Abstract
Aldose reductase (AKR1B1), which catalyzes the reduction of glucose to sorbitol and lipid aldehydes to lipid alcohols, has been shown to be involved in secondary diabetic complications including cataractogenesis. Rats have high levels of AKR1B1 in lenses and readily develop diabetic cataracts, whereas mice have very low levels of AKR1B1 in their lenses and are not susceptible to hyperglycemic cataracts. Studies with transgenic mice that over-express AKR1B1 indicate that it is the key protein for the development of diabetic complications including diabetic cataract. However, no such studies were performed in genetically altered AKR1B1 rats. Hence, we developed siRNA-based AKR1B1 knockdown rats (ARKO) using the AKR1B1-siRNA-pSuper vector construct. Genotyping analysis suggested that more than 90% of AKR1B1 was knocked down in the littermates. Interestingly, all the male animals were born dead and only 3 female rats survived. Furthermore, all 3 female animals were not able to give birth to F1 generation. Hence, we could not establish an AKR1B1 rat knockdown colony. However, we examined the effect of AKR1B1 knockdown on sugar-induced lens opacification in ex vivo. Our results indicate that rat lenses obtained from AKR1B1 knockdown rats were resistant to high glucose–induced lens opacification as compared to wild-type (WT) rat lenses. Biochemical analysis of lens homogenates showed that the AKR1B1 activity and sorbitol levels were significantly lower in sugar-treated AKR1B1 knockdown rat lenses as compared to WT rat lenses treated with 50 mM glucose. Our results thus confirmed the significance of AKR1B1 in the mediation of sugar-induced lens opacification and indicate the use of AKR1B1 inhibitors in the prevention of cataractogenesis.
Keywords: Aldose Reductase, SiRNA knock-down Rats, Lens Opacification, Cataract, Oxidative Stress
1. Introduction
Aldose reductase (AKR1B1), a member of the aldo-keto-reductase superfamily of proteins, catalyzes the first and rate-limiting step of the polyol pathway of glucose metabolism. AKR1B1 was initially discovered in seminal vesicles and has been shown to play a central role in the maintenance of cellular osmoregulation [1]. During hyperglycemia the accelerated flux of sorbitol through the polyol pathway and enhanced oxidative stress are implicated in the pathogenesis of secondary diabetic complications, such as cataractogenesis, retinopathy, neuropathy, nephropathy, and cardiovascular complications [2-6]. This view is supported by the observations that AKR1B1 inhibitors ameliorate several pleiotropic complications of diabetes, at least in experimental animal models [7-9]. Furthermore, lens-specific over-expression of AKR1B1 in transgenic mice accelerates sugar cataract, suggesting that AKR1B1 is involved in the diabetic cataractogenesis [10]. Nonetheless, the clinical utility of AKR1B1 inhibitors remains uncertain. The possible reasons behind these uncertainties are an increased polyol pathway that alters the NADPH/NADP ratio and attenuates the glutathione reductase (GR) and glutathione peroxidase system, thereby decreasing the ratio of reduced glutathione (GSH) to oxidized glutathione (GSSG) and causing oxidative stress [2, 11]. Furthermore, increased reactive oxygen species (ROS) along with elevated levels of lipid peroxidation products such as α-β-unsaturated lipid aldehyde, 4-hydroxy-trans-nonenal, that cause cellular toxicity are considered to be the intriguing contributor to various diseases including secondary diabetic complications [6]. Indeed, our previous studies indicate that antioxidants such as 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) and butylated hydroxytoluene could prevent sugar–induced lens opacification without affecting sorbitol levels [6, 12-15]. These studies suggest that oxidative stress plays a major role in sugar-induced cataract formation. However, it is not clear how hyperglycemia causes oxidative stress. It has been suggested that increased blood glucose could increase oxidative stress by autooxidation. Furthermore, in diabetic lenses, loss of cellular glutathione (GSH) could increase membrane lipid peroxidation which would contribute to oxidative stress [6]. Interestingly, treatment with AKR1B1 inhibitors restored the cellular GSH levels and prevented lipid peroxidation indicating that inhibition of AKR1B1 could prevent hyperglycemia–induced oxidative stress. Previous studies using normal rats, dogs and mice have identified that AKR1B1 inhibitors are potential drugs to prevent high glucose- and galactose-induced cataracts [6]. However, the physiological importance of AKR1B1 in diabetic cataracts is not clearly known. Studies using lens specific over-expression of AKR1B1 in mice indicated a definitive role of AKR1B1 in sugar cataracts [10]. However, the physiological levels of AKR1B1 are higher in the lenses of rats as compared to mice, and these high levels of AKR1B1 presumably makes rats more prone to diabetic cataractogenesis [16]. Furthermore, the significance of AKR1B1 in the development of diabetic complications has been supported by studies using genetically altered mice [17, 18]. Since no such studies were performed in rats, in the present study we developed siRNA-based AKR1B1 knockdown rats and examined their lenses for hyperglycemia-induced cataractogenesis.
2. Methods
2.1. Plasmid construction
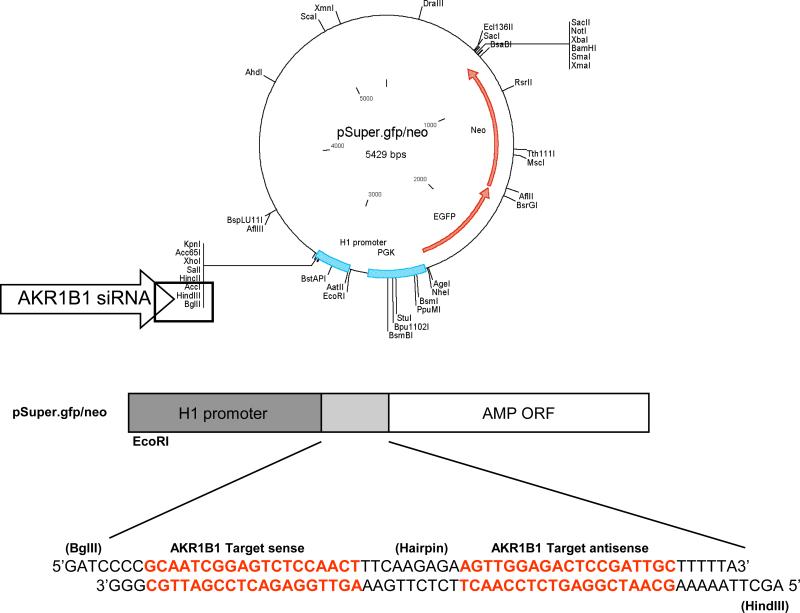
Two oligonucleotides containing sense and antisense 19 nucleotide (nt) sequences from the AKR1B1 coding region, a 9 nt spacer sequence which provides a loop structure, and a transcription termination signal of five thymidines were designed with forward and reverse sequences and subcloned between BglII and HindIII restriction sites downstream of the H1 promoter (pH1/ AKR1B1-siRNA; Fig. 1). Recombinant AKR1B1-siRNA pSuper.gfp/neo was transformed into E. coli (DH5α) cells. Ampicillin resistant E. coli positive AKR1B1-siRNA pSuper.gfp/neo clones, which yielded 287 bp fragments upon digestion with EcoRI and HindIII were selected and the presence of AKR1B1-siRNA sequence was further confirmed by DNA sequencing.
Figure 1. pSuper.gfp/neo vector map showing the multiple cloning site, AKR1B1-siRNA insertion site and the AKR1B1-siRNA sequence.
Rat specific ARSiRNA was custom designed and synthesized as described in methods. The oligo nucleotide sequence was subcloned between BglII and HindIII restriction sites downstream of the H1 promoter in the pSuper.gfp/neo vector. The figure describes the complete map of the pSuper vector from manufacturer's technical literature along with the AKR1B1-siRNA sequence.
2.2. Animals, DNA injection and genotype analysis
The AKR1B1 knockdown rats (Fischer-344) were developed as custom services at the University of Texas Health Science Center, Transgenic core facility, San Antonio, USA. The fragment with human H1 promoter AKR1B1-siRNA obtained after digesting recombinant AKR1B1-siRNA pSuper with EcoRI and HindIII, was injected into the pronuclear stage of fertilized rat embryos. The healthy embryos (14-20/rat) were immediately implanted into pseudo pregnant animals to generate transgenic AKR1B1 knockdown rats. Genotyping of rat pups was performed with tail DNA by PCR using human H1 promoter primers.
2.3. RT-PCR and Western blot analysis of AKR1B1
Total RNA from rat thoracic muscle tissue was isolated by using RNeasy kit (Qiagen) as per supplier's instructions. Aliquots of RNA (1.0 μg) isolated from each sample were reverse transcribed with Omniscript and Sensiscript reverse transcriptase one-Step RT-PCR system with HotStarTaq DNA polymerase (Qiagen) at 55 °C for 30 min followed by PCR amplification. The oligonucleotide primer sequences were as follows: AKR1B1 (f) ACTGCCATTGCAAAGGCATCGTGGT and AKR1B1 (r) CCCCCATAGGACTGGAGTTCTAAGC, 5’-TGAGACCTTCAACACCCCAG-3’ and 5’-TTCATGAGGTAGTCTGTCAGGTCC-3’ for β-actin. PCR reaction was carried out in a GeneAmp 2700 thermocycler (Applied Biosystems, Foster City, CA) under the following conditions: initial denaturation at 95 °C for 15 min; 30 cycles at 94 °C for 40 s, 62 °C at 40 s, 72 °C for 1 min, and then 72 °C for 5 min for final extension. PCR products were electrophoresed with 2% Agarose-1×TAE gels containing 0.5 μg/ml ethidium bromide. Western blot analysis was carried out to determine the expression of AKR1B1 protein by using AKR1B1 polyclonal antibodies.
2.4. Rat lens culture
The eyeballs were removed from the rats and cultured as described earlier [19]. Briefly, the eyeballs were removed and lenses were dissected in phosphate-buffered saline under sterile conditions, with the aid of a dissecting microscope. Each of the dissected lenses was immersed in a separate well of a 24-well tissue culture plate containing medium 199 supplemented with 1% penicillin-streptomycin. The lenses were divided into 4 groups with 2 lenses from a rat per group, WT control and ARKO control group were incubated with medium 199 containing 5.5 mM glucose, and the experimental groups WT+HG and ARKO+HG groups were incubated with medium 199 containing 50 mM glucose. The lenses were maintained in a humidified atmosphere of 5% CO2 and 95% air at 37 °C. The incubation medium was changed every 24 h. The incubations were staggered so that all the lenses, incubated for 1, 4 and 9 days, were ready for biochemical measurements and digital image analysis at the same time under identical conditions.
The opacity of the lenses was examined by digital image analysis, as described elsewhere [20]. Briefly, the imaging system consisted of a TV camera (Optronics Engineering, Goletta, GA) attached to the television port of an inverted microscope (Nikon). The condenser was adjusted for Köhler illumination. To view the entire lens, a 2x objective was used. The first series of images were collected under a condition in which the illumination was increased so that the center of the control (untreated) lens (on day 1) saturated the acquisition system.
2.5. Biochemical measurements
The lenses from all the groups, after the indicated days in culture, were homogenized in 0.5 mL 20 mM potassium phosphate (pH 7.0) by sonication (model W185 Heat Systems; Ultrasonics Inc., Plainview, NY). To measure GSH, 0.2 mL homogenate was mixed with 0.3 mL precipitating reagent (0.2 M glacial meta-phosphoric acid, 5.1 M NaCl and 5.9 mM EDTA) and centrifuged at 10,000g for 15 min to collect the supernatant. 0.2 mL supernatant was added to 0.8 mL 0.3 M Na2HPO4, followed by the addition of 0.1 mL of 0.04 % 5,5' dithiobis-(2-nitrobenzoicacid) in 1% sodium citrate). The change in optical density (OD) at 412 nm was recorded using a spectrophotometer (UV-Vis; Varian, Sunnyvale, CA), as described previously (Bhatnagar et al 1993). Soluble and insoluble proteins were determined by the Bradford method. Sorbitol was measured in ultrafiltered, lyophilized homogenate (YM10 Centricon; Millipore, Bedford, MA). The samples were derivatized by adding 0.1 mL of a solvent (Deriva-sil) under anhydrous condition. The amount of sorbitol present in a sample was analyzed by injecting the unknown derivatized mixture (1 μL) and known standard into a gas chromatography (GC) system (Varian 3400) using a temperature gradient (increase from 140 °C to 170 °C at 4°C/min and from 170°C to 250°C at 50 °C/min). AKR1B1 activity was determined by using an aliquot of supernatant as described by us earlier [21]. Briefly, enzyme activity was measured at room temperature in a 1 ml reaction system containing 0.1 M phosphate buffer (7.0), 10 mM glyceraldehyde, and 0.15 mM NADPH. The reaction was monitored by measuring the absorbance at 340 nm using a Varian Cary 100 Bio spectrophotometer. One unit of enzyme activity is defined as the amount of enzyme required to oxidize 1 μmol of NADPH/min.
3. Results
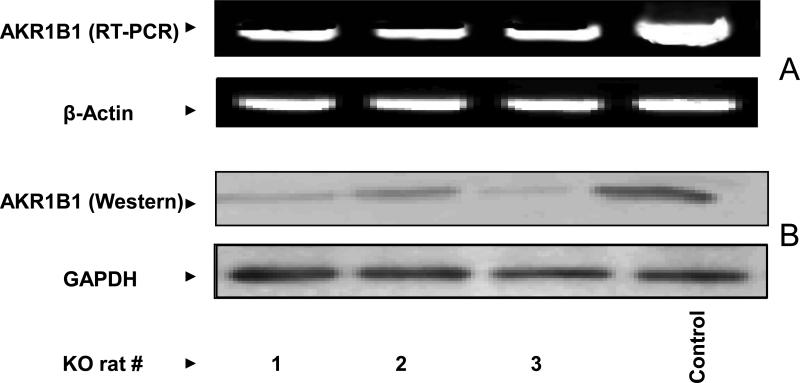
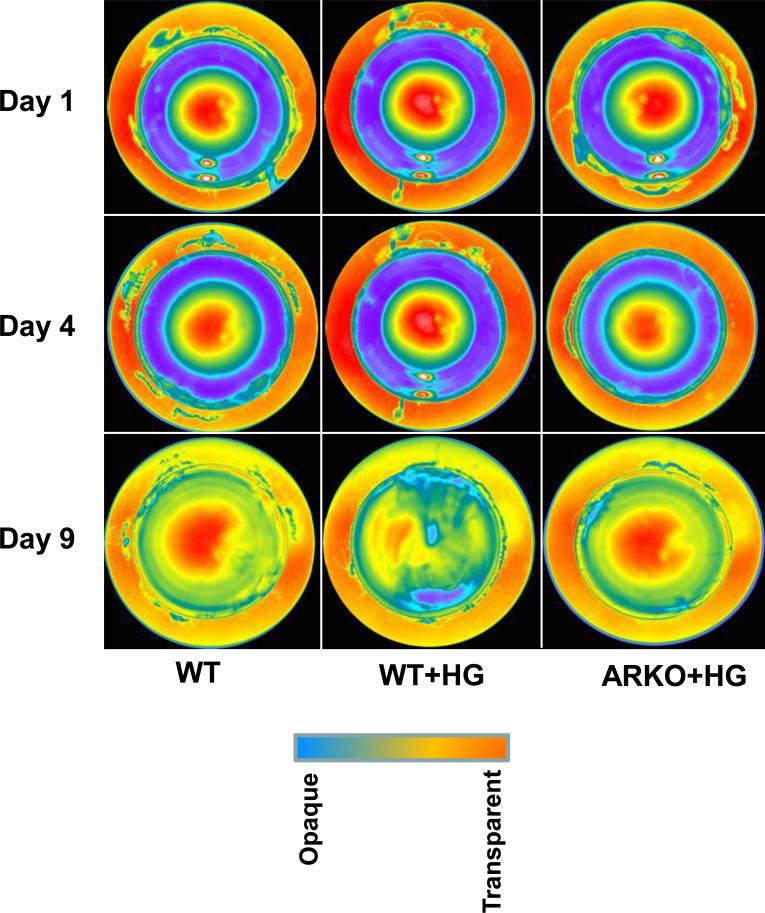
In an effort to understand the physiological functions of AKR1B1 in rats, we have attempted to develop AKR1B1 knockdown rats by using siRNA technologies. With the help of custom services at the transgenic core facility of the University of Texas Health Science Center, San Antonio, we were able to obtain 3 founder (F0) female rats. All the 9 male founder rats were born dead. The genotyping demonstrated that all three founders contained the human H1 promoter, indicating that the AKR1B1-siRNA had inserted into the cellular DNA. Expression of AKR1B1 protein in these rats was significantly (>90%) knocked down suggesting that the siRNA technology to develop AKR1B1 knockdown rats was successful (Fig. 2B). However, the results shown Fig.2A indicate that AKR1B1 mRNA levels decreased by only 50 to 60 % in ARKO rats as compared to WT rats. Perhaps the results are mis-interpreted because the PCR goes to completion (using up all NTPs) with whatever levels of mRNA are present; it may not be a measure of mRNA levels, therefore, we have considered decrease in protein levels as an index of AKR1B1 knockdown in rats. The female founder rats were not able to give birth to a F1 generation when mated with wild-type male rats. They were infertile even through in vitro fertilization experiments. At this time we are not sure how AKR1B1 knockdown in rats caused abnormalities in reproductive system. Hence, we used all the founder rats in the current studies. We examined the effect of AKR1B1 knockdown in prevention of sugar-induced lens opacification. As shown in Fig. 3, digital image analysis of lenses from WT rats clearly illustrates the increase in lens opacity in the presence of high (50 mM) glucose and the resistance of lenses from knockdown rats to sugar-induced opacification. Digital images of the lenses were taken using a Nikon inverted microscope with the light adjusted so that the central portion of the lenses on day 1, 4 and 9 saturated the image. Under these light conditions, the difference in the lens opacifications was clearly visible at the day 9 in lenses from WT rats treated with high glucose as compared to lenses from WT rats treated with normal glucose and lenses from AKR1B1 knockdown rats treated with high glucose. Each row illustrates a lens representative of each treatment as indicated in the figure legend. These results indicate that lenses from AKR1B1 knockdown rats did not develop sugar-induced lens opacification. We next measured the biochemical changes in the lenses from WT and AKR1B1 knockdown rats in culture. As shown in Table-1, there was no significant change in the ratio of soluble to insoluble proteins in the lenses of all the groups. On day 9, the glutathione (GSH) levels significantly decreased (50%) in lenses from WT rats but not in lenses from AKR1B1 knockdown rats treated with high glucose. AKR1B1 activity decreased by more than 85% in the lenses from ARKO rats cultured in high glucose compared with lenses from WT cultured in normal glucose. Similarly, the sorbitol levels in the lenses from WT rats treated with high glucose significantly increased (~8–fold) compared to those treated with normal glucose. However, in the lenses from AKR1B1 knockdown rats, there was no increase in sorbitol levels. These results indicate that the AKR1B1 knockdown provided a significant protection against glucose-induced biochemical changes in the lenses.
Figure 2. RT-PCR and Western blot confirming the knock-down of AKR1B1 by siRNA.
A) Equal amount of total RNA isolated from the thoracic muscle tissue of AKR1B1 knockdown rats was subjected to RT-PCR. B) Equal amount of protein obtained from muscle tissue homogenates was subjected to Western blot analysis using AKR1B1 antibodies. Beta–actin and GAPDH were used as internal standards for RT-PCR and Western blot analysis, respectively. #1, 2, 3 are ARKO rats, Control: Wild-type rats.
Figure 3. Hyperglycemia-induced lens opacification was not observed in ARKO rat lenses.
ARKO and age-matched control female rats (10-12 weeks; Fischer-344) were killed and the lenses were immediately dissected out and cultured in medium 199 containing 1% penicillin and streptomycin with or without high glucose (50 mM) as described in methods. On different days the lenses were periodically photographed and observed under the transmission light using a Nikon inverted microscope. The photographs show the lens opacification on days 1, 4 and 9. The opacification is represented by transmittance of light in distinct colors in decreasing order at the nuclear region of the lens. Day 1 corresponds to 24 h after the lens was incubated in the culture media. WT: Wild-type, WT+HG: Wild- type with high glucose treated, ARKO+HG, AKR1B1 knockout with high glucose treated.
Table 1.
Protein, GSH, AKR1B1 and Sorbitol levels in ARKO and wild type rat lenses.
| Group | Protein (Sol./Insol. | GSH (nmol/mg protein) | AKR1B1 (ml/mg protein) | Sorbitol (nmol/mg protein) |
|---|---|---|---|---|
| 0 day WT | 3.2 ± 1.2 | 9.5 ± 0.2 | 7.1 ± 0.4 | 1.1 ± 0.04 |
| 0 day ARKO | 3.0 ± 1.5 | 7.5 ± 0.9 | 1.2 ± 0.4 | 0.7 ± 0.01 |
| 9th day WT | 3.1 ± 1.2 | 7.5 ± 1.5 | 8.2 ± 0.2 | 1.9 ± 0.16 |
| 9th day WT+HG | 3.0 ± 1.1 | 4.8 ± 1.3 | 9.7 ± 0.9 | 16.3 ± 1.6 |
| 9th day ARKO | 2.9 ± 0.9 | 6.8 ± 0.2 | 0.8 ± 0.3 | 0.7 ± 0.02 |
| 9th day ARKO+HG | 2.8 ± 1.2 | 6.9 ± 0.2 | 0.7 ± 0.4 | 0.8 ± 0.02 |
Cultured rat lenses were homogenized and various biochemical parameters were measured as described in methods. The data represents average of two lenses from a rat. (n=2 lenses).
Discussion
Although transgenic technology in mice is well established, similar technology in rats is expensive, time consuming and the success rate is not high [22]. RNA interference technology has gained widespread application to suppress the expression of specific proteins of interest [23]. Due to its larger body of size and blood volume, the rats have advantages over mice in performing physiological studies in several disease conditions such as hyperinsulinemia and reproductive abnormalities. Further, studies by Varma and Kinoshita indicate that mice are resistant to development of sugar–induced cataracts [16]. This is because of low levels of AKR1B1 expression in their lenses. Rats have been shown to express 10 times more AKR1B1 than in mice [16]. However, no rat model of genetically altered AKR1B1 is available to investigate the role of this enzyme in diabetic cataractogenesis. Hence, our interest was to develop siRNA-based knockout rats to examine the physiological functions of AKR1B1. By using specific H1 promoter containing pSuper vector and hairpin AKR1B1-siRNA, we were able to obtain 3 female founder rats with knockdown of AKR1B1 expression to more than 90%. However, these rats were not able to produce littermates, indicating their reproductive abnormalities. In contrast, AKR1B1 null mice showed no apparent growth and reproductive abnormalities [24]. Since we failed to generate littermates, we examined the protective effect of knockdown of AKR1B1 against hyperglycemic cataractogenesis in rat lenses. Oxidative and osmotic stresses have been shown to be key biochemical changes that trigger and mediate lens opacification in hyperglycemia [6, 25]. We have therefore measured the progressive loss of light transmission by the lens that is associated with profound changes in its structure, physiology and metabolism under high glucose-containing media. Digital image analyses of rat lenses, cultured in high glucose for up to 9 days, showed that the lenses developed progressive opacity in the nuclear region as determined by transmittance measurements. In contrast, the opacification in the lenses obtained from knockdown rats and cultured in high glucose was significantly less and similar to WT control lenses cultured in normal glucose, indicating that AKR1B1 knockdown protects from hyperglycemic cataractogenesis. Consistent with our results, previous studies have also shown that transgenic overexpression of the AKR1B1 gene selectively in mice lens accelerated diabetic and galactosemic cataract formation [10]. Additionally, general over-expression of the AKR1B1 gene, using the SMV promoter, increased the rate of neuropathic changes in diabetic animals [26-27]. In addition to these studies, our results strongly indicate that AKR1B1 is an important component of high glucose-induced metabolic changes that underlie the development of secondary diabetic complications. In conclusion, we have developed siRNA-based AKR1B1 knockdown in rats which exhibited some reproductive abnormalities. Explant cultures of lenses from AKR knockdown rats demonstrated resistance to sugar-induced opacification, indicating a physiological role of AKR1B1 in the pathophysiology of hyperglycemic cataracts.
4. Acknowledgments
This study was supported in parts by NIH grants DK36118 (to S.K.S) and GM 071036; EY 018591 (to K.V.R).
Abbreviations
- AKR1B1
Aldose Reductase
- ARKO
AKR1B1 knockdown rat
- AKR1B1-siRNA
aldose reductase small interference RNA
- HG
High-glucose
- NADPH
Nicotinamide adenine dinucleotide phosphate-reduced
- NADP
Nicotinamide adenine dinucleotide phosphate
- ROS
reactive oxygen species
- HNE
4-hydroxy-trans-nonenal
- GSH
Glutathione
- GSSG
Glutathione disulfide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Authors have no conflict of interest.
5. References
- 1.Hers HG. The mechanism of the transformation of glucose in fructose in the seminal vesicles. Biochim Biophys Acta. 1956;22:202–203. doi: 10.1016/0006-3002(56)90247-5. [DOI] [PubMed] [Google Scholar]
- 2.Bhatnagar A, Srivastava SK. Biochemical aldose reductase: congenial and injurious profiles of an enigmatic enzyme. Med. Metab. Biol. 1992;48:91–121. doi: 10.1016/0885-4505(92)90055-4. [DOI] [PubMed] [Google Scholar]
- 3.Dyck PJ, Thomas PK. Diabetic Neuropathy. W. B Saunders; Philadelphia: 1999. [Google Scholar]
- 4.Brownlee M. Biochemistry and molecular biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JA, Creager MA, Libby P. Diabetic and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- 7.Hotta N, Kakuta H, Ando F, Sakamoto N. Current progress in clinical trials of aldose reductase inhibitors in Japan. Exp Eye Res. 1990;50:625–628. doi: 10.1016/0014-4835(90)90104-3. [DOI] [PubMed] [Google Scholar]
- 8.Stribling D. Clinical trials with aldose reductase inhibitors. Exp Eye Res. 1990;50:621–624. doi: 10.1016/0014-4835(90)90103-2. [DOI] [PubMed] [Google Scholar]
- 9.Pitts NE, Gundersen K, Mehta DJ, Vreeland F, Shaw GL, Peterson MJ, Collier J. Aldose reductase inhibitors in clinical practice. Preliminary studies on diabetic neuropathy and retinopathy. Drugs. 1986;32:30–35. doi: 10.2165/00003495-198600322-00008. [DOI] [PubMed] [Google Scholar]
- 10.Lee AY, Chung SK, Chung SS. Demonstration that polyol accumulation is responsible for diabetic cataract by the use of transgenic mice expressing the aldose reductase gene in the lens. Proc Natl Acad Sci USA. 1995;92:2780–2784. doi: 10.1073/pnas.92.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SM, Schade SZ, Doughty CC. Aldose reductase, NADPH and NADP in normal, galactosefed and diabetic rat lens. Biochim Biophys Acta. 1985;841:247–253. doi: 10.1016/0304-4165(85)90065-0. [DOI] [PubMed] [Google Scholar]
- 12.Ross WM, Creighton MO, Trevithick JR, Stewart-DeHaan PJ, SanwaI M. Modelling cortical cataractogenesis: VI. Induction by glucose in vitro or in diabetic rats: prevention and reversal by glutathione. Exp Eye Res. 1983;37:559–573. doi: 10.1016/0014-4835(83)90132-x. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava SK, Ansari NH. Prevention of sugar-induced cataractogenesis in rats by butylated hydroxytoluene. Diabetes. 1988;37:1505–1508. doi: 10.2337/diab.37.11.1505. [DOI] [PubMed] [Google Scholar]
- 14.Ansari NH, Srivastava SK. Aliopurinol promotes and butylated hydroxy toluene prevents sugarinduced cataractogenesis. Biochem. Biophys. Res. Commun. 1990;168:939–943. doi: 10.1016/0006-291x(90)91119-d. [DOI] [PubMed] [Google Scholar]
- 15.Richer S. Antioxidants and the eye. Int Ophthalmol Clin. 2000;40:1–16. doi: 10.1097/00004397-200010000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Varma SD, Kinoshita JH. The absence of cataracts in mice with congenital hyperglycemia. Exp Eye Res. 1974;19:577–582. doi: 10.1016/0014-4835(74)90095-5. [DOI] [PubMed] [Google Scholar]
- 17.M Yeung C, Lo AC, Cheung AK, Chung SS, Wong D, Chung SK. More severe type 2 diabetes-associated ischemic stroke injury is alleviated in aldose reductase-deficient mice. J Neurosci Res. 2010;88:2026–2034. doi: 10.1002/jnr.22349. [DOI] [PubMed] [Google Scholar]
- 18.Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- 19.Chandra D, Ramana KV, Wang L, Christensen BN, Bhatnagar A, Srivastava SK. Inhibition of fiber cell globulization and hyperglycemia-induced lens opacification by aminopeptidase inhibitor bestatin. Invest Ophthalmol Vis Sci. 2002;43:2285–2292. 2002. [PubMed] [Google Scholar]
- 20.Bhatnagar A, Ansari NH, Zacarias A, Srivastava SK. Digital image analysis of cultured rat lens during oxidative stress-induced cataractogenesis. Exp. Eye. Res. 1993;57:385–391. doi: 10.1006/exer.1993.1139. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava S, Chandra A, Wang LF, Seifert WE, DaGue BB, Ansari NH, Srivastava SK, Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydoxy-trans-2-nonenal, in isolated perfused rat heart. J Biol Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charreau B, Tesson L, Soulillou J, Pourcel C, Anegon I. Transgenesis in rats: Technical aspects and models. Transgenic Research. 1996;5:223–234. doi: 10.1007/BF01972876. [DOI] [PubMed] [Google Scholar]
- 23.Hasuwa H, Kaseda K, Einarsdottir T, Okabe M. Small interfering RNA and Gene silencing in transgenic mice and rats. FEBS Lett. 2002;532:227–230. doi: 10.1016/s0014-5793(02)03680-3. [DOI] [PubMed] [Google Scholar]
- 24.Ho HT, Chung SK SK, Law JW, Ko BC, Tam SC, Brooks HL, Knepper MA, Chung SS. Aldose reductase-deficient mice develop nephrogenic diabetes insipidus. Mol. Cell. Biol. 2000;20:5840–5846. doi: 10.1128/mcb.20.16.5840-5846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinoshita JH, Kador P, Catiles M. Aldose reductase in diabetic cataracts. JAMA. 1981;246:257–261. [PubMed] [Google Scholar]
- 26.Yagihashi S, Yamagishi SI, Wada RR, Baba M M, Hohman TC, Yabe-Nishimura C, Kokai Y. Neuropathy in diabetic mice overexpressing human aldose reductase and effects of aldose reductase inhibitor. Brain. 2001;124:2448–2458. doi: 10.1093/brain/124.12.2448. [DOI] [PubMed] [Google Scholar]
- 27.Song Z, Fu DT, Chan YS, Leung S, Chung SS, Chung SK SK. Transgenic mice overexpressing aldose reductase in Schwann cells show more severe nerve conduction velocity deficit and oxidative stress under hyperglycemic stress. Mol Cell Neurosci. 2003;23:638–647. doi: 10.1016/s1044-7431(03)00096-4. [DOI] [PubMed] [Google Scholar]