Abstract
The activation of cytokine and growth factor receptors associates with the development and progression of renal fibrosis. Suramin is a compound that inhibits the interaction of several cytokines and growth factors with their receptors, but whether suramin inhibits the progression of renal fibrosis is unknown. Here, treatment of cultured renal interstitial fibroblasts with suramin inhibited their activation induced by TGF-β1 and serum. In a mouse model of obstructive nephropathy, administration of a single dose of suramin immediately after ureteral obstruction abolished the expression of fibronectin, largely suppressed expression of α-SMA and type I collagen, and reduced the deposition of extracellular matrix proteins. Suramin also decreased the expression of multiple cytokines including TGF-β1 and reduced the interstitial infiltration of leukocytes. Moreover, suramin decreased expression of the type II TGF-β receptor, blocked phosphorylation of the EGF and PDGF receptors, and inactivated several signaling pathways associated with the progression of renal fibrosis. In a rat model of CKD, suramin abrogated proteinuria, limited the decline of renal function, and prevented glomerular and tubulointerstitial damage. Collectively, these findings indicate that suramin is a potent antifibrotic agent that may have therapeutic potential for patients with fibrotic kidney diseases.
Tubulointerstitial fibrosis is the final common pathway in late-stage renal disease. The pathogenesis of kidney fibrosis is characterized by overproduction and deposition of extracellular matrix (ECM), which ultimately leads to fibrotic lesions and tissue scarring.1,2 Renal interstitial fibroblasts are the principal effector cells that are responsible for ECM overproduction in the fibrotic kidney, and their activation is regarded as a key event in the pathogenesis of chronic renal fibrosis.3,4 Although the mechanism of myofibroblast activation and the fibrogenesis under various pathologic conditions have not been completely understood, activation of multiple cytokines/growth factor receptors is involved in these processes.5–7
Injury to the kidney is associated with release of cytokines/growth factors such as TGF-β, EGF, and PDGF by damaged or infiltrating cells.5,8,9 An increase in production of TGF-β is one of the most important mechanisms in the pathogenesis of renal fibrogenesis.10 TGF-β1 stimulates fibroblast cell activation and induces matrix expression through its interaction with TGF-β receptors, which are mainly composed of two protein families—type I (TβRI) and type II (TβRII) receptors.11,12 TGF-β1 binds to TβRII, which results in TβRI recruitment to form a heteromeric TGF-β receptor complex. The complex phosphorylates and activates Smad2 and Smad3, the two major Smads that mediate the profibrotic events.13 Other signaling pathways such as extracellular-regulated kinase 1/2 (ERK1/2) can also be activated in response to TGF-β receptor activation.14 Activated ERK1/2 contributes to tubular cell apoptosis in the obstructed kidney.15 Because activation of TGF-β signaling is considered to be the major mechanism that directly promotes fibroblast activation and fibrosis progression, therapeutic intervention of this pathway could be considered as a strategy to halt or prevent renal fibrosis.
Activation of the EGF receptor (EGFR) and PDGF receptor (PDGFR) are also involved in fibroblast activation and fibrogenesis after renal injury.16–19 Upon ligand engagement, EGFR and PDGFR are dimerized and phosphorylated on tyrosine residues. As a result, intracellular signaling pathways including ERK1/2 and signal transducer and activator of transcription 3 (STAT3) are activated.20,21 Our recent studies show that STAT3 was persistently activated after obstructive injury and was involved in activation of renal interstitial fibroblasts; accumulation of inflammatory cells; expression of TGF-β1 and TβRII; and increase of some proinflammatory mediators such as TNF-α, IL-1β, and intercellular adhesion molecule-1 (ICAM-1).22 Importantly, the EGFR not only transduces the fibrotic signal from its ligands, but it also has been regarded as a convergent pathway that integrates, directly or indirectly, the effects of many other fibrogenic factors. For example, angiotensin II and endothelin induce transactivation of the EGFR, and inactivation of the EGFR attenuates renal fibrosis induced by these substances.17,23 Therefore, the EGFR and PDGFR are also potential targets for the prevention and treatment of renal fibrosis.
Currently, drug discovery efforts for fighting renal fibrosis are largely focused on compounds that are specific for a particular receptor or protein kinase. Given that renal fibrogenesis is associated with increased production of multiple cytokines/growth factors and subsequent activation of their receptors and signaling pathways, it is expected that inhibitors with broad specificity might offer improved therapeutic benefit in kidney fibrotic diseases. In this context, it has been reported that suramin, a polysulfonated naphthylurea, has an ability to inhibit activation of multiple cytokine/growth factor (i.e., TGF-β1, EGF, and PDGF) receptors.24–28 The mechanism of action of suramin remains incompletely understood. It has been reported to interfere with the action of growth factors by competitive binding to cytokine/growth factor receptors.29,30 Suramin has been used to treat prostate and breast cancers in clinical settings.31,32 Recent experimental studies showed that it is also effective in attenuating muscle and liver fibrosis.33,34 However, it remains unclear whether suramin has an antifibrotic effect in the kidney.
In this study, we examined the effect of suramin on renal fibroblast activation in normally cultured rat kidney fibroblasts (NRK-49F) and assessed its therapeutic potential in renal fibrosis in animal models of obstructive nephropathy and remnant kidney disease. Furthermore, we investigated the mechanism by which suramin inhibits renal fibroblast activation and fibrogenesis.
RESULTS
Suramin Blocks TGF-β1 and Serum-Induced Expression of α-Smooth Muscle Actin and Fibronectin in Cultured NRK-49F Cells
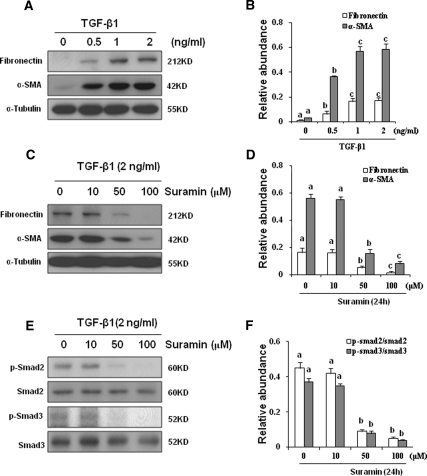
Because TGF-β1 is a major cytokine that induces transformation of quiescent renal fibroblasts to myofibroblasts through activation of Smad2/3,5,12 we first examined the effect of suramin on TGF-β1-induced activation of renal fibroblasts and phosphorylation of Smad2 and Smad3. Figure 1A shows the basal level of α-smooth muscle actin (α-SMA) in serum-starved NRK-49F cells that did not express fibronectin under starved conditions. TGF-β1 treatment dose-dependently increased expression of α-SMA and fibronectin, with the maximum induction observed at 2 ng/ml of TGF-β1. In the presence of suramin, TGF-β1-induced fibronectin expression was abolished and α-SMA expression was suppressed to the basal level (compare Figure 1A, line 1 with Figure 1C, line 4). Suramin treatment also abolished TGF-β1-induced phosphorylation of Smad2 and Smad3 (Figure 1, E and F).
Figure 1.
Suramin inhibits TGF-β1-induced expression of α-SMA and fibronectin and activation of Smad2/3. Serum-starved NRK-49F cells were incubated with various concentrations of TGF-β1 for (A) 24 hours or (C, E) 24 hours with 2 ng/ml TGF-β1 in the presence of suramin (0 to 100 μM). Cell lysates were prepared and subject to immunoblot analysis with antibodies to α-SMA, fibronectin, phosphorylated Smad2, Smad2, phosphorylated Smad3, Smad3, or α-tubulin. Representative immunoblots from three or more experiments are shown. Expression levels of the indicated proteins were quantified by densitometry and normalized with (B, D) α-tubulin or with total Smad2 or Smad3, respectively. Data are represented as the mean ± SEM. Means with different superscript letters are significantly different from one another (P < 0.05).
Multiple growth factors are produced and released to the interstitium to stimulate activation of renal fibroblasts during the course of chronic kidney injury. We further examined whether suramin had the potential to inhibit serum-induced activation of renal fibroblasts. Upon incubation with 5% FBS, α-SMA and fibronectin expression was increased in cultured NRK-49F cells. The presence of suramin inhibited their expression in a dose-dependent manner with complete blockade at 100 μM (Supplemental Figure 1, A and B). Of note, 100 μM suramin did not induce cleavage of poly(ADP-ribose) polymerase (PARP) and caspase-3, two hallmarks of apoptosis, suggesting that it does not cause apoptosis at this concentration. As a positive control, we observed that exposure of NRK-49F to 250 μM hydrogen peroxide resulted in cleavage of these two proteins (Supplemental Figure 1C).
These data collectively indicate that suramin is a potent agent in blocking activation of cultured renal interstitial fibroblasts.
Suramin Inhibits Expression of α-SMA and Fibronectin in the Obstructed Kidney
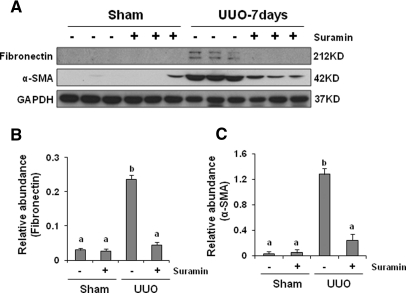
To investigate the ability of suramin to suppress myofibroblast activation in vivo, we examined the effect of suramin on expression of α-SMA and fibronectin in obstructive nephropathy, which is a model of predominantly tubulointerstitial lesions that are characterized by accumulation and activation of myofibroblasts. Because suramin has a half-life of at least 21 days,35 mice were given a single dose of suramin immediately after surgery, and kidneys were collected on days 7 and 21 after the treatment. Western blot analysis of whole kidney lysates indicated increased expression of α-SMA and fibronectin on day 7 that persisted at day 21 after unilateral urethral obstruction (UUO) injury (Figure 2 and Supplemental Figure 3). Suramin administration reduced expression of α-SMA and fibronectin in a dose- and time-dependent manner. A slight reduction of their expression was seen when 1 mg/kg was administered, and further inhibition was observed at 5 mg/kg. Administration of suramin at 10 mg/kg resulted in complete blockade of fibronectin expression and significant but incomplete suppression of α-SMA expression. Increasing the dose of suramin to 20 mg/kg did not further reduce α-SMA expression (Supplemental Figure 2). Administration of 20 mg/kg also suppressed expression of α-SMA and fibronectin by approximately 50% at 21 days after UUO injury (Supplemental Figure 3). These data, together with Figure 1, suggest that suramin has a potent capability to inhibit activation of renal interstitial fibroblasts in vitro and in vivo. A partial suppression of expression of these proteins at 21 days after treatment with suramin might be due to its long half-life.
Figure 2.
Suramin blocks on UUO-induced α-SMA and fibronectin expression. (A) Kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against α-SMA, fibronectin, or glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Expression levels of (B) fibronectin and (C) α-SMA were quantified by densitometry and normalized with GAPDH. Data are represented as the mean ± SEM (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05).
Suramin Attenuates the Progression of Renal Fibrosis and Expression of Collagen 1 after Obstructive Injury
Because the major feature of renal fibrosis is increased levels of ECM, we evaluated the effect of suramin on the expression of interstitial collagen fibrils using Masson trichrome staining. As shown in Figure 3A, kidneys with ureteral obstruction for 7 days displayed severe morphologic lesions characterized by tubular dilation with epithelial atrophy and interstitial expansion with collagen accumulation and deposition as evidenced by an increase in trichrome-positive areas within the tubulointerstitium after UUO injury. By contrast, kidneys from animals injected with suramin exhibited an obvious attenuation of these morphologic lesions with less fibrosis in the interstitium. Because collagen 1 is a major component of the interstitial matrix, we also measured collagen 1 expression, which was upregulated at day 7 after injury, and suramin treatment reduced its expression in a dose-dependent manner with the observed effect at 5 mg/kg. Suramin at a dose of 10 mg/kg resulted in a greater inhibition, and 20 mg/kg of suramin reduced collagen 1 expression to the basal level (Figure 3, B and D, and Supplemental Figure 2). This dose of suramin also partially suppressed collagen 1 expression on day 21 (Supplemental Figure 3). These data showed an efficacy of suramin for inhibiting accumulation of ECM proteins after obstructive injury.
Figure 3.
Suramin attenuates the deposition of ECM and expression of type 1 collagen in obstructed kidneys. (A) Photomicrographs illustrating Masson trichrome staining of kidney tissue after various treatments. (B) Kidney tissue lysates were subject to immunoblot analysis with specific antibodies against collagen I or GAPDH. (C) Expression levels of type 1 collagen were quantified by densitometry and normalized with GAPDH. Data are represented as the mean ± SEM (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05).
Suramin Inhibits Expression of TGF-β1 and TβRII and Phosphorylation of Smad2/3 in the Obstructed Kidney
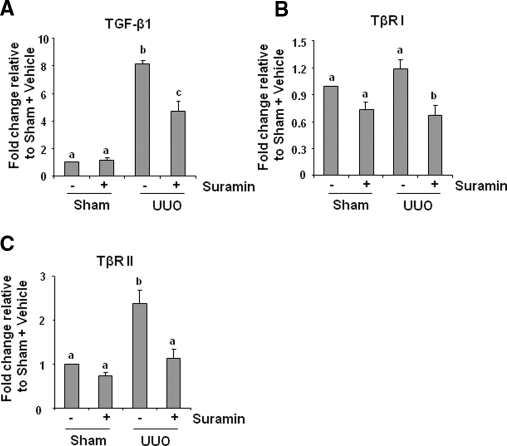
The TGF-β signaling pathway is associated with almost all forms of kidney diseases characterized by interstitial fibrosis, and an increase in the expression of TGF-β and/or its receptors has been considered as a major mechanism of renal fibrosis.1,12 We examined the level of expression of TGF-β1, TβRI, and TβRII in the obstructed kidneys by quantitative real-time PCR. As shown in Figure 4, ureteral obstruction markedly induced the expression of TGF-β1 and TβRII, and administration of suramin reduced their expression to 45% and 94%, respectively. Although UUO injury did not significantly increase expression of the TβRI, suramin treatment suppressed its basal expression in the obstructed kidney.
Figure 4.
Suramin suppresses on mRNA expression of TGF-β1 and TGF-β receptors. mRNA was extracted from sham-operated or obstructed kidneys of mice administered with/without suramin and subjected to quantitative real-time RT-PCR as described under Concise Methods. mRNA expression levels of (A) TGF-β1, (B) TβRI, and (C) TβRII were indicated as fold induction over control (sham-operated mice treated with vehicle). Data are represented as the mean ± SEM (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05).
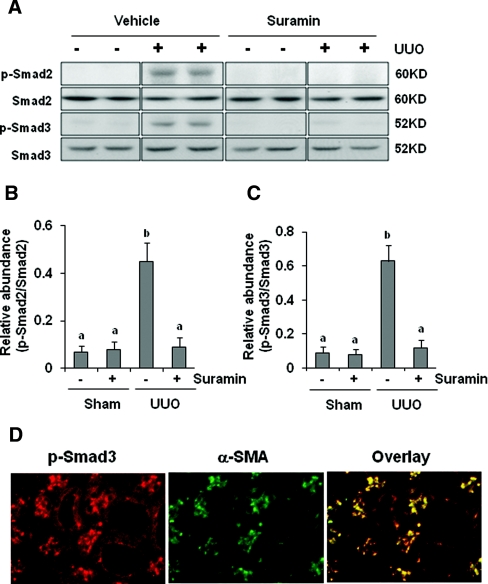
The TGF-β receptor activation triggers phosphorylation and activation of downstream signaling mediators such as Smad2 and Smad3. Phosphorylated Smad2/3 subsequently translocates into the nucleus, where it controls the transcription of TGF-β response genes required for the development of fibrosis.36,37 Western blot analysis of kidney lysates indicated that UUO injury-induced phosphorylation of Smad2 and Smad3 was abolished upon administration of suramin. Expression of total Smad2 and Smad3 was not affected by UUO injury and suramin (Figure 5A through 5C). Immunostaining showed that phosphorylated Smad3 is mainly expressed in the interstitial cells as indicated by its colocalization with α-SMA, and it was also observed, to a lesser degree, in renal tubular cells (Figure 5D). Taken together, suramin is a potent inhibitor for TGF-β1 signaling.
Figure 5.
Suramin abolishes phosphorylation of Smad2 and Smad3. (A) Kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against Smad2 and Smad3. Expression levels of (B) p-Smad2 and (C) p-Smad3 were quantified by densitometry and normalized with Smad2 and Smad3, respectively. Data are represented as the mean ± SEM (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05). (D) Kidney tissue collected at day 7 was used for co-staining with antibodies to phosphorylated Smad3 and α-SMA.
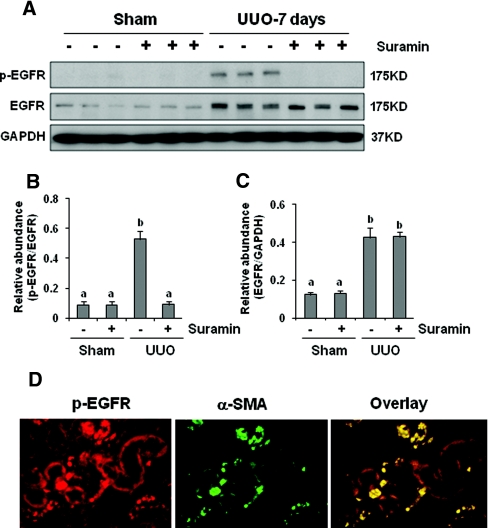
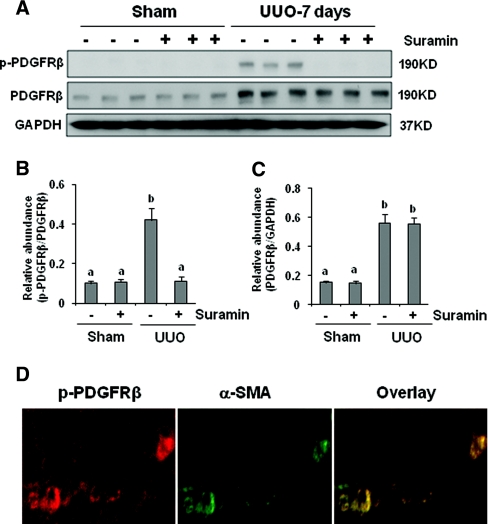
Suramin Suppresses EGFR and PDGFR Phosphorylation in the Obstructed Kidney
In addition to TGF-β signaling, activation of EGFR and PDGFR also contributes to the development/progression of renal interstitial fibrosis.16–19 We further examined the effect of suramin on the activation of these two receptors. As shown in Figures 6 and 7, increased EGFR and PDGFR phosphorylation (activation) was detected in the obstructed kidney, and suramin treatment abolished this response. UUO injury also increased expression levels of total EGFR and PDGFR; however, suramin did not have an inhibitory effect on their expression. Phosphorylated EGF receptor (p-EGFR) was localized in tubular and interstitial cells. In the latter, the p-EGFR was colocalized with α-SMA (Figure 6D), suggesting its expression in renal interstitial fibroblasts. As expected, the PDGFR was primarily localized in renal interstitial fibroblasts, as indicated by its colocalization with α-SMA (Figure 7D).
Figure 6.
Suramin abolishes phosphorylation of EGFR. Kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against (A) p-EGFR and EGFR. Expression levels of (B) p-EGFR and (C) EGFR were quantified by densitometry and normalized with GAPDH. Data are represented as the mean ± SEM (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05). (D) Kidney tissue collected at day 7 was used for co-staining with antibodies to p-EGFR and α-SMA.
Figure 7.
Suramin blocks phosphorylation of PDGFR. (A) Kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against (A) phosphorylated PDGF receptor-β (p-PDGFRβ) and PDGFR. Expression levels of (B) p-PDGFRβ and (C) PDGFRβ were quantified by densitometry and normalized with GAPDH. Data are represented as the mean ± SEM (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05). (D) Kidney tissue collected at day 7 was used for co-staining with antibodies to p-PDGFRβ and α-SMA.
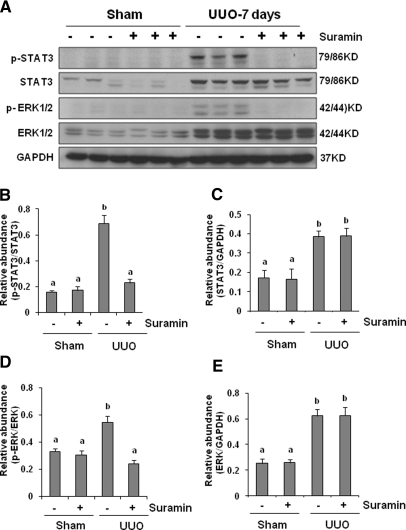
Suramin Inhibits Phosphorylation of STAT3 and ERK1/2 in the Kidney after Obstructive Injury
As a consequence of cytokine/growth factor receptor activation, some intracellular signaling pathways associated with renal fibrosis are activated. Studies from our laboratory and others have shown that the STAT3 and ERK1/2 signaling pathways are activated in the obstructed kidney and they are associated with progression of renal fibrosis in the murine model of UUO.15,22 We thus sought to determine whether suramin is able to suppress the phosphorylation of these two signaling molecules. As shown in Figure 8A, phosphorylation of STAT3 and ERK1/2 was barely detected in the sham-treated kidney, but a dramatic increase in activation of those molecules was observed in the obstructed kidney. Suramin treatment abolished phosphorylation of STAT3 and ERK1/2. The expression level of total STAT3 and ERK1/2 was also remarkably increased in UUO injury when compared with normal kidneys. However, suramin did not affect this response.
Figure 8.
Suramin blocks phosphorylation of STAT3 and ERK1/2. (A) Kidney tissue lysates were subjected to immunoblot analysis with specific antibodies against phosphorylated STAT3, STAT3, phosphorylated ERK1/2, and ERK1/2. Expression levels of phosphorylated STAT3, STAT3, phosphorylated ERK1/2, and ERK1/2 were quantified by densitometry. Activated (B) STAT3 and (D) ERK1/2 were depicted with phosphorylated STAT3/STAT3 and phosphorylated ERK/ERK ratios, respectively. Protein levels of (C) STAT3 and (E) ERK1/2 were normalized with GADPH. Data are represented as the mean ± SEM (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05).
Suramin Inhibits Phosphorylation of STAT3, ERK1/2, EGFR, and PDGFR in Cultured Renal Interstitial Fibroblasts
To specifically understand the effect of suramin on phosphorylation of STAT3, ERK1/2, EGFR, and PDGFR in renal interstitial fibroblasts, NRK-49F cells were exposed to suramin at different doses. As shown in Supplemental Figure 4, phosphorylation of all of these kinases was detected in normally cultured NRK-49F cells exposed to serum (5% FBS) and suramin treatment inhibited their phosphorylation in a dose-dependent manner with a complete inhibition at a dose of 100 μM.
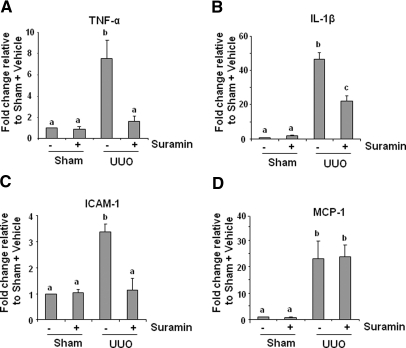
Suramin Inhibits Expression of Cytokines in the Kidney after Obstructive Injury
It has been reported that proinflammatory cytokines such as TNF-α, IL-1β, ICAM-1, and monocyte chemotactic protein-1 (MCP-1) are upregulated in the fibrotic kidney22 and contribute to the progression of renal fibrosis.5,7,38 To determine the effect of suramin on expression of these cytokines, we measured their expression levels in the fibrotic kidney of animals treated or untreated with suramin using quantitative real-time PCR. Figure 9 shows that all of these cytokines were increased after obstructive injury; suramin treatment decreased expression levels of TNF-α, IL-1β, and ICAM-1 but not MCP-1. These data suggest that suramin selectively regulates expression of proinflammatory factors in the kidney.
Figure 9.
Suramin suppresses on the expression of TNF-α, IL-1β, MCP-1, and ICAM-1. mRNA extracted from kidney tissues were subjected to quantitative real-time RT-PCR as described under Concise Methods. mRNA expression levels of (A) TNF-α, (B) IL-1β, (C) ICAM-1, and (D) MCP-1 were indicated as fold induction over control (sham with vehicle). Data are represented as the mean ± SEM (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05).
Suramin Inhibits Leukocyte Infiltration in the Kidney after Obstructive Injury
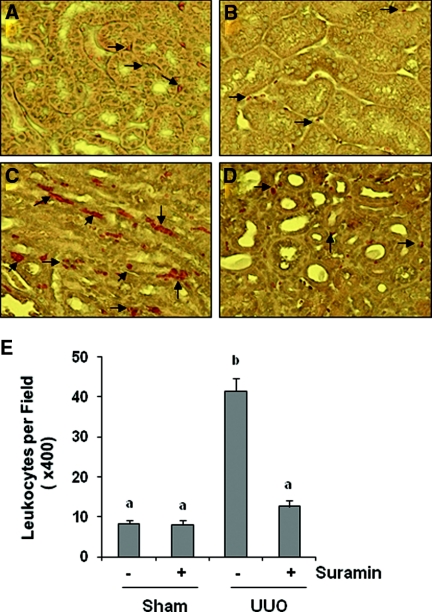
We also examined the effect of suramin on leukocyte infiltration in the kidney after obstructive injury. Staining of kidney sections with naphthol-AS-D-chloroacetate esterase showed an increase of neutrophil infiltration in the interstitium and monocytes after obstructive injury. Suramin treatment reduced leukocyte infiltration to basal levels (Figure 10).
Figure 10.
Suramin inhibits on infiltration of leukocytes. Kidney sections were evaluated for infiltration of neutrophils and monocytes with naphthol AS-D-chloroacetate esterase staining. Photomicrographs illustrate infiltration of neutrophils and monocytes (red color directed by arrows) in different groups: (A) sham with vehicle, (B) sham with suramin, (C) UUO with vehicle, and (D) UUO with suramin. (E) Infiltrated neutrophils and monocytes were counted in three random fields of each sample, and 18 fields (1 × 200) were analyzed for each condition. Data are represented as the mean ± SEM (n = 6). Means with different superscript letters are significantly different from one another (P < 0.05).
Administration of Suramin Prevents a Rise of Serum Creatinine and Proteinuria and Attenuates the Fibrotic Lesions after Renal Ablation.
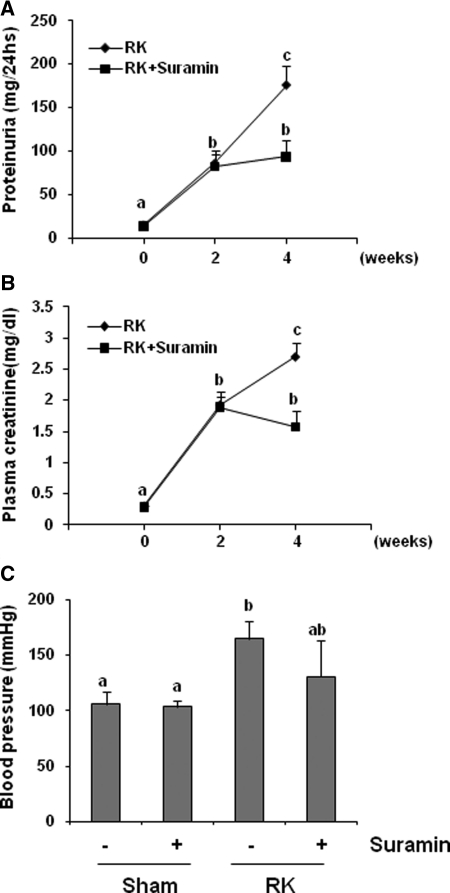
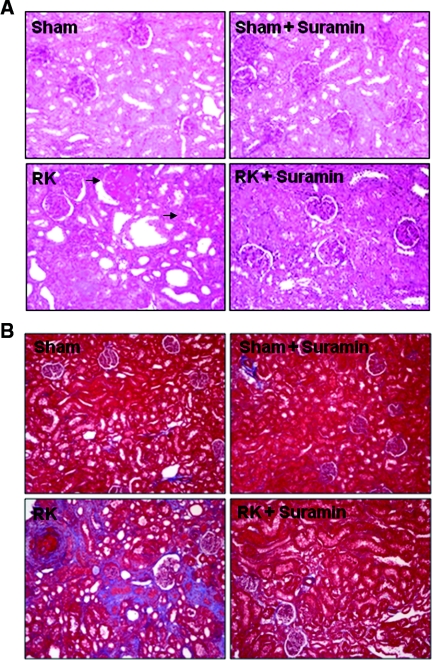
We further examined the therapeutic potential of suramin in a rat model of remnant kidney disease after 5/6 nephrectomy. Two weeks after surgery, rats developed progressive renal injury as evidenced by the significant increase in 24-hour proteinuria, serum creatinine, and mean BP (Figures 11A through 11C). Suramin administration significantly reduced proteinuria and serum creatinine; however, hypertension was not significantly affected by suramin. Histologic analysis showed the remnant kidney without suramin treatment developed severe tubulointerstitial damage (as shown by tubular atrophy and dilation and interstitial fibrosis) and glomerular damage (as displayed by severe focal and segmental glomerulosclerosis). In contrast, administration of suramin preserved kidney architecture and prevented the glomerular and tubulointerstitial damage that follows 5/6 nephrectomy. There were no obvious pathologic changes in the kidney of sham- and suramin-alone-treated animals (Figure 12). In addition, immunoblot analysis of kidney tissue indicated that suramin administration abolished the de novo expression of α-SMA and fibronectin and inhibited expression of type 1 collagen in the remnant kidney (Supplemental Figure 5). Therefore, suramin is effective in preventing the renal injury progression in rats with remnant kidney independent of changes in BP.
Figure 11.
Suramin improves renal function in 5/6 nephrectomy in rats. Rats received vehicle or suramin from day 14 to week 4 after surgery (n = 10). A parallel study was conducted in rats after sham operation (n = 6). Rats were sacrificed on day 28. (A) Twenty-four-hour urinary protein excretion, (B) serum creatinine, and (C) mean artery pressure. Data are represented as the mean ± SEM. Means with different superscript letters are significantly different from one another (P < 0.05).
Figure 12.
Surramin attenuates renal histologic damage and ECM protein deposition in 5/6 nephrectomy in rats. (A) Photomicrographs (100×) illustrating periodic-acid–Schiff-stained sections of kidney tissue on week 4 after various treatments as indicated. Sclerotic glomeruli were indicated with arrows. (B) Photomicrographs (100×) illustrating trichrome-stained kidney tissue on week 4 after various treatments. RK, remnant kidney.
DISCUSSION
Suramin is a U.S. Food and Drug Administration approved drug for treating trypanosomiaisis and selected malignancies and metastatic diseases, and it has a broad inhibitory effect on the activation of growth factor/cytokine receptors.24–28 Given the fact that numerous cytokines and growth factors are involved in activation of renal interstitial fibroblasts and progression of tubulointerstitial scarring, inhibition of a single receptor/signaling pathway may not completely block these processes. In this study, we examined the effect of suramin on the activation of renal interstitial fibroblasts and the progression of renal fibrosis. We demonstrated that suramin treatment blocks activation of renal interstitial fibroblasts in vitro and in vivo in animal models of chronic kidney disease. Suramin also reduces renal interstitial matrix deposition and inflammatory responses after UUO injury. Furthermore, suramin inhibited the progression of renal fibrosis and prevented renal function impairment in a rat model of remnant kidney disease. These findings suggest that suramin is an effective agent against renal fibrogenesis.
A key investigation was that administration of a single dose of suramin effectively inhibited activation of renal fibroblasts and attenuated the development of renal fibrosis at 7 and 21 days after UUO injury. This is in contrast to most inhibitors, which need daily administration to achieve their inhibitory effects. Such a long-term effect of suramin may be due to its accumulation in the kidney and long half-life in the body. Suramin was reported to be mostly accumulated in the kidney after injection and has a half-life of 21 days.35,39 Therefore, we speculate that the actual concentration of suramin in the kidney may be higher than in the blood and that a low dose would result in a therapeutic effect. Indeed, we observed that suramin at 5 mg/kg, a dose that is sixfold lower than the dose used for treating patients with tumors (usually 30 mg/kg),40 decreased expression of α-SMA and fibronectin and suppressed expression of collagen 1 and deposition of ECM. At 10 mg/kg, suramin also prevented the rise of 24-hour proteinuria and serum creatinine, blocked activation of renal interstitial fibroblasts, and attenuated renal fibrosis and glomerular damage in the remnant kidney disease. However, suramin administration did not significantly lower BP in this hypertensive kidney disease model, suggesting that it exerts those renoprotective effects through a mechanism independent of changes in BP.
The antifibrotic actions of suramin may be through multiple mechanisms. Because the upregulation of TGF-β1 and its receptors is an early event preceding the onset of significant renal fibrosis,1,2 we first examined the effect of suramin on their expression and activation of TGF-β signaling in the model of UUO. Our studies clearly indicated that suramin treatment suppressed the expression of TGF-β1 and TβRII and blocked phosphorylation of Smad2 and Smad3. Smad2 and Smad3 are two key downstream signaling mediators of TGF-β pathways, and their phosphorylation reflects activation of TGF-β receptors. As such, our results reveal that suramin not only inhibited expression of TGF-β1 and TβRII, but it also blocked their interaction in the obstructed kidneys. In support of this, we demonstrated that suramin blocked exogenous TGF-β-induced activation of renal fibroblasts and phosphorylation of Smad2 and Smad3 in cultured NRK-49F cells. Because Smad2 and Smad3 play a critical role in transducing TGF-β signaling to induce expression of numerous profibrotic genes, the inhibition of renal fibrosis by suramin may be mediated, in part, by the suppression of the TGF-β1 axis in the obstructed kidneys.
Inactivation of growth factor receptors may also be associated with the antifibrotic actions of suramin. Previous studies have shown that EGFR and PDGFR are implicated in the pathogenesis of renal fibrosis.16–19 In this study, we demonstrated that suramin administration abolished phosphorylation of EGFR and PDGFR in the obstructed kidney and in cultured renal interstitial fibroblasts. This action of suramin is important because activation of EGFR not only mediates fibrogenesis stimulated by its ligands, but it also mediates the fibrogenic effects of many metabolic, hormonal, and hemodynamic factors in chronic kidney disease through a mechanism of transactivation. For example, angiotensin-II-induced renal fibrosis was blocked by overexpression of a dominant negative isoform of EGFR in mice17; endothelin-induced renal vascular and glomerular fibrosis was attenuated by EGFR inhibitors.23 Moreover, tubulointerstitial lesions after ischemic renal injury can also be reduced by functional inactivation of EGFR.16 Although the PDGFR cannot transduce the fibrotic signal from other substances, it is a major growth factor receptor expressed in the fibroblast and is critically involved in the progression of renal interstitial fibrosis.41 Furthermore, PDGF is able to stimulate protein synthesis and secretion of TGF-β1 in human renal proximal tubular cells.42 Therefore, inhibition of these two receptors by suramin may have an additive or synergic therapeutic effect with inhibition of TGF-β1-Smad signaling in attenuation of progression of renal fibrosis. The antifibrotic effect of suramin may not be limited to its inhibition on those receptors. Suramin is also a potent inhibitor for purinergic receptors and sphingosine-1-phosphate receptors.34,43 Recent studies have shown that the development of renal fibrosis after UUO injury and of hepatic fibrosis after bile duct ligation is associated with P2X7 and sphingosine-1-phosphate receptors.44,45 Further investigations are needed to evaluate whether the antifibrotic action of suramin is also associated with inhibition of these two receptors in the kidney.
Inflammation is an important mechanism in initiation and maintenance of renal damage, and decreased inflammatory response results in attenuation of renal fibrosis.1,6 The mechansim(s) by which suramin reduces inflammation is currently not clear. However, our data indicate that suramin treatment is also able to suppress expression of multiple cytokines (including TNFα, IL-β1, and ICAM-1) and leukocyte infiltration,22 suggesting that inhibition of the inflammatory response is also one of the mechanisms by which suramin attenuates renal fibrosis. TGF-β1 and other growth factors can activate injured tubular cells and immune system cells to produce inflammatory cytokines and unleash an inflammatory response, which in turn activates tubule cells, fibroblasts, and myofibroblasts to produce ECM and amplifies fibrosis and tubular damage.46,47 Therefore, the beneficial effect of suramin may be mediated primarily by its ability to block activation of TGF-β and/or growth factor receptors and consequent inactivation of the STAT3 signaling pathway. This hypothesis is supported by our recent observations that the pharmacologic blockade of STAT3 signaling inhibited expression of all of those inflammatory mediators and by the data presented here showing that suramin abolished phosphorylation of Smad2/3, EGFR, PDGFR, and STAT3.
STAT3 and ERK1/2 are two pathways that are activated by different receptors and are involved in numerous events related to renal fibrosis.15,22 In particular, STAT3 is a transcriptional factor that can directly bind to the promoter region of TGF-β1, and it induces activation of its promoter activity in vitro.48 Our recent studies showed that UUO injury led to the activation of STAT3, and administration of S3I-201, a selective inhibitor, decreased activation of renal interstitial fibroblasts and reduction of expression of TGF-β1 and TβRII as well as TNF-α, IL-β, and ICAM-1 and leukocyte infiltration.22 Thus, inactivation of STAT3 may mediate the antifibrotic action of suramin. In this study, we found that suramin abolished phosphorylation of STAT3 in the obstructed kidney and in cultured renal interstitial fibroblasts, favoring our hypothesis. In addition, we found that suramin also abolished renal ERK1/2 phosphorylation after UUO injury. Because ERK1/2 activation has been reported to contribute to tubular apoptosis in the murine model of UUO,49 inactivation of the ERK1/2 pathway by suramin may reduce tubular atrophy, a degenerative response after tubular cell death. Therefore, inhibition of the STAT3 and ERK1/2 signaling pathways may be involved in the antifibrotic effect of suramin in obstructive nephropathy.
It has been documented that suramin administration in humans causes some nonspecific adverse effects when cytotoxic doses of suramin (yielding >200 μM plasma concentrations) were used.50 The most common side effects of suramin are polyneuropathy, allergic skin rash, anemia, fatigue, and nausea/vomiting.50 However, recent studies showed that administration of a low dose of suramin (10 mg/kg, twice per week) that yields 10- to 20-μM plasma concentrations enhances the antitumor effect of some anticancer drugs and is well tolerated by patients.51,52 Because effective doses of suramin in our studies were 5 to 10 mg/kg, it is expected that suramin may attenuate renal fibrosis without causing obvious adverse effects. Nonetheless, treatment of chronic fibrotic kidney disease requires a long duration of drug administration. Clinical trials are required to assess its efficacy and safety in patients with kidney diseases before clinical application.
In summary, we demonstrate for the first time that suramin inhibits activation of renal fibroblasts and prevents development and progression of renal interstitial fibrosis and glomerulosclerosis in animal models of chronic kidney disease. These antifibrotic effects of suramin are associated with inactivation of multiple cytokine/growth factor receptors and signaling pathways as well as inhibition of inflammatory responses. Because suramin inhibits renal fibrosis at low doses that have been safely used in human diseases, it has therapeutic potential for treatment of patients with chronic kidney disease.
CONCISE METHODS
Chemicals and Antibodies
Antibodies to phosphorylated STAT3, STAT3, phosphorylated Smad2, Smad2, phosphorylated Smad3, Smad3, phosphorylated ERK1/2, ERK1/2, p-EGFR, PDGFR, cleaved caspase-3, and cleaved PARP were purchased from Cell Signaling Technology (Danvers, MA). Antibodies to fibronectin, collagen 1(A2), glyceraldehyde 3-phosphate dehydrogenase, EGFR, and phosphorylated PDGFβ receptor were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Primers were synthesized from Invitrogen (Carlsbad, CA). Suramin, antibodies to α-SMA and α-tubulin, the naphthol AS-D-chloroacetate esterase kit, and all other chemicals were from Sigma (St. Louis, MO).
Cell Culture and Treatments
NRK-49F cells were cultured in DMEM (Sigma-Aldrich, St. Louis, MO) containing 5% FBS and 0.5% penicillin and streptomycin in an atmosphere of 5% carbon dioxide and 95% air at 37°C. To determine the effects of suramin on fibroblast activation, suramin was directly added to subconfluent NRK-49F cells and then incubated for the indicated time as described in the figure legends. For TGF-β1 treatment, NRK-49F cells were starved for 24 hours by incubation with 0.5% FBS containing DMEM and then exposed to TGF-β1 (0.5 to 2 ng/ml) for 24 hours.
UUO and Remnant Kidney Models and Suramin Treatment
The UUO model was established in male C57 black mice that weighed 20 to 25 g (Jackson Laboratory, Bar Harbor, ME) as described in our previous study.53 Briefly, the abdominal cavity was exposed via a midline incision and the left ureter was isolated and ligated. The contralateral kidney was used as a control. To examine the efficacy of suramin in renal fibrosis after UUO injury, a single dose of various concentrations of suramin (1, 5, 10, 20 mg/kg) in 50 μl of PBS was intraperitoneally administered immediately after ureteral ligation. The animals were sacrificed and the kidneys were removed at day 7 for protein and mRNA analysis and histologic examination. For the time-course study, a single dose of suramin (20 mg/kg) was injected and the mice were sacrificed on day 7 or 21 to collect kidneys. Six mice were used in each group. Data from mice treated with suramin (20 mg/kg) for 7 days were shown unless specifically indicated.
The remnant kidney model was created in male Sprague–Dawley rats that weighed 180 to 200 g (Charles River Laboratories, Wilmington, MA). Five-sixths of normal renal mass was surgically ablated according to our previous protocols.54 Briefly, 20 animals underwent subtotal nephrectomy involving right subcapsular nephrectomy and infarction of approximately two-thirds of the left kidney by ligation of the posterior and one or two anterior extrarenal branches of the renal artery. In addition, 12 rats underwent a sham operation (laparotomy and manipulation of the renal pedicles). On the second week after surgery, rats were randomly divided into suramin treatment and nontreatment groups. Suramin was given at 10 mg/kg once per week for two weeks. Twenty-four-hour urine samples were collected in metabolic cages at day 0 and weekly for the determination of urinary levels of protein. Blood was taken weekly for the measurement of serum creatinine. At 28 days after surgery, mean BP was measured, and then all animals were sacrificed and kidneys were collected for further analysis.
Assessment of Renal Function and Measurement of BP
Urinary protein concentrations were determined using the colorimetric Lowry assay. Serum creatinine was analyzed using a creatinine kit (Sigma). Mean BP was determined via a catheter inserted into the femoral artery attached to the Transonic Systems pressure transducer (Transonic Systems, Inc. Ithaca, NY) in anesthetized rats at the end of experiment.
Immunoblot Analysis
Immunoblot analysis of NRK-49F cells and tissue samples were conducted as described previously.53 The densitometry analysis of immunoblot results was conducted by using National Institutes of Health (NIH) Image software (NIH, Bethesda, MD).
Immunofluorescent and Immunohistochemical Staining
Immunofluorescent and immunohistochemical staining was performed according to the procedure described in our previous studies.53 For assessment of renal fibrosis, Masson trichrome staining was performed according to the protocol provided by the manufacturer (Sigma, St. Louis, MO). The collagen tissue area (blue color) was quantitatively measured using Image Pro-Plus software (Media-Cybernetics, Silver Spring, MD) by drawing a line around the perimeter of the positive staining area, and the average ratio of each microscopic field (400×) was calculated and graphed.
Quantitative Real-Time PCR
The procedure for quantitative real-time PCR and the primers used for all measurements have been described previously.22 Briefly, total RNA from mouse kidney tissue was extracted using an RNeasy kit (QIAGEN, Valencia, CA). RNA (1 μg) was reverse transcribed using the AMV First Strand cDNA Synthesis kit (Roche Diagnostics Corporation, Indianapolis, IN) and random oligodeoxynucleotide primers. Then, quantitative real-time PCR amplifications were performed. Relative mRNA abundance was determined from the nanogram ratios of specific mRNA to 18S ribosomal RNA measured in the same samples, and fold change was calculated relative to the group of sham-operated mice with vehicle treatment. Template-free reactions for each pair of primers were also included as controls.
Statistical Analysis
All of the experiments were conducted at least three times. Data depicted in graphs represent the mean ± SEM for each group. Intergroup comparisons were made using one-way ANOVA. Multiple means were compared using Tukey's test. The differences between two groups were determined by t test. The statistically significant difference between mean values was marked in each graph. P < 0.05 was considered significant.
DISCLOSURES
None.
Acknowledgments
This work was supported by grants from NIH (DK-071997 and DK-085065) and the Brown Kidney Foundation.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Wynn TA: Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neilson EG: Mechanisms of disease: Fibroblasts—A new look at an old problem. Nat Clin Pract Nephrol 2: 101–108, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Lin SL, Kisseleva T, Brenner DA, Duffield JS: Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boor P, Ostendorf T, Floege J: Renal fibrosis: Novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 6: 643–656, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Grande MT, Lopez-Novoa JM: Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol 5: 319–328, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Misseri R, Rink RC, Meldrum DR, Meldrum KK: Inflammatory mediators and growth factors in obstructive renal injury. J Surg Res 119: 149–159, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Misseri R, Meldrum KK: Mediators of fibrosis and apoptosis in obstructive uropathies. Curr Urol Rep 6: 140–145, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Couser WG, Johnson RJ: Mechanisms of progressive renal disease in glomerulonephritis. Am J Kidney Dis 23: 193–198, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Floege J, Eitner F, Alpers CE: A new look at platelet-derived growth factor in renal disease. J Am Soc Nephrol 19: 12–23, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Eddy AA: Molecular basis of renal fibrosis. Pediatr Nephrol 15: 290–301, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Bottinger EP: TGF-beta in renal injury and disease. Semin Nephrol 27: 309–320, 2007 [DOI] [PubMed] [Google Scholar]
- 12. Liu Y: Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Wang W, Koka V, Lan HY: Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology (Carlton) 10: 48–56, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Song CY, Kim BC, Hong HK, Lee HS: TGF-beta type II receptor deficiency prevents renal injury via decrease in ERK activity in crescentic glomerulonephritis. Kidney Int 71: 882–888, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Pat B, Yang T, Kong C, Watters D, Johnson DW, Gobe G: Activation of ERK in renal fibrosis after unilateral ureteral obstruction: Modulation by antioxidants. Kidney Int 67: 931–943, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Terzi F, Burtin M, Hekmati M, Federici P, Grimber G, Briand P, Friedlander G: Targeted expression of a dominant-negative EGF-R in the kidney reduces tubulo-interstitial lesions after renal injury. J Clin Invest 106: 225–234, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lautrette A, Li S, Alili R, Sunnarborg SW, Burtin M, Lee DC, Friedlander G, Terzi F: Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: A new therapeutic approach. Nat Med 11: 867–874, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Ludewig D, Kosmehl H, Sommer M, Bohmer FD, Stein G: PDGF receptor kinase blocker AG1295 attenuates interstitial fibrosis in rat kidney after unilateral obstruction. Cell Tissue Res 299: 97–103, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Bonner JC: Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev 15: 255–273, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC: Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res 67: 9066–9076, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vignais ML, Sadowski HB, Watling D, Rogers NC, Gilman M: Platelet-derived growth factor induces phosphorylation of multiple JAK family kinases and STAT proteins. Mol Cell Biol 16: 1759–1769, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pang M, Ma L, Gong R, Tolbert E, Mao H, Ponnusamy M, Chin YE, Yan H, Dworkin LD, Zhuang S: A novel STAT3 inhibitor, S3I-201, attenuates renal interstitial fibroblast activation and interstitial fibrosis in obstructive nephropathy. Kidney Int 78: 257–268, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Flamant M, Tharaux PL, Placier S, Henrion D, Coffman T, Chatziantoniou C, Dussaule JC: Epidermal growth factor receptor trans-activation mediates the tonic and fibrogenic effects of endothelin in the aortic wall of transgenic mice. FASEB J 17: 327–329, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Coffey RJ, Jr, Leof EB, Shipley GD, Moses HL: Suramin inhibition of growth factor receptor binding and mitogenicity in AKR-2B cells. J Cell Physiol 132: 143–148, 1987 [DOI] [PubMed] [Google Scholar]
- 25. Kloen P, Jennings CL, Gebhardt MC, Springfield DS, Mankin HJ: Suramin inhibits growth and transforming growth factor-beta 1 (TGF-beta 1) binding in osteosarcoma cell lines. Eur J Cancer 30A: 678–682, 1994 [DOI] [PubMed] [Google Scholar]
- 26. Fujiuchi S, Ohsaki Y, Kikuchi K: Suramin inhibits the growth of non-small-cell lung cancer cells that express the epidermal growth factor receptor. Oncology 54: 134–140, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Abdiu A, Larsson SE, Wasteson A, Walz TM: Suramin blocks growth-stimulatory effects of platelet-derived growth factor on malignant fibrous histiocytomas in vitro. Cancer Lett 146: 189–194, 1999 [DOI] [PubMed] [Google Scholar]
- 28. Stein CA, LaRocca RV, Thomas R, McAtee N, Myers CE: Suramin: An anticancer drug with a unique mechanism of action. J Clin Oncol 7: 499–508, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Zumkeller W, Schofield PN: Growth factors, cytokines and soluble forms of receptor molecules in cancer patients. Anticancer Res 15: 343–348, 1995 [PubMed] [Google Scholar]
- 30. Stein CA: Suramin: A novel antineoplastic agent with multiple potential mechanisms of action. Cancer Res 53: 2239–2248, 1993 [PubMed] [Google Scholar]
- 31. Vogelzang NJ, Karrison T, Stadler WM, Garcia J, Cohn H, Kugler J, Troeger T, Giannone L, Arrieta R, Ratain MJ, Vokes EE: A Phase II trial of suramin monthly × 3 for hormone-refractory prostate carcinoma. Cancer 100: 65–71, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Woll PJ, Ranson M, Margison J, Thomson Y, van der Water L, George N, Howell A: Suramin for breast and prostate cancer: A pilot study of intermittent short infusions without adaptive control. Ann Oncol 5: 597–600, 1994 [DOI] [PubMed] [Google Scholar]
- 33. Chan YS, Li Y, Foster W, Horaguchi T, Somogyi G, Fu FH, Huard J: Antifibrotic effects of suramin in injured skeletal muscle after laceration. J Appl Physiol 95: 771–780, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Li C, Jiang X, Yang L, Liu X, Yue S, Li L: Involvement of sphingosine 1-phosphate (SIP)/S1P3 signaling in cholestasis-induced liver fibrosis. Am J Pathol 175: 1464–1472, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McNally WP, DeHart PD, Lathia C, Whitfield LR: Distribution of [14C]suramin in tissues of male rats following a single intravenous dose. Life Sci 67: 1847–1857, 2000 [DOI] [PubMed] [Google Scholar]
- 36. Bhogal RK, Stoica CM, McGaha TL, Bona CA: Molecular aspects of regulation of collagen gene expression in fibrosis. J Clin Immunol 25: 592–603, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Kaminska B, Wesolowska A, Danilkiewicz M: TGF beta signalling and its role in tumour pathogenesis. Acta Biochim Pol 52: 329–337, 2005 [PubMed] [Google Scholar]
- 38. Bani-Hani AH, Campbell MT, Meldrum DR, Meldrum KK: Cytokines in epithelial-mesenchymal transition: A new insight into obstructive nephropathy. J Urol 180: 461–468, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Jodrell DI, Reyno LM, Sridhara R, Eisenberger MA, Tkaczuk KH, Zuhowski EG, Sinibaldi VJ, Novak MJ, Egorin MJ: Suramin: Development of a population pharmacokinetic model and its use with intermittent short infusions to control plasma drug concentration in patients with prostate cancer. J Clin Oncol 12: 166–175, 1994 [DOI] [PubMed] [Google Scholar]
- 40. Eisenberger MA, Reyno LM: Suramin. Cancer Treat Rev 20: 259–273, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Tang WW, Ulich TR, Lacey DL, Hill DC, Qi M, Kaufman SA, Van GY, Tarpley JE, Yee JS: Platelet-derived growth factor-BB induces renal tubulointerstitial myofibroblast formation and tubulointerstitial fibrosis. Am J Pathol 148: 1169–1180, 1996 [PMC free article] [PubMed] [Google Scholar]
- 42. Phillips AO, Steadman R, Topley N, Williams JD: Elevated d-glucose concentrations modulate TGF-beta 1 synthesis by human cultured renal proximal tubular cells. The permissive role of platelet-derived growth factor. Am J Pathol 147: 362–374, 1995 [PMC free article] [PubMed] [Google Scholar]
- 43. Dranoff JA, Kruglov EA, Abreu-Lanfranco O, Nguyen T, Arora G, Jain D: Prevention of liver fibrosis by the purinoceptor antagonist pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate (PPADS). In Vivo 21: 957–965, 2007 [PubMed] [Google Scholar]
- 44. Goncalves RG, Gabrich L, Rosario A, Jr, Takiya CM, Ferreira ML, Chiarini LB, Persechini PM, Coutinho-Silva R, Leite M, Jr: The role of purinergic P2X7 receptors in the inflammation and fibrosis of unilateral ureteral obstruction in mice. Kidney Int 70: 1599–1606, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Xin C, Ren S, Kleuser B, Shabahang S, Eberhardt W, Radeke H, Schafer-Korting M, Pfeilschifter J, Huwiler A: Sphingosine 1-phosphate cross-activates the Smad signaling cascade and mimics transforming growth factor-beta-induced cell responses. J Biol Chem 279: 35255–35262, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Border WA, Noble NA: TGF-beta in kidney fibrosis: A target for gene therapy. Kidney Int 51: 1388–1396, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Tamaki K, Okuda S: Role of TGF-beta in the progression of renal fibrosis. Contrib Nephrol 139: 44–65, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Ogata H, Chinen T, Yoshida T, Kinjyo I, Takaesu G, Shiraishi H, Iida M, Kobayashi T, Yoshimura A: Loss of SOCS3 in the liver promotes fibrosis by enhancing STAT3-mediated TGF-beta1 production. Oncogene 25: 2520–2530, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Rodriguez-Pena AB, Grande MT, Eleno N, Arevalo M, Guerrero C, Santos E, Lopez-Novoa JM: Activation of Erk1/2 and Akt following unilateral ureteral obstruction. Kidney Int 74: 196–209, 2008 [DOI] [PubMed] [Google Scholar]
- 50. Kaur M, Reed E, Sartor O, Dahut W, Figg WD: Suramin's development: What did we learn? Invest New Drugs 20: 209–219, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Song S, Yang F, Au JL, Wientjes MG: Nontoxic doses of suramin enhance activity of doxorubicin in prostate tumors. J Pharmacol Exp Ther 299: 426–433, 2001 [PubMed] [Google Scholar]
- 52. Lam ET, Au JL, Otterson GA, Guillaume Wientjes M, Chen L, Shen T, Wei Y, Li X, Bekaii-Saab T, Murgo AJ, Jensen RR, Grever M, Villalona-Calero MA: Phase I trial of non-cytotoxic suramin as a modulator of docetaxel and gemcitabine therapy in previously treated patients with non-small cell lung cancer. Cancer Chemother Pharmacol 66: 1019–1029, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, Zhuang S: Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 297: F996–F1005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu Y, Rajur K, Tolbert E, Dworkin LD: Endogenous hepatocyte growth factor ameliorates chronic renal injury by activating matrix degradation pathways. Kidney Int 58: 2028–2043, 2000 [DOI] [PubMed] [Google Scholar]