Abstract
Pirfenidone is an oral antifibrotic agent that benefits diabetic nephropathy in animal models, but whether it is effective for human diabetic nephropathy is unknown. We conducted a randomized, double-blind, placebo-controlled study in 77 subjects with diabetic nephropathy who had elevated albuminuria and reduced estimated GFR (eGFR) (20 to 75 ml/min per 1.73 m2). The prespecified primary outcome was a change in eGFR after 1 year of therapy. We randomly assigned 26 subjects to placebo, 26 to pirfenidone at 1200 mg/d, and 25 to pirfenidone at 2400 mg/d. Among the 52 subjects who completed the study, the mean eGFR increased in the pirfenidone 1200-mg/d group (+3.3 ± 8.5 ml/min per 1.73 m2) whereas the mean eGFR decreased in the placebo group (−2.2 ± 4.8 ml/min per 1.73 m2; P = 0.026 versus pirfenidone at 1200 mg/d). The dropout rate was high (11 of 25) in the pirfenidone 2400-mg/d group, and the change in eGFR was not significantly different from placebo (−1.9 ± 6.7 ml/min per 1.73 m2). Of the 77 subjects, 4 initiated hemodialysis in the placebo group, 1 in the pirfenidone 2400-mg/d group, and none in the pirfenidone 1200-mg/d group during the study (P = 0.25). Baseline levels of plasma biomarkers of inflammation and fibrosis significantly correlated with baseline eGFR but did not predict response to therapy. In conclusion, these results suggest that pirfenidone is a promising agent for individuals with overt diabetic nephropathy.
Diabetic nephropathy remains the leading cause of end-stage kidney disease (ESKD) in the United States, accounting for over 40% of incident ESKD cases. Diabetic nephropathy is characterized by inflammation, accumulation of mesangial matrix in established disease, marked tubulointerstitial fibrosis, and vascular hyalinosis in advanced disease. Mesangial matrix expansion and tubulointerstitial fibrosis correlate with progression of diabetic nephropathy to ESKD.1–3 The standard of care for diabetic nephropathy has been the use of inhibitors of the renin-angiotensin system (RAS), including angiotensin converting enzyme inhibitors (ACEIs)4 and angiotensin II receptor blockers (ARBs)5,6 and tight glycemic control. BP-independent benefits of RAS inhibitors may contribute to renal protection, possibly via inhibiting profibrotic factors such as TGF-β.7 However, the intensive use of RAS inhibitors is often limited by severe hyperkalemia, further reduction in the systemic BP, and decreased renal blood flow. Even when maximized, they may decrease rate of progression, but they do not arrest or reverse diabetic nephropathy. In addition, a recent large randomized clinical study found that combined ACEI/ARB therapy was associated with worse renal outcomes in diabetic and nondiabetic individuals without severe nephropathy at baseline.8 Therefore, novel approaches that block progression of nephropathy and do not rely on blocking the RAS axis may provide important additional therapies to block progressive diabetic nephropathy and renal failure.
Several growth factors or cytokines that are locally produced in the kidney appear to contribute to the extracellular matrix accumulation, inflammation, and scarring in progressive diabetic nephropathy. The TGF-β system is activated and plays a pathogenetic role in diabetic kidney disease in animal models of type 19 and type 2 diabetes.10 In addition, several studies in patients with type 1 and type 2 diabetes indicate increased renal production of TGF-β.11,12 The TNF-α system has also been recently linked with human diabetic nephropathy on the basis of circulating blood levels13 and gene expression in kidneys from patients with diabetic nephropathy.14 An orally bioavailable compound, pirfenidone, has been found to inhibit TGF-β production and consequent matrix deposition in experimental animal models of lung and kidney disease.15,16 In animal models and cell culture studies, pirfenidone also reduces TNF-α production.17,18 We have found that oral pirfenidone administered to db/db mice after the onset of established diabetic kidney disease was effective in reducing glomerulosclerosis.19 In addition, in an open-label clinical study in patients with advanced and treatment-refractory focal segmental glomerulosclerosis (FSGS), there was a 25% reduction in the rate of estimated GFR (eGFR) decline in patients on pirfenidone as compared with the rate of decline before pirfenidone.20
We hypothesized that administration of pirfenidone to type 1 and type 2 diabetic patients with established diabetic nephropathy would slow the rate of eGFR decline. We performed a double-blind, placebo-controlled, exploratory study using a dose ranging protocol. We chose to test two different doses of the drug (1200 and 2400 mg/d) on the basis of the results of the open-label FSGS study20 and information provided by the drug manufacturer (InterMune, Inc.). The 2400-mg/d dose was found to be effective in the open-label study with FSGS, but the occurrence of adverse events at this dose suggested that higher doses may not be tolerable in the diabetic population with moderate to advanced CKD. The lower dose (1200 mg/d) was included because lower doses (<2400 mg/d) may also be effective and associated with less adverse events. Of note, the same two dosage regimens (1200 and 2400 mg/d) were recently used in two phase III trials of pirfenidone in patients with idiopathic pulmonary fibrosis.
RESULTS
Subjects
Seventy-seven subjects with diabetes, reduced eGFR, and proteinuria were enrolled and randomized to one of the three study arms. All of the subjects who enrolled into the study were well matched without any statistical differences among the baseline variables between the three groups (Table 1). Of the initial 77 patients enrolled and randomized, 52 subjects completed 54 weeks of the trial. There were no statistically significant differences in demographic variables or kidney function at baseline between the completer group (n = 52) and the noncompleter group (n = 25) (Supplemental Table 1). Of the 52 study completers, baseline variables were similar across the three treatment groups except for higher diastolic BP and lower plasma albumin in the pirfenidone 2400-mg group (Table 2). Group analysis presented involves only study completers as a study group or as treatment group, except when data are specifically mentioned as pertaining to all enrolled subjects.
Table 1.
Characteristics of study participants at study entry
| Characteristics | Placebo | Pirfenidone at 1200 mg/d | Pirfenidone at 2400 mg/d | P |
|---|---|---|---|---|
| n | 26 | 26 | 25 | |
| Age (years) | 56 ± 16 | 62 ± 12 | 56 ± 13 | 0.26 |
| Male n (%) | 16 (59%) | 18 (69%) | 15 (60%) | 0.71 |
| Black n (%) | 8 (30%) | 10 (38%0 | 8 (32%) | 0.78 |
| Weight (pounds) | 207 ± 47 | 218 ± 43 | 228 ± 63 | 0.38 |
| BMI (kg/m2) | 31 ± 7 | 32 ± 5 | 34 ± 9 | 0.47 |
| Systolic BP (mmHg) | 129 ± 16 | 129 ± 12 | 130 ± 11 | 0.95 |
| Diastolic BP (mmHg) | 71 ± 9 | 72 ± 8 | 74 ± 7 | 0.38 |
| Heart rate (bpm) | 70 ± 8 | 70 ± 10 | 70 ± 13 | 0.96 |
| DM type 2, n % | 20 (74%) | 21 (81%) | 19 (76%) | 0.84 |
| DM duration (years) | 23 ± 13 | 17 ± 10 | 18 ± 11 | 0.15 |
| ACEI/ARB use n (%) | 0.89 | |||
| ACEI only | 7 (26%) | 9 (35%) | 8 (32%) | |
| ARB only | 12 (44%) | 7 (27%) | 10 (40%) | |
| ACEI + ARB | 6 (22%) | 8 (31%) | 6 (24%) | |
| Smoking n (%) | 0.49 | |||
| current | 1 (4%) | 0 (0%) | 0 (0%) | |
| former | 14 (54%) | 10 (40%) | 10 (42%) | |
| HDL-cholesterol (mg/dl) | 44 ± 11 | 43 ± 9 | 42 ± 11 | 0.83 |
| LDL-cholesterol (mg/dl) | 94 ± 26 | 103 ± 40 | 106 ± 34 | 0.46 |
| Triglycerides (mg/dl)a | 107 (70, 171) | 105 (76, 188) | 113 (87, 142) | 0.94 |
| Serum albumin (g/dl) | 4.4 ± 0.4 | 4.4 ± 0.4 | 4.2 ± 0.5 | 0.07 |
| GFR (ml/min per 1.73 m2) | 35 ± 13 | 36 ± 13 | 40 ± 15 | 0.43 |
| ACR (mg/g)a | 179 (36, 632) | 214 (93, 484) | 195 (105, 984) | 0.61 |
| Completed study n, % | 21 (78%) | 17 (65%) | 14 (56%) |
Baseline demographic, clinical, and laboratory parameters for study completers are presented. Data are presented as mean ± SD, with analysis by χ2 or Fisher's exact tests for categorical variables and ANOVA for continuous variables,
aexcept for variables for which data were not normally distributed, which are presented as median and IQR and were analyzed by Kruskal–Wallis test. BMI, body mass index; bpm, beats per minute; DM, diabetes mellitus.
Table 2.
Entry Characteristics of study participants who completed study
| Characteristics | Placebo | Pirfenidone at 1200 mg/d | Pirfenidone at 2400 mg/d | P |
|---|---|---|---|---|
| n | 21 | 17 | 14 | |
| Age (years) | 59 ± 12 | 58 ± 10 | 55 ± 13 | 0.56 |
| Male n (%) | 12 (57%) | 11 (65%) | 10 (71%) | 0.69 |
| Black n (%) | 6 (29%) | 7 (41%) | 2 (21%) | 0.48 |
| Weight (pounds) | 210 ± 50 | 206 ± 28 | 218 ± 49 | 0.74 |
| BMI (kg/m2) | 31.9 ± 7.2 | 31.5 ± 5.3 | 32.8 ± 8.2 | 0.88 |
| Systolic BP (mmHg) | 129 ± 15 | 123 ± 9 | 130 ± 10 | 0.24 |
| Diastolic BP (mmHg) | 71 ± 9 | 70 ± 8 | 78 ± 7 | 0.02 |
| Heart rate (bpm) | 71 ± 8 | 70 ± 12 | 71 ± 13 | 0.94 |
| DM type 2, n % | 16 (76%) | 13 (76%) | 11 (79%) | 0.99 |
| DM duration (years) | 24 ± 13 | 18 ± 10 | 19 ± 9 | 0.25 |
| ACEI/ARB use n (%) | 0.55 | |||
| ACEI only | 6 (29%) | 7 (41%) | 4 (29%) | |
| ARB only | 10 (48%) | 3 (18%) | 5 (36%) | |
| ACEI + ARB | 4 (19%) | 6 (35%) | 5 (36%) | |
| Smoking n (%) | 0.68 | |||
| current | 1 (5%) | 0 (0%) | 0 (0%) | |
| former | 11 (52%) | 8 (50%) | 5 (38%) | |
| Hemoglobin A1c (g/dl) | 7.3 ± 1.4 | 7.4 ± 1.2 | 7.1 ± 1.4 | 0.81 |
| HDL-cholesterol (mg/dl) | 45 ± 11 | 44 ± 9 | 43 ± 12 | 0.86 |
| LDL-cholesterol (mg/dl) | 96 ± 28 | 108 ± 39 | 104 ± 24 | 0.53 |
| Triglycerides (mg/dl)a | 104 (70, 171) | 116 (76, 188) | 109 (88, 157) | 0.83 |
| Serum albumin (g/dl) | 4.4 ± 0.3 | 4.6 ± 0.4 | 4.2 ± 0.4 | 0.04 |
| eGFR (ml/min per 1.73 m2) | 39 ± 13 | 38 ± 13 | 39 ± 13 | 0.95 |
| Urine ACR (mg/g)a | 79 (36, 514) | 131 (48, 450) | 252 (118, 984) | 0.38 |
Baseline demographic, clinical, and laboratory parameters for study completers are presented. Data are presented as mean ± SD, with analysis by χ2 or Fisher's exact tests for categorical variables and ANOVA for continuous variables,
aexcept for variables for which data were not normally distributed, which are presented as median and IQR and were analyzed by Kruskal–Wallis test.
Primary End Point
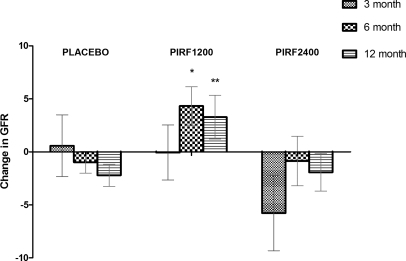
There was a significant difference in eGFR change from baseline to the end of study in the pirfenidone 1200-mg group compared with placebo (Figure 1, Table 3). The mean intergroup difference in eGFR change was +5.5 ml/min per 1.73 m2 (−2.2 ml/min per 1.73 m2 in placebo versus +3.3 ml/min per 1.73 m2 pirfenidone 1200 mg, 95% confidence interval [CI] 1.1, 9.9; P = 0.026). A post hoc two-sample t test of changes from baseline to month 6 also detected a significant difference between the pirfenidone 1200-mg and placebo groups (5.3 ml/min per 1.73 m2 [95% CI 1.3, 9.3; P = 0.02; Figure 1). At 3 months after the beginning of the study there was no significant difference in eGFR change between the placebo and the pirfenidone 1200-mg groups.
Figure 1.
Pirfenidone at 1200 mg/d, but not 2400 mg/d, improves eGFR from baseline. The change in eGFR from baseline of the completers in the three groups: PLACEBO (placebo group; n = 21), PIRF1200 (pirfenidone 1200-mg group; n = 17), and PIRF2400 (pirfenidone 2400-mg group; n = 14) at 3, 6, and 12 months. Data are expressed as mean ± SEM, *P = 0.02 when compared with placebo at 6 months, and **P = 0.026 when compared with placebo at 12 months.
Table 3.
Change in eGFR over 54 weeks by treatment group
| Treatment | eGFR Change (ml/min per 1.73 m2) | eGFR Change with Imputation (ml/min per 1.73 m2) |
|---|---|---|
| Placebo | −2.2 ± 4.8 | −3.7 ± 5.7 |
| Pirfenidone at 1200 mg/d | 3.3 ± 8.5a | 3.3 ± 8.5b |
| Pirfenidone at 2400 mg/d | −1.9 ± 6.7 | −2.1 ± 6.4 |
| Total | −0.3 ± 7.0 | −0.7 ± 9.1 |
Shown are the eGFR changes over the course of the study in the 52 study completers and in the 52 completers plus the 5 subjects who began dialysis during the study, for whom an end-of-study eGFR was imputed as 10 ml/min per 1.73 m2.
aP = 0.026 versus placebo and
bP < 0.006 versus placebo.
The mean difference in eGFR change at the end of the study between the pirfenidone 2400-mg and placebo groups was 0.3 ml/min per 1.73 m2 (95% CI −3.7, 4.2; P = 0.89). When the data were adjusted for baseline eGFR, there was a similar relationship among the groups (Supplemental Table 2, analysis of covariance [ANCOVA] P = 0.023). Comparing completers who were in the placebo arm versus receiving any pirfenidone (1200 or 2400 mg), the change in eGFR was not statistically significantly different (P = 0.085). Placebo lost 2.20 ± 4.80 ml/min per 1.73 m2 per 54 weeks, whereas the combined “any pirfenidone” arm improved slightly (gained 0.93 ± 8.05 ml/min per 1.73 m2 per 54 weeks). When all patients who enrolled were evaluated at 3 and 6 months, the trends were similar (Supplemental Figure 1); however, at 6 months of the study, the increase in eGFR in the pirfenidone 1200-mg group versus the placebo group was NS (P = 0.059).
In view of the extent of missing data, we carried out permutation tests and ANCOVA with iterated re-weighted least squares (IWLS), controlling for baseline values and their interaction with treatment. The significance of comparison of eGFR change for the pirfenidone at 1200 mg versus placebo comparison was confirmed by the permutation test (P = 0.012) and the ANCOVA with IWLS (P = 0.019). Baseline characteristics of completers versus noncompleters were balanced for clinical variables (Supplemental Table 1) with no differences reaching statistical significance.
Five subjects required initiation of dialysis during the course of the study; four subjects were in the placebo group and one subject was in the pirfenidone 2400-mg group. The decision to initiate dialysis was made by the primary provider without consultation with the study investigators. These subjects were considered to have completed the study at the time of dialysis initiation. Imputing the eGFR as 10 ml/min per 1.73 m2 for subjects requiring dialysis revealed an even greater effect of the pirfenidone 1200-mg/d regimen to slow the eGFR decline (Table 3). Of the five patients who required dialysis, three had acute kidney injury (one with infection [placebo], one from unclear etiology [placebo], and one postsurgery [pirfenidone 2400-mg group]). The remaining two patients required dialysis because of worsening of their renal function with an associated symptom attributed to advancing kidney failure (one with depression [placebo] and one with hyperkalemia [placebo]). None of the subjects in the pirfenidone 1200-mg group required dialysis.
Secondary Outcomes
Urine albumin/creatinine ratio (ACR) was evaluated as a secondary outcome. There were no significant differences among study groups in change in ACR from baseline to the end of study (P value across treatment groups = 0.19). We also found no significant change in urine TGF-β levels within the placebo or treatment groups over 54 weeks. Urine TGF-β levels increased by 1.4 pg/mg creatinine (95% CI 0.2, 6.6) over 12 months in the placebo group, numerically increased by 0.3 pg/mg (95% CI −1.6, 5.6) in the pirfenidone 1200-mg group, and numerically declined by 0.1 pg/mg (95% CI −3.4, 3.7) in the pirfenidone 2400-mg group (P value across treatment groups = 0.54).
Predictors of Response to Pirfenidone
At baseline, diastolic BP was higher in the pirfenidone 2400-mg group and plasma albumin was lower in the pirfenidone 2400-mg group (Table 2). The diastolic BP was not associated with eGFR change in any treatment group or in the total study population. However, multiple linear regression models demonstrated that higher baseline plasma albumin was associated with eGFR change over the 54 weeks among all groups (P < 0.001) (Table 4). Among individual groups, a 0.1-g/dl higher baseline plasma albumin was associated with a significant improvement in eGFR of 0.9 ml/min per 1.73 m2 in the pirfenidone 2400-mg group (95% CI 0.1, 1.7; P = 0.03), approached significance in the pirfenidone 1200-mg group (P = 0.06), but was NS in the placebo group (P = 0.21) (Table 4). However, when a multiplicative interaction term was applied with serum albumin, the interaction term was NS (P value for interaction = 0.34).
Table 4.
Association of baseline serum albumin (per 0.1 g/L higher) with 54-week eGFR change
| n | eGFR Change (95% CI) | P | |
|---|---|---|---|
| All completers | 52 | 0.9 (0.5, 1.4) | <0.001 |
| Placebo arm | 21 | 0.4 (−0.2, 1.1) | 0.21 |
| Pirfenidone at 1200 mg/d | 17 | 1.1 (0.0, 2.2) | 0.06 |
| Pirfenidone at 2400 mg/d | 14 | 0.9 (0.1, 1.7) | 0.03 |
Shown is the association of baseline serum albumin with change in eGFR over the 54-week period using linear regression. Positive values indicate that a higher serum albumin level was associated with improvement in eGFR during the course of the study.
We studied several plasma biomarkers that have correlated with kidney function decline, inflammation, or fibrosis in prior studies (baseline values shown in Supplemental Table 3).13,21–23 Interestingly, the baseline levels of many plasma biomarkers were correlated with baseline eGFR (Table 5). The strongest associations were observed with TNF, soluble TNF receptor 1 (sTNF-R1), and fibroblast growth factor-23 (FGF-23), with higher levels associated with lower baseline eGFR. We also report for the first time a highly significant correlation between FGF-23 and sTNF-R1 (r = 0.7273) and TNF (r = 0.623), P < 0.001 for both (Supplemental Table 4). Although the inflammatory plasma biomarkers were highly correlated with the baseline eGFR, none changed significantly with pirfenidone treatment (Supplemental Table 5), and none were statistically significantly associated with eGFR change in any treatment group.
Table 5.
Association of baseline plasma biomarker levels with baseline eGFR in completers
| Biomarker | n | Change GFR (95% CI) | P |
|---|---|---|---|
| IFN-γa | 48 | −5.1 (−8.7, −1.5) | 0.006 |
| IL-1a | 48 | −4.4 (−8.1, −0.8) | 0.019 |
| TNF | 48 | −6.7 (−10.0, −3.3) | <0.001 |
| sTNF-R1 | 47 | −9.0 (−11.9, −6.2) | <0.001 |
| FGF-23a | 48 | −8.1 (−11.2, −5.0) | <0.001 |
| YKL-40 | 47 | −4.8 (−8.5, −1.1) | 0.011 |
| Brain natriuretic peptidea | 46 | −0.9 (−4.8, 3.1) | 0.667 |
Shown are the cross-sectional associations of biomarkers at the baseline study visit with eGFR at the baseline study visit using linear regression. Negative values indicate that higher baseline biomarker level was associated with a lower GFR at the baseline study visit.
aSkewed variables were natural log transformed, and each biomarker was subsequently modeled as per 1 SD higher to facilitate comparison in strengths of association.
Adverse Events and Study Noncompletion
The adverse events that contributed to patients' withdrawal from the study are listed in Table 6. Adverse events were predominantly gastrointestinal, fatigue, and photosensitivity rash. None of the adverse effects were significantly more common in the two pirfenidone groups compared with the placebo group, although there was a numerically higher number of subjects who withdrew from the study because of gastrointestinal side effects and fatigue in the pirfenidone groups as compared with placebo. Of note, the incidence of gastrointestinal side effects and fatigue appeared to be dose related. One subject in the high-dose pirfenidone group was diagnosed with prostate adenocarcinoma within the first few weeks of starting drug and withdrew from the study.
Table 6.
Reasons for subject noncompletion
| Reason | Placebo (n = 26) | Pirfenidone at 1200 mg/d (n = 26) | Pirfenidone at 2400 mg/d (n = 25) | P |
|---|---|---|---|---|
| n | 5 | 9 | 11 | |
| Dialysis | 4 (15%) | 0 (0%) | 1 (4%) | 0.07 |
| Gastrointestinal symptoms | 1 (4%) | 2 (8%) | 6 (24%) | 0.06 |
| Rash | 0 | 1 (4%) | 1 (4%) | 0.59 |
| Heart failure | 0 | 1 (4%) | 0 | 0.37 |
| Aortic dilation | 0 | 1 (4%) | 0 | 0.37 |
| Bradycardia | 1 (4%) | 0 | 0 | 0.37 |
| Urinary tract infection | 0 | 0 | 1 (4%) | 0.35 |
| Fatigue | 0 | 2 (8%) | 2 (8%) | 0.34 |
| Cancer | 0 | 0 | 1 (4%) | 0.35 |
| Noncompliance | 0 | 2 (8%) | 3 (12%) | 0.21 |
| Lost to follow-up | 0 | 1 (4%) | 0 | 0.37 |
| Other (patient moved) | 0 | 1 (4%) | 0 | 0.37 |
Shown are the number of subjects randomized to each treatment group who discontinued treatment before the end of the study and the reasons for discontinuation. Several subjects had more than one adverse event that contributed to their withdrawal from the study. One patient withdrew from study for personal reasons (moved out of state) before the first post-treatment visit. The P value was calculated using Pearson's χ2 test.
DISCUSSION
Novel treatment approaches for diabetic nephropathy that reduce the rate of renal function decline are urgently needed because the number of patients with ESKD attributed to diabetes continues to increase. The available approaches to reduce the rate of renal function decline primarily work in an indirect manner via reducing hyperglycemia or BP. Although our study is limited because of the small sample size, to our knowledge this is the first randomized, double-blind study for diabetic nephropathy that uses a drug that is considered to function via its antifibrotic and cytoprotective properties. We observed a rate of decline of eGFR of −2.2 ± 4.8 ml/min per 1.73 m2 per year in the placebo group, which is lower than in other recent studies,5,6 demonstrating that conservative therapy was maximized. We observed an improvement in eGFR in the pirfenidone 1200-mg group with a net increase of +3.3 ± 8.5 ml/min per 1.73 m2 over the span of 54 weeks. The pirfenidone 2400-mg group had an intermediate change in eGFR (−1.9 ± 6.7 ml/min per 1.73 m2) that was not significantly different compared with the placebo arm.
The eGFR improvement in the pirfenidone 1200-mg group was noted as early as 6 months after treatment initiation and was maintained through the end of the study. It is possible that the early increase in eGFR may be due to a hemodynamic effect; however, there was not an increase in eGFR at 3 months with either dose of pirfenidone. The lack of an early increase in eGFR suggests that the beneficial effect of pirfenidone on the kidney required at least 3 to 6 months to manifest and therefore hemodynamic alterations may not be the likely explanation. Whether the benefit on eGFR is due to hemodynamics or a reduction of matrix expansion in the glomerulus or tubulointerstitial compartments remains unknown because biopsies were not performed in this study. The significant improvement in eGFR suggests that treatment to reduce renal fibrosis may confer some degree of regression of the disease process in diabetic nephropathy. Regression of mesangial matrix expansion has been reported in patients with established diabetic nephropathy who underwent a pancreas transplant24,25; however, a period of 5 to 10 years was required for improvement in pathology to manifest. In animal studies, a combination of ACEIs and ARBs has been found to reverse lesions of renal fibrosis.26,27 These studies provide evidence that renal fibrosis is not necessarily irreversible and a potent antifibrotic approach may be able to arrest and potentially improve renal function within a short time frame. Future studies with repeated biopsies would be informative to demonstrate whether glomerular and tubulointerstitial markers of fibrosis are reversible with innovative therapies.
Novel noninvasive biomarkers that correlate with progression and/or regression may be suitable as surrogate markers for demonstrating regression and would be of great utility in future studies evaluating antifibrotic therapies. We selected a candidate list of biomarkers within this study. Albuminuria is the classic biomarker for diabetic nephropathy and reduction in albuminuria has been found to correlate with reducing the rate of renal function decline with ARB treatment.28 Notably, albuminuria did not decrease with pirfenidone treatment. Our data are similar to what was observed in a recent open-label clinical study of pirfenidone in patients with advanced FSGS because pirfenidone treatment was associated with a reduction of the rate of renal function decline without attenuating albuminuria.20 Similarly, in an animal model pirfenidone conferred a benefit to glomerular histology and reduced gene expression of matrix molecules in the diabetic db/db mouse without lowering albuminuria.19 Urine levels of TGF-β have been found to be increased in patients with diabetic nephropathy12,29,30 and may reflect ongoing renal production.12 However, in the study presented here urine levels of TGF-β were not significantly affected by pirfenidone; this may be due to the wide variability of urine levels in patients and/or the small sample size.
Of great interest was that several biomarkers were strongly associated with eGFR at baseline. Many of these are considered to be part of the inflammatory response (TNF, sTNF-R1, IFN-γ, and IL-1) and these findings are consistent with recent data in patients with type 1 diabetes.13 Because our study included patients with type 1 and type 2 (predominantly the latter) diabetes, these inflammatory biomarkers validate the results of the prior study. Further support for inflammatory mechanisms in diabetic nephropathy is provided from gene expression of renal biopsies from patients with diabetic nephropathy.14 In this study, markers of the inflammatory network were strongly upregulated in isolated glomeruli.
Pirfenidone is a pyridone derivative that has antifibrotic and anti-inflammatory properties. There has been a growing interest in this drug to protect against various progressive fibrotic disorders.31–35 The drug has been approved for clinical use in Japan for idiopathic pulmonary fibrosis. Two phase III studies of pirfenidone for idiopathic pulmonary fibrosis in North America, Europe, and Australia reported equivocal statistical outcomes but overall reduced disease severity. The drug is currently not approved by the U.S. Food and Drug Administration for clinical use in the United States.
Our study had several important limitations. First, it was designed as an exploratory, small-scale study and our findings will need replication with a larger study. Second, the incomplete ascertainment of outcomes may have influenced the results. In this regard, change in eGFR was our primary outcome, which by definition required eGFR measurements at study entry and the end of the study. Individuals who dropped out were therefore excluded from the efficacy analysis, which may have introduced bias in either direction. It remains possible that the statistically significant benefit observed in the pirfenidone 1200-mg group was due to a type 1 error from the small sample size. A third limitation was that the lack of efficacy of high-dose pirfenidone remained unexplained. It is possible that compliance was lower with the higher dose. Adverse events that were common in patients on pirfenidone were primarily gastrointestinal, including nausea, dyspepsia, and diarrhea. This led to withdrawal from the study in 8 of 51 patients in the combined pirfenidone groups (two from the pirfenidone 1200-mg group and six from the pirfenidone 2400-mg group) versus 1 of 26 in the placebo group. Fatigue led to withdrawal of an additional four patients (two from the pirfenidone 1200-mg group and two from the pirfenidone 2400-mg group).
In conclusion, this study is the first randomized, placebo-controlled, clinical study that demonstrates that an oral antifibrotic drug has the potential to alter renal function decline in subjects with established diabetic nephropathy while on RAS blockade. The similar effect observed with the use of pirfenidone in FSGS lends further weight to this observation. On the basis of these encouraging results, we advocate for expanded clinical studies to determine if oral pirfenidone, in addition to standard therapy, will provide further renal protection and potentially induce regression of diabetic nephropathy.
CONCISE METHODS
Study Design
The study protocol was approved by the institutional review boards of the participating centers and all patients provided written consent. Trial sites were Thomas Jefferson University in Philadelphia, Pennsylvania; the Mayo Clinic in Rochester, Minnesota; and the National Institutes of Health Clinical Center in Bethesda, Maryland. At the prestudy evaluation, the potential study candidates provided a medical history and underwent a complete physical examination, including clinical laboratory determinations and the measurement of vital signs. Entry criteria included a history of type 1 or type 2 diabetes; eGFR of 20 to 75 ml/min per 1.73 m2; microalbuminuria or overt proteinuria; and BP < 140/90 mmHg on an ACEI, ARB, or a combination, if tolerated. Patients were required to be on a stable dose of the ACEI and/or ARB before entering the study. The study investigators did not change the doses of ACEI and/or ARB during the study. Exclusion criteria included other causes of kidney disease, history of photosensitivity rash, and liver disease. Patients with a known photosensitivity rash were excluded as this is a known side effect of pirfenidone. Patients with liver disease were excluded because the response to pirfenidone may be unpredictable and liver disease may also affect kidney function independent of diabetic kidney disease. A stratified block randomization scheme was used to maximize the balance of patients with type 1 and type 2 diabetes assigned to each treatment group using randomization blocks of size 4.
Eligible subjects were randomly assigned to receive pirfenidone at 1200 mg daily, pirfenidone at 2400 mg daily, or placebo for 54 weeks. Subjects received two 400-mg capsules of pirfenidone three times, one capsule of pirfenidone and one capsule of placebo three times daily, or two capsules of placebo three times daily. Subjects who developed nausea, heartburn or reflux, epigastric pain, or severe fatigue that persisted for more than 1 week had a dose reduction of 25% and then were gradually brought back to their intended dose. If symptoms persisted, the dose was kept at the lowest tolerable dose (but not <50% of the original dose) if the subject desired to continue in the protocol.
End Points
The primary end point was eGFR change in individual subjects from baseline to the end of the study. Baseline eGFR was determined from two serum creatinine measurements within 3 weeks before starting the study drug. End-of-study eGFR was determined from two serum creatinine measurements at week 52 and week 54, before stopping study medication. Serum creatinine was measured separately by the Jaffe method at each clinic site laboratory. eGFR was determined by the four-variable Modification of Diet in Renal Disease study equation.36 Secondary end points included the change in urine ACR and the change in urine TGF-β/urine creatinine. The decision to place a patient on dialysis was made by the primary physician or nephrologist. In cases in which patients were put on dialysis, the study medication was immediately stopped, patients were removed from the study, and the cause for dialysis was obtained from the primary physician or medical notes.
Biomarker Analysis
Biomarkers were analyzed in plasma and urine from samples collected at both baseline visits and both end-of-study visits with the two results averaged. Plasma biomarkers were measured by the MesoScale Discovery platform at the University of California–San Diego Clinical Translational Research Institute facility. The MesoScale Discovery platform uses electrochemiluminescence tags on specific antibodies that emit light when electrochemically stimulated. The specific blood biomarkers measured were IFN-γ, IL-1, TNF-α, sTNF-R1, YKL-40 (also called chitinase-like protein or human cartilage glycoprotein-39), brain natriuretic peptide, and TGF-β1. FGF-23 was measured by a C-terminal ELISA assay (Immutopics, San Clemente, CA). Urine biomarkers included urine TGF-β1 (measured by Quantikine, R&D Biosystems, as described previously29). Urine albumin was measured by nephelometry and urine creatinine was measured by the Jaffe method.
Statistical Methods
The prespecified primary end point analysis was the median eGFR change within each individual, comparing each pirfenidone treatment group with placebo group using the t test. Secondary end points (ACR change and urine TGF-β1 change) were similarly evaluated. When one of the two visits was missing, a single observation was used. Because of missing data, the assumption that the data points arose from an identical distribution (equal variance) was put in question. Therefore in addition to the two-sample t tests, we also performed permutation tests with 10,000 permutations of the treatment assignments, as well as an ANCOVA37 with IWLS38 as confirmatory analyses. Data are presented as mean ± SD for normally distributed variables and medians and interquartile ranges for skewed variables.
In exploratory analysis, we performed linear regression to evaluate associations between baseline eGFR as the dependent variable and each biomarker at baseline as the independent variable. Skewed variables were natural log transformed, and each biomarker was evaluated as “per SD greater” to facilitate comparisons of strengths of association. Biomarker change scores were calculated as the end-of-study value minus the baseline level. ANOVA was used to determine if the change in biomarker levels differed by randomized treatment assignment. We also evaluated whether baseline biomarker levels were associated with eGFR change using multivariable linear regression. These models were adjusted for the other biomarkers and for clinical factors that are well established markers of progressive diabetic nephropathy, including age, sex, race, systolic BP, diastolic BP, baseline ACR, and baseline eGFR.
Because some subjects did not complete the study, differences in baseline characteristics were compared between completers and noncompleters using the t test or the Mann–Whitney test for continuous variables and χ2 test or Fisher's exact test for discrete variables. An analysis based on logistic regression models was conducted to determine whether this violates the assumption of missing completely at random.39 This included using baseline characteristics to predict dropout and using eGFR values at a particular visit to predict dropout at the next visit. The latter part of the logistic regression was fitted using generalized estimating equations.40 All data analyses were conducted using R and STATA version 11.0 (College Station, TX).
DISCLOSURES
Dr. Sharma received research support from InterMune, Inc., before the completion of the clinical study. Dr. Falkner received consulting fees from Merck. Dr. McGowan is currently an employee of Johnson and Johnson. InterMune, Inc., provided study medication but had no role in study design, data analysis, data interpretation, or manuscript review.
Acknowledgments
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (R01DK063017 to K.S.); a Juvenile Diabetes Research Foundation Academic Research and Development award (to K.S.); the Intramural Research Program, NIDDK, NIH (ZO1-DK043308 to J.B.K.); the General Clinical Research Centers Program (M01 RR00827); the Clinical and Translational Science Awards Program (1UL1 RR031980-01) from NIH/National Center for Research Resources (to R.X. and M.D.); and supplemental funding from InterMune, Inc. We thank Christina Petyo, Louise Enderle, Linda Nocella, and Dr. Joseph Cheung of Thomas Jefferson University for assisting in the completion of the trial. We thank the Clinical and Translational Research Institute and SriKrishna Khandrika at the University of California–San Diego for assistance in performing the measurement of plasma biomarkers. We thank InterMune, Inc. for providing the study drug.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
See related editorial, “Trials and Tribulations of New Agents, Novel Biomarkers, and Retarding Renal Progression,” on pages 992–993.
REFERENCES
- 1. Steffes MW, Osterby R, Chavers B, Mauer SM: Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes 38: 1077–1081, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Lane PH, Steffes M, Fioretto P, Mauer S: Renal interstitial expansion in insulin-dependent diabetes mellitus. Kidney Int 43: 661–667, 1993 [DOI] [PubMed] [Google Scholar]
- 3. Fioretto P, Steffes M, Sutherland D, Mauer M: Sequential renal biopsies in insulin-dependent diabetic patients: Structural factors associated with clinical progression. Kidney Int 48: 1929–1935, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Lewis E, Hunsicker L, Bain R, Rohde R: The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 5. Brenner B, Cooper M, Zeeuw D, Keane W, Mitch W, Parving H-H, Remuzzi G, Snapinn S, Zhang Z, Shahinfar S: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Lewis E, Hunsicker L, Clarke A, Berl T, Pohl M, Lewis J, Ritz E, Atkins R, Rohde R, Raz I: Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345: 851–860, 2001 [DOI] [PubMed] [Google Scholar]
- 7. Sharma K, Eltayeb B, McGowan T, Dunn S, Alzahabi B, Rohde R, Ziyadeh F, Lewis E: Captopril-induced reduction of serum levels of transforming growth factor-β1 correlates with long-term renoprotection in insulin-dependent diabetic patients. Am J Kid Dis 34: 818–823, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Mann JF, Schmieder RE, McQueen M, Dyal L, Schumacher H, Pogue J, Wang X, Maggioni A, Budaj A, Chaithiraphan S, Dickstein K, Keltai M, Metsarinne K, Oto A, Parkhomenko A, Piegas LS, Svendsen TL, Teo KK, Yusuf S: Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): A multicentre, randomised, double-blind, controlled trial. Lancet 372: 547–553, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Sharma K, Guo J, Jin Y, Ziyadeh FN: Neutralization of TGF-β by anti-TGF-β antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes 45: 522–530, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Ziyadeh F, Hoffman B, Han D, Iglesias-de la Cruz C, Hong S, Isono M, Chen S, McGowan T, Sharma K: Long-term prevention of renal insufficiency excess matrix gene expression and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-b antibody in db/db diabetic mice. Proc Natl Acad Sci U S A 97: 8015–8020, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang C, Kim Y, Caramori ML, Fish AJ, Rich SS, Miller ME, Russell GB, Mauer M: Cellular basis of diabetic nephropathy: II. The transforming growth factor-beta system and diabetic nephropathy lesions in type 1 diabetes. Diabetes 51: 3577–3581, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Sharma K, Ziyadeh FN, Alzahabi B, McGowan TA, Kapoor S, Kurnik BRC, Kurnik PB, Weisberg LS: Increased renal production of transforming growth factor-β 1 in patients with type II diabetes. Diabetes 46: 854–859, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Niewczas MA, Ficociello LH, Johnson AC, Walker W, Rosolowsky ET, Roshan B, Warram JH, Krolewski AS: Serum concentrations of markers of TNFalpha and Fas-mediated pathways and renal function in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol 4: 62–70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lorz C, Benito-Martin A, Boucherot A, Ucero AC, Rastaldi MP, Henger A, Armelloni S, Santamaria B, Berthier CC, Kretzler M, Egido J, Ortiz A: The death ligand TRAIL in diabetic nephropathy. J Am Soc Nephrol 19: 904–914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iyer S, Gurujeyalakshmi G, Giri S: Effects of pirfenidone on transforming growth factor-beta gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther 291: 367–373, 1999 [PubMed] [Google Scholar]
- 16. Shihab F, Bennett W, Yi H, Andoh T: Pirfenidone treatment decreases transforming growth factor-beta1 and matrix proteins and ameliorates fibrosis in chronic cyclosporine nephrotoxicity. Am J Transplant 2: 111–119, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Oku H, Nakazato H, Horikawa T, Tsuruta Y, Suzuki R: Pirfenidone suppresses tumor necrosis factor-alpha, enhances interleukin-10 and protects mice from endotoxic shock. Eur J Pharmacol 446: 167–176, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Grattendick KJ, Nakashima JM, Feng L, Giri SN, Margolin SB: Effects of three anti-TNF-alpha drugs: Etanercept, infliximab and pirfenidone on release of TNF-alpha in medium and TNF-alpha associated with the cell in vitro. Int Immunopharmacol 8: 679–687, 2008 [DOI] [PubMed] [Google Scholar]
- 19. RamachandraRao SP, Zhu Y, Ravasi T, McGowan TA, Toh I, Dunn SR, Okada S, Shaw MA, Sharma K: Pirfenidone is renoprotective in diabetic kidney disease. J Am Soc Nephrol 20: 1765–1775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cho ME, Smith DC, Branton MH, Penzak SR, Kopp JB: Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2: 906–913, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Parker BD, Schurgers LJ, Brandenburg VM, Christenson RH, Vermeer C, Ketteler M, Shlipak MG, Whooley MA, Ix JH: The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: The Heart and Soul Study. Ann Intern Med 152: 640–648, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marsell R, Grundberg E, Krajisnik T, Mallmin H, Karlsson M, Mellstrom D, Orwoll E, Ohlsson C, Jonsson KB, Ljunggren O, Larsson TE: Fibroblast growth factor-23 is associated with parathyroid hormone and renal function in a population-based cohort of elderly men. Eur J Endocrinol 158: 125–129, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB: Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64: 2272–2279, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Fioretto P, Steffes MW, Sutherland DER, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 25. Fioretto P, Kim Y, Mauer M: Diabetic nephropathy as a model of reversibility of established renal lesions. Curr Opin Nephrol Hypertens 7: 489–494, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Ma LJ, Nakamura S, Whitsitt JS, Marcantoni C, Davidson JM, Fogo AB: Regression of sclerosis in aging by an angiotensin inhibition-induced decrease in PAI-1. Kidney Int 58: 2425–2436, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Ma L-J, Fogo A: Role of angiotensin II in glomerular injury. Semin Nephrol 21: 544–553, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Eijkelkamp WB, Zhang Z, Remuzzi G, Parving HH, Cooper ME, Keane WF, Shahinfar S, Gleim GW, Weir MR, Brenner BM, de Zeeuw D: Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: Post hoc analysis from the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) trial. J Am Soc Nephrol 18: 1540–1546, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Agarwal R, Siva S, Dunn SR, Sharma K: Add-on angiotensin II receptor blockade lowers urinary transforming growth factor-β levels. Am J Kidney Dis 39: 486–492, 2002 [DOI] [PubMed] [Google Scholar]
- 30. McGowan TA, Dunn SR, Falkner B, Sharma K: Stimulation of urinary TGF-β and isoprostanes in response to hyperglycemia in humans. Clin J Am Soc Nephrol 1: 263–268, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Gahl W, Brantly M, Troendle J, Avila N, Padua A, Montalvo C, Cardona H, Calis K, Gochuico B: Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genetics Metab 76: 234–242, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Bowen J, Maravilla K, Margolin S: Open-label study of pirfenidone in patients with progressive forms of multiple sclerosis. Mult Scler 9: 280–283, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Park H, Bao L, Kim Y, Cho I, Lee C, Hyun B, Margolin S, Park Y: Pirfenidone suppressed the development of glomerulosclerosis in the FGS/Kist mouse. J Korean Med Sci 18: 527–533, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giri SN, Al-Bayati M, Du X, Schelegle E, Mohr F, Margolin S: Amelioration of doxorubicin-induced cardiac and renal toxicity by pirfenidone in rats. Cancer Chemother Pharmacol 53: 141–150, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Tada S, Nakamuta M, Enjoji M, Sugimoto R, Iwamoto H, Kato M, Nakashima Y, Nawata H: Pirfenidone inhibits dimethylnitrosamine-induced hepatic fibrosis in rats. Clin Exp Pharmacol Physiol 28: 522–527, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Yang L, Tsiatis AA: Efficiency study of estimators for a treatment effect in a pretest-posttest trial. Am Statistician 55: 314–321, 2001 [Google Scholar]
- 38. Venables WN, Ripley BD: Modern Applied Statistics with S, New York, Springer, 2002 [Google Scholar]
- 39. Ridout MS: Testing for random dropouts in repeated measurement data. Biometrics 47: 1617–1619; discussion 1619–1621, 1991 [PubMed] [Google Scholar]
- 40. Zeger SL, Liang KY: Longitudinal data analysis for discrete and continuous outcomes. Biometrics 42: 121–130, 1986 [PubMed] [Google Scholar]