Abstract
Oxidative stress-induced inflammation is a major contributor to several disease conditions including sepsis, carcinogenesis and metastasis, diabetic complications, allergic asthma, uveitis and after cataract surgery posterior capsular opacification. Since reactive oxygen species (ROS)-mediated activation of redox-sensitive transcription factors and subsequent expression of inflammatory cytokines, chemokines and growth factors are characteristics of inflammatory disorders, we envisioned that by blocking the molecular signals of ROS that activate redox-sensitive transcription factors, various inflammatory diseases could be ameliorated. We have indeed demonstrated that ROS –induced lipid peroxidation-derived lipid aldehydes such as 4-hydroxy-trans-2-nonenal (HNE) and their glutathione-conjugates (e.g. GS-HNE) are efficiently reduced by aldose reductase to corresponding alcohols which mediate the inflammatory signals. Our results showed that inhibition of aldose reductase (AKR1B1) significantly prevented the inflammatory signals induced by cytokines, growth factors, endotoxins, high glucose, allergens and auto-immune reactions in cellular as well as animal models. We have demonstrated that AKR1B1 inhibitor, fidarestat, significantly prevents tumor necrosis factor-alpha (TNF-α)-, growth factors-, lipopolysachharide (LPS)-, and environmental allergens-induced inflammatory signals that cause various inflammatory diseases. In animal models of inflammatory diseases such as diabetes, cardiovascular, uveitis, asthma, and cancer (colon, breast, prostate and lung) and metastasis, inhibition of AKR1B1 significantly ameliorated the disease. Our results from various cellular and animal models representing a number of inflammatory conditions suggest that ROS-induced inflammatory response could be reduced by inhibition of AKR1B1, thereby decreasing the progression of the disease and if the therapy is initiated early, the disease could be eliminated. Since fidarestat has already undergone phase III clinical trial for diabetic neuropathy and found to be safe, though clinically not very effective, our results indicate that it can be developed for the therapy of a number of inflammation- related diseases. Our results thus offer a novel therapeutic approach to treat a wide array of inflammatory diseases.
Keywords: inflammation, oxidative stress, aldose reductase, ROS, colon cancer, uveitis, asthma
Introduction
Aldose reductase (AKR1B1, in human) that belongs to aldo-keto-reductase super family of proteins catalyzes the first and rate-limiting step of the polyol pathway of glucose metabolism. Besides reducing glucose to sorbitol, AKR1B1 reduces a wide range of aldehydes and their conjugates with glutathione [1]. Our studies also suggested a beneficial role of AKR1B1 in the detoxification of toxic lipid aldehydes generated upon oxidative stress. On the other hand, the accelerated flux of sorbitol through the polyol pathway has been implicated in the pathogenesis of the secondary diabetic complications, such as cataractogenesis, retinopathy, neuropathy, nephropathy, and cardiovascular [2,3,4,5,6,7,8]. Demonstration that AKR1B1 inhibitors decrease the sorbitol levels and ameliorate complications of diabetes such as cataract in experimental animals strongly supports this hypothesis. [9]. Although, in experimental animals AKR1B1 inhibitors have shown potential inhibition of secondary diabetic complications, none of the AKR1B1 inhibitors have passed the phase III clinical trial for the prevention of diabetic complications such as diabetic neuropathy [10]. Since, previous studies had implicated that the major cause of diabetic complications could be osmotic stress generated by polyol flux, most studies were directed towards lowering the sorbitol levels [11,12]. However, recent studies suggest that the increased polyol pathway could alter the NADPH/NADP ratio and attenuate the glutathione reductase (GR) and glutathione peroxidase (GPx) system thereby decreasing the reduced glutathione/oxidized glutathione (GSH/GSSG) ratio which would cause oxidative stress, a major cause of diabetic complications [8,13,14]. These conclusions are strongly supported by our studies showing that sugar -induced lens opacification can be significantly prevented by antioxidants such as butylated hydroxytoluene (BHT) and Trolox without decreasing highly elevated levels of sorbitol in the lens [15,16]. Patients with hyperglycemia and atherosclerosis have increased levels of oxidative stress-generated lipid peroxidation products, such as 4-hydroxy-trans-2-nonenal (HNE) and protein-HNE conjugates in their blood [17]. Further, oxidized lipids and lipoproteins are known to stimulate the cell proliferation/death and the antioxidants such as α-tocopherol, BHT, GSH-ester, curcumin, or polyphenols attenuate it [18,19,20,21,22,23,24,25,26]. Recently, our studies also suggested that AKR1B1, besides reducing glucose, efficiently reduces oxidative stress-generated lipid aldehydes with Km in micro molar range (10–30 μM) as compared to Km glucose (50–100 mM) [27]. These studies indicate the potential role of AKR1B1 in mediating oxidative stress signals since the lipid peroxidation- derived aldehydes (LDAs) such as HNE have been shown to regulate cell signals leading to cell growth or death. We have demonstrated that HNE signals rat aortic vascular smooth muscle cells (VSMC) proliferation which is attenuated by AKR1B1 inhibitor [28]. We have further demonstrated the mechanistic relationship between oxidant generation, lipid peroxidation, HNE formation, vascular cell cytotoxicity and vascular complications such as atherosclerosis [8].
The elevated levels of ROS during hyperglycemic and peroxidative stress and cytokine response are known to trigger the inflammatory response in the tissues by upregulating several redox-sensitive transcription factors such as nuclear factor-kappa B (NF-κB) and activator protein (AP)-1. Modulation of NF-κB has a great significance in the mitogenic process that is mediated by the ROS. Recently, it has been reported that hyperglycemia and TNF-α activate NF-κB and cause proliferation of VSMC and apoptosis of vascular endothelial cells (VEC) [29,30]. Since hyperglycemia activates NF-κB and cytokines such as TNF-α which besides activating NF-κB, is known to stimulate AKR1B1 gene expression, it is necessary to understand the relationship and the molecular mechanisms underlying these signals. We have investigated the mechanism(s) of cytokines- and hyperglycemia -induced NF-κB activation and proliferation/apoptosis of various cells and found that AKR1B1 is involved in the mediation of oxidation/reduction signals. These investigations are important in understanding the molecular mechanisms of various inflammatory diseases.
Detoxification and anti-oxidative roles of AKR1B1
The most obvious endogenous source of hydrophobic aldehydes is lipid peroxidation. It is well known that during free radical-mediated peroxidation of lipids, aldehydes are produced in large amounts [31]. Moreover, several of these aldehydes display high toxicity, and so could mediate some of the biological effects ascribed to their radical precursors. However, little is known about their metabolism and detoxification. Our and others observations have shown that AKR1B1 may represent an important metabolic route for the detoxification of LDA [1,32,33]. This is an important breakthrough in our understanding of the physiological role of this enzyme. Due to its involvement in diabetic complications, investigators have been pre-occupied with defining the role of AKR1B1 in glucose metabolism. However, reduction of glucose may not be AKR1B1’s primary physiological function; rather, this enzyme may have an important antioxidative role.
We have demonstrated that lipid-derived aldehydes and their conjugates with GSH are efficiently reduced by AKR1B1 with a Km of 10–30 μM in the presence of reduced nicotinamide adenine dinucleotide phosphate (NADPH) which is about a thousand-fold lower than that for glucose [27]. AKR1B1 is an excellent catalyst for the reduction of medium to long-chain unbranched saturated and unsaturated aldehydes and their conjugates with GSH. However, based on the decreased NADPH/NADP ratio, LDA could increase and bind at Cys-298. Binding of short-chain aldehydes, (propanal or acrolein) activates AKR1B1, while longer-chain aldehydes inactivates the enzyme [34]. Kinetic studies and computer modeling indicate that AKR1B1 has a specific binding site for GSH [35]. Indeed, we have solved the crystal structure of AKR1B1 bound to GSH analogue [36]. To further understand the functions of AKR1B1, we investigated the metabolism of tritiated (3H)-HNE in various tissues such as red blood cells and cardiac myocytes and found that in all these cases, AKR1B1 reduces HNE and the GS-HNE to their respective alcohols 1,4-dihydroxynonene (DHN) and glutathionyl-1,4-dihydroxynonene (GS-DHN), respectively [32,37,38]. These results not only suggested that AKR1B1 catalyzes the reduction of LDA or their metabolites both in vitro and in vivo, but also that inhibition of AKR1B1 exacerbates the toxicity of LDA in the ocular lens, isolated cardiac myocytes and VSMC. An antioxidative role of AKR1B1 is further supported by the observation that exposure of VSMC to HNE or hydrogen peroxide (H2O2) up-regulates AKR1B1 [39]. Moreover, the presence of binding sites for redox-regulated transcription factors such as AP-1 and NF-κB at the AKR1B1 gene’s promoter site further supports the view that AKR1B1 may be a significant component of antioxidant defenses involved in redox cell signaling.
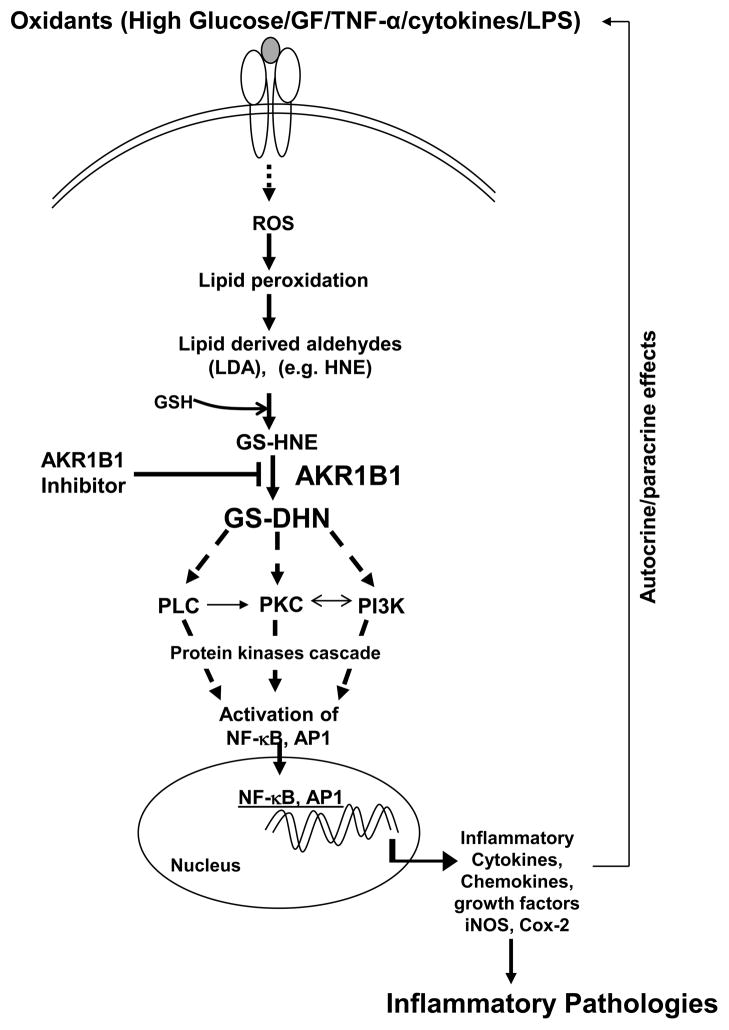
In response to growth stimuli the abundance of the AKR1B1 mRNA and protein is significantly increased. In NIH 3T3 cells, fibroblast growth factor (FGF)-1 increases both AKR1B1 and fibroblast growth factor regulated -1 protein (AKR1B8 or FR-1) mRNA, whereas serum up-regulates only AKR1B1 [40]. In astrocytes, AKR1R1 is induced by FGF-1, FGF-2, and epidermal growth factor (EGF) [41]. In addition, our studies showed that the expression of AKR1B1 in VSMC is enhanced by high glucose, TNF-α, thrombin, and FGF-2 [42]. Moreover, in several tissues, AKR1B1 is specifically induced during growth; e.g., although its expression in the adult liver is low, AKR1B1 is the most prominent tumor-associated protein in chemically induced rat hepatomas and in transformed rat liver cell lines [43]. The hepatoma-derived AKR1B1 is also stimulated by FGF-1 [44]. Even though significant levels of AKR1B1 were not detected in quiescent VSMC in the vessel wall, VSMC proliferating in situ or in culture have high levels of AKR1B1 mRNA and protein [42]. Similarly, lens fiber cells differentiating in response to FGF, show upregulation of AKR1B1, and in sugar cataracts, high expression of AKR1B1 are localized to the hyper-proliferating bow region, consistent with a growth-regulating role of the enzyme [45,46]. Further, glucose-induced hyper-proliferation and hypertrophy are decreased by inhibiting AKR1B1 [8]. In addition to growth factors, AKR1B1 is also induced by oxidants. Stimulation of VSMC with H2O2, oxidized low-density lipoprotein (oxLDL), or the lipid peroxidation product, HNE, up-regulates AKR1B1 [39,47]. AKR1B1 is also induced by HNE in lymphocytes, as well as by cytokines that generate ROS. In vivo, during giant cell arthritis, the expression of AKR1B1 is enhanced in T cells, macrophages, and VSMC in the area of high oxidative stress and HNE formation, suggesting redox regulation of the AKR1B1 gene in several tissues [48]. Interestingly, an AKR1B1-related protein (YBR49W) is markedly up-regulated as part of the adaptive response of yeast to H2O2, where the protein has been suggested to be required for scavenging lipid peroxidation-derived toxic aldehydes [49]. Thus, the sensitivity of the AKR1B1 gene to both growth factors and oxidants appears to be a phylogenetically well-conserved response [42]. Given the extensive evidence implicating ROS as mediators of cell growth (vide infra), it appears likely that growth factor-induced upregulation of AKR1B1 is due to ROS generated during mitogenic signaling. The strongest evidence that AKR1B1 is involved in mediating growth comes from our studies showing that inhibition of AKR1B1 in culture prevents proliferation of VSMC in response to FGF, thrombin, and TNF-α, and neointimal hyperplasia in balloon-injured arteries [42]. In addition, the observation that AKR1B1 inhibition prevents intimal thickening in coronary arteries of galactose fed beagle dogs provides additional support for the role of AKR1B1 in abnormal VSMC growth [50]. Nonetheless, the mechanism by which AKR1B1 facilitates cell growth remains unknown. The possible roles of AKR1B1 in lipid aldehyde detoxification, reduction of glucose, and mediation of TNF-α and other inflammatory cytokines and growth hormone signals are shown in Figure 1.
Figure 1.
Schematic presentation of role of aldose reductase (AKR1B1) in the mediation of inflammatory pathologies (for details see the text). AKR1B1, aldose reductase; GF, growth factors; TNF-α, tumor necrosis factor-alpha; LPS, Lipopolysachharide; ROS, reactive oxygen species; LDA, lipid peroxidation- derived aldehydes; HNE, 4-hydroxy-trans-2-nonenal; GS-HNE, glutathione-4-hydroxy-trans-2-nonenal; GS-DHN, glutathionyl-1,4-dihydroxynonene; PI3K, phosphoinositide 3-kinases; PKC, protein kinase C; PLC, phospholipase C; NF-κB, nuclear factor-kappa B; AP-1, activator protein 1; iNOS, inducible nitric oxide synthase; Cox-2, cycloxygenase-2; GSH, reduced glutathione;
AKR1B1 mediates oxidative stress signals
Under physiological conditions, there is a balance between the generation of ROS and their detoxification by antioxidant systems. In general, oxidative stress occurs when this balance is disrupted, either directly by infectious agents or by cytokines released from inflamed cells that may lead to increased ROS generation and/or decreased antioxidant defense. Normally, ROS are involved in signal transductions which mediate some of the essential cellular functions such as host cell defense, mitochondrial respiration, cytokine generation and cell proliferation/apoptosis [51]. There are several potential sources of ROS in inflammation, one of which is the activation of NADPH oxidase in phagocytes, monocytes and most other inflammatory cell types [52,53]. In fact, activation of NADPH oxidase has been observed upon exposure of various cell types to cytokines, growth factors and hyperglycemia [54,55]. Thus, NADPH oxidase is a key player in signal transduction. The NADPH oxidase (a membrane-bound holoenzyme with different flavocytochrome subunits) catalyzes the one electron reduction of oxygen, using NADPH or reduced nicotinamide adenine dinucleotide (NADH) as the electron donor and leading to the production of superoxide anions [56]. The superoxide anions thus generated can form other ROS (such as hydroxyl radicals and hydrogen peroxide) and cause tissue injury and alter gene expression. Several investigators have shown in multiple cell types that NADPH oxidase, rather than xanthine oxidase and other mitochondrial enzymes, is the main source of ROS [57,58,59]. LPS and various inflammatory cytokines such as TNF-α, interleukin (IL)-1 and IL-10 can activate NADPH oxidase to generate significant, sometimes toxic, amounts of ROS (initially O2−) which propagate their signals that activate transcription factors.
ROS are known to activate phospholipases and protein kinases which activate redox-sensitive transcription factors such as NF-κB and AP-1. This is supported by the observations that cytokines, growth factors and hyperglycemic-induced NF-κB signals are interrupted by such antioxidants as N-acetyl cysteine and α-tocopherol and glutathione (GSH), which can attenuate inflammation [21,23,60]. The ROS-sensitive transcription factor NF-κB is a critical inflammatory mediator which can transcribe a number of pro-inflammatory cytokines. When inactive, it is sequestered in the cytosol in a complex with its inhibitor, inhibitor-kappa B (IκB) and stimulation of protein kinases such as protein kinase C (PKC), mitogen activated protein kinases (MAPK), and inhibitor-kappa B kinase (IKK) results in the activation of NF-κB via the phosphorylation of IκB. The other major redox-sensitive transcription factor, AP-1, is formed by homo- or heterodimerization of members of the Jun and Fos families of proteins; ROS can regulate AP-1 activity via several mechanisms [61,62]. AP-1 can be regulated via the c-Jun N-terminal kinase (JNK) cascade; JNKs are part of the MAPK superfamily of serine/threonine kinases that also include the extracellular signal-regulated kinases (ERK)1/2 and p38MAPK [63]. All MAPK are activated via a cascade of phosphorylation reactions which eventually activate various transcription factors. One of the molecules involved in these processes may be HNE, which is generated by ROS-induced lipid peroxidation. It has been shown that HNE is cytotoxic, and elevated levels of HNE have been reported in various disease conditions [64,65]. Because AKR1B1 has been shown to reduce HNE and GS-HNE with a Km of 10–30 μM, we hypothesize that AKR1B1 is a key determinant of the cellular redox state and that inhibition of this enzyme will interrupt ROS signaling by increasing oxidative stress. This aldehyde is highly reactive towards free sulfhydryl groups of proteins and generate thioether adducts that undergo further cyclization and hemiacetal formation [66]. HNE also reacts with histidine and lysine residues of proteins to form stable Michael adducts [67]. This aldehyde induces heat shock proteins, inhibits cellular proliferation, and is highly toxic to cells [68,69]. However, we and other investigators have shown that low concentrations of HNE stimulate proliferation of vascular smooth muscle cells and hepatic stellate cells [42,70], whereas HNE is apoptotic in endothelial cells and lens epithelial cells [71,72]. At high concentrations, HNE displays a variety of genotoxic and mutagenic effects [73]. Cytokines, growth factors and hyperglycemia are known to increase lipid peroxidation and generate HNE. However, HNE can also regulate the activity and expression of AKR1B1, suggesting a possible feedback mechanism [74]. Interestingly, incubation of AKR1B1 with HNE in vitro causes enzyme inactivation [75], while cells treated with HNE show increased AKR1B1 mRNA synthesis and protein expression [74]. Our recent studies indicate that inhibition of AKR1B1 prevents HNE- and GS-HNE- but not GS-DHN-induced activation of NF-κB and proliferation of VSMC and apoptosis of macrophages [28,76]. These studies indicate that AKR1B1-catalyzed reduced product GS-DHN could be a mediator of oxidative stress signals.
Recent studies show that the activation of PKC in response to growth factors, cytokines or environmental stress leads to cell hypertrophy, proliferation, migration, cell growth, or apoptosis [77, 78]. The PKC isozymes are activated by many extracellular signals, including ROS, these enzymes modify the activities of multiple effectors, such as cytoskeletal proteins, MAPK, and transcription factors. Several lines of evidence suggest that PKC activation by HNE and related oxidants promote inflammation [76,79]; however, it is not known which of the PKC isozymes are responsible for inflammation, and how AKR1B1 regulates their function. Our recent studies have identified that high glucose –induced PKC-β2 and PKC-δ are significantly prevented by AKR1B1 inhibitors, suggesting that AKR1B1 inhibition worked upstream to PKC isozymes [80]. Further, we have also shown that AKR1B1 inhibition prevents the activation of phospholipase C (PLC) isozymes and formation of diacylglycerol which in turn could activate PKC isozymes [80]. Our studies performed in various cell lines indicate that AKR1B1 regulates both mitogenic and apoptotic signals. However, it is not known how AKR1B1 inhibition prevents both the processes. Interestingly, inhibition of AKR1B1 prevents TNF-α-induced proliferation of VSMC and Caco-2 cells and apoptosis of VEC, macrophages, and lens epithelial cells [76,81,82,83,84]. In all the cell lines TNF-α-induces NF-κB and AKR1B1 inhibition prevents it. Specifically in the cells undergoing apoptosis a significant activation of caspase-3 is observed and inhibition of AKR1B1 prevents it. However, no caspase-3 activation was observed in vascular and cancer cells.
AKR1B1 in the pathophysiology of inflammatory disorders
Diabetes
Based upon extensive experimental evidence showing that the inhibition of AKR1B1 prevents or delays hyperglycemic injury in several experimental models of diabetes, it has been suggested that AKR1B1 is one of the main mediators of such secondary diabetic complications as cataractogenesis, retinopathy, neuropathy, nephropathy, and microangiopathy [2,3,4,5,6,7,8]. It has been proposed that the increased flux of glucose via AKR1B1 causes osmotic and oxidative stresses, which, in turn, trigger a sequence of metabolic changes resulting in gross tissue dysfunction, altered intracellular signaling, and extensive cell death [30]. The polyol hypothesis provided a simple testable paradigm of hyperglycemic injury; however, several key observations on diabetic complications are not compatible with the accumulation of sorbitol alone as the major cause of hyperglycemic injury. For instance, in several tissues the intracellular accumulation of sorbitol is not high enough to cause significant osmotic stress [85]. Moreover, the high efficacy of antioxidants in preventing cataractogenesis, without preventing sorbitol accumulation, suggests that oxidative stress may be an important feature of hyperglycemic injury [15,16].
In addition to polyol accumulation, other metabolic changes have also been suggested to account for hyperglycemic injury. Of these, non-enzymatic glycosylation leading to the accumulation of advanced glycosylation end-products (AGEs) and alterations in PKC and myoinositol levels have received the most attention [86]. In support of these hypotheses, it has been shown that inhibition of PKC or non-enzymic glycation, as well as supplementation with myo-inositol, delays or prevents hyperglycemic tissue injury. However, recent evidence suggests that these phenomenons may be inter-related, and that AKR1B1 may represent a critical link between alterations in PKC, myo-inositol and non-enzymic glycation [8]. Because sorbitol and myo-inositol are structurally similar, the depletion of myo-inositol appears to be due in part to the inhibition of its uptake in cells accumulating sorbitol [87]. Furthermore, it has recently been demonstrated that stimulation of PKC by phorbol esters up-regulates AKR1B1 indicating that some of the injurious effects of PKC activation may be mediated by AKR1B1 [88]. Accumulating evidence suggests that effects of non-enzymatic glycosylation and AKR1B1 are inter-related [89,90]. It has been demonstrated that sorbitol-3-phosphate generated by AKR1B1 is converted to fructose 3-phosphate, which is a better glycosylating agent than glucose, so that AKR1B1-mediated catalysis can generate potent glycosylating agents [91]. This view is supported by the observations that in the galactosemic lens, AKR1B1 inhibitors suppress the accumulation of advanced Maillard reaction products such as pentosidine, stimulate the actions of aminoguanidine (an inhibitor of non-enzymic glycosylation), and inhibit the accumulation of AGEs [92]. Thus AKR1B1 may represent a common mediator of several pathological changes associated with long-term diabetes. Further support for a critical role of AKR1B1 in mediating the toxic effects of glucose is provided by acceleration of diabetic cataract by overexpression of AKR1B1 in the lens of transgenic mice [93]. It has also been demonstrated that high glucose in diabetes leads to the up-regulation of AKR1B1 in several tissues, and that treatment with AKR1B1 inhibitors prevents hyperglycemia-induced hyperplasia and hyperproliferation of vascular smooth muscle cells and diabetic cardiomyopathy [8]. Recent studies also suggest that AKR1B1 mediates hyperglycemia- induced VSMC proliferation in vitro and in vivo [42,80,81]. Further, AKR1B1 inhibition has been shown to prevent restenosis of rat carotid arteries [42]. Transgenic overexpression of AKR1B1 in mice has been shown to exacerbate diabetic cardiomyopathy [94]. These observations indicate that AKR1B1 inhibotors may be useful in preventing the pro-vasculoproliferative effects of diabetes, which still remain the major cause of morbidity and mortality associated with this disease.
Sepsis
Sepsis, an often-fatal systemic inflammatory response syndrome, develops when the initial host response to live bacteria and/or bacterial products is amplified and dysregulated [95]. Most center for disease control (CDC) category A and B pathogens such as Gram-positive and Gram-negative organisms, fungi, malarial parasites, and other microbial pathogens can cause sepsis. If not immediately treated, septic patients often develop acute multi-organ dysfunction, and shock, leading to death. A recent epidemiological study estimated that sepsis affects 18 million people worldwide annually, and kills 1400 individuals each day. In the United States alone, sepsis affects ~700,000 people annually, with an overall mortality rate of 30% [96]. Even though there have been significant advances in understanding the fundamental mechanisms of host-pathogen interactions, the clinical management of sepsis is complicated by 1) difficulties in diagnosing sepsis during its early stages; 2) multi-drug resistance of bacteria, making many antibiotics ineffective; and 3) a lack of aggressive treatment in intensive care units. After the initial host-bacterial interactions, there is widespread activation of the innate immune response involving both humoral and cellular components. This leads to the production of pro-inflammatory cytokines and chemokines that act on target cells by binding to specific receptors, activating signal transduction pathways via autocrine and paracrine mechanisms that can amplify cellular toxicity leading to multi-organ failure [97]. Although pharmacological therapies such as steroids intended to block immune response and cytokines could be lifesaving when administered with appropriate antibiotics, they have their own limitations in septic patients. Our recent studies suggest that inhibition of AKR1B1 attenuates inflammatory signaling involved in the production of cytokines and chemokines [98]. Further, inhibition of AKR1B1 decreases cytotoxic effects associated with the inflammatory mediators in tissues as well as their paracrine and endocrine effects that propagate toxicity.
Our studies show that the toxic effects of uncontrolled inflammation can be effectively prevented or significantly ameliorated by inhibiting AKR1B1, either with a pharmacological AKR1B1 inhibitor or by genetic ablation of AKR1B1 message by small interfering RNA (siRNA) [8]. We find that inhibition of AKR1B1 down-regulates bacterial endotoxin, LPS, induced signaling cascades that involve the activation of transcription factors, (such as NF-κB and AP-1) that transcribe various inflammatory cytokines (TNF-α, Interleukins), chemokines (Monocyte chemotactic protein (MCP)-1, macrophage inflammatory proteins (MIP)-1), and inflammatory mediators such as cycloxygenase-2 (Cox-2) and inducible nitric oxide synthase (iNOS) and lead to sepsis [76,99]. Our results thus support a novel anti-inflammatory therapeutic role for AKR1B1 inhibitors, and suggest that inhibition of AKR1B1 may be a clinically important strategy for controlling rampant inflammation due to physical injury, acute shock or prolonged infection. Indeed, we have shown that inhibition of AKR1B1 prevents LPS-induced: 1) secretion of cytokines in the serum, liver, kidney, spleen and heart; 2) the decrease in mouse cardiac muscle contractility that leads to cardiomyopathy; and 3) lethality in mouse model of sepsis [99]. Similarly AKR1B1 inhibition also prevents cecum-ligation puncture –induced inflammatory response in mice [98]. Thus our recently published studies suggest that AKR1B1 inhibitors, which are thought to be anti-diabetic and have been found to be safe, though not effective in ameliorating diabetic complications in clinical trials, could be used therapeutically to down-regulate septic cascades, innate immunity, septic shock and its associated lethality.
Asthma
Asthma is one of the most common chronic respiratory diseases with more than 100 million sufferers worldwide [100]. This inflammatory disorder is caused by a hypersensitive immune system that results from a number of triggers, such as dust, pollen, viruses and changes in the weather. While it is not clear how asthma is initiated in the setting of chronic inflammation, accumulating evidences strongly support the association of airway inflammation to asthma [101]. Furthermore, the increase in inflammation in bronchial epithelium leads to eosinophils infiltration, an increase in mucus production, and most importantly upregulation of cytokines such as TNF-α, IL-4, IL-5, IL-6, IL-13, chemokines such as MCP-1, MIP-1, adhesion molecules such as Inter-Cellular Adhesion Molecule (ICAM)-1, and E and P-Selectins [102,103,104]. Thus, exposure of surrounding cells to inflammatory cytokines and chemokines can trigger various autocrine/paracrine effects leading to T helper (Th)-2 immune response and inflammatory cell accumulation in the airway and lungs. Hence elucidating the mechanisms that regulate inflammatory signals is profoundly important for understanding and managing a very large array of disease processes, including asthma. Since our earlier studies indicate that AKR1B1 inhibitors could be anti-inflammatory, we have examined their efficacy in preventing airway inflammation in mice. We first sensitized and challenged the mice with ragweed pollen extract (RWE)- or carrier- treated without or with AKR1B1 inhibitor, sorbinil. Our results show that there was a robust airway inflammation as measured by accumulation of inflammatory cells in brochoalveolar lavage (BAL) fluid and subepithelium in RWE-sensitized and challenged animals [105]. In the mice treated with AKR1B1 inhibitor there was significantly less inflammation as determined by the number of eosinophils in BAL fluid. Similarly, perivascular and peribronchial inflammation and cell composition in the BAL fluid induced by RW- challenge was significantly prevented by AKR1B1 inhibitor. Further, AKR1B1 inhibition also prevented ragweed-induced mucin production and airway hyperresponsiveness in mice after methacholine challenge [105]. These results indicate that AKR1B1 inhibition significantly prevented the patho-physiological effects of a common natural allergen, RW –induced asthma in murine model. We have also examined the effectiveness of AKR1B1 inhibition in acute ovalbumin (OVA)-induced airway inflammation in mice [106]. Our results indicate that OVA-sensitization and challenge induced a clear and marked perivascular and peribronchial infiltration of eosinophils into the lungs of mice. Such infiltration of inflammatory cells into the airways of OVA-challenged mice was greatly reduced with AKR1B1 inhibitor [106]. Further, the protective effect of the AKR1B1 inhibitor against OVA-induced airway inflammation coincided with a significant reduction in the levels of Th2 cytokines including IL-4, IL-5, IL-6 and chemokines such as keratinocyte-derived chemokine (KC), granulocyte colony stimulating factor (G-CSF) and MCP-1 in BAL fluid [106]. Thus our studies indicate novel role of AKR1B1 inhibitors in the prevention of asthma.
Uveitis
Despite significant research efforts and advances in diagnosis and therapy, ocular autoimmune diseases, which cover a variety of ocular diseases with different clinical symptoms and pathogenicity, remain significant cause of visual impairment in humans. The disease may be of infectious or putative autoimmune etiology. Because uveitis frequently leads to severe vision loss and blindness with retinal vasculitis, retinal detachment, and glaucoma, it is important to elucidate the mechanisms involved in ocular inflammation. Because our earlier studies strongly suggest that inhibition of AKR1B1 prevents cytokine-, growth factor- and LPS-induced oxidative stress signals leading to production of Prostaglandin E (PGE) 2, cytokines and activation of Cox-2 and iNOS, and activation of these inflammatory markers are known to be major mediators of ocular inflammation, we investigated the effect of AKR1B1 inhibition on endotoxin-induced uveitis (EIU) in rats [107]. Inhibition of AKR1B1 prevents EIU-induced inflammatory marker levels in aqueous humor of rat eyes and also suppressed the inflammatory cells (leukocytes) infiltration and protein concentration in the aqueous humor (AqH). Similarly, the rise of TNF-α, nitric oxide (NO) and PGE2 levels in the AqH of EIU rats were significantly attenuated by AKR1B1 inhibition [107]. Furthermore, we have immunohistologically determined the levels of TNF-α at the anterior and posterior segments of eye. Increased levels of TNF-α were found in the aqueous humor, vitreous humor, choroid and retina, and were significantly prevented by AKR1B1 inhibition [107]. Similarly, the increase in iNOS and Cox-2 proteins in ciliary body, corneal epithelium and retinal wall were prevented significantly by AKR1B1 inhibition. The administration of AKR1B1 inhibitor also prevented the activation of NF-κB in the ciliary body, corneal epithelium as well as in the retinal wall of LPS-treated rat eyes suggesting that inhibition of AKR1B1 prevents EIU in rats [107]. Thus, AKR1B1 inhibitors could be used therapeutically to treat patients with uveitis and its associated complications that have the potential of stimulating the inflammatory signals.
Colon Cancer
Each year ~940,000 men and women are diagnosed with colorectal cancer (CRC) worldwide. Of these, more than 500,000 eventually die from its complications [108]. The transition of normal epithelium to adenoma to carcinoma is associated with a variety of molecular and biochemical events such as genetic alterations, intestinal epithelial cell proliferation/differentiation and inflammation. The major initiators of carcinogenesis include a) cells that suffered irreparable DNA damage due to increased free radicals, which cause activation of specific nucleases and damage DNA, RNA, proteins and lipids; b) loss of extracellular stimulation that regulates cell growth; c) upregulation of growth factors and their receptors, and d) autosomal dominant inheritance of cancer genes among the multiple family members. Dietary and environmental factors also play an important role in predisposition to carcinogenesis [109]. Furthermore, many chronic inflammatory diseases such as hepatitis, gastritis and ulcerative colitis are associated with an elevated risk of CRC [110]. Although, it is not clear how cancer is initiated in the setting of chronic inflammation, increasing evidence strongly supports the association between CRC and inflammation [111]. Also, up-regulation of cytokines such as TNF-α and IL-6, growth factors such as insulin like growth factor (IGF)-II, hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), FGF and platelet-derived growth factor (PDGF) and their receptors (HGFR, EGFR, IGFR) has been observed in CRC patients’ cells [112,113,114,115]. Exposure of cells to inflammatory cytokines and growth factors triggers upregulation of prostaglandins via Cox-2 and expands normal epithelial cells to dysplasia (precancer) and cancer [116]. Since redox-sensitive transcription factors such as NF-κB are known to promote transcription of the genes for cytokines and chemokines, the drugs and antioxidants that inhibit NF-κB are being used for intervention in CRC [117].
Our recent studies suggest that AKR1B1 is a key regulator of ROS signals induced by cytokines and growth factors (GF), leading to cell-growth and differentiation in vascular cells and in colon cancer cells [76,81,82]. RNA interference ablation of AKR1B1 or pharmacological inhibitors of AKR1B1 prevents growth factor and cytokine-induced cancer cell growth. Further, Inhibition of AKR1B1 by sorbinil or by antisense ablation prevented FGF- and PDGF-induced up-regulation of PGE2 synthesis in Caco-2 cells [112]. Inhibition of AKR1B1 also prevented GF-induced Cox-2 activity, protein, and mRNA, and significantly decreased the activation of NF-κB, PKC and phosphorylation of PKC-β2, as well as progression of Caco-2 cell growth, but had no effect on Cox-1 activity [112]. Cell cycle analysis suggested that inhibition of AKR1B1 prevents GF-induced proliferation of Caco-2 cells at S-phase. Since ROS are major culprits in the uncontrolled growth of cancer cells, we also examined the effect of AKR1B1 inhibition on ROS production. Our results suggest that AKR1B1 inhibition prevents GF-induced ROS production in cancer cells [112]. Further, we have also shown that inhibition of AKR1B1 prevents cancer cell growth by suppressing the entry of cells in G1/S phase of cell cycle via regulating the transcriptional activation of E2F transcription factors [118]. We have also examined the efficacy of AKR1B1 inhibitor or AKR1B1siRNA in the prevention of colon cancer growth in nude mice xenografts. Inhibition or siRNA ablation of AKR1B1 completely halted the growth of human adenocarcinoma cells (SW480) in nude mice xenograft tumors [118]. None of the treatments interfered with the normal weight gain of animals during the experiments. AKR1B1 inhibition also prevented the azoxymethane (AOM) -induced aberrant crypt foci (ACF) formation in mice [119]. Our studies also indicate that AKR1B1 null mice are resistant to AOM-induced ACF formation and expression of inflammatory and carcinogenic markers [119]. Several studies indicate that AKR1B1 is overexpressed in human cancers such as lung, colon, breast and prostate [120]. Further, AKR1B1 is overexpressed in hepatocarcinogenesis [121]. Previous studies also indicate that AKR1B1 inhibition prevents colon cancer cachexia [122]. These studies thus indicate that inhibition of AKR1B1 may be a novel therapeutic approach in preventing the progression of CRC.
Conclusions
Recent studies demonstrate that besides reducing glucose to sorbitol, AKR1B1 efficiently reduces lipid aldehydes and their conjugates with GSH. This has opened new dimensions in understanding the detoxification of reactive aldehydes generated during lipid peroxidation. Using kinetic, structural, and physiological studies, we and others have investigated the mechanisms by which AKR1B1 selectively recognizes and catalyzes the reduction of LDA and their GSH conjugates [1,32,35,37,38]. To our surprise, we found that AKR1B1-catalyzed LDA metabolites are mediators of cytokine-, chemokine-, growth factor- and LPS-induced cellular cytotoxicity [76,83,84,99,112,113]. Furthermore, recent studies using cell culture and animal models indicate that AKR1B1 plays a pivotal role in inflammation, which is a major contributing factor to many disorders such as cancer, asthma, sepsis and uveitis [98,99,105,106,107,112,113,118,119]. Several AKR1B1 inhibitors have already undergone Phase III clinical trials for diabetic complications such as neuropathy and found to be ineffective though clinically safe. Therefore, based upon our recent findings, AKR1B1 inhibitors could be readily developed for the prevention and therapy of a number of inflammatory disorders mentioned above.
Acknowledgments
This study was supported in parts by NIH grants GM071036 and EY015891 to KVR, and CA129383, DK36118 and American Asthma Foundation Grant AAF 08-0219 to SKS, William Bowes Scholar.
List of Abbreviations
- AKR1B1
aldose reductase
- ROS
reactive oxygen species
- RWE
ragweed pollen extract
- TNF-α
tumor necrosis factor-alpha
- LPS
Lipopolysachharide
- OVA
Ovalbumin
- LDA
lipid peroxidation- derived aldehydes
- HNE
4-hydroxy-trans-2-nonenal
- GS-HNE
glutathione-4-hydroxy-trans-2-nonenal
- GS-DHN
glutathionyl-1,4-dihydroxynonene
- BAL
brochoalveolar lavage
- iNOS
inducible nitric oxide synthase
- Cox
cycloxygenase
- siRNA
small interfering RNA
- GR
glutathione reductase
- GPx
glutathione peroxidase
- GSSG
oxidized glutathione
- GSH
reduced glutathione
- GS
glutathione
- BHT
butylated hydroxytoluene
- AGEs
advanced glycosylation end-products
- VSMC
vascular smooth muscle cells
- VEC
vascular endothelial cells
- FGF
fibroblast growth factor
- PGE2
Prostaglandin E2
- oxLDL
oxidized low-density lipoprotein
- NO
nitrtic oxide
- IL
interleukin
- G-CSF
Granulocyte colony-stimulating factor
- KC
keratinocyte-derived chemokine
- MCP-1
Monocyte chemotactic protein-1
- MIP
macrophage inflammatory proteins
- NADP
nicotinamide adenine dinucleotide phosphate
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NADH
reduced nicotinamide adenine dinucleotide
- PKC
protein kinase C
- PLC
phospholipase C
- MAPK
mitogen activated protein kinases
- JNK
c-Jun N-terminal kinase
- IKK
inhibitor-kappa B kinase
- IκB
inhibitor-kappa B
- NF-κB
nuclear factor-kappa B
- AP-1
activator protein 1
- ERK1/2
extracellular-signal-regulated kinases
- PI3K
phosphoinositide 3-kinases
- ICAM
Inter-Cellular Adhesion Molecule
- Th2
T helper cell type 2
- EIU
endotoxin-induced uveitis
- AqH
aqueous humor
- CRC
colorectal cancer
- IGF
insulin like growth factor
- HGF
hepatocyte growth factor
- VEGF
vascular endothelial growth factor
- PDGF
platelet-derived growth factor
- GF
growth factors
- AOM
azoxymethane
- ACF
aberrant crypt foci
Footnotes
Conflict of Interest: the Authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Srivastava S, Chandra A, Bhatnagar A, Srivastava SK, Ansari NH. Lipid peroxidation product, 4-hydroxynonenal and its conjugate with GSH are excellent substrates of bovine lens aldose reductase. Biochem Biophys Res Commun. 1995;217:741–746. doi: 10.1006/bbrc.1995.2835. [DOI] [PubMed] [Google Scholar]
- 2.Obrosova IG, Chung SS, Kador PF. Diabetic cataracts: mechanisms and management. Diabetes Metab Res Rev. 2010;26:172–180. doi: 10.1002/dmrr.1075. [DOI] [PubMed] [Google Scholar]
- 3.Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038. doi: 10.1155/2007/61038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oates PJ. Aldose reductase, still a compelling target for diabetic neuropathy. Curr Drug Targets. 2008;9:14–36. doi: 10.2174/138945008783431781. [DOI] [PubMed] [Google Scholar]
- 5.Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int Suppl. 2000;77:S3–12. doi: 10.1046/j.1523-1755.2000.07702.x. [DOI] [PubMed] [Google Scholar]
- 6.Chung SS, Chung SK. Aldose reductase in diabetic microvascular complications. Curr Drug Targets. 2005;6:475–486. doi: 10.2174/1389450054021891. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Hwang YC, Ananthakrishnan R, Oates PJ, Guberski D, Ramasamy R. Polyol pathway and modulation of ischemia-reperfusion injury in Type 2 diabetic BBZ rat hearts. Cardiovasc Diabetol. 2008;7:33. doi: 10.1186/1475-2840-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 9.Lee AY, Chung SK, Chung SS. Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci U S A. 1995;92:2780–2784. doi: 10.1073/pnas.92.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzen S, Buyukbingol E. Recent studies of aldose reductase enzyme inhibition for diabetic complications. Curr Med Chem. 2003;10:1329–1352. doi: 10.2174/0929867033457377. [DOI] [PubMed] [Google Scholar]
- 11.Narayanan S. Aldose reductase and its inhibition in the control of diabetic complications. Ann Clin Lab Sci. 1993;23:148–158. [PubMed] [Google Scholar]
- 12.Zenon GJ, 3rd, Abobo CV, Carter BL, Ball DW. Potential use of aldose reductase inhibitors to prevent diabetic complications. Clin Pharm. 1990;9:446–457. [PubMed] [Google Scholar]
- 13.Lee AY, Chung SS. Contributions of polyol pathway to oxidative stress in diabetic cataract. FASEB J. 1999;13:23–30. doi: 10.1096/fasebj.13.1.23. [DOI] [PubMed] [Google Scholar]
- 14.Chung SS, Ho EC, Lam KS, Chung SK. Contribution of polyol pathway to diabetes-induced oxidative stress. J Am Soc Nephrol. 2003;14:S233–6. doi: 10.1097/01.asn.0000077408.15865.06. [DOI] [PubMed] [Google Scholar]
- 15.Ansari NH, Bhatnagar A, Fulep E, Khanna P, Srivastava SK. Trolox protects hyperglycemia-induced cataractogenesis in cultured rat lens. Res Commun Chem Pathol Pharmacol. 1994;84:93–104. [PubMed] [Google Scholar]
- 16.Srivastava SK, Ansari NH. Prevention of sugar-induced cataractogenesis in rats by butylated hydroxytoluene. Diabetes. 1988;37:1505–1508. doi: 10.2337/diab.37.11.1505. [DOI] [PubMed] [Google Scholar]
- 17.Carini M, Aldini G, Facino RM. Mass spectrometry for detection of 4-hydroxy-trans-2-nonenal (HNE) adducts with peptides and proteins. Mass Spectrom Rev. 2004;23:281–305. doi: 10.1002/mas.10076. [DOI] [PubMed] [Google Scholar]
- 18.Galle J, Heermeier K, Wanner C. Atherogenic lipoproteins, oxidative stress, and cell death. Kidney Int Suppl. 1999;71:S62–65. doi: 10.1046/j.1523-1755.1999.07116.x. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld ME. Inflammation, lipids, and free radicals: lessons learned from the atherogenic process. Semin Reprod Endocrinol. 1998;16:249–261. doi: 10.1055/s-2007-1016285. [DOI] [PubMed] [Google Scholar]
- 20.Martinet W, Kockx MM. Apoptosis in atherosclerosis: focus on oxidized lipids and inflammation. Curr Opin Lipidol. 2001;12:535–541. doi: 10.1097/00041433-200110000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Uemura M, Manabe H, Yoshida N, Fujita N, Ochiai J, Matsumoto N, Takagi T, Naito Y, Yoshikawa T. Alpha-tocopherol prevents apoptosis of vascular endothelial cells via a mechanism exceeding that of mere antioxidation. Eur J Pharmacol. 2002;456:29–37. doi: 10.1016/s0014-2999(02)02639-0. [DOI] [PubMed] [Google Scholar]
- 22.Evensen SA, Galdal KS, Nilsen E. LDL-induced cytotoxicity and its inhibition by anti-oxidant treatment in cultured human endothelial cells and fibroblasts. Atherosclerosis. 1983;49:23–30. doi: 10.1016/0021-9150(83)90004-7. [DOI] [PubMed] [Google Scholar]
- 23.Obrador E, Navarro J, Mompo J, Asensi M, Pellicer JA, Estrela JM. Regulation of tumour cell sensitivity to TNF-α-induced oxidative stress and cytotoxicity: role of glutathione. Biofactors. 1998;8:23–26. doi: 10.1002/biof.5520080105. [DOI] [PubMed] [Google Scholar]
- 24.Kanitkar M, Gokhale K, Galande S, Bhonde RR. Novel role of curcumin in the prevention of cytokine-induced islet death in vitro and diabetogenesis in vivo. Br J Pharmacol. 2008;155:702–713. doi: 10.1038/bjp.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Premanand C, Rema M, Sameer MZ, Sujatha M, Balasubramanyam M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Invest Ophthalmol Vis Sci. 2006;47:2179–2184. doi: 10.1167/iovs.05-0580. [DOI] [PubMed] [Google Scholar]
- 26.Babu BI, Malleo G, Genovese T, Mazzon E, Di Paola R, Crisafulli C, Caminiti R, Siriwardena AK, Cuzzocrea S. Green tea polyphenols ameliorate pancreatic injury in cerulein-induced murine acute pancreatitis. Pancreas. 2009;38:954–967. doi: 10.1097/MPA.0b013e3181b28d11. [DOI] [PubMed] [Google Scholar]
- 27.Dixit BL, Balendiran GK, Watowich SJ, Srivastava S, Ramana KV, Petrash JM, Bhatnagar A, Srivastava SK. Kinetic and structural characterization of the glutathione-binding site of aldose reductase. J Biol Chem. 2000;275:21587–21595. doi: 10.1074/jbc.M909235199. [DOI] [PubMed] [Google Scholar]
- 28.Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- 29.Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Srivastava SK. Aldose reductase mediates the mitogenic signals of cytokines. Chem Biol Interact. 2003;143–144:587–596. doi: 10.1016/s0009-2797(02)00194-1. [DOI] [PubMed] [Google Scholar]
- 30.Chandra D, Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Role of aldose reductase in TNF-alpha-induced apoptosis of vascular endothelial cells. Chem Biol Interact. 2003;143–144:605–612. doi: 10.1016/s0009-2797(02)00191-6. [DOI] [PubMed] [Google Scholar]
- 31.Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Srivastava S, Dixit BL, Cai J, Sharma S, Hurst HE, Bhatnagar A, Srivastava SK. Metabolism of lipid peroxidation product, 4-hydroxynonenal (HNE) in rat erythrocytes: role of aldose reductase. Free Radic Biol Med. 2000;29:642–651. doi: 10.1016/s0891-5849(00)00351-8. [DOI] [PubMed] [Google Scholar]
- 33.Vander Jagt DL, Kolb NS, Vander Jagt TJ, Chino J, Martinez FJ, Hunsaker LA, Royer RE. Substrate specificity of human aldose reductase: identification of 4-hydroxynonenal as an endogenous substrate. Biochim Biophys Acta. 1995;1249:117–126. doi: 10.1016/0167-4838(95)00021-l. [DOI] [PubMed] [Google Scholar]
- 34.Bhatnagar A, Liu SQ, Ueno N, Chakrabarti B, Srivastava SK. Human placental aldose reductase: role of Cys-298 in substrate and inhibitor binding. Biochim Biophys Acta. 1994;1205:207–214. doi: 10.1016/0167-4838(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 35.Ramana KV, Dixit BL, Srivastava S, Bhatnagar A, Balendiran GK, Watowich SJ, Petrash JM, Srivastava SK. Characterization of the glutathione binding site of aldose reductase. Chem Biol Interact. 2001;130–132:537–548. doi: 10.1016/s0009-2797(00)00297-0. [DOI] [PubMed] [Google Scholar]
- 36.Singh R, White MA, Ramana KV, Petrash JM, Watowich SJ, Bhatnagar A, Srivastava SK. Structure of a glutathione conjugate bound to the active site of aldose reductase. Proteins. 2006;64:101–110. doi: 10.1002/prot.20988. [DOI] [PubMed] [Google Scholar]
- 37.Srivastava S, Chandra A, Wang LF, Seifert WE, Jr, DaGue BB, Ansari NH, Srivastava SK, Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J Biol Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava S, Chandra A, Ansari NH, Srivastava SK, Bhatnagar A. Identification of cardiac oxidoreductase(s) involved in the metabolism of the lipid peroxidation-derived aldehyde-4-hydroxynonenal. Biochem J. 1998;329:469–475. doi: 10.1042/bj3290469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spycher SE, Tabataba-Vakili S, O’Donnell VB, Palomba L, Azzi A. Aldose reductase induction: a novel response to oxidative stress of smooth muscle cells. FASEB J. 1997;11:181–188. doi: 10.1096/fasebj.11.2.9039961. [DOI] [PubMed] [Google Scholar]
- 40.Hsu DK, Guo Y, Peifley KA, Winkles JA. Differential control of murine aldose reductase and fibroblast growth factor (FGF)-regulated-1 gene expression in NIH 3T3 cells by FGF-1 treatment and hyperosmotic stress. Biochem J. 1997;328:593–598. doi: 10.1042/bj3280593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacquin-Becker C, Labourdette G. Regulation of aldose reductase expression in rat astrocytes in culture. Glia. 1997;20:135–144. doi: 10.1002/(sici)1098-1136(199706)20:2<135::aid-glia5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Ruef J, Liu SQ, Bode C, Tocchi M, Srivastava S, Runge MS, Bhatnagar A. Involvement of aldose reductase in vascular smooth muscle cell growth and lesion formation after arterial injury. Arterioscler Thromb Vasc Biol. 2000;20:1745–1752. doi: 10.1161/01.atv.20.7.1745. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi M, Fujii J, Miyoshi E, Hoshi A, Taniguchi N. Elevation of aldose reductase gene expression in rat primary hepatoma and hepatoma cell lines: implication in detoxification of cytotoxic aldehydes. Int J Cancer. 1995;62:749–754. doi: 10.1002/ijc.2910620617. [DOI] [PubMed] [Google Scholar]
- 44.Zeindl-Eberhart E, Jungblut PR, Otto A, Kerler HM, Rabes R. Further characterization of a rat hepatoma-derived aldose-reductase-like protein--organ distribution vand modulation in vitro. Eur J Biochem. 1997;247:792–800. doi: 10.1111/j.1432-1033.1997.t01-1-00792.x. [DOI] [PubMed] [Google Scholar]
- 45.Yadav UC, Ighani-Hosseinabad F, van Kuijk FJ, Srivastava SK, Ramana KV. Prevention of posterior capsular opacification through aldose reductase inhibition. Invest Ophthalmol Vis Sci. 2009;50:752–759. doi: 10.1167/iovs.08-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi S, Bekhor I. Abnormal expression of aldose reductase mRNA in fiber cells of cataractous rat lens. Analysis by in situ hybridization. Mol Cell Biochem. 1994;131:35–41. doi: 10.1007/BF01075722. [DOI] [PubMed] [Google Scholar]
- 47.Srivastava S, Conklin DJ, Liu SQ, Prakash N, Boor PJ, Srivastava SK, Bhatnagar A. Identification of biochemical pathways for the metabolism of oxidized low-density lipoprotein derived aldehyde-4-hydroxy trans-2-nonenal in vascular smooth muscle cells. Atherosclerosis. 2001;158:339–350. doi: 10.1016/s0021-9150(01)00454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rittner HL, Hafner V, Klimiuk PA, Szweda LI, Goronzy JJ, Weyand CM. Aldose reductase functions as a detoxification system for lipid peroxidation products in vasculitis. J Clin Invest. 1999;103:1007–1013. doi: 10.1172/JCI4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Godon C, Lagniel G, Lee J, Buhler JM, Kieffer S, Perrot M, Boucherie H, Toledano MB, Labarre J. The H2 O2 stimulon in Saccharomyces cerevisiae. J Biol Chem. 1998;273:22480–22489. doi: 10.1074/jbc.273.35.22480. [DOI] [PubMed] [Google Scholar]
- 50.Kasuya Y, Ito M, Nakamura J, Hamada Y, Nakayama M, Chaya S, Komori T, Naruse K, Nakashima E, Kato K, Koh N, Hotta N. An aldose redutase inhibitor prevents the intimal thickening in coronary arteries of galactose-fed beagle dogs. Diabetologia. 1999;42:1404–1409. doi: 10.1007/s001250051310. [DOI] [PubMed] [Google Scholar]
- 51.Halliwell B. Mechanisms involved in the generation of free radicals. Pathol Biol (Paris) 1996;44:6–13. [PubMed] [Google Scholar]
- 52.Forman HJ, Torres M. Redox signaling in macrophages. Mol Aspects Med. 2001;22:189–216. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- 53.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453–462. doi: 10.1161/01.RES.0000204727.46710.5e. [DOI] [PubMed] [Google Scholar]
- 55.Wu CH, Wu CF, Huang HW, Jao YC, Yen GC. Naturally occurring flavonoids attenuate high glucose-induced expression of proinflammatory cytokines in human monocytic THP-1 cells. Mol Nutr Food Res. 2009;53:984–995. doi: 10.1002/mnfr.200800495. [DOI] [PubMed] [Google Scholar]
- 56.El-Benna PM, Dang J, Gougerot-Pocidalo MA, Marie JC, Braut-Boucher F. p47phox, the phagocyte NADPH oxidase/NOX2 organizer: structure, phosphorylation and implication in diseases. Exp Mol Med. 2009;41:217–225. doi: 10.3858/emm.2009.41.4.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mustapha NM, Joanna MT, Kohner EM, Chibber R. NADPH Oxidase versus Mitochondria-Derived ROS in Glucose-Induced Apoptosis of Pericytes in Early Diabetic Retinopathy. J Ophthalmol. 2010;2010 doi: 10.1155/2010/746978. Article ID 746978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murdoch CE, Zhang M, Cave AC, Shah AM. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res. 2006;71:208–215. doi: 10.1016/j.cardiores.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 59.Seno T, Inoue N, Gao D, Okuda M, Sumi Y, Matsui K, Yamada S, Hirata KI, Kawashima S, Tawa R, Imajoh-Ohmi S, Sakurai H, Yokoyama M. Involvement of NADH/NADPH oxidase in human platelet ROS production. Thromb Res. 2001;103:399–409. doi: 10.1016/s0049-3848(01)00341-3. [DOI] [PubMed] [Google Scholar]
- 60.Ho E, Chen G, Bray TM. Supplementation of N-acetylcysteine inhibits NFkappaB activation and protects against alloxan-induced diabetes in CD-1 mice. FASEB J. 1999;13:1845–1854. [PubMed] [Google Scholar]
- 61.Gius D, Botero A, Shah S, Curry HA. Intracellular oxidation/reduction status in the regulation of transcription factors NF-kappaB and AP-1. Toxicol Lett. 1999;106:93–106. doi: 10.1016/s0378-4274(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 62.Karin M, Shaulian E. AP-1: linking hydrogen peroxide and oxidative stress to the control of cell proliferation and death. IUBMB Life. 2001;52:17–24. doi: 10.1080/15216540252774711. [DOI] [PubMed] [Google Scholar]
- 63.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 64.Poli G, Schaur RJ. 4-Hydroxynonenal in the pathomechanisms of oxidative stress. IUBMB Life. 2000;50:315–321. doi: 10.1080/713803726. [DOI] [PubMed] [Google Scholar]
- 65.Parola M, Bellomo G, Robino G, Barrera G, Dianzani MU. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid Redox Signal. 1999;1:255–284. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]
- 66.Asselin C, Bouchard B, Tardif JC, Des Rosiers C. Circulating 4-hydroxynonenal-protein thioether adducts assessed by gas chromatography-mass spectrometry are increased with disease progression and aging in spontaneously hypertensive rats. Free Radic Biol Med. 2006;41:97–105. doi: 10.1016/j.freeradbiomed.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 67.Nadkarni DV, Sayre LM. Structural definition of early lysine and histidine adduction chemistry of 4-hydroxynonenal. Chem Res Toxicol. 1995;8:284–291. doi: 10.1021/tx00044a014. [DOI] [PubMed] [Google Scholar]
- 68.Cajone F, Crescente M. In vitro activation of heat shock transcription factor by 4-hydroxynonenal. Chem Biol Interact. 1992;84:97–112. doi: 10.1016/0009-2797(92)90071-r. [DOI] [PubMed] [Google Scholar]
- 69.Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am J Clin Nutr. 1993;57(suppl 5):779S–785S. doi: 10.1093/ajcn/57.5.779S. [DOI] [PubMed] [Google Scholar]
- 70.Zamara E, Novo E, Marra F, Gentilini A, Romanelli RG, Caligiuri A, Robino G, Tamagno E, Aragno M, Danni O, Autelli R, Colombatto S, Dianzani MU, Pinzani M, Parola M. 4-Hydroxynonenal as a selective pro-fibrogenic stimulus for activated human hepatic stellate cells. J Hepatol. 2004;40:60–68. doi: 10.1016/s0168-8278(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 71.Lee JY, Je JH, Kim DH, Chung SW, Zou Y, Kim ND, Ae Yoo M, Suck Baik H, Yu BP, Chung HY. Induction of endothelial apoptosis by 4-hydroxyhexenal. Eur J Biochem. 2004;271:1339–1347. doi: 10.1111/j.1432-1033.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- 72.Choudhary S, Zhang W, Zhou F, Campbell GA, Chan LL, Thompson EB, Ansari NH. Cellular lipid peroxidation end-products induce apoptosis in human lens epithelial cells. Free Radic Biol Med. 2002;32:360–369. doi: 10.1016/s0891-5849(01)00810-3. [DOI] [PubMed] [Google Scholar]
- 73.Eckl PM. Genotoxicity of HNE. Mol Aspects Med. 2003;24:161–165. doi: 10.1016/s0098-2997(03)00010-4. [DOI] [PubMed] [Google Scholar]
- 74.Spycher S, Tabataba-Vakili S, O’Donnell VB, Palomba L, Azzi A. 4-hydroxy-2,3-trans-nonenal induces transcription and expression of aldose reductase. Biochem Biophys Res Commun. 1996;226:512–516. doi: 10.1006/bbrc.1996.1386. [DOI] [PubMed] [Google Scholar]
- 75.Martin HJ, Maser E. Role of human aldo-keto-reductase AKR1B10 in the protection against toxic aldehydes. Chem Biol Interact. 2009;178:145–150. doi: 10.1016/j.cbi.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 76.Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- 77.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554–562. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
- 78.Musashi M, Ota S, Shiroshita N. The role of protein kinase C isoforms in cell proliferation and apoptosis. Int J Hematol. 2000;72:12–19. [PubMed] [Google Scholar]
- 79.Marinari UM, Nitti M, Pronzato MA, Domenicotti C. Role of PKC-dependent pathways in HNE-induced cell protein transport and secretion. Mol Aspects Med. 2003;24:205–211. doi: 10.1016/s0098-2997(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 80.Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54:818–829. doi: 10.2337/diabetes.54.3.818. [DOI] [PubMed] [Google Scholar]
- 81.Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. J Biol Chem. 2002;277:32063–32070. doi: 10.1074/jbc.M202126200. [DOI] [PubMed] [Google Scholar]
- 82.Tammali R, Ramana KV, Srivastava SK. Aldose reductase regulates TNF-alpha-induced PGE2 production in human colon cancer cells. Cancer Lett. 2007;252:299–306. doi: 10.1016/j.canlet.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramana KV, Bhatnagar A, Srivastava SK. Aldose reductase regulates TNF-alpha-induced cell signaling and apoptosis in vascular endothelial cells. FEBS Lett. 2004;570:189–194. doi: 10.1016/j.febslet.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 84.Ramana B, Friedrich KV, Bhatnagar A, Srivastava SK. Aldose reductase mediates cytotoxic signals of hyperglycemia and TNF-alpha in human lens epithelial cells. FASEB J. 2003;17:315–317. doi: 10.1096/fj.02-0568fje. [DOI] [PubMed] [Google Scholar]
- 85.King GL, Shiba T, Oliver J, Inoguchi T, Bursell SE. Cellular and molecular abnormalities in the vascular endothelium of diabetes mellitus. Annu Rev Med. 1994;45:179–188. doi: 10.1146/annurev.med.45.1.179. [DOI] [PubMed] [Google Scholar]
- 86.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 87.Cammarata PR, Chen HQ, Yang J, Yorio T. Modulation of myo-[3H]inositol uptake by glucose and sorbitol in cultured bovine lens epithelial cells. II. Characterization of high- and low-affinity myo-inositol transport sites. Invest Ophthalmol Vis Sci. 1992;33:3572–3580. [PubMed] [Google Scholar]
- 88.Kang ES, Kim HJ, Paek KS, Jang HS, Chang KC, Lee JH, Nishinaka T, Yabe-Nishimura C, Seo HG. Phorbol ester up-regulates aldose reductase expression in A549 cells: a potential role for aldose reductase in cell cycle modulation. Cell Mol Life Sci. 2005;62:1146–1155. doi: 10.1007/s00018-005-5024-4. [DOI] [PubMed] [Google Scholar]
- 89.Yamaoka T, Oda A, Bannai C, Itakura M, Yamashita K. The effect of non-enzymatic glycation on recombinant human aldose reductase. Diabetes Res Clin Pract. 1995;27:165–169. doi: 10.1016/0168-8227(95)01055-i. [DOI] [PubMed] [Google Scholar]
- 90.Dan Q, Wong R, Chung SK, Chung SS, Lam KS. Interaction between the polyol pathway and non-enzymatic glycation on aortic smooth muscle cell migration and monocyte adhesion. Life Sci. 2004;76:445–459. doi: 10.1016/j.lfs.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 91.Hamada Y, Araki N, Koh N, Nakamura J, Horiuchi S, Hotta N. Rapid formation of advanced glycation end products by intermediate metabolites of glycolytic pathway and polyol pathway. Biochem Biophys Res Commun. 1996;228:539–543. doi: 10.1006/bbrc.1996.1695. [DOI] [PubMed] [Google Scholar]
- 92.Nagaraj RH, Prabhakaram M, Ortwerth BJ, Monnier VM. Suppression of pentosidine formation in galactosemic rat lens by an inhibitor of aldose reductase. Diabetes. 1994;43:580–586. doi: 10.2337/diab.43.4.580. [DOI] [PubMed] [Google Scholar]
- 93.Lee AY, Chung SK, Chung SS. Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci U S A. 1995;92:2780–2784. doi: 10.1073/pnas.92.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hwang YC, Kaneko M, Bakr S, Liao H, Lu Y, Lewis ER, Yan S, Ii S, Itakura M, Rui L, Skopicki H, Homma S, Schmidt AM, Oates PJ, Szabolcs M, Ramasamy R. Central role for aldose reductase pathway in myocardial ischemic injury. FASEB J. 2004;18:1192–1199. doi: 10.1096/fj.03-1400com. [DOI] [PubMed] [Google Scholar]
- 95.Sessler CN, Shepherd W. New concepts in sepsis. Curr Opin Crit Care. 2002;8:465–472. doi: 10.1097/00075198-200210000-00016. [DOI] [PubMed] [Google Scholar]
- 96.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 97.Waage A, Brandtzaeg P, Espevik T, Halstensen A. Current understanding of the pathogenesis of gram-negative shock. Infect Dis Clin North Am. 1991;5:781–791. [PubMed] [Google Scholar]
- 98.Reddy AB, Srivastava SK, Ramana KV. Anti-inflammatory effect of aldose reductase inhibition in murine polymicrobial sepsis. Cytokine. 2009;48:170–176. doi: 10.1016/j.cyto.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;114:1838–1846. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- 100.Braman SS. The global burden of asthma. Chest. 2006;130(1 Suppl):4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 101.Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M, Djukanović R. The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161:9–16. doi: 10.1164/ajrccm.161.1.9802048. [DOI] [PubMed] [Google Scholar]
- 102.Hamid Q, Tulic M. Immunobiology of asthma. Annu Rev Physiol. 2009;71:489–507. doi: 10.1146/annurev.physiol.010908.163200. [DOI] [PubMed] [Google Scholar]
- 103.Fireman P. Understanding asthma pathophysiology. Allergy Asthma Proc. 2003;24:79–83. [PubMed] [Google Scholar]
- 104.Björnsdottir US, Cypcar DM. Asthma: an inflammatory mediator soup. Allergy. 1999;54:55–61. doi: 10.1111/j.1398-9995.1999.tb04389.x. [DOI] [PubMed] [Google Scholar]
- 105.Yadav UC, Ramana KV, Aguilera-Aguirre L, Boldogh I, Boulares HA, Srivastava SK. Inhibition of aldose reductase prevents experimental allergic airway inflammation in mice. PLoS One. 2009;4:e6535. doi: 10.1371/journal.pone.0006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yadav UC, Naura AS, Aguilera-Aguirre L, Ramana KV, Boldogh I, Sur S, Boulares HA, Srivastava SK. Aldose reductase inhibition suppresses the expression of Th2 cytokines and airway inflammation in ovalbumin-induced asthma in mice. J Immunol. 2009;183:4723–4732. doi: 10.4049/jimmunol.0901177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yadav UC, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2007;48:4634–4642. doi: 10.1167/iovs.07-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stewart BW, Kleihues P. World Cancer Report. IARCPress; Lyon: 2003. [Google Scholar]
- 109.Alcantara EN, Speckmann EW. Diet, nutrition, and cancer. Am J Clin Nutr. 1976;29:1035–1047. doi: 10.1093/ajcn/29.9.1035. [DOI] [PubMed] [Google Scholar]
- 110.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217–226. [PubMed] [Google Scholar]
- 111.Kraus S, Arber N. Inflammation and colorectal cancer. Curr Opin Pharmacol. 2009;9:405–410. doi: 10.1016/j.coph.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 112.Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–9713. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- 113.Weaver SA, Russo MP, Wright KL, Kolios G, Jobin C, Robertson DA, Ward SG. Regulatory role of phosphatidylinositol 3-kinase on TNF-α-induced cyclooxygenase 2 expression in colonic epithelial cells. Gastroenterol. 2001;120:1117–1127. doi: 10.1053/gast.2001.23257. [DOI] [PubMed] [Google Scholar]
- 114.Atreya R, Neurath MF. Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol. 2005;28:187–196. doi: 10.1385/CRIAI:28:3:187. [DOI] [PubMed] [Google Scholar]
- 115.Liu W, Reinmuth N, Stoeltzing O, Parikh AA, Tellez C, Williams S, Jung YD, Fan F, Takeda A, Akagi M, Bar-Eli M, Gallick GE, Ellis LM. Cyclooxygenase-2 is up-regulated by interleukin-1 beta in human colorectal cancer cells via multiple signaling pathways. Cancer Res. 2003;63:3632–3636. [PubMed] [Google Scholar]
- 116.Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135:2993S–3001S. doi: 10.1093/jn/135.12.2993S. [DOI] [PubMed] [Google Scholar]
- 117.Ramana KV, Tammali R, Srivastava SK. Inhibition of aldose reductase prevents growth factor-induced G1-S phase transition through the AKT/phosphoinositide 3-kinase/E2F-1 pathway in human colon cancer cells. Mol Cancer Ther. 2010;9:813–824. doi: 10.1158/1535-7163.MCT-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tammali R, Reddy AB, Ramana KV, Petrash JM, Srivastava SK. Aldose reductase deficiency in mice prevents azoxymethane-induced colonic preneoplastic aberrant crypt foci formation. Carcinogenesis. 2009;30:799–807. doi: 10.1093/carcin/bgn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saraswat M, Mrudula T, Kumar PU, Suneetha A, Rao Rao TS, Srinivasulu M, Reddy B. Overexpression of aldose reductase in human cancer tissues. Med Sci Monit. 2006;12:CR525–529. [PubMed] [Google Scholar]
- 120.Lee KW, Ko BC, Jiang Z, Cao D, Chung SS. Overexpression of aldose reductase in liver cancers may contribute to drug resistance. Anticancer Drugs. 2001;12:129–132. doi: 10.1097/00001813-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 121.Kawamura I, Lacey E, Yamamoto N, Sakai F, Takeshita S, Inami M, Nishigaki F, Naoe Y, Tsujimoto S, Manda T, Shimomura K, Goto T. Ponalrestat, an aldose reductase inhibitor, inhibits cachexia syndrome induced by colon26 adenocarcinoma in mice. Anticancer Res. 1999;19:4105–4111. [PubMed] [Google Scholar]