Abstract
Tenofovir disoproxil fumarate (TDF) is an oral prodrug and acyclic nucleotide analog of adenosine monophosphate that inhibits HIV-1 (HIV) reverse transcriptase. A growing subset of TDF-treated HIV+ individuals presented with acute renal failure, suggesting tenofovir-associated kidney-specific toxicity. Our previous studies using an HIV transgenic mouse model (TG) demonstrated specific changes in renal proximal tubular mitochondrial DNA (mtDNA) abundance. Nucleosides are regulated in biological systems via transport and metabolism in cellular compartments. In this study, the role(s) of organic anion transporter type 1 (OAT1) and multidrug-resistant protein type 4 (MRP4) in transport and regulation of tenofovir in proximal tubules were assessed. Renal toxicity was assessed in kidney tissues from OAT1 knock-out (KO) or MRP4 KO compared to wild-type (WT, C57BL/6) mice following treatment with TDF (0.11 mg/day), didanosine (ddI, a related adenosine analog, 0.14 mg/day) or vehicle (0.1 M NaOH) daily gavage for 5 wks. Laser-capture microdissection (LCM) was used to isolate renal proximal tubules for molecular analyses. mtDNA abundance and ultrastructural pathology were analyzed. mtDNA abundance in whole-kidneys from both KO and WT was unchanged regardless of treatment. Renal proximal tubular mtDNA abundance from OAT1 KO also remained unchanged, suggesting prevention of TDF toxicity due to loss of tenofovir transport into proximal tubules. In contrast, renal proximal tubules from MRP4 KO exhibited increased mtDNA abundance following TDF treatment compared to WT littermates, suggesting compensation. Renal proximal tubules from TDF treated WT and MRP4 KO exhibited increased numbers of irregular mitochondria with sparse, fragmented cristae compared to OAT1 KO. Treatment with ddI had a compensatory effect on mtDNA abundance in OAT1 KO but not in MRP4 KO. Both OAT1 and MRP4 have a direct role in transport and efflux of tenofovir, regulating levels of tenofovir in proximal tubules. Disruption of OAT1 activity prevents tenofovir toxicity but loss of MRP4 can lead to increased renal proximal tubular toxicity. These data help explain mechanisms of human TDF renal toxicity.
INTRODUCTION
Tenofovir disoproxil fumarate (TDF) is an oral prodrug of tenofovir (TFV), an acyclic nucleotide analog of adenosine monophosphate, a reverse transcriptase inhibitor of human immunodeficiency virus type 1 (HIV-1) (1). Clinical studies found TDF safe, with low incidence (1–3 %) of renal impairment characterized by elevated serum creatinine or hypophosphatemia (2–4). Several subsequent reports (5) uncovered a subset of TDF-treated patients that presented with acute kidney injury (AKI) (6–10) in which kidney damage reversed upon TDF discontinuation (11, 12). These reports raised questions about potential kidney-specific toxicity and TFV-associated increased risk of tubulopathy, which appears similar to reported toxicity with other related acyclic nucleoside phophonate antivirals such as cidofovir and adefovir (13).Drug uptake and efflux of antiretrovirals in cells is regulated by their transport and metabolism/elimination in compartments (14). The transport of nucleoside analogs across the gastrointestinal epithelial barrier is mediated by transporters (reviewed in (15)). TFV transport from blood into proximal tubule epithelial cells is most likely facilitated by human organic anion transporter (OAT) types 1 and 3, with an efficiency similar to that for other acyclic nucleotides (16, 17). OAT types are part of a superfamily that mediate transmembrane transport of a wide range of amphipathic endogenous and exogenous organic compounds (reviewed in (18, 19)). TFV efflux from the proximal tubule cells and secretion into the tubular lumen is likely facilitated by multidrug-resistant protein type 4 (MRP4), which acts as an efflux pump and is highly expressed in the apical membranes of proximal tubules (20, 21).
An OAT1 hemizygous depleted murine model decreases organic anion transport and limits concentrations of endogenous organic anions in renal proximal tubules (22). Decreased transport of TFV in this OAT1 depleted model could potentially alter its tissue-specific pharmacokinetics.
An Mrp4−/ − KO murine model has decreased efflux activity of organic anions across the apical membrane of renal proximal tubules (23). In this case, decreased efflux of TFV could increase TFV concentrations in proximal tubules and increase the potential for toxicity. Studies here systematically determined the role(s) of OAT1 and/or MRP4 in regulation of TFV and proximal tubular mitochondrial toxicity. Identifying the mechanisms of TFV regulation is fundamental to developing ways to circumvent related toxicity and improving on drug delivery approaches to treat for HIV-1/AIDS.
MATERIALS AND METHODS
Animals
Hemizygous OAT1 KO mice were generated from offspring of the colony established by Sanjay Nigam (22) on a C57/BL6 background. Mrp4 −/ − mice were generated from offspring of the colony established by John Schuetz and colleagues (23), also on a C57/BL6 background. For this study, a “2 by 2” factorial protocol included KOs and wild-type (WT; C57/BL6 background) mice treated with vehicle or NRTI. KO mice were routinely identified for each generation of offspring using dot blot analysis and real-time PCR as reported previously (24).
Treatment protocols
Procedures complied with Emory IACUC and NIH guidelines. WT and KO littermates of both gender (male and female) were age-matched (8–12 weeks) at the start of the treatment regimen. Food and water were provided ad libitum. Antiretroviral drugs were obtained from the manufacturers through the Emory Center for AIDS Research Pharmacology Core. Dosing was done by daily gavage at doses that resemble human therapy on a mg/kg/d basis. Treatments included tenofovir disoproxyl fumarate (TDF, 0.11 mg/day), didanosine (ddI, 0.14 mg/day) or vehicle (0.1 M NaOH). Morbidity and mortality from the procedure were negligible. After 5 weeks treatment, body weights were measured, animals terminated, and samples retrieved and stored for DNA extraction and analysis.
mtDNA and nuclear DNA (nDNA) quantitation in heart tissue using real time PCR
Methods employed were modifications used by us in the past (24, 25). Total DNA was extracted from kidney (~10 mg wet weight) using a MagNA Pure System and reagents (Roche Life Sciences, Indianapolis, IN). Alternatively, DNA was extracted from proximal tubules isolated with laser-capture microscopy (LCM, see procedure below).
DNA sequences for primers and probes used for quantitation of mitochondrial and nuclear DNA were described previously (25). Real-Time PCR was performed in duplicate for each amplicon. Amplification was performed using Light Cycler LC 480 (Roche). PCR products of mtDNA and nDNA were quantified using the corresponding external standard DNA curves.
Laser-captured micro-dissection (LCM)
The inherent complexity of organs causes heterogeneity of tissues (in the case of the kidney, glomeruli, proximal and distal tubule epithelial cells, and arterioles, and others) can affect the outcome and interpretation of molecular studies. An ideal approach would allow us to identify and isolate selected tissue elements in the kidney in a homogeneous way and obviate mixing of cell types. LCM, a novel technique developed at the National Cancer Institute allows for specific, single-cell isolation from fixed heterogenous tissues. Briefly, proximal renal tubular cells were isolated using the Arcturus LCM 1110 system (Arcturus Biosciences Inc., Mountain View, CA) and a protocol previously established (26). DNA was extracted from pooled renal tubular epithelial cells (~100 cells) isolated from each embedded sample using PicoPure DNA Extraction Kit (MDS Analytical Technologies, Sunnyvale, CA).
Fine structure of kidney tissues (EM)
EM was evaluated using methods employed regularly in the Lewis lab (27). Sections (0.5μ) were cut with glass knives and stained with Toluidine Blue for orientation. Ultrathin (900 Å) sections were cut with a diamond knife, stained with uranyl acetate and lead citrate and examined by EM (Philips Morgagni Model 201, Philips, Eindhoven, Amsterdam, NL) and evaluated and photographed. Each EM photomicrograph was reviewed independently by two investigators. Parameters included presence of structurally abnormal mitochondria, increased numbers of mitochondrial profiles per field, intra-mitochondrial lamellar bodies, abnormal cristae density, cristae reduplication, mitochondrial swelling, and intra-mitochondrial paracrystals (28).
Statistical analysis
Data expressed as the ratio of mean values for mtDNA to the mean values of nDNA (× 10−3). Resultant values expressed as mean ± standard error, normalized to untreated WT mean (set at 1.0). A value of p < 0.05 was considered statistically significant. Echocardiographic determinations from all groups were compared by 1-way ANOVA (29).
RESULTS
General
All KO and WT cohorts maintained overall health with normal body weights and levels of activity throughout the study duration, regardless of treatment.
mtDNA abundance in whole kidney tissues following treatment
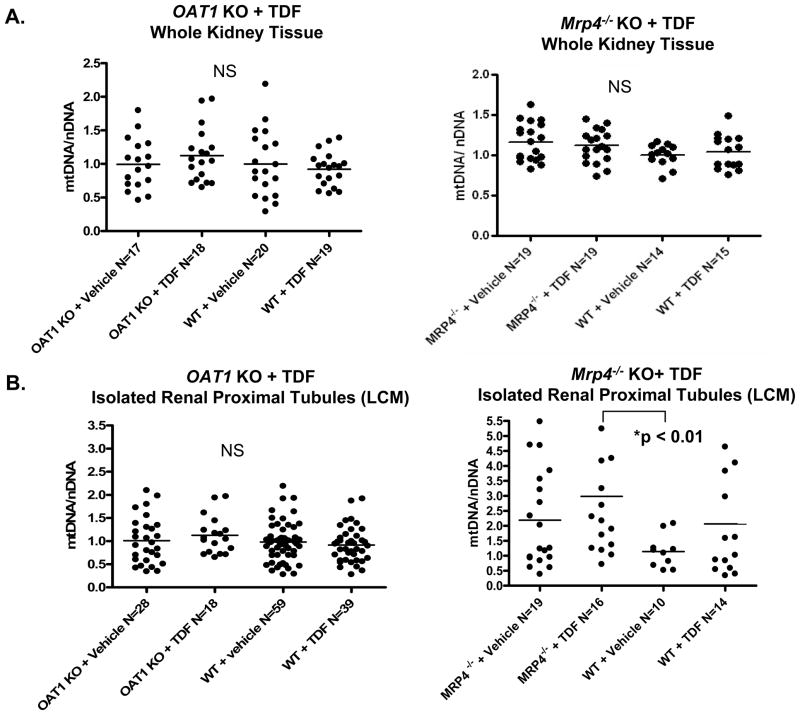
To determine the role of OAT1 or MRP4 on TFV-associated mitochondrial toxicity, mtDNA abundance was assessed using DNA extracts from whole kidney tissues of either OAT1 KO or Mrp4−/ − KO compared to WTs following 5 weeks treatment with TDF or vehicle. Analysis of whole kidney tissue extractions detected no change in mtDNA abundance (expressed as ratio of mtDNA to nDNA) following TDF treatment of WTs compared to their vehicle-treated littermates (Figure 1A). In addition, whole kidney tissue extracts from OAT1 KO or Mrp4−/ − KO treated with TDF similarly demonstrated no detectable changes in mtDNA abundance compared to WTs (Figure 1A, left and right graphs, respectively).
Figure 1.
Changes in mtDNA abundance after TDF treatment of OAT1 KO or Mrp4−/ − KO. mtDNA abundance (mtDNA/nDNA) was determined from whole kidney extracts (A) or isolated renal proximal tubules using LCM (B). No changes in mtDNA abundance were detected in whole tissues. The isolation of tubules using the LCM improved sensitivity and revealed changes that appear compensatory in samples from Mrp4−/ − KO treated with TDF compared to untreated WT (p < 0.01). TDF treated Mrp4−/ − KO in proximal tubules also had a trend for increased mtDNA compared to untreated Mrp4−/ − KO or TDF treated WTs, but with no statistical significance (p > 0.05).. In contrast, TDF had no measureable impact on mtDNA abundance in OAT1 KO compared to untreated or WT controls (p > 0.05, not significant, NS).
LCM-isolated proximal tubular mtDNA abundance
In effort to increase the homogeneity of cells being sampled, proximal tubules were micro-dissected from kidney sections using LCM to determine the effect(s) of TFV on this histological compartment. Proximal tubules were microscopically defined by their brush border with intense PAS positive staining from glycoproteins therein. TDF treatment of WTs caused minimal change to mtDNA abundance of LCM-isolated renal proximal tubules compared to tubules from vehicle-treated WTs (p > 0.05) (Figure 1B). mtDNA abundance in OAT1 KO treated with TDF remained unchanged from vehicle-treated OAT1 KO and WT controls (Figure 1B, left graph).
In contrast, the mtDNA abundance of proximal tubules from Mrp4−/ − KO increased significantly following TDF treatment, compared to vehicle-treated WTs (p < 0.01) (Figure 1B, right). Notably, TDF treatment in WTs as well as untreated Mrp4−/ − KO also demonstrated a trend of increased mtDNA abundance compared to vehicle-treated WTs, although neither was statistically significant (p>0.05). Only the combined effect of both the Mrp4−/ − KO and the TDF treatment in proximal tubules increased mtDNA abundance high enough to reach significance.
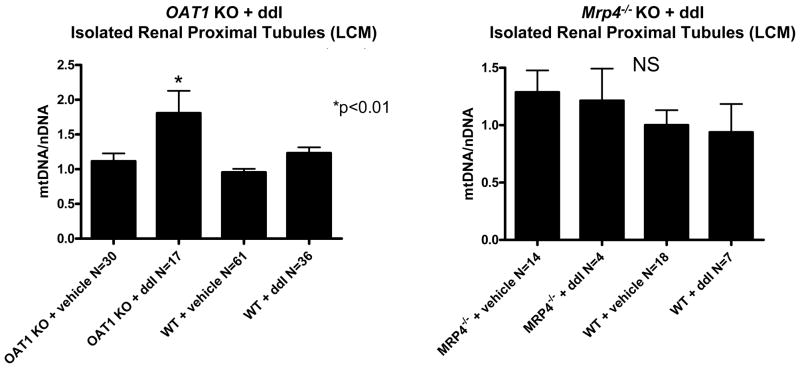
To further unravel the mechanism, a related antiretroviral drug, ddI, was tested similarly in these animal models. mtDNA abundance from whole kidney exhibited no change in either OAT1 or Mrp4−/ − KO with ddI treatment (1.12 ± 0.25 and 1.05 ± 0.2, respectively) or without (0.98 ± 0.18 and 1.0 ± 0.14, respectively) compared to vehicle-treated WTs (1.0 ± 0.16) (p > 0.05). LCM was performed to select for renal proximal tubules and assess cell-specific changes in mtDNA abundance with ddI. In contrast to the observations with TDF, ddI treatment of OAT1 KO resulted in a significant increase in mtDNA abundance in proximal tubules (p < 0.01) (Figure 2, left graph). Treatment with ddI had no effect on mtDNA abundance in proximal tubules from Mrp4−/ − KO (p > 0.05) (Figure 2, right graph), which was in stark contrast to results obtained with TDF treatment (Figure 1B, right graph).
Figure 2.
Changes in mtDNA abundance after ddI treatment of OAT1 KO or Mrp4−/ − KO. mtDNA abundance was determined from isolated renal proximal tubules using LCM. ddI treatment increased mtDNA abundance in renal proximal tubules of OAT1 KO (p < 0.01) compared to other three groups (untreated OAT1 KO, untreated WTs, and ddI treated WTs). ddI treatment had no impact on WT or Mrp4−/ − KO (p > 0.05, not significant, NS).
EM features of mitochondria in tubular epithelium
To investigate organelle-specific structural changes in renal proximal tubules that were caused by TFV or ddI treatment in the mouse, ultrastructural evaluations of were performed. Vehicle-treated WTs demonstrated characteristic, oval mitochondria with densely packed cristae (Figure 3, top left panel). TDF-treated WTs exhibited mitochondria with irregular shape, and sparse, fragmented cristae (Figure 3, top center panel). In contrast, EM profiles from WTs treated with ddI appeared unchanged from vehicle-treated littermates (Figure 3, top right panel).
Figure 3.
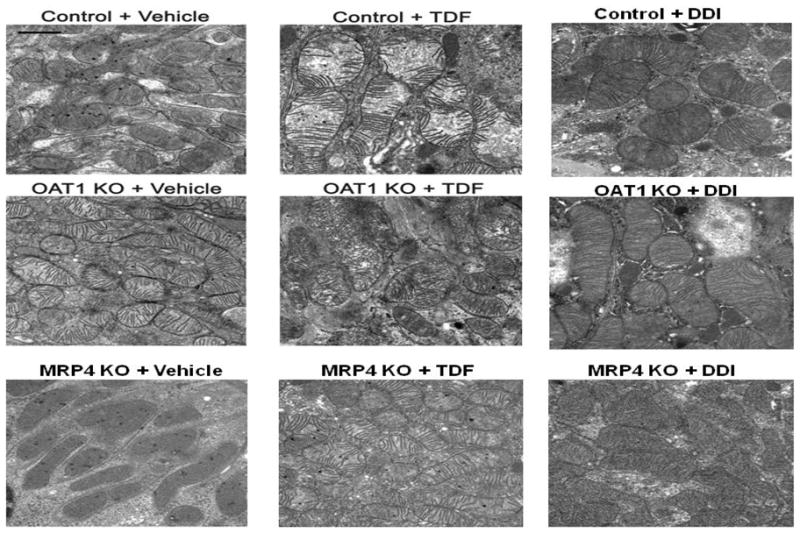
Mitochondrial ultrastructural changes in OAT1 KO, Mrp4−/ − KO, and WT treated with TDF, ddI, or vehicle. Control and Mrp4−/ − KO treated with TDF showed increased mitochondria, irregular shape and disrupted cristae (top center panel and bottom center panel, respectively). Mitochondria from TDF-treated OAT1 KO appeared normal (middle center panel). Changes after treatment with ddI in OAT1 KO, Mrp4−/ − KO and WT were unremarkable (right panels). (Original magnification: x 22300, marker indicates 1 μm).
Mitochondria from vehicle-treated OAT1 KO were oblong (Figure 3, middle left panel), but resembled vehicle-treated WTs with respect to the mitochondrial cristae. Treatment with either TDF or ddI resulted in no significant changes in the size and number of mitochondria (Figure 3, middle center and right panels). Likewise, vehicle-treated Mrp4−/ − KO appeared similar to vehicle-treated WTs (Figure 3, bottom left panel). TDF-treated Mrp4−/ − KO, however, exhibited increased number of mitochondria, with irregular mitochondrial shape, and sparse, fragmented cristae (Figure 3, bottom center panel). Treatment with ddI had no apparent effect on mitochondria from Mrp4−/ − KO (Figure 3, bottom right panel).
DISCUSSION
TFV is a highly effective antiretroviral agent used to treat HIV-1. Reports raised questions about potential renal toxicity and tubulopathy (reviewed in (30)). Since renal excretion plays a major role in TFV elimination, it follows logically that kidney cells are routinely exposed to high TFV concentrations. Mechanisms of TFV cellular accumulation are unclear. Transporters belonging to the SLC22 family of solute carriers (e.g., OAT1) and members of the ATP-binding cassette (ABC) transporter family (e.g., MRP4) are implicated in the renal secretion of toxins, metabolites and drugs, including antivirals (31, 32). OAT1 is located on the basolateral membrane of the proximal renal tubule and is therefore primarily responsible for renal uptake. For example, OAT1 has been shown to be the major transporter for adefovir (33). Therefore, OAT1 is likely to have a rate-limiting role in regulating the distribution of other related antiretrovirals, such as TDF or ddI, between blood and urine. OAT-mediated tubular accumulation of some drugs, including certain antiretrovirals, can result in nephrotoxicity (34).
Previously, we found that TDF treatment results in renal proximal tubular toxicity with disruption of mitochondrial biogenesis (decreased mtDNA abundance) in an AIDS murine model (26). In this study, TDF treatment (5 wks) in Mrp4−/− KO resulted in a statistically significant increase in mtDNA abundance. Similarly, an increasing trend in mtDNA abundance occurred in proximal tubules from TDF treated WTs and in vehicle-treated MRP4−/− KOs alone, although not statistically significant. An increase in mtDNA abundance seems counterintuitive as decreased mtDNA is characteristically reported in mitochondrial dysfunction. The observed increase here may be an attempt by mitochondria to compensate for sirupted mtDNA replication, as is suggested in some mitochondrial genetic disorders. This observed increase in mtDNA abundance has been reported previously (25, 35, 36). This compensational response which is detected as an apparent increase in mtDNA abundance may represent an “early” marker for disruption of mtDNA biogenesis ultimately progressing to complete mitochondrial toxicity with hallmark features including mtDNA depletion, mtDNA mutations, and inhibition of the electron transfer chain function.
In these studies, OAT1 KO was used to pinpoint the contribution of this transporter in TFV-related mitochondrial toxicity. Absence of OAT1 in the KO model prevented mitochondrial disruption following TDF treatment, suggesting that OAT1 may be the major transporter for TDF. Follow up studies with a related antiretroviral, ddI, resulted in an unanticipated outcome. OAT1 KO treated with ddI resulted in a significant increase in renal proximal tubular mtDNA abundance, suggesting a compensatory response. This type of response was previously found associated with TFV-related mitochondrial toxicity. Yet, ddI in WT or AIDS model had no impact on renal proximal tubular mitochondrial biogenesis (26).
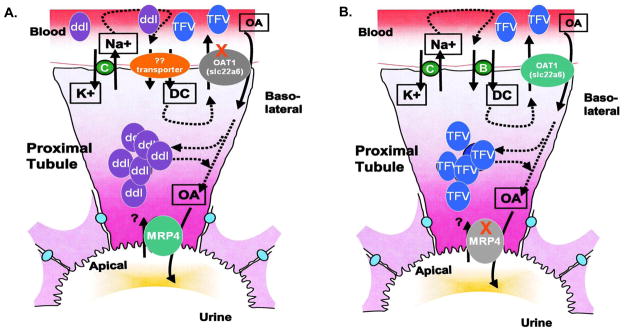
Other studies demonstrated that in the absence of a specific transporter, compensatory changes in functionally related transporters occur (37). In this case, it may be possible that the absence of OAT1 expression (rendered in the OAT1 KO) resulted in an up-regulation of an alternate transporter with increased affinity for ddI. This could lead to increased transport of ddI into the proximal tubules and promote its concentration therein. In turn, this could disrupt the intracellular nucleotide pool balance and lead to mitochondrial dysfunction in cells. This concept is summarized in Figure 4A.
Figure 4.
Schematic diagram of mechanisms of OAT1 and MRP4 in the transport of antiretrovirals within renal proximal tubules. (A) The potential block of TFV transport across the basolateral membrane of the proximal tubule in OAT1 KO is shown. An unidentified alternate transporter may be up-regulated in the absence of OAT1. This moiety may have affinity for ddI transport, and cause accumulation of ddI in proximal tubules with mitochondrial toxicity. (B) Increased accumulation of TFV in proximal tubules results from OAT1 transport across the basolateral membrane but lack of efflux on the apical membrane in Mrp4−/ − KO, leading to increased mitochondrial toxicity.
Equally important in the regulation of antiretrovirals in proximal tubules is the role of the ABC transporters in facilitating efflux of these drugs to urine. MRP4 is a member of this family of transporters that is located on the apical membrane of proximal tubules. Studies have suggested that TFV is a substrate for MRP4 (38, 39). Furthermore, it has been reported that intracellular concentrations of TFV in HIV-infected patients may be related to MRP4 genetic determinants (40). Here, Mrp4−/ − KO were used to systematically determine its role in TFV renal proximal tubular mitochondrial toxicity. Results suggested Mrp4−/ − KO are more susceptible to TFV mitochondrial toxicity as mtDNA abundance increased following TDF treatment compared to WTs. The observed increase in mtDNA may be an attempt by mitochondria to compensate for disrupted mtDNA replication (35). These data support the hypothesis that MRP4 has a critical role in the efflux of TFV from renal proximal tubules (summarized in Figure 4B), and may underscore the importance of efflux rather than influx in the mechanisms of toxicity of TDF. Furthermore, MRP4 does not appear to have a critical role in efflux of ddI as mtDNA abundance in Mrp4−/ − KO treated with ddI was unchanged from WTs.
Clinical outcomes with TFV are varied. Some individuals treated with TFV develop mitochondrial damage and AKI, while many individuals appear to tolerate TFV treatment with little or no apparent short-term side effects. This clinical dichotomy in outcomes may be genetically linked, in part, as there is a higher incidence of reported toxic side effects in African-American males with reports that this group have higher risk for HIVAN and end-stage renal disease (41–43).
These studies elucidate drug-specific regulatory roles of OAT1 and MRP4 to renal proximal tubular mitochondrial toxicity from nucleoside analogs used to treat HIV. Results suggest that OAT1 regulates the transport of TDF from the blood into the proximal tubules. Subsequently, MRP4 plays a critical role in the efflux of TFV out into the urine, preventing the accumulation of TFV inside the proximal tubule. Expression of these kidney transporters may vary in individuals. Genetic regulation and/or down-regulation by TFV of genes coding for transporter proteins involved in renal excretion has yet to be fully identified and is crucial for understanding the nephrotoxicity of antiretrovirals.
Acknowledgments
The studies were supported by a grant from Emory University Research Committee (URC) to JJK and R01 HL059798, HL079867 and DA030996 to WL. JJK is a recipient of K01 DK078513.
References
- 1.Lyseng-Williamson KA, Reynolds NA, Plosker GL. Tenofovir disoproxil fumarate: a review of its use in the management of HIV infection. Drugs. 2005;65(3):413–432. doi: 10.2165/00003495-200565030-00006. [DOI] [PubMed] [Google Scholar]
- 2.Izzedine H, Isnard-Bagnis C, Hulot JS, Vittecoq D, Cheng A, Jais CK, et al. Renal safety of tenofovir in HIV treatment-experienced patients. Aids. 2004;18(7):1074–1076. doi: 10.1097/00002030-200404300-00019. [DOI] [PubMed] [Google Scholar]
- 3.Schooley RT, Ruane P, Myers RA, Beall G, Lampiris H, Berger D, et al. Tenofovir DF in antiretroviral-experienced patients: results from a 48-week, randomized, double-blind study. AIDS. 2002;16(9):1257–1263. doi: 10.1097/00002030-200206140-00008. [DOI] [PubMed] [Google Scholar]
- 4.Padilla S, Gutierrez F, Masia M, Canovas V, Orozco C. Low frequency of renal function impairment during one-year of therapy with tenofovir-containing regimens in the real-world: a case-control study. AIDS Patient Care STDS. 2005;19(7):421–424. doi: 10.1089/apc.2005.19.421. [DOI] [PubMed] [Google Scholar]
- 5.Nelson MR, Katlama C, Montaner JS, Cooper DA, Gazzard B, Clotet B, et al. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: the first 4 years. Aids. 2007;21(10):1273–1281. doi: 10.1097/QAD.0b013e3280b07b33. [DOI] [PubMed] [Google Scholar]
- 6.Barrios A, Garcia-Benayas T, Gonzalez-Lahoz J, Soriano V. Tenofovir-related nephrotoxicity in HIV-infected patients. Aids. 2004;18(6):960–963. doi: 10.1097/00002030-200404090-00019. [DOI] [PubMed] [Google Scholar]
- 7.Breton G, Alexandre M, Duval X, Prie D, Peytavin G, Leport C, et al. Tubulopathy consecutive to tenofovir-containing antiretroviral therapy in two patients infected with human immunodeficiency virus-1. Scand J Infect Dis. 2004;36(6–7):527–528. doi: 10.1080/00365540310016169. [DOI] [PubMed] [Google Scholar]
- 8.James CW, Steinhaus MC, Szabo S, Dressier RM. Tenofovir-related nephrotoxicity: case report and review of the literature. Pharmacotherapy. 2004;24(3):415–418. doi: 10.1592/phco.24.4.415.33182. [DOI] [PubMed] [Google Scholar]
- 9.Lochet P, Peyriere H, Le Moing V, Blayac JP, Hansel S, Reynes J. Assessment of renal abnormalities in 107 HIV patients treated with tenofovir. Therapie. 2005;60(2):175–181. doi: 10.2515/therapie:2005022. [DOI] [PubMed] [Google Scholar]
- 10.Peyriere H, Reynes J, Rouanet I, Daniel N, de Boever CM, Mauboussin JM, et al. Renal tubular dysfunction associated with tenofovir therapy: report of 7 cases. J Acquir Immune Defic Syndr. 2004;35(3):269–273. doi: 10.1097/00126334-200403010-00007. [DOI] [PubMed] [Google Scholar]
- 11.Zimmermann AE, Pizzoferrato T, Bedford J, Morris A, Hoffman R, Braden G. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin Infect Dis. 2006;42(2):283–290. doi: 10.1086/499048. [DOI] [PubMed] [Google Scholar]
- 12.Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D'Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78(11):1171–1177. doi: 10.1038/ki.2010.318. [DOI] [PubMed] [Google Scholar]
- 13.Izzedine H, Launay-Vacher V, Isnard-Bagnis C, Deray G. Drug-induced Fanconi's syndrome. Am J Kidney Dis. 2003;41(2):292–309. doi: 10.1053/ajkd.2003.50037. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Chan WK. Transport, metabolism and elimination mechanisms of anti-HIV agents. Adv Drug Deliv Rev. 1999;39(1–3):81–103. doi: 10.1016/s0169-409x(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 15.Balimane PV, Sinko PJ. Involvement of multiple transporters in the oral absorption of nucleoside analogues. Adv Drug Deliv Rev. 1999;39(1–3):183–209. doi: 10.1016/s0169-409x(99)00026-5. [DOI] [PubMed] [Google Scholar]
- 16.Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999;56(3):570–580. doi: 10.1124/mol.56.3.570. [DOI] [PubMed] [Google Scholar]
- 17.Miller DS. Nucleoside phosphonate interactions with multiple organic anion transporters in renal proximal tubule. J Pharmacol Exp Ther. 2001;299(2):567–574. [PubMed] [Google Scholar]
- 18.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447(5):653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 19.Eraly SA, Bush KT, Sampogna RV, Bhatnagar V, Nigam SK. The molecular pharmacology of organic anion transporters: from DNA to FDA? Mol Pharmacol. 2004;65(3):479–487. doi: 10.1124/mol.65.3.479. [DOI] [PubMed] [Google Scholar]
- 20.Izzedine H, Hulot JS, Villard E, Goyenvalle C, Dominguez S, Ghosn J, et al. Association between ABCC2 gene haplotypes and tenofovir-induced proximal tubulopathy. J Infect Dis. 2006;194(11):1481–1491. doi: 10.1086/508546. [DOI] [PubMed] [Google Scholar]
- 21.Takenaka K, Morgan JA, Scheffer GL, Adachi M, Stewart CF, Sun D, et al. Substrate overlap between Mrp4 and Abcg2/Bcrp affects purine analogue drug cytotoxicity and tissue distribution. Cancer Res. 2007;67(14):6965–6972. doi: 10.1158/0008-5472.CAN-06-4720. [DOI] [PubMed] [Google Scholar]
- 22.Eraly SA, Vallon V, Vaughn DA, Gangoiti JA, Richter K, Nagle M, et al. Decreased Renal Organic Anion Secretion and Plasma Accumulation of Endogenous Organic Anions in OAT1 Knock-out Mice. J Biol Chem. 2006;281(8):5072–5083. doi: 10.1074/jbc.M508050200. [DOI] [PubMed] [Google Scholar]
- 23.Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24(17):7612–7621. doi: 10.1128/MCB.24.17.7612-7621.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis W, Day BJ, Kohler JJ, Hosseini SH, Chan SS, Green EC, et al. Decreased mtDNA, oxidative stress, cardiomyopathy, and death from transgenic cardiac targeted human mutant polymerase gamma. Lab Invest. 2007;87(4):326–335. doi: 10.1038/labinvest.3700523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosseini SH, Kohler JJ, Haase CP, Tioleco N, Stuart T, Keebaugh E, et al. Targeted Transgenic Overexpression of Mitochondrial Thymidine Kinase (TK2) Alters Mitochondrial DNA (mtDNA) and Mitochondrial Polypeptide Abundance: Transgenic TK2, mtDNA, and Antiretrovirals. Am J Pathol. 2007;170(3):865–874. doi: 10.2353/ajpath.2007.060655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohler JJ, Hosseini SH, Hoying-Brandt A, Green E, Johnson DM, Russ R, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Invest. 2009;89(5):513–519. doi: 10.1038/labinvest.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis W, Grupp IL, Grupp G, Hoit B, Morris R, Samarel AM, et al. Cardiac dysfunction occurs in the HIV-1 transgenic mouse treated with zidovudine. Lab Invest. 2000;80(2):187–197. doi: 10.1038/labinvest.3780022. [DOI] [PubMed] [Google Scholar]
- 28.Dalakas MC, Illa I, Pezeshkpour GH, Laukaitis JP, Cohen B, Griffin JL. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med. 1990;322(16):1098–1105. doi: 10.1056/NEJM199004193221602. [DOI] [PubMed] [Google Scholar]
- 29.Lewis W, Haase CP, Raidel SM, Russ RB, Sutliff RL, Hoit BD, et al. Combined antiretroviral therapy causes cardiomyopathy and elevates plasma lactate in transgenic AIDS mice. Laboratory Investigation. 2001;81(11):1527–1536. doi: 10.1038/labinvest.3780366. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez-Novoa S, Alvarez E, Labarga P, Soriano V. Renal toxicity associated with tenofovir use. Expert Opin Drug Saf. 2010;9(4):545–559. doi: 10.1517/14740331003627458. [DOI] [PubMed] [Google Scholar]
- 31.Truong DM, Kaler G, Khandelwal A, Swaan PW, Nigam SK. Multi-level analysis of organic anion transporters 1, 3, and 6 reveals major differences in structural determinants of antiviral discrimination. J Biol Chem. 2008;283(13):8654–8663. doi: 10.1074/jbc.M708615200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cihlar T, Laflamme G, Fisher R, Carey AC, Vela JE, Mackman R, et al. Novel nucleotide human immunodeficiency virus reverse transcriptase inhibitor GS-9148 with a low nephrotoxic potential: characterization of renal transport and accumulation. Antimicrob Agents Chemother. 2009;53(1):150–156. doi: 10.1128/AAC.01183-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Servais A, Lechat P, Zahr N, Urien S, Aymard G, Jaudon MC, et al. Tubular transporters and clearance of adefovir. Eur J Pharmacol. 2006;540(1–3):168–174. doi: 10.1016/j.ejphar.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 34.Eraly SA, Blantz RC, Bhatnagar V, Nigam SK. Novel aspects of renal organic anion transporters. Curr Opin Nephrol Hypertens. 2003;12(5):551–558. doi: 10.1097/00041552-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Divi RL, Haverkos KJ, Humsi JA, Shockley ME, Thamire C, Nagashima K, et al. Morphological and molecular course of mitochondrial pathology in cultured human cells exposed long-term to Zidovudine. Environ Mol Mutagen. 2007;48(3–4):179–189. doi: 10.1002/em.20245. [DOI] [PubMed] [Google Scholar]
- 36.Kohler JJ, Hosseini SH, Cucoranu I, Hoying-Brandt A, Green E, Johnson D, et al. Murine cardiac mtDNA: effects of transgenic manipulation of nucleoside phosphorylation. Lab Invest. 2009;89(2):122–130. doi: 10.1038/labinvest.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chu XY, Strauss JR, Mariano MA, Li J, Newton DJ, Cai X, et al. Characterization of mice lacking the multidrug resistance protein MRP2 (ABCC2) J Pharmacol Exp Ther. 2006;317(2):579–589. doi: 10.1124/jpet.105.098665. [DOI] [PubMed] [Google Scholar]
- 38.Imaoka T, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Functional involvement of multidrug resistance-associated protein 4 (MRP4/ABCC4) in the renal elimination of the antiviral drugs adefovir and tenofovir. Mol Pharmacol. 2007;71(2):619–627. doi: 10.1124/mol.106.028233. [DOI] [PubMed] [Google Scholar]
- 39.Ray AS, Cihlar T, Robinson KL, Tong L, Vela JE, Fuller MD, et al. Mechanism of active renal tubular efflux of tenofovir. Antimicrob Agents Chemother. 2006;50(10):3297–3304. doi: 10.1128/AAC.00251-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47(3):298–303. doi: 10.1097/qai.0b013e31815e7478. [DOI] [PubMed] [Google Scholar]
- 41.Norris KC, Agodoa LY. Unraveling the racial disparities associated with kidney disease. Kidney Int. 2005;68(3):914–924. doi: 10.1111/j.1523-1755.2005.00485.x. [DOI] [PubMed] [Google Scholar]
- 42.Daugas E, Rougier JP, Hill G. HAART-related nephropathies in HIV-infected patients. Kidney Int. 2005;67(2):393–403. doi: 10.1111/j.1523-1755.2005.67096.x. [DOI] [PubMed] [Google Scholar]
- 43.Franey C, Knott D, Barnighausen T, Dedicoat M, Adam A, Lessells RJ, et al. Renal impairment in a rural African antiretroviral programme. BMC Infect Dis. 2009;9:143. doi: 10.1186/1471-2334-9-143. [DOI] [PMC free article] [PubMed] [Google Scholar]