Abstract
IL-1β is an important inflammatory mediator of type 2 diabetes (T2D). Here we show that oligomers of islet amyloid polypeptide (IAPP), a protein that forms amyloid deposits in the pancreas during T2D, trigger the Nlrp3 inflammasome and generate mature interleukin (IL)-1β. A T2D therapy, glyburide, suppresses IAPP-mediated IL-1β production in vitro. Processing of IL-1β initiated by IAPP first requires priming, a process that involves glucose metabolism and can be facilitated by minimally oxidized low density lipoprotein. Finally, mice transgenic for human IAPP have increased IL-1β in pancreatic islets, which colocalizes with amyloid and macrophages. Our findings reveal novel mechanisms in the pathogenesis of T2D and treatment of pathology caused by IAPP.
Introduction
Type 2 diabetes (T2D) is characterized by insulin resistance and islet beta cell dysfunction, with the progressive loss of insulin release being responsible for ever increasing glucose concentrations in the absence of treatment1. Obesity is frequently the basis for insulin resistance in T2D, and is associated with increased concentrations of oxidized low density lipoprotein (LDL)2, free fatty acids (FFAs), and pro-inflammatory cytokines 1. One cytokine in particular, interleukin-1 beta (IL-1β), has been found to have profound effects on the function of pancreatic beta cells, inducing them to undergo apoptosis 3. There is an abundant literature describing the involvement of IL-1β in type 1 diabetes (T1D), however IL-1β is also a risk factor for T2D 4, and a recent clinical trial of IL-1 blockade in T2D has been very encouraging5. These data clearly suggest a role for IL-1β in the pathogenesis of T2D, however the events leading to increased concentrations of active, secreted IL-1β in this disease remain unclear.
Recently a large number of publications have elucidated mechanisms by which biologically active IL-1β is made in the cell. This usually involves activation of a protein complex termed the inflammasome, which is formed by a nucleating sensor protein (typically a member of the nucleotide-binding domain and leucine-rich repeat containing, NLR, family) that oligomerises the adaptor protein ASC to dimerise and activate caspase-1 and thus cleave pro-IL-1β into the processed, secreted form. Before this can happen, certain cell types require priming to induce suitable amounts of pro-IL-1β and NLR proteins, this is sometimes referred to as ‘signal 1’. The ‘signal 2’ factors that can activate the nucleating receptor of the complex are still being determined. These include microbial DNA, RNA, cell wall components and toxins. With reference to inflammatory diseases such as gout and fibrosing disorders, endogenous factors and environmental contaminants have also been discovered, such as uric acid crystals 6 and asbestos or silica respectively7. Most of these molecules activate the inflammasome complex nucleated by Nlrp3 (also known as Nalp3 or cryopyrin), however there is no structural similarity to indicate that they would bind to it directly. Instead Nlrp3 is probably a sensor of some homeostatic intracellular process that, if perturbed, will activate the inflammasome. Certainly a large number of the Nlrp3 activating agents seem to perturb the lysosomal vacuole, some due to their size through a process that has been termed “frustrated phagocytosis”, and others that are not degraded normally once phagocytosed8. This prompted us to ask if there might also be an endogenous factor in T2D that could activate the Nlrp3 inflammasome in this way.
A hallmark feature of T2D is the deposition of amyloid in the pancreas of most patients, with this morphological change being associated with the loss of insulin-producing beta cells 9. The unique polypeptide constituent of amyloid found in pancreatic islets is islet amyloid polypeptide (IAPP, also known as amylin)10,11, a 37 amino acid peptide co-secreted by the beta cell with insulin. There are a number of lines of evidence that strongly implicate this deposition in disease progression. First there is species specificity whereby IAPP in certain animals, such as rodents, is unable to form amyloid due to the alteration of certain critical residues, however when a human IAPP transgene is expressed in mice or rats, amyloid is deposited within pancreatic islets, leading to loss of beta cell mass 12–14. Second, experiments have been performed using non-human primates in which the concentration of amyloid in the pancreas clearly correlates with the severity of disease and beta cell function 15. Third, in humans a rare polymorphism in IAPP, S20G, is associated with increased incidence or severity of T2D in some populations 16, and this version of the protein has a higher propensity to form amyloid 17. IAPP oligomerization might also have a role in preventing successful islet transplantation, which is a potential treatment for T1D. Amyloid deposition is observed when human IAPP transgenic mouse islets, or human islets are transplanted into mice 18,19, and this could also be associated with the failure of islet transplantation in humans 20.
Initially it was established that IAPP is cytotoxic 21, which could be due to effects at the cell membrane 22. Mechanistically, this might also involve reactive oxygen species (ROS) because ROS inhibition prevents IAPP-mediated beta cell apoptosis in vitro 23. The process of IAPP incorporation into amyloid is thought to proceed via the aggregation of prefibrillar oligomers. These soluble oligomers, rather than mature fibrils, could be the cytotoxic component24. Amyloid builds up extracellularly but phagocytosed IAPP can be detected within the lysosomal compartment of human pancreatic macrophages in vivo, where due to its amyloid structure, it is not degraded normally 25,26. There are also reports showing induction of IL-1β by IAPP 27,28, however these studies were performed before the identification of the inflammasome, and did not examine islets or primary macrophages and dendritic cells (DC), the latter two of which could be the main source of pathogenic IL-1β in the pancreas. This observation prompted us to evaluate the potential for IAPP to activate the Nlrp3 inflammasome through the mechanism of phagosomal destabilization 8. We show that amyloidogenic human IAPP, but not the non-amyloidogenic rat form of IAPP can trigger inflammasome activation and IL-1β production in lipopolysacharride- (LPS) primed macrophages or DC, and that this was dependent on Nlrp3. This then allowed us to investigate the effect of glyburide, a common treatment in T2D, which is known to inhibit the inflammasome 29. We also observe that minimally oxidized LDL could prime for IL-1β processing, and that priming (signal 1) was dependent on glucose metabolism. Finally, we employed a mouse model where the human IAPP gene is expressed in islet beta cells, and found increased IL-1β expression that broadly co-localizes with amyloid and also macrophages. Our results therefore provide a possible mechanism for IL-1β activation in T2D.
Results
IAPP activates the inflammasome to produce mature IL-1β
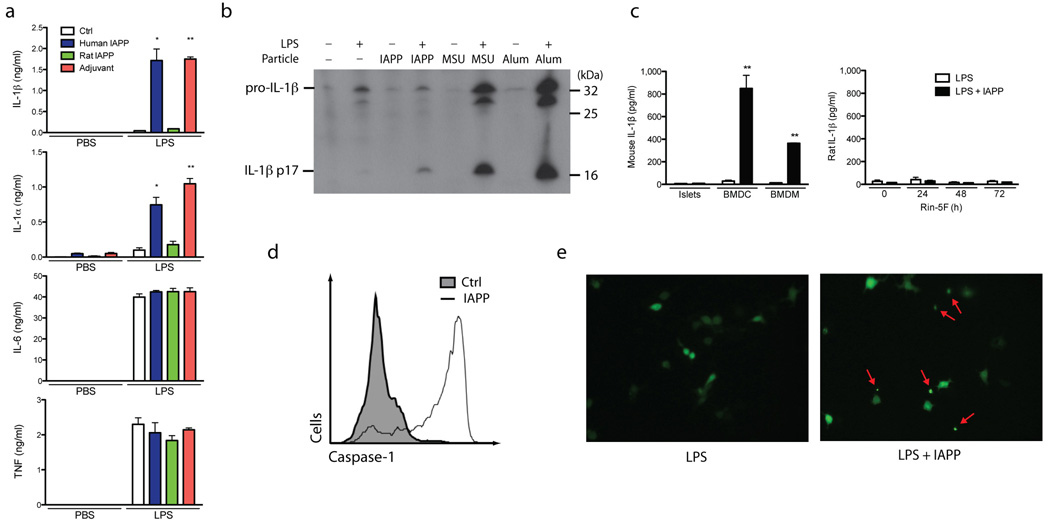
Bone marrow-derived dendritic cells (BMDC) and bone marrow-derived macrophages (BMDM) are commonly used to investigate the inflammasome because of their high expression of pro-IL-1β following LPS priming. We compared the ability of IAPP to stimulate production of mature IL-1β from LPS primed BMDC with known inflammasome-activating agents like particulate adjuvants 30. Overnight stimulation with human IAPP was able to generate comparable amounts of IL-1β to these adjuvants, but rat IAPP which is unable to form amyloid did not stimulate IL-1β release (Fig. 1a). Human IAPP also stimulated the release of IL-1α which, like IL-1β, has been shown to rely on the activity of caspase-131, but could also represent paracrine induction of IL-1α by IL-1β. IL-1α in cell lysates was not decreased, suggesting that full length IL-1α is not released by cell lysis (Supplementary Figure 1a). TNF and IL-6 production (Fig. 1a) were undetectable without LPS stimulation, indicating that the IAPP preparations were not contaminated with LPS. The concentrations of TNF and IL-6 were not altered by the presence of IAPP, indicating that there was a specific effect on IL-1β production and not a general increase in pro-inflammatory cytokine production, and that there was no obvious cell death before these cytokines were produced.
Figure 1. Inflammasome activation and IL-1β production induced by human IAPP.
(a) IAPP from different species or polystyrene beads (adjuvant) were incubated overnight with BMDC with and without 3 hours priming using LPS. Supernatants were then analyzed for IL-1β, IL-1α, IL-6 and TNF by ELISA. (b) Immunoblotting for IL-1β was performed using supernatants from BMDC primed with LPS, then activated with IAPP, MSU or Alum for 6 hours. (c) IL-1β secretion from BMDM and BMDC compared to purified mouse pancreatic islets or Rin-5F cells (a pancreatic beta cell line), stimulated with LPS and IAPP. Rin-5F cells were also cultured for different times with elevated glucose (25 mM D-glucose). (d) Flow cytometric analysis of BMDC stimulated with IAPP for 1 hour then treated with FAM-YVAD-fmk which covalently binds to active caspase-1 and fluoresces. Control is shaded under the curve, IAPP stimulated is unshaded. (e) Immortalized BMDM expressing YFP-ASC were primed with LPS, then activated with IAPP overnight and imaged with a fluorescent microscope (40x objective). Arrows indicates speck formation. Means ± SD, * p<0.01, ** p<0.001. All data representative of three independent experiments.
Immunoblotting was used to confirm that the detected IL-1β was the cleaved 17.5 kDa fragment. Cleaved IL-1β was increased in the presence of IAPP and the inflammasome-activating agents uric acid crystals (MSU) and ATP (Fig. 1b). Measurement of lactate dehydrogenase (LDH) in the supernatants was carried out along with the measurement of IL-1α and IL-1β; this confirmed that there was no obvious cytotoxicity under these conditions (Supplementary Figure 1a–d). Both DCs and macrophages are found in pancreatic islets, and these cells have been observed to phagocytose IAPP in T2D 26. Like BMDC, BMDM also produced a significant amount of IL-1β in response to IAPP. In comparison, the production of IL-1β by purified mouse pancreatic islets ex vivo and the rat beta cell line Rin-5F was very low, even when these cells were cultured under hyperglycemic conditions (Fig. 1c). These results show that amyloidogenic human IAPP can trigger processing of IL-1β from BMDM and BMDC.
IAPP activates caspase-1 and initiates ASC speck formation
Caspase-1 is the protease activated in the inflammasome complex to cleave IL-1β and we measured active caspase-1 with a fluorescent cell-permeable probe that binds activated caspase-1 (FAM-YVAD-fmk). Flow cytometry indicated a large increase in the percentage of BMDC that contain active caspase-1 after being stimulated with IAPP for 1 hour (Fig. 1d). The mechanism by which caspase-1 is activated to produce mature IL-1β requires the apoptosis-associated speck-like protein containing a CARD (ASC), and its ability to form the multimeric inflammasome complex. Normally ASC is evenly distributed throughout the cell cytoplasm, but when the inflammasome complex becomes activated, ASC aggregates to a single foci within the cell, known as a speck. We used immortalized BMDM that stably express a fluorescent ASC protein to measure speck formation. LPS-treated cells that were activated with IAPP induced formation of an intense, single fluorescent speck in the cell, indicative of inflammasome activation (Fig. 1e, Supplementary Figure 1e). This documents that the along with IL-1β processing, IAPP causes activation of caspase-1 and the ASC inflammasome complex.
IAPP oligomers activate the Nlrp3 inflammasome
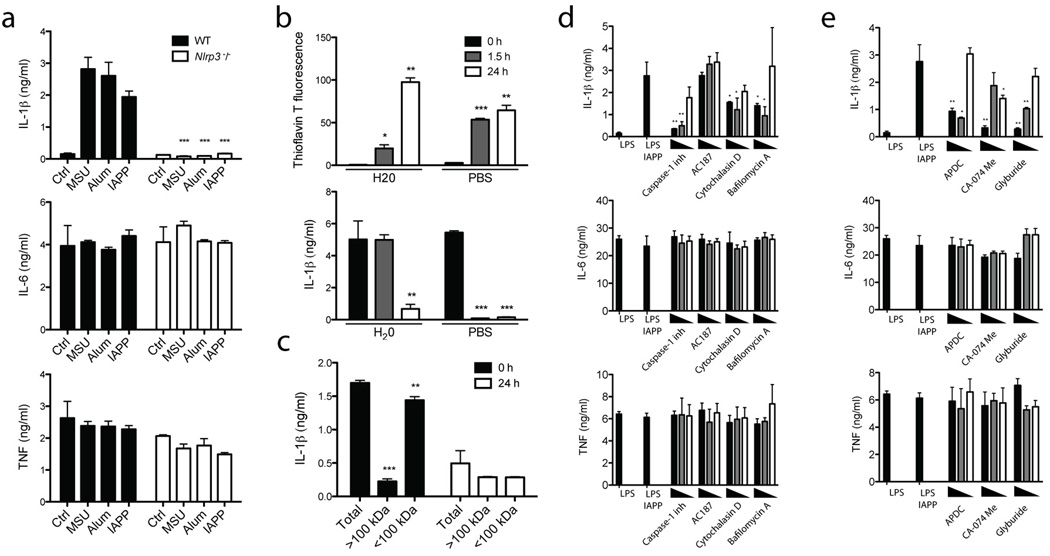
Having shown that an inflammasome complex containing caspase-1 and ASC is activated by IAPP to cleave IL-1β, we next examined which NLR protein nucleates this inflammasome complex. We elected to study Nlrp3, which is required for inflammasome activation by particles, rather than IPAF, Nlrp1, Nod1 or Nod2 which are not. Accordingly, for Nlrp3-deficient BMDC, mature IL-1β production was completely abrogated in response to IAPP (Fig. 2a). These cells still produced TNF and IL-6, attesting to the specificity of Nlrp3 deletion, and IL-1β mRNA expression was not decreased (Supplementary Figure 1f). To determine whether oligomers or IAPP fibrils activate the Nlrp3 inflammasome, we employed the organic solvent HFIP to dissolve amyloid fibrils. The HFIP was then removed using nitrogen gas and the IAPP was reconstituted in H2O or PBS and incubated at room temperature. Thioflavin T fluorescence was used to indicate fibrillar content. IAPP fibrils were formed over 24 h and this was accelerated when reconstituted in PBS compared to H2O (Fig. 2b, top panel). This increase in fibrillar content coincided with a decrease in IL-1β production from BMDC (Fig. 2b, lower panel). To further elucidate the active species, preparations of IAPP were subjected to size fractionation, generating samples with predominantly fibrillar (>100 kDa) or oligomeric (<100 kDa) species. We found that the IL-1β activating potential was predominantly retained in the oligomeric fraction <100 kDa from freshly reconstituted IAPP (Fig. 2c). Collectively this data suggests that the Nlrp3-activating constituent is a soluble oligomer of IAPP, rather than a highly fibrillar species of high molecular weight.
Figure 2. IAPP oligomers activate the Nlrp3 inflammasome which is prevented by glyburide, and inhibitors targeting phagocytosis, ROS and Cathepsin B.
(a) WT or Nlrp3 deficient (Nlrp3−/−) BMDM were primed with LPS and then activated with uric acid crystals (MSU), Alum or IAPP overnight after which IL-1β, TNF and IL-6 production was measured. (b) Thioflavin T fluorescence was used to indicate IAPP fibrillar content after reconstitution in H20 or PBS for different times at room temperature. These different preparations of IAPP were then used to activate BMDC as in (a), and IL-1β was measured. (c) IAPP reconstituted in H20 for different times was separated based on size into fractions >100 kDa and <100 kDa, then used to activate BMDC as in (a), and IL-1β was measured. (d) BMDC were treated as in (a) for IAPP, with the addition of various inhibitors 1 hour before inflammasome activation; 1 µg/ml caspase-1 inhibitor, 5 µM cytochalasin D, 250 µM bafilomycin A or 40 µM IAPP receptor inhibitor peptide (AC187). Starting at the highest concentration indicated, two 10 fold dilutions of the inhibitor were also tested, or in the case of AC187, two 4 fold dilutions. (e) BMDC were treated as in (a) for IAPP, with the addition of various inhibitors 1 hour before inflammasome activation; 1 µg/ml ROS inhibitor (APDC), 10 µM cathepsin B inhibitor (CA-074 Me) and 50 µM glyburide. Starting at the highest concentration indicated, two 10 fold dilutions of the inhibitor were also tested, or in the case of glyburide, two 4 fold dilutions. Means ± SD, * p<0.05, ** p<0.01, *** p<0.001. All data representative of three independent experiments.
IAPP inflammasome activation involves the phagolysosome
To confirm the role of caspase-1 and support the use of inhibitors to investigate mechanisms of inflammasome activation, a caspase-1 inhibitor was added to BMDC before IAPP activation. Caspase-1 inhibition reduced IL-1β production in a does-dependent manner (Fig. 2d). IAPP is thought to signal through a complex of receptor activity-modifying proteins with the calcitonin receptor, and the biological effects of IAPP can be blocked at this point using a specific peptide inhibitor (AC187)32. To determine if recognition of IAPP by the receptor complex is required for inflammasome activation a titration of up to 40 µM AC187 (a 4 fold molar excess) was used, however this had no effect on IL-1β (Fig. 2d). Alternatively IAPP could be phagocytosed by BMDM and thereby activate the inflammasome in a manner similar to other species of amyloid33. Inhibition ofIL-1β production following the addition of cytochalasin D confirmed that inflammasome activation was dependent on phagocytosis (Fig. 2d). Furthermore, inhibition of the vacuolar H+ ATPase by bafilomycin A restricted the effect of IAPP on IL-1β secretion (Fig. 2d). Taken together this suggests that phagolysosomal processes downstream of acidification are altered to trigger Nlrp3 activation.
IAPP inflammasome activation is prevented by glyburide
Two mechanisms have been proposed to account for pertubations in phagocytic processes that activate the inflammasome. Previously cathepsin B was implicated in this process for amyloid beta 33 while IAPP 23 and MSU 6, have been found to induce ROS. We used the ROS inhibitor (2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylic acid (APDC) and a cathepsin B inhibitor (CA-074 Me) to confirm their role in IAPP activation of the inflammasome (Fig. 2e). Glyburide is a second generation sulfonylurea, an agonist of the potassium channel composed of SUR1 and KIR6.2 that signals for increased insulin secretion. Additionally, this small molecule agonist inhibits the inflammasome and IL-1β production, but this is independent of its interaction with the SUR1, KIR6.2 complex 29,34. We found that activation of the Nlrp3 inflammasome by IAPP was also inhibited by glyburide (Fig. 2e).
Txnip is not involved in IAPP activation of IL-1β
The thioredoxin interacting protein Txnip was recently identified as a redox sensitive ligand of Nlrp3 35. This study suggested that an increased concentration of glucose alone could induce pancreatic beta cell Txnip to activate the inflammasome, and that glyburide could prevent this. However it was also shown that expression of Txnip in macrophages is constitutively high, so it was unlikely that this would be the mechanism of inflammasome inhibition by glyburide in macrophages. Nevertheless, we formally examined Txnip mRNA levels both before and after LPS priming in BMDM, and found no difference due to glyburide in either setting (Supplementary Figure 2a). Furthermore, LPS decreased expression of Txnip arguing against this protein as the instigator of Nlrp3 activation in BMDM. We then tested Txnip-deficient macrophages to see if this Nlrp3 ligand was required for IL-1β production in response to IAPP (Supplementary Figure 2b). We could find no difference in IL-1β secretion in response to IAPP, or indeed other inflammasome activators such as MSU or ATP in BMDM ex vivo. In addition we directly compared BMDM from mice lacking Txnip or Nlrp3 for IL-1β secretion and caspase-1 activation and found that these parameters were only decreased in the Nlrp3−/− mice (Supplementary Figure 2c–f). Therefore our studies to date do not find role for Txnip regulating the effect of glyburide on the inflammasome, or IL-1β production by the inflammasome.
Glucose metabolism is required to prime the inflammasome
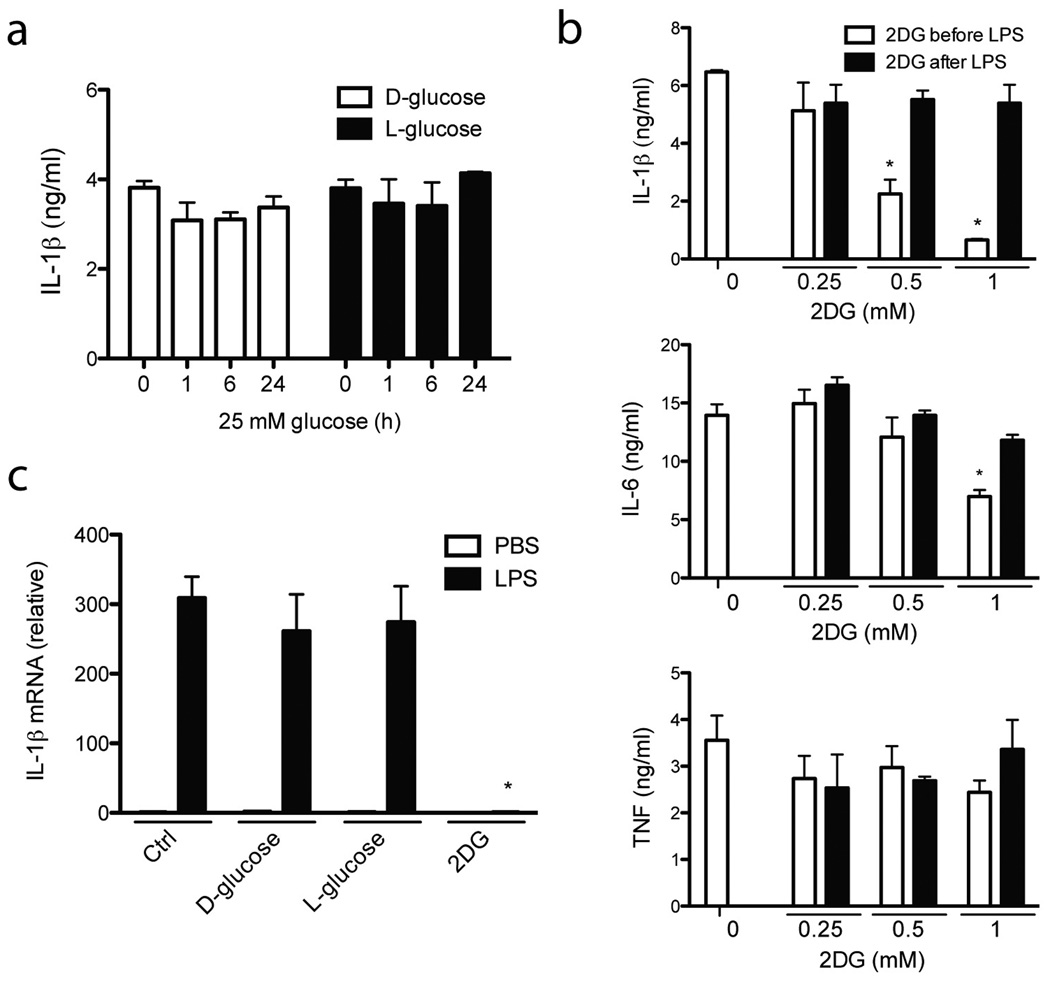
Elevated concentrations of D-glucose, similar to those encountered in T2D, can induce pro-IL-1β 36,37 although this is debated 38. Growing BMDM in different concentrations of glucose influenced cell proliferation, to which BMDC were more resistant. We therefore tested BMDC in hyperglycemic conditions (25 mM D-glucose) for up to 24 h either before or after priming with LPS. We saw no significant difference in IL-1β production after IAPP stimulation (Fig. 3a). We also tested D-glucose analogs; one that is not metabolized by the cell and provides a similar osmotic environment to D-glucose (L-glucose), and another that actually inhibits the processing of D-glucose and subsequent metabolic processes in the cell (2-deoxy-D-glucose, 2DG). L-glucose had no effect (Fig. 3a), however we found that low concentrations of 2DG prevented IL-1β release due to IAPP in a dose dependent manner (Fig. 3b). 2DG had no effect when added after the addition of LPS, but only before the addition of IAPP (Fig. 3b), and we found that 2DG prevented IL-1β mRNA induction during LPS priming of the inflammasome (Fig. 3c). There was also a small decrease in the production of IL-6 at the highest concentration of 2DG (Fig. 3b), however the production of TNF and the viability of cells in general were not perturbed (Fig. 3b and Supplementary Fig. 3a). This documents the in vitro requirement for sufficient glucose metabolism during priming (signal 1) of the inflammasome activation process.
Figure 3. Priming the inflammasome requires glucose metabolism.
(a) BMDC were treated with 25 mM D- or L-glucose for different periods of time prior to LPS priming and inflammasome activation with IAPP, after which IL-1β production was measured. (b) BMDC were treated with increased amounts of 2-deoxy-D-glucose (2DG) either 3 hours before or 3 hours after priming with LPS. Cells were then activated with IAPP and the cytokines IL-1β, IL-6 and TNF were measured. (c) IL-1β mRNA levels were determined after 3 hours LPS stimulation of BMDC in the presence of increased amounts of D-glucose and its analogues for a total of 6 hours. Means ± SD, * p<0.001. All data representative of three independent experiments.
mmLDL primes the inflammasome activated by IAPP
Our studies to this point showed that glucose metabolism is required for LPS to prime the inflammasome after which IAPP can activate Nlrp3, caspase-1 and ASC, leading to IL-1β processing. The question then arose as to what the endogenous priming agent of the Nlrp3 inflammasome might be i.e. a potential host factor as opposed to LPS. It has been reported that a particular form of LDL, minimally oxidized LDL (minimally modified LDL or mmLDL), can engage Toll-like receptor 4 (TLR4) in a manner similar to LPS, while extensively oxidized LDL does not39. Specifically, mmLDL can be elevated in individuals with T2D 40,41, and can cause apoptosis and a loss of insulin secretion by islets in culture 42. We therefore tested preparations of LDL that had been oxidized to different extents. Although the level of IL-1β produced after IAPP activation of the inflammasome was low when first primed with mmLDL, it was clearly higher than priming with more extensively oxidized species of LDL, or LDL that was not oxidized (Fig. 4a). mmLDL recognition depended on TLR4, because C3H/HeJ mice which have a non-functional TLR4, were unable to prime the inflammasome via LPS or mmLDL. mmLDL also resulted in the dose-dependent production of TNF and IL-6 (Fig. 4b). Although LPS was not detected in mmLDL preparations, an LPS inhibitor, polymyxin B, inactivated mmLDL (Supplementary Figure 3b). While trace amounts of LPS might be specifically presented to TLR4 by mmLDL, it is also possible that polymyxin B neutralizes mmLDL in the same way as LPS. There are thought to be two prerequisites for inflammasome priming, the induction of pro-IL-1β, and Nlrp3 43, however we observed that mmLDL only induced IL-1β mRNA, in a time-dependent manner (Fig. 4c). This lack of Nlrp3 upregulation might help explain the relatively low level of IL-1β activation when compared to LPS priming. Nevertheless these data suggest that mmLDL can promote IL-1β by providing signal 1 (priming) for the inflammasome, which may be particularly relevant in T2D.
Figure 4. Minimally oxidized LDL can prime for inflammasome activation by IAPP.
(a) IAPP activation of the inflammasome after 3 hrs priming with LPS, human plasma LDL, or LDL oxidized to different extents. LDL oxidation is quantified by relative electrophoretic mobility (REM). BMDM from C3H/HeN mice were compared to those from C3H/HeJ mice that do not have functional TLR4. (b) Dose response for minimally oxidized LDL (mmLDL) priming of IAPP inflammasome activation and IL-1β production. IL-6 and TNF are also produced in a dose-dependent fashion. (c) IL-1β and Nlrp3 mRNA levels were measured after priming with mmLDL for different times. Means ± SD, * p<0.05, ** p<0.01, *** p<0.001. All data representative of three independent experiments.
IAPP induces IL-1β in vivo
As mouse IAPP is not amyloidogenic, mice that are transgenic for the expression of human IAPP have been generated and extensively characterized as a rodent model of islet amyloid formation 44. In particular, when these mice are fed a high fat diet, amyloid deposition within islets of the pancreas occurs and is associated with the impaired function of insulin-producing cells 45. We compared wild-type mice (control) and IAPP transgenic mice, both fed on a high fat diet for one year (the length of time required in this model), and then determined whether insulin and IL-1β were present in pancreatic islets by immunofluoresence (Fig. 5a). Although variable, IAPP transgenic mice had marked regions within the islet that did not stain for insulin, and instead these regions displayed evidence of IL-1β immunoreactivity. However the IL-1β staining co-localized to regions where amyloid was deposited, as determined by staining with thioflavin S (Fig. 5b). To identify a cell type in the islet that produces this IL-1β we stained with an antibody that is particularly useful for detecting peripheral tissue macrophages, MoMa2 (Fig. 5c). Most of the macrophages in the islet were positive for IL-1β, but there was still a large area staining for IL-1β outside these cells. When quantified, a statistically significant decrease in the area of insulin-producing cells was observed (Fig. 5d), with a concurrent increase in the amount of amyloid (Fig 5e), and the area of IL-1β expression (Fig. 5f) within pancreatic islets of IAPP transgenic mice. There was no significant difference in the area of MoMa2 reactivity within the islet (Fig. 5g). This mouse model highlights that the presence of IAPP amyloid promotes IL-1β in vivo.
Figure 5. Increased IL-1β expression in islets of mice transgenic for human IAPP.
(a) Immunofluorescent analysis (20x objective) of sections from the pancreas of wild-type (WT) and IAPP transgenic (IAPP-TG) mice, both fed a high fat diet for one year. Sections were stained for insulin (red) and IL-1β (green). (b) Pancreatic sections from IAPP-TG mice stained for amyloid (red) and IL-1β (green). (c) Pancreatic sections from WT and IAPP-TG mice stained for macrophages (red) and IL-1β (green). All images representative of three individual mice. (d) The area within the islet expressing insulin was quantified for multiple islets of three individual mice. (e) The area of amyloid deposition in the islet, measured as for insulin. (f) The islet area expressing IL-1β, measured as for insulin. (g) The islet area stained for macrophages, measured as for insulin. Means ± SD, * p=0.0427, ** p=0.0261, n=3.
Discussion
This work describes the mechanism whereby an endogenous molecule that is deposited in the pancreas during T2D can potentiate the processing of IL-1β, a pathogenic cytokine that causes beta cell death. We also saw release of IL-1α due to IAPP, which could augment sterile inflammation in pancreatic islets. Our observation that IAPP can activate the Nlrp3 inflammasome is consistent with similar activation from a different amyloidogenic peptide, amyloid beta33, and literature showing that the disease-causing species of IAPP might be oligomers 46. Macrophages and DC are professional phagocytic cells that take up IAPP in pancreatic islets, and we found that these cell types produce significant amounts of IL-1β in response to IAPP oligomers. Using multiple inhibitors we showed that pertubation of the phagolysosomal pathway seems to be involved in Nlrp3 activation by IAPP, however the role of ROS may be complex. Recently a number of papers have shown that patients deficient for ROS production still activate the inflammasome, to an even higher degree than healthy controls with normal ROS production 47. This suggests that the effect of the ROS inhibitor that we used might be non-specific, and indeed this is a caveat raised against the use of inhibitors in general. Although the effects of the inhibitors we used probably point towards a common mechanism of inflammasome activation during the phagocytosis of IAPP, their precise target in this pathway remains to be elucidated.
Unexpectedly, we found no evidence that the signaling cascade leading to Nlrp3 activation requires Txnip. Particularly with regard to T2D where Txnip is known to be a ROS-sensitive molecule implicated in disease, we had hypothesized that IAPP-induced changes would utilize this pathway. While it is possible that our in vitro experiments have not fully recapitulated in vivo mechanisms of disease, they clearly show that Nlrp3 is a critical mediator ofIL-1β release while Txnip is not. Certainly cell type differences might account for our observation since Txnip mRNA levels are not altered by glyburide in macrophages, while others report that glyburide might prevent Txnip induction in beta cells 35. Because IAPP can induce significant IL-1β production from macrophages and this is inhibited by glyburide while Txnip mRNA levels remain unchanged, a separate mechanism for this inhibition must exist, and is sure to attract interest in the future. Although we found that glyburide can inhibit IAPP-induced IL-1β in vitro at concentrations above 5 µg/ml, it is unlikely that the concentration of glyburide currently used clinically would reach this level. New small molecules that can target this inflammatory pathway in vivo will likely be attractive therapeutic candidates.
Identification of IAPP as a possible trigger for inflammasome activation in T2D still leaves open the question as to which endogenous factors prime the inflammasome in this disease. High glucose alone was not able to replace LPS as the priming agent, or influence IL-1β mRNA levels. We found that mmLDL could prime the inflammasome through TLR4, however extensively oxidized LDL, which does not engage this receptor, could not. Potentially this is a mechanism by which mmLDL might be associated with T2D, and other IL-1β-dependent manifestations such as atherosclerosis 48. Recently, a further involvement of LDL in inflammasome activation was also described, where it facilitates cholesterol crystal deposition in the vessel wall 48. While the role of mmLDL in T2D has not been extensively characterized, FFAs are commonly thought of as potentially pathogenic in T2D. Based on the literature, we would expect that FFAs could also prime the inflammasome by interaction with TLRs 49.
Although increasing glucose concentration had no effect on IL-1β activation in our in vitro assays, we found that glucose metabolism is absolutely required for LPS to induce the expression of pro-IL-1β in macrophages. To inhibit glucose metabolism we used 2DG, which has also been found to reduce IL-1β in vivo, as for atherosclerosis- prone, insulin-resistant rats 50. Clearly the in vivo effects of IL-1β with regard to fever and inflammation are highly energy-dependent processes. Thus it would be appropriate for cells to prevent IL-1β production unless there is sufficient glucose that can be metabolized to mediate these biological responses. A small decrease in IL-6 production was observed, however it is likely that the factors controlling IL-1β synthesis that are inhibited by 2DG are also shared in some part by other pro-inflammatory cytokines. Because we have found glucose metabolism to be a limiting step in inflammasome priming, this could be a physiologically relevant mechanism in the pathogenesis of T2D where persistent hyperglycemia might abolish any such limits. Indeed the beta cell loss in high fat fed IAPP transgenic mice is greatly increased when they develop amyloid deposition45, and we now report that this is associated with a significant increase in IL-1β immunostaining. The IL-1β observed in islets of IAPP transgenic mice co-localized with macrophages, and also with amyloid. We could find no clear evidence of beta cells producing IL-1β, and if they do it is likely to be at a low level, or a rare event. While IL-1β staining in macrophages might represent intracellular pro-IL-1β, staining outside these cells that is coincident with amyloid could represent the secreted, processed form, as there are few live cells in these regions. Our analysis is somewhat limited in this respect, as the antibody we used to detect IL-1β would recognize both the processed and pro-IL-1β species.
Our data is unambiguous as to the ability of IAPP to activate the Nlrp3 inflammasome and generate processed IL-1β. This might be a key mechanism for IL-1β production in T2D. It could be that amyloid deposition in the pancreas only happens later in the disease process, and some have argued that this would be as a result, and not as a cause of disease. However it is also possible that even if this buildup is secondary to the initiation of disease, that it nevertheless aids and abets in the progressive loss of beta cell function that is a key component of T2D. Future studies on patients should identify if targeting IL-1 has most benefit only later in the disease progression or for those with more severe disease pathology. It is also becoming apparent that IAPP could contribute to the death of transplanted islets being used as therapy in T1D 18–20. The current Edmonton protocol of immunosuppression used during this transplant procedure could conceivably be improved with anti-inflammatory therapy targeting IL-1β activated by IAPP. To conclude, our findings highlight new roles for well-known entities in the pathogenesis and treatment of diabetes and will hopefully spur new research targeted at the mechanisms of this debilitating, chronic disease.
Materials and Methods
Cell culture
Bone marrow from C57BL/6 mice was differentiated for 10 days in GM-CSF (4% J588 myeloma cell supernatant) or 7 days in M-CSF (20% L929 cell supernatant) in typical media preparations to make bone marrow derived dendritic cells (BMDC) and macrophages (BMDM) respectively Nlrp3 KO mice were from Jurg Tschopp (University of Lausanne) or Millennium Pharmaceuticals. Pancreatic islet cells were obtained from collagenase digested mouse pancreatic islets and cultured overnight 51. The Rin-5F cell line was from ECACC and cultured according to their protocol.
Materials
Unless stated, cells were primed for 3 hrs with 100 ng/ml LPS (Alexis) or 10 µg/ml human plasma LDL oxidized with copper(II) sulphate for different times (Kalen biomedical), then activated with 10 µM human IAPP (Sigma), 10 µM rat IAPP (Bachem), 20 µg/ml MSU (Opsona), 100 µg/ml Alum (Brenntagbiosector) or 0.6 mg/ml 430 nm polystyrene beads (Corpuscular) for 16 hours. The following inhibitors or glucose analogs were added 2 hours after LPS priming, 1 hour before inflammasome activation at the highest concentration indicated, unless stated; 1 µg/ml Caspase-1 inhibitor VI (Calbiochem), 5 µM cytochalasin D, 250 µM bafilomycin A, 10 µM cathepsin B inhibitor (CA-074 Me), 1 µg/ml (2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylic acid (APDC), 50 µM N-acetyl-cysteine (NAC), 50 µM glyburide, 25 mM D-glucose and its analogs (all from Sigma), and 40 µM IAPP receptor inhibitor peptide AC187 (Tocris biosciences). 100 µg/ml Polymyxin B (Sigma) was also added to 1 µg/ml LPS or 100 µg/ml mmLDL overnight at 4° C before use in some experiments with a 1/10 dilution.
Measurement of caspase-1 activity
BMDC were stimulated with IAPP for 1 hour before the addition of FAM-YVAD-fmk according to manufacturer’s instructions (Immunochemistry Technologies). Fluorescent cells were measured by flow cytometry.
Analysis of ASC speck formation
Immortalized BMDM expressing YFP-ASC were generated as described previously8. These cells were primed with LPS then activated with IAPP overnight and imaged using a fluorescent microscope with a 40x objective lens.
Immunoblotting
To blot for IL-1β in supernatants, BMDM grown in 24 well plates in 0.5 ml media containing 1% FCS were stimulated, supernatants collected and precipitated with 1 volume methanol, 1/4 volume chloroform, then the precipitate was washed in 1 volume methanol and finally resuspended in 60 µl SDS loading buffer for running on 15% Tris-glycine polyacrylamide gels. Proteins were transferred to PVDF membranes, blocked in 5% skimmed milk then probed with goatanti-mIL-1β (RnD systems, AF-401) and detected with HRP-donkey anti-goat antibody (Upstate).
Alternatively, cell lysates were prepared by lysis in RIPA buffer, followed by electrophoresis and blotting as above, then probed with goat anti-IL-1α (RnD systems, AF-400), rabbit anti-ASC (Alexis), mouse anti-Txnip (MBL international) or mouse anti-caspase-1 (developed in-house) followed by a suitable detection antibody. Immunoblots were quantified using ImageJ software.
Cytokine measurements
For cytokine measurements, BMDM or BMDC were cultured in 96 well plates, stimulated in triplicate, supernatants were then collected and ELISA performed for mIL-1β, mIL-1α, mIL-6 and mTNF (RnD systems).
Q-PCR
BMDM were cultured in 24 well plates, stimulated in triplicate, RNA prepared using the RNeasy kit (Qiagen) and then Q-PCR performed using Taqman probes specific for IL-1β (Mm01336189_m1), Nlrp3 (Mm00840904_m1) or Txnip (Mm00452393_m1).
Cytotoxicity assay
Cytotoxicity was determined by an LDH assay (Promega) according to manufacturer’s instructions.
Thioflavin T fluorescence and size fractionation
IAPP was dissolved in HFIP (Sigma) which was subsequently removed using nitrogen gas. Aliquots were then resuspended at 500 µM in H20 or PBS for different times. This was diluted to 10 µM in RPMI and thioflavin T added to a concentration of 20 µM after which fluorescence measurements were recorded according to protocol 52 using a FP6200 fluorescence spectrometer (JASCO). To separate fractions based on size, resuspended IAPP was diluted to 20 µM in RPMI and quickly filtered through a 100 kDa cut-off membrance (Amicon). The concentrated fraction greater than 100 kDa was supplemented to the original volume with RPMI and both fractions were diluted 1/2 onto BMDC for a final IAPP concentration of 10 µM.
Immunofluorescent analysis of sections from IAPP transgenic mice
IAPP transgenic mice were fed a high fat diet for one year then the pancreas was sectioned as described 45. Immunofluorescent staining was performed using thioflavin S (Sigma), mouse anti-insulin (Sigma, I2018), rabbit anti-IL-1β (Santa Cruz Biotechnology, sc-7884) and specific fluorescently-conjugated secondary antibodies (Molecular Probes), or Alexa647 conjugated anti-MoMa2 (AdbSerotech). Analysis of images performed using ImageJ software.
Statistical analysis
Data is presented as mean ± SD. Significance was determined by two-tailed unpaired t-test with the exception of insulin staining in pancreatic islets, for which a one-tailed unpaired t-test was used as the direction of the outcome can be anticipated based on similar experiments we have published previously.
Supplementary Material
Acknowledgements
The authors thank Andes Mori for assistance with Nlrp3−/− mice, Prof. Jurg Tschopp for providing Nlrp3−/− mice, and Prof. Eicke Latz for providing YFP-ASC BMDM. S.L.M. was supported by an NHMRC Overseas Biomedical Fellowship (516783). Work performed at Trinity College Dublin was supported by Science Foundation Ireland and at VA Puget Sound Health Care System by the United States Department of Veterans Affairs and National Institutes of Health grant DK-75998 (to S.E.K.). L.F. was supported by a fellowship from the Crohn’s and Colitis Foundation. Work performed at the University of Michigan was supported by National Institutes of Health grant AI063331.
Footnotes
Author contributions
S.L.M. designed and performed experiments, analyzed data and wrote the paper; L.A.J.O. and E.C.L. conceived ideas and oversaw research; A.D., S.L.S., R.L.H., G.M.T., F.A.S., C.B., L.F., E.Y., Z.C., N.M., L.A.M., J.H. and R.C.C., performed experiments; K.H.G.M, K.H.M., P.N., G.N., J.Y., and S.E.K. provided advice and reagents.
The authors declare no competing financial interests.
References
- 1.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 2.Njajou OT, et al. Association between oxidized LDL, obesity and type 2 diabetes in a population-based cohort, the Health, Aging and Body Composition Study. Diabetes Metab Res Rev. 2009;25:733–739. doi: 10.1002/dmrr.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendtzen K, et al. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986;232:1545–1547. doi: 10.1126/science.3086977. [DOI] [PubMed] [Google Scholar]
- 4.Spranger J, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 5.Larsen CM, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 6.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 7.Dostert C, et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320:674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clark A, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 10.Cooper GJ, et al. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westermark P, et al. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler AE, et al. Diabetes due to a progressive defect in beta-cell mass in rats transgenic for human islet amyloid polypeptide (HIP Rat): a new model for type 2 diabetes. Diabetes. 2004;53:1509–1516. doi: 10.2337/diabetes.53.6.1509. [DOI] [PubMed] [Google Scholar]
- 13.Janson J, et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:7283–7288. doi: 10.1073/pnas.93.14.7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verchere CB, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1996;93:3492–3496. doi: 10.1073/pnas.93.8.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard CF., Jr Longitudinal studies on the development of diabetes in individual Macaca nigra. Diabetologia. 1986;29:301–306. doi: 10.1007/BF00452067. [DOI] [PubMed] [Google Scholar]
- 16.Seino S. S20G mutation of the amylin gene is associated with Type II diabetes in Japanese. Study Group of Comprehensive Analysis of Genetic Factors in Diabetes Mellitus. Diabetologia. 2001;44:906–909. doi: 10.1007/s001250100531. [DOI] [PubMed] [Google Scholar]
- 17.Ma Z, et al. Enhanced in vitro production of amyloid-like fibrils from mutant (S20G) islet amyloid polypeptide. Amyloid. 2001;8:242–249. doi: 10.3109/13506120108993820. [DOI] [PubMed] [Google Scholar]
- 18.Udayasankar J, et al. Amyloid formation results in recurrence of hyperglycaemia following transplantation of human IAPP transgenic mouse islets. Diabetologia. 2009;52:145–153. doi: 10.1007/s00125-008-1185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westermark P, Eizirik DL, Pipeleers DG, Hellerstrom C, Andersson A. Rapid deposition of amyloid in human islets transplanted into nude mice. Diabetologia. 1995;38:543–549. doi: 10.1007/BF00400722. [DOI] [PubMed] [Google Scholar]
- 20.Westermark GT, Westermark P, Berne C, Korsgren O. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359:977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 21.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 22.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 23.Zraika S, et al. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zraika S, et al. Toxic oligomers and islet beta cell death: guilty by association or convicted by circumstantial evidence? Diabetologia. 53:1046–1056. doi: 10.1007/s00125-010-1671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Badman MK, Pryce RA, Charge SB, Morris JF, Clark A. Fibrillar islet amyloid polypeptide (amylin) is internalised by macrophages but resists proteolytic degradation. Cell Tissue Res. 1998;291:285–294. doi: 10.1007/s004410050998. [DOI] [PubMed] [Google Scholar]
- 26.de Koning EJ, et al. Macrophages and pancreatic islet amyloidosis. Amyloid. 1998;5:247–254. doi: 10.3109/13506129809007297. [DOI] [PubMed] [Google Scholar]
- 27.Gitter BD, Cox LM, Carlson CD, May PC. Human amylin stimulates inflammatory cytokine secretion from human glioma cells. Neuroimmunomodulation. 2000;7:147–152. doi: 10.1159/000026432. [DOI] [PubMed] [Google Scholar]
- 28.Yates SL, et al. Amyloid beta and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J Neurochem. 2000;74:1017–1025. doi: 10.1046/j.1471-4159.2000.0741017.x. [DOI] [PubMed] [Google Scholar]
- 29.Lamkanfi M, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharp FA, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:870–875. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 32.Hay DL, Christopoulos G, Christopoulos A, Poyner DR, Sexton PM. Pharmacological discrimination of calcitonin receptor: receptor activity-modifying protein complexes. Mol Pharmacol. 2005;67:1655–1665. doi: 10.1124/mol.104.008615. [DOI] [PubMed] [Google Scholar]
- 33.Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamon Y, et al. Interleukin-1beta secretion is impaired by inhibitors of the Atp binding cassette transporter, ABC1. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- 35.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 36.Boni-Schnetzler M, et al. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93:4065–4074. doi: 10.1210/jc.2008-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maedler K, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–860. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welsh N, et al. Is there a role for locally produced interleukin-1 in the deleterious effects of high glucose or the type 2 diabetes milieu to human pancreatic islets? Diabetes. 2005;54:3238–3244. doi: 10.2337/diabetes.54.11.3238. [DOI] [PubMed] [Google Scholar]
- 39.Miller YI, et al. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J Biol Chem. 2003;278:1561–1568. doi: 10.1074/jbc.M209634200. [DOI] [PubMed] [Google Scholar]
- 40.Apolinario E, et al. Minimally modified (electronegative) LDL- and Anti-LDL-autoantibodies in diabetes mellitus and impaired glucose tolerance. Int J Atheroscler. 2006;1:42–47. [Google Scholar]
- 41.Yano M, et al. Increased electronegative charge of serum low-density lipoprotein in patients with diabetes mellitus. Clin Chim Acta. 2004;340:93–98. doi: 10.1016/j.cccn.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 42.Abderrahmani A, et al. Human high-density lipoprotein particles prevent activation of the JNK pathway induced by human oxidised low-density lipoprotein particles in pancreatic beta cells. Diabetologia. 2007;50:1304–1314. doi: 10.1007/s00125-007-0642-z. [DOI] [PubMed] [Google Scholar]
- 43.Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J. 2006;47:225–233. doi: 10.1093/ilar.47.3.225. [DOI] [PubMed] [Google Scholar]
- 45.Hull RL, et al. Increased dietary fat promotes islet amyloid formation and beta-cell secretory dysfunction in a transgenic mouse model of islet amyloid. Diabetes. 2003;52:372–379. doi: 10.2337/diabetes.52.2.372. [DOI] [PubMed] [Google Scholar]
- 46.Butler AE, Janson J, Soeller WC, Butler PC. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes. 2003;52:2304–2314. doi: 10.2337/diabetes.52.9.2304. [DOI] [PubMed] [Google Scholar]
- 47.van de Veerdonk FL, et al. Reactive oxygen species-independent activation of the IL-1{beta} inflammasome in cells from patients with chronic granulomatous disease. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.0914795107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell JC, Proctor SD. Increased insulin sensitivity and reduced micro and macro vascular disease induced by 2-deoxy-D-glucose during metabolic syndrome in obese JCR: LA-cp rats. Br J Pharmacol. 2007;151:216–225. doi: 10.1038/sj.bjp.0707226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Michalska M, Wolf G, Walther R, Newsholme P. The effects of pharmacologic inhibition of NADPH oxidase or iNOS on pro-inflammatory cytokine, palmitic acid or H2O2 -induced mouse islet or clonal pancreatic beta cell dysfunction. Biosci Rep. 2010 doi: 10.1042/BSR20090138. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson MR. Techniques to study amyloid fibril formation in vitro. Methods. 2004;34:151–160. doi: 10.1016/j.ymeth.2004.03.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.