SUMMARY
Infection or vaccination confers heightened resistance to pathogen re-challenge due to quantitative and qualitative differences between naïve and primary memory T cells. Herein, we show that secondary (boosted) memory CD8+ T cells were better than primary memory CD8+ T cells in controlling some, but not all acute infections with diverse pathogens. However, secondary memory CD8+ T cells were less efficient than an equal number of primary memory cells at preventing chronic LCMV infection and are more susceptible to functional exhaustion. Importantly, localization of memory CD8+ T cells within lymph nodes, which is reduced by antigen re-stimulation, was critical for both viral control in lymph nodes and for the sustained CD8+ T cell response required to prevent chronic LCMV infection. Thus, repeated antigen-stimulation shapes memory CD8+ T cell populations to either enhance or decrease per cell protective immunity in a pathogen-specific manner, a concept of importance in vaccine design against specific diseases.
INTRODUCTION
The ability to quickly and specifically eliminate recurring infections is a hallmark of immunological memory. Thus, the generation of quality memory CD8+ T cells is an appealing goal for vaccine design against a variety of infectious diseases (Harty and Badovinac, 2008; Kaech et al., 2002; Prlic et al., 2007). Although many studies have demonstrated the enhanced protective capacity of primary memory CD8+ T cells compared to naïve cells, much less is understood about the function and properties of memory CD8+ T cells that have been exposed to additional rounds of antigenic stimulation through either recurring infections or from booster immunizations.
Recent experimental evidence has suggested that antigen re-stimulation can dramatically impact both the phenotype and function of the ensuing memory CD8+ T cell population (Badovinac et al., 2003; Grayson et al., 2002; Jabbari and Harty, 2006; Masopust et al., 2006; Unsoeld and Pircher, 2005). Specifically, secondary memory CD8+ T cells populations express increased amounts of Granzyme B, exhibit increased cytolytic activity, and are more protective than primary memory cells against acute infection with Listeria monocytogenes (LM) (Jabbari and Harty, 2006). This suggests that additional antigen encounters can not only increase the overall number of antigen-specific CD8+ T cells, but also result in specific biological changes that impact the per cell protective capacity of the memory populations. However, it is unknown whether antigen re-stimulation increases the per cell protective capacity of memory CD8+ T cells against pathogens other than LM.
Infection with a pathogenic agent can be broadly defined as either being acute or chronic. Microbes such as LM, Vaccinia Virus (VacV), Lymphocytic Choriomeningitis Virus (LCMV) Armstrong, and Mouse Hepatitis Virus (MHV) cause acute infections, where the pathogen is either eliminated or causes mortality. In non-lethal infections with these pathogens, antigen-specific CD8+ T cell numbers peak after clearance and, after contraction, progress into long-lived primary memory populations. In contrast, infection with agents that result in chronic infection, such as LCMV clone 13, causes antigen-specific CD8+ T cells undergoing a primary response to become functionally exhausted during the disease course (Wherry et al., 2007). However, it is currently unknown whether primary or secondary memory CD8+ T cells are more efficient at controlling different types of acute or chronic infections. In addition, it is also unknown whether memory CD8+ T cells exhibit similar characteristics of functional exhaustion compared to naïve CD8+ T cells during chronic infection.
Ideally, booster immunizations will result in the generation of increased numbers of memory CD8+ T cells (Woodland, 2004), because this number strongly correlates with providing host protection (Badovinac et al., 2003; Harty and Badovinac, 2008; Kaech et al., 2002; Schmidt et al., 2008). In contrast to laboratory animal studies, the ability to reach large numbers of antigen-specific memory CD8+ T cells in vaccinated humans has proven difficult (Hill et al., 2010; Masopust, 2009). In addition, a recent study in our laboratory demonstrated that repeated antigenic stimulations have a profound impact on the overall gene expression profile of the ensuing memory CD8+ T cell populations (Wirth et al., 2010). Collectively, this suggests that antigen re-stimulation associated changes in memory CD8+ T cell populations may not always accompany large increases in cell number. Thus, it is critical to determine whether multiple antigen encounters alter the per cell protective capacity of memory cell populations against diverse pathogens, because these changes in gene expression may influence the ‘quality’, and therefore, the ‘threshold number’ of memory CD8+ T cells required to provide host protection (Schmidt et al., 2008).
RESULTS
Secondary Antigen Encounter Impacts Pathogen-Specific Memory CD8+ T Cell-Mediated Protective Immunity
Our previous studies using the OT-I CD8+ TCR-tg model demonstrated that secondary memory CD8+ T cells protect better against LM expressing ovalbumin (LM-OVA) than primary memory cells (Jabbari and Harty, 2006). These data raised the question of whether enhanced protection by secondary memory CD8+ T cells was universal or specific for certain types of pathogens or antigens. To address this, primary and secondary memory P14 TCR-tg CD8+ T cells (specific for LCMV gp33-41) (Pircher et al., 1989) were generated using adoptive transfers and LCMV Armstrong infections (Figure S1A). At 60+ days post-infection, both populations of memory cells were analyzed for expression of a variety of phenotypic markers, cytokine production, and sensitivity to antigen (Figure S1B–F). Specifically, primary memory CD8+ T cell populations contained more CD62Lhi cells and produced more IL-2 than secondary memory T cells, but both populations were equal producers of IFNγ and TNFα. Importantly, using a transfer model of bulk polyclonal CD8+ T cells and LCMV Armstrong infections, similar functional characteristics including cytokine production and sensitivity to antigen were observed in endogenous, primary and secondary polyclonal memory CD8+ T cell populations specific for multiple LCMV-derived antigens (JCN and JTH, data not shown). Thus, primary and secondary memory P14 TCR-tg CD8+ T cells recapitulate both the phenotype and functionality observed with endogenous populations of memory CD8+ T cells.
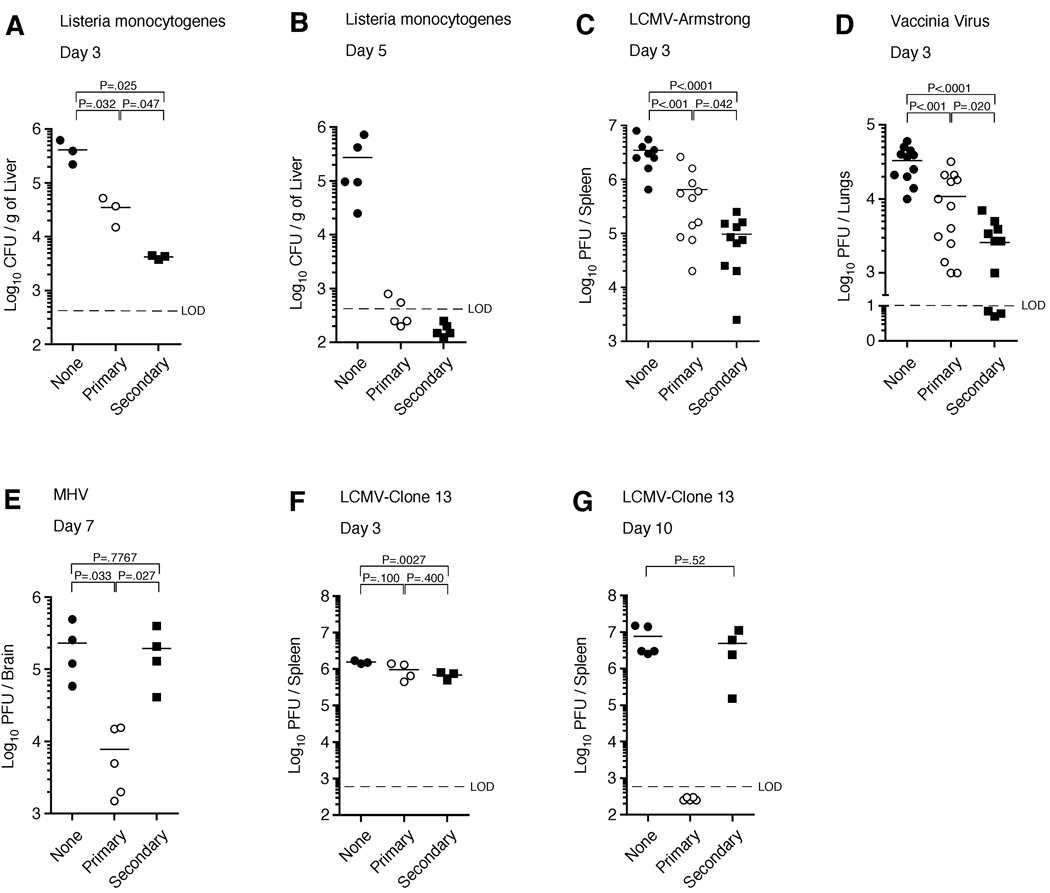
We next determined if the enhanced protection by secondary memory OT-I CD8+ T cells seen against LM-OVA could be extended to P14 CD8+ T cells and the gp33 epitope from LCMV. To test this, equal numbers of primary and secondary memory P14 CD8+ T cells were purified and transferred into naïve recipients prior to infection with LM-gp33. In agreement with our previous data (Jabbari and Harty, 2006), secondary memory P14 CD8+ T cells were better than an equal number of primary memory CD8+ T cells at reducing bacterial load following LM infection on day 3 post-infection, although both populations efficiently cleared the pathogen by day 5 (Figure 1A,B). To determine whether this finding could be generalized, we also compared the protective capacity of memory CD8+ T cells using three other acute infection models. Indeed, secondary memory CD8+ T cells were also better at reducing viral load during a systemic, acute viral infection with LCMV-Armstrong (Figure 1C) and during an acute lung infection with VacV-gp33 (Figure 1D). In contrast, primary memory CD8+ T cells protected better during a uniformly lethal infection with the neurotropic strain of MHV-gp33 on day 7 post-infection (Figure 1E), suggesting that primary memory CD8+ T cells may be important for controlling acute infections at later time-points. These data show that secondary antigen encounter may either increase or decrease memory CD8+ T cell-mediated protective immunity during acute infection.
Figure 1. Secondary memory CD8+ T cells provide better protection against infections with LM, LCMV-Armstrong, and VacV, but decreased protection against MHV and LCMV clone 13.
(A,B) Naïve B6 mice receiving either no cells (None) or Thy1.1 purified 2.5 × 105 memory P14 TCR-tg CD8+ T cells (Primary or Secondary) were challenged with 1 × 105 CFU of virulent LM-gp33. Bacterial burdens were analyzed in the liver on (A) day 3 or (B) day 5 post-infection. (C) Same as (A) except 5.0 × 105 memory cells were transferred and mice were challenged with 2 × 105 PFU of LCMV Armstrong. Viral titers were analyzed in the spleen on day 3 post-infection. (D) Same as (A) except 3.0 × 105 memory cells were transferred and mice were infected intranasally with 1 × 107 PFU of VacV-gp33. Viral titers were analyzed in the lung on day 3 post-infection. (E) Same as (C) except mice were infected intranasally with 1 × 105 PFU of MHV-gp33 and titers were analyzed in the brain on day 7 post-infection. (F,G) Same as (A) except mice were challenged with 2 × 106 PFU of LCMV clone 13. Viral titers were analyzed in the spleen on (F) day 3 and (G) day 10 post-infection. Dashed line indicates limit of detection (LOD). Statistical analyses employed the student’s t test. (see also Figure S1).
Booster immunizations are often administered to provide enhanced protection against a number of acute infections in humans. However, attempts to create a memory CD8+ T cell population capable of preventing chronic infections, such as HIV and Hepatitis C Virus, have been less successful (Autran et al., 2004; Berzofsky et al., 2004; Klenerman and Hill, 2005). To this end, we next tested whether a second antigen stimulation would also increase the per cell ability of a CD8+ T cell population to prevent chronic LCMV infection. Naïve mice infected with LCMV clone 13 exhibit high viral titers in the spleen on both day 3 and 10 post-infection, indicative of an established chronic infection. In agreement with our acute infection models, secondary memory CD8+ T cells modestly reduced viral levels in the spleen on day 3 post-infection, whereas primary memory CD8+ T cells did not (Figure 1F). However, by day 10 post-infection, primary memory CD8+ T cells completely prevented the establishment of the chronic infection, whereas an equal number of secondary memory CD8+ T cells failed to clear the infection and viral titers in the spleen resembled those of naïve mice receiving no memory CD8+ T cells (Figure 1G). These data demonstrate that secondary antigenic stimulation decreased the per cell ability of the resulting memory CD8+ T cell population to prevent chronic LCMV infection. Collectively, these results suggest the intriguing notion that qualitative changes in the memory CD8+ T cell populations resulting from repeated antigen stimulation could either improve or limit pathogen-specific immunity.
Secondary Memory CD8+ T cells Become Functionally Exhausted During Chronic LCMV Infection
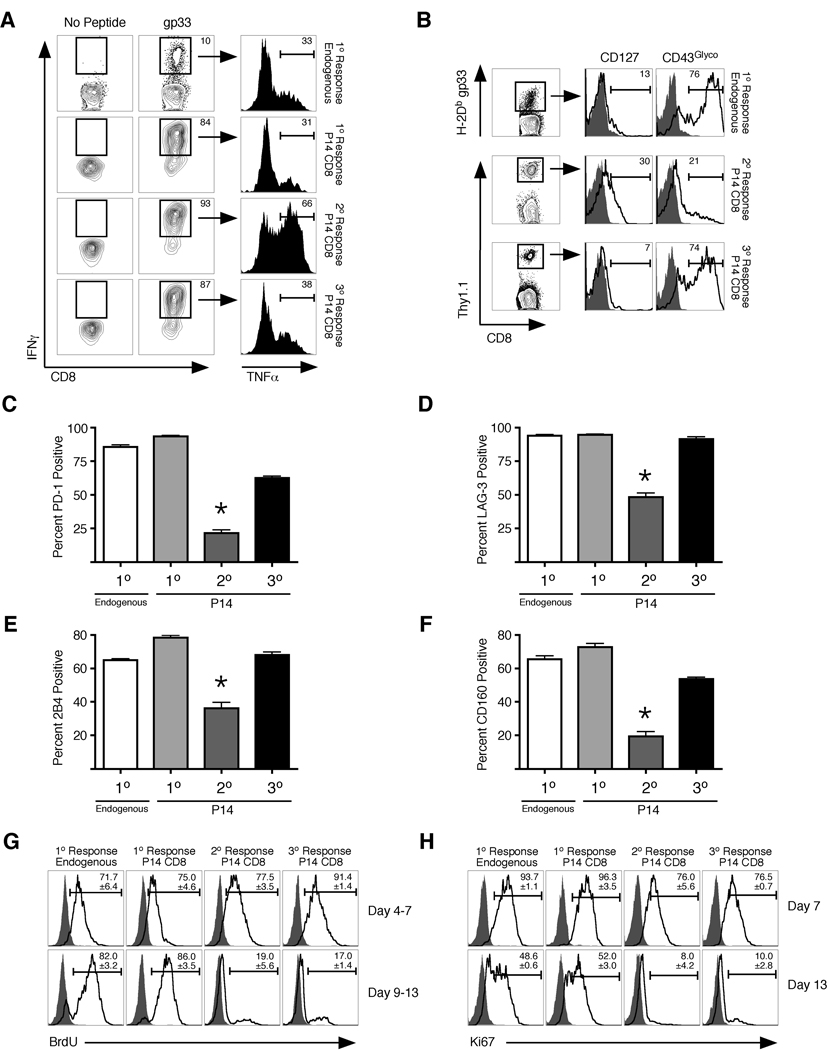
The LCMV clone 13 infection model has revealed that chronic infection profoundly influences the responding T cells in ways that are also observed in chronic infections of humans (Day et al., 2006). The effects of chronic infection on a CD8+ T cell population responding to LCMV clone 13 in a previously naïve host include loss of cytokine production (such as TNFα), expression of inhibitory receptors, and a dependence on antigen and proliferation for CD8+ T cell survival (Shin et al., 2007; Shin and Wherry, 2007; Wherry et al., 2004; Wherry et al., 2003a; Wherry et al., 2007). Because secondary memory CD8+ T cells were less efficient than primary memory cells at preventing chronic LCMV infection, we next analyzed whether this cell population exhibited characteristics of functional exhaustion during the infection. On day 7 following LCMV clone 13 infection, endogenous CD8+ T cells specific for gp33 and adoptively transferred naïve P14 CD8+ T cells undergoing a primary response exhibited functional exhaustion, with only about 35% of the IFNγ+ population also producing TNFα (Figure 2A, top two rows). In contrast, a large fraction (65%) of primary memory P14 CD8+ T cells undergoing a secondary response, which clear the virus from the spleen by day 10 (Figure 1F), were capable of producing TNFα at the same timepoint (Figure 2A, third row). However, secondary memory CD8+ T cells undergoing a tertiary response, which fail to clear the virus, were markedly impaired in TNFα production (Figure 2A, bottom row). This result demonstrates that secondary memory CD8+ T cells undergoing a tertiary response become functionally exhausted during early stages of chronic viral infection.
Figure 2. Secondary memory CD8+ T cells develop a unique phenotype of functional exhaustion during chronic LCMV infection.
(A) Naïve mice receiving either no T cells (1° Response, Endogenous), 500 naïve P14’s (1° Response, P14 CD8+), 2.5 × 105 primary (2° Response, P14 CD8+) or secondary memory P14’s (3° Response, P14 CD8+) were infected with 2 × 106 PFU of LCMV clone 13. On day 7 post-infection, cells were obtained from blood of infected animals and stimulated ex vivo with gp33 peptide for 5 hours. IFNγ and TNFα positive cells were identified using intracellular staining. (B) Adoptive transfers and infections were performed as in (A). On day 25 post-infection, cells were obtained from spleen and P14 CD8+ T cells or endogenous gp33-specfic T cells were analyzed for expression of CD127 and the glycosylated isoform of CD43. (C–F) Adoptive transfers and infections were performed as in (A). On day 15 post-infection, expression of (C) PD-1, (D) LAG-3, (E) 2B4, and (F) CD160 was analyzed on the indicated CD8+ T cells in spleen. Representative histograms are shown in Figure S2. For (C–F), *P<0.001 compared to all other groups using the students t test. (G) Same as (A). Mice were then pulsed with BrdU on days 4–7 or 9–13. Representative BrdU incorporation profiles are shown from P14 CD8+ T cells from spleen. (H) Same as (G) except expression of Ki67 was assessed on day 7 or 13 post-infection. For (G) and (H), numbers indicate mean and std. dev. from 3 independent mice per group. (see also Figure S2).
Along with the loss of cytokine production, exhausted T cells also exhibit a phenotype that includes molecular indications of continued antigenic stimulation and increases in inhibitory receptor expression (Blackburn et al, 2009; Mueller and Ahmed, 2009). Indeed, secondary memory P14’s undergoing a tertiary response are impaired in re-expression of CD127 and continue to express the glycosylated isoform of CD43 (Figure 2B). In addition, a similarly high percentage of CD8+ T cells expressing a variety of inhibitory receptors (Blackburn et al., 2009) including PD-1, LAG-3, 2B4, and CD160 (Figure 2C–F and S2A) were observed in tertiary responses compared with exhausted CD8+ T cells undergoing a primary response. In contrast, expression of these receptors on CD8+ T cells during a secondary response is substantially reduced. Collectively, these data demonstrate that secondary memory CD8+ T cells display a phenotype of functional exhaustion during a tertiary response when they fail to prevent chronic LCMV infection.
We next determined if secondary memory CD8+ T cells were also more susceptible to functional exhaustion than primary memory cells when antigen load remained constant. To test this, a small number of Thy1-disparate primary and secondary memory P14 CD8+ T cells were co-transferred into naïve animals and subsequently infected with LCMV clone 13. Importantly, this number of mixed memory CD8+ T cells is unable to prevent the chronic infection (Figure S2B). In this scenario, primary memory CD8+ T cells underwent much greater expansion than secondary memory following infection (Figure S2C). In addition, on day 7 post-infection, the percentage of secondary memory CD8+ T cells undergoing a tertiary response that were able to produce IFNγ and TNFα was substantially lower than primary memory cells undergoing a secondary response in the same host (Figure S2D, E). However, both populations became functionally exhausted by day 13 post-infection. High expression of inhibitory receptors was found on all cell populations regardless of antigen exposure history (Figure S2F–I). Thus, secondary memory CD8+ T cells are inherently more ‘exhaustible’ than primary memory cells.
Secondary Memory CD8+ T cells Do Not Continue to Proliferate During Chronic LCMV Infection
Besides displaying increased expression of a variety of inhibitory receptors and losing the ability to produce cytokines, prior studies have demonstrated that CD8+ T cells undergoing a primary response continue to proliferate during the course of chronic viral infection (Shin et al., 2007; Wherry et al., 2004). However, it remains unclear if sustained proliferation is required for the molecular changes associated with CD8+ T cell exhaustion. During the early stages of LCMV clone 13 infection (days 4–7), all CD8+ T cell populations proliferated, as demonstrated by BrdU incorporation and Ki67 expression (a nuclear marker of cellular proliferation) during that time period. In agreement with previous studies (Shin et al., 2007; Wherry et al., 2004), both naïve endogenous and P14 CD8+ T cells continued to proliferate during the primary response following the peak day of expansion (days 9–13) (Figure 2G, H). In contrast, primary memory CD8+ T cells undergoing a secondary response undergo minimal proliferation during that time frame, because the infection has been cleared in these animals (Figure 1F). However, secondary memory CD8+ T cells undergoing a tertiary response did not exhibit sustained proliferation despite failure to clear the chronic infection. Differences in sustained proliferation of memory CD8+ T cells were also observed in the same host during established chronic infection (Figure S2B), where primary memory CD8+ T cells continue to proliferate more than secondary memory cells following the peak of expansion (Day 9–13) (Figure S2J). These data demonstrate that although secondary memory CD8+ T cells display several signs of becoming functionally exhausted during LCMV clone 13 infection, they do not continue to proliferate. These data show that CD8+ T cell exhaustion in response to chronic infection is not intrinsically linked to sustained proliferation.
CD62Lhi Primary and Secondary Memory CD8+ T cells Both Prevent Chronic LCMV Infection
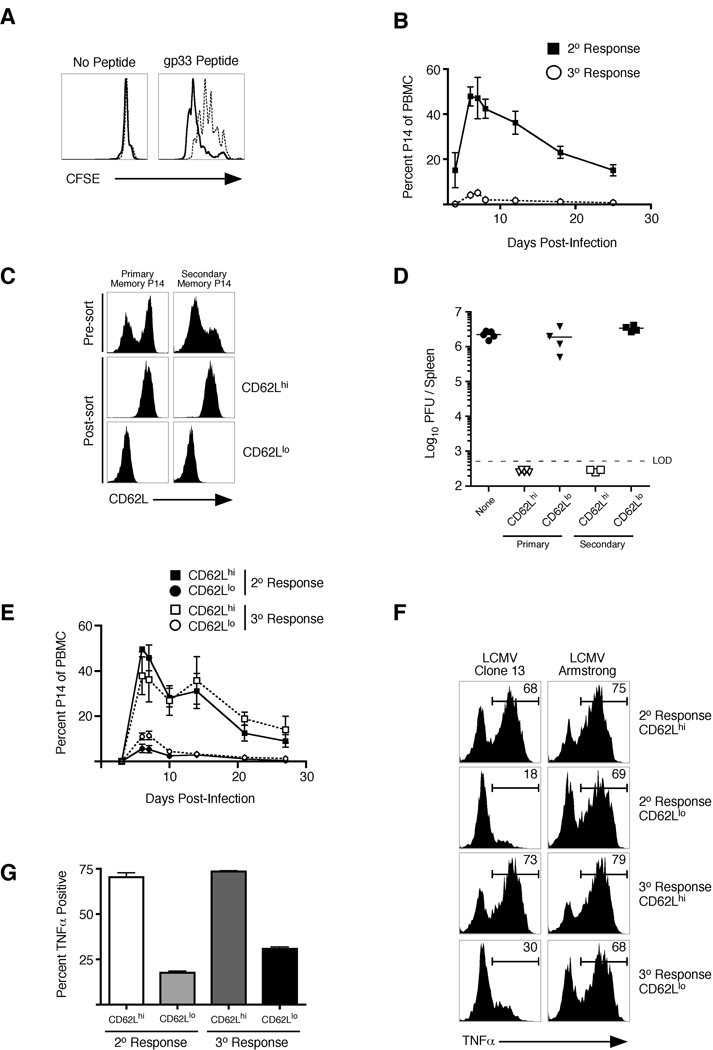
In order to determine the mechanism(s) by which primary memory CD8+ T cells are more protective against LCMV clone 13 infection than secondary memory CD8+ T cells, we first analyzed the proliferative capacity of each memory population. When memory P14 CD8+ T cells were stimulated directly ex vivo with gp33 peptide, primary memory CD8+ T cells underwent more rounds of cell division than secondary memory cells (Figure 3A). Secondary memory CD8+ T cells produce less IL-2 than primary memory CD8+ T cells, which could impact differences in TCR-mediated proliferation. However, addition of excess exogenous IL-2 did not rescue the differences in proliferation observed in the two populations (Figure S3A). In addition, primary memory CD8+ T cells also underwent greater expansion than secondary memory CD8+ T cells in response to infections with LCMV clone 13, LM, and LCMV-Armstrong (Figure 3B and S3B,C). Collectively, these data suggest that primary memory CD8+ T cells undergo more vigorous proliferation than secondary memory CD8+ T cells regardless of the infectious agent. However, in contrast to some previous suggestions (Wherry et al., 2003b), but not others (Huster et al., 2006; Lauvau et al., 2001), this difference in proliferative potential does not always translate into providing greater protection by a memory CD8+ T cell population, because secondary memory CD8+ T cells proliferate less, but protect better against acute infections with LM, LCMV, or VacV (Figure 1).
Figure 3. CD62Lhi memory CD8+ T cells are equally protective against chronic viral infection regardless if they are primary or secondary.
(A) 2 × 106 total splenocytes from mice containing either primary (solid line histogram) or secondary (dashed line histogram) memory P14 CD8+ T cells were labeled with CFSE and were incubated with or without gp33 peptide for 60 hours. Representative CFSE profiles of Thy1.1 memory P14 CD8+ T cells with and without the addition of antigen are shown. (B) 2.5 × 105 primary and secondary memory P14 CD8+ T cells (Thy1.1) were purified and adoptively transferred into naïve recipients (Thy1.2) which were subsequently infected with LCMV clone 13. Kinetics of the secondary or tertiary CD8+ T cell response were analyzed in the blood following infection using CD8+ and Thy1.1 staining to identify the adoptively transferred population. Error bars represent std. dev. of 3–5 mice at each time point. (C) Analysis of sorted CD62L high (CD62Lhi) and low (CD62Llo) expressing P14 CD8+ T cells from bulk primary and secondary memory populations. (D) 1.5 × 105 cells from each of the groups in (C) were transferred into naïve recipients subsequently infected with LCMV clone 13. Viral titers were analyzed in the spleen on day 10 postinfection. (E) Same as (D) except kinetics of T cell expansion were monitored over the indicated time frame using CD8+ and Thy1.1 staining to identify the adoptively transferred populations. Error bars represent std. dev. of 3 samples for each time point. (F) Adoptive transfer of memory populations was performed as in (B) and recipient mice were subsequently infected with either LCMV clone 13 or LCMV Armstrong. On day 14 post-infection, TNFα production by IFNγ+ P14’s was assessed using intracellular stain following ex vivo stimulation with gp33 peptide. (G) Cumulative data of triplicate samples shown in (F). Data are representative of two independent experiments. (see also Figure S3).
It has been demonstrated that primary CD62Lhi (central memory; Tcm) CD8+ T cells are better than primary CD62Llo (effector memory; Tem) cells at clearing LCMV clone 13 (Wherry et al., 2003b). To test whether the reduced number of CD62hi cells in the bulk secondary memory population impacted viral clearance, CD62Lhi and CD62Llo populations of both primary and secondary memory CD8+ T cells were purified (Figure 3C), transferred into naïve recipients, and subsequently infected with LCMV clone 13. CD62Lhi memory cells underwent robust expansion, regardless if they originated from primary or secondary memory, and were equally efficient in preventing chronic LCMV infection (Figure 3D,E). In contrast, the CD62Llo populations of both groups expanded less and failed to prevent the chronic infection. Finally, similar to what was seen with secondary memory CD8+ T cells, CD62Llo populations of both primary and secondary memory cells were impaired in TNFα production following LCMV clone 13 infection (Figure 3F,G), indicating enhanced susceptibility to exhaustion. However, this is not an intrinsic characteristic of the population, as all responding CD8+ T cell populations efficiently produced TNFα in response to acute LCMV Armstrong infection (Figure 3F). Collectively, these data suggest that the reduced frequency of CD62Lhi cells in secondary memory CD8+ T cell populations likely accounted for their failure to prevent chronic LCMV clone 13 infection.
Memory CD8+ T cell Localization into Lymph Nodes is Required to Prevent Chronic LCMV Infection
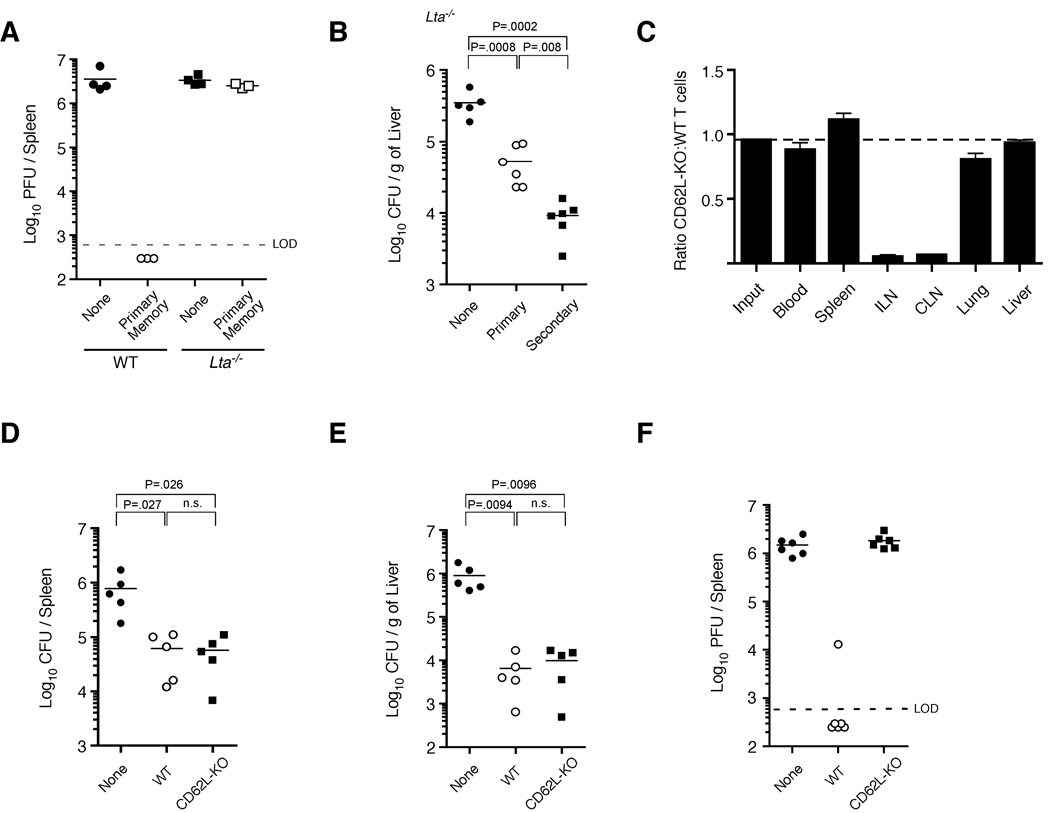
It has been argued that primary Tcm are more protective than Tem during chronic LCMV infection due to their increased proliferative capacity (Wherry et al., 2003b). However, it has not been addressed whether differential trafficking patterns of these subsets can also impact protective immunity, as Tcm localize to lymph nodes more efficiently than Tem. To initially determine whether the localization of memory CD8+ T cells into lymph nodes impacts the ability of these cells to prevent chronic LCMV infection, primary memory P14 CD8+ T cells were transferred into WT or Lymphotoxin-α (Lta)−/− mice (which lack lymph nodes) (De Togni et al., 1994) and subsequently infected with LCMV clone 13. As demonstrated previously, primary memory CD8+ T cells transferred into WT mice prevent chronic LCMV infection. However, the same number of primary memory CD8+ T cells transferred into Lta−/− mice did not prevent the chronic infection (Figure 4A). In contrast, both primary and secondary memory CD8+ T cells were able to decrease bacterial burden in Lta−/− mice following LM infection (Figure 4B). These data demonstrate that lymph node-deficient Lta−/− mice are specifically unable to prevent chronic LCMV infection, even when a sufficient primary memory population of CD8+ T cells is present.
Figure 4. Lymph node homing of memory CD8+ T cells is required for prevention of chronic LCMV infection.
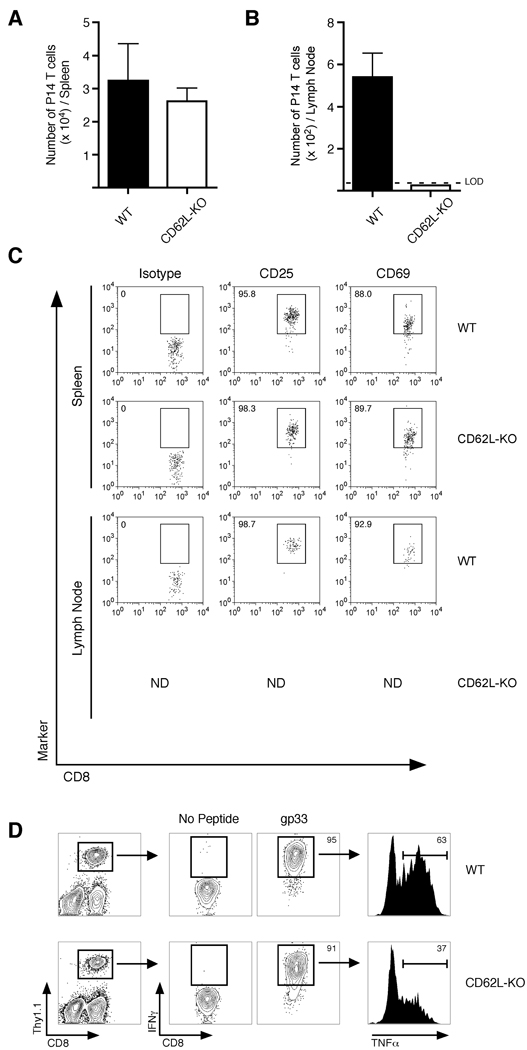
(A) 2.5 × 105 primary memory P14 TCR-tg CD8+ T cells were transferred into either WT or Lta−/− mice, followed by infection with LCMV clone 13. Viral burden was analyzed from spleens on day 10 post-infection. Data are representative of two independent experiments. (B) 2.5 × 105 primary or secondary memory P14 CD8+ T cells were transferred into Lta−/− mice and subsequently infected with virulent LM-gp33. Bacterial burden was analyzed in the liver on day 3 postinfection. (C) Equal numbers (2 × 106) of WT (Thy1.1/1.1) and CD62L-deficient (CD62L-KO) (Thy1.1/1.2) primary memory P14 TCR-tg CD8+ T cells were transferred into naïve (Thy1.2/1.2) recipients. 48 hours later, organ-specific localization of both cell populations was determined. Representative histograms are shown in Figure S4H. (ILN, inguinal lymph node; CLN, cervical lymph node). (D,E) 2.5 × 105 WT or CD62L–deficient primary memory P14 CD8+ T cells were purified and transferred into naïve recipients followed by infection with virulent LM-gp33. Bacterial burden was measured in the (E) spleen and (F) liver on day 3 post-infection. (F) Same as (D,E) except mice were infected with LCMV clone 13 and viral burden was analyzed in the spleen on day 10 post-infection. (see also Figure S4).
Whereas these data suggest that lymph nodes are critical for memory CD8+ T cell-mediated prevention of chronic LCMV infection, Lta−/− mice also exhibit other biological and anatomical defects including alterations in splenic architecture (Banks et al., 1995). To more directly address whether lymph node localization was specifically required by the memory CD8+ T cell compartment in order to prevent chronic LCMV infection, we generated primary memory P14 CD8+ T cells that lacked CD62L (CD62L–deficient) (Xu et al., 1996). At 60 days post-infection with LCMV-Armstrong, CD62L–deficient P14’s exhibited a similar phenotype (other than CD62L expression) compared to WT cells (Figure S4A). In addition, both WT and CD62L–deficient cells were equally efficient at producing IFNγ, TNFα and IL-2, underwent similar ex vivo proliferation in response to gp33 peptide, and expressed equal amounts of CCR7 (Figure S4B–G). However, loss of CD62L dramatically impaired localization of these cells into lymph nodes, without affecting distribution into other tissues (Figure 4C and S4H). Thus, naïve CD62L–deficient CD8+ T cells are able to become functional memory cells following LCMV-Armstrong infection, although their ability to localize into lymph nodes is dramatically impaired.
Because CD62L is required for efficient lymph node localization (Arbones et al., 1994; Catalina et al., 1996; Steeber et al., 1996), we next determined if expression of CD62L provided any benefit to primary memory CD8+ T cell populations with regards to protective immunity during both acute and chronic infections. To test this, equal numbers of both WT and CD62L–deficient primary memory P14’s were transferred into naïve recipients and subsequently infected with LM-gp33. WT and CD62L–deficient primary memory CD8+ T cells provided equal protection against LM (Figure 4D,E), demonstrating that CD62L on memory CD8+ T cells does not impact their ability to protect against this acute infection. In contrast, CD62L–deficient primary memory CD8+ T cells failed to prevent chronic LCMV infection compared to an equal number of WT cells (Figure 4F). Thus, expression of CD62L on primary memory CD8+ T cells and the ensuing ability to enter lymph nodes directly impacts the ability of these cells to prevent chronic LCMV infection.
Lymph Node Primed Memory CD8+ T cells Are Required to Prevent Chronic LCMV Infection
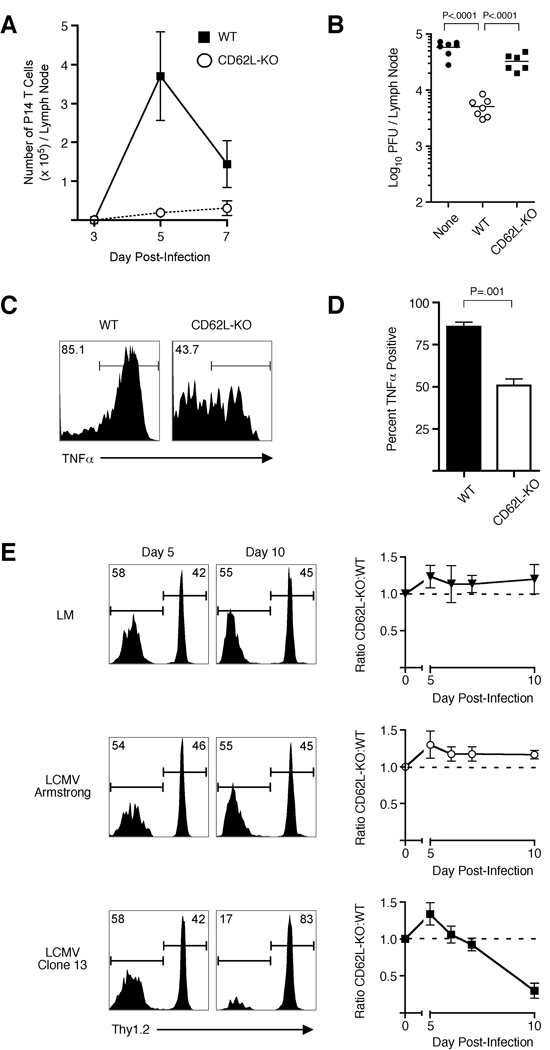
To determine mechanistically why lymph node localization is required to prevent chronic LCMV infection, we first analyzed organ-specific activation of both WT and CD62L–deficient primary memory CD8+ T cells following LCMV clone 13 infection. On day 3 post-infection, equal numbers of both cell populations could be found in the spleen and expressed similar levels of the early activation markers CD25 and CD69 (Figure 5A – C). In contrast, CD62L–deficient cells could not be found in the lymph nodes at this time point, whereas activated WT cells were readily detected. Furthermore, by day 7 post-infection, CD62L–deficient memory CD8+ T cells in the spleen became functionally exhausted and produced less TNFα than WT cells (Figure 5D). Thus, these data suggest that activation of memory CD8+ T cells in lymph nodes is required for the prevention of chronic LCMV infection. Furthermore, WT primary memory CD8+ T cells underwent dramatic expansion in lymph nodes following LCMV clone 13 infection (Figure 6A), which also resulted in decreased viral burden in the lymph node (Figure 6B). In addition, responding WT memory CD8+ T cells produced significantly more TNFα than CD62L–deficient cells that emigrated into the lymph node and became detectable on day 7 following infection (Figure 6C,D). Therefore, these data demonstrate that activation and expansion of memory CD8+ T cells in the lymph nodes results in both decreased viral burden and generation of ‘quality’ effector cells following LCMV clone 13 infection.
Figure 5. CD62L-deficient primary memory CD8+ T cells become activated in the spleen following LCMV clone 13 infection, but become functionally exhausted.
(A,B) 2.5 × 105 WT or CD62L-deficient (CD62L-KO) primary memory P14 TCR-tg CD8+ T cells were transferred into naïve B6 mice and subsequently infected with LCMV clone 13. Total numbers of P14 CD8+ T cells were determined in the (A) spleen or (B) inguinal lymph node on day 3 post-infection. (C) P14 CD8+ T cells from (A) were analyzed for expression of CD25 and CD69. (D) Adoptive transfers and infection was performed as in (A). On day 7 post-infection, cells were obtained from blood of infected animals and stimulated ex vivo with gp33 peptide for 5 hours. IFNγ and TNFα positive cells were identified using intracellular staining. Data are representative of two independent experiments.
Figure 6. Lymph node localization of memory CD8+ T cells results in enhanced viral clearance and sustained effector response during LCMV clone 13 Infection.
(A) 2.0 × 105 WT or CD62L-deficient (CD62L-KO) primary memory CD8+ T cells were transferred into naïve recipients and subsequently infected with LCMV clone 13. On days 3, 5 and 7, total number of transferred cells was quantified in the inguinal lymph nodes. (B) Viral burden in the inguinal lymph node from (A) was determined on day 5 post-infection. (C,D) Same as (A) except on day 7 post-infection, lymph node cells were stimulated with gp33 peptide and TNFα production was analyzed on IFNγ+ cells using intracellular staining. (E) Equal numbers (1 × 104) of WT (Thy1.1/1.2) or CD62L-deficient (Thy1.1/1.1) primary memory P14 TCR-tg CD8+ T cells were transferred into naïve B6 mice and subsequently infected with virulent LM-gp33, LCMV-Armstrong, or LCMV clone 13. On days 5, 6, 7, and 10, representation of each cell population was determined in the blood using Thy1.1 and Thy1.2 staining. Representative histograms from days 5 and 10 show representation of CD62L–deficient (Thy1.2-negative) and WT (Thy1.2-positive) P14 CD8+ T cells. Dashed line indicates input ratio. Error bars represent std. dev. of individual samples at each time point and data are representative of two independent experiments with 3 mice per group.
The robust expansion of WT primary memory CD8+ T cells in the lymph node following LCMV clone 13 infection suggested that once activated, these cells may play an important role in combating the systemic infection. To test this, equal numbers of WT and CD62L–deficient primary memory CD8+ T cells were co-transferred and recipient mice were infected with LM, LCMV Armstrong, or LCMV clone 13 (Figure 6E). During early time points (day 5) following all infections, more CD62L–deficient than WT T cells could be found in the blood, further demonstrating that early activation and proliferation of CD62L–deficient memory T cells were not impaired. Following LCMV Armstrong or LM infection, the representation of each population remained constant until day 10 pot infection. However, by day 10 post-infection with LCMV clone 13, nearly all of the detectable cells in the blood were WT (Figure 6E). These data show that the impaired lymph node localization of CD62L–deficient primary memory T cells does prevented a sustained response from this population following LCMV clone 13 infection. This suggests that in addition to impaired viral clearance in lymph nodes, the inability of CD62L–deficient memory CD8+ T cells to prevent chronic LCMV infection also resulted from the lack of a lymph-node dependent sustained CD8+ T cell response.
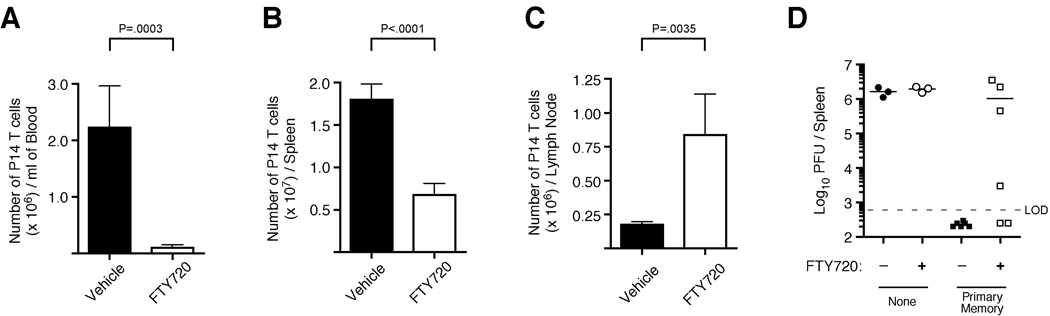
To further evaluate the requirement for egress of lymph node primed memory CD8+ T cells to prevent chronic LCMV infection, we utilized the sphingosine-1-phosphate receptor agonist FTY720, which inhibits T cell egress from lymph nodes (Brinkmann et al., 2002; Matloubian et al., 2004). Indeed, following LCMV clone 13 infection of mice seeded with primary memory P14 cells, treatment with FTY720 caused an accumulation of activated memory CD8+ T cells within lymph nodes and decreased numbers in both the spleen and blood (Figure 7A–C and S5). Importantly, FTY720-mediated trapping of responding primary memory CD8+ T cells in the lymph node also impaired clearance of LCMV clone 13 (Figure 7D). Although the effect of FTY720 treatment on viral clearance in mice harboring primary memory CD8+ T cells was not absolute, these data demonstrate that altering the capacity of re-activated memory CD8+ T cells to leave lymph nodes can dramatically impact their ability to prevent chronic viral infection. Collectively, these data suggest that memory CD8+ T cell entry into lymph nodes, activation, expansion, and subsequent egress are required for the sustained CD8+ T cell response necessary to prevent chronic infection with LCMV clone 13. Thus, the altered tissue localization of either CD62L–deficient primary or of boosted secondary memory CD8+ T cells directly impacts the per cell protective capacity of these populations against a specific pathogen.
Figure 7. Blocking activated memory CD8+ T cell egress from lymph nodes following LCMV clone 13 infection results in impaired viral clearance.
(A–C) 1.5 × 105 primary memory P14 TCR-tg CD8+ T cells were transferred into naïve B6 mice and subsequently infected with LCMV clone 13. Mice were then treated with FTY720 or vehicle control on days 0, 2, and 4 post-infection. On day 6 post-infection, numbers of P14 CD8+ T cells were determined in the (A) blood, (B) spleen, or (C) inguinal lymph node. Representative dot plots for each organ are shown in Figure S7. For A–C, error bars represent std. dev. and statistical analysis was performed using the student’s t test. Data are representative of two independent experiments with 5 mice per group. (D) Same as (A) except mice were treated with FTY720 or vehicle control on days 0, 2, 4, 6, and 8 post-infection. On day 10 post-infection, viral burden was determined in the spleens of infected animals. Cumulative data from two independent experiments is shown. (see also Figure S5).
DISCUSSION
Although a great deal of effort has been dedicated to the understanding of the factors and mechanisms that ultimately lead to the generation of primary memory CD8+ T cells, much less is known about memory CD8+ T cell populations that are generated following additional antigen re-stimulations. Herein, we demonstrate that antigen re-stimulation increases the ability for a memory CD8+ T cell population to protect against acute infections with several pathogens. However, antigen re-stimulation decreases the per cell ability of memory CD8+ T cells to protect against acute, lethal MHV infection and prevent chronic LCMV infection. Because the model of chronic LCMV infection has provided a wealth of knowledge to the field of experimental immunology, we went on to delineate the individual factors that contribute to both the ability for primary memory CD8+ T cells to effectively control the infection and those that contribute to the impaired protection by secondary memory CD8+ T cells.
Previous studies report that re-expression of CD62L is delayed in memory populations generated by multiple antigen encounters (Jabbari and Harty, 2006; Masopust et al., 2006). However, because expression of this molecule also tracks with the differentiation state of primary memory CD8+ T cells (Tcm vs. Tem), it was unknown whether localization alone or other genetic factors associated with the specific differentiation state were the driving force behind the ability for Tcm to efficiently prevent chronic LCMV infection (Wherry et al., 2003b). We show that CD62L–deficient primary memory CD8+ T cells are similar to WT cells with regards to function, phenotype, and ability to protect against virulent LM infection. However, genetic ablation of this single molecule specifically inhibits memory CD8+ T cell distribution into lymph nodes, but not to other tissues of the body. Therefore, our data strongly argue that, from a mechanistic standpoint, CD62L-dependent lymph node localization of memory CD8+ T cells is critical for maximum defense (both viral control in lymph nodes and sustained recall response) against chronic LCMV infection.
In this report, we also identify three key aspects of memory CD8+ T cell biology that change following a subsequent antigen encounter that impair their ability to prevent chronic LCMV infection. First, on a population level, secondary memory CD8+ T cells not only become ‘exhausted’ during chronic LCMV infection, but are inherently more ‘exhaustible’ than a primary memory population. Second, the ability to mount a robust recall response is diminished in secondary memory CD8+ T cells. Third, the ability to localize to lymph nodes decreases in secondary memory cells due to delayed re-expression of CD62L. Although we provide substantial, additional evidence that lymph node localization is critical for conferring host protection against chronic LCMV infection, this finding is complicated by the fact that hundreds of genes are differentially regulated between primary and secondary memory CD8+ T cells (Wirth et al., 2010). Thus, it is highly likely that several linked mechanisms act in concert to cause the overall failure of secondary memory CD8+ T cells to prevent chronic LCMV infection.
Current vaccination strategies have proven successful for the prevention of a variety of acute infections (de Quadros, 2002). In these cases, the establishment of a sufficient memory immune repertoire limits pathogen spread and replication, and elimination of the infectious agent occurs without onset of severe disease symptoms. However, vaccines against infectious agents that cause persistent or chronic infections have been far less successful (Berzofsky et al., 2004). Chronic viral infections are a major cause of not only a number of human diseases (HIV, Hepatitis C Virus), but are also thought to contribute to the development of a variety of human cancers (EBV—B cell lymphomas, Human papillomavirus virus—cervical cancer). In virtually all these cases, failure to completely eliminate the pathogen leads to the generation of CD8+ T cells that are functionally exhausted. In fact, it has been demonstrated that human CD8+ T cells specific for antigens of HIV, EBV, and CMV express inhibitory receptors and are CD62Llo (Chen et al., 2001; Day et al., 2006). However, other aspects of pathogen biology, such as rapid mutation, also probably contribute to the establishment of chronic infection in humans. In addition, our data also suggest that the duration of antigen or pathogen load during infection (such as in MHV and chronic LCMV infection) may also be a determining factor as to which type of memory CD8+ T cell population is most protective. Although it is unclear if entry into lymph nodes will be an essential feature of protective CD8+ T cells against all chronic infections, it is possible that driving memory CD8+ T cells away from lymph nodes through repeated antigen encounters may be advantageous to the persistence of these types of pathogens. Clearly, the ‘best’ memory CD8+ T cell population is going to vary from pathogen to pathogen, dependent upon variables including the site of infection, microbial persistence, and cellular targets of the pathogen.
In conclusion, our data demonstrate that booster immunizations may not only increase the overall number of memory CD8+ T cells, but also leads to biologically relevant functional alterations that can either increase or decrease the per cell protective capacity of the memory cell population for specific pathogens. Because vaccines against chronic infections have been difficult to develop, these data provide direct evidence that both the quality and quantity of a memory CD8+ T cell population must be taken into account when developing vaccination strategies that are able to prevent specific diseases.
EXPERIMENTAL PROCEDURES
Mice and Pathogens
C57BL/6 mice (Thy1.2) were obtained form the National Cancer Institute and used for experiments at 6–10 weeks of age. P14 transgenic mice (Thy1.1) (Pircher et al., 1989) were provided by Dr. Michael Bevan and B6.PL (Thy1.1) were obtained from Jackson Laboratories and maintained by sibling x sibling mating. Lymphotoxin-α (Lta)-deficient mice (De Togni et al., 1994) and CD62L (Sell)-deficient mice (Xu et al., 1996) have been previously described. LCMV Armstrong and LCMV clone 13 were propagated according to standard protocols. LCMV Armstrong (2 × 105 PFU) was injected i.p. as indicated. LCMV clone 13 (2 × 106 PFU) was injected i.v. Vaccinia virus expressing full-length LCMV glycoprotein (VacV-gp) was kindly provided by Dr. Eric Butz and was propagated according to standard protocols. VV-gp was given intranasally in 40 µl of saline. Virulent Listeria monocytogenes expressing gp33 (LM-gp33) (Kaech and Ahmed, 2001) was grown and injected i.v. Recombinant MHV expressing gp33 (Kim and Perlman, 2003) was kindly provided by Dr. Stanley Perlman, and was given intranasally. All animal experiments followed approved Institutional Animal Care and Use Committee protocols.
Cell Purification and Adoptive Transfer
For generation of primary memory P14 CD8+ T cells, naïve Thy1.1 P14 CD8+ T cells (5 × 103) were obtained from peripheral blood and were injected i.v. into naïve Thy1.2 WT recipients. Mice were infected 24 hours later with 2 × 105 PFU of LCMV-Armstrong i.p. To generate secondary memory P14 CD8+ T cells, total splenocytes from mice containing primary memory were stained with PE-anti-Thy1.1 antibody (Clone OX-7, BD Pharmingen) and purified with anti-PE magnetic bead sorting using standard AutoMacs protocols. Following purification, primary memory Thy1.1 P14 CD8+ T cells were injected i.v. into naïve Thy1.2 recipients and the recipients were infected 24 hours later with LCMV-Armstrong i.p. Primary and secondary memory P14 CD8+ T cell populations were used at the same timepoint (60+ days) following LCMV-Armstrong infection. For sorting of CD62L high and low expressing cells, memory P14 CD8+ T cells were purified with magnetic bead sorting as described above. Purified populations were then stained with anti-CD62L antibody and sorted using a FACSDiva (BD Biosciences) cell sorter.
Analysis of Bacterial and Viral Burden
For analysis of bacterial burden following LM infection, primary or secondary memory P14 CD8+ T cells were purified as described above and adoptively transferred into naïve recipients. The recipients and naïve control mice were infected 24 hours later with virulent LM-gp33. On day 3 post-infection, liver or spleen samples were obtained and bacterial content was analyzed as previously described (Harty and Bevan, 1995). For analysis of viral burdens, memory P14 CD8+ T cells were adoptively transferred as indicated. The recipients and naïve control mice were infected 24 hours later with LCMV Armstrong (2 × 105, i.p.), LCMV clone 13 (2 × 106, i.v.), VacV-gp (1 × 107 i.n.), or MHV-gp33 (1 × 105 i.n.). On the peak day of infection, the appropriate organ was obtained, homogenized, and viral titers were quantified using standard plaque assaying on VERO cells as previously described (Shen et al., 1998). For VacV-gp titers, lung homogenates underwent 3 rounds of freeze-thaw before being applied to VERO cells. MHV viral load was quantified as previously described (Kim and Perlman, 2003).
In vitro proliferation
Spleen samples were obtained from mice containing either primary or secondary memory P14 CD8+ T cell populations. Total splenocytes were stained with 1 µM CFSE for 15 minutes and thoroughly washed with RPMI containing 10% fetal calf serum. 2 × 106 total splenocytes were then incubated for 60 hours with our without 50 nM gp33 peptide. Recombinant human IL-2 was used at a concentration of 100 U/ml. Proliferation was analyzed by CFSE dilution of the Thy1.1 memory P14 CD8+ T cell population.
Ex vivo cytokine production
Analysis of IFNγ, TNFα, and IL-2 production by antigen specific CD8+ T cells was performed essentially as previously described (Badovinac et al., 2002). Briefly, 2 × 106 total splenocytes were incubated for 5 hours with or without gp33 peptide in the presence of Brefeldin A. Intracellular cytokine staining was then performed using the BD Cytofix/Cytoperm kit according to the manufacturers protocol. In cases where cytokine production was analyzed from blood samples, 2 × 105 EL4 (H-2b) cells were added to maximize antigen presentation to responding T cells.
FTY720 Preparation and Treatment
FTY720 (Caymen Chemical, Ann Arbor, Michigan) was dissolved in DMSO at a concentration of 10 mg/ml and stored at −20°C. Stock solutions of FTY720 were then diluted in sterile saline before i.v. administration at a dose of 1 mg/kg. Following LCMV clone 13 infection, mice received FTY720 treatments on days 0, 2, 4, 6, and 8.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (AI42767, AI46653, AI50073, AI59752 , JTH) and a Career Development Award from The Leukemia and Lymphoma Society (JCN). The authors would like to thank members of the Harty Lab for helpful discussions and Vladimir Badovinac, Stanley Perlman, and Steve Varga for critical review of the manuscript. We thank Stanley Perlman and Jason Netland for reagents and technical assistance using MHV and Lecia Epping, Lisa Hancox and Jemmie Hoang for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
JCN designed and performed experiments, analyzed data, and wrote the paper. JTH designed experiments, analyzed data, and wrote the paper.
REFERENCES
- Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Autran B, Carcelain G, Combadiere B, Debre P. Therapeutic vaccines for chronic infections. Science. 2004;305:205–208. doi: 10.1126/science.1100600. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Messingham KA, Hamilton SE, Harty JT. Regulation of CD8+ T cells undergoing primary and secondary responses to infection in the same host. J Immunol. 2003;170:4933–4942. doi: 10.4049/jimmunol.170.10.4933. [DOI] [PubMed] [Google Scholar]
- Badovinac VP, Porter BB, Harty JT. Programmed contraction of CD8(+) T cells after infection. Nat Immunol. 2002;3:619–626. doi: 10.1038/ni804. [DOI] [PubMed] [Google Scholar]
- Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin-alpha-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol. 1995;155:1685–1693. [PubMed] [Google Scholar]
- Berzofsky JA, Ahlers JD, Janik J, Morris J, Oh S, Terabe M, Belyakov IM. Progress on new vaccine strategies against chronic viral infections. J Clin Invest. 2004;114:450–462. doi: 10.1172/JCI22674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, et al. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- Catalina MD, Carroll MC, Arizpe H, Takashima A, Estess P, Siegelman MH. The route of antigen entry determines the requirement for L-selectin during immune responses. J Exp Med. 1996;184:2341–2351. doi: 10.1084/jem.184.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Shankar P, Lange C, Valdez H, Skolnik PR, Wu L, Manjunath N, Lieberman J. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 2001;98:156–164. doi: 10.1182/blood.v98.1.156. [DOI] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- de Quadros CA. History and prospects for viral disease eradication. Med Microbiol Immunol. 2002;191:75–81. doi: 10.1007/s00430-002-0120-7. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, et al. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Grayson JM, Harrington LE, Lanier JG, Wherry EJ, Ahmed R. Differential sensitivity of naive and memory CD8+ T cells to apoptosis in vivo. J Immunol. 2002;169:3760–3770. doi: 10.4049/jimmunol.169.7.3760. [DOI] [PubMed] [Google Scholar]
- Harty JT, Badovinac VP. Shaping and reshaping CD8+ T-cell memory. Nat Rev Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- Hill AV, Reyes-Sandoval A, O'Hara G, Ewer K, Lawrie A, Goodman A, Nicosia A, Folgori A, Colloca S, Cortese R, et al. Prime-boost vectored malaria vaccines: progress and prospects. Hum Vaccin. 2010;6:78–83. doi: 10.4161/hv.6.1.10116. [DOI] [PubMed] [Google Scholar]
- Huster KM, Stemberger C, Busch DH. Protective immunity towards intracellular pathogens. Curr Opin Immunol. 2006;18:458–464. doi: 10.1016/j.coi.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Harty JT. Secondary memory CD8+ T cells are more protective but slower to acquire a central-memory phenotype. J Exp Med. 2006;203:919–932. doi: 10.1084/jem.20052237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- Kim TS, Perlman S. Protection against CTL escape and clinical disease in a murine model of virus persistence. J Immunol. 2003;171:2006–2013. doi: 10.4049/jimmunol.171.4.2006. [DOI] [PubMed] [Google Scholar]
- Klenerman P, Hill A. T cells and viral persistence: lessons from diverse infections. Nat Immunol. 2005;6:873–879. doi: 10.1038/ni1241. [DOI] [PubMed] [Google Scholar]
- Lauvau G, Vijh S, Kong P, Horng T, Kerksiek K, Serbina N, Tuma RA, Pamer EG. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 2001;294:1735–1739. doi: 10.1126/science.1064571. [DOI] [PubMed] [Google Scholar]
- Masopust D. Developing an HIV cytotoxic T-lymphocyte vaccine: issues of CD8 T-cell quantity, quality and location. J Intern Med. 2009;265:125–137. doi: 10.1111/j.1365-2796.2008.02054.x. [DOI] [PubMed] [Google Scholar]
- Masopust D, Ha SJ, Vezys V, Ahmed R. Stimulation history dictates memory CD8 T cell phenotype: implications for prime-boost vaccination. J Immunol. 2006;177:831–839. doi: 10.4049/jimmunol.177.2.831. [DOI] [PubMed] [Google Scholar]
- Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, Allende ML, Proia RL, Cyster JG. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427:355–360. doi: 10.1038/nature02284. [DOI] [PubMed] [Google Scholar]
- Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Prlic M, Williams MA, Bevan MJ. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr Opin Immunol. 2007;19:315–319. doi: 10.1016/j.coi.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Schmidt NW, Podyminogin RL, Butler NS, Badovinac VP, Tucker BJ, Bahjat KS, Lauer P, Reyes-Sandoval A, Hutchings CL, Moore AC, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci U S A. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Miller JF, Fan X, Kolwyck D, Ahmed R, Harty JT. Compartmentalization of bacterial antigens: differential effects on priming of CD8 T cells and protective immunity. Cell. 1998;92:535–545. doi: 10.1016/s0092-8674(00)80946-0. [DOI] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med. 2007;204:941–949. doi: 10.1084/jem.20061937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Steeber DA, Green NE, Sato S, Tedder TF. Lyphocyte migration in L-selectin-deficient mice. Altered subset migration and aging of the immune system. J Immunol. 1996;157:1096–1106. [PubMed] [Google Scholar]
- Unsoeld H, Pircher H. Complex memory T-cell phenotypes revealed by coexpression of CD62L and CCR7. J Virol. 2005;79:4510–4513. doi: 10.1128/JVI.79.7.4510-4513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci U S A. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003a;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003b;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- Wirth TC, Xue HH, Rai D, Sabel JT, Bair T, Harty JT, Badovinac VP. Repetitive antigen stimulation induces stepwise transcriptome diversification but preserves a core signature of memory CD8(+) T cell differentiation. Immunity. 2010;33:128–140. doi: 10.1016/j.immuni.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland DL. Jump-starting the immune system: prime-boosting comes of age. Trends Immunol. 2004;25:98–104. doi: 10.1016/j.it.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Xu J, Grewal IS, Geba GP, Flavell RA. Impaired primary T cell responses in L-selectin-deficient mice. J Exp Med. 1996;183:589–598. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.