-IV conduct disorder (obtained at MM1 and MM2) and antisocial personality disorder (MM2) (APA, 1994), and twin report of antisocial personality disorder in their co-twin and father using Family History-Research Diagnostic Criteria (Muffler et al. 1991) (MM2). The contribution of each measure to the total genetic risk for externalizing disorders was based on the modified ridit score as described above. The product-moment correlation between our measures of the genetic risk for AUDs and for externalizing disorders was +0.39.
Church attendance was measured by the response to the item ‘When you were between the ages of x and y, how often would you attend religious services ?’ asked in our MM3 interview for ages 12–14 and 15–17. Response options were ‘more than once a week’, ‘once a week’, ‘a few times a month’, ‘once a month’, ‘less than once a month’ and ‘never’. Test–retest reliability, as assessed by the intraclass correlation coefficient (ICC), of this variable for these two time periods was +0.77 and +0.83 respectively.
Parental monitoring was assessed as the sum of three items asked (MM3) for ages 12–14 and 15–17 based on previous work examining parental effects on risk of drug use and delinquency (Steinberg et al. 1994; Kerr & Stattin, 2000): how much parents really knew about: who the twin’s friends were, how the twin spent his money, what he did with his free time. Response options were they ‘didn’t know’, ‘knew a little’ or ‘knew a lot’. Reliability (ICC) of this variable for these two time periods was +0.75 and +0.80 respectively.
Peer group deviance was assessed by two validated instruments (Johnston et al. 1982; Tarter & Hegedus, 1991) that evaluated the proportion of the respondent’s friends, at ages 12–14, 15–17, 18–21 and 22–25, who were engaged in a range of deviant behaviors (MM3) (see Kendler et al. 2007 for details). Test–retest reliability (ICC) was, for the four age groups, +0.75, +0.81, +0.78 and +0.73 respectively. Alcohol availability was assessed by a single item from the Monitoring the Future study (Johnston et al. 1982), which asked subjects at ages 12–14, 15–17 and 18–21, on a four-point scale, how easy it would have been to get alcohol if they wanted to use it. Reliability (ICC) of this item at these three time periods was +0.65, +0.70 and +0.62 respectively.
Parent–child bonding was assessed by the sum of two items asking for the average frequency of ‘unpleasant disagreements or conflicts’ (reverse coded) separately with mother and with father and the overall emotional closeness felt between the subject and his mother and father at ages 12–14 and 15–17. Reliability (ICC) of this assessment at both time periods equaled +0.83.
Prosocial behavior was assessed at ages 12–14 and 15–17 by the sum of responses to four items assessing frequency of participation in ‘organized sports activities’, ‘school activities like clubs and bands’, ‘community activities like YMCA and scouting’ and ‘church activities like Sunday school or a church youth group’. Reliability (ICC) of this variable at these two time periods equaled +0.88 and +0.89 respectively.
Church attendance, parental monitoring, parent–child bonding and prosocial behaviors were all measured up to age 17 and most subjects were living in the home of origin. Alcohol availability was assessed up to age 21 when it becomes legally available. Only peer deviance could be meaningfully assessed up to age 25.
Following the methods of Cohen et al. (2003), the actual calendar completed with the subjects contained columns for each year of the subject’s life. The first rows, completed early in the interview, documented key changes in living situation in addition to major educational, employment and interpersonal mile-stones. Toward the end of the interview, after completion of drug sections assessing standard questions about age at first use, maximal lifetime use and symptoms of abuse and dependence, we returned to the calendar. For alcohol, we asked subjects, starting with the age at which they reported using alcohol, the average number of times per month they consumed alcohol and the average number of drinks they consumed per day when drinking. We defined a drink as ‘one bottle of beer, one glass of wine or one shot of liquor’. We then moved forward in time year by year up to their present age, asking if their drinking patterns had changed and, if so, asked them to document the new pattern. If necessary, the interviewers would use other memory prompts from the information previously recorded on the calendar to ‘cue’ the respondent into the relevant ‘memory files’. The test–retest reliability of our assessments of average monthly alcohol consumption (calculated as the product of the average number of days per month of drinking and the average number of drinks per day when drinking) has been presented in detail elsewhere (Kendler et al. 2008, Fig. 2) and exceeded +0.85 for the time period considered here. The key dependent variable for our analyses was the maximum reported yearly alcohol use for each of the age periods examined.
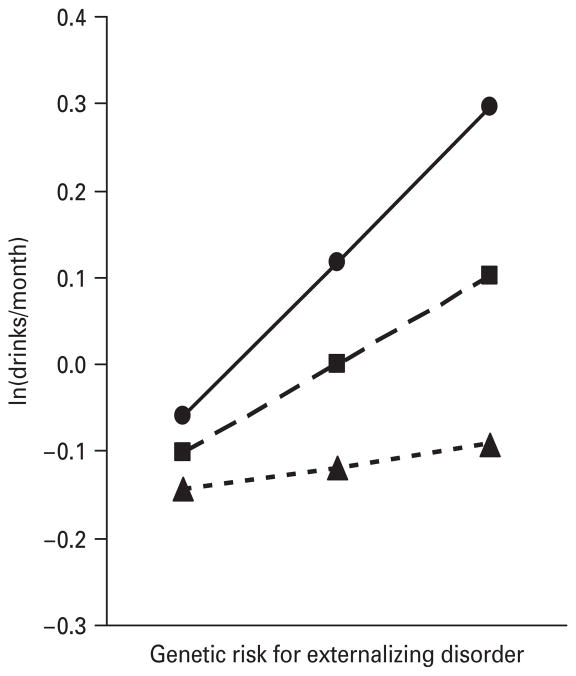
Fig. 2.
The prediction of the maximal yearly alcohol consumption from ages 12–14, as measured by standardized monthly intake, by the genetic risk for externalizing disorders (Ext Dis), alcohol availability (Alc Avl) and their interaction. The results, from the best-fit regression model with parameter estimates as outlined in Table 1, are depicted for three hypothetical individuals with a moderately high level of alcohol availability [–●–, values 1 standard deviation (S.D.) above the mean], an average level of alcohol availability (– –■– –, mean value) and a moderately low level of alcohol availability (- -▲- -, values 1 S.D. below the mean). Maximal yearly alcohol consumption is expressed by the average monthly alcohol consumption in that year, standardized so that, at the mean level of genetic risk and the mean level of alcohol availability, the score is approximately zero. The y-axis then depicts this mean score in standard deviation units.
Statistical analysis
The original distribution of the alcohol use data was highly right skewed. We explore possible optimal Box–Cox transformations in PROC TRANSREG in SAS (SAS Institute, 2008). The results indicated that a log transformation was optimal at stabilizing the variance (i.e. minimizing heteroscedasticity).
As the residual correlation within twin pairs was substantial and stronger in MZ twin pairs, regression models were run as hierarchical linear models using PROC MIXED in SAS (SAS Institute, 2008), with twin pairs and individuals within twin pairs being treated as separate levels. Random intercepts were used to estimate family-level variance components. Both the family-level variance and residual variance were estimated separately for MZ and DZ twin pairs. This yields parameter estimates and test statistics for regression coefficients that are adjusted for the residual ICCs for MZ and DZ twins. All explanatory variables were grand mean centered.
Models for dichotomous outcomes were performed as relative risk regressions using a quasi-likelihood approach in SAS PROC GENMOD (SAS Institute, 2008). The predicted outcome was p (the probability of being a drinker) while the residual variance was allowed to be heteroscedastic, estimated as p/(1 − p). Predicted outcomes were constrained to lie in the interval (0,1).
Results
Developmental effects of two forms of genetic risk
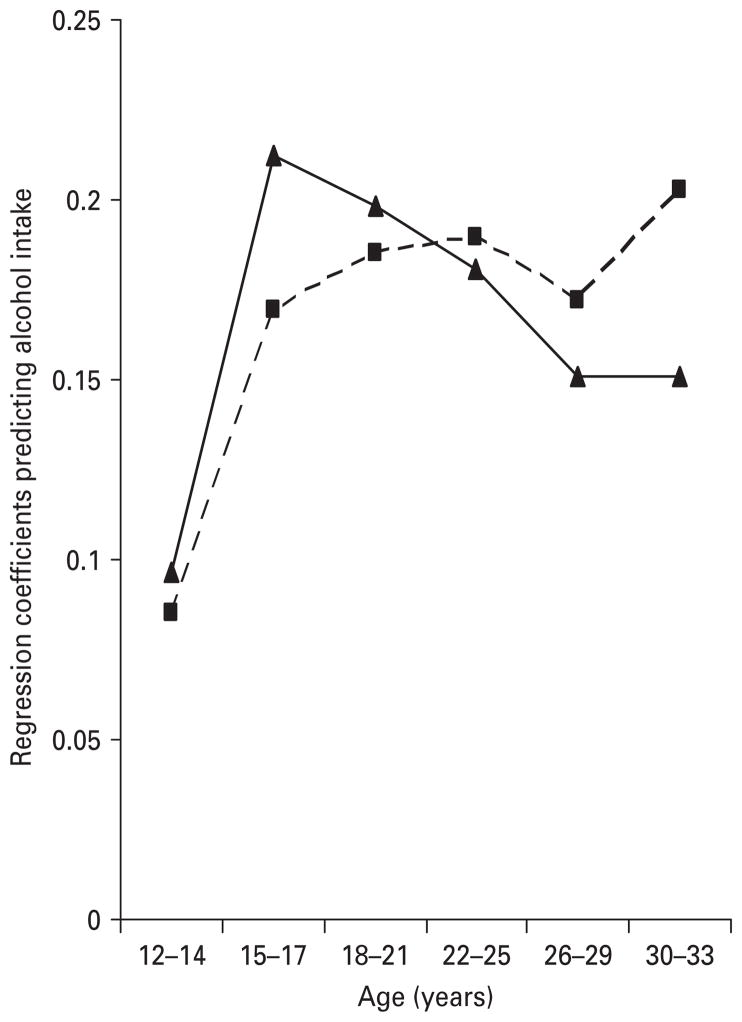
The relationship between genetic risk for AUDs (GR-AUD) and genetic risk for externalizing disorders (GR-ExtD) and maximal alcohol consumption over development are depicted in Fig. 1. For GR-ExtD, the regression coefficient begins at +0.10 at ages 12–14, rises rapidly to +0.21 at ages 15–17, and then declines slowly to +0.15 at ages 26–33. The pattern is somewhat different for GR-AUD, which also starts at a relatively low value at ages 12–14 (+0.09) and then rises first quickly and then more slowly (aside from an anomalous dip at ages 26–29), reaching a peak value of +0.20 at ages 30–33.
Fig. 1.
The strength of association, operationalized as a regression coefficient, between the genetic risk for alcohol use disorders (- -■- -; GR-AUD) and the genetic risk for externalizing disorders (–▲–, GR-ExtD) and maximal alcohol consumption as a function of age. For GR-AUD, all of these regression coefficients are significant at p<0.0001. For GR-ExtD, the regression coefficients for ages 12–14, 15–17, 18–21 and 22–25 are all significant at p<0.0001 and are significant at p=0.006 for ages 26–29 and p=0.001 for ages 30–33.
Prediction of alcohol intake from genetic and environmental risks and their interaction
Table 1 presents the results of hierarchical linear regression models predicting maximal normalized levels of alcohol consumption across four age periods from the main effects of GR-AUD and GR-ExtD, the main effects of the specified environmental risk factors and the interaction between them. For ages 12–14, each of the environmental risk factors significantly predicted alcohol consumption, with peer group deviance having the strongest effect followed by low parental monitoring and alcohol availability. Low church attendance had the weakest effect. Strong interactions were seen between both GR-AUD and GR-ExtD and peer group deviance and alcohol availability. A strong interaction in the prediction of alcohol consumption was also seen between low prosocial behavior and GR-ExtD and a weaker interaction was evident with GR-AUD. Low parental monitoring interacted with GR-ExtD but not GR-AUD in the prediction of alcohol intake.
Table 1.
Prediction of maximal alcohol consumption from ages 12 to 25 years from environmental risk factors, specific and non-specific genetic risk factors and their interaction
| Age range (years) | Environmental risk | Main effects (with 95% CIs) |
Interactions (with 95% CIs) |
|||
|---|---|---|---|---|---|---|
| Environmental risk | GR-AUD | GR-ExtD | GR-AUD | GR-ExtD | ||
| 12–14 | None | – | +0.08**** (0.05–0.12) | +0.10**** (0.06–0.13) | – | – |
| Alcohol availability | +0.11**** (0.08–0.14) | +0.08**** (0.04–0.11) | +0.08**** (0.05–0.12) | +0.08**** (0.05–0.11) | +0.08**** (0.05–0.11) | |
| Low church attendance | +0.06*** (0.03–0.09) | +0.08**** (0.05–0.12) | +0.09**** (0.06–0.12) | +0.01 (−0.02 to 0.04) | +0.03 (−0.00 to 0.06) | |
| Peer group deviance | +0.22**** (0.19–0.25) | +0.07**** (0.04–0.10) | +0.04* (0.01–0.07) | +0.08**** (0.05–0.11) | +0.09**** (0.06–0.11) | |
| Low prosocial behaviors | +0.07**** (0.04–0.10) | +0.08**** (0.05–0.12) | +0.09**** (0.05–0.12) | +0.03* (0.00–0.06) | +0.07**** (0.04–0.10) | |
| Low parental bonding | +0.09**** (0.05–0.12) | +0.08**** (0.05–0.11) | +0.08**** (0.05–0.12) | +0.02 (−0.01 to 0.05) | +0.02 (−0.01 to 0.05) | |
| Low parental monitoring | +0.14**** (0.11–0.17) | +0.07**** (0.04–0.11) | +0.06*** (0.03–0.10) | +0.02 (−0.01 to 0.05) | +0.05*** (0.02–0.08) | |
| 15–17 | None | – | +0.17**** (0.10–0.24) | +0.21**** (0.14–0.28) | – | – |
| Alcohol availability | +0.32**** (0.26–0.39) | +0.15**** (0.08–0.22) | +0.19**** (0.12–0.26) | +0.07* (0.00–0.13) | +0.11** (0.04–0.17) | |
| Low church attendance | +0.24**** (0.17–0.31) | +0.16**** (0.09–0.23) | +0.17**** (0.10–0.24) | −0.04 (−0.10 to 0.02) | +0.04 (−0.03 to 0.10) | |
| Peer group deviance | +0.57**** (0.51–0.63) | +0.13** (0.04–0.17) | +0.09*** (0.03–0.16) | +0.06* (0.00–0.11) | +0.06* (0.01–0.12) | |
| Low prosocial behaviors | +0.19*** (0.12–0.25) | +0.16**** (0.09–0.23) | +0.18**** (0.10–0.25) | +0.04 (−0.02 to 0.10) | +0.08** (0.02–0.14) | |
| Low parental bonding | +0.20**** (0.13–0.26) | +0.15**** (0.08–0.22) | +0.18**** (0.11–0.25) | +0.04 (−0.02 to 0.10) | −0.01 (−0.08 to 0.05) | |
| Low parental monitoring | +0.41**** (0.35–0.48) | +0.15**** (0.09–0.22) | +0.13*** (0.06–0.20) | 0.03 (−0.02 to 0.09) | −0.00 (−0.06 to 0.05) | |
| 18–21 | None | – | +0.18**** (0.10–0.27) | +0.20**** (0.11–0.28) | – | – |
| Alcohol availability | +0.32**** (0.25–0.39) | +0.18**** (0.10–0.26) | +0.22**** (0.13–0.30) | −0.02 (−0.11 to 0.07) | +0.01 (−0.06 to 0.08) | |
| Peer group deviance | +0.71**** (0.63–0.78) | +0.15**** (0.08–0.23) | +0.06 (−0.02 to 0.14) | −0.05 (−0.12 to 0.02) | −0.05 (−0.11 to 0.02) | |
| 22–25 | None | – | +0.19**** (0.10–0.27) | +0.18**** (0.09–0.27) | – | – |
| Peer group deviance | +0.82**** (0.74–0.89) | +0.14*** (0.07–0.22) | +0.01 (−0.07 to 0.09) | +0.01 (−0.06 to 0.07) | −0.06 (−0.12 to 0.01) | |
CI, Confidence interval; GR-AUD, genetic risk for alcohol use disorders; GR-ExtD, genetic risk for externalizing disorders.
The first three columns of the results in this table depict the main effects of the specific environmental risk factor examined and our two genetic risk factors: GR-AUD and GR-ExtD. These main effects are calculated without the presence of interactions. In the last two columns, we depict the interactions between the specific environmental risk factors and GR-AUD and GR-ExtD. These interactions, along with all the main effects, were run separately.
p<0.05,
p<0.01,
p<0.001,
p<0.0001.
The main effects on maximal alcohol consumption of all of the environmental risk factors increased substantially in strength from ages 12–14 to ages 15–17, as did the effects of both GR-AUD and GR-ExtD. The pattern of interactions remained relatively similar to that seen at ages 12–14 except that the weaker interactions between GR-AUD and low prosocial behavior, and between GR-Ext and low parental monitoring, disappeared. Our environmental measures were much more limited at ages 18–21 and 22–25 when most of the twins had moved out of the home. Despite strong main effects of alcohol availability and peer group deviance on alcohol consumption at these ages, no gene×environment interactions were observed.
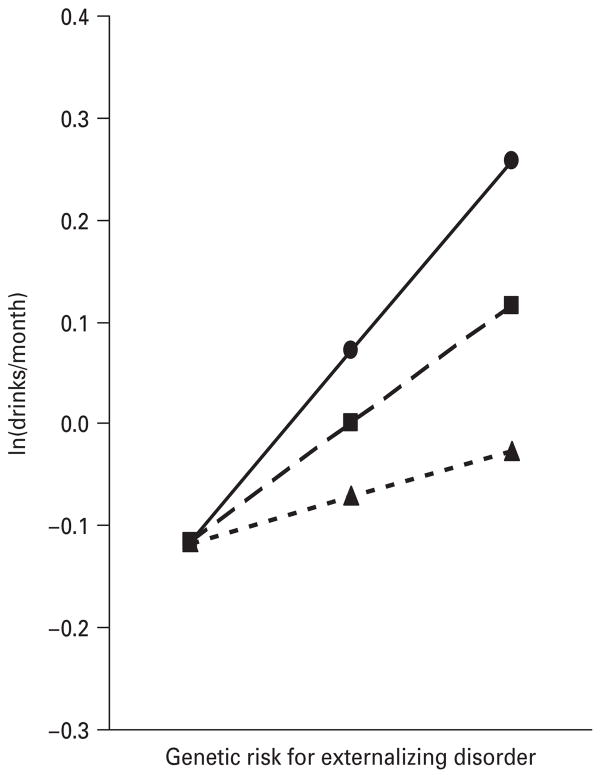
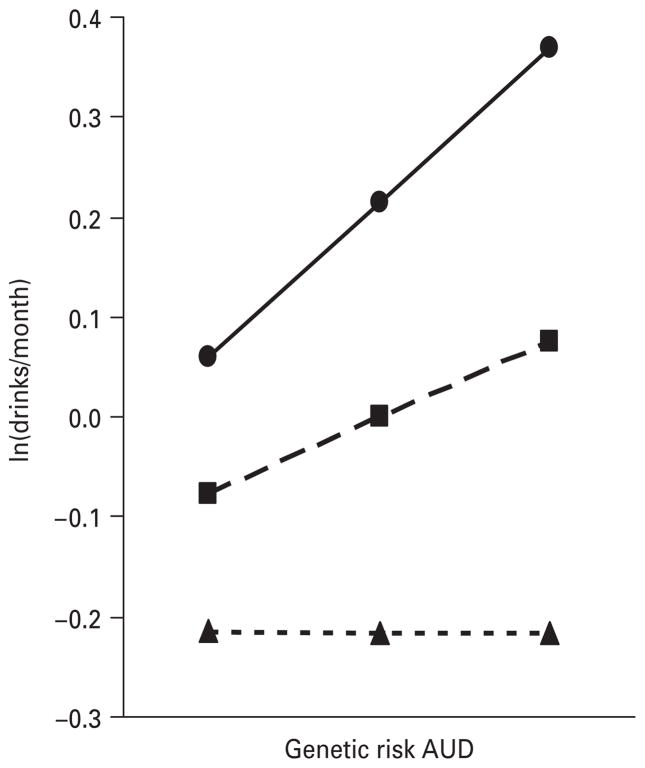
We illustrate three different patterns of genotype–environment interaction, all at ages 12–14, in our analyses in Figs 2–4. Fig. 2 depicts the interaction of GR-ExtD and alcohol availability. The maximal monthly intake of alcohol over this age period increased with increasing levels of genetic risk at all levels of alcohol availability. However, at low levels of availability, the increase was fairly modest whereas at high levels the increase was much stronger. Fig. 3 shows the interaction of GR-AUD and peer group deviance in the prediction of maximal alcohol consumption. At low levels of peer deviance, genetic risk had no effective impact on the level of alcohol consumption. By contrast, at high levels of peer deviance, levels of alcohol consumption were strongly related to level of genetic risk. Fig. 4 depicts the results for the interaction of GR-ExtD and prosocial behaviors. At low levels of genetic risk, alcohol consumption was low and unrelated to level of prosocial behaviors. However, as the level of genetic risk for externalizing disorders increased, there was a greater effect of involvement in prosocial behavior.
Fig. 4.
The prediction of the maximal yearly alcohol consumption from ages 12–14, as measured by standardized monthly intake, by the genetic risk for externalizing disorders (Ext Dis), level of prosocial activities (LPSA), reverse coded, and their interaction. The results, from the best-fit regression model with parameter estimates as outlined in Table 1, are depicted for three hypothetical individuals with a moderately low levels of prosocial activities [–●–, values 1 standard deviation (S.D.) above the mean], an average level of prosocial activities (– –■– –, mean value) and a moderately high level of prosocial activities (- -▲- -, values 1 S.D. below the mean).
Fig. 3.
The prediction of the maximal yearly alcohol consumption from ages 12–14, as measured by standardized monthly intake, by the genetic risk for alcohol use disorders (AUD), peer group deviance (PGD) and their interaction. The results, from the best-fit regression model with parameter estimates as outlined in Table 1, are depicted for three hypothetical individuals with moderately high peer group deviance [–●–, values 1 standard deviation (S.D.) above the mean], an average level of peer group deviance (– –■– –, mean value) and a moderately low level of peer group deviance (- -▲- -, values 1 S.D. below the mean).
Discussion
The first goal of this study was to examine developmentally the impact of two different classes of genetic risk factors on alcohol consumption: those specific to risk for AUDs and those reflecting a more general liability to externalizing disorders. We found a temporal progression in their effects. The non-specific genetic risk factors rose rapidly in importance in early to mid-adolescence, peaking in influence at ages 15–17 and then declining gradually in impact from late adolescence onward. By contrast, the alcohol-specific risk factors did not have maximal impact on alcohol consumption until ages 30–33. These results suggest that, in early adolescence, genetic influences on alcohol intake are preponderantly non-specific and may reflect a more general picture of largely adolescent-limited externalizing behaviors (Moffitt, 1993; Moffitt et al. 2002). However, the specific genetic risk factors become more important than non-specific influences in early to mid-adulthood, a typical time for the onset of serious alcohol problems (Schuckit et al. 1995). These results provide a good example of genetic and developmental equifinality (Cicchetti & Rogosch, 1996) in which the same phenotype at different developmental stages can be influenced by different combinations of distinct genetic risk factors.
These results also provide potential developmental insight into the etiology of alcohol dependence. Given that heavy alcohol exposure is a necessary precondition to the development of dependence (Koob & Le Moal, 2006), our findings suggest two potential path-ways by which genes influence the development of dependence: an early onset path driven in part by high genetic risk to externalizing disorders and a later onset path influenced largely by genetic risk factors more specific to alcohol disorders. These two path-ways to alcohol dependence are broadly analogous to several prior influential subtyping systems for alcoholism in postulating early versus late onset forms of illness, with the former demonstrating more prominent externalizing symptoms than the latter (Cloninger et al. 1981; Babor & Dolinsky, 1988; Babor et al. 1992).
The second aim of this study was to explore environmental moderation of genetic effects on alcohol consumption. These analyses yielded three particularly noteworthy results. First, environmental moderation of genetic effects was much more pronounced in early and mid-adolescence than in later periods. Genetic effects on alcohol use seem to be much more environmentally sensitive at this early developmental stage. As use patterns become more established, genetic effects may become less flexible and open to environmental moderation. These results have obvious implications for prevention efforts. Environmental interventions that occur in adolescence might be capable of substantially attenuating the effect of high genetic risk. Similar interventions in early to mid-adulthood may be much less efficacious as the system has become more developmentally canalized.
Second, somewhat more interactions with environmental risk factors were detected for GR-ExtD than for GR-AUD. This would suggest that the genetic vulnerability for externalizing disorder is more environmentally malleable than the genetic risks for AUDs. This finding is broadly consistent with the large literature that emphasizes the strong role of social–environmental factors in the etiology of externalizing disorders (e.g. Thornberry et al. 1993; Dishion et al. 1995; Hawkins et al. 1998; Farrington, 2005; Granic & Patterson, 2006).
Third, our findings are congruent with prior studies in suggesting that when significant gene×environment interaction effects are found, they show genetic effects on alcohol use to increase when social constraints are minimized (e.g. low parental monitoring, low prosocial behaviors and low parental bonding), or when the environment permits easy access to alcohol and/or encourages its use (e.g. high alcohol availability or high peer deviance) (Kendler, 2001; Shanahan & Hofer, 2005). For example, previous twin studies have demonstrated that genetic influences on adolescent substance use and externalizing behavior are magnified under conditions of lower parental monitoring (Dick et al. 2007b) and higher peer substance use/deviance (Button et al. 2007; Dick et al. 2007a). More recently, this has also been demonstrated with respect to specific genes, showing stronger genetic effects in the presence of lower parental monitoring (Dick et al. 2009) and higher peer deviance (Latendresse et al., unpublished observations).
Limitations
These results should be interpreted in the context of seven potentially important methodological limitations. First, we used regression rather than structural equal modeling in our analyses. We used regression models because they allowed us to easily incorporate data on parental psychopathology in our measures of genetic risk and because of their greater simplicity, flexibility and ease of interpretation. However, as we have used them, they are less precise in their ability to separate genetic from familial environment effects. Our measures of genetic risk could include environmental parental influences, although a prior study from this cohort showed no evidence of environmental transmission of risk for AUDs from parents to children (Kendler et al. 1994). In constructing our measures of genetic risk, we weighted contributions from MZ twins twice as strongly as from DZ twins, thereby protecting ourselves from any shared environmental influences ‘leaking into’ our measures of genetic risk.
Second, the assessment of interactions can be sensitive to the distributional properties of the dependent variable, here alcohol consumption. The major problem is heteroscadasticity, which can produce overly influential data points, typically as the high end of a rightward skewed distribution. We explored optimal transformations of our data to minimize heteroscadasticity, and the logarithmic transformation, a standard approach in the analysis of alcohol intake (Viken et al. 2007), performed best.
Third, we were concerned about the degree to which our interactions resulted from the prediction of drinking versus not drinking versus the quantity of alcohol consumed given drinking. We explored this question for the ages 12–14 and 15–17 where we observed our interactions. At ages 12–14, most but not all of the interactions resulted from the drinker–non-drinker dichotomy (analyzed on a scale of direct probability using a quasi-likelihood approach, not by logistic regression). At ages 15–17, approximately equal proportions of the interaction arose from the drinker–non-drinker dichotomy and from the quantity consumed among drinkers. A substantial rise in levels of alcohol use from ages 12–14 to ages 15–17 (Fig. 1) probably contributed to greater power to observe interactions in the quantity of alcohol consumed at the later age.
Fourth, despite its flexibility, our regression model does not allow us to unconfound the effects of gene– environment interaction and gene–environment correlation. We know, for example, that the two exposures that had the most robust evidence for interaction – alcohol availability and peer group deviance – both have heritable components (Gillespie et al. 2007; Kendler et al. 2007). Perhaps the observed interaction with GR-ExtD and GR-AUD in the prediction of alcohol consumption results in part or entirely from genetic correlations between these variables. However, the pattern of our findings argues strongly against such an interpretation. For example, the genetic influences on both alcohol availability and peer deviance are considerably stronger at ages 18–21 than at ages 12–14 (Gillespie et al. 2007; Kendler et al. 2007). If our evidence for gene–environment interaction resulted from genetic effects on these variables, we would expect stronger evidence for interactions at ages 18–21 than at ages 12–14. However, the observed pattern is exactly the opposite.
Fifth, a quarter of the eligible twins for this study did not participate. Might this have biased our findings? Using data collected at the second interview wave, a multiple logistic regression analysis showed participation in the third wave to be strongly predicted by educational status but not by age, zygosity, level of alcohol use or alcohol abuse/dependence, or by several DSM-IV adult antisocial symptoms. With respect to vulnerability to the alcohol and externalizing symptoms, this sample was likely to be broadly representative of the original twin cohort.
Sixth, no formal corrections were made for multiple testing. However, each of the variables chosen in our analysis has strong prior evidence of an association with alcohol consumption. The pattern of findings is not consistent with stochastic effects, although it is always possible that a few of our significant results arose from chance.
Seventh, information on our environmental risk factors and alcohol intake were collected retrospectively from adults. Some of our findings could arise solely from retrospective recall bias. For example, subjects may exaggerate the resemblance between their own level of deviant behavior and that of their peers. However, we consider it unlikely that such biases would produce the major findings in this study. In particular, it is difficult to construct a plausible pattern of recall bias that would result in our pattern of interactions. We demonstrated excellent test–retest reliability for our measures. Furthermore, we used a life history calendar in our assessments. This method, designed to capitalize on the structure of autobiographical memory and promote sequential retrieval within memory networks, has been shown to improve substantially the completeness and accuracy of retrospective recall (Freedman et al. 1988; Belli, 1998; Yoshihama et al. 2002; Cook et al. 2003).
Acknowledgments
This study was supported in part by grants AA-011408, DA-011287 and MH-49492 from the NIH. D.M.D. is supported by AA015416.
L. Corey provided assistance with the ascertainment of twins from the Virginia Twin Registry, now part of the Mid-Atlantic Twin Registry (MATR). The MATR, currently directed by J. Silberg, has received support from the National Institutes of Health (NIH), the Carman Trust, and the W. M. Keck, John Templeton, and Robert Wood Johnson Foundations. L. Murrelle, P. Sullivan and C. Prescott contributed to the design and implementation of this study. C. Prescott also provided helpful comments on an earlier version of this manuscript.
Footnotes
Declaration of Interest
The NIH played no direct role in the design or conduct of the study or in the collection, management, analysis and interpretation of the data, and did not review or approve this manuscript. K.S.K. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Babor TF, Dolinsky ZS. Alcoholic typologies: historical evolution and empirical evaluation of some common classification schemes. In: Rose R, Barrett J, editors. Alcoholism: Origins and Outcome. Raven Press, Ltd; New York: 1988. pp. 245–264. [Google Scholar]
- Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B. Types of alcoholics. I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Archives of General Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Belli RF. The structure of autobiographical memory and the event history calendar: potential improvements in the quality of retrospective reports in surveys. Memory. 1998;6:383–406. doi: 10.1080/741942610. [DOI] [PubMed] [Google Scholar]
- Button TM, Corley RP, Rhee SH, Hewitt JK, Young SE, Stallings MC. Delinquent peer affiliation and conduct problems: a twin study. Journal of Abnormal Psychology. 2007;116:554–564. doi: 10.1037/0021-843X.116.3.554. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. Equifinality and multifinality in developmental psychopathology. Development and Psychopathology. 1996;8:597–600. [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Archives of General Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cohen P, Kasen S, Chen H, Hartmark C, Gordon K. Variations in patterns of developmental transitions in the emerging adulthood period. Developmental Psychology. 2003;39:657–669. doi: 10.1037/0012-1649.39.4.657. [DOI] [PubMed] [Google Scholar]
- Cook LS, White JL, Stuart GC, Magliocco AM. The reliability of telephone interviews compared with in-person interviews using memory aids. Annals of Epidemiology. 2003;13:495–501. doi: 10.1016/s1047-2797(03)00039-5. [DOI] [PubMed] [Google Scholar]
- Dick DM, Latendresse SJ, Lansford JE, Budde JP, Goate A, Dodge KA, Pettit GS, Bates JE. Role of GABRA2 in trajectories of externalizing behavior across development and evidence of moderation by parental monitoring. Archives of General Psychiatry. 2009;66:649–657. doi: 10.1001/archgenpsychiatry.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Pagan JL, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Changing environmental influences on substance use across development. Twin Research and Human Genetics. 2007a;10:315–326. doi: 10.1375/twin.10.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Rose RJ, Viken RJ, Kaprio J, Koskenvuo M. Exploring gene-environment interactions: socioregional moderation of alcohol use. Journal of Abnormal Psychology. 2001;110:625–632. doi: 10.1037//0021-843x.110.4.625. [DOI] [PubMed] [Google Scholar]
- Dick DM, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. Journal of Abnormal Psychology. 2007b;116:213–218. doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishion TJ, Andrews DW, Crosby L. Antisocial boys and their friends in early adolescence: relationship characteristics, quality, and interactional process. Child Development. 1995;66:139–151. doi: 10.1111/j.1467-8624.1995.tb00861.x. [DOI] [PubMed] [Google Scholar]
- Farrington D. Childhood origins of antisocial behavior. Clinical Psychology and Psychotherapy. 2005;12:177–190. [Google Scholar]
- Freedman D, Thornton A, Camburn D, Alwin D, Young-DeMarco L. The life history calendar: a technique for collecting retrospective data. Sociological Methodology. 1988;18:37–68. [PubMed] [Google Scholar]
- Gillespie NA, Kendler KS, Prescott CA, Aggen SH, Gardner CO, Jr, Jacobson K, Neale MC. Longitudinal modeling of genetic and environmental influences on self-reported availability of psychoactive substances: alcohol, cigarettes, marijuana, cocaine and stimulants. Psychological Medicine. 2007;37:947–959. doi: 10.1017/S0033291707009920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granic I, Patterson GR. Toward a comprehensive model of antisocial development: a dynamic systems approach. Psychological Review. 2006;113:101–131. doi: 10.1037/0033-295X.113.1.101. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Herrenkohl T, Farrington DP, Brewer D, Catalano RF, Harachi TW. A review of predictors of youth violence. In: Loeber R, Farrington DP, editors. Serious and Violent Juvenile Offenders: Risk Factors and Successful Interventions. Sage Publications, Inc; London, UK: 1998. pp. 106–146. [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: a longitudinal twin study. Journal Abnormal Psychology. 2007;116:433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, McGue M, Patrick CJ. Family transmission and heritability of externalizing disorders: a twin-family study. Archives of General Psychiatry. 2004;61:922–928. doi: 10.1001/archpsyc.61.9.922. [DOI] [PubMed] [Google Scholar]
- Hicks BM, South SC, DiRago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Archives of General Psychiatry. 2009;66:640–648. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, Bachman JG, O’Malley PM. Monitoring the Future: Questionnaire Responses from the Nation’s High School Seniors, 1981. Institute for Social Research; Ann Arbor, MI: 1982. [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: an update. Archives of General Psychiatry. 2001;58:1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Eaves LJ. Models for the joint effect of genotype and environment on liability to psychiatric illness. American Journal of Psychiatry. 1986;143:279–289. doi: 10.1176/ajp.143.3.279. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Gardner CO, Gillespie NA, Aggen SH, Prescott CA. Creating a social world: a developmental study of peer deviance. Archives of General Psychiatry. 2007;64:958–965. doi: 10.1001/archpsyc.64.8.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Archives of General Psychiatry. 2000;57:261–269. doi: 10.1001/archpsyc.57.3.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Heath AC, Kessler RC, Eaves LJ. A twin-family study of alcoholism in women. American Journal Psychiatry. 1994;151:707–715. doi: 10.1176/ajp.151.5.707. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA. Genes, Environment, and Psychopathology: Understanding the Causes of Psychiatric and Substance Use Disorders. Guilford Press; New York: 2006. [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Archives of General Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt JE, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M, Stattin H. What parents know, how they know it, and several forms of adolescent adjustment: further support for a reinterpretation of monitoring. Developmental Psychology. 2000;36:366–380. [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Alcohol. In: Koob GF, Le Moal M, editors. Neurobiology of Addiction. Academic Press; London, UK: 2006. pp. 173–242. [Google Scholar]
- Macgregor S, Lind PA, Bucholz KK, Hansell NK, Madden PA, Richter MM, Montgomery GW, Martin NG, Heath AC, Whitfield JB. Associations of ADH and ALDH2 gene variation with self report alcohol reactions, consumption and dependence: an integrated analysis. Human Molecular Genetics. 2009;18:580–593. doi: 10.1093/hmg/ddn372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens MP, Neighbors C, Dams-O’Connor K, Lee CM, Larimer ME. The factor structure of a dichotomously scored Rutgers Alcohol Problem Index. Journal of Studies on Alcohol and Drugs. 2007;68:597–606. doi: 10.15288/jsad.2007.68.597. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: a developmental taxonomy. Psychology Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE. The new look of behavioral genetics in developmental psychopathology: gene-environment interplay in antisocial behaviors. Psychological Bulletin. 2005;131:533–554. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M, Silva PA. Sex Differences in Antisocial Behavior: Conduct Disorder, Delinquency, and Violence in the Dunedin Longitudinal Study. Cambridge University Press; Cambridge, UK: 2002. [Google Scholar]
- Muffler J, Langrod J, Larson DB. ‘There is a balm in Gilead’: religion and substance abuse rehabilitation. In: Lowinson J, Ruiz P, editors. Comprehensive Textbook of Substance Abuse. Williams & Wilkins; Baltimore, MD: 1991. pp. 584–595. [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene–environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcoholism: Clinical and Experimental Research. 2001;25:637–643. [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene–environment interplay and psychopathology: multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS OnlineDoc Version 9.2. SAS Institute, Inc; Cary, NC: 2008. [Google Scholar]
- Schuckit MA, Anthenelli RM, Bucholz KK, Hesselbrock VM, Tipp J. The time course of development of alcohol-related problems in men and women. Journal of Studies on Alcohol. 1995;56:218–225. doi: 10.15288/jsa.1995.56.218. [DOI] [PubMed] [Google Scholar]
- Shanahan MJ, Hofer SM. Social context in gene-environment interactions: retrospect and prospect. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2005;60:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Sigvardsson S, Bohman M, Cloninger CR. Replication of the Stockholm Adoption Study of alcoholism. Confirmatory cross-fostering analysis. Archives of General Psychiatry. 1996;53:681–687. doi: 10.1001/archpsyc.1996.01830080033007. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Fletcher A, Darling N. Parental monitoring and peer influences on adolescent substance use. Pediatrics. 1994;93:1060–1064. [PubMed] [Google Scholar]
- Tarter RE, Hegedus A. The Drug Use Screening Inventory: evaluation and treatment of alcohol and drug abuse. Alcohol Health & Research World. 1991;15:65–75. [Google Scholar]
- Thornberry TP, Krohn MD, Lizotte AJ, Chard-Wierschem D. The role of juvenile gangs in facilitating delinquent behavior. Journal of Research in Crime and Delinquency. 1993;30:55–87. [Google Scholar]
- Viken RJ, Kaprio J, Rose RJ. Personality at ages 16 and 17 and drinking problems at ages 18 and 25: genetic analyses of data from Finn Twin16–25. Twin Research and Human Genetics. 2007;10:25–32. doi: 10.1375/twin.10.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihama M, Clum K, Crampton A, Gillespie B. Measuring the lifetime experience of domestic violence: application of the life history calendar method. Violence and Victims. 2002;17:297–317. doi: 10.1891/vivi.17.3.297.33663. [DOI] [PubMed] [Google Scholar]