Abstract
The burst of reactive oxygen species (ROS) during reperfusion of ischemic tissues can trigger the opening of the mitochondrial permeability transition (MPT) pore, resulting in mitochondrial depolarization, decreased ATP synthesis, and increased ROS production. Rapid recovery of ATP upon reperfusion is essential for survival of tubular cells, and inhibition of oxidative damage can limit inflammation. SS-31 is a mitochondria-targeted tetrapeptide that can scavenge mitochondrial ROS and inhibit MPT, suggesting that it may protect against ischemic renal injury. Here, in a rat model of ischemia-reperfusion (IR) injury, treatment with SS-31 protected mitochondrial structure and respiration during early reperfusion, accelerated recovery of ATP, reduced apoptosis and necrosis of tubular cells, and abrogated tubular dysfunction. In addition, SS-31 reduced medullary vascular congestion, decreased IR-mediated oxidative stress and the inflammatory response, and accelerated the proliferation of surviving tubular cells as early as 1 day after reperfusion. In summary, these results support MPT as an upstream target for pharmacologic intervention in IR injury and support early protection of mitochondrial function as a therapeutic maneuver to prevent tubular apoptosis and necrosis, reduce oxidative stress, and reduce inflammation. SS-31 holds promise for the prevention and treatment of acute kidney injury.
Acute kidney injury (AKI) develops in 5% of hospitalized patients and is associated with significant morbidity. Ischemia is the most common cause of AKI. Despite our current knowledge of the pathophysiology underlying renal ischemia-reperfusion (IR) injury, pharmacologic interventions have not reduced the mortality and morbidity associated with AKI.
Rapid recovery of ATP after ischemia is essential for cell survival after IR injury. A profound reduction in intracellular ATP occurs early after onset of ischemia and leads to cytoskeletal derangements, membrane alterations, and cell death by apoptosis and necrosis.1 Disruption of the cytoskeleton leads to redistribution of integrins and Na+,K+-ATPase from the basal membrane, resulting in detachment of viable cells from the basement membrane and impairment of Na+ reabsorption. The mode of cell death depends on the duration of ischemia and the region of the nephron. Cell death is usually restricted to the outer medullary region where oxygen tension drops precipitously at the corticomedullary junction.2 The proximal tubules are particularly susceptible to IR injury because they have minimal glycolytic capacity and must rely on mitochondrial metabolism for ATP synthesis.3–5
Ischemia causes damage to all components of the mitochondrial electron transport chain (ETC), resulting in decreased oxidative phosphorylation upon reperfusion.6 In addition, mitochondria are the primary source of reactive oxygen species (ROS).6,7 Mitochondria can undergo further damage upon reperfusion because of mitochondrial permeability transition (MPT). During ischemia, elevated mitochondrial Ca2+, increased ROS, and high inorganic phosphate (Pi) prime the opening of the MPT pore.8 This MPT pore is composed of cyclophilin D (CypD), voltage-dependent anion channels, and adenine nucleotide translocase. Opening of the MPT pore is triggered by a burst of mitochondrial ROS upon reperfusion and leads to mitochondrial depolarization, uncoupling of the respiratory chain, matrix swelling, outer membrane rupture, and release of cytochrome c into the cytosol. Sustained opening of the MPT pore would result in the failure of mitochondria to generate ATP after reperfusion and apoptosis.
MPT as a target for pharmacologic intervention in IR injury is supported by preclinical and clinical studies.9,10 CypD gene ablation protected mice from cardiac and renal IR injury.11–14 Treatment with cyclosporin A (CsA), a CypD inhibitor, reduced renal IR injury in mice and rats.15,16 CsA was recently reported to reduce infarct size in patients undergoing percutaneous coronary intervention after acute myocardial infarction.17 However, the nephrotoxic profile of CsA makes clinical application of this drug less practical for renal IR injury.18–20
We recently developed a series of mitochondria-targeting tetrapeptides (Szeto-Schiller peptides) that scavenge ROS and inhibit MPT.21 SS-31 (d-Arg-dimethylTyr-Lys-Phe-NH2) specifically targets the inner mitochondrial membrane and inhibits MPT induced by Ca2+ and Pi. The dimethyltyrosine residue provides added antioxidant properties. This small peptide with dual function has been reported to significantly increase cardiac ATP content, decrease oxidative stress, and reduce myocardial infarct size in rats.22,23 In this study, we examined the therapeutic potential of SS-31 to prevent AKI in rats caused by warm IR injury.
RESULTS
Effects of SS-31 on AKI Caused by IR
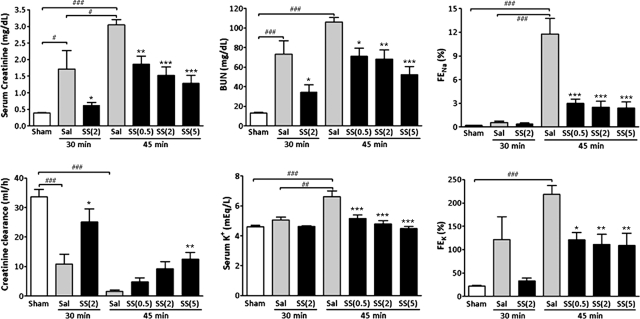
SS-31 or saline was administered subcutaneously to rats 30 minutes before bilateral occlusion of renal blood flow for 30 or 45 minutes. Treatment was repeated at the onset of reperfusion and 2 hours later. Renal function was determined at 24 hours, the time of maximal dysfunction in rodents.24–26 This model resulted in significant renal dysfunction as measured by several biomarkers (ANOVA P < 0.0001) (Figure 1). The extent of injury was directly correlated with the duration of ischemia. Thirty-minute ischemia did not alter serum K+ or fractional excretion of Na+ (FENa) and K+ (FEK). With 30-minute ischemia, SS-31 (2 mg/kg) significantly improved all biomarkers when compared with the saline group, and SS-31 rats were not different from Sham rats. With 45-minute ischemia, SS-31 dose-dependently improved all biomarkers compared with saline, and serum K+ and FENa were not statistically different from Sham at all dose levels. Serum creatinine was normalized by 5 mg/kg SS-31.
Figure 1.
SS-31 reduced AKI 24 hours after IR injury. Rats were subcutaneously treated with saline or SS-31 (0.5, 2, or 5 mg/kg) 30 minutes before bilateral occlusion of renal blood flow for 30 or 45 minutes. Treatment was repeated just before onset of reperfusion and at 2 hours after reperfusion. Significant differences were found in all biomarkers among the treatment groups (ANOVA, P < 0.0001). Data are presented as mean ± SEM; n = 6 to 14 in each group. #P < 0.05 and ###P < 0.001 compared with Sham-operated control. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with corresponding saline group.
Effects of SS-31 on Tubular Injury after IR
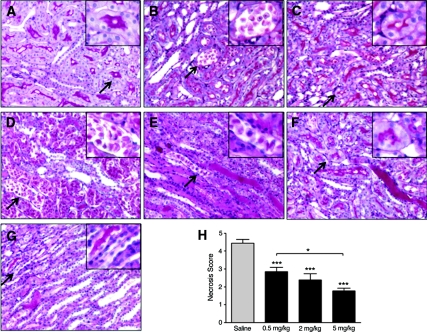
Kidney sections obtained after 24-hour reperfusion from Sham, saline IR, and SS-31 IR rats were stained with periodic acid–Schiff (PAS) and examined by light microscopy (Figure 2). Renal architecture was normal in the Sham group, with brush borders evident in the proximal tubules in the outer stripe of the outer medulla (OSOM) (Figure 2A). Focal areas of necrosis were observed in the OSOM in the saline IR group 24 hours after 30-minute ischemia (Figure 2B). In contrast, treatment with SS-31 reduced focal necrosis and preserved the brush border of the tubular cell (Figure 2C).
Figure 2.
SS-31 reduced tubular injury after ischemia. Rats were subcutaneously treated with saline or SS-31 (0.5, 2 or 5 mg/kg) 30 minutes before bilateral occlusion of renal blood flow for 30 or 45 minutes. Treatment was repeated just before onset of reperfusion and at 2 hours after reperfusion. Kidney sections were stained with PAS. Representative sections from the outer medulla are shown in panels A through G, Magnification: ×200. Inset shows higher magnification of area indicated by the arrow. (A) Kidneys from Sham-operated rats showed normal architecture in the OSOM with prominent brush borders in the proximal tubules (see inset). (B) Focal necrosis was found in the OSOM of saline-treated animals after 30-minute ischemia (inset shows cell sloughing). (C) Necrosis was rare in the SS-31-treated animals after 30-minute ischemia, and brush borders were preserved in many proximal tubules (see inset). (D) Extensive tubular necrosis was found in the OSOM of saline-treated animals after 45-minute ischemia. (E) Architecture of the ISOM was greatly distorted in saline-treated animals after 45 minutes ischemia, and tubules were filled with hyaline casts and sloughed cells. (F) Only focal necrotic areas were observed in the OSOM of SS-31-treated animals after 45-minute ischemia. (G) Architecture of the ISOM was greatly protected by SS-31, with few sloughed cells and occasional casts after 45-minute ischemia. (H) Cell necrosis score after 45-minute ischemia showing dose-dependent protection provided by SS-31 treatment. n = 12 to 14 in each group. ANOVA revealed significant differences among the four groups (P < 0.0001). ***P < 0.001 compared with saline; *P < 0.05 between groups as indicated.
Prolonging ischemia time to 45 minutes resulted in extensive tubular necrosis and cell sloughing in the OSOM in the saline IR group (Figure 2D). Necrotic cell debris and hyaline casts filled many tubules in the inner stripe of the outer medulla (ISOM) (Figure 2E). In contrast, cellular architecture was remarkably well preserved in all regions of the kidney in the SS-31 IR group (Figure 2, F and G). SS-31 significantly reduced tubular necrosis in the OSOM, and the brush border was preserved in the proximal tubules (Figure 2F). Tubular architecture was preserved in the ISOM with few detached or swollen cells (Figure 2G). The cell necrosis score was significantly reduced by all three doses of SS-31, and 5 mg/kg was significantly better than 0.5 mg/kg (Figure 2H).
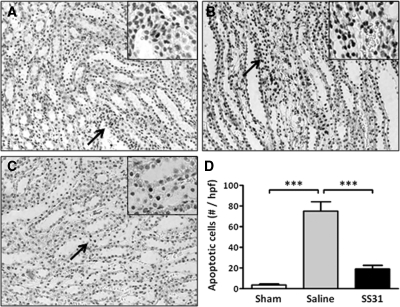
A significant increase in apoptosis was observed in the outer medulla with shorter ischemia time (30 minutes). Apoptotic cells were infrequent in the Sham group (Figure 3A), but they were greatly increased in the saline IR group (Figure 3B) and dramatically reduced in the SS-31 IR group (Figure 3C). The number of apoptotic cells was significantly reduced by SS-31 treatment (Figure 3D).
Figure 3.
SS-31 reduced tubular apoptosis after 30-minute ischemia. Rats were subcutaneously treated with saline or SS-31 (2 mg/kg) 30 minutes before bilateral occlusion of renal blood flow for 30 minutes. Treatment was repeated just before onset of reperfusion and at 2 hours after reperfusion. Apoptotic cells were determined after 24-hour reperfusion by TUNEL stain (brown nuclei). Representative sections from the outer medulla are shown for (A) Sham, (B) saline-treated IR, and (C) SS-31-treated IR rats (magnification: ×200). Inset shows higher magnification of the area indicated by the arrow. (D) Total number of TUNEL-positive cells per high-power field. ***P < 0.001.
Effects of SS-31 on Mitochondrial Structure and Function
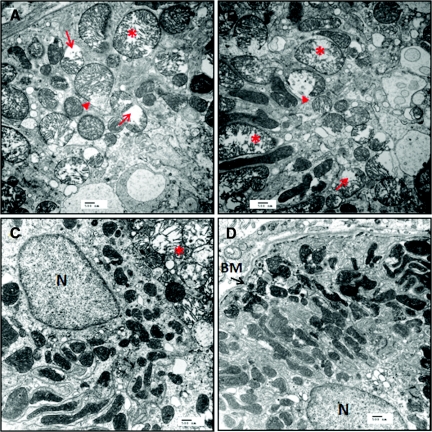
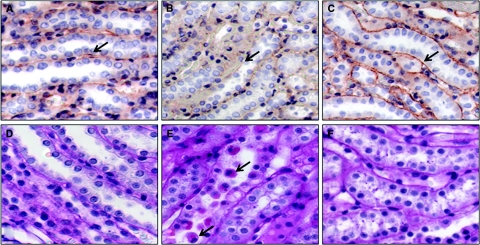
Electron microscopy revealed extensive damage to mitochondria in proximal tubular cells after 45-minute ischemia. Figure 4, a and b, shows round, swollen mitochondria with loss of cristae in two representative samples obtained from the OSOM of a saline IR rat after 20-minute reperfusion. Some mitochondria show disrupted membranes and release of matrix material into the cytoplasm. In contrast, the representative samples from a SS-31 IR rat (Figure 4, c and d) show many elongated mitochondria with preservation of cristae structure within intact membrane infoldings in the basal side of the tubular cell. A few swollen mitochondria were detected in some cells.
Figure 4.
SS-31 protects kidney mitochondrial structure after IR injury. Rats were subcutaneously treated with saline or SS-31 (2 mg/kg) 30 minutes before occlusion of renal blood flow for 45 minutes. Treatment was repeated just before onset of reperfusion. Ultrastructural studies of kidney sections obtained after 20-minute reperfusion were carried out using transmission electron microscopy. Representative sections from the outer medulla of a saline-treated rat show disruption of normal tubular cytoarchitecture (A and B). Note many rounded, swollen mitochondria with disrupted cristae architecture (*). Some mitochondria show disruption of membranes and release of matrix materials into the cytosol (arrow head). Other swollen mitochondria show complete loss of cristae (arrow). Some normal elongated mitochondria with dense matrix can be observed (B). Representative sections from the outer medulla of a SS-31-treated rat appear quite normal with numerous mitochondria within membrane infoldings near the basement membrane (BM) (C and D). Although a few swollen mitochondria can be found in some cells (*), most mitochondria were elongated with well preserved cristae structure. All images were magnified ×15,000. Scale bar = 500 nm.
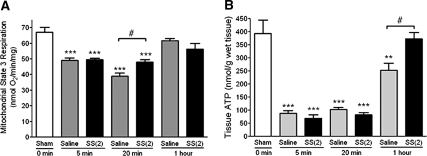
Mitochondria were isolated from 20-minute postischemic kidneys for assessment of respiratory function. Oxygen consumption was measured using complex I substrates (glutamate/malate) because complex-I-initiated respiration is inhibited in IR.27 Compared with the Sham-operated group, state 3 respiration was significantly depressed in the saline IR group at 5 minutes and decreased further by 20 minutes before recovery to Sham level by 60 minutes (Figure 5A). Mitochondrial respiration was similarly depressed at 5 minutes in the SS-31 IR animals. However, SS-31 IR animals did not show the further deterioration of mitochondrial respiration at 20 minutes, resulting in significantly higher oxygen consumption when compared with saline animals. The respiration rate in SS-31 IR animals was 71.6% of Sham levels as compared with 58.2% for the saline IR group.
Figure 5.
SS-31 improves kidney mitochondrial function after IR injury. Rats were subcutaneously treated with saline or SS-31 (2 mg/kg) 30 minutes before occlusion of renal blood flow for 45 minutes. Treatment was repeated just before onset of reperfusion. Mitochondria were freshly isolated from kidney tissue obtained at 5, 20, or 60 minutes after reperfusion. ATP was determined from whole kidney tissue obtained at the same time points. (A) Mitochondrial respiration was measured by oxygen consumption using isolated mitochondria with complex I substrates (glutamate/malate) and ADP was added to initiate state 3 respiration. (B) Whole kidney tissue ATP content was determined using the luciferase assay. n = 4 to 8 in each group. **P < 0.01 and ***P < 0.001 compared with Sham; #P < 0.05 comparing saline and SS-31 treatment.
ATP levels in ischemic renal tissues were dramatically lower than Sham levels after 5 minutes of reperfusion and did not improve after 20 minutes of reperfusion (Figure 5B). ATP levels only partially recovered after 60 minutes (approximately 63%) and remained significantly lower than in Sham-treated rats. Treatment with SS-31 did not improve ATP recovery during the first 20 minutes of reperfusion, but complete recovery of ATP was found after 1 hour of reperfusion (Figure 5B). At 1 hour, ATP in the SS-31 IR group was significantly higher (48%) compared with the saline group.
SS-31 Minimized Tubular Cell Detachment
Loss of ATP after IR injury led to redistribution of β1-integrin from the basal surface of tubular epithelia (Figure 6A) into the cytoplasm (Figure 6B), resulting in the detachment of viable cells from the basement membrane (Figure 6E). In contrast, β1-integrin remained localized to the basal membrane in animals that were treated with SS-31 (Figure 6C) and tubular cells remained attached to the basement membrane (Figure 6F).
Figure 6.
SS-31 minimizes renal tubular cell detachment at 1 hour after IR injury. Rats were subcutaneously treated with saline or SS-31 (2 mg/kg) 30 minutes before occlusion of renal blood flow for 45 minutes. Treatment was repeated just before onset of reperfusion, and kidneys were sectioned and stained for β1-integrin by immunohistochemisty and for cell detachment using PAS stain. Panels a through c show β1-integrin staining (reddish-brown stain as shown by arrow) in (A) Sham, (B) saline-treated IR, and (C) SS-31-treated IR rats. β1-integrin was clearly localized to the basal membrane of the tubular cell in Sham and SS-31-treated IR animals, whereas it was diffused throughout the cell in saline-treated IR animals. Panels D through F show that tubular cells are attached to the basement membrane in (D) Sham and (F) SS-31-treated IR animals, but detached cells, some viable (shown by arrow), were clearly found within the tubular lumen of (E) saline-treated IR animals. Magnification: ×600.
SS-31 Reduced Medullary Microvascular Congestion
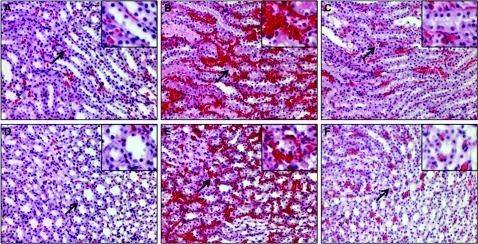
Reperfusion is also associated with microvascular congestion and trapping of erythrocytes in the inner medullary region.28,29 Extensive trapping of erythrocytes was seen in the ISOM (Figure 7B) and inner medulla (Figure 7E) of saline-treated animals after 1-hour reperfusion after 45-minute ischemia. The trapping of erythrocytes was greatly reduced in ISOM (Figure 7C) and the inner medulla (Figure 7F) of SS-31 IR animals.
Figure 7.
SS-31 reduces renal medullary vascular congestion 1 hour after IR injury. Rats were subcutaneously treated with saline or SS-31 (2 mg/kg) 30 minutes before occlusion of renal blood flow for 45 minutes. Treatment was repeated just before onset of reperfusion, and kidney sections were stained with hemotoxylin and eosin. Representative sections from the ISOM are shown in panels A through C, and representative sections from the inner medulla are shown in panels D through F (magnification: ×200). Inset shows higher magnification of the area identified by the arrow. Kidneys from Sham-operated rats showed minimal erythrocyte trapping in ISOM and the inner medulla (A, D). Extensive trapping of erythrocytes was found in ISOM and the inner medulla in the saline-treated IR group (B, E). Erythrocyte trapping was greatly reduced in the SS-31-treated IR group (C, F).
SS-31 Reduced IR-Mediated Oxidative Stress
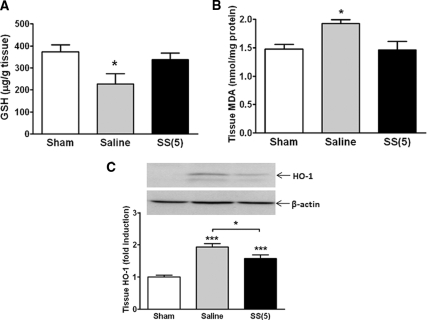
Oxidative stress occurs in the kidney during IR as a result of excess ROS production and inhibition of antioxidant enzyme activities.30,31 The effect of SS-31 on oxidative stress was assessed using three different parameters: intracellular glutathione (GSH), lipid hydroperoxides (as measured by malondialdehyde [MDA]), and heme oxygenase-1 (HO-1). Within 1 hour of reperfusion after 45-minute ischemia, there was significant depletion of GSH in kidney tissues from the saline IR animals that was completely prevented by SS-31 treatment (Figure 8A). The significant increases in kidney MDA and HO-1 expression were also prevented by SS-31 (Figure 8, B and C).
Figure 8.
SS-31 reduces renal oxidative stress caused by IR. Rats were subcutaneously treated with saline or SS-31 (2 mg/kg) 30 minutes before occlusion of renal blood flow for 45 minutes. (A) Effects of SS-31 on renal GSH level after 1-hour reperfusion. n = 6 to 8 in each group. ANOVA, P < 0.05. *P < 0.05 compared with Sham. GSH level in the SS-31-treated IR animals was not different from Sham. (B) Effects of SS-31 on lipid hydroperoxides after 24-hour reperfusion as measured by MDA in renal medullary tissues. n = 3 to 4 in each group. ANOVA, P = 0.01. *P < 0.05 compared with Sham-operated animals. (C) Effects of SS-31 on HO-1 expression at 24 hours after IR injury. Representative western blot for HO-1 from Sham, saline IR, and SS-31 IR animals is shown with β-actin as a loading control. n = 11 to 12 in each group. ANOVA, P < 0.0001. HO-1 protein expression was significantly increased in saline and SS-31 IR animals (***P < 0.001 compared with Sham); however, HO-1 expression in SS-31 animals was significantly lower than saline (*P < 0.05).
SS-31 Limited IR-Mediated Inflammatory Response
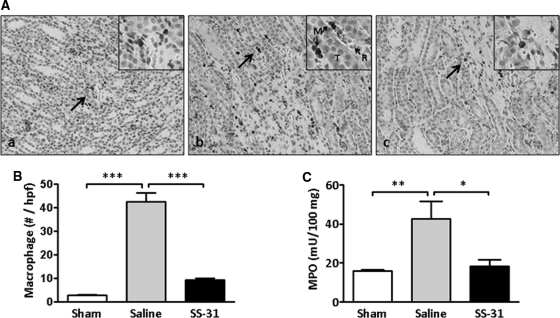
Macrophages migrate to the outer medulla of kidneys after IR injury in response to apoptotic cell death. A significant increase in macrophage infiltration was observed in saline rats 24 hours after being subjected to 30-minute ischemia (Figure 9, A and B). SS-31-treated animals showed no significant increase in macrophage number when compared with Sham-treated rats (Figure 9, A and B).
Figure 9.
SS-31 reduces inflammation response to IR injury. (A) Macrophage infiltration in medullary region of (a) Sham, (b) saline-treated IR, and (c) SS-31-treated IR rats. Rats were subcutaneously treated with saline or SS-31 (2 mg/kg) 30 minutes before occlusion of renal blood flow for 30 minutes. Treatment was repeated just before onset of reperfusion and 2 hours later. Kidney sections obtained after 24-hour reperfusion were stained for macrophage using anti-CD68 antibody (brown stain); magnification: ×200. Inset shows area indicated by arrow at higher magnification. Note the presence of macrophages (M) and erythrocytes (R) in capillaries adjacent to tubular cells (T) in the saline IR group. (B) The number of macrophages per high power field were few in the Sham but significantly increased in the saline-treated IR group. SS-31 treatment completely prevented macrophage infiltration. n = 4 to 6 in each group. ANOVA P < 0.0001. ***P < 0.001 between groups. (C) Effects of SS-31 on neutrophil activation 24 hours after 45-minute ischemia as measured by the release of MPO. n = 11 to 13 in each group. ANOVA, P = 0.005, *P < 0.05 and **P < 0.01 between groups.
Necrotic cell injury triggers an acute inflammatory response characterized by activation of neutrophils that subsequently release myeloperoxidase (MPO) and hydrogen peroxide upon reperfusion of ischemic tissues.32 MPO was significantly elevated in the saline IR group 24 hours after 45-minute ischemia (Figure 9C). The increase in MPO was completely prevented by SS-31, suggesting that SS-31 can prevent the IR-mediated inflammatory response.
SS-31 Accelerated Tubular Regeneration
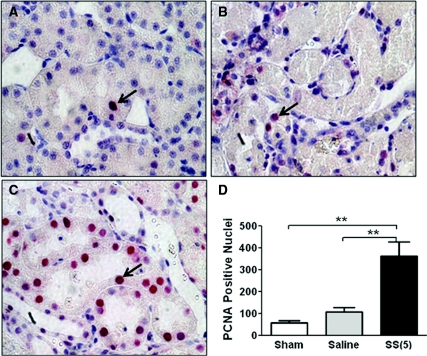
Injured proximal tubular cells that do not detach or die from IR injury undergo repair and regeneration to restore nephron structure and function.33 The cell regeneration marker proliferating cell nuclear antigen (PCNA) increases as early as 24 hours after reperfusion.34,35 In this study, PCNA-positive (PCNA+) cells were few in renal sections obtained from the Sham group (Figure 10A). A small increase in PCNA+ nuclei was observed in the saline IR group at 24 hours (Figure 10, B and D), but the number of PCNA+ nuclei was significantly greater in the SS-31 IR group (Figure 10, C and D).
Figure 10.
SS-31 accelerates proximal tubular cell regeneration at 24 hours after IR injury. Rats were subcutaneously treated with saline or SS-31 (2 mg/kg) 30 minutes before occlusion of renal blood flow for 45 minutes. Treatment was repeated just before onset of reperfusion and 2 hours later. Representative kidney sections showing immunostaining of PCNA from (A) Sham, (B) saline-treated IR, and (C) SS-31-treated IR animals. Sections were developed using alkaline phosphatase staining, and PCNA+ cells are shown as red nuclei (arrows). PCNA+ cells were very few in the Sham animals and slightly increased 24 hours after IR injury in the saline treatment group, but they were significantly increased in the SS-31 treatment group (D). n = 4 in each group. **P < 0.01 between groups.
DISCUSSION
Renal tubules in the medullary ray and OSOM are especially vulnerable to ischemic injury because of low blood flow in this region of the nephron, high ATP demands of Na+ transport, and the absence of glycolysis for generating ATP. This study shows that SS-31 protected mitochondrial structure and prevented deterioration of mitochondrial function in early reperfusion, thus resulting in accelerated ATP recovery. Faster ATP recovery rescued tubular cells from reversible cellular swelling and prevented tubular apoptosis and necrosis. Rapid re-establishment of renal epithelial polarity preserved tubular function as demonstrated by reduced FENa and FEK. Furthermore, SS-31 significantly reduced oxidative stress and inflammation after IR injury and promoted proliferation of surviving tubular cells. These results suggest that SS-31 acts upstream of the initial ischemic cell injury and can prevent subsequent inflammatory damage associated with reperfusion.
Kidney ATP levels fall to <10% of preischemic values within 5 to 10 minutes of ischemia and remain low for the duration of ischemia.36–38 ATP levels can return to control values upon reperfusion if ischemia is <20 minutes.39 If the ischemic interval is extended beyond 25 minutes, there is only a 50% to 60% recovery of tissue ATP even after 2-hour reperfusion.36–39 Our results are consistent with these reports. After 45 minutes of ischemia, ATP content remained very low (approximately 20%) throughout the first 20 minutes of reperfusion, with only partial recovery (approximately 60%) after 1-hour reperfusion. In contrast, ATP was completely restored by 1 hour with SS-31 treatment.
The accelerated recovery of ATP by SS-31 suggests that it is acting by protecting mitochondria during IR. Oxidative phosphorylation is inhibited during hypoxia and deteriorates upon reoxygenation.27,40 The progressive deterioration of mitochondrial function during reperfusion may result from MPT triggered by the normalization of intracellular pH and burst of ROS activity.41 Opening of the MPT pore would cause immediate collapse of the mitochondrial membrane potential and inhibits ATP synthesis. In this study, electron microscopic evaluation clearly demonstrated many swollen and damaged mitochondria in the outer medulla 20 minutes after prolonged ischemia. This is consistent with the deterioration of oxygen consumption determined in mitochondria isolated at that time, which significantly limited restoration of ATP after 1-hour reperfusion. SS-31 has been shown to inhibit MPT induced by Ca2+ and Pi in isolated mitochondria as well as prevent mitochondrial depolarization in intact cells caused by ETC inhibitors.21,23 Indeed, electron microscopy revealed significant protection of proximal tubular mitochondria after IR injury in rats that received SS-31 treatment. Most mitochondria were elongated and retained normal cristae architecture, and swollen mitochondria were far fewer than in saline IR samples. Indeed, mitochondria isolated from animals pretreated with SS-31 did not undergo functional deterioration upon reperfusion. Prevention of additional mitochondrial damage during early reperfusion allowed for complete recovery of tissue ATP by 1 hour.
The protection of mitochondrial structure provided by SS-31 is greater than that suggested by the respiration studies. This is likely because the more severely damaged mitochondria with broken membranes in the saline IR tissues were not picked up by the conventional isolation procedure, which is based on differential centrifugation to separate the various organelles and membranes. Thus the measurement of oxygen consumption would primarily reflect the better mitochondria that were isolated and underestimates the degree of mitochondrial damage. The preservation of mitochondria integrity by SS-31 was sufficient to allow full recovery of ATP content at 1 hour after reperfusion. The apparent lack of temporal correlation between mitochondrial respiration and ATP content can be explained by the fact that mitochondrial respiration measures the ability of ATP synthesis, whereas tissue ATP content reflects ATP synthesis and expenditure. The lack significant difference in ATP content between SS-31 and saline IR groups at 20 minutes may be due to increased ATP expenditure in the SS-31 group for maintenance of cellular cytoarchitecture, as suggested by the β1-integrin findings.
Ischemic defects in the ETC increase ROS production, especially from complex I and complex III. Reintroduction of oxygen during reperfusion leads to a dramatic increase in ROS within seconds.42 This burst of ROS plays a critical role in triggering MPT8 and leads to a positive feedback loop of ROS-induced ROS release.43 By protecting the ETC during ischemia, SS-31 reduces mitochondrial ROS production upon reperfusion. In addition, SS-31 can directly scavenge mitochondrial ROS.21,23 SS-31 clearly reduced oxidative stress associated with renal IR injury, as demonstrated by GSH depletion, lipid peroxidation, and upregulation of HO-1.
Ischemia can cause cell death by apoptosis or necrosis. Shorter ischemia time (30 minutes) primarily resulted in apoptosis, whereas prolonged ischemia (45 minutes) led to extensive necrosis. The rapid recovery of ATP and prevention of oxidative stress provided by SS-31 significantly reduced apoptosis and necrosis of tubular cells and preserved tubular function. SS-31 completely prevented renal dysfunction caused by 30-minute ischemia and significantly reduced AKI from 45-minute ischemia.
ATP depletion leads to rearrangement of the actin cytoskeleton, loss of cell polarity and cell-to-cell contact, and internalization of Na+,K+-ATPase.44–46 Rapid ATP recovery is essential for restoration of cytoskeletal architecture.47 With 30-minute ischemia there was no increase in FENA, but 45-minute ischemia led to a dramatic increase in FENa that was prevented by SS-31 treatment. Improved ATP recovery with SS-31 led to β1-integrin being retained on the basal membrane even after 45-minute ischemia, thus minimizing loss of tubular cells, preserving epithelial barrier function, and minimizing backflow of creatinine.
Medullary microvascular congestion plays an important role in limiting reflow in the postischemic kidney.48 The trapping of blood cells is thought to be due to loss of endothelial cell-to-cell contact, increased microvascular permeability, breakdown of the perivascular matrix, and increased vascular reactivity.49 ATP depletion and oxidative stress play a role in microvascular congestion.48 Similar to the tubular cell, ATP depletion leads to disruption of the actin cytoskeleton of the renal microvascular endothelial cell,50 loss of cell-to-cell contact, and cell detachment.48,51 The rapid recovery of ATP provided by SS-31 appears to protect the microvascular endothelial cell and dramatically reduce microvascular congestion, providing better reflow to the medulla and improved creatinine clearance.
By acting upstream of epithelial and endothelial injury, SS-31 was able to prevent the inflammatory reaction that normally accompanies AKI. Macrophage infiltration and neutrophil activation normally contribute to further cellular injury in late reperfusion. Activated neutrophils release hydrogen peroxide and MPO and produce hypochlorous acid, which has been shown to cause mitochondrial dysfunction and cell death that could be prevented by SS-31.52 Thus, SS-31 may prevent downstream inflammatory events that can lead to chronic kidney injury after AKI.
Finally, recovery of the tubular epithelia after IR injury requires proliferation and repolarization of surviving epithelial cells.53 Cell division, an ATP-dependent process, is suppressed after ischemia and does not peak until 3 to 4 days after reperfusion.54 ROS release after IR injury also inhibits the regeneration of tubular cells.55 Treatment with SS-31 significantly accelerated the rate of tubular cell regeneration as early as 1 day after IR injury by improving ATP recovery and reducing ROS. It is also possible that there are fewer viable tubular cells to undergo proliferation in the saline group.
Recent pharmacologic interventions against IR injury have concentrated on antioxidants and anti-inflammatory compounds. SS-31 is the first compound that acts upstream of the initial cell injury by protecting mitochondrial function and oxidative stress during reperfusion. By accelerating ATP recovery and reducing oxidative damage, SS-31 protects epithelial and endothelial cell viability, maintains cell polarity and cell attachment, reduces no-reflow, and minimizes inflammation. These findings suggest that SS-31 may be useful in minimizing AKI induced by shock or cardiovascular surgery and in minimizing early allograft dysfunction to improve long-term allograft survival.
CONCISE METHODS
Animals
The study was performed using male Sprague–Dawley rats (Charles River Laboratories International, Inc., Wilmington, MA) weighing 250 to 300 g. Animals were housed in a light-controlled room with a 12:12-hour light-dark cycle and allowed free access to water and standard rat chow. Care of the rats before and during the experimental procedures was conducted in accordance with the policies of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All protocols had received prior approval by the Cornell University Institutional Animal Care and Use Committee.
Materials
Unless otherwise specified, reagents and assay kits were purchased from Sigma (St. Louis, MO). Anti-HO-1 goat polyclonal IgG (R&D Systems, Minneapolis, MN), anti-β1-integrin (CD29), anti-CD68 (ED-1), mouse monoclonal IgG (Abcam, Cambridge, MA), anti-PCNA mouse monoclonal IgG (Dako, Carpinteria, CA), anti-β-actin mouse monoclonal IgG (Santa Cruz Biotechnology, Santa Cruz, CA), horseradish peroxidase (HRP)-conjugated rabbit anti-goat IgG and goat anti-mouse IgG (Pierce, Rockford, IL), alkaline-phosphatase-conjugated rabbit anti-goat IgG, and horse anti-mouse IgG (Vector Laboratories Inc, Burlingame, CA) were commercially obtained.
Experimental Protocol
Rats were anesthetized with a ketamine/xylazine mixture (90 mg/kg ketamine and 4 mg/kg xylazine). Bilateral renal ischemia was induced by the application of nontraumatic microvascular clamps around both left and right renal pedicles. Ischemia was confirmed by blanching of the kidneys. After 30- or 45-minute ischemia, the clamps were removed and reperfusion was confirmed visually. Sham-operated animals were not subjected to ischemia. During the ischemic interval, the animals were kept hydrated with normal saline instilled intraperitoneally and were kept on warm heating pads to maintain body temperature. After ischemia, the animals were allowed to recover in individual metabolic cages with free access to food and water.
Animals were randomly assigned to the following groups: Sham-operated, IR with saline, or IR with SS-31 (0.5, 2.0, or 5.0 mg/kg). Treatment was administered subcutaneously 30 minutes before onset of ischemia, at the onset of reperfusion, and at 2 hours after reperfusion. Serum and urine samples were collected at 24 hours and stored at −20°C until analysis. Kidneys were harvested at different times after onset of reperfusion (5, 20, or 60 minutes or 24 hours) for assessment of mitochondrial respiration, ATP content, oxidative markers, and histopathology by light and electron microscopy.
Renal Function Assessment
Serum and urine samples obtained 24 hours after reperfusion were analyzed by the ALX Laboratory at the Animal Medical Center in New York. FENa and FEK were calculated as follows: FENa = (UNa × SCr/SNa × UCr) × 100% and FEK = (UK × SCr/SK × UCr) × 100%, where U represents urinary, S represents serum, and Cr represents creatinine.
Kidney Histopathology
Kidney samples were fixed in 10% neutral-buffered formalin, embedded in paraffin wax, and sectioned at 3-μm thickness. The sections were stained with PAS or hemotoxylin and eosin and examined by light microscopy (Nikon Eclipse, TE2000-U). For quantification of tubular injury score, sections were assessed by counting the percentage of tubular necrosis in the OSOM using the following scoring criteria: 0 = normal, 1 = <10%, 2 = 10% to 25%, 3 = 26% to 50%, 4 = 51% to 75%, and 5 = >75%. A total of ten fields were scored from each sample and averaged.
TUNEL Assay for Apoptosis
Deparaffinized and rehydrated tissue sections were prepared according to standard protocols. Slides were incubated with proteinase K for 20 minutes at RT. TUNEL labeling was carried out using an in situ cell death detection kit (Roche, Indianapolis, IN) according to the manufacturer's instructions, and color was developed using the diaminobenzidine substrate kit (Vector, Burlingame, CA). The number of apoptotic cells in the outer medulla were counted from ten different fields for each sample and were averaged.
Immunohistochemical Staining for β1-Integrin, Macrophages, and PCNA
Kidney sections (4 μm) were deparaffinized and rehydrated by xylene, a graded alcohol series, and double-deionized water. Immunostaining was based on protocols from the VECTASTAIN ABC kit (Vector, Burlingame, CA). Briefly, sections were blocked in blocking buffer for 30 minutes at room temperature; incubated with anti-β1-integrin, anti-CD68 (ED-1), or anti-PCNA mouse monoclonal IgG; and incubated with the secondary antibodies of HRP-conjugated rabbit anti-mouse IgG or alkaline-phosphatase-conjugated horse anti-mouse IgG. The number of CD68+ cells or PCNA+ cells in the outer medulla were counted from five different fields for each sample and then averaged.
Electron Microscopy
Pieces of renal cortical and medullary tissue were fixed in 4% paraformaldehyde, postfixed in 1% osmium tetroxide, dehydrated in graded alcohols, and embedded in Epon. Ultrathin sections (200 to 400 Å) were cut on nickel grids, stained with uranyl acetate and lead citrate, and examined using a digital electron microscope with a 2.0 CCD camera (Hitachi H-7500, Pleasanton, CA).
Whole-Tissue ATP Content Assay
Kidney tissue was placed in 5 ml of ice-cold 5% TCA (containing 10 mM dithiothreitol and 2 mM EDTA), cut to small pieces, homogenized, and incubated for 10 minutes on ice. Tissue homogenate was centrifuged for 10 minutes at 2000 × g, neutralized with 1 N potassium hydroxide (KOH) to pH 7.6, centrifuged again for 10 minutes at 2000 × g, and stored at −20°C. ATP was determined using an ATP bioluminescent assay kit.
Isolation of Renal Mitochondria
Excised kidneys were cut and incubated in wash buffer (200 mM mannitol, 10 mM sucrose, 5 mM HEPES, 1 g/L fatty-acid-free BSA, to pH to 7.4 with KOH) on ice for 10 minutes. Samples were washed 2 times in isolation buffer (wash buffer with 1 mM EGTA), homogenized for 3 minutes, and then centrifuged in 20 ml of isolation buffer at 900 × g for 10 minutes. The white fatty acid layer was then removed and the pellet was discarded. The supernatant was centrifuged at 11,000 × g for 10 minutes, and the pellet was resuspended in 800 μl of wash buffer and kept on ice for further analysis.
Mitochondria Oxygen Consumption Assay
Oxygen consumption was measured at 30°C using a Clark electrode (Hensatech, Norfolk, UK). Isolated kidney mitochondria (200 μg) were added to 1 ml of respiration buffer (3.25 mM potassium dihydrogen phosphate, 320 mM sucrose, 7.5 mM HEPES, 0.5 mM EGTA, pH adjusted to 7.4 with KOH and hydrochloric acid) and allowed to equilibrate at 30°C for 1 minute. Glutamate (5 mM) and malate (5 mM) were added as substrates, and state 3 respiration was initiated by the addition of ADP (400 μM).
GSH Assay
GSH levels in kidney tissue homogenates were quantified using the enzymatic recycling (GSH reductase) method described previously.56 The assay for GSH is based on the reaction of GSH with 5,5′-dithiobis(2-nitrobenzoic acid), which produces the 2-nitro-5-thiobenzoic acid chromophore that can be monitored at 412 nm.
MDA Assay
MDA was determined using the TBARS assay kit according to the manufacturer's instructions (Cayman Chemicals, Ann Arbor, MI). The MDA-TBAR adduct was formed under high temperature (90 to 100°C) in acidic conditions and measured colorimetrically at 532 nm using a plate reader (Molecular Devices, Sunnyvale, CA).
Western Blot Analysis for HO-1
Tissue homogenate was prepared according to the lysis protocol for radioimmunoprecipitation assay buffer (Santa Cruz Biotechnology, Santa Cruz, CA). Homogenate (35 μg) was suspended in loading buffer and subjected to a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis. The resolved proteins were transferred to a polyvinylidene fluoride membrane. After electroblotting, the membrane was incubated overnight with anti-HO-1 (0.5 μg/ml) antibody or for 1 hour with anti-β-actin (1:5000). Membranes were further incubated for 1 hour with their respective HRP-conjugated antibodies. Protein bands were detected with an enhanced chemiluminescence detection system (Cell Signaling, Danvers, MA) and autoradiography. Bands were evaluated for integrated density values on Flurochem 8900 software.
MPO Assay
Tissue homogenates were prepared and solubilized according to instructions for the Fluoro MPO detection kit (Cell Technology Inc, Mountain View, CA). N-ethylmaleimide, catalase inhibitor, and erythropoietin inhibitor were added to minimize interference. The fluorescence signal was measured at an excitation of 530 nm and an emission of 590 nm using a Gemini XPS fluorescence plate reader.
Statistical Analysis
Results are expressed as means ± SEM. Statistical analysis was carried out using Prism software (GraphPad Software, Inc., San Diego, CA). Multiple group comparisons were performed using ANOVA followed by a Tukey post hoc test. A t test was used for comparison between two groups. A P value <0.05 was considered statistically significant.
DISCLOSURES
The SS peptides technology has been licensed for commercial development to Stealth Peptides, Inc., by the Cornell Research Foundation (CRF), and CRF and H.H.S. have financial interests.
Acknowledgments
This study was supported by funds from Stealth Peptides Inc. and Weill Cornell Medical College (H.H.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Pores for Thought: New Strategies to Re-energize Stressed Mitochondria in Acute Kidney Injury,” on pages 986–988.
REFERENCES
- 1. Devarajan P: Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol 17: 1503–1520, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Brezis M, Rosen S, Silva P, Epstein FH: Selective vulnerability of the medullary thick ascending limb to anoxia in the isolated perfused rat kidney. J Clin Invest 73: 182–190, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wirthensohn G, Guder WG: Renal substrate metabolism. Physiol Rev 66: 469–497, 1986 [DOI] [PubMed] [Google Scholar]
- 4. Klein KL, Wang MS, Torikai S, Davidson WD, Kurokawa K: Substrate oxidation by isolated single nephron segments of the rat. Kidney Int 20: 29–35, 1981 [DOI] [PubMed] [Google Scholar]
- 5. Uchida S, Endou H: Substrate specificity to maintain cellular ATP along the mouse nephron. Am J Physiol 255: F977–F983, 1988 [DOI] [PubMed] [Google Scholar]
- 6. Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ: Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol 292: C137–C147, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ: Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol 294: C460–C466, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Halestrap AP, Pasdois P: The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta 1787: 1402–1415, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Javadov S, Karmazyn M, Escobales N: Mitochondrial permeability transition pore opening as a promising therapeutic target in cardiac diseases. J Pharmacol Exp Ther 330: 670–678, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Morin D, Assaly R, Paradis S, Berdeaux A: Inhibition of mitochondrial membrane permeability as a putative pharmacological target for cardioprotection. Curr Med Chem 16: 4382–4398, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD: Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y: Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature 434: 652–658, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Devalaraja-Narashimha K, Diener AM, Padanilam BJ: Cyclophilin D gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol 297: F749–F759, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Hu W, Chen Z, Ye Z, Xia D, Xia Z, Ma J, Zhu M, Chen G: Knockdown of cyclophilin D gene by RNAi protects rat from ischemia/reperfusion-induced renal injury. Kidney Blood Press Res 33: 193–199, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Yang CW, Ahn HJ, Han HJ, Kim WY, Li C, Shin MJ, Kim SK, Park JH, Kim YS, Moon IS, Bang BK: Pharmacological preconditioning with low-dose cyclosporine or FK506 reduces subsequent ischemia/reperfusion injury in rat kidney. Transplantation 72: 1753–1759, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Singh D, Chander V, Chopra K: Cyclosporine protects against ischemia/reperfusion injury in rat kidneys. Toxicology 207: 339–347, 2005 [DOI] [PubMed] [Google Scholar]
- 17. Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M: Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 359: 473–481, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Ysebaert DK, De Greef KE, Nouwen EJ, Verpooten GA, Eyskens EJ, De Broe ME: Influence of cyclosporin A on the damage and regeneration of the kidney after severe ischemia/reperfusion injury. Transplant Proc 29: 2348–2351, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Goncalves GM, Cenedeze MA, Feitoza CQ, de Paula CB, Marques GD, Pinheiro HS, de Paula Antunes Teixeira V, Antonia dos Reis M, Pacheco-Silva A, Camara NO: The role of immunosuppressive drugs in aggravating renal ischemia and reperfusion injury. Transplant Proc 39: 417–420, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Magnasco A, Rossi A, Catarsi P, Gusmano R, Ginevri F, Perfumo F, Ghiggeri GM: Cyclosporin and organ specific toxicity: Clinical aspects, pharmacogenetics and perspectives. Curr Clin Pharmacol 3: 166–173, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH: Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem 279: 34682–34690, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Cho J, Won K, Wu D, Soong Y, Liu S, Szeto HH, Hong MK: Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis 18: 215–220, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Szeto HH: Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal 10: 601–619, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Vercauteren SR, Ysebaert DK, De Greef KE, Eyskens EJ, De Broe ME: Chronic reduction in renal mass in the rat attenuates ischemia/reperfusion injury and does not impair tubular regeneration. J Am Soc Nephrol 10: 2551–2561, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Yoshida T, Kurella M, Beato F, Min H, Ingelfinger JR, Stears RL, Swinford RD, Gullans SR, Tang SS: Monitoring changes in gene expression in renal ischemia-reperfusion in the rat. Kidney Int 61: 1646–1654, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Basireddy M, Isbell TS, Teng X, Patel RP, Agarwal A: Effects of sodium nitrite on ischemia-reperfusion injury in the rat kidney. Am J Physiol Renal Physiol 290: F779–F786, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Weinberg JM, Venkatachalam MA, Roeser NF, Saikumar P, Dong Z, Senter RA, Nissim I: Anaerobic and aerobic pathways for salvage of proximal tubules from hypoxia-induced mitochondrial injury. Am J Physiol Renal Physiol 279: F927–F943, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mason J, Welsch J, Torhorst J: The contribution of vascular obstruction to the functional defect that follows renal ischemia. Kidney Int 31: 65–71, 1987 [DOI] [PubMed] [Google Scholar]
- 29. Hellberg PO, Bayati A, Kallskog O, Wolgast M: Red cell trapping after ischemia and long-term kidney damage. Influence of hematocrit. Kidney Int 37: 1240–1247, 1990 [DOI] [PubMed] [Google Scholar]
- 30. De Vecchi E, Lubatti L, Beretta C, Ferrero S, Rinaldi P, Galli Kienle M, Trazzi R, Paroni R: Protection from renal ischemia-reperfusion injury by the 2-methylaminochroman U83836E. Kidney Int 54: 857–863, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Sehirli AO, Sener G, Satiroglu H, Ayanoglu-Dulger G: Protective effect of N-acetylcysteine on renal ischemia/reperfusion injury in the rat. J Nephrol 16: 75–80, 2003 [PubMed] [Google Scholar]
- 32. Matthijsen RA, Huugen D, Hoebers NT, de Vries B, Peutz-Kootstra CJ, Aratani Y, Daha MR, Tervaert JW, Buurman WA, Heeringa P: Myeloperoxidase is critically involved in the induction of organ damage after renal ischemia reperfusion. Am J Pathol 171: 1743–1752, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nony PA, Schnellmann RG: Mechanisms of renal cell repair and regeneration after acute renal failure. J Pharmacol Exp Ther 304: 905–912, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Witzgall R, Brown D, Schwarz C, Bonventre JV: Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest 93: 2175–2188, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Delbridge MS, Shrestha BM, Raftery AT, El Nahas AM, Haylor JL: Reduction of ischemia-reperfusion injury in the rat kidney by FTY720, a synthetic derivative of sphingosine. Transplantation 84: 187–195, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Siegel NJ, Avison MJ, Reilly HF, Alger JR, Shulman RG: Enhanced recovery of renal ATP with postischemic infusion of ATP-MgCl2 determined by 31P-NMR. Am J Physiol 245: F530–F534, 1983 [DOI] [PubMed] [Google Scholar]
- 37. Gaudio KM, Stromski M, Thulin G, Ardito T, Kashgarian M, Siegel NJ: Postischemic hemodynamics and recovery of renal adenosine triphosphate. Am J Physiol 251: F603–F609, 1986 [DOI] [PubMed] [Google Scholar]
- 38. Arnold PE, Van Putten VJ, Lumlertgul D, Burke TJ, Schrier RW: Adenine nucleotide metabolism and mitochondrial Ca2+ transport following renal ischemia. Am J Physiol 250: F357–F363, 1986 [DOI] [PubMed] [Google Scholar]
- 39. Vogt MT, Farber E: On the molecular pathology of ischemic renal cell death. Reversible and irreversible cellular and mitochondrial metabolic alterations. Am J Pathol 53: 1–26, 1968 [PMC free article] [PubMed] [Google Scholar]
- 40. Veitch K, Hombroeckx A, Caucheteux D, Pouleur H, Hue L: Global ischaemia induces a biphasic response of the mitochondrial respiratory chain. Anoxic pre-perfusion protects against ischaemic damage. Biochem J 281: 709–715, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Griffiths EJ, Halestrap AP: Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J 307: 93–98, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Becker LB: New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res 61: 461–470, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ: Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bacallao R, Garfinkel A, Monke S, Zampighi G, Mandel LJ: ATP depletion: A novel method to study junctional properties in epithelial tissues. I. Rearrangement of the actin cytoskeleton. J Cell Sci 107: 3301–3313, 1994 [DOI] [PubMed] [Google Scholar]
- 45. Mandel LJ, Doctor RB, Bacallao R: ATP depletion: A novel method to study junctional properties in epithelial tissues. II. Internalization of Na+,K(+)-ATPase and E-cadherin. J Cell Sci 107: 3315–3324, 1994 [DOI] [PubMed] [Google Scholar]
- 46. Van Why SK, Mann AS, Ardito T, Siegel NJ, Kashgarian M: Expression and molecular regulation of Na(+)-K(+)-ATPase after renal ischemia. Am J Physiol 267: F75–F85, 1994 [DOI] [PubMed] [Google Scholar]
- 47. Weinberg JM, Venkatachalam MA, Roeser NF, Senter RA, Nissim I: Energetic determinants of tyrosine phosphorylation of focal adhesion proteins during hypoxia/reoxygenation of kidney proximal tubules. Am J Pathol 158: 2153–2164, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brodsky SV, Yamamoto T, Tada T, Kim B, Chen J, Kajiya F, Goligorsky MS: Endothelial dysfunction in ischemic acute renal failure: Rescue by transplanted endothelial cells. Am J Physiol Renal Physiol 282: F1140–F1149, 2002 [DOI] [PubMed] [Google Scholar]
- 49. Sutton TA: Alteration of microvascular permeability in acute kidney injury. Microvasc Res 77: 4–7, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sutton TA, Fisher CJ, Molitoris BA: Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int 62: 1539–1549, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Sutton TA, Mang HE, Campos SB, Sandoval RM, Yoder MC, Molitoris BA: Injury of the renal microvascular endothelium alters barrier function after ischemia. Am J Physiol Renal Physiol 285: F191–F198, 2003 [DOI] [PubMed] [Google Scholar]
- 52. Whiteman M, Spencer JP, Szeto HH, Armstrong JS: Do mitochondriotropic antioxidants prevent chlorinative stress-induced mitochondrial and cellular injury? Antioxid Redox Signal 10: 641–650, 2008 [DOI] [PubMed] [Google Scholar]
- 53. Liu KD, Brakeman PR: Renal repair and recovery. Crit Care Med 36: S187–S192, 2008 [DOI] [PubMed] [Google Scholar]
- 54. Biggar KK, Storey KB: Perspectives in cell cycle regulation: Lessons from an anoxic vertebrate. Curr Genomics 10: 573–584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jang HS, Kim J, Park YK, Park KM: Infiltrated macrophages contribute to recovery after ischemic injury but not to ischemic preconditioning in kidneys. Transplantation 85: 447–455, 2008 [DOI] [PubMed] [Google Scholar]
- 56. Rahman I, Kode A, Biswas SK: Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1: 3159–3165, 2006 [DOI] [PubMed] [Google Scholar]