Abstract
Although T and B cell alloimmunity contribute to transplant injury, autoimmunity directed at kidney-expressed, non-HLA antigens may also participate. Because the specificity, prevalence, and importance of antibodies to non-HLA antigens in late allograft injury are poorly characterized, we used a protein microarray to compare antibody repertoires in pre- and post-transplant sera from several cohorts of patients with and without transplant glomerulopathy. Transplantation routinely induced changes in antibody repertoires, but we did not identify any de novo non-HLA antibodies common to patients with transplant glomerulopathy. The screening studies identified three reactivities present before transplantation that persisted after transplant and strongly associated with transplant glomerulopathy. ELISA confirmed that reactivity against peroxisomal-trans-2-enoyl-coA-reductase strongly associated with the development of transplant glomerulopathy in independent validation sets. In addition to providing insight into effects of transplantation on non-HLA antibody repertoires, these results suggest that pretransplant serum antibodies to peroxisomal-trans-2-enoyl-coA-reductase may predict prognosis in kidney transplantation.
The immune response to a transplanted organ is driven by T and B cell alloimmunity directed at donor MHC,1,2 but evolving evidence from animal models3–7 and human transplant recipients8,9 indicates that autoreactivity also participates. When transplantation is performed in patients with primary organ failure caused by immune-mediated destruction of normal tissue (e.g. type 1 diabetes), recurrent post-transplant autoimmunity can contribute to graft failure.10,11 Tissue damage accompanying end-stage organ failure, regardless of etiology, could expose physiologically sequestered antigens to the immune system, breaking self-tolerance.12 Mounting associative evidence suggests that such pretransplant autoimmunity to myosin, among other antigens, contributes to post-transplant graft injury.13,14
In addition to pre-existing autoimmunity, ischemia reperfusion injury and tissue healing necessitated by transplant surgery, along with anti-donor alloimmunity, result in inflammation15–18 and exposure of cryptic or sequestered self-antigens to the immune system.19–21 These processes overcome self-tolerance, resulting in de novo pathogenic autoimmunity. One example of this phenomenon in humans is the development of de novo post-transplant antibodies and T cell reactivity to lung-expressed type V collagen in lung transplant patients with bronchiolitis obliterans.8 Other associations include anti-angiotensin II receptors9 or anti-agrin22 antibodies in patients with kidney transplant rejection.
The development of a protein microarray platform has permitted large-scale antibody screening to non-HLA antigens.23 Using such arrays, others showed that serum obtained from children with well-functioning kidney allografts contained antibodies to kidney-expressed antigens,24 some transplant recipients develop autoantibodies with acute kidney rejection and allograft loss,25 and autoantibodies are found in patients with chronic humoral rejection.26 Whether antibodies to non-HLA antigens are pathogenic and/or whether they can be used as biomarkers for transplant outcome remains unclear.
Herein, we used a protein microarray to screen non-HLA antibody repertoires in kidney-transplant recipients with transplant glomerulopathy (TG), a histopathologically distinct manifestation of chronic allograft injury27,28 generally considered to be immune-mediated.29,30 We compared non-HLA antibody profiles of patients with TG to those with stable kidney function after transplantation. Our results, using test and validation sets and confirmed with ELISA assays, indicate that (1) transplantation induces antibodies reactive to a wide assortment of non-HLA antigens, but these reactivities are unique to the individual transplant recipient; and (2) pretransplant detection of antibodies reactive to a specific kidney-expressed target, peroxisomal-trans-2-enoyl-coA-reductase (PECR), is strongly associated with late development of TG.
RESULTS
Protein Array Measures Antibody Repertoires in Human Serum
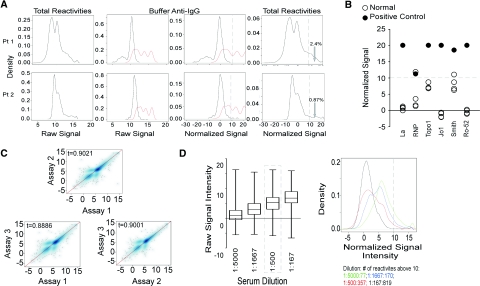
We studied non-HLA antibody repertoires in kidney-transplant recipients by screening serum samples for reactivity to a protein array containing approximately 9000 antigens. We initially performed experiments aimed at understanding assay performance. Figure 1A (left panels) depicts representative raw data derived from testing the serum of two individuals. The signal intensity (log2) and density of reactivities (percentage of all target proteins) are shown on the X and Y axes, respectively. To compare samples from different patients performed at different times and with various array lots and to define a positive threshold, we exploited the fact that each array contains negative (buffer), and positive control (human IgG), spots. The second and third panels in Figure 1A depict only these reactivities to the positive (red, ≈300 spots) or negative controls (black, ≈300 spots). We normalized the results among arrays so that the mean anti-IgG (positive) value was identical for each array (see Concise Methods and Figure 1A). Using the normalized data, we chose a stringent threshold to define a positive result at a signal intensity of log210 (1024, dotted line in Figure 1A, third panels), which reduced the false-positive detection of negative control spots to essentially zero. At this threshold, serum samples from normal volunteers and transplant recipients reacted to 0.07 to 27% of antigens (Figure 1A, right panels).
Figure 1.
Protein microarray is a screening tool that reproducibly detects serums reactivities to non-HLA antigens. (A) Data normalization approach for two representative serum samples from two different patients. Raw signals are shown on the left. Unmanipulated positive control (red) and negative control (black) signals are shown in the next panel. The third panel depicts the signals for normalized positive and negative controls. The final panel on the right shows post normalization histogram for all reactivities. The vertical dotted line in the right two panels is drawn at log210. The numbers refer to percentages of reactivities above log210 threshold. (B) Assay validation with positive control sera. Normalized signal values are plotted for positive control serum (closed circles) and four healthy control serum samples (open circles). The horizontal dotted line is drawn at the significance level of log2 10. (C) Assay variability. Representative Spearman plots of a single serum sample tested on three separate arrays. Spearman plots comparing pairs of arrays with a mean tau of 0.90. The experiment was repeated twice with comparable results (data not shown). (D) Effects of serum dilution. The left panel shows box plots (median, 25th and 75th percentiles) for four different dilutions of the same serum sample tested on four separate arrays. The dashed box surrounds the dilution recommended by the manufacturer. Median reactivity for each array titrates down with serum dilution. The right panel shows signal histograms with numbers of significant reactivities >10 for each dilution.
As validation of our analytic methods, we tested commercially purchased pooled serum containing antibodies to Ro-52, La, Jo-1, TopoI, RNP, and Smith antigens for reactivity to the array (Figure 1B). The positive control serum reacted significantly to all six autoantigens. Signals for each reactivity were among the strongest detected (>98th percentile of 9000 antigens on the array). Serum from two of the normal volunteers reacted to none of the test antigens (all reactivities < log2 10 with percentile ranks ≤87%). The other two sera each reacted with only one of six control autoantigens (Figure 1B).
To test intra-assay variability, we performed three replicate assays using a single serum sample (Figure 1C). These comparisons revealed Spearman tau correlations coefficients of 0.88 to 0.93, indicating approximately 10% intra-assay variability.
To assess how well the array detected quantitative differences in antibody concentration, we performed side-by-side assays on serially-diluted serum samples (Figure 1D). These experiments showed that median signal intensities (left panel) fell with the dilution, as did the number of positive reactivities (right panel).
Kidney Transplantation Induces Changes in Antibody Repertoires Reactive to Non-HLA Antigens
Next we tested whether kidney transplantation alters antibody repertoires to non-HLA antigens. We obtained sera pretransplant and 12 months post-transplant from six stable kidney-transplant recipients (cohort 1; Table 1). Data from this ongoing trial indicate that these patients had serum creatinine values ≤1.8 mg/dl at 12 months post-transplant, no acute rejection episodes ≥ Banff 1A grade, and no detectable serum anti-HLA antibodies. We measured the non-HLA antibody repertoires by array and compared pre- and post-transplant results for each patient (representative result in Figure 2A and see Supplemental Figure 1). We found that Spearman tau coefficients averaged 0.55 (0.43 to 0.64; Supplemental Figure 1), indicating significant differences between pre- and post-transplant serum antibody repertoires for each recipient. Table 2 quantifies, for each patient and group, the numbers of weak pretransplant reactivities, within the lowest 10th or 30th percentile, respectively) that became strongly positive post-transplant (≥90th percentile).
Table 1.
Patient characteristics
| Patient Cohort (Number of Patients) | Median Age in Years (Range) | Diagnosis | Median Time Since Transplant in Years (Range) | Donor-specific Anti-HLA Antibodiesa | Median Proteinuria by Dipstick or g/24 h | Median Serum Creatinine in mg/dl (Range) |
|---|---|---|---|---|---|---|
| 1 (n = 6)b | 54 (11 to 80) | Stable kidney function | 1 | 0/6 | Not available | 1.3 (1.0 to 1.8) |
| 2 (n = 5) | 44.5 (40 to 57) | TG | 4 (3 to 19) | 1/5 | 2.4 g/24 h | 2.2 (2 to 3.2) |
| 3 (n = 7) | 49.5 (37 to 72) | TG | 5.4 (1.6 to 13.8) | 3/7 | 171 mg/24 h (n = 3)c | 2.7 (1.6 to 3.8) |
| 4 (n = 9) | 60 (33 to 74) | TG | 6 (3 to 22) | 2/9 | 100 (dipstick) | 2.2 (1 to 5) |
| 5 (n = 8) | 64 (39 to 77) | Stable kidney function | 6.5 (3 to 10) | Not tested | Neg (dipstick) | 1.15 (0.8 to 1.8) |
aNumber of patients with anti-donor HLA antibody/total number of patients in the group.
bThe data derive from an ongoing study (CTOT01, www.ctot.org), and the results are unvalidated.
cNo available data on four patients.
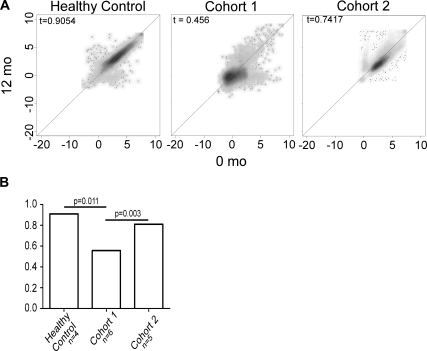
Figure 2.
Transplantation induces new antibodies reactive to non-HLA antigens. (A) Representative Spearman plots depicting antigen reactivities in two serum samples obtained 12 months apart from a normal volunteer (left panel) and pretransplant and 12 months post-transplant from an individual transplant recipient with stable kidney function (cohort 1, middle panel) and an individual transplant recipient with TG (cohort 2, right panel). (B) Median spearman tau correlation coefficients for each group. The P values were determined by Mann-Whitney U test.
Table 2.
New antibody reactivities detected after transplantation in patients with stable renal function (cohort 1)
| Patient | Number of Pretransplant <10th Percentile to Posttransplant >90th Percentilea | Number of Pretransplant <30th Percentile to Posttransplant >90th Percentile |
|---|---|---|
| 1 | 2 | 18 |
| 2 | 4 | 17 |
| 3 | 0 | 9 |
| 4 | 3 | 24 |
| 5 | 8 | 24 |
| 6 | 4 | 15 |
| Total | 21 | 107 |
aNo common reactivities are shared between any two patients.
When we compared the results of serum samples obtained 12 months apart from normal volunteers (Figure 2A, left panel), we found essentially no change in the repertoires (Spearman tau, 0.91). Neither did we observe any reactivities that commonly increased from the lower percentile ranks pretransplant to above the positive threshold 12 months later (not shown).
Autoantibody Repertoires in Kidney Transplant Recipients with TG
We next examined autoantibody profiles in patients with biopsy proven TG, a well-defined pathologic entity commonly associated with serum-donor HLA-specific antibodies.27–30 We evaluated pre- and post-transplant serum samples from five patients followed at Hospital 12 de Octubre in Madrid Spain (cohort 2) and seven patients from the Ohio State University (cohort 3). Clinical characteristics are summarized in Table 1 and Supplemental Tables 1 (A and B). Sera from four of 12 patients with TG contained donor-reactive antibodies by single-antigen testing. Serum samples were obtained a median of 4 years and 5.4 years post-transplant from the two cohorts, respectively.
When we compared pre- and post-transplant repertoires for each patient (Figure 2A and Supplemental Figure 2), we found that Spearman tau coefficients ranged from 0.74 to 0.93. Few (range, 3 to 46) pretransplant reactivities within the lowest 10th percentile became strongly positive (> 90th percentile) in the post-transplant sample (Tables 3 and 4). We found essentially no overlap in the antigenic targets among the 12 patients (Tables 3 and 4). Ingenuity analysis revealed that these proteins mapped to diverse pathways (not shown). Reanalyzing the data with less stringent thresholds (<30th to >90th percentile) increased the numbers of reactivities for each patient but did not reveal any common targets (not shown). Together, the results suggest that TG is associated with changes in non-HLA antibody repertoires, but the induced alterations seem to be unique to the individual.
Table 3.
New reactivities detected after transplantation in patients with TG (cohort 2)
| Patient | Number of Pretransplant <10th Percentile to Posttransplant >90th Percentilea | Number of Pretransplant <30th Percentile to Posttransplant >90th Percentile |
|---|---|---|
| 1 | 5 | 27 |
| 2 | 46 | 53 |
| 3 | 3 | 3 |
| 4 | 3 | 4 |
| 5 | 7 | 12 |
| Total | 64 | 99 |
aThree reactivities are shared between two individual patients. BC016330.1-RAD51-associated protein 1 (RAD51AP1) and NM_032349.1-nudix (nucleoside diphosphate-linked moiety X)-type motif 16-like 1 (NUDT16L1) are shared between patients 2 and 5. BC014394.1-A. T hook DNA-binding motif-containing protein 1 is shared between patients 2 and 3.
Table 4.
New reactivities detected after transplantation in patients with TG (cohort 3)
| Patient | Number of Pretransplant <10th Percentile to Posttransplant >90th Percentilea | Number of Pretransplant <30th Percentile to Posttransplant >90th Percentile |
|---|---|---|
| 1 | 3 | 5 |
| 2 | 3 | 3 |
| 3 | 2 | 8 |
| 4 | 2 | 4 |
| 5 | 4 | 17 |
| 6 | 4 | 8 |
| 7 | 2 | 6 |
| Total | 20 | 51 |
aTwo antigen reactivities are shared between two individual patients. NM_024068.1-oligonucleotide/oligosaccharide-binding fold containing 2B (OBFC2B) is shared between patients 8 and 10 and BC021622.1-phosphoinositide-3-kinase, regulatory subunit 3 (p55, gamma) (PIK3R3) is shared between patients 3 and 4.
Pretransplant Autoantibody Repertoires in Kidney Transplant Recipients Who Develop TG
Primary organ failure is commonly caused by and/or associated with autoreactivity directed at antigens expressed in the failing organ.31,32 Because pre-existing autoimmune memory likely persists post-transplant, it could negatively affect transplant outcome. To address this, we reanalyzed our data and assessed whether pretransplant autoantibodies that persisted post-transplant were strongly associated with the development of TG.
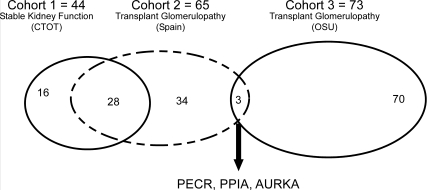
We identified 65 reactivities with signal intensities >90th percentile that were detectable in both pre- and post-transplant serum samples of all five (cohort 2) patients with TG and 44 reactivities common to all stable patients (cohort 1; Figure 3). Thirty-seven of 65 antigens common to the five TG patients (cohort 2) were unique (not found in a single stable patient serum; Figure 3). When we compared these 37 reactivities with those common to the seven other patients with TG (cohort 3, 73 individual reactivities), we discovered three candidate biomarker reactivities common to all 12 TG patients and absent in all stable kidney transplant recipients; Figure 3). These antigens (Table 5) are peptidyl-prolyl-isomerase-A (PPIA or cyclophilin A), PECR, and serine threonine kinase 6 (AURKA). They are intracellular proteins and are detectable within kidney tissue (Table 5). PECR is a peroxisomal protein involved in fatty-acid synthesis and has not been studied in transplantation.33 PPIA is a T cell–signaling molecule that when bound to cyclosporine is inhibited from interacting with calcineurin.34 AURKA is required for normal mitosis.35,36
Figure 3.
Microarray analysis identifies three putative biomarkers for transplant glomerulopathy. Shown is a Venn diagram depicting the overlapping reactivities in sera from patients with stable kidney function (Cohort 1, left oval) and from two different cohorts of patients with TG (cohorts 2 and 3, middle dashed oval and right oval, respectively). Thirty-seven reactivities were found in cohort 2 but not detected in any of the cohort 1 patients. Of those 37 reactivities, three were also detected in each of the patients in cohort 3.
Table 5.
Antigen reactivities specific to all cohort 2 and 3 patients with TG
| Accession Number | Protein | Known Function | Known Tissue Expression Patterna |
|---|---|---|---|
| BC005982.1 | PPIA | Also called cyclophilin A, PPIA is the molecular target of cyclosporine A involved in T cell activation. The cyclosporine-cyclophilin A complex inhibits a calcium/calmodulin-dependent phosphatase, calcineurin, the inhibition of which is thought to suppress organ rejection by halting the production of the pro-inflammatory molecules TNFα and interleukin-2. PPIs also catalyze the cis-trans isomerization of proline imidic peptide bonds in oligopeptides and accelerate the folding of proteins.55 | Kidney, vascular smooth muscle cells, fibroblasts macrophages53,56 |
| BC006423.1 | AURKA | The cell cycle-regulated kinase appears to be involved in microtubule formation and/or stabilization at the spindle pole during chromosome segregation; Aurora A dysregulation has been associated with a high occurrence of cancer.35,36 | Kidney, liver, but highest expression in lymphoblasts and endothelial cells, colon adenocarcinoma56 |
| NM018441.2 | PECR | PECR is a peroxisomal NADPH-specific trans-2-enoyl-CoA reductase that catalyzes the reduction of trans-2-enoyl-CoAs of varying chain lengths from 6:1 to 16:1, having maximum activity with 10:1 CoA.33,57 | CD4/CD8 T cells, B cells, dendritic cells, endothelial cells, smooth muscle, kidney, liver, adipocytes56 |
aTissue expression patterns found by GenBank/GeneCards search and http://biogps.gnf.org; http://www.ncbi.nlm.nih.gov/geoprofiles (GEO ID# GDS899, GDS3397, GDS1453, GDS2534, and GDS1892).
Post- and Pretransplant Reactivity to PECR by ELISA Strongly Associates with TG
To validate the data derived from the array, we tested pre- and post-transplant sera from each patient for reactivity to the three candidate antigens, PECR, PPIA, and AURKA by ELISA. We measured total IgG levels in a subset of 28 patients (nine with TG, eight with stable kidney function, and 11 patients on the transplant waiting list) and found no difference among the groups (not shown).
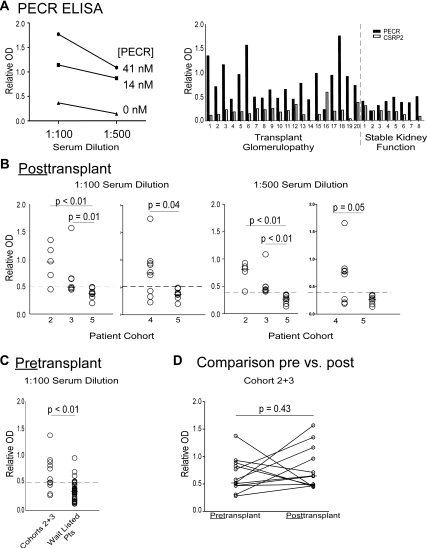
Representative anti-PECR ELISA results from one patient (Figure 4A, left panel) show that the reactivity titrates with serum dilution (1:100 to 1:500) and antigen concentration. In patients with TG, reactivity to PECR was stronger than to a control protein (cysteine- and glycine-rich protein 2 [CSRP2]; Figure 4A, right panel, and Supplemental Figure 3), indicating specificity.
Figure 4.
Patients with TG specifically have serum antibodies reactive to PECR. (A) Anti-PECR ELISA. (Left panel) Serum from one representative patient with TG was diluted 1:100 and 1:500 and tested in an anti-PECR ELISA in which plates were coated with PECR (41 or 14 nM) or without PECR (0 nM). The mean values of triplicate wells are shown. (Right panel) Sera (1:100 dilutions) from 28 individual patients (20 with TG and eight with stable kidney function) were tested for reactivity to two PECR and a control protein, CSRP2 at 14 nM. (B) Anti-PECR reactivity detected post-transplant. Individual ELISA results for anti-PECR reactivity tested at 1:100 dilution (left panels) or 1:500 dilution (right panels) in patients with late TG (cohorts 2, 3, and 4) and stable kidney function (cohort 5). The wells were coated with 14 nM PECR. Arbitrary thresholds for positive values are shown by the dashed lines (OD 0.5 at 1:100 dilution and 0.4 at 1:500 dilution). (C) Pretransplant anti-PECR ELISA. Pretransplant sera obtained from patients with late TG (cohorts 2 and 3) and a randomly selected group of 37 patients on the transplant waiting list were tested for anti-PECR reactivity (1:100 serum dilution, 14 nM PECR). The dashed horizontal line represents the threshold of OD 0.5. Medians were compared by Mann-Whitney U test. (D) Individual pre- and post-transplant anti-PECR reactivity by ELISA. Each line represents the pre- and post-transplant serum reactivity for PECR for the 12 patients from cohorts 2 and 3 (extracted from panel C).
Post-transplant anti-PECR ELISA results (Figure 4B) for cohorts 2 and 3 with TG and eight post-transplant, time-matched stable transplant recipients (cohort 5; Table 1 and Supplemental Table 1D) reveal stronger responses in each of the TG cohorts (P < 0.01 and P = 0.01, respectively).
To strengthen our validation, we tested post-transplant serum samples from an additional cohort of nine patients with TG followed at Mount Sinai Hospital in New York (cohort 4; Table 1 and Supplemental Table 1C). We observed stronger reactivity in the separate TG cohort 4 compared with those with stable kidney function (P = 0.04), thus confirming the finding in a validation set (Figure 4B). Using a candidate threshold of 0.5 units (1:100 dilution), we calculated a positive predictive value of 62% with a negative predictive value of 100%. At the 1:500 dilution and a lower candidate threshold of 0.4, the positive predictive value was 86%, and the negative predictive value was 100%.
When we correlated the pathologic grade of TG with the strength of the anti-PECR response in the 21 patients with TG (cohorts 2 to 4), we did not observe a significant relationship (Supplemental Table 1). Nor did we find a relationship between the presence of pre- or post-transplant anti-PECR immunity and the cause of the primary kidney failure (Supplemental Table 1 and Supplemental Figure 4).
To test whether pretransplant anti-PECR antibodies are associated with the development of late TG, we performed anti-PECR ELISAs on stored sera obtained before transplantation from the patients who ultimately developed this histology (cohorts 2 and 3). The ideal control would be pretransplant sera obtained from time-matched patients to the TG groups (approximately 6 years) with biopsies negative for TG, but such samples were not available (surveillance biopsies are not routinely obtained 5 to 10 years post-transplant). Instead we obtained and tested serum samples from a cohort of 37 wait-listed kidney transplant candidates as controls (Figure 4C). We reasoned that because TG occurs in approximately 20% of transplant recipients at 5 years,37 for pretransplant anti-PECR antibodies to be predictive of TG, the strength of the pretransplant anti-PECR responses should be significantly higher in those with documented TG than in the randomly selected controls on the waiting list. We observed that the median anti-PECR reactivity was higher in those destined to develop TG (P < 0.01) compared with the 37 randomly-selected, wait-listed transplant candidates. At a proposed threshold of 0.5, we found eight of 12 (75%) patients who eventually developed TG to have anti-PECR immunity. At this same cutoff, eight of 37 (21%) wait-listed transplant candidates had anti-PECR reactivity (Figure 4C; P = 0.01 by χ2).
To assess whether transplantation affects the anti-PECR response, we compared pre- and post-transplant results for the 12 patients with TG in cohorts 2 to 3 (Figure 4D). The median values did not differ (P = 0.43). The response increased in seven of 12 post-transplant. Two patients with negative values pretransplant (<0.5) became positive post-transplant. Reactivity fell in three patients post-transplant but did not fall below the 0.5 threshold.
In contrast to the results for anti-PECR antibodies, when we tested sera for anti-PPIA reactivity post-transplant, we did not observe a correlation between the strength of the response and the presence of TG (Supplemental Figure 5). Nor did ELISAs for AURKA performed on a subset of 12 patients with TG and 17 patients without TG reveal differences between the groups (not shown).
DISCUSSION
Using a screening microarray, we showed that serum from two independent cohorts with TG contained IgG reactive to three non-HLA antigens (Figure 3). ELISAs confirmed reactivity to one of these, PECR, in the two cohorts (Figure 4). The post-transplant anti-PECR immunity was stronger than in recipients with stable kidney function, and the finding was confirmed using post-transplant serum from an independent fell in three patients posttransplant cohort 4 with biopsy proven TG. Reactivity was titratable and specific because sera from those with anti-PECR immunity did not react to another tested antigen (Figure 4A), limiting the likelihood that the responses represent polyreactive antibodies38 or low affinity autoantibodies found in normal individuals.39
In addition to the post-transplant correlations, our analyses revealed that sera obtained pretransplant from patients who ultimately developed TG reacted significantly more to PECR than pretransplant sera from a randomly selected cohort wait-listed transplant candidates (Figure 4C), of whom only a minority will develop TG.40 Acknowledging the small sample size and the need for larger prospective studies, our results raise the possibility that both pre- and post-transplant antibodies to this kidney-expressed, non-HLA antigen could be used as a biomarker for the development of TG.
PECR is a peroxisomal reductase involved in fatty acid elongation and to our knowledge has not been previously reported as a target autoantigen in any disease state. Although a pathogenic role for PECR in kidney disease is unknown, when we performed a GEO database analysis of gene array profiling studies to determine expression profiles for PECR (http://www.ncbi.nlm.nih.gov/geoprofiles), the analysis revealed strong expression in glomerular mesangial cells and renal tubules (Table 5). Together with reports that intracellular fatty acid accumulation can be a feature of kidney disease,41–45 the glomerular location of PECR makes a pathogenic role for anti-PECR antibodies plausible. Fatty acid accumulation could aggravate lipid loading of glomerular and tubular cells driven by increased endogenous synthesis and that may contribute to progression of kidney failure.41,42 We speculate that upregulated expression and release of PECR could occur as a consequence of primary kidney disease and if presented in a proinflammatory environment could result in B cell and/or T cell autoimmunity that could participate in the pathogenesis of allograft injury.
Once induced, memory autoimmunity is long lived, high affinity, and rapidly engaged after another antigen exposure and resistant to immunosuppression.46,47 Autoimmunity to islet antigens portends poor outcome after pancreas or islet transplantation, anti-cardiac myosin antibodies detectable in patients with heart failure persist after transplantation, and their presence increases the risk of severe acute rejection.10–12 Our data indicate that anti-PECR immunity could function analogously in the context of kidney transplantation but will require further study.
Whether antibodies reactive to PECR, an intracellular protein, participate in the pathogenesis of chronic injury or are nonparticipatory bystanders cannot be ascertained from our work. Studies by others revealed that apoptosis, induced by a variety of mechanisms, can reorient intracellular proteins such that they are expressed on cell surface blebs.48,49 In the context of lupus, this mechanism can result in concentrated and altered presentation of intracellular antigens to the immune system, stimulating autoantibody formation, specifically anti-SSA/SSB antibodies that are pathogenic mediators of congenital heart block, thus providing a context through which such antibodies to intracellular antigens could mediate injury.50 An alternative explanation for the association between anti-PECR immunity and TG is the detected IgG is a surrogate for anti-PECR T cell immunity that mediates the disease.
TG has traditionally been described29,30 as an antibody-associated process that affects 5% of renal transplant recipients per year, decreasing allograft half-life by 50%.37,40 We and others have previously reported that TG can occur in the absence of donor-specific anti-HLA antibodies or C4d staining,51–53 a result supported by the findings in this study in which only six of 21 patients with documented TG had detectable donor-reactive antibodies. In contrast, we found that serum from the majority of patients with this pathology contained antibodies reactive to PECR. Moreover, of seven transplants from our cohorts in which C4d staining was reported to be positive within the glomeruli, sera from all seven contained anti-PECR antibodies and only three of seven contained donor-specific antibody.
We acknowledge that the pathology of kidney allografts with TG, including those studied herein, also includes some elements of interstitial fibrosis and tubular atrophy. Although we do not have access to time-post-transplant–matched serum samples from patients with biopsy proven Interstitial Fibrosis/Tubular Atrophy (IF/TA) without TG as a control, the literature54 supports the idea that allografts from the stable patients have some degree of IF/TA, yet our data indicate that these clinically stable patients do not have serum reactivity to PECR.
The microarray data indicate that transplantation alters antibody repertoires (Figure 2), but we found essentially no overlap among the patients with TG with regard to de novo non-HLA antibodies. The findings support results by another group suggesting that autoimmune profiles induced by transplantation are unique to the recipient.26
Our study highlights the strengths and limitations of large throughput protein microarray screening for identifying transplant associated biomarkers. Whereas the data indicate that such studies can be informative, high false-positive rates (only one of three antigens identified was informative) and cost of array experiments require careful selection of well-defined cohorts and novel analytical methods to enhance signal to noise. Further data mining and reanalysis of previously published studies in publicly available databases using such analytical approaches may provide additional valuable information.
CONCISE METHODS
Human Subjects and Sera
All serum samples were collected with informed consent and ethics approval by all local institutional review boards. After collection, all of the serum samples were stored in aliquots at −80°C.
We obtained and tested two different serum samples from four normal healthy controls 12 months apart. The median age of the normal subjects was 37.5 years (age, 23 to 50).
We obtained pretransplant sera from 37 patients participating in the Clinical Trials in Organ Transplantation CTOT01 observational trial (www.ctot.org). In six of these patients, we also obtained serum samples 1 year post-transplant. Unvalidated clinical data from this ongoing trial indicated that these six patients had stable renal function with serum creatinine ≤1.8 mg/dl at 1 year and no serum anti-HLA antibodies at 1 year, and none experienced an episode of acute cellular rejection. These six stable patients are termed cohort 1.
We obtained pre- and post-transplant serum samples from two independent cohorts of patients diagnosed with biopsy proven TG (Banff 1997 criteria). Samples from five patients (cohort 2) were obtained at the Hospital 12 de Octubre (Madrid, Spain), and samples from seven patients (cohort 3) were obtained from the Ohio State University transplant program (Columbus, OH). Donor-specific anti-HLA reactivity was detected in one of five patients by Luminex in the Spanish cohort and in three of seven patients in the Ohio State cohort by GenProbe single-antigen testing (see Table 1 and Supplemental Table 1, A through E) for detailed demographics.
We obtained post-transplant serum from nine patients at Mount Sinai Medical Center with biopsy-proven (Banff 1997) TG (cohort 4) and from eight patients with stable kidney function (cohort 5) time matched to cohort 4. Donor specific antibodies were determined by Luminex single-antigen testing.
Protein Microarray Methods
We used the ProtoArray microarray platform versions 4.1 and 5.0 (Invitrogen, Carlsbad, CA), which contain duplicate spots of approximately 9000 glutathione S-transferase-tagged human proteins printed on a nitrocellulose slide. The antigens include a selection of intra- and extracellular proteins of known function, multiple proteins derived from open reading frames of unknown significance, and human immunoglobulins (see http://www.invitrogen.com/site/us/en/home/Products-and-Services/Applications/Protein-Expression-and-Analysis/Biomarker-Discovery/ProtoArray.html for details). The proteins expressed on the array were selected by the manufacturer and not chosen for their putative relationship to transplantation immunology.
As per manufacturer instructions, the microarrays were stored at −20°C and were equilibrated for 15 minutes at 4°C immediately before use. After a 1-hour block in 50 mM Hepes (pH 7.5), 200 mM NaCl, 0.08% Triton X-100, 25% glycerol, 20 mM reduced glutathione, 1× Synthetic block, 1 mM dTT, (pH 7.5), deionized water (www.Invitrogen.com), serum diluted 1:500 in wash buffer (1× PBS, 0.1% Tween 20, 1× Synthetic block, deionized water) was added and incubated for 90 minutes. The arrays were washed five times with wash buffer at room temperature, followed by incubation with an AlexaFluor 647-conjugated anti-human IgG (1 μg/ml) (Invitrogen) for 90 minutes at 4 °C. After an additional five washes at room temperature, the arrays were dried and scanned with an Axon 4300A (Axon, Toronto, CA). Scanned images from individual arrays were converted into digital signals, which were analyzed. The raw data files are available upon request from the authors.
Bioinformatics Analysis of Protein Microarray Results
Although the manufacturer provides an analysis tool, Prospector Analyzer, the limitations of the program are that data can only be compared from arrays of the same lot and version. We therefore performed an independent analysis using internally developed software on the R platform (http://www.r-project.org/). We determined strict signal thresholds, quality checked each array by examining true positive and true negative signal distributions, summarized protein expression from replicate spots, and normalized intensities using IgG and buffer signals. Software and analysis algorithms are available upon request from the authors. An overview of our analysis strategy follows.
All of the arrays contained 300+ buffer spots (no antigen) and 300 anti-IgG-positive controls. GPR (GenePix Result) files were generated and read, and the raw signal values were converted to log2 scale. Noise channel values (between antigen spots) were discarded. Using only buffer and anti-IgG spot values, we estimated positive and true negative cutoffs for each array. Modified median-shift normalization was performed (the arrays were scaled so that the difference between the median of the true negatives and the median of the true positives was identical for each array). The signal values for each array were shifted so that the mean anti-IgG (defined as positive) value was identical for each array (Figure 1A). All of the reactivities were also assigned a percentile rank (0 to 100%). Signals above the 90th percentile were considered to be significant, corresponding to a normalized signal value of 1024 on arrays. This value was chosen because it minimized the false-positive rate. Although the stringent threshold elevated the false-negative rate, we chose to sacrifice potential loss of data so as to limit the numbers of antigenic targets required for expensive validation studies. Rank shifts between pre- and post-transplant samples were then compared. Patients sharing a clinical phenotype (i.e. stable kidney function or TG) were analyzed on the same array version to minimize version to version variability. Normalized signal values and percentile rank shifts were used as dual methods to stringently stratify differences between groups when the groups were tested on different versions of the array. When assessing signal reactivities between array versions, only normalized signal intensities/percentile ranks were studied for antigens with similar amounts spotted between versions (within two-fold of each other) to minimize false positives.
ELISA
ELISAs were developed to detect IgG reactive to PECR, PPIA, and CSRP2, which were manufactured in a baculovirus system and purchased from the array manufacturer (Invitrogen). Immulon 4HBX plates were coated with 41 and 14 nM of antigen diluted in bicarbonate coating buffer (Sigma) at 50 μl/well in triplicate. The plates were incubated overnight at 4°C on a plate shaker (120 RPM) and washed. All of the wash steps were performed with 1× TBST at 300 μl/well five times. The plates were blocked for 1 hour in 1× TBST and 2% dry milk at 100 μl/well and then washed. Serum was diluted in 1× TBST and 2% dry milk at 1:100, 1:500, and incubated for 2 hours at room temperature, followed by five washes. Secondary HRP-conjugated goat-α human IgG (Santa Cruz) was added to all of the wells at a 1:4000 dilution at 50 μl/well for 1 hour and incubated at 4°C. After another five washes, tetramethylbenzide liquid substrate (Sigma) was added to all of the wells at 100 μl/well and allowed to develop for 30 minutes. Stop solution (1 N sulfuric acid) was added to all wells at 100 μl/well, and the plates were read at 450 nm. Background serum reactivity in the absence of protein antigen served as an internal control and subtracted from reactivity against protein.
Statistical Analyses
Microarray results were compared using Spearman tau correlations in which results are expressed as 0 to 1 with a perfect correlation being 1.0. The distribution of anti-PECR reactivity was tested for normality using the Kolmogorov-Smirnov and Shapiro-Wilk tests of normality. OD values of reactivity values were compared by nonparametric tests on the basis of data distribution. Median reactivities were initially compared among all groups using a Kruskal-Wallis one-way ANOVA and thereafter among individual groups using a Mann-Whitney U test or X square. P values of <0.05 were considered statistically significant. Statistical analysis was performed with the SPSS version 18.0 software package (SPSS, Inc., Chicago, IL).
DISCLOSURES
None.
Acknowledgments
The authors acknowledge the efforts of the Clinical Trials in Organ Transplantation investigators, including D. Hricik (Case Western, Cleveland, OH), E. Poggio, (Cleveland Clinic, Cleveland, OH), R. Formica (Yale University, New Haven, CT), K. Newell (Emory University, Atlanta, GA), P. Nickerson and D. Rush (University of Manitoba, Winnipeg, Canada), and J. Gobel (University of Cincinnati, Cincinnati, OH), for collecting patient samples that were used as part of the work and thank Denise Peace for her technical guidance.
This work was supported by National Institutes of Health Grant U19AI063603 awarded to D.R.S. and an American Recovery and Reinvestment Act (ARRA) supplement to National Institutes of Health Grant U01AI63594 awarded to P.S.H.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Lechler RI, Sykes M, Thomson AW, Turka LA: Organ transplantation: How much of the promise has been realized? Nat Med 11: 605–613, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Nankivell BJ, Alexander SI: Rejection of the kidney allograft. N Engl J Med 363: 1451–1462, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Rolls HK, Kishimoto K, Dong VM, Illigens BM, Sho M, Sayegh MH, Benichou G, Fedoseyeva EV: T-cell response to cardiac myosin persists in the absence of an alloimmune response in recipients with chronic cardiac allograft rejection. Transplantation 74: 1053–1057, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Benichou G, Alessandrini A, Charrad RS, Wilkes DS: Induction of autoimmunity after allotransplantation. Front Biosci 12: 4362–4369, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G: De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol 162: 6836–6842, 1999 [PubMed] [Google Scholar]
- 6. Fedoseyeva EV, Kishimoto K, Rolls HK, Illigens BM, Dong VM, Valujskikh A, Heeger PS, Sayegh MH, Benichou G: Modulation of tissue-specific immune response to cardiac myosin can prolong survival of allogeneic heart transplants. J Immunol 169: 1168–1174, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Yamada Y, Sekine Y, Yoshida S, Yasufuku K, Petrache I, Benson HL, Brand DD, Yoshino I, Wilkes DS: Type V collagen-induced oral tolerance plus low-dose cyclosporine prevents rejection of MHC class I and II incompatible lung allografts. J Immunol 183: 237–245, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, Meyer KC, Hayney MS, Braun RK, Greenspan DS, Gopalakrishnan B, Cai J, Brand DD, Yoshida S, Cummings OW, Wilkes DS: IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest 117: 3498–3506, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dragun D, Muller DN, Brasen JH, Fritsche L, Nieminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schonemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G: Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 352: 558–569, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Braghi S, Bonifacio E, Secchi A, Di Carlo V, Pozza G, Bosi E: Modulation of humoral islet autoimmunity by pancreas allotransplantation influences allograft outcome in patients with type 1 diabetes. Diabetes 49: 218–224, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Vendrame F, Pileggi A, Laughlin E, Allende G, Martin-Pagola A, Molano RD, Diamantopoulos S, Standifer N, Geubtner K, Falk BA, Ichii H, Takahashi H, Snowhite I, Chen Z, Mendez A, Chen L, Sageshima J, Ruiz P, Ciancio G, Ricordi C, Reijonen H, Nepom GT, Burke GW, 3rd, Pugliese A: Recurrence of type 1 diabetes after simultaneous pancreas-kidney transplantation, despite immunosuppression, is associated with autoantibodies and pathogenic autoreactive CD4 T-cells. Diabetes 59: 947–957, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warraich RS, Pomerance A, Stanley A, Banner NR, Dunn MJ, Yacoub MH: Cardiac myosin autoantibodies and acute rejection after heart transplantation in patients with dilated cardiomyopathy. Transplantation 69: 1609–1617, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Laguens RP, Vigliano CA, Argel MI, Chambo JG, Rozlosnik JA, Perrone SV, Favaloro RR: Anti-skeletal muscle glycolipid antibodies in human heart transplantation as predictors of acute rejection: Comparison with other risk factors. Transplantation 65: 1345–1351, 1998 [DOI] [PubMed] [Google Scholar]
- 14. Nath DS, Ilias Basha H, Tiriveedhi V, Alur C, Phelan D, Ewald GA, Moazami N, Mohanakumar T: Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J Heart Lung Transplant 29: 1277–1285, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rauen U, de Groot H: New insights into the cellular and molecular mechanisms of cold storage injury. J Investig Med 52: 299–309, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Yang B, Jain S, Ashra SY, Furness PN, Nicholson ML: Apoptosis and caspase-3 in long-term renal ischemia/reperfusion injury in rats and divergent effects of immunosuppressants. Transplantation 81: 1442–1450, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Land WG: The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation 79: 505–514, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Suzuki C, Isaka Y, Takabatake Y, Tanaka H, Koike M, Shibata M, Uchiyama Y, Takahara S, Imai E: Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun 368: 100–106, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Ciubotariu R, Liu Z, Colovai AI, Ho E, Itescu S, Ravalli S, Hardy MA, Cortesini R, Rose EA, Suciu-Foca N: Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. J Clin Invest 101: 398–405, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Obata F, Yoshida K, Ikeda Y, Ohkubo M, Saito T, Takeuchi Y, Shinohara N, Endo T, Baba S: Clonality analysis of T cells mediating acute and chronic rejection in kidney allografts. Transpl Immunol 13: 233–237, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K: Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol 11: 729–766, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Joosten SA, Sijpkens YW, van Ham V, Trouw LA, van der Vlag J, van den Heuvel B, van Kooten C, Paul LC: Antibody response against the glomerular basement membrane protein agrin in patients with TG. Am J Transplant 5: 383–393, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Zhu H, Bilgin M, Bangham R, Hall D, Casamayor A, Bertone P, Lan N, Jansen R, Bidlingmaier S, Houfek T, Mitchell T, Miller P, Dean RA, Gerstein M, Snyder M: Global analysis of protein activities using proteome chips. Science 293: 2101–2105, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Li L, Wadia P, Chen R, Kambham N, Naesens M, Sigdel TK, Miklos DB, Sarwal MM, Butte AJ: Identifying compartment-specific non-HLA targets after renal transplantation by integrating transcriptome and “antibodyome” measures. Proc Natl Acad Sci U S A 106: 4148–4153, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sutherland SM, Li L, Sigdel TK, Wadia PP, Miklos DB, Butte AJ, Sarwal MM: Protein microarrays identify antibodies to protein kinase Czeta that are associated with a greater risk of allograft loss in pediatric renal transplant recipients. Kidney Int 76: 1277–1283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Porcheray F, DeVito J, Yeap BY, Xue L, Dargon I, Paine R, Girouard TC, Saidman SL, Colvin RB, Wong W, Zorn E: Chronic humoral rejection of human kidney allografts associates with broad autoantibody responses. Transplantation 89: 1239–1246, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Habib R, Broyer M: Clinical significance of allograft glomerulopathy. Kidney Int Suppl 43: S95–S98, 1993 [PubMed] [Google Scholar]
- 28. Habib R, Zurowska A, Hinglais N, Gubler MC, Antignac C, Niaudet P, Broyer M, Gagnadoux MF: A specific glomerular lesion of the graft: Allograft glomerulopathy. Kidney Int Suppl 42: S104–S111, 1993 [PubMed] [Google Scholar]
- 29. Mauiyyedi S, Pelle PD, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, Williams WW, Cosimi AA, Schneeberger EE, Colvin RB: Chronic humoral rejection: Identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol 12: 574–582, 2001 [DOI] [PubMed] [Google Scholar]
- 30. Regele H, Bohmig GA, Habicht A, Gollowitzer D, Schillinger M, Rockenschaub S, Watschinger B, Kerjaschki D, Exner M: Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: A contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol 13: 2371–2380, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Miao D, Yu L, Eisenbarth GS: Role of autoantibodies in type 1 diabetes. Front Biosci 12: 1889–1898, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Nussinovitch U, Shoenfeld Y: Autoimmunity and heart diseases: Pathogenesis and diagnostic criteria. Arch Immunol Ther Exp 57: 95–104, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Gloerich J, Ruiter JP, van den Brink DM, Ofman R, Ferdinandusse S, Wanders RJ: Peroxisomal trans-2-enoyl-CoA reductase is involved in phytol degradation. FEBS Lett 580: 2092–2096, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Brazin KN, Mallis RJ, Fulton DB, Andreotti AH: Regulation of the tyrosine kinase Itk by the peptidyl-prolyl isomerase cyclophilin A. Proc Natl Acad Sci U S A 99: 1899–1904, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sen S, Zhou H, White RA: A putative serine/threonine kinase encoding gene BTAK on chromosome 20q13 is amplified and overexpressed in human breast cancer cell lines. Oncogene 14: 2195–2200, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Crane R, Gadea B, Littlepage L, Wu H, Ruderman JV: Aurora A, meiosis and mitosis. Biol Cell 96: 215–229, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Gloor JM, Sethi S, Stegall MD, Park WD, Moore SB, DeGoey S, Griffin MD, Larson TS, Cosio FG: TG: Subclinical incidence and association with alloantibody. Am J Transplant 7: 2124–2132, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Zhou ZH, Tzioufas AG, Notkins AL: Properties and function of polyreactive antibodies and polyreactive antigen-binding B cells. J Autoimmun 29: 219–228, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zelenay S, Moraes Fontes MF, Fesel C, Demengeot J, Coutinho A: Physiopathology of natural auto-antibodies: The case for regulation. J Autoimmun 29: 229–235, 2007 [DOI] [PubMed] [Google Scholar]
- 40. Cosio FG, Gloor JM, Sethi S, Stegall MD: TG Am J Transplant 8: 492–496, 2008 [DOI] [PubMed] [Google Scholar]
- 41. Abrass CK: Cellular lipid metabolism and the role of lipids in progressive renal disease. Am J Nephrol 24: 46–53, 2004 [DOI] [PubMed] [Google Scholar]
- 42. Kasiske BL, O'Donnell MP, Cleary MP, Keane WF: Treatment of hyperlipidemia reduces glomerular injury in obese Zucker rats. Kidney Int 33: 667–672, 1988 [DOI] [PubMed] [Google Scholar]
- 43. Keane WF, Mulcahy WS, Kasiske BL, Kim Y, O'Donnell MP: Hyperlipidemia and progressive renal disease. Kidney Int Suppl 31: S41–S48, 1991 [PubMed] [Google Scholar]
- 44. Zager RA, Andoh T, Bennett WM: Renal cholesterol accumulation: A durable response after acute and subacute renal insults. Am J Pathol 159: 743–752, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zager RA, Johnson AC, Hanson SY: Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int 67: 111–121, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Valujskikh A, Li XC: Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. J Am Soc Nephrol 18: 2252–2261, 2007 [DOI] [PubMed] [Google Scholar]
- 47. Valujskikh A, Lakkis FG: In remembrance of things past: Memory T cells and transplant rejection. Immunol Rev 196: 65–74, 2003 [DOI] [PubMed] [Google Scholar]
- 48. Casciola-Rosen LA, Anhalt G, Rosen A: Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med 179: 1317–1330, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Clancy RM, Buyon JP: More to death than dying: Apoptosis in the pathogenesis of SSA/Ro-SSB/La-associated congenital heart block. Rheum Dis Clin North Am 30: 589–602, 2004 [DOI] [PubMed] [Google Scholar]
- 50. Buyon JP, Clancy RM: Autoantibody-associated congenital heart block: TGFbeta and the road to scar. Autoimmun Rev 4: 1–7, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Akalin E, Dinavahi R, Dikman S, de Boccardo G, Friedlander R, Schroppel B, Sehgal V, Bromberg JS, Heeger P, Murphy B: TG may occur in the absence of donor-specific antibody and C4d staining. Clin J Am Soc Nephrol 2: 1261–1267, 2007 [DOI] [PubMed] [Google Scholar]
- 52. Vongwiwatana A, Gourishankar S, Campbell PM, Solez K, Halloran PF: Peritubular capillary changes and C4d deposits are associated with TG but not IgA nephropathy. Am J Transplant 4: 124–129, 2004 [DOI] [PubMed] [Google Scholar]
- 53. Al Aly Z, Yalamanchili P, Cortese C, Salinas-Madrigal L, Bastani B: C4d peritubular capillary staining in chronic allograft nephropathy and TG: An uncommon finding. Transpl Int 18: 800–805, 2005 [DOI] [PubMed] [Google Scholar]
- 54. Nankivell BJ, Borrows RJ, Fung CL, O'Connell PJ, Allen RD, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 [DOI] [PubMed] [Google Scholar]
- 55. Jin ZG, Lungu AO, Xie L, Wang M, Wong C, Berk BC: Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol 24: 1186–1191, 2004 [DOI] [PubMed] [Google Scholar]
- 56. Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, 3rd, Su AI: BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 10: R130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Das AK, Uhler MD, Hajra AK: Molecular cloning and expression of mammalian peroxisomal trans-2-enoyl-coenzyme A reductase cDNAs. J Biol Chem 275: 24333–24340, 2000 [DOI] [PubMed] [Google Scholar]