Abstract
Introduction:
Studies suggest that in smokers attempting to quit smoking, the occurrence of stressful events is associated with smoking relapse. The purpose of this study was to determine the effect of bupropion (an agent known to increase smoking cessation rates) on the craving, withdrawal, and mood response to stressful tasks administered in a laboratory setting.
Methods:
Response to three tasks (a speech, math, and cold pressor task) was measured in 65 smokers during ad libitum smoking. Smokers were then randomized to either bupropion or placebo. Fourteen days after starting medication, 43 subjects (28 receiving bupropion and 15 receiving placebo) quit smoking and laboratory procedures were repeated on the third day of abstinence.
Results:
Prior to cessation, stressors presented in a laboratory setting increased craving, nicotine withdrawal symptoms, and subjective distress but decreased positive affect. Thirty minutes of relaxation after the stressors did not result in these measures returning to prestress levels. During the nicotine withdrawal period, stress-induced responses were generally smaller than during the precessation period. Bupropion (relative to placebo) reduced overall levels of craving and withdrawal symptoms but did not have significant effects on response to stress during the nicotine withdrawal period.
Conclusions:
This study demonstrates that stress results in sustained increases in craving and withdrawal symptoms and changes in mood symptoms and that bupropion affects overall levels of these symptoms. Further research is needed to determine if modifying response to stress is predictive of an effective treatment for facilitating smoking cessation.
Introduction
Exposure to stressful situations is a commonly cited smoking trigger with approximately 40%–50% of smokers indicating that during a smoking cessation attempt, stress contributed to a smoking lapse (Borland, 1990; Cummings, Jaen, & Giovino, 1985; Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996; Swan et al., 1988). Consistent with this, laboratory studies have found that stress increases craving and withdrawal symptoms and affects measures of smoking topography such as the number of cigarettes smoked and inhalation length per puff (al’Absi, Wittmers, Erickson, Hatsukami, & Crouse, 2003; Cherek, 1985; Niaura, Shadel, Britt, & Abrams, 2002; Perkins & Grobe, 1992; Pomerleau & Pomerleau, 1987; Rose, Ananda, & Jarvik, 1983).
Medications known to increase smoking cessation rates (e.g., nicotine replacement therapy, bupropion, varenicline) have been shown to decrease craving and withdrawal symptoms during a smoking cessation attempt (Mooney & Sofuoglu, 2006; Shiffman, Ferguson, Gwaltney, Balabanis, & Shadel, 2006; West, Baker, Cappelleri, & Bushmakin, 2008), but there is little data regarding their effect on stress-induced changes in craving and withdrawal symptoms. Since stress is consistently cited as an important precipitant to smoking, it is important to determine if effective medications increase cessation rates (at least in part) by altering how smokers respond to stress. Determining mechanisms by which current medications are effective can lead to better methods to identify and test promising new smoking cessation therapies. The objective of this study is to determine how bupropion affects stress-induced changes in craving, withdrawal, and mood symptoms.
Methods
Study Design
Tobacco craving, withdrawal symptoms, and mood were assessed at each of two laboratory sessions at five timepoints (i.e., prior to an initial 30-min relaxation period, immediately following a speech task, a mental arithmetic task, a cold pressor, and following a final 30-min relaxation period). Thirty minutes separated the three tasks. Prior to the first session, smokers could smoke ad libitum (however, they could not smoke after arrival at the laboratory session). Subjects were then randomized to receive either bupropion, bupropion with behavioral counseling, or matching placebo. Subjects quit smoking after 14 days of medications, and the second laboratory session occurred on the morning of the third day of cessation. Only subjects who abstained from smoking for the required time period (as verified by exhaled CO concentrations <10 ppm) completed the second laboratory session. Bupropion was dosed at 150 mg once daily for 3 days followed by 150 mg twice daily. Subjects were blinded regarding drug assignment, and investigators were blinded regarding drug assignment in those not receiving counseling.
Eligible subjects for the study were between the ages of 18 and 65 years, smoked (on average) ≥ 15 cigarettes/day, were in generally good health, had no contraindications to bupropion, and were not taking any medications likely to interact with bupropion or to interfere with measures being assessed in the study. Subjects agreed to abstain from smoking for 3 days prior to the second laboratory session but were not necessarily intending to quit permanently. The study was approved by the University of Minnesota Institutional Review Board, and written informed consent was obtained from all subjects. Additional details of the study design and results from physiological measures assessed have been presented previously (Kotlyar et al., 2006).
Outcome Measures
Craving, withdrawal, and mood symptoms scores were calculated from the Subjective State Scale in which subjects rated 24 items on a scale from 0 to 7 (al’Absi, Hatsukami, Davis, & Wittmers, 2004). Consistent with previous studies (al’Absi et al., 2003), Cronbach’s alpha scores (averaged for the three stress tasks) in the current study for positive affect and distress were .86 and .83 respectively. Urge to smoke was also measured using the 32-item Questionnaire on Smoking Urges (QSU) prior to the first rest period and at the end of the last rest period. A score for Factor 1 (reflecting primarily an intention and desire to smoke and anticipation of pleasure from smoking) and Factor 2 (reflecting anticipation of relieve from negative affect) was calculated (Tiffany & Drobes, 1991).
Analysis
Data were analyzed using a mixed effects approach (SAS v. 9.1, PROC MIXED) examining overall change in response to the stress task as well as differences by session and treatment group and the interactions among group, session and response to stressor. The model was fitted with a random interaction term (subject level) and treatment group, session, and time as well as interaction terms as fixed effects. No significant differences in any measure were observed between the two bupropion groups (i.e., with or without counseling); therefore, they were collapsed for analysis purposes.
Results
Sixty-five subjects completed the first laboratory session, and 43 subjects completed both laboratory sessions (28 subjects in the bupropion groups and 15 in the placebo group). Baseline characteristics (mean ± SD) of those completing the first laboratory session included an average age of 39.8 ± 12.0, women comprised 48% of the subjects, the average number of cigarettes smoked per day was 20.5 ± 5.2, and average score on the Fagerström Test for Nicotine Dependence was 5.0 ± 1.9. No significant differences in these measures were found between those who completed both laboratory sessions and those who only completed the first laboratory session.
Effect of Stressors on Subjective Measures
In those who completed the first laboratory session, significant time effects were observed for all four measures (p values < .001). Compared with the measures following the initial baseline period, mean scores were significantly higher (all p values < .001) after each of the three stress tasks for craving (1.89 at baseline, 2.53 after speech, 2.78 after math, and 3.42 after cold), withdrawal (5.35 at baseline, 8.92 after speech, 9.71 after math, and 10.12 after cold), and distress (3.66 at baseline, 8.54 after speech, 6.83 after math, and 7.64 after cold). Scores for positive affect were significantly lower (17.74 at baseline, 15.31 after speech, 14.35 after math, and 14.12 after cold) compared with baseline. After the last relaxation period, scores for all measures continued to be significantly higher (all p values < .001) than following the initial baseline period (scores of 3.34, 9.06, and 6.42 for craving, withdrawal, and distress, respectively) and lower for positive affect (15.15), although withdrawal and distress scores did decrease significantly during the final 30-min relaxation period from the levels observed after the cold pressor task; t(64) = −2.06, p = .044 and t(64) = 2.8, p < .006. Urge to smoke as measured by the QSU found consistent results. Both Factor 1 and Factor 2 scores were significantly higher following the last relaxation period than prior to the first relaxation period; t(54) = 7.9 and 6.8, respectively, both p < .001). Factor 1 score (mean ± SD) increased from 4.5 ± 1.1 to 5.6 ± 1.1 and Factor 2 score increased from 3.0 ± 1.2 to 3.8 ± 1.5.
Comparing the first laboratory session results of the 43 subjects who completed both laboratory sessions with the 22 subjects who completed only the first session found no significant Group × Time Effect differences for any of the measures assessed and found a significant group effect only for distress that was higher overall in those who did not complete the entire study (mean = 7.0) than in those who did; mean = 5.0, F(1, 63) = 3.9, p < .05.
Effect of Bupropion on Stress-Induced Changes in Subjective Measures
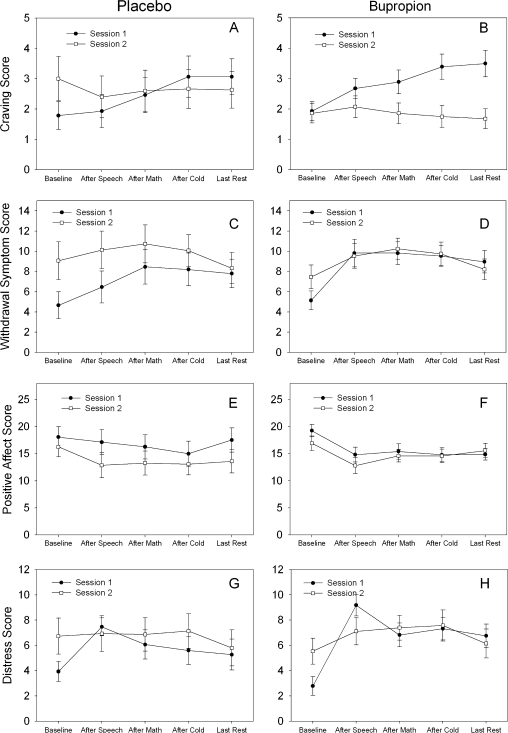
Measures of craving, withdrawal, positive affect, and distress at each laboratory session are illustrated in Figure 1 for those receiving placebo (panels A, C, E, and G) or bupropion (panels B, D, F, and H). For both craving and withdrawal scores, a significant Session × Group interaction was observed; F(1, 41) = 19.2, p < .001 and F(1, 41) = 7.7, p < .01; however, the pattern of response was different in these two measures. In those receiving bupropion, craving score during the second laboratory session was significantly lower than during the first laboratory session; t(41) = 6.1, p < .001, whereas withdrawal symptoms were not significantly different between the two laboratory sessions; t(41) = 0.84, p < .41. In those receiving placebo, however, craving was not significantly different between the two laboratory sessions; t(41) = 0.98, p < .33, whereas withdrawal symptoms were significantly higher during the second laboratory session; t(41) = 4.00, p < .001.
Figure 1.
Craving, withdrawal symptom, positive affect, and distress scores (means ± SE) in subjects receiving placebo (panels A, C, E, and G) or bupropion (panels B, D, F, and H) during laboratory sessions conducted prior to and 3 days after subjects quit smoking.
A significant Session × Time interaction; F(4, 163) = 5.1, p < .001; was observed for craving but not withdrawal symptoms scores. Craving increased during the first laboratory session, whereas little increase was observed during the second laboratory session. Withdrawal symptoms increased in response to stressors during both laboratory sessions, although there was a trend toward a smaller increase in the second laboratory session compared with the first (maximum increase in Laboratory 1 = 4.2 and Laboratory 2 = 2.2; F(4, 164) = 2.1, p < .09).
Urge to smoke as measured by the QSU found similar results. For both Factor 1 and Factor 2 scores, a significant Session × Group interaction; F(1, 37) = 7.9, p < .008 and F(1, 37) = 6.5, p < .02; and Session × Time interaction; F(1, 31) = 27.8, p < .001 and F(1, 31) = 18.4, p < .001; was found. In those receiving bupropion (but not in those receiving placebo), urge scores were lower in the second laboratory session than in the first. As with the single-item craving score, an increase in smoking urges during the laboratory session was observed at the first laboratory session; t(31) = 3.0, p < .006; but not at the second; t(31) = 1.2, p < .26.
Although both groups had lower positive affect scores during the second laboratory session, the decline for the bupropion group was smaller (15.8–14.9; t(41) = 2.32, p = .02) than that observed for the placebo group (16.8–13.8; t(41) = 5.47, p = .0001). The magnitude of the decline was significantly different as indicated by a significant Session × Group effect; F(4, 41) = 9.2, p < .005. For distress, we observed a significant Session × Time Effect; F(4, 164) = 4.1, p < .004. Whereas a large increase in distress was observed after the first stressor in the first laboratory session; t(164) = 6.7, p < .001, this was not observed during the second laboratory session; t(164) = 1.2, p < .23; regardless of treatment assignment.
For all four measures, baseline values at the second laboratory session were significantly different from baseline values at the first session (i.e., higher craving, withdrawal, and distress scores but lower positive affect scores at the second laboratory session; all p values < .05). No significant Session × Treatment × Time Effect was observed for any of the four measures assessed.
Discussion
This study found that a speech, math, and cold pressor stressor when presented to ad libitum smokers in a laboratory setting increases craving, withdrawal symptoms, and subjective distress while decreasing positive affect. Thirty minutes of relaxation after the stressors did not completely reverse these effects. During the nicotine withdrawal period, bupropion (relative to placebo) reduced overall levels of craving and withdrawal symptoms. Bupropion did not significantly alter stress-induced changes during the nicotine withdrawal period; however, this may be due to the smaller stress-induced responses observed during the second laboratory session compared with the first. It should be noted that data regarding the effect of bupropion are available only from subjects who completed both laboratory sessions. Those who completed both sessions were similar in most measures to those who dropped out after the first laboratory session; however, differences in overall levels of distress were found, and it is therefore possible that those who dropped out would have had a different pattern of medication response.
Our results demonstrating that stress in a laboratory session increases craving, withdrawal, and mood symptoms in smokers are consistent with previously published literature (al’Absi, Amunrud, & Wittmers, 2002; al’Absi, Hatsukami, & Davis, 2005; al’Absi et al., 2003). Previous studies have similarly found that subjective measures remain elevated for prolonged periods of time after completion of a stress task (al’Absi et al., 2003; Childs & de Wit, 2009). If situational stressors outside the laboratory setting also result in a prolonged increase in craving, withdrawal, and mood symptoms, it may be difficult for smokers to resist smoking for such an extended time period. Since a control group that was not exposed to stress was not utilized, it is possible that the changes observed were due to the increasing time elapsed from when the subjects last smoked (rather than exposure to a stressor). This seems unlikely, however, since withdrawal symptoms and distress decreased during the final relaxation period despite the passage of additional time.
Our study found that approximately 60 hr after smoking cessation, stress results in an attenuated change in craving, withdrawal, and mood symptoms compared with the effects of stressors presented during ad libitum smoking. One possible explanation for this finding is that habituation to the stressor occurs such that an attenuated response is observed at the second session. Other possibilities are that the nicotine withdrawal period is associated with a blunted stress response or that during the nicotine withdrawal period, a ceiling effect was reached such that stressors did not result in further increases in symptoms. The effects of repeated stressor administration on craving, withdrawal, and mood response in smokers have not been extensively studied, although one study found that the response is largely maintained over two sessions (al’Absi et al., 2002). Previous studies evaluating subjective response to stressors during the nicotine withdrawal period have demonstrated that a response does occur, although those studies were conducted after shorter abstinence periods (i.e., 18–24 hr) than in the current study (al’Absi et al., 2002, 2003, 2005). It is therefore unknown if greater habituation occurred in subjective response in the current study than previously reported or if nicotine withdrawal is a dynamic process with the magnitude of response to a stressor differing depending on when in the withdrawal process the stress tasks are presented.
Previously reported physiological response data from the current study demonstrated significant increases in blood pressure, heart rate, and plasma epinephrine concentrations during both laboratory sessions and in those receiving bupropion a similar magnitude of response at both laboratory sessions (Kotlyar et al., 2006). This is consistent with other studies finding relative stability in cardiovascular response over two laboratory stress sessions (as opposed to the greater habituation seen in measures of hypothalamic–pituitary–adrenal response; Al’Absi et al., 1997; Grissom & Bhatnagar, 2009; Hamer, Gibson, Vuononvirta, Williams, & Steptoe, 2006; Schommer, Hellhammer, & Kirschbaum, 2003). Therefore, it appears that, in the current study, either habituation affected cardiovascular response differently than subjective response or that nicotine withdrawal and bupropion administration have differential effects on cardiovascular compared with subjective responses to stress. The possibility that a ceiling effect contributed to the observed attenuation in stress response during the nicotine withdrawal period is supported by the observed differences in the baseline values in the measures of interest. Future studies assessing reasons for the attenuated response during the second laboratory session are needed in order to more fully interpret the current results regarding the effects of bupropion. The overall decrease in craving and withdrawal symptoms observed in those taking bupropion (relative to those taking placebo) is consistent with other research demonstrating that bupropion decreases craving and withdrawal symptoms during a smoking cessation attempt (Mooney & Sofuoglu, 2006).
In summary, this study demonstrated that during ad libitum smoking, stress tasks presented in a laboratory setting result in at least 30 min of increased craving, withdrawal symptoms, and subjective distress as well as decreased positive affect. These changes in subjective measures are attenuated or absent approximately 60 hr after smoking cessation. Bupropion decreased overall levels of craving and withdrawal and increased positive affect (relative to placebo).
Funding
Supported by University of Minnesota Academic Health Center Faculty Research Development Grant #01-01, Grant #M01-RR00400 from the General Clinical Research Centers program of the National Center for Research Resources, and Grant #K23DA017307 (PI: MK) and Grants R03DA013435, R21CA088272, and R01 DA016351 to Dr. MaA from the National Institutes of Health.
Declaration of Interests
DEA has received honoraria from AstraZeneca, Forest Labs, Pfizer, and Merck. DKH has received research funding from Nabi Biopharmaceuticals
References
- al’Absi M, Amunrud T, Wittmers LE. Psychophysiological effects of nicotine abstinence and behavioral challenges in habitual smokers. Pharmacology, Biochemistry, and Behavior. 2002;72:707–716. doi: 10.1016/s0091-3057(02)00739-6. doi:10.1016/S0091-3057(02)00739-6. [DOI] [PubMed] [Google Scholar]
- Al’Absi M, Bongard S, Buchanan T, Pincomb GA, Licinio J, Lovallo WR. Cardiovascular and neuroendocrine adjustment to public speaking and mental arithmetic stressors. Psychophysiology. 1997;34:266–275. doi: 10.1111/j.1469-8986.1997.tb02397.x. doi:10.1111/j.1469-8986.1997.tb02397.x. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology. 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. doi:10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug and Alcohol Dependence. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. doi:10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacology, Biochemistry, and Behavior. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. doi:10.1016/S0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Borland R. Slip-ups and relapse in attempts to quit smoking. Addictive Behaviors. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. doi:10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- Cherek DR. Effects of acute exposure to increased levels of background industrial noise on cigarette smoking behavior. International Archives of Occupational and Environmental Health. 1985;56:23–30. doi: 10.1007/BF00380697. doi:10.1007/BF00380697. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology. 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. doi:10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KM, Jaen CR, Giovino G. Circumstances surrounding relapse in a group of recent exsmokers. Preventive Medicine. 1985;14:195–202. doi: 10.1016/0091-7435(85)90035-0. doi:10.1016/0091-7435(85)90035-0. [DOI] [PubMed] [Google Scholar]
- Grissom N, Bhatnagar S. Habituation to repeated stress: Get used to it. Neurobiology of Learning and Memory. 2009;92:215–224. doi: 10.1016/j.nlm.2008.07.001. doi:10.1016/j.nlm.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Gibson EL, Vuononvirta R, Williams E, Steptoe A. Inflammatory and hemostatic responses to repeated mental stress: Individual stability and habituation over time. Brain, Behavior, and Immunity. 2006;20:456–459. doi: 10.1016/j.bbi.2006.01.001. doi:10.1016/j.bbi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Kotlyar M, Brauer LH, al’absi M, Adson DE, Robiner W, Thuras P. Effect of bupropion on physiological measures of stress in smokers during nicotine withdrawal. Pharmacology, Biochemistry, and Behavior. 2006;83:370–379. doi: 10.1016/j.pbb.2006.02.017. doi:10.1016/j.pbb.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Sofuoglu M. Bupropion for the treatment of nicotine withdrawal and craving. Expert Review of Neurotherapeutics. 2006;6:965–981. doi: 10.1586/14737175.6.7.965. doi:10.1586/14737175.6.7.965. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Britt DM, Abrams DB. Response to social stress, urge to smoke, and smoking cessation. Addictive Behaviors. 2002;27:241–250. doi: 10.1016/s0306-4603(00)00180-5. doi:10.1016/S0306-4603(00)00180-5. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE. Increased desire to smoke during acute stress. British Journal of Addiction. 1992;87:1037–1040. doi: 10.1111/j.1360-0443.1992.tb03121.x. doi:10.1111/j.1360-0443.1992.tb03121.x. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF. The effects of a psychological stressor on cigarette smoking and subsequent behavioral and physiological responses. Psychophysiology. 1987;24:278–285. doi: 10.1111/j.1469-8986.1987.tb00295.x. doi:10.1111/j.1469-8986.1987.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME. Cigarette smoking during anxiety-provoking and monotonous tasks. Addictive Behaviors. 1983;8:353–359. doi: 10.1016/0306-4603(83)90035-7. doi:10.1016/0306-4603(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosomatic Medicine. 2003;65:450–460. doi: 10.1097/01.psy.0000035721.12441.17. doi:10.1097/01.PSY.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Ferguson SG, Gwaltney CJ, Balabanis MH, Shadel WG. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology. 2006;184:637–644. doi: 10.1007/s00213-005-0184-3. doi:10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. doi:10.1037/0022-006X.64.2.366. [DOI] [PubMed] [Google Scholar]
- Swan GE, Denk CE, Parker SD, Carmelli D, Furze CT, Rosenman RH. Risk factors for late relapse in male and female ex-smokers. Addictive Behaviors. 1988;13:253–266. doi: 10.1016/0306-4603(88)90052-4. doi:10.1016/0306-4603(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. doi:10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion sr on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. 2008;197:371–377. doi: 10.1007/s00213-007-1041-3. doi:10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]