Abstract
Physical activity is one of the most important determinants of cardiac function. The ability of the heart to increase delivery of oxygen and metabolic fuels relies on an array of adaptive responses necessary to match bodily demand while avoiding exhaustion of cardiac resources. The ATP-sensitive potassium (KATP) channel has the unique ability to adjust cardiac membrane excitability in accordance with ATP and ADP levels, and up-regulation of its expression that occurs in response to exercise could represent a critical element of this adaption. However, the mechanism by which KATP channel expression changes result in a beneficial effect on cardiac excitability and function remains to be established. Here, we demonstrate that an exercise-induced rise in KATP channel expression enhanced the rate and magnitude of action potential shortening in response to heart rate acceleration. This adaptation in membrane excitability promoted significant reduction in cardiac energy consumption under escalating workloads. Genetic disruption of normal KATP channel pore function abolished the exercise-related changes in action potential duration adjustment and caused increased cardiac energy consumption. Thus, an expression-driven enhancement in the KATP channel-dependent membrane response to alterations in cardiac workload represents a previously unrecognized mechanism for adaptation to physical activity and a potential target for cardioprotection.
Keywords: KATP, K-ATP, remodeling, oxygen consumption, heart rate, exercise
1. Introduction
Physical activity is one of the most important determinants of cardiac function. The ability of the heart to increase delivery of oxygen and metabolic fuels relies on an array of adaptive responses necessary to match bodily demand while avoiding exhaustion of cardiac energetic and functional resources [1]. Yet, the molecular mechanisms of these adaptive changes are not fully understood. The ATP-sensitive potassium (KATP) channel, one of the most abundant cardiac membrane protein complexes, has the unique ability to adjust membrane excitability in response to changes in ATP and ADP levels [2-6]. The channel complex is formed through physical association of the pore-forming inwardly rectifying potassium channel, Kir6.x, with the regulatory sulfonylurea receptor, SUR [2,5,7-12]. KATP channels in ventricular myocytes are composed primarily of Kir6.2 and SUR2A subunits, while in atria the combination of Kir6.2 or 6.1 with SUR1 and SUR2A has been reported [2,5-10]. The metabolic sensing of KATP channels occurs through modulation of the ATP sensitivity of the pore-forming subunit by the regulatory sulfonylurea receptor, closely integrated with cellular energetic networks [6,13-24]. When activated by reduced [ATP] to [ADP] ratio that occurs with increased cellular metabolic demand, KATP channel-dependent potassium efflux shortens cardiac action potentials [3,5,25,26]. This potassium efflux limits sodium and calcium entry into the cell and thus reduces energy requirements for ion homeostasis and contraction, as well as prolongs the diastolic interval that supports myocardial relaxation and replenishment of resources [4,5,21,25-41]. In this way, cardiac KATP channels serve a critical role in myocardial protection in response to vigorous exercise and heart rate acceleration [25,26], mandatory for optimal exercise capacity [25,29]. Furthermore, KATP channel expression up-regulation has been shown to be part of exercise-induced cardiac remodeling and has been linked to enhanced myocardial resistance to ischemic injury [42-45]. However, since the opening of only 1% of sarcolemmal KATP channels is sufficient to cause significant cardiac action potential shortening [5,16,19,46], it remains to be established how an increase in the expression of the already dense population of KATP channels could have a sufficient impact on cardiac excitability and energy balance.
Here, we demonstrate that an exercise-induced rise in KATP channel expression enhances the rate and magnitude of action potential shortening that occurs in response to heart rate acceleration, and that this adaptation in membrane excitability is critical to optimize cardiac energy consumption under conditions of increased demand. Thus, this study defines increased KATP channel membrane expression as part of the phenomenon of exercise-induced cardiac conditioning, and determines how this dynamic expression change is translated into the maintenance of cardiac energy homeostasis.
2. Materials and methods
2.1 Generation of genetically modified mice
Transgenic Tg[CX1-eGFP-Kir6.1AAA] mice (Fig. 2) were created on the FVB/N mouse strain using a non-conducting Kir6.1AAA pore mutant where a Gly-Phe-Gly motif critical for K+ permeation was replaced by Ala-Ala-Ala [26,29,47]. Kir6.1AAA was subcloned downstream of the loxP site-flanked eGFP coding region and a stop codon, in such a way that dominant negative suppression of Kir6.2/SUR2A potassium efflux could be initiated by expression of Cre recombinase. Accordingly, cardiac-specific reduction of KATP channel activity was achieved by crossing Tg[CX1-eGFP-Kir6.1AAA] and Tg[αMHC-Cre] mice that express Cre recombinase under control of the αMHC promoter [48]. The resulting offspring are notated Tg[αMHC-Kir6.1AAA] to indicate cardiac specific expression of the dominant negative, non-functioning, pore-forming KATP channel subunit. Male WT, Tg[CX1-eGFP-Kir6.1AAA], and Tg[αMHC-Kir6.1AAA] littermate mice, aged 8-12 weeks, with and without exposure to 5 days treadmill exercise, were used for all experiments. Protocols were approved by the University of Iowa Institutional Animal Care and Use Committee.
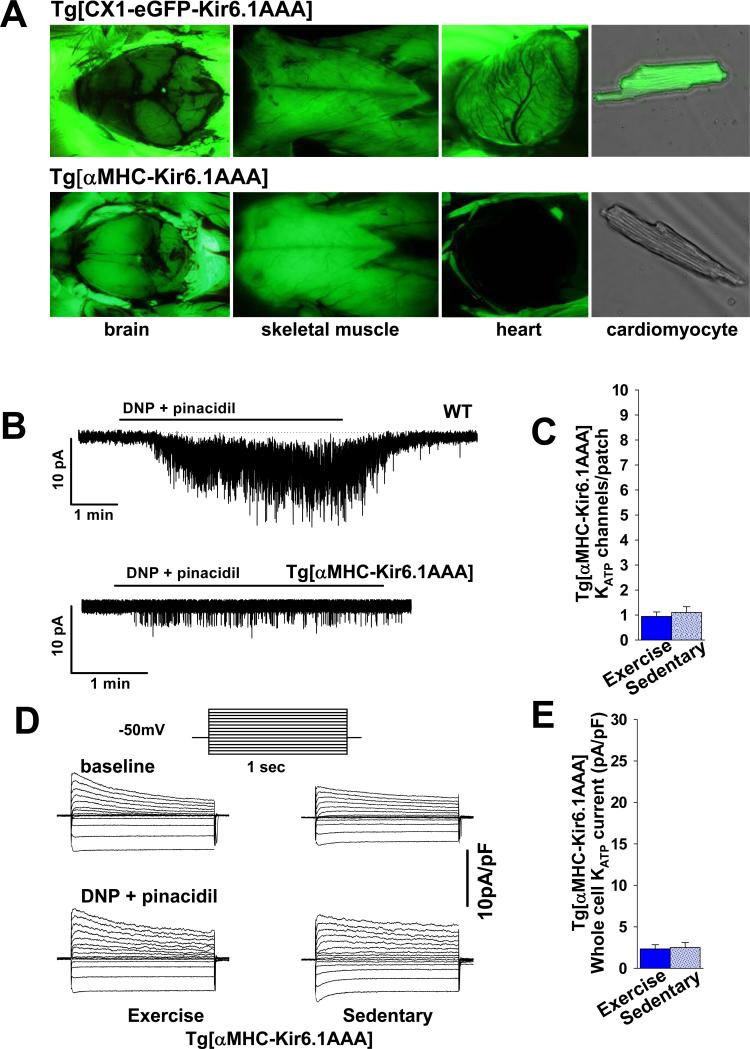
Figure 2. Expression of a dominant negative KATP channel pore-forming subunit results in significant reduction of functional cardiac channels and interference with exercise-induced KATP current up-regulation.
A. Representative images of tissues from transgenic mice under UV light. Tg[CX1-eGFP-Kir6.1AAA] mice express eGFP in all cells (top panel). Crossing Tg[CX1-eGFP-Kir6.1AAA] with Tg[αMHC-Cre] generated Tg[αMHC-Kir6.1AAA] progeny with cardiomyocyte specific Kir6.1AAA transgene expression tracked by selective elimination of eGFP fluorescence in the heart only (bottom panel). B. Representative cell-attached KATP channel recordings in response to DNP 200μM and pinacidil 100μM in the perfusate. C. Summary statistics indicating the number of KATP channels/patch in cell-attached recordings following exposure to DNP 200μM and pinacidil 100μM performed in cardiomyocytes isolated from exercised (n=5 mice and 17 patches) vs. sedentary (n=5 mice, 10 patches) Tg[αMHC-Kir6.1AAA] animals (p=NS). The patch pipette tip size was not significantly different between groups (4.62±.09 vs. 4.44±.07 MΩ, respectively, p=NS). D. Representative whole cell KATP channel current recordings in cardiomyocytes isolated from hearts of exercised and sedentary Tg[αMHC-Kir6.1AAA] mice in response to DNP 50μM and pinacidil 100μM in the perfusate. Inset shows stimulation pattern. Current is normalized to cell capacitance (pA/pF). E. Summary statistics of whole cell KATP channel current normalized to cell capacitance (pA/pF) in response to DNP 50μM and pinacidil 100μM for cardiomyocytes isolated from hearts of exercised (n= 3 mice and 11 patches) vs. sedentary (n= 3 mice and 11 patches) Tg[αMHC-Kir6.1AAA] animals (p=NS).
2.2 Isolated heart studies
Isolated hearts were retrogradely perfused at 90 mmHg with Krebs–Henseleit buffer bubbled with 95% O2/5% CO2, at 37°C and pH 7.4 [25,26,40]. The AV node was me chanically dissociated and hearts were paced (Bloom Electrophysiology, Fischer Imaging Corp., Denver, CO) using a bipolar platinum pacing catheter positioned in the right ventricular apex (NuMed; Hopkinton, NY).
A monophasic action potential (MAP) probe (EP Technologies; Sunnyvale, CA) was maintained at a single stable position on the LV epicardium, and amplified signals (IsoDam; World Precision Instruments; Sarasota, FL) were acquired at 2 kHz [25,26,40]. Using custom software, (MATLAB, Mathworks, Natick, MA) monophasic action potential recordings were analyzed for duration at 90% repolarization (APD90). Changes in APD90 (ΔAPD90) were calculated as the absolute change in milliseconds of the APD90 using the duration of the last action potential at the previous pacing cycle length as a reference. The pacing protocol for ΔAPD90 calculations was 12 minutes of continuous pacing consisting of 3 minutes at each of the following sequential cycle lengths: 150, 130, 100 and 80 msec. Monophasic action potentials were only analyzed from tracings in which the MAP probe remained in a stable position throughout the entire pacing protocol, and in which pacing capture was maintained throughout the protocol without interruption. All calculations were manually reviewed to exclude artifacts.
Cardiac oxygen consumption was calculated based on Henry's Law as the difference between the oxygen partial tension measured in the perfusate prior (PO2pre) and after (PO2post) heart passage (Model 210, Instech Laboratories, Plymouth Meeting, PA). The rate of oxygen consumption (V̇O2) was calculated as: V̇O2 = (PO2cntr – PO2)·α·ν/mh, where ν is rate of coronary flow (T402, Transonic System Inc., Ithaca, NY); α=0.024 ml O2 per ml H2O)/760 mmHg denotes the Bunsen's solubility coefficient [26,49]; and mh is heart wet weight. Measurements were taken at pacing cycle lengths of 150, 100 and 80 msec.
2.3 Patch-clamp studies
Single ventricular cardiomyocytes were enzymatically isolated as previously described [20,23]. Briefly, hearts were cannulated in situ then rapidly excised and retrogradely perfused at 90 mmHg for 5 min with Hepes buffer (Medium 199, Sigma), 1 min with a “low-calcium” medium (in mM): NaCl 100, KCl 10, KH2PO4 1.2, MgSO4 5, glucose 20, taurine 50, HEPES 10, supplemented with (in mM) CaCl2 0.13, EGTA 2.1, then 13 min with “low-calcium” medium supplemented with 1% bovine serum albumin, CaCl2 0.2 mM, collagenase (type IV, 22 U/ml, Worthington) and pronase (100 μg/ml, Serva). Ventricles were dissected away, cut into pieces (~ 3×3 mm), and incubated at 37°C for 15 min in the enzyme solution with gentle stirring until dissociated cardiomyocytes could be collected.
Patch clamp studies were performed using an Axopatch 200B (Molecular Devices, Sunnyvale, CA) amplifier integrated with a Nikon TE2000-U microscope. Experiments were performed at 33-35°C using a temperature controller TC2r (Cell MicroControls, Norfolk, VA).
For whole-cell recording, pipettes (3-4 MΩ) were filled with internal solution (in mM): KCl 140, MgCl2 1, EGTA 5, ATP 5, HEPES-KOH 5 (pH 7.3). Cardiomyocytes were superfused with Tyrode solution (in mM): NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, glucose 5.5, HEPES-NaOH 5.5 (pH 7.4). Whole-cell current traces were obtained in response to rectangular pulses from a holding potential of −50 mV to test potentials from -100 to +40 mV. For quantification, whole cell KATP channel current was measured as the difference between baseline and stimulated current recorded just before the end of a 1-second applied voltage step from -50 to +40mV. For cell attached and inside-out single channel recording, myocytes were bathed in internal solution (mM) KCl 140, MgCl2 1, EGTA 5, HEPES-KOH 5 (pH 7.3), supplemented with glucose (1 g/liter). Pipettes (4-5 MΩ) were filled with “Electrode Solution” (in mM): KCl 140, CaCl2 1, MgCl2 1, HEPES-KOH 5 (pH 7.3). Pipette potential was +40 mV. All recordings were monitored, sampled and analyzed using pCLAMP software (Molecular Devices, Sunnyvale, CA).
2.4 Exercise protocol
Three days before exercise, mice were acclimated daily for 45 min on a nonmoving treadmill (Columbus Instruments, Columbus, OH) followed by 15 min at a velocity of 3.5 m/min. After this training period mice were exercised daily for 5 consecutive days at a speed of 12 m/min and inclination of 15° for 45 minutes. Mice were sacrificed for further studies on the last day of the exercise protocol.
2.5 Experimental Protocols
Quantitative real-time PCR
Total RNA was isolated from liquid N2 freeze-clamped left ventricles using a commercial kit (RNeasy, Mini Kit, Qiagen) according to the manufacturer's instructions. Strand cDNA was synthesized with random hexanucleotides from 1 μg of total RNA using a reverse transcription system kit (Superscript III First Strand Synthesis, Invitrogen). Quantitative PCR was done using Biorad's iQ SYBR Green Supermix (iCycler, Biorad). The primer sets were as follows: Kir6.2 Forward: GAA-GCC-TGT-ACC-GGG-TTA-TT, Reverse: GCT-TTA-GAG-GCC-CTG-AAC-CT, 118bp product; SUR2A Forward: GAG-TGT-CAG-ACC-TGC-GCT-TCT, Reverse: GCT-GCT-CAG-CAG-GAT-TGG-TCT-C, 251bp product; HPRT Forward: GAA-CCT-CTC-GAA-GTG-TTG-GAT-AC, Reverse: GCT-CAT-CTT-AGG-CTT-TGT-ATT-TGG-CT, 165bp product. The quality of the RT-qPCR product was routinely checked by the thermal denaturation curve following RT-qPCR reactions. The expression of RNA encoding the channel subunits was normalized to Hypoxanthine-Guanine Phosphoribosyltransferase (HPRT) and quantification of relative mRNA levels was performed by △CT method.
Western blotting
Protein extracts were prepared by homogenizing ventricular tissue in NaCl 150 mM, Tris-HCl 50 mM (pH 7.8), supplemented with 1% Triton X-100, protease and phosphatase inhibitors (Roche). Electrophoresis was performed on 3-8% gradient Nu-Page Tris-Acetate gel and transferred to 0.2μm Sequi-Blot PVDF membranes (Bio-Rad). Rabbit polyclonal antibodies against SUR2, goat polyclonal antibodies against Kir6.2, and secondary anti-rabbit and anti-goat HP-conjugated antibodies were used (Santa Cruz Biotechnology). Densitometric analysis of the Western blot was performed using Adobe Photoshop (Adobe Systems, San Jose, CA).
2.6 Statistical analysis
Results are expressed as mean±SEM. Comparisons between two groups were made using the 2-sided Student's t-test and between more than two groups using analysis of variance (ANOVA). A p value <0.05 was considered statistically significant.
3. Results
3.1. Exercise increases the expression of functional KATP channels
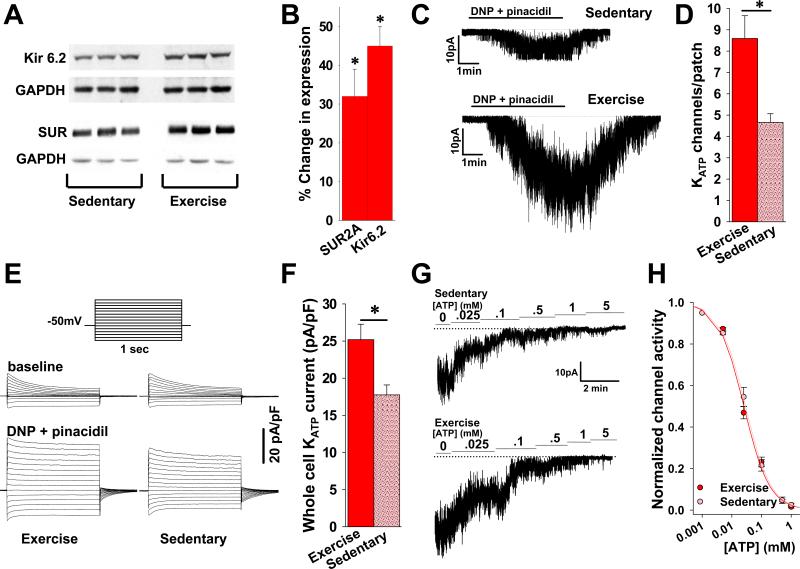
Exercise causes significant cardiac remodeling [50]. We used short-term exposure to exercise training to evaluate if increased cardiac KATP channel expression is part of activity-induced cardiac remodeling. A densitometric analysis of western blots (Fig. 1A, B) revealed a significant increase in the expression of Kir6.2 and SUR2A subunits in protein extracts from ventricles of mice with exposure to 5 days exercise compared to sedentary controls (45±5% increase for Kir6.2 and 32±7% increase for SUR2A, Fig. 1B). These changes in protein expression were accompanied by a gain in the number of functional KATP channels at the sarcolemma and an increase in the KATP channel current stimulated by application of the KATP channel specific opener pinacidil, and mitochondrial uncoupler 2,4-dinitrophenol (DNP). Specifically, membrane patches on ventricular cardiomyocytes from exercised animals had on average 8.59±1.07 channels vs. 4.66±.41 channels in sedentary animals (p<.05, Fig. 1C, D). Similarly, whole cell KATP channel current in myocytes isolated from hearts of mice after exposure to exercise training was 25.19±2.09 pA/pF vs. 17.75±1.34 pA/pF in sedentary controls (p<.05, Fig. 1E, F). No changes in sensitivity to ATP, single channel conductance or open channel probability were detected in response to exercise (Fig. 1G, H). The [IC50] for ATP in the exercise group was 26±2.1μM vs. 31±1.1 μM for the sedentary group (p=NS). The Hill coefficient was 1.0±.3 μM for exercise vs. 1.1±.2 μM for sedentary (p=NS). Single channel conductance was 70.9±.5 pS (n=5 patches) for exercise vs. 71.4±.6 (n=4 patches) for sedentary conditions (p=NS). Open probability was .84±.05 (n=5 patches) for exercise vs. .82±.03 (n=4 patches) for sedentary conditions (p=NS). Thus, short-term exposure to exercise caused a significant increase in the expression of functional KATP channels without altering their ATP-dependent gating properties or conductance.
Figure 1. Short-term exercise increases cardiac expression of functional KATP channels.
A. Representative Western blots of KATP channel subunits Kir6.2 and SUR2A in protein extracts from ventricles of mice under sedentary and exercise conditions. B. Summary statistics indicating increased expression of Kir6.2 and SUR2A subunits over baseline in response to exercise (both n=10 and *p<.05 vs. baseline). C. Representative cell-attached KATP channel recordings in ventricular myocytes isolated from hearts of sedentary and exercised mice. Channel opening is stimulated by 2,4-dinitrophenol (DNP) 200μM and pinacidil 100μM in the perfusate. D. Summary statistics indicating the number of KATP channels/patch detected in cardiomyocytes isolated from exercised (n=22 patches, 5 mice) and sedentary (n=29 patches, 6 mice) animals (*p<.001). The patch pipette tip size was not significantly different between groups (4.71±.31 vs. 4.65±.22 MΩ, respectively, p=NS). E. Representative whole cell KATP current recordings in cardiomyocytes isolated from hearts of exercised and sedentary mice in response to DNP 50μM and pinacidil 100μM in the perfusate. Inset: stimulation pattern. Current is normalized to membrane capacitance (pA/pF). F. Summary statistics of whole cell KATP channel current normalized to cell capacitance (pA/pF) in response to DNP 50μM and pinacidil 100μM for cardiomyocytes isolated from hearts of exercised (n=19 patches, 5 mice) vs. sedentary (n=10 patches, 6 mice) animals (* p<.05). G. Representative inside-out patch recordings of KATP channel activity in isolated cardiomyocytes from hearts of exercised and sedentary mice in response to escalating concentrations of ATP in the perfusate. H. KATP channel activity in membrane patches (inside-out configuration), calculated relative to activity in the absence of ATP, and fitted by the Hill equation (y=[IC50]/([IC50]h + [ATP]h), where h is the Hill coefficient, [ATP] is the ATP concentration, and [IC50] is the half-maximal inhibitory ATP concentration (n=10 exercise and 9 sedentary).
3.2. Exercise-induced increase in KATP channel current capacity is eliminated by cardiac-specific expression of a non-functional KATP channel pore-forming subunit
Cardiac remodeling may be beneficial independently from changes in KATP channel expression. In order to determine if changes in KATP channel expression after exposure to exercise effect cardiac function and energetics, we explored a model of cardiac-specific disruption of KATP channel function as a control. Tg[CX1-eGFP-Kir6.1AAA] mice, which express eGFP in all somatic cells (Fig. 2A, upper panel) but are otherwise phenotypically normal [26,47], were crossed with αMHC-Cre transgenic animals [48]. The resulting Tg[αMHC-Kir6.1AAA] progeny displayed cardiac-specific potassium impermeable Kir6.1AAA expression tracked by selective cardiac elimination of eGFP fluorescence (Fig. 2A, bottom). The eGFP fluorescence was not eliminated in any other tissue, including brain (Fig. 2A, bottom, left panel), skeletal muscle (Fig. 2A, bottom, middle panel), vasculature, or pancreas (not shown), consistent with previous reports [47,48], and supporting the expression of the dominant negative Kir6.1AAA construct only in cardiomyocytes.
There was no difference in the number of KATP channels/patch (20.88±1.18pA/pF, n=6, p=NS) or whole cell KATP channel current in cardiomyocytes from sedentary Tg[CX1-eGFP-Kir6.1AAA] (4.5±.64, n=10, p=NS, tip size 4.74±.21mΩ) compared to sedentary WT littermates (data in section 3.1). However, cardiac expression of the mutant Kir6.1-AAA subunit significantly reduced the number of functional KATP channels per patch (Fig. 2B, C) and whole cell KATP channel current (Fig. 2D, E). Specifically, when sedentary Tg[αMHC-Kir6.1AAA] mice were compared to their sedentary WT or Tg[CX1-eGFP-Kir6.1AAA] littermates, the number of functional KATP channels/patch was reduced by 76% (Fig. 1C, Dvs. 2B, C; p<.05) and the whole cell KATP channel current by 86% (Fig. 1E, Fvs. 2D, E).
Formation of KATP channels requires 4 pore-forming subunits, and since inclusion of only one mutant Kir6.1AAA subunit is sufficient to eliminate K+ conductance, an increase in Kir6.2 expression is not expected to easily overcome the dominant-negative effect of the Kir6.1AAA transgene. As such, expression of the non-functional pore-forming subunit not only reduced the KATP channel current capacity in sedentary animals, but also prevented an exercise-induced increase in the number of functional KATP channels and KATP channel current capacity. Specifically, patch clamp assays performed on ventricular myocytes isolated from Tg[αMHC-Kir6.1AAA] with and without exposure to exercise revealed .94±.18 and 1.1±.23 channels per patch and whole cell current densities of 2.3±.5 and 2.5±.6 pA/pF, respectively (Fig. 2C-E, p=NS). Thus, cardiac-specific transgenic expression of the nonfunctional Kir6.1AAA subunit significantly reduced KATP channel current capacity and prevented its increase by exercise.
3.3. Increased KATP channel expression promotes action potential shortening in response to heart rate acceleration
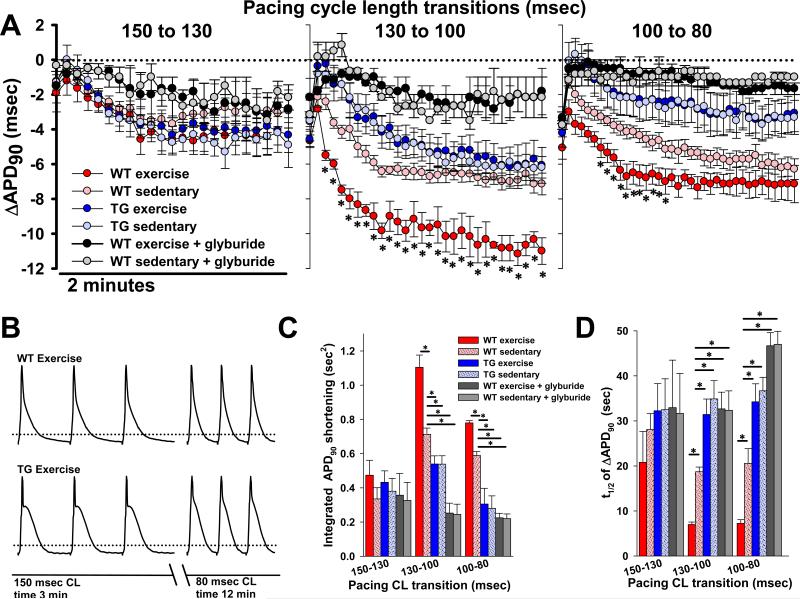
During exercise, bodily energy consumption increases 20-100 times over the basal level [51]. The ability of the cardiovascular system to deliver required oxygen and other metabolic fuels is critical for such physical performance, but is limited by the capacity to achieve and maintain sufficient heart rate and stroke volume [1]. Acceleration of heart rate requires shortening of the action potential duration to adjust myocyte refractoriness and to balance augmented cardiac contractile function with relaxation and resource replenishment [4,5,21,25-32]. We have previously shown that this action potential shortening in response to an increase in heart rate has a prominent KATP channel-dependent component [26]. Therefore, to determine if the witnessed exercise-induced increase in functional KATP channel expression is sufficient to have a measurable and physiologically relevant impact on the regulation of cardiac excitability, we evaluated action potential duration changes in response to heart rate acceleration. Specifically, we measured monophasic action potential duration in retrogradely perfused hearts isolated from exercised and sedentary mice, paced over a range of heart rates (450-720 beats/min corresponding to cycle lengths of 150-80 msec). When the change in APD90 (ΔAPD90) was tracked as a function of time following heart rate acceleration, we found that ΔAPD90 follows a complex function with an immediate decrease of several milliseconds, followed by a rapid rebound back towards baseline, and then a slower (1-2 min) decline to a steady state. Wild type hearts exposed to exercise, associated with an increase in KATP channel expression, demonstrated enhanced APD90 shortening at higher heart rates (Fig. 3). Specifically, for the transitions 130-to-100, and 100-to-80 msec cycle length (Fig. 3A middle and left panels), there was a significantly faster and greater extent of APD90 shortening in hearts with increased KATP channel expression. There was no significant effect of exercise on the extent of APD90 shortening at the slower cycle length transition, 150-to-130 msec, which apparently did not increase energy consumption to a degree sufficient to promote KATP channel opening (Fig. 3A first panel). To quantify the magnitude and rate of the ΔAPD90 response to heart rate transitions, we examined the area above the ΔAPD90-time curve or “integrated APD90 shortening” as a parameter which reflects both the magnitude and the rate of change. We also calculated the half-time of the ΔAPD90 response in order to assess rate alone (Fig. 3C, D). The 130-to-100 and 100-to-80 msec cycle length transitions resulted in a consistently greater integrated APD90 shortening and shorter response half times for hearts from exercised compared with sedentary mice (Fig. 3C, D, p<.05). APD90 reduction in response to heart rate increase was significantly blunted in both exercise and sedentary group hearts perfused with the specific KATP channel blocker glyburide (Fig. 3A) at dose of 10μM, sufficient to completely block cardiac KATP channel current [52].
Figure 3. Exercise-induced increase in KATP channel expression translates to enhanced action potential shortening in response to heart rate acceleration.
A. Summary plots of changes in monophasic action potential shortening (ΔAPD90) as a function of time following each of three pacing cycle length transitions in isolated, perfused hearts from WT exercised (n=7), WT sedentary (n=8), TG exercised (n=7), and TG sedentary (n=7) mice (*p<.05 for WT exercise vs. WT sedentary). Also graphed are glyburide (10μM) treated hearts from WT exercise (n=3) and WT sedentary mice (n=3). Each graph indicates shortening as compared to the steady state APD90 measured for the previous pacing cycle length. There is no difference in steady state APD90 after 3 minutes of pacing at 150 msec. (59.3±3.5, 57.4±3, 59.6±3.3, 57.4±4.1, 60.3±4.1 and 59.5±3.8 msec, respectively, p=NS). The ΔAPD90 of the first action potential following the transition is graphed, followed by every 40th action potential thereafter for 2 minutes. B. Representative examples of monophasic action potentials recorded from the left ventricular epicardium of isolated, perfused hearts from exercised WT and Tg[αMHC-Kir6.1AAA] mice after 3 minutes of pacing at the displayed cycle length, in accordance with the 12 minute protocol of progressive cycle length shortening. Dotted lines indicate the 90% repolarization level. C. Summary statistics showing the integrated APD90 shortening, defined as the area above the curve of the ΔAPD90 time course (n = same as in Fig. 3A, *p<.05 compared to WT sedentary). D. Summary statistics showing the half time (t1/2) of ΔAPD90 response (n = same as in Fig. 3A, *p<.05 compared to WT sedentary). WT = wild-type, TG = Tg[αMHC-Kir6.1AAA].
Furthermore, to establish that the differential effect of heart rate acceleration on action potential shortening between exercise and sedentary groups is indeed a result of augmented KATP expression, and not due to other beneficial effects of exercise, we examined ΔAPD90 in the hearts of Tg[αMHC-Kir6.1AAA], in which expression of the dominant negative Kir6.1AAA pore-forming subunit prevented an increase in KATP channel current capacity after exposure to exercise (Fig. 2, 3). These experiments revealed a significantly decreased integrated ΔAPD90 shortening and half time of ΔAPD90 for the 130-to-100 and 100-to-80 msec cycle length transitions in the transgenic hearts compared to the hearts of WT with or without exposure to exercise (Fig. 3A, C). The total shortening of APD90 at steady state was similar in Tg[αMHC-Kir6.1AAA] and sedentary WT hearts after the 130-to-100 msec transition, indicating that the reduced KATP channel current capacity in the Tg[αMHC-Kir6.1AAA] decelerated but did not eliminate APD90 adjustment at this level of energy demand (Fig. 3A). There was no difference in the APD90 shortening between Tg[αMHC-Kir6.1AAA] hearts with and without exercise training (Fig. 3A, C). Thus, an exercise-induced increase in cardiac KATP channel expression enhanced the rate and magnitude of action potential shortening in response to heart rate acceleration.
3.4. Exercise-induced increase in KATP channel expression limits escalation of cardiac oxygen consumption during heart rate acceleration
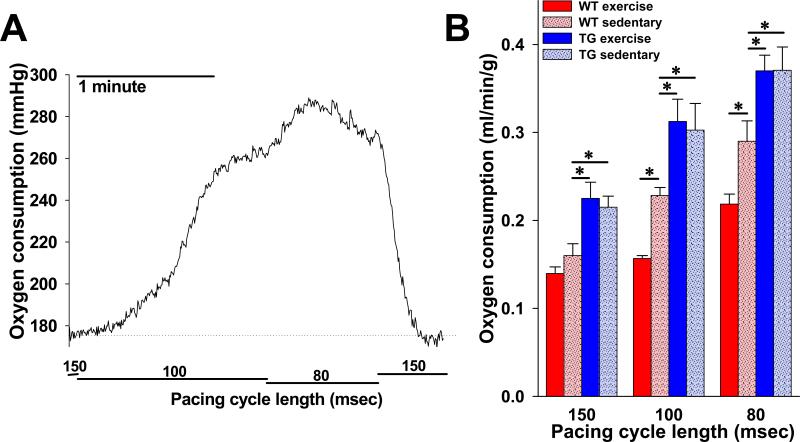
KATP channels are critical regulators of myocyte energy consumption. To investigate whether the increase in KATP channel expression and consequent enhanced action potential shortening in response to acceleration of heart rate is sufficient to impact cardiac energetic status, we compared the cardiac oxygen consumption of isolated hearts extracted from exercised and sedentary WT and Tg[αMHC-Kir6.1AAA] littermates (Fig. 4). For WT animals, oxygen consumption, normalized to heart weight and coronary flow, was comparable when hearts were paced at a cycle length of 150 msec. Shortening the pacing cycle length from 150 to 100 msec, or 100 to 80 msec, produced an expected increase in oxygen consumption but significantly less so for the exercise group compared to the sedentary group (Fig. 4B). Specifically, the oxygen consumption of sedentary WT mice was .17±.01, .23±.01, and .29±.02 ml/min/g at cycle lengths of 150, 100, and 80 msec, respectively, while oxygen consumption for exercised mice was .14±.02, .16±.003, and .22±.01 at the same cycle lengths (p<.05 for 100 and 80 msec cycle lengths). By comparison, hearts from sedentary Tg[αMHC-Kir6.1AAA] animals had significantly greater oxygen consumption at every cycle length (.22±.01, .30±.03, and .37±.03, p<.05 for each cycle length) and exercised Tg[αMHC-Kir6.1AAA] animals did not demonstrate a reduction in oxygen consumption (.23±.02, .31±.03, and .37±.02, p=NS compared to sedentary). These data confirm that normal KATP channel current capacity is necessary to properly regulate cardiac energy use, and that augmentation of KATP channel expression increases cardiac energetic efficiency at higher heart rates.
Figure 4. Rate of cardiac oxygen consumption in response to heart rate acceleration is reduced in animals with increased KATP channel expression following exercise.
A. Representative tracing of raw oxygen consumption data (perfusate oxygen tension – effluent oxygen tension) in response to pacing cycle length transitions in an isolated perfused heart from a WT sedentary animal. B. Summary statistics indicating steady state oxygen consumption rate (V̇O2), normalized to heart weight and coronary flow (see Methods), at each of three pacing cycle lengths in isolated perfused hearts from WT exercise (n=7), WT sedentary (n=7), TG exercise (n=4) and TG sedentary (n=5) mice (*p<.05 compared to WT sedentary). WT = wild-type, TG = Tg[αMHC-Kir6.1AAA].
3.5. Exercise increases transcription of ABCC9 encoding the SUR2A regulatory subunit
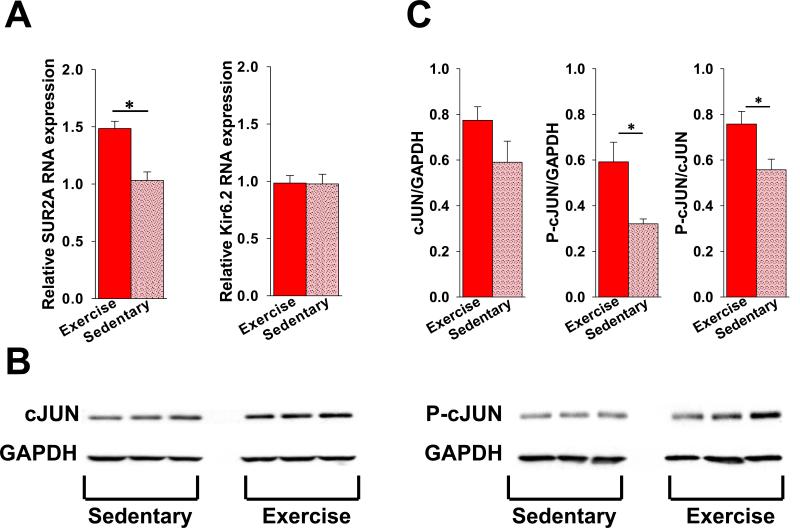
Exercise is a well-established inducer of cardioprotective gene reprogramming [50]. To investigate whether exercise-activated transcription of ABCC9 and KCNJ11 genes encoding KATP channel subunits underlies changes in the number of plasmalemmal KATP channels, Kir6.2 and SUR2A mRNA were measured using RT-PCR. Exposure to exercise caused an approximately 1.5-fold increase in the level of SUR2A mRNA, while Kir6.2 mRNA did not change (Fig. 5A). This is in agreement with the previously established role of transcription of the ABCC9 gene, encoding the SUR2A regulatory subunit, as a rate limiting factor in the assembly of functional KATP channels [53-55].
Figure 5. Increased cardiac KATP channel expression in response to exercise is associated with increased transcription of the SUR2A encoding gene.
A. Summary of the relative levels of SUR2A (left bar graph) and Kir6.2 (right bar graph) mRNA in extracts from ventricles isolated from hearts of exercised and sedentary mice (n=9 for each group, *p<.05). B. Representative western blots of cJUN and P-cJUN compared to GAPDH in extracts from ventricles isolated from hearts of exercised and sedentary mice. C. Summary of cJUN and phorphorylated cJUN (P-cJUN) expression relative to GAPDH in extracts from ventricles of hearts from exercised (n=3) and sedentary (n=3) mice (*p<.05 for Pc-JUN and Pc-JUN/cJUN exercise vs. sedentary).
The transcription of ABCC9 has been shown to be increased by the activator protein-1 (AP-1) transcription factor complex [53]. The activity of this complex is upregulated by exposure to short-term exercise [56-58] and depends on the presence of phosphorylated cJUN kinase [59]. In our model, activation of ABCC9 transcription in hearts exposed to exercise training was paralleled by an increased myocardial content of phosphorylated cJUN (P-cJUN), while the level of total cJUN was not significantly changed (Fig. 5B, C). Specifically, densitometry of western blots revealed a relative P-cJUN density of .59±.09 for exercise and .32±.02 for sedentary groups (p<.05), and a P-cJUN/cJUN ratio of .76±.06 and .56±.05, respectively (p<.05). Thus increased KATP channel expression after exposure to exercise was associated with increased transcription of the SUR2A encoding gene.
4. Discussion
KATP channels are unique membrane protein complexes critical for preservation of myocardial energy balance under normal and disease conditions [4,5,7,21,25,26,40]. Such a basic physiologic role in cardiac adaptation to increased workload is consistent with the dense expression of these channels in cardiomyocytes as well as their evolutionary conservation across a broad range of species [4-7,19,21,25,26]. Here we demonstrated the significance of physiologic regulation of KATP channel expression for cardiac energy homeostasis. Short-term exposure to exercise caused significant up-regulation of functional KATP channels at the cardiomyocyte sarcolemma. This increase in KATP channel expression enhanced APD90 shortening and reduced cardiac energy consumption in response to workload imposed by heart rate acceleration. Disruption of exercise-induced KATP channel current up-regulation in Tg[αMHC-Kir6.1AAA] transgenic mice eliminated this adaptive energy saving response.
Several earlier studies associated exposure to exercise with augmented KATP channel expression and reduced susceptibility to ischemic injury [42-45]. Our data corroborate these findings and provide a mechanistic explanation for this phenomenon. Here, enhanced expression of KATP channels increased the magnitude and rate of action potential shortening in response to workload intensification. Metabolic sensing by the channel occurs through modulation of the K+ pore ATP-sensitivity by the SUR subunit, which is also required for channel activation by MgADP and potassium channel openers as well as inhibition by sulfonylurea drugs [2-24,60]. Nucleotide exchange between the SUR2A ATPase and intracellular metabolic pathways provides a mechanism coupling KATP channel function with cellular energetics and allows precise modulation of membrane potential-dependent cellular functions in response to changes in cellular energetics [13-15,20,23,61]. However, since the bulk cellular ATP/ADP ratio remains unchanged under all but the most severe metabolic insults, the concept of local energetic signaling has been proposed [26,62]. Specifically, compartmentalization of the intracellular milieu provides conditions such that membrane ATPases may reduce the local ATP concentration, and elevate the local ADP concentration, significantly, without bulk ATP/ADP changes. Thus, KATP channels could be differentially activated according to their proximity to these ATPases [26,62,63]. We hypothesize that the findings described in the current study may be explained by the compartmentalized cellular environment in which increased expression of KATP channels could result in a greater number of channels under the influence of ATP consuming sites, thus accounting for an increased magnitude of response. Furthermore, in the presence of a larger number of channels, the probability is increased that KATP channels would be located in a closer proximity to such sites, which may explain the increased rate of action potential shortening. A faster response in the potassium efflux-driven repolarization, following an increase in heart rate, would accelerate action potential shortening and thus improve the energy efficiency of myocyte contraction and preservation of ion homeostasis [26]. This change in KATP channel-dependent membrane response, and the consequent beneficial effect on myocardial oxygen consumption, may also provide a mechanistic explanation for the previously reported increased resistance to ischemic injury of hearts from rats with exercise-induced enhancement of KATP channel expression [42-45].
Action potential shortening in response to heart rate acceleration is critical to reduce refractory period and preserve diastolic filling time [30-32]. This response includes an immediate change (first 100 milliseconds) and a longer-term (1-2 minutes) phase [30,64]. The molecular mechanisms of this response are not yet fully understood, but likely embrace complex changes in the balance between inward and outward ion currents [30-32]. The phenomenon of immediate change in action potential duration in response to a sudden increase in stimulation frequency has been attributed to incomplete recovery of ion currents from the prior cardiac cycle, such as ongoing inactivation of L-type Ca++ channels and continuing decay of outward K+ fluxes [30-32]. The next, more prolonged phase of action potential shortening, has been linked to activation of the Na+/Ca++ exchanger and the Na+/K+ ATPase as a result of changes in intracellular sodium and calcium content, as well as extracellular potassium accumulation [30-32]. Recently, we reported in a murine model that action potential adjustment in response to heart rate acceleration also depends on opening of the KATP channel [26]. The current study confirms these previous findings and also reveals the time course of these changes in the APD. An immediate APD shortening occurred with the first beat after heart rate acceleration and was similar in all experimental groups without relation to exercise or disruption of the KATP channel current. This was followed by a rapid rebound of the APD, possibly associated with activation of calcium-dependent calmodulin kinase (CaMKII) and increased calcium influx [65], which was counterbalanced by a prolonged and more prominent APD shortening phase that depended substantially on KATP channel current. The observed KATP channel-dependent APD shortening took approximately 1-2 minutes before reaching steady state. As expected, the effect was more prominent at high heart rates, likely due to an increase in Ca++ and Na+ turnover and activation of Na+/K+ and Ca++ ATPases [26,63]. We interpret these changes as confirmation that KATP channels are critical for myocyte excitability adjustment under normal physiologic dynamics of cardiac workload and that increased KATP channel expression accelerates and augments this response as part of the physiologic adaptation to exercise training.
Beneficial effects of exercise involve a broad variety of molecular targets and mechanisms [1,50]. To dissect the KATP channel-dependent mechanisms we used a transgenic model, Tg[α-MHC-Kir6.1AAA], with cardiac-specific functional disruption of sarcolemmal KATP channel potassium conductance. Expression of the dominant negative Kir6.1AAA mutant subunit has been successfully used to disrupt normal potassium conductance of sarcolemmal KATP channels in the heart, skeletal muscles and endothelium [26,29,47]. Of note, no expression of this transgene has been found in the mitochondria [29], and therefore disruption of mitochondrial KATP channels in the Tg[α-MHC-Kir6.1AAA] mice can be excluded. In this study, we use transgenic mice with mutant Kir6.1AAA expression sufficient to reduce KATP channel current density by 80%, but not to completely eliminate it. Accordingly, this reduction in KATP channel current capacity does not eliminate KATP channel-dependent APD shortening (compare to glyburide-treated hearts), but rather significantly attenuates it – an effect achieved in part by slowing the KATP channel-dependent response rate. Furthermore, exposure to exercise did not significantly change the cardiac KATP channel current capacity, APD90 response, or oxygen consumption with heart rate acceleration in Tg[α-MHC-Kir6.1AAA] mice. In fact, the oxygen consumption of Tg[α-MHC-Kir6.1AAA] hearts was greater than WT at all cycle lengths, even following the 150-to-130 msec transition when significant differences in ΔAPD90 were not found. This is in agreement with our previously published findings [26], and may reflect a difference in the resolution of the MAP vs. oxygen consumption techniques. Indeed, monophasic action potential recordings in murine hearts may correlate with, but not exactly reproduce, the absolute transmembrane action potential duration [66]. Here, we used each MAP position as its own control, and examined changes, rather than absolute duration, in order to eliminate this potential source of error. However, when true transmembrane action potentials were measured in isolated ventricular cardiomyocytes expressing Kir6.1AAA, the action potentials were found to be significantly longer than in WT, even during resting conditions, and this was corroborated by longer ventricular refractory periods during pacing studies in isolated hearts [29]. Therefore, an overall increase in APD could account for the differences in oxygen consumption seen at slower heart rates. Also, similar to previously published transmembrane action potentials in ventricular myocytes from Tg[αMHC-Kir6.1AAA mice [29], we see a more prominent action potential plateau. This suggests increased calcium entry, increased calcium release, and thus increased SERCA2A activity to restore diastolic calcium concentrations. This, possibly coupled with increased contraction, would be expected to increase ATP hydrolysis and oxygen consumption. Thus, the data reported in the current study support a previously unrecognized homeostatic feedback loop for myocardial energy conservation: exercise-related increase in cardiac energy demand triggers an increase in KATP channel expression which, in turn, enhances APD shortening and thus limits workload-driven energy consumption.
Defining this mechanism further expands our understanding of exercise-induced cardiovascular conditioning leading to improved cardiovascular performance [1]. Indeed, exposure to exercise causes a broad spectrum of beneficial changes which promote cardiac and general stress resistance [1,50]. One of the reported consequences of exercise-related gene reprogramming associated with short-term exposure to exercise (up to 5 days) is activation of the c-Jun/NH2-terminal kinase signaling cascade [56,58], critical for activation of SUR2A encoding gene transcription [53]. Indeed, our data indicate that the exercise-induced increase in phosphorylated cJUN was paralleled by a 50% increase in the presence of SUR2A encoding mRNA, comparable with the change in functional KATP channel expression. No change was evident in the level of Kir6.2 encoding mRNA in agreement with previously reported data which indicate that Kir6.2 mRNA is present in relative abundance to SUR encoding mRNA, and does not correlate with KATP channel complex expression [53]. Although detailed definition of the mechanism underlying up-regulation of KATP channels by exercise will require future study, we interpret the current findings as an indication that elevated transcription of ABCC9 promotes production of SUR2A, driving the increased expression of functional KATP channels in response to short-term exposure to exercise.
A limitation of this study is that we did not measure energy consumption by membrane ATPases or sub-membrane ATP/ADP levels. These future experiments would provide a more comprehensive understanding of KATP channel responses under physiologic or pathophysiologic conditions. It will also be critical to investigate the role of KATP channels in APD adaptation to heart rate acceleration in other animal models to exclude this as a species-specific phenomenon related to the unique composition of ion channel currents comprising the murine action potential.
In this study we examined KATP channel-dependent changes associated with short-term exposure to exercise. It has previously been reported that long-term exposure to exercise also augments KATP channel expression [42]. It is possible that these changes in KATP channel expression are driven by different mechanisms and not by activation of SUR2A transcription, as AP1 activity is known to be reduced in chronically trained hearts [56,58]. It would be important to evaluate KATP channel subunit stability, localization, trafficking, endocytosis, and degradation, as well as AP1 independent transcription activation. It will also be important to define the duration of KATP channel expression and cardiac energy consumption changes in response to exercise.
5. Conclusions
This study demonstrates that dynamic regulation of cardiac KATP channel expression modulates the speed and magnitude of the membrane electrical response to changes in the metabolic state of the cell. This mechanism allows the cardiomyocyte to adapt energy consumption to available resources more efficiently, and thereby maintain cardiac well-being under a range of physiologic stresses. Further understanding of the mechanisms responsible for KATP channel regulation provides novel insights into endogenous cardioprotection, and the development of strategies for the management and prevention of cardiovascular injury and diseases.
Acknowledgments
This work was supported by NIH grant HL092286 and the Carver Trust Young Investigator Award to D.H-Z., by NIH grant HL093368 and the Carver Pilot Grant to L.Z., and by NIH grant HL085820 to W.A.C. We are grateful to Dr. Michael D. Schneider for Tg[αMHC-Cre] mice.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None declared
References
- 1.Guyton AC, Hall JE. Sport physiology. In: Guyton AC, Hall JE, editors. Textbook of medical physiology. Elsevier Saunders; Philadelphia, PA: 2006. pp. 1062–1062-1065. [Google Scholar]
- 2.Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr Rev. 1999;20:101–35. doi: 10.1210/edrv.20.2.0361. [DOI] [PubMed] [Google Scholar]
- 3.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 4.Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–76. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- 5.Flagg TP, Enkvetchakul D, Koster JC, Nichols CG. Muscle KATP channels: recent insights to energy sensing and myoprotection. Physiol Rev. 2010;90:799–829. doi: 10.1152/physrev.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675–86. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- 7.Ashcroft FM. Adenosine 5'-triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- 8.Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–7. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 9.Inagaki N, Gonoi T, Clement JP, 4th, Namba N, Inazawa J, Gonzalez G, et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–70. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 10.Lefer DJ, Nichols CG, Coetzee WA. Sulfonylurea receptor 1 subunits of ATP-sensitive potassium channels and myocardial ischemia/reperfusion injury. Trends Cardiovasc Med. 2009;19:61–7. doi: 10.1016/j.tcm.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clement JP, 4th, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, et al. Association and stoichiometry of K(ATP) channel subunits. Neuron. 1997;18:827–38. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 12.Schwappach B, Zerangue N, Jan YN, Jan LY. Molecular basis for K(ATP) assembly: transmembrane interactions mediate association of a K+ channel with an ABC transporter. Neuron. 2000;26:155–67. doi: 10.1016/s0896-6273(00)81146-0. [DOI] [PubMed] [Google Scholar]
- 13.Abraham MR, Selivanov VA, Hodgson DM, Pucar D, Zingman LV, Wieringa B, et al. Coupling of cell energetics with membrane metabolic sensing. Integrative signaling through creatine kinase phosphotransfer disrupted by M-CK gene knock-out. J Biol Chem. 2002;277:24427–34. doi: 10.1074/jbc.M201777200. [DOI] [PubMed] [Google Scholar]
- 14.Bienengraeber M, Alekseev AE, Abraham MR, Carrasco AJ, Moreau C, Vivaudou M, et al. ATPase activity of the sulfonylurea receptor: a catalytic function for the KATP channel complex. FASEB J. 2000;14:1943–52. doi: 10.1096/fj.00-0027com. [DOI] [PubMed] [Google Scholar]
- 15.Carrasco AJ, Dzeja PP, Alekseev AE, Pucar D, Zingman LV, Abraham MR, et al. Adenylate kinase phosphotransfer communicates cellular energetic signals to ATP-sensitive potassium channels. Proc Natl Acad Sci U S A. 2001;98:7623–8. doi: 10.1073/pnas.121038198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lederer WJ, Nichols CG. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Rourke B, Ramza BM, Marban E. Oscillations of membrane current and excitability driven by metabolic oscillations in heart cells. Science. 1994;265:962–6. doi: 10.1126/science.8052856. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki N, Sato T, Marban E, O'Rourke B. ATP consumption by uncoupled mitochondria activates sarcolemmal K(ATP) channels in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2001;280:H1882–8. doi: 10.1152/ajpheart.2001.280.4.H1882. [DOI] [PubMed] [Google Scholar]
- 19.Weiss JN, Venkatesh N. Metabolic regulation of cardiac ATP-sensitive K+ channels. Cardiovasc Drugs Ther. 1993;7(Suppl 3):499–505. doi: 10.1007/BF00877614. [DOI] [PubMed] [Google Scholar]
- 20.Zingman LV, Alekseev AE, Bienengraeber M, Hodgson D, Karger AB, Dzeja PP, et al. Signaling in channel/enzyme multimers: ATPase transitions in SUR module gate ATP-sensitive K+ conductance. Neuron. 2001;31:233–45. doi: 10.1016/s0896-6273(01)00356-7. [DOI] [PubMed] [Google Scholar]
- 21.Zingman LV, Alekseev AE, Hodgson-Zingman DM, Terzic A. ATP-sensitive potassium channels: metabolic sensing and cardioprotection. J Appl Physiol. 2007;103:1888–93. doi: 10.1152/japplphysiol.00747.2007. [DOI] [PubMed] [Google Scholar]
- 22.Zingman LV, Hodgson DM, Alekseev AE, Terzic A. Stress without distress: homeostatic role for K(ATP) channels. Mol Psychiatry. 2003;8:253–4. doi: 10.1038/sj.mp.4001323. [DOI] [PubMed] [Google Scholar]
- 23.Zingman LV, Hodgson DM, Bienengraeber M, Karger AB, Kathmann EC, Alekseev AE, et al. Tandem function of nucleotide binding domains confers competence to sulfonylurea receptor in gating ATP-sensitive K+ channels. J Biol Chem. 2002;277:14206–10. doi: 10.1074/jbc.M109452200. [DOI] [PubMed] [Google Scholar]
- 24.Shyng S, Ferrigni T, Nichols CG. Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J Gen Physiol. 1997;110:643–54. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–83. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alekseev AE, Reyes S, Yamada S, Hodgson-Zingman DM, Sattiraju S, Zhu Z, et al. Sarcolemmal ATP-sensitive K(+) channels control energy expenditure determining body weight. Cell Metab. 2010;11:58–69. doi: 10.1016/j.cmet.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34:1259–71. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- 28.Moore RL. Myocardial KATP channels are critical to Ca2+ homeostasis in the metabolically stressed heart in vivo. Am J Physiol Heart Circ Physiol. 2007;292:H1692–3. doi: 10.1152/ajpheart.00076.2007. [DOI] [PubMed] [Google Scholar]
- 29.Tong X, Porter LM, Liu G, Dhar-Chowdhury P, Srivastava S, Pountney DJ, et al. Consequences of cardiac myocyte-specific ablation of KATP channels in transgenic mice expressing dominant negative Kir6 subunits. Am J Physiol Heart Circ Physiol. 2006;291:H543–51. doi: 10.1152/ajpheart.00051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisner DA, Dibb KM, Trafford AW. The mechanism and significance of the slow changes of ventricular action potential duration following a change of heart rate. Exp Physiol. 2009;94:520–8. doi: 10.1113/expphysiol.2008.044008. [DOI] [PubMed] [Google Scholar]
- 31.Carmeliet E. Action potential duration, rate of stimulation, and intracellular sodium. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S2–7. doi: 10.1111/j.1540-8167.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 32.Carmeliet E. Intracellular Ca(2+) concentration and rate adaptation of the cardiac action potential. Cell Calcium. 2004;35:557–73. doi: 10.1016/j.ceca.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Yamada S, Kane GC, Behfar A, Liu XK, Dyer RB, Faustino RS, et al. Protection conferred by myocardial ATP-sensitive K+ channels in pressure overload-induced congestive heart failure revealed in KCNJ11 Kir6.2-null mutant. J Physiol. 2006;577:1053–65. doi: 10.1113/jphysiol.2006.119511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kane GC, Behfar A, Dyer RB, O'Cochlain DF, Liu XK, Hodgson DM, et al. KCNJ11 gene knockout of the Kir6.2 KATP channel causes maladaptive remodeling and heart failure in hypertension. Hum Mol Genet. 2006;15:2285–97. doi: 10.1093/hmg/ddl154. [DOI] [PubMed] [Google Scholar]
- 35.Liu XK, Yamada S, Kane GC, Alekseev AE, Hodgson DM, O'Cochlain F, et al. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes. 2004;53(Suppl 3):S165–8. doi: 10.2337/diabetes.53.suppl_3.s165. [DOI] [PubMed] [Google Scholar]
- 36.Kane GC, Behfar A, Yamada S, Perez-Terzic C, O'Cochlain F, Reyes S, et al. ATP-sensitive K+ channel knockout compromises the metabolic benefit of exercise training, resulting in cardiac deficits. Diabetes. 2004;53(Suppl 3):S169–75. doi: 10.2337/diabetes.53.suppl_3.s169. [DOI] [PubMed] [Google Scholar]
- 37.Gumina RJ, O'Cochlain DF, Kurtz CE, Bast P, Pucar D, Mishra P, et al. KATP channel knockout worsens myocardial calcium stress load in vivo and impairs recovery in stunned heart. Am J Physiol Heart Circ Physiol. 2007;292:H1706–13. doi: 10.1152/ajpheart.01305.2006. [DOI] [PubMed] [Google Scholar]
- 38.Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O'Cochlain F, Gao F, et al. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–7. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu X, Xu X, Huang Y, Fassett J, Flagg TP, Zhang Y, et al. Disruption of sarcolemmal ATP-sensitive potassium channel activity impairs the cardiac response to systolic overload. Circ Res. 2008;103:1009–17. doi: 10.1161/CIRCRESAHA.107.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hodgson DM, Zingman LV, Kane GC, Perez-Terzic C, Bienengraeber M, Ozcan C, et al. Cellular remodeling in heart failure disrupts K(ATP) channel-dependent stress tolerance. EMBO J. 2003;22:1732–42. doi: 10.1093/emboj/cdg192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, et al. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002;109:509–16. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown DA, Chicco AJ, Jew KN, Johnson MS, Lynch JM, Watson PA, et al. Cardioprotection afforded by chronic exercise is mediated by the sarcolemmal, and not the mitochondrial, isoform of the KATP channel in the rat. J Physiol. 2005;569:913–24. doi: 10.1113/jphysiol.2005.095729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chicco AJ, Johnson MS, Armstrong CJ, Lynch JM, Gardner RT, Fasen GS, et al. Sex-specific and exercise-acquired cardioprotection is abolished by sarcolemmal KATP channel blockade in the rat heart. Am J Physiol Heart Circ Physiol. 2007;292:H2432–7. doi: 10.1152/ajpheart.01301.2006. [DOI] [PubMed] [Google Scholar]
- 44.Brown DA, Moore RL. Perspectives in innate and acquired cardioprotection: cardioprotection acquired through exercise. J Appl Physiol. 2007;103:1894–9. doi: 10.1152/japplphysiol.00464.2007. [DOI] [PubMed] [Google Scholar]
- 45.Jew KN, Moore RL. Exercise training alters an anoxia-induced, glibenclamide-sensitive current in rat ventricular cardiocytes. J Appl Physiol. 2002;92:1473–9. doi: 10.1152/japplphysiol.00513.2001. [DOI] [PubMed] [Google Scholar]
- 46.Ferrero JM, Jr, Saiz J, Ferrero JM, Thakor NV. Simulation of action potentials from metabolically impaired cardiac myocytes. Role of ATP-sensitive K+ current. Circ Res. 1996;79:208–21. doi: 10.1161/01.res.79.2.208. [DOI] [PubMed] [Google Scholar]
- 47.Malester B, Tong X, Ghiu I, Kontogeorgis A, Gutstein DE, Xu J, et al. Transgenic expression of a dominant negative K(ATP) channel subunit in the mouse endothelium: effects on coronary flow and endothelin-1 secretion. FASEB J. 2007;21:2162–72. doi: 10.1096/fj.06-7821com. [DOI] [PubMed] [Google Scholar]
- 48.Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–79. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinaasappel M, Donkersloot C, van Bommel J, Ince C. PO2 measurements in the rat intestinal microcirculation. Am J Physiol. 1999;276:G1515–20. doi: 10.1152/ajpgi.1999.276.6.G1515. [DOI] [PubMed] [Google Scholar]
- 50.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–15. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spriet LL, Hargreaves M. Overview of exercise metabolism. In: Hargreaves M, Spriet LL, editors. Exercise Metabolism. Human Kinetics; Champaign, IL: 2006. pp. 1–1-7. [Google Scholar]
- 52.Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–91. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 53.Crawford RM, Jovanovic S, Budas GR, Davies AM, Lad H, Wenger RH, et al. Chronic mild hypoxia protects heart-derived H9c2 cells against acute hypoxia/reoxygenation by regulating expression of the SUR2A subunit of the ATP-sensitive K+ channel. J Biol Chem. 2003;278:31444–55. doi: 10.1074/jbc.M303051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Du Q, Jovanovic S, Clelland A, Sukhodub A, Budas G, Phelan K, et al. Overexpression of SUR2A generates a cardiac phenotype resistant to ischemia. FASEB J. 2006;20:1131–41. doi: 10.1096/fj.05-5483com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jovanovic A, Jovanovic S. SURA2 targeting for cardioprotection? Curr Opin Pharmacol. 2009;9:189–93. doi: 10.1016/j.coph.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Boluyt MO, Loyd AM, Roth MH, Randall MJ, Song EY. Activation of JNK in rat heart by exercise: effect of training. Am J Physiol Heart Circ Physiol. 2003;285:H2639–47. doi: 10.1152/ajpheart.00596.2003. [DOI] [PubMed] [Google Scholar]
- 57.Iemitsu M, Maeda S, Jesmin S, Otsuki T, Kasuya Y, Miyauchi T. Activation pattern of MAPK signaling in the hearts of trained and untrained rats following a single bout of exercise. J Appl Physiol. 2006;101:151–63. doi: 10.1152/japplphysiol.00392.2005. [DOI] [PubMed] [Google Scholar]
- 58.Wakatsuki T, Schlessinger J, Elson EL. The biochemical response of the heart to hypertension and exercise. Trends Biochem Sci. 2004;29:609–17. doi: 10.1016/j.tibs.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 60.D'hahan N, Moreau C, Prost AL, Jacquet H, Alekseev AE, Terzic A, et al. Pharmacological plasticity of cardiac ATP-sensitive potassium channels toward diazoxide revealed by ADP. Proc Natl Acad Sci U S A. 1999;96:12162–7. doi: 10.1073/pnas.96.21.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Crawford RM, Ranki HJ, Botting CH, Budas GR, Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. FASEB J. 2002;16:102–4. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selivanov VA, Alekseev AE, Hodgson DM, Dzeja PP, Terzic A. Nucleotide-gated KATP channels integrated with creatine and adenylate kinases: amplification, tuning and sensing of energetic signals in the compartmentalized cellular environment. Mol Cell Biochem. 2004;256-257:243–56. doi: 10.1023/b:mcbi.0000009872.35940.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yaniv Y, Juhaszova M, Nuss HB, Wang S, Zorov DB, Lakatta EG, et al. Matching ATP supply and demand in mammalian heart: in vivo, in vitro, and in silico perspectives. Ann N Y Acad Sci. 2010;1188:133–42. doi: 10.1111/j.1749-6632.2009.05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franz MR, Swerdlow CD, Liem LB, Schaefer J. Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady-state frequencies. J Clin Invest. 1988;82:972–9. doi: 10.1172/JCI113706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu Y, Shintani A, Grueter C, Zhang R, Hou Y, Yang J, et al. Suppression of dynamic Ca(2+) transient responses to pacing in ventricular myocytes from mice with genetic calmodulin kinase II inhibition. J Mol Cell Cardiol. 2006;40:213–23. doi: 10.1016/j.yjmcc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 66.Knollmann BC, Katchman AN, Franz MR. Monophasic action potential recordings from intact mouse heart: validation, regional heterogeneity, and relation to refractoriness. J Cardiovasc Electrophysiol. 2001;12:1286–94. doi: 10.1046/j.1540-8167.2001.01286.x. [DOI] [PubMed] [Google Scholar]