Abstract
The putative transcription factor AF17 upregulates the transcription of the epithelial sodium channel (ENaC) genes, but whether AF17 modulates sodium homeostasis and BP is unknown. Here, we generated Af17-deficient mice to determine whether deletion of Af17 leads to sodium wasting and low BP. Compared with wild-type mice, Af17-deficient mice had lower BP (11 mmHg), higher urine volume, and increased sodium excretion despite mildly increased plasma concentrations of aldosterone. Deletion of Af17 led to increased dimethylation of histone H3 K79 and reduced ENaC function. The attenuated function of ENaC resulted from decreased ENaC mRNA and protein expression, fewer active channels, lower open probability, and reduced effective activity. In contrast, inducing high levels of plasma aldosterone by a variety of methods completely compensated for Af17 deficiency with respect to sodium handling and BP. Taken together, these data identify Af17 as a potential locus for the maintenance of sodium and BP homeostasis and suggest that a particular histone modification is directly linked to these processes. Af17-mediated regulation of BP is largely, but not exclusively, the result of modulating ENaC, suggesting it has potential as a therapeutic target for the control of BP.
Abnormal regulation of BP leads to hypertension or hypotension. Hypertension is a major risk factor for cardiovascular diseases such as heart attack, congestive heart failure, stroke, peripheral vascular disease, and ESRD. Hypotension can deprive the brain and other vital organs of oxygen and nutrients, leading to shock, a life-threatening condition.
Enhanced Na+ reabsorption is long thought to increase blood volume and thus systemic arterial BP. Molecular characterization of monogenic forms of hypertension has confirmed this idea. These disorders are exemplified by mineralocorticoid excess, glucocorticoid-remedial aldosteronism, pseudohypoaldosteronism type II (PHAII), and Liddle's syndrome. They result from inactivated 11-β-HSD2,1 fused 11-hydroxylase/aldosterone (aldo) synthase (AS),2 gain-of-function mutations in the mineralocorticoid receptor (MR),3 or in the with-no-lysine (K) kinases WNK1 and WNK44,5 or gain-of-function mutations in β and γ subunits of the epithelial sodium channel (ENaC),6,7 respectively. All of these mutations impinge on salt absorptive mechanisms in the kidney. WNK1 and WNK4 have been shown to regulate ENaC.8,9
A number of inherited disorders characterized by salt wasting and hypotension are caused by loss-of-function mutations impairing salt homeostasis. Examples include Bartter's and Gitelman's syndromes, with defects in six genes encoding renal tubular transporters and ion channels (reviewed in ref. 10), and autosomal recessive PHAI, with loss-of-function mutations in the three subunits of ENaC.11,12
However, the etiology of abnormal BP is not completely defined. First, BP is a complex trait determined by the interactions of multiple genetic and environmental factors. Epigenetic regulation through chromatin modifications should be important in BP control. To date, a direct link between a particular gene impinging histone modifications to BP has not been clearly established. Second, numerous mouse models lacking ENaC and their direct or indirect regulators such as AS,13 angiotensinogen (Agt),14 angiotensin-converting enzyme (ACE),15 MR,16 Sgk1,17 WNK1,18 WNK4,19 and Period20 have been reported. However, the role of these models in showing the epigenetic control of BP is still unclear. This is because, to our knowledge, the function of these genes in chromatin regulation remains unknown. Finally, ENaC is tightly regulated at multiple levels by aldo. Most studies concerning ENaC and aldo focus on posttranslational events (reviewed in21), leaving a gap in knowledge between chromatin modifications and regulation of ENaC transcription, Na+ retention and BP.
We have described that aldo activates ENaC transcription in part by inhibiting Dot1a-Af9–mediated histone H3 K79 methylation in IMCD3 cells22 and that ALL-1 partner at 17q21 (AF17) also decreases H3 K79 methylation to upregulate these genes, but by competing with Af9 to bind Dot1a and facilitating Dot1a nuclear export in 293T cells.23 The Af17-harboring 17q21 has been previously implicated in BP variation.24 Although highly suggestive, these studies exclude the possibility of determining if and how AF17 regulates BP. Here, we use Af17−/− mice to address this question. We showed for the first time that a particular histone modification is directly linked to Na+ reabsorption and BP control, defined Af17 as a novel regulator of Na+ balance and BP, and showed that Af17-mediated regulation can be aldo-resistant or -sensitive, depending on plasma [aldo]. This type of mechanism may also be applicable to other hormones and transcription factors under various physiologic conditions. Af17 may be explored as a new therapeutic target of BP control.
RESULTS
Af17−/− Mice Fed a Normal Na+ Diet Have Impaired Renal Water and Electrolyte Retention and Decreased BP
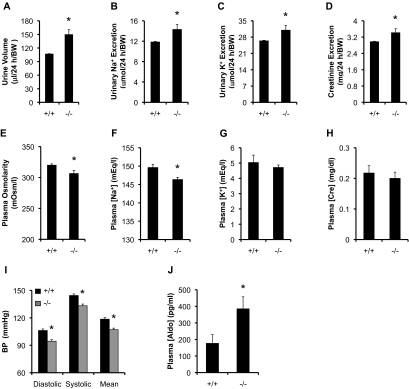
Generation of Af17−/− mice has been described.25 As shown in Supplementary Figure S1, analyses of 37 Af17−/− and 34 Af17+/+ mice on a normal Na+ diet showed subtle, but nonsignificant differences in body weight, urinary osmolarity, [Na+], [K+], [creatinine], [Na+]/[creatinine], and [K+]/[creatinine] between the two groups. The mutant urine volume and excretion of Na+, K+, and creatinine were significantly increased by 15 to 38% versus control, respectively (Figure 1, A–D). These changes were associated with a significant decrease in plasma osmolarity and [Na+] and a nonsignificant reduction in plasma [K+] and [creatinine] (Figure 1, E–H). Extensive BP measurements with 57 Af17+/+ mice and 67 mutants showed that the mutants display significantly lowered diastolic, systolic, and mean BP by 11 to 12 mmHg (Figure 1I), which was coupled with increased plasma [aldo] from 176 pg/ml in Af17+/+ mice to 385 pg/ml (Figure 1J). The increased [aldo] is possibly caused by reduced BP.26 Obviously, such [aldo] was not high enough to fully compensate for the loss of Af17 (see below). These results suggest that Af17−/− mutants show impaired renal function and decreased BP, a phenotype observed in several of other knockout mice under the same dietary conditions (see Discussion).
Figure 1.
Af17−/− mice fed the normal Na+ diet showed impaired renal function and decreased BP, despite mildly increased plasma aldo. Af17+/+ (n = 34) and Af17−/− (n = 37) mice fed the normal Na+ diet in metabolic cages were analyzed for the parameters as indicated. For BP measurement, n = 57 mice for Af17+/+ and n = 65 mice for Af17−/−. For all other parameters, n = 6 to 37 mice/group. For additional parameters, see Supplemental Figure S1. In all cases, *P < 0.05 versus Af17+/+.
Active Channel Number, Open Probability, and Effective Activity of ENaC Were Lower in Af17−/− than Af17+/+ Mice on the Normal Na+ Diet
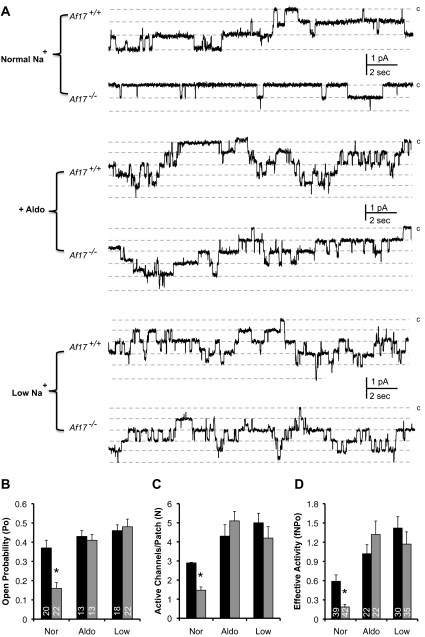
To determine whether the Na+ disturbance is a result of attenuated ENaC function, we performed cell-attached experiments to directly assess the basal ENaC activity on the apical plasma membrane of principal cells in split-open aldo-sensitive distal nephron. Figure 2A contains representative current traces. In parallel with dramatically decreased ENaC open probability (Po) and the number of active channels within a patch (N), Af17−/− mice on the normal Na+ diet have markedly reduced effective ENaC activity (fNPo), which is defined as NPo multiplied by the probability of observing patches with at least one active channel (f) (Figure 2, B–D). Although the decrease in N can be attributed to the impaired ENaC transcription (see below), the decrease of the Po points to an unexpected new role of Af17 in regulating ENaC activity.
Figure 2.
Impairment and restoration of ENaC activity in Af17−/− mice. (A) Representative current traces from cell-attached patches monitoring ENaC activity under conditions as indicated. Dashed lines indicate the respective current state with a c denoting the closed state. (B–D) Summary graph of ENaC Po (B), active channels within a patch (C), and effective ENaC activity (D). In all cases, *P < 0.05 versus Af17+/+.
Af17−/− Mice Fed the Normal Na+ Diet Display Impaired ENaC Expression and Increased H3 K79 Methylation in Whole Kidney Lysate
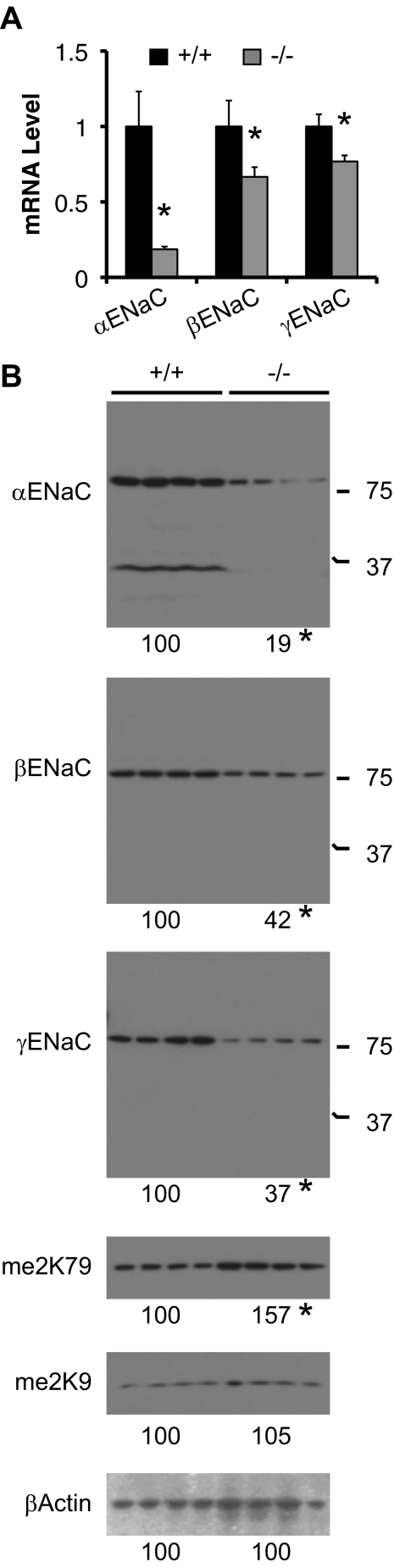
To investigate whether the decreased active channel number of ENaC results from impaired ENaC expression in kidney, we performed real-time RT-qPCR and Western blot analyses. Af17−/− mice display decreased expression of α, β, and γENaC to about 19, 67, and 76% of control at the mRNA level and to about 19, 42, and 37% of control at the protein level, respectively (Figure 3). As predicted,23 histone H3 K79 dimethylation (me2K79) was increased to 157% of control, and H3 K9 dimethylation (me2K9) remained constant with Af17 deletion (Figure 3).
Figure 3.
Af17 regulates mRNA and protein expression of ENaC. (A) Real-time RT-qPCR for expression of ENaC genes in kidney of mice fed the normal Na+ diet, with β-actin as internal control. n = 3 mice/group. (B) Western blots for expression of proteins as indicated, with β-actin as internal control. For αENaC, the approximately 30-kD band was excluded in data analyses. me2K79 and me2K9: histone H3 dimethylated K79 and K9, respectively. n = 4 mice/group. In all cases, *P < 0.05 versus Af17+/+.
Af17 Is Expressed in Aqp2-Expressing Renal Collecting Duct Principal Cells
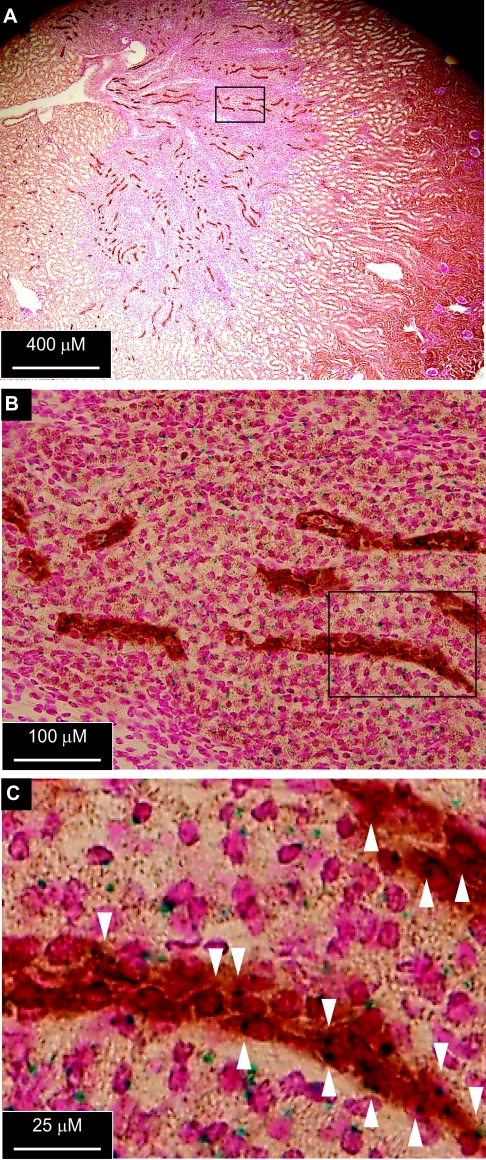
To test whether Af17 is expressed in aldo-sensitive distal nephron, we analyzed the expression of β-geo reporter driven by the Af17 promoter in the Af17−/− kidney,25 with Aqp2 as a marker of principal cells. X-gal staining was detected in Aqp2-expressing and many other cells (Figure 4). Therefore, Af17 is expressed in the principal cells, providing a molecular basis for directly regulating ENaC expression and activity.
Figure 4.
Af17 is expressed in aldo-sensitive distal nephron. (A and B) Sections of Af17−/− kidney were stained with X-gal and an antibody against the principal cell marker Aqp2. Boxed regions in A and B were amplified in C and D, respectively. The arrowheads indicate cells apparently expressing Aqp2 and β-geo reporter driven by the Af17 promoter.
Af17−/− Mice Fed a Normal Na+ Diet Display H3 K79 Hypermethylation at the αENaC Promoter
To show that Af17 regulates αENaC transcription through modulation of H3 K79 methylation in vivo, chromatin immunoprecipitation (ChIP) assay coupled with real-time qPCR was conducted with primers specific for amplification of the αENaC promoter regions (Figure 5A and refs. 22,27,28). ChIP with anti-me2K79 detected no substantial signals in Ra and R1 regions but robust signals from R0, R2, and R3 in wild-type (WT) mice (Figure 5B). Despite no difference in Ra, Af17−/− mice significantly enhanced me2K79 in all four other regions, with >170-fold in R1 versus WT control (Figure 5B). No obvious differences in total H3 or H3 me2K9 between the two groups over the entire αENaC 5′-flanking region were observed (Figure 5C). Furthermore, ChIP with normal rabbit IgG yielded barely detectable background signals. In brief, deletion of Af17 causes specific changes in H3 me2K79 associated with the αENaC promoter, but not a generalized effect on histone H3 in vivo in mouse kidney, indicating that Af17 upregulates αENaC mRNA expression by modulating H3 m2K79 at the αENaC promoter.
Figure 5.
Af17 deletion causes H3 K79 hypermethylation at the αENaC promoter in kidney. (A) Diagram of the αENaC promoter.12–14 (B and C) ChIP assay showing increased H3me2K79 associated with R0-R3, but not with Ra subregion of the αENaC promoter. Kidney chromatin was prepared from four WT and four mutant mice, pooled into two groups according to genotype, and analyzed by ChIP with the antibodies indicated, and followed by real-time qPCR with primers amplifying the subregions of αENaC promoter as shown in A. Relative H3me2K79 abundance was set to 1 in R0 from WT kidneys and was calculated accordingly for all other samples. *P < 0.05 versus Af17+/+. n = 4 mice/group (B). Representative agarose gel analyses of the final qPCR products were shown to verify the specificity of qPCR for each sample (C).
Aldo-Perfused Af17−/− Mice Display Similar Renal Physiology and BP to Af17+/+ Control
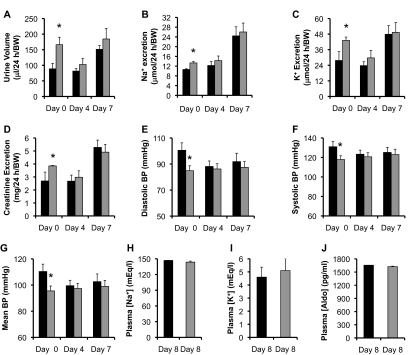
To directly test the hypothesis that high levels of aldo antagonizes the effect of Af17 deletion on H3 K79 methylation and thus ameliorates the renal disfunction of Af17−/− mice, Af17+/+ and Af17−/− (n = 10 to 11 mice/genotype) animals were perfused with aldo at 10 μg/100 g per day. Urine collection and BP measurements were conducted on days 0, 4, and 7. For convenience, animals were treated one more day before tissue harvest.
Although Af17−/− mice had significantly higher urine volume, excretion of Na+, K+, and creatinine, and lower diastolic, systolic, and mean BP than control at day 0 (Figure 6 and Supplementary Figure S2), these parameters were no longer significantly different between the two groups after 4- or 7-day aldo perfusion (Figure 6). Other urinary values ([Na+], [K+], [creatinine], [Na+]/[creatinine], and [K+]/[creatinine]) were similar at all of these three time points (Supplementary Figure S2). The plasma [Na+], [K+], and [aldo] at day 8 showed no significant difference between the mutants and WT, each with >1600 pg/ml of plasma aldo (Figure 6E and Supplementary Figure S2).
Figure 6.
Aldo perfusion largely compensated for the loss of Af17 function in renal physiology. Twenty-four-hour urine volume, urinary electrolyte excretion, and BP were examined before (day 0) and after aldo perfusion as indicated. n = 10 to 11 mice/genotype. Dark bar: Af17+/+. Gray bar: Af17−/−. For additional parameters, see Supplemental Figure S2. In all cases, *P < 0.05 versus Af17+/+.
Compared with the normal Na+ diet, aldo perfusion significantly increased the number of active channel, Po, and effective activity of ENaC in both sets of mice except WT Po to various degrees ranging from 16 to about 600% (Figure 2, A–D). However, all of these parameters were no longer significantly different between the two groups. Consistently, no significant difference in protein expression of α, β, and γENaC and H3 m2K79 was observed between Af17−/− and Af17+/+ mice (Supplementary Figure S3).
On a Low Na+ Diet, Af17−/− Mice Exhibited Similar Renal Physiology and BP to Af17+/+ Control in a Time-Dependent Manner
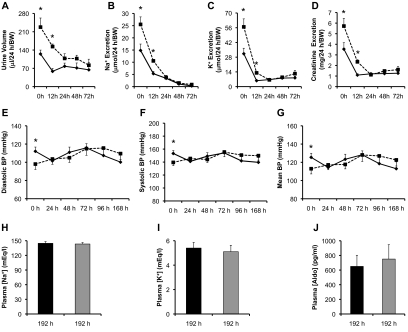
Low Na+ diet is known to increase [aldo].29 To test whether low Na+ diet-induced aldo excess also rescues Af17 phenotype, we challenged Af17+/+ and Af17−/− (n = 6 to 20 mice/group) animals with a low Na+ diet (0.02% Na+). Urine was collected at 12 hours and then every 24 hours. After 72 hours, urinary Na+ was barely detectable. Therefore, additional urinary analyses beyond this time point were omitted. BP was measured for 7 days. At day 8, mice were terminated for further analyses. All mice survived the treatment, eliminating the possibility of biased data because of differences in the animal tolerance to the challenge.
Both groups had greatly decreased urine volume and excretion of Na+, K+, and creatinine to levels ranging from 17 to 69% of their pretreatment values at 12 hours. For these parameters, Af17−/− mice maintained significantly higher levels than control (Figure 7 and Supplementary Figure S4). However, such differences became nonsignificant as the treatment proceeded. No differences were also observed in urinary [Na+], [K+], [creatinine], [Na+]/[creatinine], and [K+]/[creatinine], except [Na+] at 12 hours and [creatinine] at 12 and 24 hours (Supplementary Figure S4). The diastolic, systolic, and mean BPs in the Af17−/− were normalized in all days examined (Figure 7 and Supplementary Figure S4). Similar to aldo perfusion, dietary salt restriction for 8 days caused a pronounced elevation of ENaC activity for both genotypes compared with those on the normal Na+ diet (Figure 2). Importantly, we no longer observed an inhibitory effect of Af17 deletion on ENaC Po, the functional channel numbers, and the effective ENaC activity (fNPo) for Af17+/+ and Af17−/−, respectively. The plasma [Na+], [K+], and [aldo] were statistically indistingushible between the two genotypes (Figure 7 and Supplementary Figure S4). However, the [aldo] was 750 pg/ml in Af17−/− and 650 pg/ml in Af17+/+ mice (Figure 7E), which was significantly higher than that on the normal Na+ diet (Figure 1J).
Figure 7.
Af17−/− and Af17+/+ mice show similar renal physiology and BP in a time-dependent manner on the low Na+ diet. Af17+/+ (solid line or dark bar) and Af17−/− (dotted line or gray bar) mice were fed the low Na+ diet (0.02% Na+) in metabolic cages and analyzed for the parameters before (day 0) and after treatment at various time points as indicated. For BP measurement, n = 17 to 21 mice/group. For all other parameters, n = 4 to 14 for Af17+/+ mice and n = 6 to 14 for Af17−/− mice. For additional parameters, see Supplemental Figure S4. In all cases, *P < 0.05 versus Af17+/+.
High Dietary Potassium Attenuated the Effect of Af17 Deletion on Renal Function and BP
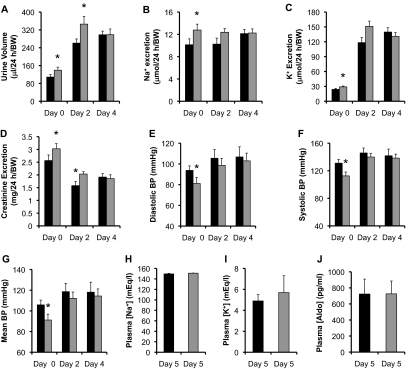
High KCl diets are also known to significantly increase plasma aldo in mice and rats,30 so we fed Af17+/+ and Af17−/− (n = 7 to 26 mice/group) with a 6% K+ diet for 5 days. Although the urine volume and excretion of Na+, K+, and creatinine at day 0 on the normal Na+ diet was significantly higher in the mutant mice than the Af17+/+ control, these differences were blunted after 2 and 4 days of K+ loading, with only two exceptions (Figure 8 and Supplementary Figure S5). The urine volume and creatinine excretion were still significantly higher in Af17−/− than Af17+/+ mice at day 2 (Figure 8 and Supplementary Figure S5). Similar to the normal Na+ diet, the high K+ diet did not differentiate the two groups in all other urinary parameters ([Na+], [K+], [creatinine], [Na+]/[creatinine], and [K+]/[creatinine]; Supplementary Figure S5). However, it should be noted that, compared with the normal Na+ diet at day 0, in both genotypes, the treatment for 2 and 4 days led to a more than two-fold increase in urine volume, no significant changes in Na+ excretion, a more than four-fold increase in K+ excretion, and a two-fold increase in urinary [K+] (Figure 8 and Supplementary Figure S5). In contrast, the urinary [Na+] was significantly decreased to about 40% of the pretreatment in the two genotypes (Supplementary Figure S5). Consistently, the significant differences between the two genotypes in diastolic, systolic, and mean BP at day 0 were diminished at day 2 and day 4 (Figure 8 and Supplementary Figure S5). Plasma analyses unearthed comparable levels of [Na+], [K+], and [aldo] at day 5, with [aldo] being dramatically increased to 723 pg/ml in the Af17+/+ and 725 pg/ml in the Af17−/− mice (Figure 8).
Figure 8.
On the high K+ diet, Af17−/− and Af17+/+ mice had similar renal physiology and BP on the high K+ diet. Af17+/+ and Af17−/− mice were fed the high K+ diet (6% K+) for up to 5 days in metabolic cages and analyzed for the parameters before (day 0) and after treatment as indicated. n = 8 to 26 for Af17+/+ mice and n = 7 to 25 for Af17−/− mice. For additional parameters, see Supplemental Figure S5. In all cases, *P < 0.05 versus Af17+/+.
DISCUSSION
Strong evidence suggests the presence of BP loci at 17p11–21 around marker D17S250 on human chromosome 17.24,31 Mutations in WNK4 within this interval are the causative factor for PHAII.5 AF17 also lies in this segment only 290 kb away from D17S250. The rat and mouse syntenic fragments on chromosomes 10 and 11, respectively, contain an AF17 homolog and are strongly linked to hypertension.32,33 It is worthy to see whether mutations of Af17 occur and account for BP variation in the general population.
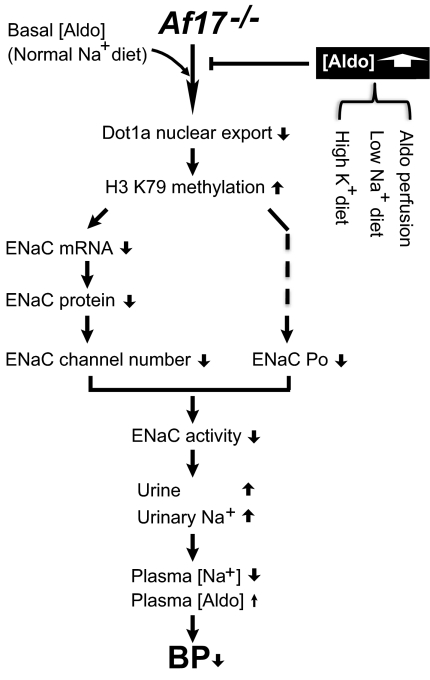
The observed renal phenotype in Af17−/− mice is not simply caused by a defect in feeding and drinking behavior, but at least partially results from decreased ENaC activity. Under basal conditions, Af17 may downregulate H3me2K79 and upregulate ENaC by multiple mechanisms, ranging from expression of mRNA, protein, and active channels to Po (Figure 9). Although the mechanism is elusive at this moment, identification of Af17 as an ENaC Po regulator is a surprise and points to a new aspect of Af17 function. Sgk1 can upregulate ENaC Po,34 possibly by phosphorylating inducible nitric oxide synthase (iNOS) to limiting NO production and its inhibitory effect on Po.35 Af17 may interact with Sgk123 to upregulate Po by forming an Af17–Sgk1–iNOS complex to restrict the NO production. Deletion of Af17 permanently removes these regulatory mechanisms, leading to renal dysfunction. We do not know whether the increased urinary creatinine excretion and the decreased plasma [creatinine] in Af17−/− animals result from an increase in either GFR and/or the creatinine secretion. Like Af17, aldo also decreases H3me2K79.22 Accordingly, on treatments that dramatically increase aldo, the role of Af17 in diminishing H3me2K79 becomes less important, resulting in a similar phenotype between Af17−/− and Af17+/+ mice, as seen with aldo perfusion and Na+ restriction.
Figure 9.
Model for impairment and restoration of Na+ balance and BP in Af17−/− mice. Under basal conditions such as the normal Na+ diet, deletion of Af17 leads to significantly increased H3 K79 methylation, and subsequently impaired ENaC activity, disturbance of Na+ balance, and decreased BP. The mildly increased plasma [aldo] is not sufficient to antagonize the effect of Af17 loss on H3 K79 methylation and thus fails to rescue the Af17−/− phenotype. Under aldo excess such as aldo perfusion or the low Na+ diet dramatically increased aldo levels efficiently decrease H3 K79 methylation regardless of the presence or absence of Af17, leading to comparable levels of H3 K79 methylation and a renal physiology and BP phenotype indistinguishable between Af17+/+ and Af17−/− mice. For clarity, detailed mechanisms by which Af1723 and aldo22 inhibit H3 K79 methylation are not shown. The dotted line indicates unknown mechanisms.
Af17−/− mice share the low BP phenotype on a normal Na+ diet with Ren1c−/−,36 AS−/−,13 Agt−/−,14 Ace−/−,15 and WNK1±18 mice. Ren1c, AS, Agt, and Ace are key components of the renin-angiotensin-aldo system and thus are upstream regulators of ENaC. WNK1 and WNK4 can upregulate ENaC activity through SGK1 and Nedd4–2.8,9 Although the BP phenotype of Period−/− mice has not been reported, these mice fed the standard diet also had higher Na+ excretion and lower αENaC expression than WT controls.20
Another feature not limited to Af17−/− mice is that the changes in Na+ reabsorption are not accompanied by an inverse effect on K+, indicating that the impaired ENaC function can not account for the complete panoply of the renal dysfunction. Indeed, such coupling was not observed in Sgk1−/−,17,37 AS−/−,13 αENaC(−/−)Tg,38 and βENaCm/m mice.39 Although Sgk1+/+ and Sgk1−/− mice did not display a significant difference in urinary Na+ excretion on a normal Na+ diet, their plasma [K+] was significantly different.40 Morever, the significantly increased Na+ excretion was not coupled with markedly decreased K+ excretion and was instead paralleled by increased K+ excretion during the early phases of Na+ restriction in Sgk1−/− versus Sgk1+/+ mice.17,37 αENaC(−/−)Tg mice showed significant salt-wasting [Na+] without significantly decreasing urinary [K+].38 Similarly, significantly elevated urinary [Na+]/[creatinine] and plasma [K+] in βENaCm/m mice on a low Na+ diet were not accompanied with the expected changes in the urinary excretion of Na+, K+, and plasma [Na+].39
The most striking feature differing Af17−/− from others identified thus far lies in its response to aldo. The renal physiology and BP phenotype in Af17−/− mice is [aldo] dependent. Nevethereless, dramatically elevated plasma [aldo] can rescue neither the renal physiology and BP phenotype of Sgk1−/− nor the salt wasting of MR−/− mice.40,41 A high intake of dietary NaCl can largely restore Na+ reabsorption, but not the low BP in AS−/− mice.13 These observations highlight the importance of the interactions between the genetic (such as Af17 mutations) and behavioral risk factors (salt intake) impacting BP. The availability of Af17−/− mice will offer a valuable model for the further exploration of these mechanisms.
CONCISE METHODS
Reagents
Antibodies against dimethyl histone H3 K79 and dimethylated histone H3 K9 were purchased from Millipore. Antibodies specific for α, β, or γENaC were either purchased from SantaCruz or kindly provided by Dr. Ryoichi Teruyama, who purified these antibodies originally generated by Dr. Mark Knepper's group. The chicken anti-Aqp2 antibody is a generous gift from Dr. James Wade (University of Maryland, College Park, MD).
Serum, Plasma, and Urine Measurements
The concentrations of Na+, K+, and creatinine in urine or serum were measured with an analyzer (Roche Cobas Integra 400 plus) in the Clinical Pathology Laboratory, Department of Veterinary Medicine and Surgery, University of Texas MD Anderson Cancer Center. This analyzer measures Na+ and K+ using ion selective potentiometry and creatinine by absorbance photometry. Plasma aldosterone was specifically measured using a 125I-Coat-A-Count RIA kit (Siemens, Los Angeles, CA).42 Because the aldosterone-specific antibody used in this kit was generated in rabbit, mouse samples can be directly measured, eliminating large variations and possible aldosterone loss created from additional extraction steps. Plasma and urine osmolarity was measured by vapor pressure (Wescor Vapro Vapor Pressure Osmometer 5520; Scimetrics, Houston, TX).
Metabolic Balance Studies
Two- to to 4-month-old Af17+/+ mice and their mutant littermates were used for the study. They were acclimated for 3 to 7 days in Tecniplast metabolic cages with free access to water and normal laboratory diet containing 0.4% Na+. After that, mice were continued on the same normal Na+ diet for at least 3 more days. During these days, systolic, diastolic, and mean BPs were measured daily with the CODA tail-cuff BP system (Kent Scientific, Torrington, CT) as reported.43 All mice were subjected to at least one cycle of measurement containing 20 to 30 individual readings for each parameter each day. Twenty-four-hour urine was collected daily unless otherwise indicated. For each mouse, data from multiple days were pooled to calculate the final average to represent that mouse and counted as 1 (n = 1). To minimize circadian effects, BP measurements and urine collection were conducted around 5:00 p.m. each day. In other experiments, mice examined as above with the normal Na+ diet were subsequently implanted subcutaneously with ALZET 1007D micro-osmotic pumps preloaded with aldosterone. The infusion was conducted for 8 days at 10 μg/100 g per day.29 Alternatively, the mice were challenged with the low Na+ diet (0.02% Na+) for 8 days or the high potassium diet (6% K+) for 5 days.
In all cases, urine volume and urinary electrolyte excretion were normalized to two parameters: (1) body weight at the start point of collection and (2) 24 hours based on actual hours between the two collection time points. The ethics of all animal experiments were reviewed and approved by the Animal Welfare Committee, The University of Texas Health Science Center at Houston.
Electrophysiology, Real-Time RT-qPCR, X-gal Staining, and IHC
These assays have been described previously22,25,44 and are detailed in Supplemental Information.
Statistical Analysis
Quantitative data were presented as mean ± SEM. A t test was used for all comparisons, with the statistical significance set at P < 0.05.
DISCLOSURES
None.
Acknowledgments
We thank Dr. Mark Knepper, Dr. Ryoichi Teruyama, and Dr. James Wade for sharing the ENaC and Aqp2 antibodies. This work was funded by National Institutes of Health Grants R01 DK080236 (to W.Z.Z.), the American Heart Association (Grant 0865271F to W.Z.Z. and Grant 09SDG2230391 to O.P.), and an American Society of Nephrology Carl W. Gottschalk Research Scholar grant (to W.Z.Z.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Wilson RC, Krozowski ZS, Li K, Obeyesekere VR, Razzaghy-Azar M, Harbison MD, Wei JQ, Shackleton CH, Funder JW, New MI: A mutation in the HSD11B2 gene in a family with apparent mineralocorticoid excess. J Clin Endocrinol Metab 80: 2263–2266, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Lifton RP, Dluhy RG, Powers M, Rich GM, Cook S, Ulick S, Lalouel JM: A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 355: 262–265, 1992 [DOI] [PubMed] [Google Scholar]
- 3. Geller DS, Farhi A, Pinkerton N, Fradley M, Moritz M, Spitzer A, Meinke G, Tsai FT, Sigler PB, Lifton RP: Activating mineralocorticoid receptor mutation in hypertension exacerbated by pregnancy. Science 289: 119–123, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Rafestin-Oblin ME, Souque A, Bocchi B, Pinon G, Fagart J, Vandewalle A: The severe form of hypertension caused by the activating S810L mutation in the mineralocorticoid receptor is cortisone related. Endocrinology 144: 528–533, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP: Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Shimkets RA, Warnock DG, Bositis CM, Nelson-Williams C, Hansson JH, Schambelan M, Gill JR, Jr, Ulick S, Milora RV, Findling JW, Canessa CM, Rossier BC, Lifton RP: Liddle's syndrome: Heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell 79: 407–414, 1994 [DOI] [PubMed] [Google Scholar]
- 7. Yamashita Y, Koga M, Takeda Y, Enomoto N, Uchida S, Hashimoto K, Yamano S, Dohi K, Marumo F, Sasaki S: Two sporadic cases of Liddle's syndrome caused by de novo ENaC mutations. Am J Kidney Dis 37: 499–504, 2001 [PubMed] [Google Scholar]
- 8. Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ, Lee BH, English JM, Ortega B, Huang CL, Cobb MH: WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci USA 102: 10315–10320, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heise CJ, Xu BE, Deaton SL, Cha SK, Cheng CJ, Earnest S, Sengupta S, Juang YC, Stippec S, Xu Y, Zhao Y, Huang CL, Cobb MH: Serum and glucocorticoid-induced kinase (SGK) 1 and the epithelial sodium channel are regulated by multiple with no lysine (WNK) family members. J Biol Chem 285: 25161–25167, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Naesens M, Steels P, Verberckmoes R, Vanrenterghem Y, Kuypers D: Bartter's and Gitelman's syndromes: From gene to clinic. Nephron Physiol 96: 65–78, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP: Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet 12: 248–253, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Adachi M, Tachibana K, Asakura Y, Abe S, Nakae J, Tajima T, Fujieda K: Compound heterozygous mutations in the gamma subunit gene of ENaC (1627delG and 1570–1G–>A) in one sporadic Japanese patient with a systemic form of pseudohypoaldosteronism type 1. J Clin Endocrinol Metab 86: 9–12, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Makhanova N, Lee G, Takahashi N, Sequeira Lopez ML, Gomez RA, Kim HS, Smithies O: Kidney function in mice lacking aldosterone. Am J Physiol Renal Physiol 290: F61–F69, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Tanimoto K, Sugiyama F, Goto Y, Ishida J, Takimoto E, Yagami K, Fukamizu A, Murakami K: Angiotensinogen-deficient mice with hypotension. J Biol Chem 269: 31334–31337, 1994 [PubMed] [Google Scholar]
- 15. Krege JH, John SW, Langenbach LL, Hodgin JB, Hagaman JR, Bachman ES, Jennette JC, O'Brien DA, Smithies O: Male-female differences in fertility and blood pressure in ACE-deficient mice. Nature 375: 146–148, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Ronzaud C, Loffing J, Bleich M, Gretz N, Grone HJ, Schutz G, Berger S: Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18: 1679–1687, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Huang DY, Wulff P, Volkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V: Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol 15: 885–891, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Zambrowicz BP, Abuin A, Ramirez-Solis R, Richter LJ, Piggott J, BeltrandelRio H, Buxton EC, Edwards J, Finch RA, Friddle CJ, Gupta A, Hansen G, Hu Y, Huang W, Jaing C, Key BW, Jr., Kipp P, Kohlhauff B, Ma ZQ, Markesich D, Payne R, Potter DG, Qian N, Shaw J, Schrick J, Shi ZZ, Sparks MJ, Van Sligtenhorst I, Vogel P, Walke W, Xu N, Zhu Q, Person C, Sands AT: Wnk1 kinase deficiency lowers blood pressure in mice: A gene-trap screen to identify potential targets for therapeutic intervention. Proc Natl Acad Sci USA 100: 14109–14114, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP: Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Gumz ML, Stow LR, Lynch IJ, Greenlee MM, Rudin A, Cain BD, Weaver DR, Wingo CS: The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J Clin Invest 119: 2423–2434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kleyman TR, Carattino MD, Hughey RP: ENaC at the cutting edge: Regulation of epithelial sodium channels by proteases. J Biol Chem 284: 20447–20451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang W, Xia X, Reisenauer MR, Rieg T, Lang F, Kuhl D, Vallon V, Kone BC: Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest 117: 773–783, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Reisenauer MR, Anderson M, Huang L, Zhang Z, Zhou Q, Kone BC, Morris AP, Lesage GD, Dryer SE, Zhang W: AF17 competes with AF9 for binding to Dot1a to up-regulate transcription of epithelial Na+ channel {alpha}. J Biol Chem 284: 35659–35669, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mansfield TA, Simon DB, Farfel Z, Bia M, Tucci JR, Lebel M, Gutkin M, Vialettes B, Christofilis MA, Kauppinen-Makelin R, Mayan H, Risch N, Lifton RP: Multilocus linkage of familial hyperkalaemia and hypertension, pseudohypoaldosteronism type II, to chromosomes 1q31–42 and 17p11–q21. Nat Genet 16: 202–205, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Zhang Z, Huang L, Reisenauer MR, Wu H, Chen L, Zhang Y, Xia Y, Zhang W: Widely Expressed Af17 is likely not required for embryogenesis, hematopoiesis, and animal survival. Genesis 48: 693–706, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bleich M, Warth R, Schmidt-Hieber M, Schulz-Baldes A, Hasselblatt P, Fisch D, Berger S, Kunzelmann K, Kriz W, Schutz G, Greger R: Rescue of the mineralocorticoid receptor knock-out mouse. Pflugers Arch 438: 245–254, 1999 [DOI] [PubMed] [Google Scholar]
- 27. Zhang W, Xia X, Jalal DI, Kuncewicz T, Xu W, Lesage GD, Kone BC: Aldosterone-sensitive repression of ENaCalpha transcription by a histone H3 lysine-79 methyltransferase. Am J Physiol Cell Physiol 290: C936–C946, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang W, Xia X, Reisenauer MR, Hemenway CS, Kone BC: Dot1a-AF9 complex mediates histone H3 Lys-79 hypermethylation and repression of ENaC{alpha} in an aldosterone-sensitive manner. J Biol Chem 281: 18059–18068, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hou J, Speirs HJ, Seckl JR, Brown RW: Sgk1 gene expression in kidney and its regulation by aldosterone: Spatio-temporal heterogeneity and quantitative analysis. J Am Soc Nephrol 13: 1190–1198, 2002 [PubMed] [Google Scholar]
- 30. Grimm PR, Irsik DL, Settles DC, Holtzclaw JD, Sansom SC: Hypertension of Kcnmb1−/− is linked to deficient K secretion and aldosteronism. Proc Natl Acad Sci USA 106: 11800–11805, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levy D, DeStefano AL, Larson MG, O'Donnell CJ, Lifton RP, Gavras H, Cupples LA, Myers RH: Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the framingham heart study. Hypertension 36: 477–483, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Dukhanina OI, Dene H, Deng AY, Choi CR, Hoebee B, Rapp JP: Linkage map and congenic strains to localize blood pressure QTL on rat chromosome 10. Mamm Genome 8: 229–235, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Salzler HR, Griffiths R, Ruiz P, Chi L, Frey C, Marchuk DA, Rockman HA, Le TH: Hypertension and albuminuria in chronic kidney disease mapped to a mouse chromosome 11 locus. Kidney Int 72: 1226–1232, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alvarez de la Rosa D, Paunescu TG, Els WJ, Helman SI, Canessa CM: Mechanisms of regulation of epithelial sodium channel by SGK1 in A6 cells. J Gen Physiol 124: 395–407, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Helms MN, Yu L, Malik B, Kleinhenz DJ, Hart CM, Eaton DC: Role of SGK1 in nitric oxide inhibition of ENaC in Na+-transporting epithelia. Am J Physiol Cell Physiol 289: C717–C726, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Takahashi N, Lopez ML, Cowhig JE, Jr, Taylor MA, Hatada T, Riggs E, Lee G, Gomez RA, Kim HS, Smithies O: Ren1c homozygous null mice are hypotensive and polyuric, but heterozygotes are indistinguishable from wild-type. J Am Soc Nephrol 16: 125–132, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Fejes-Toth G, Frindt G, Naray-Fejes-Toth A, Palmer LG: Epithelial Na+ channel activation and processing in mice lacking SGK1. Am J Physiol Renal Physiol 294: F1298–F1305, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Hummler E, Barker P, Talbot C, Wang Q, Verdumo C, Grubb B, Gatzy J, Burnier M, Horisberger JD, Beermann F, Boucher R, Rossier BC: A mouse model for the renal salt-wasting syndrome pseudohypoaldosteronism. Proc Natl Acad Sci USA 94: 11710–11715, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pradervand S, Barker PM, Wang Q, Ernst SA, Beermann F, Grubb BR, Burnier M, Schmidt A, Bindels RJ, Gatzy JT, Rossier BC, Hummler E: Salt restriction induces pseudohypoaldosteronism type 1 in mice expressing low levels of the beta-subunit of the amiloride-sensitive epithelial sodium channel. Proc Natl Acad Sci USA 96: 1732–1737, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wulff P, Vallon V, Huang DY, Volkl H, Yu F, Richter K, Jansen M, Schlunz M, Klingel K, Loffing J, Kauselmann G, Bosl MR, Lang F, Kuhl D: Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest 110: 1263–1268, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schutz G: Mineralocorticoid receptor knockout mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci USA 95: 9424–9429, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sallstrom J, Carlstrom M, Jensen BL, Skott O, Brown RD, Persson AE: Neuronal nitric oxide synthase-deficient mice have impaired renin release but normal blood pressure. Am J Hypertens 21: 111–116, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellems RE, Xia Y: Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med 14: 855–862, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bugaj V, Pochynyuk O, Stockand JD: Activation of the epithelial Na+ channel in the collecting duct by vasopressin contributes to water reabsorption. Am J Physiol Renal Physiol 297: F1411–F1418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]