Abstract
Airway inflammation induced by reactive oxygen species-mediated activation of redox-sensitive transcription factors is the hallmark of asthma, a prevalent chronic respiratory disease. In various cellular and animal models, we have recently demonstrated that, in response to multiple stimuli, aldose reductase (AKR1B1) regulates the inflammatory signals via NF-kappa B activation. Since NF-κB activation is implicated in asthma pathogenesis, we investigated whether AKR1B1 inhibition could prevent ovalbumin (Ova)- and ragweed pollen extract (RWE)-induced airway inflammation and hyper-responsiveness in mice models and tumor necrosis factor-alpha (TNF-α)-, lipopolysachharide (LPS)- and RWE-induced cytotoxic and inflammatory signals in primary human small airway epithelial cells (SAEC). Sensitization and challenge with Ova or RWE caused airway inflammation and production of inflammatory cytokines, accumulation of eosinophils in airways and sub-epithelial regions, mucin production in the bronchoalveolar lavage fluid, airway hyperresponsiveness, elevated IgE levels and release of Th2 cytokines in the airway and treatment with AKR1B1 inhibitors markedly reduced these pathological changes in mice. In SAEC, treatment with TNF-α, LPS or RWE induced apoptosis, reactive oxygen species generation, synthesis of inflammatory markers IL-6, IL-8, and PGE2 and activation of NF-κB and AP-1. Pharmacological inhibition prevented these changes suggesting that AKR1B1 mediates ROS induced inflammation in small airway epithelial cells. Our results indicate that AKR1B1 inhibitors may offer a novel therapeutic approach to treat inflammatory airway diseases such as asthma.
Keywords: airway inflammation, aldose reductase, ROS, asthma, ragweed pollen extract
1. Introduction
Airway inflammation is one of the main characteristic features of asthma, which affects approximately 300 million people worldwide [1]. In the US, approximately 8% of the population suffers from asthma and according to an estimate the annual expenditure on asthma treatment was approximately 37 billion dollars in 2007 [2,3]. In spite of progress made in the therapy and management of the disease, there has been increase in the prevalence of asthma in last few decades which suggests the lack of clear understanding of the pathophysiology of asthma [4]. The ever increasing evidences suggest that decrease in the antioxidant capacity of the airway and resultant oxidative stress due to either external stimuli such as environmental pollutants, allergens, gases, particulate matters, pets, exercise, cold etc or intrinsic factors such as inappropriate immune response, genetic predisposition or compromised host-pathogen interaction could be significant player in asthma pathogenesis [5,6,7,8,9,10]. The decrease in antioxidant capacity of the lung results from an enhanced production of reactive oxygen species (ROS) upon exposure to the extrinsic or intrinsic stimulants results in the generation of inflammatory mediators that drive the pathophysiology of asthma [11, 12]. The altered redox status of the airway cells due to overwhelming level of ROS activates the cascade of molecular signals involving an array of intermediate protein kinases eventually leading to activation of transcription factors that transcribe a number of inflammatory genes including cytokines, chemokines and other mediators such as prostaglandins, leukotrienes, nitric oxide, adhesion molecules, and proteases [13]. The inflammatory mediators, when released in the local microenvironment, attract an entire array of immune cells such as eosinophils, macrophages, neutrophils and other leukocytes, which further secrete inflammatory cytokines and chemokines starting a cycle of events resulting in tissue damage, cytotoxicity, and remodeling which contribute to the progression of asthma [14].
There has been increased correlation between the markers of oxidative stress including malondialdehyde, thiobarbituric acid reactive products, and oxidized glutathione (GSSG) in the urine, plasma, sputum, and BAL fluid of patients with severity of asthma [15]. The increased ROS levels lead to lipid peroxidation resulting in the formation of lipid-derived aldehydes such 4-hydroxynonenal (HNE), which could readily combine with the cellular reduced glutathione (GSH) to form GS-lipid aldehydes conjugates thereby trapping cellular glutathione and increasing the oxidative stress even further [16]. In addition, we have shown that both lipid derived aldehydes such as HNE as well as their glutathione conjugates (GS-HNE) are the excellent substrates (Km=10–30 μM) for aldose reductase, an enzyme linked to diabetic complications for its role in the reduction of glucose to sorbitol via polyol pathway [17,18]. We have demonstrated that the reduced product of GS-lipid aldehydes i.e. GS-lipid alcohol (such as GS-DHN) is an important inducer of signaling cascade that activates the signaling kinases including PKC, PLC, MAPKs in different cellular models eventually activating transcription factors leading to transcription of various genes involved in inflammation and pathologies [19,20] and inhibition of AKR1B1 prevents ROS-induced inflammatory changes in cellular and animal models probably by blocking the formation of GS-lipid alcohol species and breaks the cycle of events that results in the pathological development [18,19,20]. Based on these evidences, we hypothesized that since ROS and ROS-mediated lipid peroxidation products are involved in asthma pathogenesis, blocking the ROS-mediated activation of inflammatory signals by AKR1B1 inhibition could prevent airway inflammation.
Here we have investigated whether stimulants such as RWE, bacterial endotoxin, LPS or TNF-α-induced inflammatory changes in human primary small airway epithelial cells could be prevented by pharmacological inhibition or siRNA ablation of AKR1B1. Further, we have also used animal models of Ova- or RWE-induced asthma in mice to further confirm our hypothesis. Our results demonstrate that inhibition of AKR1B1 in airway cells prevented various oxidants – induced cytotoxicity, ROS levels, inflammatory mediators, activation of signaling intermediates and airway resistance, cytokines and chemokines synthesis in mouse model of Ova- or RWE-induced airway inflammation. These results suggest important role of AKR1B1 in asthma pathogenesis and that the use of AKR1B1 inhibitors could be a potential therapeutic approach for airway inflammation in asthma.
2. Materials and Methods
2.1 Reagents
The media and reagent pack for cell culture such as small airway epithelial basal medium (SABM), and small airway epithelial growth media (SAGM™) bulletkit and Reagent pack containing Trypsin and EDTA in the ratio of 0.025% : 0.01%, Trypsin neutralizing solution and HEPES buffered saline solution were purchaged from Lonza Walkersville Inc. (Walkersville, MD). Two structurally different AKR1B1 inhibitors, Sorbinil and Zopolrestat, were obtained as gifts from Pfizer (New York, NY). Dimethyl sulfoxide (DMSO) was purchased from Fischer scientific (Pittsburg, PA). LPS from Escherichia coli was from Sigma (Sigma-Aldrich, Saint Louise, MO), Recombinant TNF-α was purchased from Research diagnostics Inc (Concord, MA). Nitrite/Nitrate and PGE2 assay kits were from Cayman Chemical Inc (Ann Arbor, MI). Human IL-6 ELISA kits was from Diaclone (Stamford, CT) and and IL-8 ELISA kit was from R&D systems (Minneapolis, MN). The ROS sensitive dihydroethidium (DHE) fluorescent dye was from Molecular Probes, Invitrogen (Carlsbad, CA). All other reagents used were of analytical grade.
2.2. Cell Culture
The human primary small airway epithelial cells (SAEC) were obtained from Lonza Walkersville Inc. (Walkersville, MD) and cultured according to the supplier’s instructions at 37°C in humidified incubator with 95% O2 and 5% CO2 in SABM supplemented with 52 μg/ml bovine pituitary extract, 0.5 ng/ml human recombinant epidermal growth factor (EGF), 0.5 μg/ml epinephrine, 1 μg/ml hydrocortisone, 10 μg/ml transferrin, 5 μg/ml insulin, 0.1 ng/ml retinoic acid (RA), 6.5 ng/ml triiodothyronine, 50 μg/ml Gentamicin/Amphotericin-B (GA-1000), and 50 μg/ml fatty acid-free bovine serum albumin (BSA). The cells were passaged using reagent pack supplied by the vendor and cells from passages 3–7 were used in the experiments.
2.3. Cell Viability Assays
To assess the cell viability 5000 cells/well in a 96-well plate were seeded. The cells were growth-arrested for 24 h by replacing complete medium with fresh basal medium containing AKR1B1 inhibitors sorbinil (in all experiments with TNF-α or LPS) or zopolrestat (in all experiments with RWE) (20 μM) or carrier. The cells were incubated with TNF-α (2 nM), LPS (1 μg/mL) or carrier for an additional 24 h, after which 10 uL of MTT (5 mg/ml) was added to each well and incubated at 37°C for 2 h. The medium was removed and the formazan granules obtained were dissolved in 100% DMSO. Absorbance was read at 570 nm using a 96-well ELISA plate reader. Manual cell counting using hemocytometer was performed in experiments with RWE because high level of NADPH oxidase is present RWE which interfered with the MTT assay. Further, FITC labeled Annexin-V and propidium iodide (PI) staining of the cells after treatment with the stimulants was used to confirm the apoptotic cell death. Approximately 2×105 SAEC per well plated in 6-well plates for overnight. The medium was replaced with serum-free SABM with or without AKR1B1 inhibitors sorbinil or zopolrestat (20 μM) and incubated for 24 h. The cells were treated with RWE (150 μg/ml), TNF-α (2 nM) or LPS (1 μg/mL) in a fresh medium containing AKR1B1 inhibitors or carrier and incubated for additional 18 h. Apoptotic cell death was examined using the annexin-V FITC/PI, (molecular probes, Invitrogen) according to the manufacturer’s instructions. Twenty thousand events for each sample were acquired and analyzed by flow cytometry using the LYSIS II software (FACScan, BD Pharmingen) and results are expressed as percent annexin-V positive cells.
2.4. Detection of superoxide
For the detection of ROS in the cells in response to stimulant challenge, approximately 1×105 SAEC were seeded on chambered slides and incubated for over-night at 37°C. The cells were starved in serum-free basal medium containing AKR1B1 inhibitors (20 μM) or carrier for 24 h. The cells were then stimulated with RWE, TNF-α, LPS or carrier for 16 h. Subsequently, the cells were washed with cold PBS twice and incubated with DHE fluorescent dye at the final concentration of 2.5 μmol/L in PBS at 37°C in a humidified chamber for 15 min. The cells were washed in PBS twice and mounted with fluorsave mounting medium containing DAPI (Vector Laboratories Inc., Burlingame, CA). The photomicrographs were obtained and analyzed with a Nikon epifluorescence microscope with a 585 nm long-pass filter. The results are presented as relative fluorescence units.
2.5. ELISA for cytokines in cell-culture medium
For the determination of inflammatory mediators such as PGE2, IL-6 and IL-8, approximately 2×105 SAEC were seeded per well in 6-well plates in triplicate for each group and incubated for overnight. The cells were starved in serum-free basal medium containing AKR1B1 inhibitors (20 μM) or carrier. The growth-arrested cells were treated with either RWE (150 μg/ml), TNF-α (2 nM), LPS (1 μg/mL) or carrier in serum-free medium for another 24 h. The media were collected, cleared by centrifugation and the supernatant was analyzed for PGE2 (Cayman Chemical Co., Ann Arbor, Michigan); IL-6 (Diaclone, Stamford, CT) and IL-8 (R&D systems Inc, Minneapolis, MN) by using respective kits following manufacturer’s instructions.
2.6. Secretory alkaline phosphatase (SEAP) reporter Assay
The activation of NF-κB was assayed using a highly sensitive reporter assay. The SAEC were serum starved without or with AKR1B1 inhibitors for 24 h and transiently transfected with pNF-κB-SEAP construct or control plasmid pTAL-SEAP DNA (Clontech, Palo Alto, CA) using the LipofectAMINE Plus reagent. After 6 h, transfection medium was replaced with fresh medium and cells were incubated with TNF-α (2 nM), LPS (1 μg/ml) or RWE (50 μg/ml) or carrier for 48 h. The cell culture medium was harvested and SEAP activity was analyzed using a 96-well chemiluminescence plate reader, essentially as described by the manufacturer (Clontech, Palo Alto, CA).
2.6. Animals
Mice (Balb/c and C57BL/6; wild type) from Harlan Sprague-Dawley (San Diego, CA, USA) were bred in a specific-pathogen free facility at Louisiana State University Health Sciences Center, New Orleans, LA, and allowed unlimited access to sterilized chow and water. Maintenance, experimental protocols, and procedures were all approved by the University of Texas Medical Branch Animal Care and Use Committee and the Louisiana State University Health Sciences Center Animal Care & Use Committee. All animal experiments were performed according to the National Institutes of Health Guide for Care and Use of Experimental Animals.
2.7. Sensitization, challenge and administration of drugs
Six-Eight weeks old mice were sensitized with two intraperitoneal administrations of endotoxin-free RWE (Greer Laboratories, Lenoir, NC, USA), 150 μg/100 μl/injection, combined in a 3:1 ratio with Alum adjuvant (Pierce Laboratories, Rockford, IL, USA) on days 0 and 4. On days 9 and 10, animals were administered with AKR1B1 inhibitor zopolrestat (i.p., 25 mg per kg) every 12 h for total duration of 48 h. On day 11, mice (n = 6–8) were challenged intranasally with RWE (100 μg), Control groups of mice were challenged with equivalent volumes of PBS. For sensitization with Ova, six-eight-weeks old C57BL/6 wild type mice were sensitized with injections (i.p.) of 100 μg Grade-V chicken ovalbumin (Sigma-Aldrich, St. Louis MO), mixed with 2 mg aluminum hydroxide in saline on day 1 and 7. On day 14 the mice were challenged by placing them in groups of six in a Plexiglas chamber and were exposed for 30 min to aerosolized Ova (3% Ova in saline). The Ova-aerosol was generated by a Bennett nebulizer (DeVilbiss, PA). The mice in AKR1B1 inhibitor-treated group received an injection of 25 mg AKR1B1 inhibitor sorbinil (i.p.)/kg body weight prior to challenge. Control groups were not sensitized or challenged.
2.8. Evaluation of eosinophils in BAL during allergic airway inflammation
To evaluate inflammation, RWE- and Ova- challenged animals were euthanized after 72 h and 48 h, respectively (unless mentioned otherwise) either with overdose of ketamine (135 mg/kg body wt) and xylazine (15 mg/kg body wt) or CO2 asphyxiation, and the lungs were lavaged with two 0.8 ml aliquots of ice-cold PBS. The cells were collected by centrifugation (1000 g, for 10 min at 4°C) and resuspended in one ml of PBS, and total cell counts were determined. Differential cell counts were performed on cytocentrifuge preparations stained with hematoxylin and eosin.
2.9. Cytokine assessment in BAL
The concentrations of Th2 cytokines IL-4, IL-5, IL-6, and IL-10 and chemokines G-CSF, KC and MCP-1 in BAL fluids were determined using the Bio-Rad Bioplex System for mouse according to the manufacturer’s instructions.
2.10. Statistical analysis
For the cell culture experiments data presented are mean ± SD and p values were determined by unpaired student’s t test. For animal studies, data were analyzed by ANOVA, followed by Bonferroni post-hoc analyses for least significant difference. p<0.05 was considered as statistically significant.
3. Results
3.1. TNF-α-, LPS- and RWE-induced ROS levels prevented by AKR1B1 inhibition in SAEC
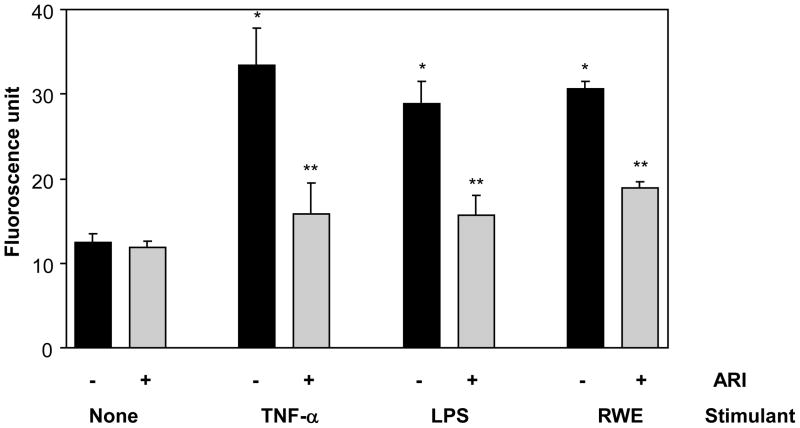
First we examined if SAEC showed increased generation of ROS in response to the treatment with TNF-α, LPS or RWE. As shown in Fig 1, approximately 2-fold increase in the level of ROS was observed after stimulation with TNF-α, LPS or RWE compared to control cells not stimulated with any of these agents and treatment with AKR1B1 inhibitor prevented the increase in ROS levels in SAEC.
Figure 1. AKR1B1 inhibition prevents TNF-α-, LPS- and RWE-induced ROS generation in SAEC.
Relative ROS levels in RWE-, TNF-α-, or LPS-treated SAEC without or with ARI are expressed as a change in fluorescence (arbitrary units). The bars represent mean ± SD (n = 6); *p<0.01 vs control; **p<0.02 vs TNF-α/LPS/RWE. ARI, AKR1B1 inhibitor
3.2. TNF-α-, LPS- and RWE-induced cellular apoptosis prevented by AKR1B1 inhibition
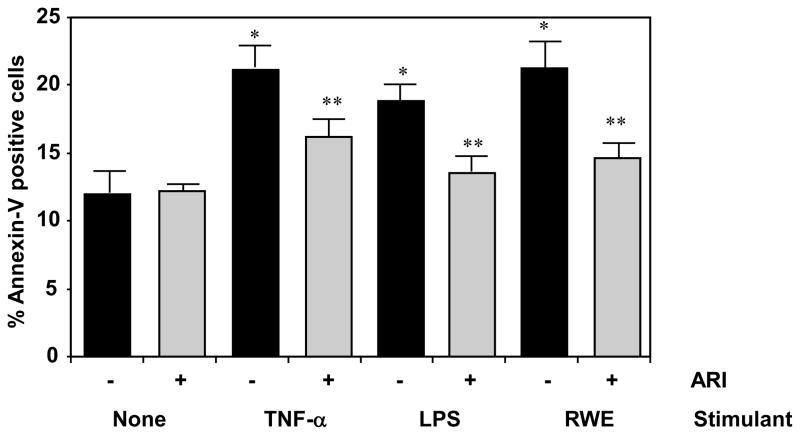
Since ROS – induction is well known to induce cytotoxic effects resulting in the altered cell fate, we determined the cell viability by MTT assay and cell count which showed reduced number of cells in TNF-α-, LPS- or RWE- treated groups probably due to increased cell death as untreated cells kept growing (results not shown). To asses the cause of cell death we determined the early apoptotic events such as membrane inversion by annexin-V staining. As shown in Fig 2, there was increased number of annexin-V positive cells in stimulant-treated cells compared to unstimulated control cells and AKR1B1 inhibitor-treated cells showed significantly reduced percentage of annexin-V positive cells. These results suggest that inhibition of AKR1B1 prevented the TNF-α-, LPS- and RWE-induced apoptosis in SAEC. Further, the levels of inflammatory markers such as PGE2, IL-6 and IL-8 in the SAEC culture media which were markedly elevated by RWE, TNF-α, and LPS challenge and treatment with AKR1B1 inhibitors significantly prevented the increase (data not shown), suggesting that AKR1B1 mediates the release of various inflammatory markers and in airway epithelial cells.
Figure 2. AKR1B1 inhibition prevents TNF-α-, LPS- and RWE-induced apoptosis in SAEC.
The increase (%) in the annexin-V positive cells in RWE-, TNF-α-, or LPS-treated SAEC without or with ARI is shown as mean ± SD and presented as bar diagram (n=4); *p < 0.01 Vs Control; **p < 0.05 Vs RWE/TNF-α/LPS. ARI, AKR1B1 inhibitor
3.3. TNF-α-, LPS- and RWE-induced activation of NF-κB prevented by AKR1B1 inhibition
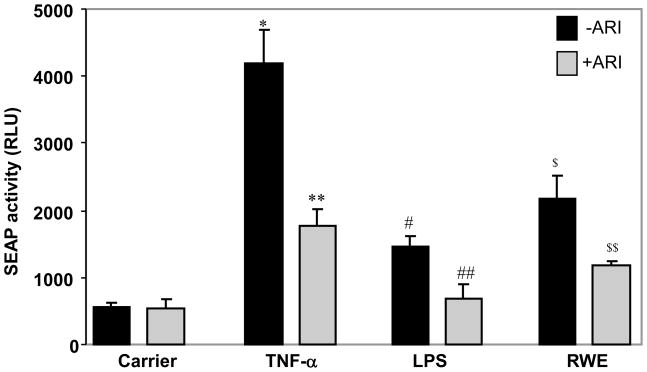
Since increased ROS levels are known to alter the redox status of the cells resulting in the activation of redox signaling events that eventually activate redox-sensitive transcription factor such NF-κB [21], we next investigated the effect of AKR1B1 inhibition on the activation of NF-κB by SEAP activity assay which corresponds to the NF-κB activation. As shown in the Fig 3, Stimulation of SAEC with TNF-α, LPS and RWE resulted in a varied level of SEAP activity approximately 7-fold by TNF-α, 3-fold by LPS and 4-fold by RWE which were significantly (p<0.001 vs TNF-α; p<0.01 vs LPS and RWE) prevented by treatment of the cells with AKR1B1 inhibitor. The control cells that were not stimulated showed minimal SAEP activity.
Figure 3. AKR1B1 inhibition prevents TNF-α-, LPS- and RWE-induced activation of redox- sensitive transcription factor NF-κB in SAEC.
Increase in the SEAP activity (RLU, relative luminescent unit) in SAEC media in response to RWE-, TNF-α-, or LPS-treatment without or with ARI is presented as Mean±SD (n=6). *p<0.001 vs control; **p<0.001 vs TNF-α; #p<0.01 vs control; ##p<0.01 vs LPS; $p<0.001 Vs. Control; $$p<0.01 Vs RWE. ARI, AKR1B1 inhibitor
3.4. RWE- and Ova-induced eosinophils infiltration prevented by AKR1B1 inhibition in mice
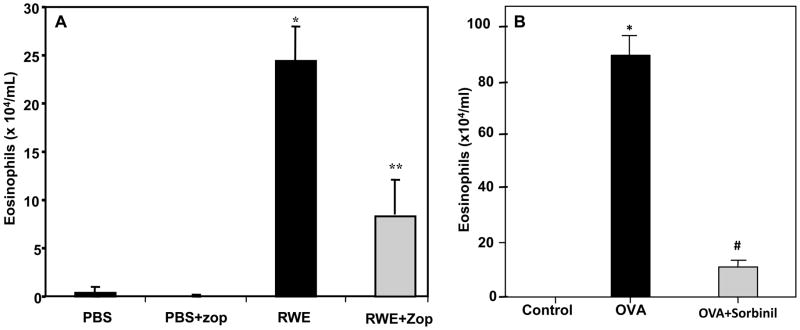
Since oxidative stress and ROS have been implicated in the allergic airway inflammation during allergic asthma [22,23], next we examined the effect of AKR1B1 inhibition using two different animal models i.e. RWE and Ova-induced asthma in mouse. The RWE and Ova-sensitization and challenge in mice resulted in robust infiltration of eosinophils in the broncho-alveolar lavage (BAL) of the mice that was prevented in AKR1B1 inhibitor-treated groups, while control did not show any infiltration of eosinophils (Fig 4). These results suggest that inhibition of AKR1B1 is crucial in the prevention of oxidative stress-induced inflammatory cells infiltration in lung caused by various stimulants including RWE and Ova.
Figure 4. AKR1B1 inhibition prevents eosinophils accumulation in BALF of RWE- and Ova- sensitized and -challenged mice.
The number of eosinophils in the BALF of (A) RWE- or (B) Ova-sensitized and challenged mice with or without ARIs zopolrestat (A) and sorbinil (B) is presented as bar diagram showing Mean±SD (n=6–8). *p <0.001 vs control; **p<0.002 vs RWE #p <0.005 vs Ova-challenge. RWE, ragweed pollen extract; PBS, phosphate buffered saline; Zop, zopolrestat,
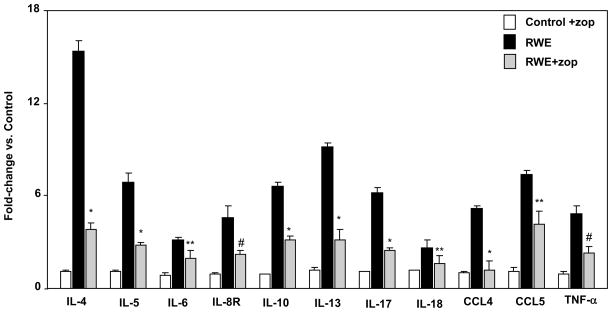
3.5. RWE- and Ova-induced expression of Th2 cytokines and chemokines prevented by AKR1B1 inhibition in mice
Since we demonstrated earlier that excessive oxidative stress results in the activation of transcription factor such as NF-κB which transcribe inflammatory marker genes and results in the secretion of various cytokines and chemokines [24,25], we next investigated the effect of AKR1B1 inhibition on the levels of cytokines and chemokines expression in RWE-sensitized and challenged mice lungs. As shown in Fig 5, several fold increase in the expression of Th2 cytokines and chemokines at the mRNA levels was observed in RWE-sensitized and a challenged mouse lungs compared to control mice which were significantly prevented in the AKR1B1 inhibitor-treated mice. Similarly in Ova-challenged mice there was significant increase in the Th2 cytokines (IL-4, IL-5, IL-10, and IL13) and chemokines including MCP-1, G-CSF, and KC which were significantly prevented by AKR1B1 inhibition in mice (data not shown). These results suggest that AKR1B1 inhibition could prevent oxidative stress-induced airway inflammation and resultant pathological changes in mice model of allergic asthma.
Figure 5. AKR1B1 inhibition prevents expression of cytokines and chemokines in lung of RWE -sensitized and -challenged mice.
The relative expression of mRNA was determined by qRT-PCR and data are presented in the form of bar diagram representing fold-change (SEM±SD) over the control (n=6). *p<0.001 vs RWE; **p<0.05 vs RWE #p<0.01 vs RWE-challenge. RWE, ragweed pollen extract; zop, zopolrestat.
4. Discussion
Epidemiological studies suggest multiple interacting risk factors for asthma such as inhaled pollutants, environmental tobacco smoke, particulate matter, oxides of nitrogen, ozone, and repeated respiratory virus exposures [26–28]. These factors, directly or indirectly, induce and/or augment ROS generation in the airways [27]. Excessive immune response to a particular antigen resulting in the chemo-attraction of inflammatory cells in the lung also results in the increased ROS generation locally which could damage the cellular integrity by attacking the cell membrane on one hand and on the other hand by producing lipid aldehydes by lipid peroxidation which alter the cellular redox status [27]. Enhanced ROS levels in the airway epithelium of murine lungs is known to increase the levels of oxidized glutathione (GSSG), 4-HNE, and malondialdehyde in the lung tissue which cause cytotoxicity by inducing cellular apoptosis resulting in cell-death and tissue damage [29]. Further, ROS is also implicated in the exacerbation of asthma as they disturb the cellular redox status and induce signaling intermediates that eventually activate transcription factors such as NF-κB leading to the expression of a number of inflammatory mediators such as cytokines, chemokines, PGE2, NO, and adhesion molecules which promote inflammatory cells infiltration, tissue remodeling and pathophysiology of asthma [13,30,31]. Therefore, regulating the ROS levels is critical in the management and amelioration of asthma. It has been suggested that antioxidant(s) or the compounds that could block the ROS-induced inflammatory signals could be excellent drugs for asthma [32–34]. Further, several pharmacological anti-inflammatory agents that inhibit specific signaling kinases such as MAPK (p38, ERK1/2), PI3K, GSK-3β and transcription factors such as NF-κB and AP-1 have been suggested for the amelioration of airway inflammation in asthma [35–40]. Although these approaches appear to work in cell culture and animal models, they have not been translated into clinical intervention for asthma probably because of either inherent redundancy of the signaling pathways, where inhibition of one kinase is compensated by other, or the side effects resulting from these molecules. Since in our studies we have recently demonstrated that inhibition of a polyol pathway enzyme, AKR1B1, blocks the oxidative stress-induced signaling upstream of the signaling kinases and transcription factors, it would be an ideal approach to prevent and ameliorate ROS-induced airway inflammation in asthma. Indeed, our results from a number of cellular and animal models support our view that AKR1B1 inhibition could efficiently prevent the transcription of cytokines and chemokines by blocking the signals downstream of ROS that activate transcription factors NF-κB and AP-1 [19, 41,42,43], which would block the inflammation during asthma. Our present results also show that TNF-α-, LPS- or RWE- induced ROS levels and cell death/apoptosis in SAEC were significantly prevented by pharmacological inhibition of AKR1B1, which corroborates our earlier findings. Further, the increased activation of NF-κB in response to stimulation with TNF- α, LPS or RWE was also blocked significantly by inhibition of AKR1B1 suggesting that the transcription of inflammatory intermediates would be stopped which would then block the synthesis of cytokines and chemokines that cause disease pathogenesis in autocrine and paracrine manner.
The results observed in cell culture model using airway epithelial cells were confirmed in the well established murine models of RWE- and Ova-induced allergic asthma. These models exhibit a typical airway hyperresponsiveness, secretion of inflammatory cytokines (particularly Th2 type) and chemokines, infiltration of inflammatory cells such as eosinophils, and tissue remodeling etc which bear similarity to allergic asthma pathogenesis in human. We used these models to test the efficacy of AKR1B1 inhibitors in living system and as expected we observed that AKR1B1 inhibition significantly (p<0.01) reduced the infiltration of eosinophils in the BAL fluid as well as in the perivascular and peribronchial spaces and also significantly prevented the transcription of Th-2 cytokines IL-4, IL-5, IL-6, IL-10 IL-13, Il-17 and IL-18 and chemokines CCL-4, CCL-5 and TNF-α in the lung tissues of RWE-sensitized and -challenged mice. Similarly, administration of AKR1B1 inhibitor in Ova-sensitized and -challenged mice significantly prevented the release of Th-2 cytokines IL-4, IL-5, IL-6 and IL-10 and chemokines KC, G-CSF and MCP-1 in the BAL fluid (Data not shown). These results strongly support our hypothesis that inhibition of AKR1B1 could be antioxidative and anti-inflammatory and could be an excellent approach to treat allergic asthma, especially because AKR1B1 inhibitors have been found to be safe and passed FDA approved clinical trials for diabetic neuropathy in the US and are clinically used in Japan to treat diabetic neuropathy [44, 45]. Although, the exact molecular mechanism of AKR1B1 inhibitors in the prevention and amelioration of inflammation is not yet clear, we have identified that ROS induces generation of lipid aldehydes such as HNE which readily conjugates with GSH to form GS-HNE, and both are reduced to form dihydronone (DHN) and glutathiolated-dihydrononene (GS-DHN), respectively catalyzed by AKR1B1 [17, 18]. We have also demonstrated that GS-DHN activates upstream kinases including PLC/PKC/MAPK in several cellular models eventually activating redox sensitive transcription factors such as NF-κB and AP-1 [19], which enter the nucleus and enhance the transcription of a number of inflammatory cytokines and chemokines and AKR1B1 itself. Therefore, blocking the AKR1B1 activity or decreasing the level of the enzyme in the cells by siRNA ablation would result in the decreased levels of GS-DHN thereby block the ROS-induced signaling cascade and prevent/ameliorate inflammation. However, more studies are required to ascertain the precise role of AKR1B1 in allergic airway inflammation.
Acknowledgments
This study was supported in parts by American Asthma Foundation Grant AAF 08-0219 to William Bowes Scholar SKS and NIH grant GM-71036 to KVR.
Abbreviations
- AKR1B1
aldose reductase
- ARI
aldose reductase inhibitor
- ROS
reactive oxygen species
- RWE
ragweed pollen extract
- TNF-α
tumor necrosis factor – alpha
- LPS
Lipopolysachharide
- Ova
Ovalbumin
- PBS
phosphate buffered saline
- DMSO
Dimethyl sulfoxide
- SAEC
small airway epithelial cell
- HNE
4-hydroxynonenal
- GS-HNE
glutathione-4-hydroxynonenal
- GS-DHN
glutathionyl-1,4-dihydroxynonene
- SABM
small airway epithelial basal medium
- DHE
dihydroethidium
- MTT
3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide
- EDTA
Ethylenediaminetetraacetic acid
- FITC
Fluorescein isothiocyanate
- PI
propidium iodide
- SEAP
secretary alkaline phosphatase
- BAL
brochoalveolar lavage
- iNOS
inducible nitric oxide synthase
- siRNA
small interfering RNA. GSSG, oxidized glutathione
- GSH
reduced glutathione
- GS
glutathione
- PGE2
Prostaglandin E2
- NO
nitrtic oxide
- IL
interleukin
- CCL
Chemokine (C-C motif) ligand
- G-CSF
Granulocyte colony-stimulating factor
- KC
keratinocyte-derived chemokine
- MCP-1
Monocyte chemotactic protein-1
- NADPH
nicotinamide adenine dinucleotide phosphate
- PKC
protein kinase C
- PLC
phospholipase C
- MAPK
mitogen activated protein kinases
- NF-kB
nuclear factor-kappa B
- AP-1
activated protein 1
- ERK1/2
extracellular-signal-regulated kinases
- PI3K
phosphoinositide 3-kinases
- GSK-3β
Glycogen synthase kinase 3 beta
Footnotes
Conflict of Interest: The Authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami L Office of Analysis and Epidemiology. [(accessed Aug 31, 2010)];CDC National Center for Health Statistics E-stat, Asthma Prevalence, Health Care Use and Mortality: United States. 2003–05 http://www.cdc.gov/nchs/data/hestat/asthma03-05/asthma03-05.htm-fig1.
- 3.Kamble S, Bharmal MJ. Incremental direct expenditure of treating asthma in the U.S. J Asthma. 2009;46:73–80. doi: 10.1080/02770900802503107. [DOI] [PubMed] [Google Scholar]
- 4.Nauta AJ, Engels F, Knippels LM, Garssen J, Nijkamp FP, Redegeld FA. Mechanisms of allergy and asthma. Eur J Pharmacol. 2008;585:354–360. doi: 10.1016/j.ejphar.2008.02.094. [DOI] [PubMed] [Google Scholar]
- 5.D’Amato G, Cecchi L, D’Amato M, Liccardi G. Urban air pollution and climate change as environmental risk factors of respiratory allergy: an update. J Investig Allergol Clin Immunol. 2010;20:95–102. [PubMed] [Google Scholar]
- 6.Agrawal DK, Shao Z. Pathogenesis of allergic airway inflammation. Curr Allergy Asthma Rep. 2010;10:39–48. doi: 10.1007/s11882-009-0081-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons JP, Mastronarde JG. Exercise-induced asthma. Curr Opin Pulm Med. 2009;15:25–28. doi: 10.1097/MCP.0b013e32831da8ab. [DOI] [PubMed] [Google Scholar]
- 8.Robinson DS. Regulatory T cells and asthma. Clin Exp Allergy. 2009;39:1314–23. doi: 10.1111/j.1365-2222.2009.03301.x. [DOI] [PubMed] [Google Scholar]
- 9.Holloway JW, Yang IA, Holgate ST. Genetics of allergic disease. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S81–94. doi: 10.1016/j.jaci.2009.10.071. [DOI] [PubMed] [Google Scholar]
- 10.Hopkin J. Immune and genetic aspects of asthma, allergy and parasitic worm infections: evolutionary links. Parasite Immunol. 2009;31:267–273. doi: 10.1111/j.1365-3024.2009.01104.x. [DOI] [PubMed] [Google Scholar]
- 11.Yao H, Yang SR, Kode A, Rajendrasozhan S, Caito S, Adenuga D, Henry R, Edirisinghe I, Rahman I. Redox regulation of lung inflammation: role of NADPH oxidase and NF-kappaB signaling. Biochem Soc Trans. 2007;35:1151–1155. doi: 10.1042/BST0351151. [DOI] [PubMed] [Google Scholar]
- 12.Fujisawa T. Role of oxygen radicals on bronchial asthma. Curr Drug Targets Inflamm Allergy. 2005;4:505–509. doi: 10.2174/1568010054526304. [DOI] [PubMed] [Google Scholar]
- 13.Henricks PA, Nijkamp FP. Reactive oxygen species as mediators in asthma. Pulm Pharmacol Ther. 2001;14:409–420. doi: 10.1006/pupt.2001.0319. [DOI] [PubMed] [Google Scholar]
- 14.Holgate ST. Pathogenesis of asthma. Clin Exp Allergy. 2008;38:872–897. doi: 10.1111/j.1365-2222.2008.02971.x. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick AM, Teague WG, Holguin F, Yeh M, Brown LA. Severe Asthma Research Program, Airway glutathione homeostasis is altered in children with severe asthma: evidence for oxidant stress. J Allergy Clin Immunol. 2009;123:146–152. doi: 10.1016/j.jaci.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav UC, Ramana KV, Awasthi YC, Srivastava SK. Glutathione level regulates HNE-induced genotoxicity in human erythroleukemia cells. Toxicol Appl Pharmacol. 2008;227:257–264. doi: 10.1016/j.taap.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivastava S, Chandra A, Bhatnagar A, Srivastava SK, Ansari NH. Lipid peroxidation product, 4-hydroxynonenal and its conjugate with GSH are excellent substrates of bovine lens aldose reductase. Biochem Biophys Res Commun. 1995;217:741–746. doi: 10.1006/bbrc.1995.2835. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 19.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- 20.Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidationderived aldehydes 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 21.Janssen-Heininger YM, Poynter ME, Aesif SW, Pantano C, Ather JL, Reynaert NL, Ckless K, Anathy V, van der Velden J, Irvin CG, van der Vliet A. Nuclear factor kappaB, airway epithelium, and asthma: avenues for redox control. Proc Am Thorac Soc. 2009;6:249–255. doi: 10.1513/pats.200806-054RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park CS, Kim TB, Lee KY, Moon KA, Bae YJ, Jang MK, Cho YS, Moon HB. Increased oxidative stress in the airway and development of allergic inflammation in a mouse model of asthma. Ann Allergy Asthma Immunol. 2009;103:238–247. doi: 10.1016/S1081-1206(10)60188-3. [DOI] [PubMed] [Google Scholar]
- 23.Talati M, Meyrick B, Peebles RS, Jr, Davies SS, Dworski R, Mernaugh R, Mitchell D, Boothby M, Roberts LJ, 2nd, Sheller JR. Oxidant stress modulates murine allergic airway responses. Free Radic Biol Med. 2006;40:1210–1219. doi: 10.1016/j.freeradbiomed.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava SK, Ramana KV. Focus on molecules: nuclear factor-kappaB. Exp Eye Res. 2009;88:2–3. doi: 10.1016/j.exer.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation, Cold Spring Harb. Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subbarao P, Mandhane PJ, Sears MR. Asthma: epidemiology, etiology and risk factors. CMAJ. 2009;181:E181–190. doi: 10.1503/cmaj.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122:456–468. doi: 10.1016/j.jaci.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mochizuki H, Todokoro M, Arakawa H. RS virus-induced inflammation and the intracellular glutathione redox state in cultured human airway epithelial cells. Inflammation. 2009;32:252–264. doi: 10.1007/s10753-009-9128-0. [DOI] [PubMed] [Google Scholar]
- 29.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yadav UC, Ramana KV, Aguilera-Aguirre L, Boldogh I, Boulares HA, Srivastava SK. Inhibition of aldose reductase prevents experimental allergic airway inflammation in mice. PLoS One. 2009;4:e6535. doi: 10.1371/journal.pone.0006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav UC, Naura AS, Aguilera-Aguirre L, Ramana KV, Boldogh I, Sur S, Boulares HA, Srivastava SK. Aldose reductase inhibition suppresses the expression of Th2 cytokines and airway inflammation in ovalbumin-induced asthma in mice. J Immunol. 2009;183:4723–4732. doi: 10.4049/jimmunol.0901177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman I. Oxidative stress and gene transcription in asthma and chronic obstructive pulmonary disease: antioxidant therapeutic targets. Curr Drug Targets Inflamm Allergy. 2002;1:291–315. doi: 10.2174/1568010023344607. [DOI] [PubMed] [Google Scholar]
- 33.Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111:476–494. doi: 10.1016/j.pharmthera.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Park SJ, Lee YC. Antioxidants as novel agents for asthma. Mini Rev Med Chem. 2006;6:235–240. doi: 10.2174/138955706775475948. [DOI] [PubMed] [Google Scholar]
- 35.Duan W, Chan JH, Wong CH, Leung BP, Wong WS. Antiinflammatory effects of mitogen-activated protein kinase kinase inhibitor U0126 in an asthma mouse model. J Immunol. 2004;172:7053–7059. doi: 10.4049/jimmunol.172.11.7053. [DOI] [PubMed] [Google Scholar]
- 36.Duan W, Aguinaldo Datiles AM, Leung BP, Vlahos CJ, Wong WS. An anti-inflammatory role for a phosphoinositide 3-kinase inhibitor LY294002 in a mouse asthma model. Int Immunopharmacol. 2005;5:495–502. doi: 10.1016/j.intimp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Bao ZS, Lim S, Liao W, Lin Y, Thiemermann C, Leung BP, Wong WS. Glycogen synthase kinase-3β inhibition attenuates asthma in mice. Am J Respir Crit Care Med. 2007;176:431–438. doi: 10.1164/rccm.200609-1292OC. [DOI] [PubMed] [Google Scholar]
- 38.Duan W, Chan JH, McKay K, Crosby JR, Choo HH, Leung BP, Karras JG, Wong WS. Inhaled p38α mitogen-activated protein kinase antisense oligonucleotide attenuates asthma in mice. Am J Respir Crit Care Med. 2005;171:571–578. doi: 10.1164/rccm.200408-1006OC. [DOI] [PubMed] [Google Scholar]
- 39.Henderson WR, Jr, Chi EY, Teo JL, Nguyen C, Kahn M. A small molecule inhibitor of redox-regulated NF-κB and activator protein-1 transcription blocks allergic airway inflammation in a mouse asthma model. J Immunol. 2002;169:5294–5299. doi: 10.4049/jimmunol.169.9.5294. [DOI] [PubMed] [Google Scholar]
- 40.Chue SC, Seow CJ, Duan W, Yeo KS, Koh AH, Wong WS. Inhibitor of p42/44 mitogen-activated protein kinase, but not p38 MAPK, attenuated antigen challenge of guinea pig airways in vitro. Int Immunopharmacol. 2002;4:1089–1098. doi: 10.1016/j.intimp.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nulcear factor-_B by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;53:2910–2920. doi: 10.2337/diabetes.53.11.2910. [DOI] [PubMed] [Google Scholar]
- 42.Ramana KV, Bhatnagar A, Srivastava SK. Aldose reductase regulates TNF-α-induced cell signaling and apoptosis in vascular endothelial cells. FEBS Lett. 2004;570:189–194. doi: 10.1016/j.febslet.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 43.Pladzyk A, Reddy AB, Yadav UC, Tammali R, Ramana KV, Srivastava SK. Inhibition of aldose reductase prevents lipopolysaccharide-induced inflammatory response in human lens epithelial cells. Invest Ophthalmol Visual Sci. 2006;47:5395–5403. doi: 10.1167/iovs.06-0469. [DOI] [PubMed] [Google Scholar]
- 44.Hotta N, Toyota T, Matsuoka K, Shigeta Y, Kikkawa R, Kaneko T, Takahashi A, Sugimura K, Koike Y, Ishii J, Sakamoto N. SNK-860 Diabetic Neuropathy Study Group, Clinical efficacy of fidarestat, a novel aldose reductase inhibitor, for diabetic peripheral neuropathy: a 52-week multicenter placebo-controlled double-blind parallel group study. Diabetes Care. 2001;24:1776–1782. doi: 10.2337/diacare.24.10.1776. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez MA, Borja NL. Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy. 2008;28:646–655. doi: 10.1592/phco.28.5.646. [DOI] [PubMed] [Google Scholar]