Abstract
The long-term outcome of kidney transplantation in patients infected with hepatitis C virus (HCV) and end stage renal disease (ESRD) is not well described. We retrospectively identified 230 HCV-infected patients using enzyme immunoassay and nucleic acid testing obtained during the transplant evaluation. Of 207 patients who had a liver biopsy before transplant, 44 underwent 51 follow-up liver biopsies at approximately 5-year intervals either while on the waitlist for a kidney or after kidney transplantation. Advanced fibrosis was present in 10% of patients biopsied, identifying a population that may warrant consideration for combined liver-kidney transplantation. Kidney transplantation does not seem to accelerate liver injury; 77% of kidney recipients who underwent follow-up biopsies showed stable or improved liver histology. There was a higher risk for death during the first 6 months after transplant, but undergoing transplantation conferred a long-term survival advantage over remaining on the waitlist, which was evident by 6 months after transplant (HR, 0.32; 95% CI, 0.17 to 0.62). Furthermore, the risk for death resulting from infection was significantly higher during the first 6 months after transplant (HR, 26.6; 95% CI, 5.01 to 141.3), whereas there was an early (≤6 months) and sustained decrease in the risk for cardiovascular death (HR, 0.20; 95% CI, 0.08 to 0.47). In summary, these data suggest the importance of liver biopsy before transplant and show that kidney transplantation confers a long-term survival benefit among HCV-infected patients with ESRD compared with remaining on the waitlist. Nevertheless, the higher incidence of early infection-related deaths after transplant calls for further study to determine the optimal immunosuppressive protocol.
Twenty years after its identification, hepatitis C virus (HCV) is a global health problem that affects approximately 170 million people worldwide while also being a major cause of end-stage liver disease leading to liver transplantation.1,2 The prevalence of HCV infection in patients with chronic kidney disease (CKD) exceeds the general population,3 and HCV is not only causative of kidney disease4–10 but also contributes to increased morbidity and mortality in patients with established CKD.11–15 Recent guidelines from the Kidney Disease: Improving Global Outcomes workgroup16 provided recommendations for diagnosis and treatment of HCV in patients with CKD; however, many of the guidelines are largely opinion-based because of limitations of available data. By design, the major published studies on the natural history of HCV infection2,17,18 and the important prospective trials of IFN and ribavirin therapy excluded patients with CKD.19–22
Kidney transplantation is recognized as the renal replacement therapy of choice for patients with ESRD. Wolfe et al.23 showed that transplantation was associated with a 68% reduction in long-term mortality compared with remaining on the kidney waiting list. This benefit was present despite an early (0 to 3 months) increase in mortality in the transplanted cohort. Several studies have suggested that HCV-infected ESRD patients on maintenance hemodialysis11 and after kidney transplantation12 have an increased relative risk of death compared with HCV-negative controls. Of importance, however, is that the survival advantage associated with transplantation is still present in HCV-infected ESRD patients. Pereira et al.14 showed that anti-HCV–positive patients receiving a kidney transplant had a relative risk of death of 0.31 in the 7- to 48-month post-transplant period compared with patients remaining on the waitlist. Because this was a registry analysis, liver biopsies and nucleic acid testing (NAT) were not available. Nevertheless, the striking benefit of transplantation on mortality was consistent with the findings of Wolfe et al.23 for the general kidney transplant population.
This study is a single center analysis of clinical outcomes over approximately 20 years in 230 consecutive patients determined to be HCV-infected during their pretransplant evaluation. The pretransplant process included baseline liver histology, NAT, and longitudinal follow-up in all patients except those lost to follow-up. Sequential liver biopsies were obtained in approximately 25% of the waitlisted and transplanted cohort. Because cause of death was documented, we were able to assess the impact of transplantation on both cause-specific and all-cause mortality.
RESULTS
Patient Characteristics at Referral
HCV infection was identified in 230 patients. Patient demographics are reported in Table 1 and are unremarkable except for a higher percentage of Hispanic patients than national averages. Of note, 14 patients (6%) were Enzyme-linked Immunosorbent Assay (EIA) negative but NAT positive at referral. Baseline liver histology (n = 207) showed that 80 (166/207), 10 (20/207), 6 (12/207), and 4% (9/207) of the patients had stages 0/1, 2, 3, and 4 (cirrhosis), respectively. Importantly, all of the stage 3/4 patients (n = 21) had clinically well-compensated liver disease at the time of referral.
Table 1.
Patient demographicsa
| Variable | Total (n = 230) | Candidates (n = 175) | Transplanted (n = 110) |
|---|---|---|---|
| Age (years)b | 45.9 (11 to 74) | 47.6 (12 to 76) | 48 (12 to 74) |
| Race (%) | |||
| white | 32.6 | 37.1 | 37.3 |
| Hispanic | 27.8 | 29.7 | 28.2 |
| black | 37.8 | 32.6 | 33.6 |
| Male gender (%) | 70.4 | 69.1 | 68.2 |
| Prior transplant (%) | 30.9 | 30.9 | 20.0 |
| PRA > 40 (%) | 14.0 | 15.3 | 5.0 |
| Cause of ESRD (%) | |||
| diabetes (1 and 2) | 27.0 | 26.3 | 30.0 |
| hypertension | 33.5 | 29.1 | 26.4 |
| glomerulonephritis | 23.5 | 25.7 | 26.4 |
| Dialysis time (mo)c | 14.9 (0 to 302) | 31.6 (0 to 318) | 41 (0 to 246) |
PRA, panel reactive antibody.
aData presented as median (with range) for continuous variables, and percentage with characteristic for categorical variables.
bAge (years) at referral, listing, or transplant.
cTime (months) from starting dialysis to referral, listing, or transplant.
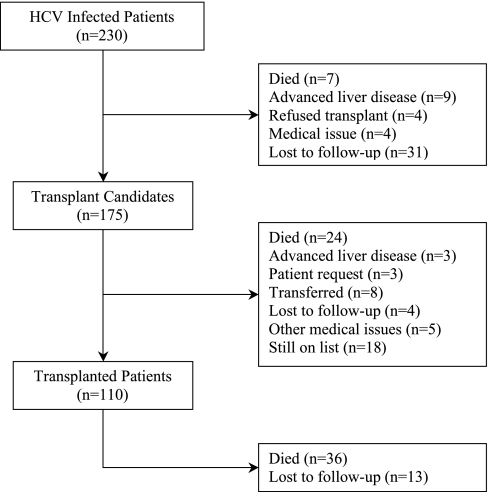
Of the 230 patients diagnosed with HCV infection, 159 were eventually listed with United Network Organ Sharing, and an additional 16 received a living donor transplant without being listed (Figure 1). There were 32 patients biopsied during the pretransplant process that were never listed or transplanted. In this group, the distribution of liver disease from stage 0 to 4 was 43.7, 12.5, 15.6, 9, and 18.8%, respectively. Thirteen patients received anti-viral treatment for HCV infection at some time before wait listing; however, all were NAT positive when listed. Four patients tested positive for hepatitis B surface antigen, two of whom were listed but never transplanted.
Figure 1.
Patient flow diagram describing the reasons for not being considered a transplant candidate or not having received a transplant.
Pretransplant Outcomes
Of the 175 patients who completed their pretransplant evaluation (Table 1), 110 (63%) were subsequently transplanted (Figure 1), including 19 living donor, 91 deceased donor, and 14 simultaneous kidney-pancreas (SPK) transplants. Twenty-eight of 175 patients (16%) died before transplant (24 while still listed and 4 others after being removed from the list) secondary to cardiovascular (n = 19), infection (n = 2), liver failure (n = 1), other (n = 3), and unknown (n = 3) causes. Stepwise Cox regressions of pretransplant any-cause and cardiovascular death rates showed that being listed for a SPK transplant (i.e., having type 1 diabetes; P < 0.001) and older age at listing (P = 0.02 for any cause and P < 0.001 for cardiovascular death) were associated with an increased mortality.
Post-Transplant Outcomes
Death-censored kidney graft failure occurred in 15% (17/110) of the patients. One additional patient had primary nonfunction. Causes of graft failure were chronic allograft nephropathy (n = 10), membranoproliferative glomerulonephritis (n = 4), acute rejection (n = 1), diabetic nephropathy (n = 1), and hepatorenal syndrome (n = 1). Biopsy-proven acute rejection occurred in 16/110 patients; median time-to-biopsy-proven acute rejection (range) was 7 (0.3 to 93) months. Of the patients with rejection, 8/16 (50%) ultimately developed graft failure in contrast to 9.6% (9/94) of those without rejection. Median time-to-graft failure was 64 (12 to 98) months.
Death with a functioning graft (DWFG) occurred in 29/110 (26%) patients at a median of 37 (1 to 111) months after transplant. Causes of DWFG were infection (n = 12; lung infection = 7, other sepsis = 5), cardiovascular (n = 11), malignancy (n = 2, one hepatocellular carcinoma), liver failure (n = 1), and other (n = 3). In multivariable analysis, receiving a SPK transplant (P = 0.01), age at transplant ≥55 years (P = 0.02), and white race (P = 0.03) were associated with a higher hazard rate of DWFG. For the entire group, induction therapy was not associated with the DWFG rate because of infection. In addition, there were seven deaths that occurred after graft failure from infection (n = 2), cardiovascular (n = 2), liver failure (n = 2), and unknown (n = 1). Median time-to-death after graft failure was 4 months.
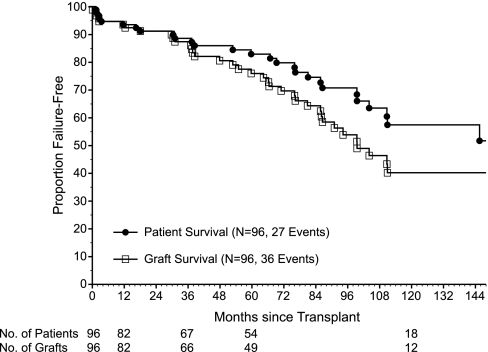
Several other outcomes were of clinical interest. New-onset diabetes after transplant occurred in 19/77 (25%) kidney-alone recipients who were not diabetic before transplant. Of note, only one post-transplant patient was treated with IFN, and this patient developed antibody-mediated rejection resulting in graft failure. Patient survival at 1, 5, and 10 years after transplant for kidney-alone patients was 94 ± 3, 83 ± 4, and 57 ± 7%, and graft survival (death uncensored) was 94 ± 3, 76 ± 5, and 40 ± 7%, respectively (Figure 2).
Figure 2.
Patient and graft survival in kidney alone recipients. Kaplan-Meier patient and graft (death uncensored) survival for kidney alone transplant recipients (n = 96: 27 deaths and 36 graft failures or deaths).
Postlisting Predictors of Survival (Combining Pre- and Post-Transplant Follow-Up)
In multivariable analysis, three factors were found to be associated with significantly higher rates of any-cause and cardiovascular death: being listed for a SPK transplant (i.e., having type 1 diabetes; P = 0.002 and <0.001), older age at transplant (≥55 years for any-cause, P = 0.002; as a continuous variable for cardiovascular death, P < 0.001), and white race (P = 0.03 in both models). Being listed for a SPK transplant (P = 0.02) was the only factor associated with a significantly higher infection death rate.
Effect of Transplant Status on Survival
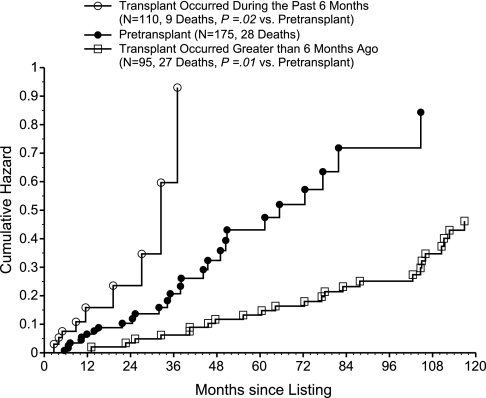
We tested the effect of transplant status as a time-dependent covariate on the hazard rate of death for the 175 transplant candidates. Because likelihood ratio tests did not support the use of smaller post-transplant intervals, the final Cox model included only three distinct time period effects of transplantation (0 to 6, 7 to 84, and >84 months). During the first 6 months after transplant (Table 2), the hazard ratio (HR) of death adjusted for SPK listing, age ≥55 years, and white race (the variables found to be associated with a higher rate of death because of any cause) was 2.51 (95% confidence interval [CI], 1.12, 5.66; P = 0.03). In contrast, the post-transplant effect during the 7- to 84-month period was notably favorable with an HR of death of 0.32 (95% CI, 0.17, 0.62; P < 0.001). For the period >84 months, the observed HR of 0.74 did not achieve significance (95% CI, 0.19, 2.92; P = 0.66). A cumulative hazard plot of the impact of transplantation (without adjustment for covariates; Figure 3) shows that the death rate (slope of the curve) during the first 6 months after transplant was significantly higher, whereas the death rate beyond 6 months after transplant was significantly lower in comparison with before transplant.
Table 2.
Impact of kidney transplantation on the hazard rate of death (n = 175, 64 deaths: 28 pretransplant and 36 post-transplant)
| Predictor Variables in the Modela | Cox Model (Coefficient ± SE) | Wald Test (P) | Adjusted Relative Risk [95% Confidence Interval] |
|---|---|---|---|
| Post-Tx effect during 0 to 6 monthsb | 0.922 ± 0.414 | 0.03 | 2.51 [1.12 to 5.66] |
| Post-Tx effect >6 and ≤84 monthsbc | −1.140 ± 0.336 | 0.0007 | 0.32 [0.17 to 0.62] |
| Post-Tx effect >84 monthsc | −0.307 ± 0.704 | 0.66 | 0.74 [0.19 to 2.92] |
| Listed for kidney/pancreas Txd | 1.160 ± 0.355 | 00.001 | 3.19 [1.59 to 6.39] |
| Age at listing ≥55 years | 0.864 ± 0.296 | 0.004 | 2.37 [1.33 to 4.24] |
| White race | 0.589 ± 0.262 | 0.02 | 1.80 [1.08 to 3.01] |
Tx, transplant.
aThree distinct post-transplant effect time periods, adjusting for three significant baseline covariates. Of note, the effect of kidney transplantation during six distinct post-transplant time periods was initially considered, i.e., 0 to 3, 4 to 6, 7 to 12, 13 to 48, 49 to 84, and >84 months. Likelihood ratio tests of the impact of transplantation were not significantly different (1) during the first two periods (i.e., 0 to 6 months, P = 0.20) and (2) during the next three periods (i.e., 7 to 84 months, P = 0.73). Among the 36 post-transplant deaths, there were 9, 17, and 10 deaths that occurred during 0 to 6, 6 to 84, and >84 months after transplantation, respectively.
bLikelihood ratio test (with 1 df) for equality of the post-transplant effects during 0 to 6 and 6 to 84 months after transplant yielded P < 0.001.
cLikelihood ratio test (with 1 df) for equality of the post-transplant effects during 6 to 84 and >84 months after transplant yielded P = 0.22.
dAll patients listed for a kidney-pancreas transplant were type 1 diabetics.
Figure 3.
Transplant recipients whose transplant occurred more than 6 months ago have the lowest hazard rate of death post-listing compared to patients whose transplant occurred during the past 6 months or those in the pretransplant state. Cumulative hazard plot of the hazard rate of death after listing, using time-dependent covariate methodology to compare three patient states: transplant occurred during the past 6 months (n = 110, 9 deaths; P = 0.02 versus pretransplant), pretransplant (n = 175, 28 deaths), and transplant occurred >6 months ago (n = 95, 27 deaths; P = 0.01 versus pretransplant).
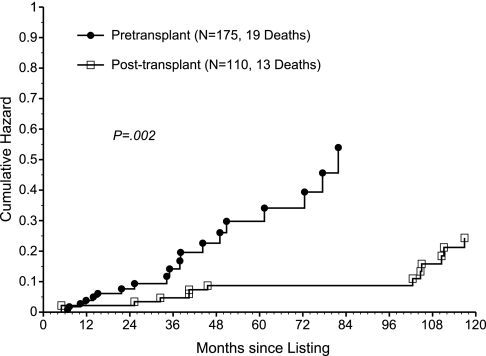
To more precisely identify the impact of kidney transplantation on survival, cause-specific hazard rates of death were analyzed. During the first 6 months after transplant, the HR of death caused by infection (adjusted for SPK listing) was 26.6 (95% CI, 5.01, 141.3; P < 0.001; Table 3). Although the HR remained >1 beyond 6 months after transplant, this effect was not significant (P = 0.54). In contrast, a significantly favorable and consistent impact of transplantation on the HR of cardiovascular death was observed, with the overall hazard rate of death (adjusted for SPK listing, age at listing, and white race) being 0.20 (95% CI, 0.08, 0.47; P < 0.001; Table 4; Figure 4). Regarding the HR of death caused by other causes, no significant impact of transplantation was observed (overall HR, 0.86; P = 0.82). Thus, the unfavorable impact of transplantation on all-cause mortality during the first 6 months after transplant seems to be caused by a significantly higher infection death rate during that period. Conversely, the favorable impact of transplantation on all-cause mortality beyond 6 months after transplant is explained by a significantly lower cardiovascular death rate that takes effect early after transplant and remains consistent over time.
Table 3.
Impact of kidney transplantation on the hazard rate of death caused by infection (n = 175; 16 deaths: 2 pretransplant and 14 post-transplant)
| Predictor Variables in the Modela | Cox Model (Coefficient ± SE) | Wald Test (P) | Adjusted Relative Risk [95% Confidence Interval] |
|---|---|---|---|
| Post-Tx effect during 0 to 6 monthsb | 3.281 ± 0.852 | 0.0001 | 26.62 [5.01 to 141.3] |
| Post-Tx effect >6 monthsb | 0.524 ± 0.861 | 0.54 | 1.69 [0.31 to 9.14] |
| Listed for kidney/pancreas Txc | 1.116 ± 0.607 | 00.07 | 3.05 [0.93 to 10.02] |
Tx, transplant.
aTwo distinct post-transplant effect time periods, adjusting for the single significant baseline covariate. Among the 14 post-transplant infection deaths, the numbers occurring during 0 to 6 and >6 months after transplantation were 7 and 7, respectively.
bLikelihood ratio test (with 1 df) for equality of the post-transplant effects during 0 to 6 and >6 months after transplant yielded P < 0.001.
cAll patients listed for a kidney-pancreas transplant were type 1 diabetics.
Table 4.
Impact of kidney transplantation on the hazard rate of death caused by a cardiovascular event (n = 175; 32 deaths: 19 pretransplant and 13 post-transplant)
| Predictor Variables in the Modela | Cox Model (Coefficient ± SE) | Wald Test (P) | Adjusted Relative Risk [95% Confidence Interval] |
|---|---|---|---|
| Overall post-Tx effect | −1.632 ± 0.443 | 0.0002 | 0.20 [0.08 to 0.47] |
| Listed for kidney/pancreas Txb | 2.110 ± 0.495 | 0.00002 | 8.25 [3.13 to 21.78] |
| Age at listing (continuous) | 0.063 ± 0.018 | 0.0003 | 1.07 [1.03 to 1.10] |
| White race | 0.727 ± 0.391 | 0.06 | 2.07 [0.96 to 4.45] |
Tx, transplant.
aOne overall effect of transplantation over time, adjusting for three significant baseline covariates. Of note, by allowing three distinct time period effects during 0 to 6, 7 to 84, and >84 months after transplant, the estimated hazard ratio was <1 during each period with no significant differences (P = 0.45). Among the 13 post-transplant cardiovascular deaths, there were 1, 6, and 6 deaths that occurred during 0 to 12, 12 to 84, and >84 months after transplantation, respectively.
bAll patients listed for a kidney-pancreas transplant were type 1 diabetics.
Figure 4.
The cumulative hazard rate of death due to a cardiovascular event post listing is significantly lower in the post transplant patients compared to those in the pretransplant state. Cumulative hazard plot of the hazard rate of death caused by a cardiovascular event after listing, using time-dependent covariate methodology to compare two patient states: pretransplant (n = 175, 19 deaths) versus post-transplant (n = 110, 13 deaths; P = 0.002).
Liver Disease Progression
More than one liver biopsy was performed in 44 patients (7 patients had three biopsies). Median (range) time between biopsies were 57 (8 to 117) and 76 (27 to 139) months for patients in whom the follow-up biopsy was obtained while the patient was still waitlisted (n = 13) versus after transplant (n = 31), respectively. The mean change in fibrosis (±SD) was 0.28 ± 0.64 versus 0.04 ± 0.26 per year (P = 0.08) for the pretransplant versus post-transplant follow-up biopsies, respectively. Although this difference did not achieve significance, it is important to note that 16% (5/31) of the liver biopsies obtained after transplant showed histologic improvement when compared with the baseline pretransplant sample, whereas only 23% (7/31) showed progression of liver injury. This result is in distinct contrast to the ongoing wait listed patients in whom 62% (8/13) of the follow-up liver biopsies showed interval worsening of the fibrosis score (P = 0.03). Daclizumab was the sole induction agent used in 14 of the 31 kidney recipients who underwent a repeat post-transplant liver biopsy. In this subgroup, the fibrosis progression rate was 0.18 ± 0.28 per year compared with −0.08 ± 0.18 in the remaining 17 patients who (in all but one case) received a lymphocyte-depleting agent (P = 0.005). In a separate analysis of maintenance immunosuppressive medication, we did not find an association between the progression of liver disease and receiving any particular medication.
DISCUSSION
This is the largest single-center study and first to our knowledge of HCV-infected ESRD patients where baseline pretransplant liver histology was available for comparison to subsequent biopsies obtained on ongoing wait-listed (n = 13) and post-transplant patients (n = 31). Importantly, we were able to show that, despite many years of immunosuppression, liver histology remained stable (or even improved) in the majority of re-biopsied transplant patients, whereas liver injury progressed more commonly in patients who remained on the waitlist and had a follow-up biopsy. In addition, the results showed that for the HCV-infected ESRD patient, kidney transplantation is associated with a survival advantage over remaining on dialysis. Because this was not a registry analysis, we were able to determine the impact of transplantation on cause-specific death in this cohort; these data were not previously available.
Several studies have shown that HCV infection confers an increased mortality risk to both dialysis and kidney transplant patients.11,12,24–26 In a meta analysis, Fabrizi et al. demonstrated an independent, significant impact of HCV on mortality in both dialysis11 and transplant patients,12 with summary relative risk estimates of 1.57 (95% CI, 1.33, 1.86) and 1.79 (95% CI, 1.57, 2.03), respectively. Nevertheless, the survival advantage conferred by kidney transplantation in the general ESRD population23 is still present in the subset of HCV-infected patients.14,27–29 Our results confirmed these findings and furthermore showed that, although there was an increased HR of death during the first 6 months after transplant, this was followed by an unequivocal survival advantage out to 7 years. In addition, our 10-year patient and graft survival rates of 57 and 40% are only slightly less than the outcomes available in the Scientific Registry of Transplant Recipients of 65 and 43%, respectively.30
Although prior studies have shown an overall increased risk of death from infection in HCV-infected kidney transplant recipients,3,31,32 this study showed that the increased death rate from infection occurs in the early (<6 months) post-transplant period. This raises as yet unanswered questions about the contribution of HCV infection to this increased mortality and suggests that one approach may be to modify the immunosuppression administered during this period. In contrast, the estimated HR of death from a cardiovascular event was 0.20 (95% CI, 0.08, 0.47; P < 0.001), an effect that became evident early after transplantation and remained consistent over time. No other published study evaluating the impact of kidney transplantation in HCV-infected patients has, to our knowledge, shown cause-specific effects for each of the major causes of death, particularly this apparently dramatic relationship between kidney transplantation and reduction in cardiovascular mortality.
Pretransplant liver biopsies from 207 patients showed that, although the majority of patients had no fibrosis, approximately 10% had stage 3/4 disease. This is in concordance with other published studies33–37 and emphasizes the importance of the pretransplant liver biopsy in identifying well-compensated ESRD patients with advanced liver disease. As recommended by the Kidney Disease: Improving Global Outcomes workgroup,16 patients with advanced liver disease should be cautiously considered for kidney-alone transplant and perhaps referred for combined liver-kidney transplantation.
The natural progression of HCV-associated liver disease in the immunocompetent population without kidney disease has been well described.18,38 In contrast, the histologic course of liver disease in HCV-infected ESRD patients is less well defined. Data in the dialysis population are scant, and there are conflicting results from studies examining sequential post-transplant biopsies without the benefit of pretransplant samples. Using a matched immunocompetant control group, Zylberberg et al.39 reported more rapid progression of liver fibrosis in renal transplant recipients, whereas Alric et al.40 found just the opposite, with slower progression in the transplanted cohort. Interestingly, Kamar et al.41 reported variable outcomes including progression, stability, and even improvement in liver fibrosis among patients who underwent more than one post-transplant liver biopsy. It is important to emphasize that, unlike this analysis, none of the available studies had baseline pretransplant liver histology available for comparative purposes.
This study describes the progression of liver fibrosis both before and after kidney transplantation in a subset of patients who agreed to follow-up liver biopsies. Interestingly, 16% of the post-transplant biopsies showed histologic improvement compared with the pretransplant baseline sample, whereas 62% of the sequential pretransplant biopsies showed interval worsening of the fibrosis score, a finding found in only 23% of the post-transplant biopsies. Of note, in a subset analysis of patients who received daclizumab, the progression rate was significantly worse than in patients who had received lymphocyte-depleting induction therapy. Similar results using daclizumab have previously been reported in orthotopic liver transplant recipients, although not in comparison with a lymphocyte-depleting agent.42 The full impact of the immunosuppressive burden administered to a transplant recipient cannot be fully assessed if limited to comparing only sequential post-transplant biopsies without a pretransplant baseline sample, as this study provides in a subset of the patients.
This study has several limitations. The data were retrospectively obtained and thus lacks power to avoid potential selection bias. Second, the sample size limits our ability to draw conclusions about small-to-moderate differences with good statistical power. In this context, there were only 14 SPK recipients, with 6/14 having a history of a previous transplant; thus, outcomes in this subgroup must be interpreted cautiously. Finally, only 44 of the 175 transplant candidates had a follow-up liver biopsy, either because of patient refusal, being lost to follow-up, or insufficient time elapsed since the first biopsy. Although our study represents the largest reported cohort of patients with pretransplant liver biopsies, the number of patients with follow-up biopsies must be taken into consideration when interpreting the results.
In conclusion, the results of this study showed that kidney transplantation is the preferred renal replacement therapy for HCV-infected ESRD patients. A thorough pretransplant screening for HCV infection that includes both EIA and NAT is necessary to identify EIA-negative, NAT-positive patients.43,44 Importantly, a liver biopsy is essential so that clinically well-compensated patients with advanced liver injury can be identified and referred for combined liver-kidney transplantation. The increased early HR of death (0 to 6 months) in our study is similar to that reported for the general kidney transplant population23; however, we were able to identify infection deaths as the explanation for the increased early mortality. This finding should focus the attention of further studies on determining the safest combination of induction and maintenance immunosuppression for the HCV-infected kidney and SPK recipient. Our data suggest that most patients with untreated HCV infection do not have significant progression of liver disease after successful kidney transplantation.
CONCISE METHODS
Patients
Starting in 1990, all patients being evaluated at the University of Miami/Jackson Memorial Hospital Kidney Transplant Program were screened for anti-HCV antibody using first-generation and then subsequent versions of the EIA assay as they became available. Beginning in 1995, all candidates were screened for HCV viremia by nucleic acid testing. Stored serum samples from patients seen and evaluated between 1990 and 1995 were also tested by NAT. All patients were tested for hepatitis B surface antigen.
Patients with a positive EIA and/or NAT had a liver biopsy as part of their pretransplant evaluation. One patient with a stage 0 biopsy at the time of listing had progressed to stage 4 liver disease on a clinically indicated repeat liver biopsy but remained on the list and received a kidney-alone transplant nonetheless. This patient died of liver failure at 18 months after transplant. Two other patients with stage 4 liver disease were listed early-on in our clinical experience but were delisted because of medical complications and were never transplanted. Subsequent to the poor outcome in the above described case, all patients with stage 4 liver disease were referred for consideration of combined liver-kidney transplantation. Patients with stage 3 liver disease were felt to be acceptable kidney-alone candidates. Patients were asked to have a follow-up liver biopsy at approximately 5-year intervals while on the waitlist or after transplantation, although biopsies may have been obtained earlier if clinically indicated. None of the patients received a kidney from an HCV-infected donor. All patients listed for a SPK transplant had type 1 diabetes as documented by stimulated C-peptide measurement. SPK recipients received similar immunosuppression as the kidney-alone recipients.
New-onset diabetes after transplant was defined as a consistent fasting blood sugar ≥126 mg/dl in a transplanted patient not previously known to be diabetic. Patient information was collected from time of referral until death, loss to follow-up, or February 1, 2009, whichever occurred earlier. The study was approved by the University of Miami Institutional Review Board.
Immunosuppression
Antibody induction therapy was administered to 104/110 recipients. Patients transplanted in the early 1990s received anti-lymphocyte globulin or OKT3 (Muromonab-CD3; Ortho Biotech, Bridgewater, NJ). Subsequently, daclizumab (Zenapax; Roche, Nutley, NJ), anti-thymocyte globulin (Thymoglobulin; Genzyme, Cambridge, MA), or a combination of both agents were used. In the early years, patients received cyclosporine, azathioprine, and corticosteroids as maintenance immunosuppression. When tacrolimus and mycophenolate mofetil became available, these agents combined with low-dose corticosteroids became the primary maintenance immunosuppressive protocol at our center.45,46 Rejection episodes were documented by biopsy and treated with high-dose corticosteroids and/or antibody as clinically indicated.
Hepatic Histology
Change in liver histology in patients having more than one biopsy were assessed by determining the change in the stage of fibrosis as per the METAVIR scale (scored as 0 to 4, with stage 0 having no fibrosis and stage 4 representing cirrhosis) over time.47 The rate of progression was calculated as the absolute change in stage from baseline to most current biopsy divided by the number of years elapsed between biopsies.
Statistical Analysis
Variables analyzed for their prognostic value via Cox stepwise regression multivariable models included date of listing (or transplant), age at listing (or transplant), time from listing to transplant, race/ethnicity, gender, primary transplant versus retransplant, original disease (types 1 and 2 diabetes, glomerulonephritis, etc.), listed (received) kidney alone versus SPK, received deceased donor (versus living donor) kidney, donor age, developed delayed graft function (yes/no), number of HLA mismatches, panel reactive antibody, initial dialysis date, age at start of dialysis, time from start of dialysis to listing (or transplant), induction antibody received, initially received tacrolimus (yes/no), initially received mycofenolate mofetil (yes/no), time from baseline liver biopsy to listing (or transplant), and baseline liver biopsy stage of fibrosis and grade of inflammation. These variables were used to determine the significant prognostic factors for the postlisting hazard rate of pretransplant death, post-transplant hazard rates of kidney graft failure (censored for death and primary nonfunction) and DWFG, and the postlisting hazard rate of death, combining pre- and post-transplant follow-up. By using the stepwise procedure in the Cox model, only the statistically significant baseline predictors would be retained in a multivariable model. Cause-specific HR analyses of the primary causes of death (cardiovascular, infection, and other) were also performed. The impact of transplantation was assessed using a Cox model with months since listing as the time variable and time-dependent covariates to represent the effect of transplantation during distinct post-transplant time periods while simultaneously adjusting for the significant baseline covariates.23,48–50 Nonparametric graphical display of the impact of transplantation was performed using Nelson-Aalen cumulative hazard plots, allowing correct time-dependent accounting of each patient's follow-up within the various pre- and post-transplant states. Last, to use all patients who successfully completed their pretransplant evaluation in analyzing the impact of kidney transplantation, the 16 patients who received a living donor kidney transplant without being placed on the United Network for Organ Sharing list were assigned a pretransplant waiting time of 2 months for purposes of analyses (the approximate time required to prepare for a living donor transplant at our center).
DISCLOSURES
None.
Acknowledgments
This work was presented in part at the American Transplant Congress, May 2–5, 2010, San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton ML: Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244: 359–362, 1989 [DOI] [PubMed] [Google Scholar]
- 2. Alter HJ, Seeff LB: Recovery, persistence, and sequelae in hepatitis C virus infection: A perspective on long-term outcome. Semin Liver Dis 20: 17–35, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Tsui JI, Vittinghoff E, Shlipak MG, O'Hare AM: Relationship between hepatitis C and chronic kidney disease: Results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 17: 1168–1174, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Pereira BJ, Levey AS: Hepatitis C virus infection in dialysis and renal transplantation. Kidney Int 51: 981–999, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Dalrymple LS, Koepsell T, Sampson J, Louie T, Dominitz JA, Young B, Kestenbaum B: Hepatitis C virus infection and the prevalence of renal insufficiency. Clin J Am Soc Nephrol 2: 715–721, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Fabrizi F, Pozzi C, Farina M, Dattolo P, Lunghi G, Badalamenti S, Pagano A, Locatelli F: Hepatitis C virus infection and acute or chronic glomerulonephritis: An epidemiological and clinical appraisal. Nephrol Dial Transplant 13: 1991–1997, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Gumber SC, Chopra S: Hepatitis C: A multifaceted disease. Review of extrahepatic manifestations. Ann Intern Med 123: 615–620, 1995 [DOI] [PubMed] [Google Scholar]
- 8. Johnson RJ, Gretch DR, Yamabe H, Hart J, Bacchi CE, Hartwell P, Couser WG, Coreu L, Wener MH, Alpers CE, Willson R: Membranoproliferative glomerulonephritis associated with hepatitis C virus infection. N Engl J Med 328: 465–470, 1993 [DOI] [PubMed] [Google Scholar]
- 9. D'Amico G: Renal involvement in hepatitis C infection: Cryoglobulinemic glomerulonephritis. Kidney Int 54: 650–671, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Sabry AA, Sobh MA, Irving WL, Grabowska A, Wagner BE, Fox S, Kudesia G, El Nahas M: A comprehensive study of the association between hepatitis C virus and glomerulopathy. Nephrol Dial Transplant 17: 239–245, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G: Meta-analysis: Effect of hepatitis C virus infection on mortality in dialysis. Aliment Pharmacol Ther 20: 1271–1277, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G: Hepatitis C virus antibody status and survival after renal transplantation: Meta-analysis of observational studies. Am J Transplant 5: 1452–1461, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Miller LG, Daar ES, Gjerston SW, Kopple JD, Greenland S: Hepatitis C virus and death risk in hemodialysis patients. J Am Soc Nephrol 18: 1584–1593, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Pereira BJ, Natov SN, Bouthot BA, Murthy BVR, Ruthhazer R, Schmid CH, Levey AS: Effects of hepatitis C infection and renal transplantation on survival in end-stage renal disease. The New England Organ Bank Hepatitis C Study Group. Kidney Int 53: 1374–1381, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Stehman-Breen CO, Emerson S, Gretch D, Johnson RJ: Risk of death among chronic dialysis patients infected with hepatitis C virus. Am J Kidney Dis 32: 629–634, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Kidney disease: Improving global outcomes KDIGO Clinical Practice Guidelines for the Prevention, Diagnosis, Evaluation and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney Int 73[Suppl 109]: S1–S99, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Kenny-Walsh E: Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin: Irish Hepatology Research Group. N Engl J Med 340: 1228–1233, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Seeff LB: Natural history of chronic hepatitis C. Hepatology 36: S35–S46, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Hoofnagle JH, Seeff LB: Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med 355: 2444–2451, 2006 [DOI] [PubMed] [Google Scholar]
- 20. McHutchison JG, Gordon SC, Schiff ER, Schiffman ML, Lee WM, Rustgi VK, Goodman ZD, Ling M-H, Cort S, Albrecht JK. for the Hepatitis Interventional Therapy Group: Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med 339: 1485–1492, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcole J, Zeumen S, Trepo C, Albrecht J: Randomized trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 352: 1426–1432, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J: Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 347: 975–982, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LYC, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Hanafusa T, Ichikawa Y, Kishikawa H, Kyo MI, Fukunishi T, Kokado Y, Okuyama A, Shinji Y, Nagamo S: Retrospective study on the impact of hepatitis C virus infection on kidney transplant patients over 20 years. Transplantation 66: 471–476,1998 [DOI] [PubMed] [Google Scholar]
- 25. Legendre C, Garrigue V, Le Bihan C, Manzer-Bruneel MT, Chaix ML, Landais P, Kreis H, Pol S: Harmful long-term impact of hepatitis C virus infection in kidney transplant recipients. Transplantation 65: 667–670, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Luan FL, Schaubel DE, Zhang H, Jia X, Pettetier SJ, Port FK, Magee JC, Sung RS: Impact of immunosuppressive regimen on survival of kidney transplant recipients with hepatitis C. Transplantation 85: 1601–1606, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Bloom RD, Sayer G, Fa K, Constantinescu S, Abt P, Reddy R: Outcome of hepatitis C virus-infected kidney transplant candidates who remain on the waiting list. Am J Transplant 5: 139–144, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Knoll GA, Tankersley MR, Lee JY, Julian BA, Curtis JJ: The impact of renal transplantation on survival in hepatitis C-positive end-stage renal disease patients. Am J Kidney Dis 29: 608–614, 1997 [DOI] [PubMed] [Google Scholar]
- 29. Maluf DG, Fisher RA, King AL, Gibney EL, Mas VR, Coterell AH, Shiffman ML, Sterling RK, Behnke M, Posner MP: Hepatitis C virus infection and kidney transplantation: Predictors of patient and graft survival. Transplantation 83: 853–857, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Scientific Registry of Transplant Recipients. Available online at http://www.ustransplant.org/ Accessed September 22, 2010
- 31. Pereira BJ, Wright TL, Schmid CH, Levey AS: The impact of pretransplantation hepatitis C infection on the outcome of renal transplantation. Transplantation 60: 799–805, 1995 [PubMed] [Google Scholar]
- 32. Batty DS, Jr, Swanson SJ, Kirk AD, Ko CW, Agodoa LY, Abbott KC: Hepatitis C virus seropositivity at the time of renal transplantation in the United States: Associated factors and patient survival. Am J Transplant 1: 179–184, 2001 [PubMed] [Google Scholar]
- 33. Martin P, Carter D, Fabrizi F, Dixit V, Conrad AJ, Artinian L, Peacock V, Han S, Wilkinson A, Lassman CR, Danovitch G: Histopathological features of hepatitis C in renal transplant candidates. Transplantation 69:1479–1484, 2000 [DOI] [PubMed] [Google Scholar]
- 34. Sterling RK, Sanyal AJ, Luketic VA, Stravitz RT, King AL, Post AB, Mills AS, Contros MJ, Schiffman ML: Chronic hepatitis C infection in patients with end stage renal disease: Characterization of liver histology and viral load in patients awaiting renal transplantation. Am J Gastroenterol 94: 3576–3582, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Caramelo C, Ortiz A, Aguilera B, Porres JC, Navas S, Marriott E, Alberola MC, Alamo C, Galera A, Garron MP: Liver disease patterns in hemodialysis patients with antibodies to hepatitis C virus. Am J Kidney Dis 22: 822–828, 1993 [DOI] [PubMed] [Google Scholar]
- 36. Glicklich D, Thung SN, Kapoian T, Tellis V, Reinus JF: Comparison of clinical features and liver histology in hepatitis C-positive dialysis patients and renal transplant recipients. Am J Gastroenterol 94: 159–163, 1999 [DOI] [PubMed] [Google Scholar]
- 37. Pol S, Romeo R, Zins B, Driss F, Lebkiri B, Garnot F, Berthelot P, Brechot C: Hepatitis C virus RNA in anti-HCV positive hemodialyzed patients: Significance and therapeutic implications. Kidney Int 44: 1097–2000, 1993 [DOI] [PubMed] [Google Scholar]
- 38. Lauer GM, Walker BD: Hepatitis C virus infection. N Engl J Med 345: 41–52, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Zylberberg H, Nalpas B, Carnot F, Skhiri H, Fontaine H, Legendre C, Kreis H, Brechot C, Pol S: Severe evolution of chronic hepatitis C in renal transplantation: A case control study. Nephrol Dial Transplant 17: 129–133, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Alric L, Di-Martino V, Selves J, Cacoub P, Charlotte F, Reynaud D, Piette J-C, Peron J-M, Vinel J-P, Durand D, Izopet J, Poynard T, Duffaut M, Rostaing L: Long-term impact of renal transplantation on liver fibrosis during hepatitis C virus infection. Gastroenterology 123: 1494–1499, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Kamar N, Rostaing L, Selves J, Sandres-Saune K, Alric L, Durant D, Izopet J: Natural history of hepatitis C virus-related liver fibrosis after renal transplantation. Am J Transplant 5: 1704–1712, 2005 [DOI] [PubMed] [Google Scholar]
- 42. Nelson D, Soldevila-Pico C, Reed A, Abdelmalek MF, Hemming AW, van de Werf WJ, Howard R, Davis GL: Anti-interleukin-2 receptor therapy in combination with mycophenolate is associated with more severe hepatitis C recurrence after liver transplantation. Liver Transplant 7: 1064–1070, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Hanuka N, Sikuler E, Tovbin D, Mostoslavsky M, Hausman M, Orgel M, Years A, Shemer-Avni Y: Hepatitis C virus infection in renal failure patients in the absence of anti-hepatitis C virus antibodies. J Viral Hepat 9: 141–145, 2002 [DOI] [PubMed] [Google Scholar]
- 44. Garinis G, Spanakis N, Theodorou V, Manolis E, Karameris A, Valis D: Comparison of the enzyme-linked immunosorbant assay III, recombinant immunoblot third generation assay, and polymerase chain reaction method in the detection of hepatitis C virus infection in haemodialysis patients. J Clin Lab Anal 13: 122–125, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ciancio G, Burke GW, Suzart K, Roth D, Kupin W, Rosen A, Olson L, Esquanazi V, Miller J: Daclizumab induction, tacrolimus, mycophenolate mofetil and steroids as an immunosuppression regimen for primary kidney transplant recipients. Transplantation 73: 1100–1106, 2002 [DOI] [PubMed] [Google Scholar]
- 46. Ciancio G, Burke G, Gaynor J, Ruiz P, Roth D, Kupin W, Rosen A, Miller J: A randomized long-term trial of tacrolimus/sirolimus versus tacrolimus/mycophenolate versus cyclosporine/sirolimus in renal transplantation: Three-year analysis. Transplantation 81: 845–852, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Bedossa P, Poynard T: An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24: 289–293, 1996 [DOI] [PubMed] [Google Scholar]
- 48. Kalbfleisch JD, Prentice RL: The Statistical Analysis of Failure Time Data, New York, John Wiley and Sons, 1980 [Google Scholar]
- 49. Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K: Comparison of survival probabilities for dialysis patients vs. cadaveric renal transplant recipients. JAMA 270: 1339–1343, 1993 [PubMed] [Google Scholar]
- 50. Mauger EA, Wolfe RA, Port F: Transient effects in the Cox proportional hazards regression model. Stat Med 14: 1553–1565, 1995 [DOI] [PubMed] [Google Scholar]