Abstract
Several comparisons of peritoneal dialysis (PD) and hemodialysis (HD) in incident patients with ESRD demonstrate superior survival in PD-treated patients within the first 1 to 2 years. These survival differences may be due to higher HD-related mortality as a result of high rates of incident central venous catheter (CVC) use or due to an initial survival advantage conferred by PD. We compared the survival of incident PD patients with those who initiated HD with a CVC (HD-CVC) or with a functional arteriovenous fistula or arteriovenous graft (HD-AVF/AVG). We used multivariable piece-wise exponential nonproportional and proportional hazards models to evaluate early (1 year) mortality as well as overall mortality during the period of observation using an intention-to-treat approach. We identified 40,526 incident adult dialysis patients from the Canadian Organ Replacement Register (2001 to 2008). Compared with the 7412 PD patients, 1-year mortality was similar for the 6663 HD-AVF/AVG patients but was 80% higher for the 24,437 HD-CVC patients (adjusted HR, 1.8; 95% confidence intervals [CI], 1.6 to 1.9). During the entire period of follow-up, HD-AVF/AVG patients had a lower risk for death, and HD-CVC patients had a higher risk for death compared with patients on PD. In conclusion, the use of CVCs in incident HD patients largely accounts for the early survival benefit seen with PD.
The survival benefits of peritoneal dialysis (PD) versus hemodialysis (HD) in the treatment of patients with end-stage renal disease continue to be debated. In HD, vascular access type is significantly associated with patient survival. The use of a central venous catheter (CVC) is associated with a substantially greater risk of sepsis, hospitalization, and mortality when compared with the use of an arteriovenous fistula (AVF) or an arteriovenous graft (AVG).1–5 This association may directly relate to CVC-associated infectious and noninfectious complications. However, the association may also be confounded by case-mix differences between patients initiating HD with either a CVC (HD-CVC) or an AVF/AVG (HD-AVF/AVG). These differences may include: the acuity of dialysis initiation, the absence of timely access to predialysis care, the presence of comorbid conditions, and surgical vascular access eligibility, all of which may be independently associated with patient survival.
Case-mix differences between patients treated with PD and HD have limited the interpretation of studies that have examined the effect of dialysis modality on patient survival. Although several observational studies have used robust statistical techniques to account for confounding, none have accounted for the role of HD vascular access at the time of dialysis initiation.6–16 We speculate that compared with patients initiating HD with a CVC, patients initiating HD with an AVF or an AVG are more likely to share characteristics similar to those of incident PD patients. These features include ambulatory initiation of dialysis, timely access to predialysis care, and willingness to make decisions regarding dialysis modality and vascular access choice. In this regard, patients starting HD with an AVF or AVG may serve as more appropriate comparators for PD patients. In this report, our objective was to use data from the Canadian Organ Replacement Register (CORR) to compare survival between PD and HD patients with the latter stratified by HD vascular access type at dialysis initiation. We also sought to test our hypothesis that the early relative survival benefits attributed to PD are attenuated when compared with HD that is initiated with a functioning AVF or AVG.
RESULTS
Baseline Characteristics
40,526 incident chronic dialysis patients were registered in CORR between 2001 and 2008. Over 95% (n = 38,512) of patients had documentation of both dialysis modality and incident HD vascular access. Among these patients, PD was the initial dialysis modality for 19% (n = 7412). Among HD patients, 21.4% (n = 6 663) initiated dialysis with an AVF or AVG, whereas the remainder initiated HD with a CVC.
Table 1 lists the baseline characteristics of the study population. Over the course of the study period, there was a trend toward increased CVC use (P < 0.0001) and decreased PD utilization (P = 0.02). Compared with PD patients, HD-CVC patients were more likely to be older; to be Caucasian; to have a higher frequency of diabetes mellitus, coronary artery disease, and peripheral vascular disease; and to have a history of malignancy. Compared with PD patients, HD-CVC patients were also more likely to be referred late to a nephrologist (49.7% versus 15.2%) and initiate dialysis with lower hemoglobin, serum albumin, and estimated GFR (eGFR).
Table 1.
Baseline patient characteristics at dialysis initiation in Canada, 2001 to 2008
| PD (n = 7,412) | HD-AVF/AVG (n = 6,663) | HD-CVC (n = 24,437) | P | |
|---|---|---|---|---|
| Era of dialysis initiation (%) | <0.0001 | |||
| 2001 to 2004 | 19.7 | 18.1 | 62.3 | |
| 2005 to 2008 | 18.9 | 16.6 | 64.6 | |
| Age (%) | ||||
| 18 to 44 years | 15.4 | 9.5 | 11.0 | |
| 45 to 54 years | 16.7 | 12.5 | 11.1 | |
| 55 to 64 years | 22.6 | 20.2 | 19.4 | |
| 65 to 74 years | 25.2 | 29.1 | 26.9 | |
| 75+ years | 20.0 | 28.6 | 31.5 | |
| Race (%) | <0.0001 | |||
| Caucasian | 70.4 | 76.5 | 75.8 | |
| Asian | 8.6 | 5.8 | 5.0 | |
| black | 3.4 | 2.8 | 3.4 | |
| other | 12.6 | 10.0 | 11.2 | |
| unknown | 5.0 | 4.9 | 4.6 | |
| Female gender (%) | 42.7 | 34.4 | 41.7 | |
| Primary renal diagnosis (%) | <0.0001 | |||
| glomerulonephritis | 16.7 | 12.3 | 10.1 | |
| diabetes | 36.2 | 38.4 | 35.4 | |
| renal vascular disease | 17.2 | 20.2 | 20.1 | |
| polycystic kidney disease | 6.9 | 7.7 | 2.2 | |
| other | 11.8 | 11.2 | 18.5 | |
| unknown | 11.2 | 10.2 | 13.7 | |
| Comorbidities (%) | ||||
| diabetes mellitus | 42.6 | 47.3 | 46.5 | <0.0001 |
| coronary artery diseasea | 24.8 | 32.0 | 36.1 | <0.0001 |
| peripheral vascular disease | 13.5 | 17.8 | 20.8 | <0.0001 |
| malignancy | 7.4 | 10.6 | 12.6 | <0.0001 |
| lung disease | 6.6 | 12.3 | 14.1 | <0.0001 |
| pulmonary edema | 12.9 | 18.6 | 28.6 | <0.0001 |
| hypertension | 85.4 | 86.6 | 80.1 | <0.0001 |
| current smoker | 12.1 | 12.0 | 13.8 | <0.0001 |
| BMI (median, IQR) (kg/m2) | 26.0 (22.9, 29.6) | 27.1 (23.6, 31.6) | 25.9 (22.6, 30.3) | <0.0001 |
| Late referral (%) | 15.2 | 3.6 | 49.7 | <0.0001 |
| Time from referral to dialysis initiation (median, IQR) (days) | 637 (212, 1490) | 851 (399, 1620) | 188 (11, 784) | <0.0001 |
| Hemoglobin (g/L) | 111 (101, 120) | 108 (98, 119) | 98 (87, 110) | <0.0001 |
| eGFR (ml/min per 1.73 m2) | 9.1 (7.1, 11.9) | 8.9 (7.0, 11.4) | 8.6 (6.3, 11.8) | <0.0001 |
| Serum albumin (g/L) | 36 (32, 40) | 35 (32, 39) | 31 (26, 36) | <0.0001 |
IQR, interquartile range; eGFR, eGFR as determined by the modification of diet in renal disease formula.33
aCoronary artery disease was determined from the presence of a history of at least one of the following: coronary artery bypass grafting, previous myocardial infarction, or previous angina.
Compared with PD patients, HD-AVF/AVG patients were more likely to be older and Caucasian and have more extensive comorbidity. HD-AVF/AVG and PD patients initiated dialysis with similar levels of serum hemoglobin, serum albumin, and eGFR, but HD-AVF/AVG patients were less likely to be referred late to a nephrologist (3.6% versus 15.2%).
Patient Survival by Dialysis Modality and Hemodialysis Vascular Access
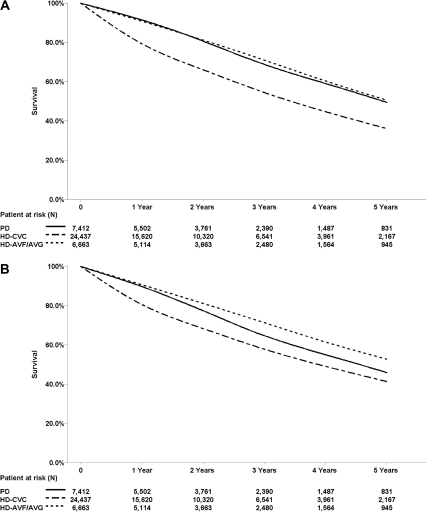
15,327 patients died over the course of follow-up. Among the 11,369 who had available information regarding cause of death, cardiovascular causes remained the most common cause of death (40.6% PD, 32.3% HD-CVC, and 34.4% HD-AVF/AVG), whereas the second most common cause was death caused by infection (11.5% PD, 11.7% HD-CVC, and 11.5% HD-AVF/AVG). Table 2 summarizes the results from the primary analysis. HD patients had higher adjusted 1-year mortality compared with PD patients (adjusted hazard ratio [AHR], 1.5; 95% CI, 1.4 to 1.7). When HD patients were stratified by incident vascular access type, HD-CVC patients had a higher unadjusted 1-year mortality (HR, 2.7; 95% CI, 2.4 to 2.9) and higher adjusted 1-year mortality (AHR, 1.8; 95% CI, 1.6 to 1.9) compared with PD patients. In contrast, 1-year mortality risk was similar in HD-AVF/AVG patients compared with PD patients (HR, 1.1; 95% CI, 1.0 to 1.3; and AHR, 0.9; 95% CI, 0.8 to 1.1). During the initial 5 years of follow-up, cumulative mortality remained higher among HD-CVC patients (AHR, 1.2; 95% CI, 1.1 to 1.2) and lower among HD-AVF/AVG patients, relative to PD patients (AHR, 0.80; 95% CI, 0.8 to 0.9) (Figure 1). After the first year, HD-CVC patients had a time-dependent mortality risk similar to that of PD patients. Over the entire course of follow-up, unadjusted cumulative mortality was 31% (PD), 44.1% (HD-CVC), and 33.9% (HD-AVF/AVG). During this time, mortality was greater in HD-CVC patients (AHR, 1.2; 95% CI, 1.1 to 1.2), and risk of death was lower in HD-AVF/AVG patients (AHR, 0.8; 95% CI, 0.8 to 0.9) relative to PD patients. Irrespective of vascular access type, patients who started HD were less likely to receive a kidney transplant over the course of follow-up compared with those initiating PD (HD-CVC [AHR, 0.8; 95% CI, 0.8 to 0.9] and HD-AVF/AVG [AHR, 0.9; 95% CI, 0.8 to 0.9]).
Table 2.
Results of the piecewise proportional hazards model for the relationship between dialysis modality and death
| Adjustedb Time dependenta HR [95% CI] | Univariate Time dependenta HR [95% CI] | Adjustedb Time dependenta HR [95% CI] | Adjustedb Time averagec HR [95% CI] | ||
|---|---|---|---|---|---|
| Overalld | |||||
| PD | 1.0 | PD | 1.0 | 1.0 | 1.0 |
| HD | 1.0 [1.0, 1.1] | HD-CVC | 1.7 [1.6, 1.7] | 1.2 [1.1, 1.2] | 1.2 [1.1 1.2] |
| HD-AVF/AVG | 1.1 [1.0, 1.1] | 0.8 [0.8, 0.9] | 0.8 [0.8,0.9] | ||
| Year 1 | |||||
| PD | 1.0 | PD | 1.0 | 1.0 | 1.0 |
| HD | 1.5 [1.4,1.7] | HD-CVC | 2.7 [2.4, 2.9] | 1.8 [1.6, 1.9] | 1.6 [1.5, 1.8] |
| HD-AVF/AVG | 1.1 [1.0, 1.3] | 0.9 [0.8, 1.1] | 0.9 [0.8,1.1] | ||
| Year 2 | |||||
| PD | 1.0 | PD | 1.0 | 1.0 | 1.0 |
| HD | 1.0 [0.9, 1.1] | HD-CVC | 1.5 [1.4, 1.6] | 1.1 [1.0, 1.2] | 1.4 [1.3, 1.5] |
| HD-AVF/AVG | 0.9 [0.8, 1.0] | 0.8 [0.7, 0.9] | 0.8 [0.8,0.9] | ||
| Year 3 | |||||
| PD | 1.0 | PD | 1.0 | 1.0 | 1.0 |
| HD | 0.8 [0.7, 0.9] | HD-CVC | 1.2 [1.1, 1.4] | 0.9 [0.8, 1.0] | 1.2 [1.2, 1.3] |
| HD-AVF/AVG | 0.9 [0.8, 1.0] | 0.7 [0.6, 0.8] | 0.8 [0.7, 0.9] | ||
| Year 4 | |||||
| PD | 1.0 | PD | 1.0 | 1.0 | 1.0 |
| HD | 0.8 [0.7,1.0] | HD-CVC | 1.3 [1.1, 1.5] | 0.9 [0.8, 1.0] | 1.2 [1.1, 1.2] |
| HD-AVF/AVG | 1.1 [0.9, 1.3] | 0.8 [0.7, 1.0] | 0.8 [0.7,0.9] | ||
| Year 5 | |||||
| PD | 1.0 | PD | 1.0 | 1.0 | 1.0 |
| HD | 0.8 [0.7, 0.9] | HD-CVC | 1.2 [1.1, 1.5] | 0.9 [0.7, 1.0] | 1.2 [1.1,1.2] |
| HD-AVF/AVG | 1.1 [0.9, 1.3] | 0.8 [0.7, 1.0] | 0.8 [0.8,0.9] |
aTime-dependent hazard ratios within each year were used to assess annual mortality risk.
bIntention to treat, adjusted for age, race, gender, era of dialysis initiation, end-stage renal disease comorbidity index, primary renal diagnosis, serum albumin, estimated glomerular filtration rate, province of treatment, and late referral.
cTime-averaged hazard ratios from a proportional hazards model were used to assess the cumulative treatment effect from day 0 through the end of years 1 to 5, respectively.
dOverall model and time average models constructed using 29,647 subjects using proportional hazards model, remainder of time-dependent models using nonproportional hazards model.
Figure 1.
Survival curves for HD-CVC (short-dashed line), HD-AVF/AVG (long-dashed line), and PD (solid line) demonstrate higher 1-year mortality in HD-CVC patients. (A) Unadjusted. (B) Adjusted on the basis of a stratified Cox proportional Hazards model stratified by HD-CVC, PD, and HD-AVF/AVG and adjusted for age, race, gender, era of dialysis initiation, end-stage renal disease comorbidity index, primary renal diagnosis, serum albumin, eGFR, province of treatment, and late referral.
Sensitivity Analyses
Table 3 summarizes the results of the sensitivity analyses. Referral timing, eGFR, and albumin were missing in 7, 9, and 15% of patients, respectively. Imputation of values for these missing results did not appreciably change the direction and magnitude of our results. Mortality within 90 days of dialysis initiation was highest among HD-CVC patients (15.6% for HD-CVC, 6.1% for HD-AVF/AVG, and 7.4% for PD; P < 0.001). After exclusion of patients who died within 90 days of starting dialysis, the increased 1-year mortality risk persisted among HD-CVC-treated patients relative to PD patients. Similar results were seen in the models that excluded patients who were referred late and after censoring patients 60 days or more after a change in dialysis modality. Using the inverse probability of treatment and censoring weighting analysis led to similar results compared with the primary model. The models used to derive the propensity score demonstrated reasonable prediction efficiency with an area under the receiver operating characteristic of 0.8 for HD-CVC versus PD and 0.7 for HD-AVF/AFG versus PD.
Table 3.
Results of the sensitivity analysis, piecewise proportional hazards model for the relationship between dialysis modality and death
| Censored at 60 Days after Modality Switcha | Modality at 90 Days after Dialysis Initiationa | Multiple Imputation of Missing Dataa,b | IPTCWa,c | Exclusion of Late-referral Patientsa,d | |
|---|---|---|---|---|---|
| Overall | |||||
| PD | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| HD-CVC | 1.2 (1.1, 1.2) | 1.1 (1.0, 1.1) | 1.2 (1.1, 1.2) | 1.1 (1.0, 1.1) | 1.1 (1.1, 1.2) |
| HD-AVF/AVG | 0.8 (0.8, 0.9) | 0.8 (0.7, 0.9) | 0.8 (0.8, 0.9) | 0.7 (0.6, 0.8) | 0.8 (0.8, 0.9) |
| Year 1 | |||||
| PD | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| HD-CVC | 1.8 (1.6, 2.0) | 1.4 (1.3, 1.6) | 1.8 (1.6, 1.9) | 1.3 (1.1, 1.5) | 1.6 (1.5, 1.8) |
| HD-AVF/AVG | 1.0 (0.9, 1.2) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) | 0.7 (0.6, 0.9) | 1.0 (0.9, 1.1) |
| Year 2 | |||||
| PD | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| HD-CVC | 1.1 (1.0, 1.3) | 1.1 (1.0, 1.2) | 1.0 (0.9, 1.1) | 1.1 (1.0, 1.3) | 1.0 (0.9, 1.2) |
| HD-AVF/AVG | 0.9 (0.8, 1.0) | 0.8 (0.7, 0.9) | 0.7 (0.7, 0.8) | 0.8 (0.6, 1.1) | 0.8 (0.7, 0.9) |
| Year 3 | |||||
| PD | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| HD-CVC | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) | 0.9 (0.7, 1.0) | 0.9 (0.8, 1.0) |
| HD-AVF/AVG | 0.7 (0.6, 0.8) | 0.7 (0.6, 0.8) | 0.7 (0.6, 0.8) | 0.5 (0.4, 0.7) | 0.7 (0.6, 0.8) |
| Year 4 | |||||
| PD | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| HD-CVC | 0.9 (0.7, 1.0) | 0.9 (0.8, 1.0) | 0.9 (0.8, 1.0) | 0.9 (0.7, 1.1) | 0.9 (0.8, 1.1) |
| HD-AVF/AVG | 0.8 (0.6, 1.0) | 0.8 (0.7, 0.9) | 0.9 (0.7, 1.0) | 0.8 (0.5, 1.2) | 0.8 (0.7, 0.9) |
| Year 5 | |||||
| PD | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| HD-CVC | 0.7 (0.6, 0.9) | 0.8 (0.7, 1.0) | 0.9 (0.7, 1.0) | 1.0 (0.8, 1.3) | 0.9 (0.8, 1.1) |
| HD-AVF/AVG | 0.7 (0.6, 1.0) | 0.8 (0.6, 1.0) | 0.9 (0.7, 1.0) | 0.9 (0.6, 1.4) | 0.8 (0.6, 0.9) |
IPTCW, inverse probability of treatment and censoring weighting.
aAdjusted for age, race, gender, era of dialysis initiation, end-stage renal disease comorbidity index, primary renal diagnosis, serum albumin, estimated glomerular filtration rate, province of treatment, and late referral.
bAssuming monotone missing pattern, the predictive mean matching method was used to impute missing values.
cPairwise PD-HD(CVC) and PD-HD(AVF/AVG) propensity scores were used.
dExclusion of 11,076 HD-CVC, 1126 PD, and 240 HD-AVF/AVG patients who had 3 months or less of predialysis care by a nephrologist.
Prespecified Interactions
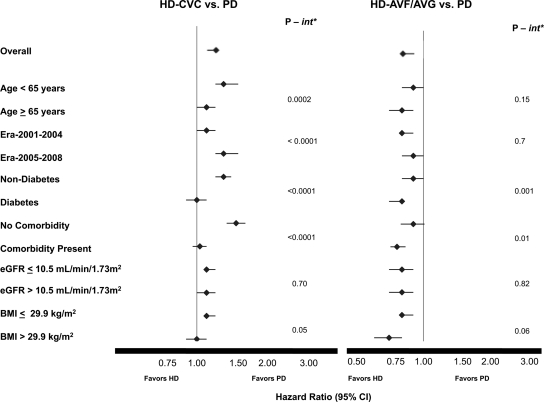
Figure 2 demonstrates the results of the prespecified subgroup analyses. A higher overall mortality risk was seen in HD-CVC-treated patients relative to PD patients in those less than 65 years of age compared with those over the age of 65. Moreover, the era of dialysis initiation (2005 to 2008 versus 2001 to 2004) modified survival comparisons only between HD-CVC- and PD-treated patients but not between HD-AVF/AVG- and PD-treated patients. In this regard, even lower survival in HD-CVC-treated patients was seen relative to PD patients in the more contemporary era compared with the prior era. Diabetes as a cause of ESRD modified the relationship between HD-CVC and HD-AVF/AVG and PD (Table 4). The mortality risk of diabetic HD-CVC patients relative to diabetic PD patients (AHR, 1.0; 95% CI, 0.9 to 1.1) was attenuated compared with the relationship in nondiabetics (AHR, 1.3; 95% CI, 1.2 to 1.4). Similarly, compared with HD-AVF/AVG patients without diabetes (AHR, 0.9; 95% CI, 0.8 to 1.0), diabetic HD-AVF/AVG patients had a significantly lower risk of death compared with diabetic PD patients (AHR, 0.8; 95% CI, 0.7 to 0.8). No significant interactions were seen between eGFR, Body mass index (BMI), and dialysis modality.
Figure 2.
Hemodialysis vascular access affects the association between modality and survival in selected subgroups. *P value for interaction (int). The models were adjusted for age, race, gender, era of dialysis initiation, ESRD comorbidity index, primary renal diagnosis, serum albumin, estimated GFR, province of treatment and late referral.
Table 4.
Results stratified by diabetes and era of dialysis initiation
| Patient Subgroup | HD-CVC versus PD |
HD-AVF/AVG versus PD |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Diabetes | 1.0 (0.9, 1.1) | 0.6 | 0.8 (0.7, 0.8) | <0.0001 |
| Nondiabetes | 1.3 (1.2, 1.4) | <0.0001 | 0.9 (0.8, 1.0) | 0.04 |
| Era 2001 to 2004 | 1.1 (1.0, 1.2) | 0.02 | 0.8 (0.8, 0.9) | <0.0001 |
| Era 2005 to 2008 | 1.3 (1.2, 1.5) | <0.0001 | 0.9 (0.8, 1.0) | 0.02 |
The values are adjusted for age, race, gender, era of dialysis initiation, end-stage renal disease comorbidity index, primary renal diagnosis, serum albumin, estimated glomerular filtration rate, province of treatment, and late referral.
DISCUSSION
In this registry-based, observational cohort study, we identified the important influence of HD vascular access type on survival comparisons between incident HD and PD patients. Patients starting HD using a CVC had a higher risk of death in the first year compared with those who started PD, whereas there was no difference in survival between HD-AVF/AVG and PD patients. These relationships persisted over a 5-year follow-up with a small survival benefit in the HD-AVF/AVG group.
Our findings should prompt a reconsideration of conclusions drawn from previous studies comparing HD and PD. Large registry-based studies,7,9,14,16,17 including a previous analysis of this Canadian registry,7 have demonstrated a survival advantage with PD over HD during the first 1 to 2 years of therapy with similar or inferior survival thereafter. Greater relative preservation of residual kidney function with the use of PD in the initial period after dialysis initiation has been cited as a possible mechanism for this finding.18 However, we found that vascular access type significantly modified this early survival benefit because it was only observed in PD patients when compared with the subgroup of patients who initiated HD with a CVC. This suggests that vascular access-related morbidity/mortality and case-mix differences that coincide with HD vascular access type are more likely to explain the higher early mortality attributed to HD.
Higher 1-year mortality in incident HD patients compared with PD patients has recently been reported by the Australian and New Zealand Dialysis and Transplant (ANZDATA) registry14 and by the United States Renal Data System (USRDS).16 These studies did not adjust for vascular access type. However, in the USRDS study, 1-year survival was similar between HD- and PD-treated patients, once deaths within the first 90 days of dialysis initiation were excluded. Although the USRDS analysis did not directly account for vascular access type, HD patients who were successfully matched to PD patients had characteristics that were likely associated with incident AVF/AVG use as compared with their unmatched counterparts. In the United States, initiatives such as Fistula First may have resulted in the stabilization of prevalent and incident CVC use.19 In contrast, Canada has one of the highest rates of CVC use among developed countries,20 and this may be contributing to early HD-related mortality as CVC use continues to increase.
In addition to the direct effects of CVC use on morbidity and mortality, initiation of HD with a CVC is a proxy for both measured and unmeasured comorbid patient characteristics that are associated with reduced survival among dialysis patients. HD-CVC patients were older, had a greater comorbidity profile, and had less exposure to predialysis care as compared with PD and HD-AVF/AVG patients. Not surprisingly, patients initiating HD with a CVC were more likely to die within 90 days of dialysis initiation. Despite extensive and robust adjustment for case-mix differences, large unmeasured differences likely persist with respect to the severity of comorbidities between CVC- and AVF/AVG-treated HD patients. This would imply that AVF or AVG use at dialysis initiation would be associated with healthier HD patients. Comparing incident PD patients to HD patients who initiated dialysis with an AVF/AVG offered a unique opportunity to assess the effect of dialysis modality in a more homogeneous cohort of incident dialysis patients. Both groups shared similar laboratory profiles including similar serum albumin levels and fewer comorbidities relative to HD-CVC patients. With this analysis, we were unable to demonstrate any early survival differences between PD patients and HD-AVF/AVG patients within the first year of dialysis. Perhaps most importantly, the ability to commence dialysis with PD or HD using an AVF or AVG suggests that exposure to some form of predialysis care is associated with improved early survival, which was likely lacking in many patients who started HD with a CVC. Predialysis care is an important determinant of survival and hospitalization, particularly in the early ESRD period.21,22
After the first year of dialysis, we found that HD-AVF/AVG patients had consistently improved survival compared with PD patients. This finding persisted even after accounting for the effect of a change in modality in a sensitivity analysis that censored patients at the time of a change in dialysis modality. Possible reasons may relate to unmeasured case-mix differences between HD-AVF/AVG and PD patients, which persisted despite extensive multivariable adjustment. It is possible that the very ability to create an AVF or AVG is associated with favorable vascular health and that the inability to create an AVF or AVG may have been a factor in the selection of PD for some patients.23 However, in our cohort, HD-AVF/AVG patients had improved survival despite being older and having a higher burden of documented comorbidities as compared with PD patients. Moreover, our findings remained robust to several sensitivity analyses. It is also possible that survival differences between HD-AVF/AVG patients and PD patients may be due to the effects of informative censoring. Both in this study and in others, higher rates of kidney transplantation have been observed among PD patients relative to HD patients.24,25 Although patients were censored at the time of kidney transplantation, selective removal of a population of transplant-eligible, healthy patients from the PD cohort may have led to reduced survival among the remaining PD patients, many of whom may have been ineligible for transplantation. We partially accounted for this bias by performing an inverse probability of treatment and censoring weight analysis that exhibited little deviation in either the direction or magnitude of the results from our primary analysis.
Many studies have demonstrated that dialysis modality-related survival is modified in particular subgroups of patients.8,10,14–17,26–28 In keeping with previous studies, we found that PD was generally associated with more favorable outcomes in patients ≤65 years old, those without diabetes, and those without additional comorbidities7,14–17,26. Temporal trends toward improving survival in PD patients relative to HD patients have been observed in several studies.25,29 Potential reasons have included both technologic advances in PD connectology, PD solutions, and favorable changes in PD-related practices.29 In comparing two eras (2005 to 2008 versus 2001 to 2004), we found that the relative risk of death among HD-CVC-treated patients compared with PD patients was higher in the more recent era. In contrast, era did not modify survival differences in comparisons between PD and HD-AVF/AVG comparisons. We speculate that survival differences over time between HD and PD patients in Canada reflect a more contemporary HD patient population characterized by both a higher burden of comorbidities and higher rates of incident CVC use.
The study has several limitations. The major threat to validity is selection bias introduced by nonrandom allocation of patients to both dialysis modality and incident HD vascular access. Residual confounding may remain on the basis of unmeasured differences between patients that may influence both incident vascular access and dialysis modality choice while at the same time being associated with survival. Large administrative datasets such as the one that we used are subject to limitations arising from data validity and the availability of data elements that may be germane to the research question being posed. Comorbidities captured within CORR have been recently validated30 and are therefore likely to offer reliable information.31 Several data elements were incomplete. We partially accounted for this by performing multiple data imputation, which demonstrated little change in either the direction or the effect size of our primary results. Changes in vascular access type were not recorded. We were therefore unable to perform as-treated analyses that accounted for: (1) vascular access immediately after PD technique failure; (2) conversion to a functional AVF or AVG among incident HD-CVC patients; and (3) vascular access failure among HD-AVF/AVG patients. It is possible that the conversion to an AVF or AVG in a subset of patients who initiated HD with a CVC may explain the absence of a mortality difference between the HD-CVC and PD patients after the second year of follow-up.
Notwithstanding these limitations, we have demonstrated that incident HD vascular access type at the time of dialysis initiation is an important modifier of the relationship between dialysis modality and survival among incident Canadian dialysis patients. These findings need to be confirmed among other patient populations where regional practice patterns related to HD vascular access and dialysis modality selection may vary. The adverse effects of starting HD with a CVC may have largely driven the relative survival benefits that have been previously attributed to PD. Initiation of HD with an optimal vascular access may be associated with reduced overall mortality as compared with initiating dialysis with PD, but this observation requires confirmation via further prospective studies. In a subset of patients who would otherwise start HD with a CVC because of late referral or ineligibility for a surgical vascular access or who defer a dialysis modality choice or surgical vascular access creation, PD offers the opportunity to avoid HD initiation with a CVC. In this regard, the adverse effects of starting HD with a CVC may be largely driving the relative survival benefits associated with PD.
CONCISE METHODS
Study Design
This is an observational study of consecutive adult patients (age, 18 years or older at the start of chronic dialysis) who registered in the CORR and initiated their first form of dialysis between January 1, 2001 and December 31, 2008.
Data Source, Definitions, and Collection
Patients were identified from the CORR, a national registry that, during the period studied, captured the incidence, prevalence, treatment changes, and outcomes of over 99% of chronic dialysis and solid organ transplant patients in Canada.31 The data were collected by completion of a registration form by the dialysis provider on each patient at dialysis initiation and yearly thereafter. A change of status form is completed to document patient death, transplantation, or a switch in dialysis modality. CORR data has recently been validated.30 We restricted our analysis to patients with documented incident dialysis modality (PD versus HD) and incident vascular access type reported as an AVF, AVG, or CVC (any type). Only patients undergoing 3 to 5 hours of conventional HD three times weekly were included in the primary analysis. Because of the limited number of patients who initiated HD with an AVG (n = 660), we combined AVF or AVG into one category. All of the subtypes of PD (continuous ambulatory PD and automated PD) were included. Three cohorts of incident patients were established: PD, HD-CVC, and HD-AVF/AVG.
Baseline comorbidities were documented by the individual facilities using the CORR registration forms. Information on the presence or absence of coronary artery disease (angina, myocardial infarction, and coronary artery bypass surgery), peripheral vascular disease, hypertension, diabetes mellitus, and cerebrovascular disease were categorized as “yes,” “no,” and “unknown.” The unknowns were combined into the “no” group. Diabetes was classified as a single variable including diabetes as a comorbidity or a cause of end-stage renal disease. Current smokers were documented as those having smoked in the last 3 months. Late referral was defined as never having been seen by a nephrologist before dialysis initiation or first seeing a nephrologist within 3 months before starting dialysis. BMI was calculated using the height and weight collected at the start of dialysis. Baseline laboratory parameters included hemoglobin, serum albumin, and serum creatinine measured as the value closest to but preceding the initial dialysis treatment. eGFR was calculated using the four-variable Modification of Diet in Renal Disease equation.32
Outcome
The primary outcome was mortality at 1 year from the time of first dialysis. Secondary outcomes included overall mortality during the study period and annual mortality risk within the first 5 years after dialysis initiation. Annual mortality risk was assessed using time-dependent hazard ratios within each year. Time-averaged hazard ratios from a proportional hazards model were used to assess the cumulative treatment effect from day 0 through the end of years 1 to 5, respectively. Patients were censored at kidney transplantation, loss to follow-up, or at the end of the observation period (December 31, 2008).
Statistical Analyses
Categorical variables were compared using the chi-squared test. The Kruskal-Wallis test was used to analyze differences among continuous variables. In the primary analysis, study subjects were analyzed in an intention-to-treat manner, using complete-case analysis. Prespecified interactions with the exposure of interest included age (<65 versus ≥65 years), the presence or absence of diabetes, the presence or absence of any comorbidities, BMI (≤29 kg/m2 versus >29 kg/m2), eGFR above and below the median value (≤10.5 ml/min per 1.73 m2 versus >10.5 ml/min per 1.73 m2), and era of dialysis initiation (2001 to 2004 versus 2005 to 2008).
Proportional and nonproportional piecewise exponential survival models were used to compare mortality between PD, HD-CVC, and HD-AVF/AVG patients within sequential 12-month intervals during the first 60 months. Average or time-independent hazard ratios of death for PD compared with HD-CVC and HD-AVF/AVG patients were estimated using a proportional hazards model, whereas time-dependent relative risks were estimated using a nonproportional hazards model. Hazard ratios and corresponding 95% CI were adjusted for case-mix differences in the cohorts including: age, gender, race, cause of ESRD, weighting of comorbidities (diabetes mellitus, coronary artery disease, peripheral vascular disease, malignancy, lung disease, and pulmonary edema) on the basis of a validated ESRD comorbidity index,33 body mass index, eGFR, serum albumin, late referral, province of treatment, and era of dialysis initiation.
Several additional analyses were performed to test the robustness of our findings. First, to account for the effect of missing data on our results, an analysis was performed assigning values for missing data via multiple data imputation using the predictive mean matching method. This strategy has been used successfully in previous studies to avoid exclusion of patients with missing values.34 An additional analysis excluded deaths that occurred after patients were established on a new dialysis modality by censoring patients at 60 days after a change in dialysis modality. To limit the potential for selection bias, an analysis was performed excluding patients who died within 90 days of dialysis initiation. In order to minimize confounding caused by the strong association between late referral and CVC use, a separate analysis was also performed excluding those patients who were referred late.
In addition to traditional multivariable adjustment, outcomes were also compared using a marginal structural model with inverse probability of treatment and censoring weighting. This technique25,35 allowed us to adjust for measured covariates in a single summary propensity score and simultaneously adjust for the effect of informative censoring caused by potential differences in the rates of kidney transplantation between PD patients compared with HD-AVF/AVG and HD-CVC patients. In the first step, propensity scores (PS) were determined as an estimate of each study subject's probability of initial PD treatment. Because our exposure of interest was not binary (i.e. three levels: HD-CVC versus HD-AVF/AVG versus PD]), we used two separate multivariable logistic regression models (PD versus HD-CVC and PD versus HD-AVF/AVG) using all available covariates to calculate our PS. The areas under receiver operating characteristic curves were evaluated to test the discriminatory capacity of each model. In the second step, we determined stabilized censoring weights by estimating the probability of remaining transplant free for each individual in successive 1-year time intervals. Each observation was then weighted both by the inverse probability of treatment with PD (1/PS) for each individual and by the stabilized censoring weights. All of the analyses were performed using SAS version 9.1.3 (Cary, NC).
DISCLOSURES
J.P. has received speaking honoraria from Amgen Canada and Baxter Healthcare Canada and holds an unrestricted educational fellowship from Baxter Healthcare Canada. P.M. has received speaking honoraria from Biovail, Boehringer Ingelheim, Bristol Myers Squibb, GlaxoSmithKline, Merk, Novartis, and Sanofi-Aventis and has served on advisory boards for Amgen Canada, Baxter Healthcare Canada, Biovail, Boehringer Ingelheim, Bristol Myers Squibb, Fresenius, Merk Novartis, Ortho-Biotech, Sanofi-Aventis, and Schering. R.W. has served on advisory boards for Amgen, Gilead, and Fresenius Kabi and receives an unrestricted educational fellowship from Amgen. J.B. has served on advisory boards for Amgen, Takeda, and Hospira and has received speaking honoraria from Baxter Healthcare Canada, Amgen Canada, and Genzyme Canada. E.V. has served as a consultant for Baxter Healthcare. V.J. has served on advisory boards for Amgen Canada and has received speaking honoraria from Baxter Healthcare Canada. L.M. has served on advisory boards for Amgen Canada and Merck Frosst.
Acknowledgments
The authors gratefully acknowledge the staff at CORR for maintaining the database and the renal units throughout Canada for submitting information to CORR.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Differential Outcomes Between Dialysis Modalities: Purely a Reflection of Selection,” on pages 989–990.
REFERENCES
- 1. Oliver MJ, Rothwell DM, Fung K, Hux JE, Lok CE: Late creation of vascular access for hemodialysis and increased risk of sepsis. J Am Soc Nephrol 15: 1936–1942, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Moist LM, Trpeski L, Na Y, Lok CE: Increased hemodialysis catheter use in Canada and associated mortality risk: Data from the Canadian Organ Replacement Registry 2001–2004. Clin J Am Soc Nephrol 3: 1726–1732, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Polkinghorne KR: Vascular access practice in hemodialysis: Instrumental in determining patient mortality. Am J Kidney Dis 53: 359–362, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Lacson E, Jr., Wang W, Hakim RM, Teng M, Lazarus JM: Associates of mortality and hospitalization in hemodialysis: Potentially actionable laboratory variables and vascular access. Am J Kidney Dis 53: 79–90, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, Rayner HC, Saito A, Sands JJ, Saran R, Gillespie B, Wolfe RA, Port FK: Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: An instrumental variable analysis. Am J Kidney Dis 53: 475–491, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Abbott KC, Glanton CW, Trespalacios FC, Oliver DK, Ortiz MI, Agodoa LY, Cruess DF, Kimmel PL: Body mass index, dialysis modality, and survival: Analysis of the United States Renal Data System Dialysis Morbidity and Mortality Wave II Study. Kidney Int 65: 597–605, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Fenton SS, Schaubel DE, Desmeules M, Morrison HI, Mao Y, Copleston P, Jeffery JR, Kjellstrand CM: Hemodialysis versus peritoneal dialysis: a comparison of adjusted mortality rates. Am J Kidney Dis 30: 334–342, 1997 [DOI] [PubMed] [Google Scholar]
- 8. Ganesh SK, Hulbert-Shearon T, Port FK, Eagle K, Stack AG: Mortality differences by dialysis modality among incident ESRD patients with and without coronary artery disease. J Am Soc Nephrol 14: 415–424, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Heaf JG, Lokkegaard H, Madsen M: Initial survival advantage of peritoneal dialysis relative to haemodialysis. Nephrol Dial Transplant 17: 112–117, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Inrig JK, Sun JL, Yang Q, Briley LP, Szczech LA: Mortality by dialysis modality among patients who have end-stage renal disease and are awaiting renal transplantation. Clin J Am Soc Nephrol 1: 774–779, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Jaar BG, Coresh J, Plantinga LC, Fink NE, Klag MJ, Levey AS, Levin NW, Sadler JH, Kliger A, Powe NR: Comparing the risk for death with peritoneal dialysis and hemodialysis in a national cohort of patients with chronic kidney disease. Ann Intern Med 143: 174–183, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Korevaar JC, Feith GW, Dekker FW, van Manen JG, Boeschoten EW, Bossuyt PM, Krediet RT: Effect of starting with hemodialysis compared with peritoneal dialysis in patients new on dialysis treatment: A randomized controlled trial. Kidney Int 64: 2222–2228, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Liem YS, Wong JB, Hunink MG, de Charro FT, Winkelmayer WC: Comparison of hemodialysis and peritoneal dialysis survival in The Netherlands. Kidney Int 71: 153–158, 2007 [DOI] [PubMed] [Google Scholar]
- 14. McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR: Relationship between dialysis modality and mortality. J Am Soc Nephrol 20: 155–163, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vonesh EF, Snyder JJ, Foley RN, Collins AJ: The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int 66: 2389–2401, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Weinhandl ED, Foley RN, Gilbertson DT, Arneson TJ, Snyder JJ, Collins AJ: Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J Am Soc Nephrol 21: 499–506, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vonesh EF, Moran J: Mortality in end-stage renal disease: A reassessment of differences between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol 10: 354–365, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, Hulbert-Shearon T, Jones CA, Bloembergen WE: Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 11: 556–564, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Spergel LM: Has the Fistula First Breakthrough Initiative caused an increase in catheter prevalence? Semin Dial 21: 550–552, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Mendelssohn DC, Ethier J, Elder SJ, Saran R, Port FK, Pisoni RL: Haemodialysis vascular access problems in Canada: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS II). Nephrol Dial Transplant 21: 721–728, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Goldstein M, Yassa T, Dacouris N, McFarlane P: Multidisciplinary predialysis care and morbidity and mortality of patients on dialysis. Am J Kidney Dis 44: 706–714, 2004 [PubMed] [Google Scholar]
- 22. Black C, Sharma P, Scotland G, McCullough K, McGurn D, Robertson L, Fluck N, MacLeod A, McNamee P, Prescott G, Smith C: Early referral strategies for management of people with markers of renal disease: A systematic review of the evidence of clinical effectiveness, cost-effectiveness and economic analysis. Health Technol Assess 14: 1–184 [DOI] [PubMed] [Google Scholar]
- 23. Wasse H, Hopson SD, McClellan W: Racial and gender differences in arteriovenous fistula use among incident hemodialysis patients. Am J Nephrol 32: 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Snyder JJ, Kasiske BL, Gilbertson DT, Collins AJ: A comparison of transplant outcomes in peritoneal and hemodialysis patients. Kidney Int 62: 1423–1430, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E: Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med 171: 110–118, 2011 [DOI] [PubMed] [Google Scholar]
- 26. Winkelmayer WC, Glynn RJ, Mittleman MA, Levin R, Pliskin JS, Avorn J: Comparing mortality of elderly patients on hemodialysis versus peritoneal dialysis: A propensity score approach. J Am Soc Nephrol 13: 2353–2362, 2002 [DOI] [PubMed] [Google Scholar]
- 27. Stack AG, Molony DA, Rahman NS, Dosekun A, Murthy B: Impact of dialysis modality on survival of new ESRD patients with congestive heart failure in the United States. Kidney Int 64: 1071–1079, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Stack AG, Murthy BV, Molony DA: Survival differences between peritoneal dialysis and hemodialysis among “large” ESRD patients in the United States. Kidney Int 65: 2398–2408, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Mehrotra R, Kermah D, Fried L, Kalantar-Zadeh K, Khawar O, Norris K, Nissenson A: Chronic peritoneal dialysis in the United States: Declining utilization despite improving outcomes. J Am Soc Nephrol 18: 2781–2788, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Canadian Institute for Health Information, Data Quality Study on the Canadian Organ Replacement Register. Ottawa, Ontario, Canada, Canadian Institute for Health Information, 2009 [Google Scholar]
- 31. Karamadoukis L, Ansell D, Foley RN, McDonald SP, Tomson CR, Trpeski L, Caskey FJ: Towards case-mix-adjusted international renal registry comparisons: How can we improve data collection practice? Nephrol Dial Transplant 24: 2306–2311, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Levey AS, Greene T, Kusek J, Beck G: A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 33. Hemmelgarn BR, Manns BJ, Quan H, Ghali WA: Adapting the Charlson Comorbidity Index for use in patients with ESRD. Am J Kidney Dis 42: 125–132, 2003 [DOI] [PubMed] [Google Scholar]
- 34. van der Heyden GJ, Donders AR, Stynen T, Moons KG: Imputation of missing values is superior to complete case analysis and the missing indicator method in multivariable diagnostic research: A clinical example. J Clin Epidemiol 59: 1102–1109, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Hernan MA, Brumback B, Robins JM: Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 11: 561–570, 2000 [DOI] [PubMed] [Google Scholar]