Abstract
Hepatocyte transplantation to treat liver disease is largely limited by the availability of useful cells. Amniotic epithelial cells (hAECs) from term human placenta express surface markers and genes characteristic of embryonic stem cells and have the ability to differentiate into all three germ layers, including tissues of endodermal origin (i.e. liver). Thus, hAECs could provide a source of stem cell-derived hepatocytes for transplantation. We investigated the differentiation of hAECs in vitro and after transplantation into the liver of SCID/Beige mice. Moreover, we tested the ability of rat amniotic epithelial cells (rAECs) to replicate and differentiate upon transplantation into a syngenic model of liver repopulation. In vitro results indicate that the presence of extracellular matrix proteins together with a cocktail of growth factors, cytokines and hormones are required for differentiation of hAECs into hepatocyte-like cells. Differentiated hAECs expressed hepatocyte markers at levels comparable to those of fetal hepatocytes. They were able to metabolize ammonia, testosterone and 17α-hydroxyprogesterone caproate, and expressed inducible fetal cytochromes. After transplantation into the liver of Retrorsine (RS) treated SCID/beige mice, naïve hAECs differentiated into hepatocyte-like cells which expressed mature liver genes such as cytochromes, plasma proteins, transporters and other hepatic enzymes at levels equal to adult liver tissue. When transplanted in a syngenic animal pretreated with RS, rAECs were able to engraft and generate a progeny of cells with morphology and protein expression typical of mature hepatocytes.
Conclusion
amniotic epithelial cells possess the ability to differentiate into cells with characteristics of functional hepatocytes, in vitro and in vivo, thus representing a useful and non controversial source of cells for transplantation.
Keywords: Placenta, Stem Cells, Hepatocyte-like, Hepatocytes
INTRODUCTION
Regenerative medicine is a growing research field, which attempts to exploit and maximize the potential for repair and/or regeneration in organs and tissues. As part of this strategy, isolated cells, including stem cells, are increasingly being considered as a possible therapeutic tool for the management of human disease, including liver disease. Currently, the only effective therapy for end-stage liver disease is whole organ transplantation; however, this clinical procedure involves high costs, high morbidity and is severely limited by the shortage of donors
Hepatocyte transplantation has been proposed as a method to support hepatic function in acute or chronic liver failure and as a cell therapy for metabolic diseases in the liver (1).
An impediment to clinical hepatocyte transplantation is the limited availability of hepatocytes. The normal source of cells for hepatocyte transplants are livers with > 50% steatosis, vascular plaques or other factors which render the tissue unsuitable for whole organ transplantation (2–7). The isolation of viable and useful cells from discarded organs has made possible the small proof of concept studies in humans (2, 3, 8). A wider use of hepatocyte transplants will require alternative and more reliable sources of cells. Xenotransplants (9), immortalized human hepatocytes (10, 11) and stem cell or induced pluripotent stem cell-derived hepatocytes (12–15) have been proposed as alternative sources of cells for clinical transplants, research and toxicology studies (16).
The placenta represents a promising source of cells for regenerative medicine because of the phenotypic plasticity of the cell types that can be isolated from this tissue (17–19). We previously reported that human amniotic epithelial cells (hAECs) from term placenta have stem cell characteristics typical of embryonic stem cells (ESCs) (20). Under defined culture conditions hAECs differentiate into cell types normally originating from all three germ layers (20, 21).
The placenta is a non-controversial source for stem cells that is readily available. Moreover, unlike ESCs, hAECs are not tumorigenic upon transplantation (20). Several reports indicate that the amniotic membrane and amniotic epithelial cells do not induce immune reaction when transplanted (22, 23). These are evident advantages for the potential clinical use of this stem cell source.
In the last decade, several reports have described differentiation, to different extents, of various stem cell types towards a hepatocyte-like phenotype (13–15). However, differentiation of hAECs into functional hepatocytes has not been reported so far.
The aim of this study is to investigate the ability of hAECs to differentiate into functional hepatocytes. To this end, responsiveness of hAECs to various treatments in culture was tested. In vivo transplants of naïve amnion-derived cells of human or rat origin were also evaluated.
MATERIALS AND METHODS
Isolation and maintenance of hAECs
hAECs were isolated and cultured as previously described (24). Discarded placentas from uncomplicated caesarean resections at 37–40 weeks of gestational age were obtained from Magee-Women’s Hospital, Pittsburgh, with University of Pittsburgh institutional review board approval. Viability ranged from 90 to 97%. hAECs were cultured in Dulbecco's Modified Eagle Medium (DMEM high glucose, Lonza, Walkersville, MD) with standard supplements (Std) defined as follows: 2 mM L-glutamine, 1% non-essential amino acids, 55 μM 2-mercaptoethanol, 1 mM sodium pyruvate (Gibco, Grand Island, NY). For maintenance of hAECs, DMEM Std was also supplemented with 10% fetal bovine serum (FBS) and 10 ng/ml Epidermal growth factor (EGF, BD Bioscience, Franklin Lakes, NJ).
Pretreatment with Activin-A
hAECs were kept for 3 days in DMEM Std + 10% FBS + 10ng/mL EGF right after isolation, then seeded on 6-well plates at a density of 1.5 × 106 cells/well and treated for endodermal differentiation in serum free DMEM Std ± 100ng/mL Activin-A (Peprotech, Rocky Hill, NJ) for two days, and 0.2% BCS for two more days. Samples were harvested for real-time Reverse Transcriptase-PCR (qRT-PCR). In a second experiment, after Activin-A pretreatment, hAECs were treated for hepatic differentiation in Iscove's Modified Dulbecco's Medium (IMDM, Lonza) Std + 5% FBS + 10ng/mL EGF + 10ng/mL basic Fibroblast Growth Factor (FGF-2) + 10ng/mL Hepatocyte Growth Factor (HGF) (both from Peprotech) + 10−6M Dexamethasone (Dex, Lonza) for 28 days. Samples were harvested at different time points for qRT-PCR.
Mouse co-culture
C57BL/6 Mouse Hepatocytes (mHeps) were isolated with a two-step collagenase perfusion as previously described (25). mHeps were seeded on collagen-coated 6-well plates at a density of 0.5 × 106 cells/well. After 2hrs the medium was removed and plates were washed. Freshly isolated hAECs were seeded on top of mHeps at a density of 0.75 × 106 cells/well and kept in IMDM Std + 5%FBS + 10ng/ml EGF + 1uM Dex for 48hrs. Medium was then switched to MGM, a modified version of Hepatocyte Growth Medium (HGM) optimized for mouse hepatocyte replication and maintenance. This medium is MEM-based, rather than DMEM-based, contains no nicotinamide and 1/10 the Dex of HGM. Cultures were kept for a total of 16 days. Cells were then treated for Cytochrome P450 (CYP) induction and testosterone metabolism was measured. Samples were then harvested for qRT-PCR.
Hepatic differentiation with Extracellular Matrix substrates
Porcine liver-derived extracellular matrices (L-ECM) was prepared as previously described (26). 12-well plates were coated with 200μl (6mg/ml) of either Matrigel (BD Biosciences) or L-ECM. Gels were allowed to polymerize and hAECs were seeded at a density of 0.75 × 106 cells/well in DMEM Std + 10% FBS + 10ng/ml EGF and kept for 24 hrs. The cultures were then overlayed each with 0.44mg/ml of the respective matrix and kept for another 24 hrs. At day 2 the medium was switched to IMDM Std + 10% FBS + 10ng/ml EGF + 10ng/ml FGF-2 for 48 hrs and then supplemented with 20ng/ml HGF, 1μM Dex, 1X Insulin/Transferrin/Selenium (Gibco) for the following 5 days. For an additional week the treatment was maintained with the exception of FGF-2 which was replaced by 20ng/ml Oncostatin-M (Peprotech). In some experiments, cells were then treated for a further week for CYP induction and metabolic assays were performed. Samples were harvested at different time points for qRT-PCR.
Metabolic assays
Cytocrome P450 induction and metabolic activity in differentiated hAECs were assessed as described in supplemental material.
Animals
All animals were maintained on daily cycles of alternating 12h light-darkness with food and water available ad libitum. They were fed Purina Rodent Lab Chow diet throughout the experiment and received humane care according to the criteria outlined in the National Institutes of Health Publication 86-23, revised 1985. Animal studies were reviewed and approved by the University of Pittsburgh's Institutional Animal Care and Use Committee (mouse experiments) and by the University of Cagliari Ethical Comitee (rat experiments).
Mouse Transplants
SCID/Beige male mice 8–9 week-old were given three intraperitoneal (IP) injections of 70mg/kg Retrorsine (RS) (Sigma-Aldrich, St. Louis, MO) 1 week apart. Four weeks later, 60% hepatectomy was performed and 0.5 × 106 freshly isolated hAECs were injected via spleen the following day. Recipient animals were sacrificed 6 months after cell transplant. Livers were snap-frozen and utilized for DNA and RNA analysis. DNA analysis was performed as previously described (27).
Rat transplants
To follow the fate of donor cells into the recipient liver, the dipeptidyl peptidase type IV-deficient (DPPIV−) rat model was used (28). Donor amniotic epithelial cells (rAECs) were isolated from Fisher 344 (F344) wild type (DPPIV-expressing) pregnant rats at 16–18 days of gestational age. Recipient animals, DPPIV− F344 female rats 4 weeks old, were given two IP injections of 60mg/kg RS, 2 weeks apart. Four weeks later, 2/3 hepatectomy was performed and 3 × 106 freshly isolated rAECs were injected via a mesenteric vein. Animals were sacrificed 2, 6 and 12 months after cell transplant. Livers were snap-frozen and utilized for immunofluorescence analysis.
RNA Isolation, RT-PCR and Real-Time qPCR
Total RNA was isolated, and analyzed as described in supplemental material.
Immunofluorescence
Immunofluorescent staining of frozen liver tissue sections was performed as described in supplemental material.
RESULTS
Activin-A pretreatment is not required for hepatic differentiation of hAECs
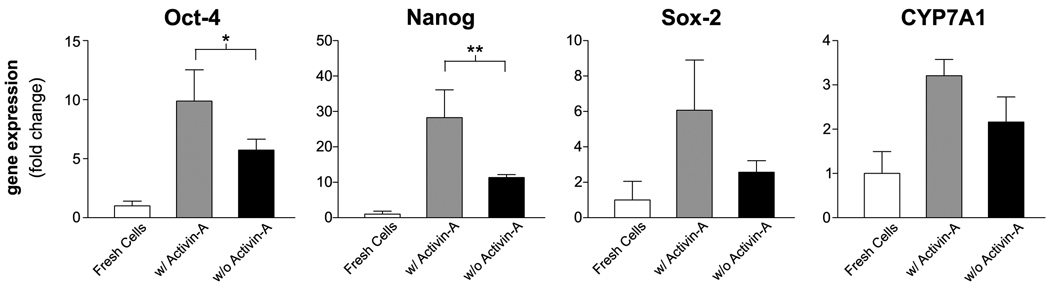
We examined the effects of a four day treatment with Activin-A on the endodermal commitment of hAECs (29). The expression of endodermal markers such us FOXA2, SOX17, or the mesendodermal marker Brachyury was not detected. Stem cell marker gene expression was not decreased but rather enhanced in hAECs after Activin-A exposure (Figure 1). The expression of CYP7A1, a mark of definitive endoderm (30), was slightly increased after Activin-A treatment. We also examined the long term effects of the Activin-A pretreatment on a 35-day hepatic-differentiation protocol. The results showed no improvement in gene expression of liver specific genes as compared with the untreated control (Supplemental Figure 1).
Figure 1. Activin-A pretreatment is not required for hepatic differentiation of hAECs.
Gene expression of hAECs after a 4 day treatment in the presence or absence of 100ng/ml Activin-A. mRNA levels are expressed as arbitrary numbers normalized to Cyclophilin-A and relative to freshly isolated cells. *P < 0.05; **P < 0.005.
Co-culture of hAECs with Mouse hepatocytes improves hepatic differentiation of hAECs
In order to determine the effects of liver microenvironment on hepatic commitment, hAECs were cultured with adult mouse hepatocytes for 16 days.
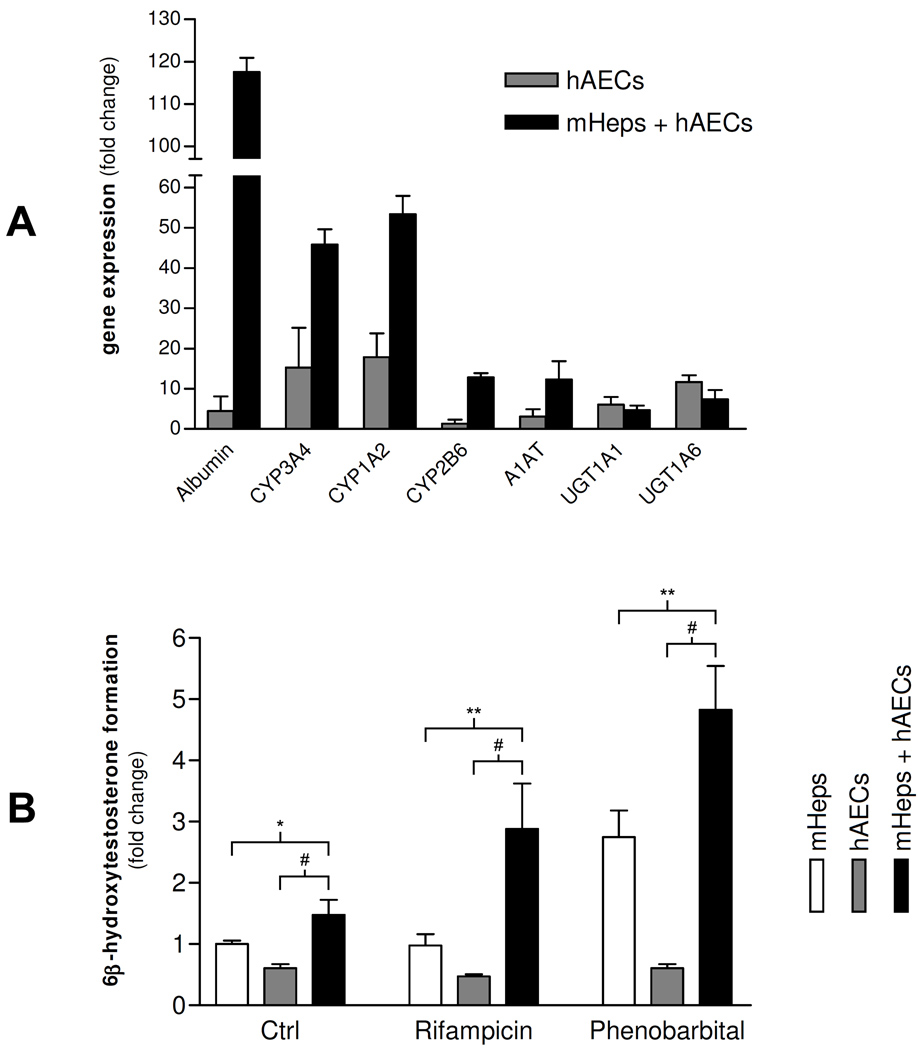
Gene expression analysis was performed at the end of the experiment, utilizing human specific primer/probes. mRNA levels for mature liver genes such as Albumin, CYP3A4, 1A2, 2B6 and alpha-1 anti-trypsin (A1AT) were strongly increased in samples co-cultured with hepatocytes as compared to hAECs alone (Figure 2A).
Figure 2. Co-culture of hAECs with Mouse hepatocytes improves hepatic differentiation of hAECs.
(A) Gene expression of hAECs after co-culture with mouse hepatocytes (mHeps) at day 16. mRNA levels expressed as arbitrary numbers normalized to Cyclophilin-A and relative to mRNA levels at day 1. (B) Testosterone metabolism of hAECs after co-culture with mHeps at day 20, after 3-days induction with Rifampicin and Phenobarbital. Results measured by HPLC and expressed as 6β-hydroxytestosterone metabolite formation, relative to mHep control sample. *P < 0.05; **P < 0.005; #P < 0.001.
To determine if the cells possessed metabolic activity, testosterone (TE) metabolism to its 6β-hydroxy metabolite was measured (Figure 2B). This is a CYP3A4 mediated activity which is expressed in mature hepatocytes and is induced in mature human hepatocytes by prior exposure to Rifampicin (Rif) or Phenobarbital (PB) (31).
After a 3-day induction, TE metabolism by mouse hepatocytes alone was induced by PB, while Rif-treated samples showed TE metabolism levels comparable to those of untreated controls. Rif, in fact, is a poor inducer of 3A activity in rodent hepatocytes (32) and it is a specific inducer for human hepatocytes. hAECs alone showed no difference in metabolism between treated and untreated samples. However, when hAECs were co-cultured with mouse hepatocytes and then exposed to the inducing agents, samples treated with Rif and PB displayed increased TE metabolism as compared to untreated controls, demonstrating the presence of mature metabolic enzyme activity in differentiated hAECs.
Basement membrane matrix proteins influence hepatic differentiation of hAECs
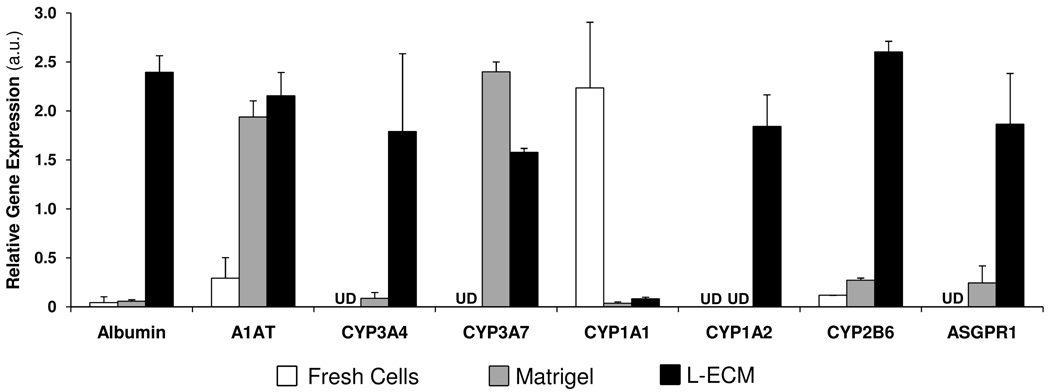
We examined the effects of different extracellular matrix (ECM) preparations on hepatic differentiation of hAECs. After a 3-week differentiation protocol, gene expression of major liver genes, such as Albumin, A1AT, CYP3A4, 3A7, 1A2, 2B6 and the Asialoglycoprotein receptor 1 (ASGPR1) was up-regulated in treated samples, as compared to freshly isolated hAECs (Figure 3). Particularly those samples that were cultured on L-ECM showed the highest levels of expression of mature liver genes. CYP1A1is a gene expressed at low levels in most adult liver samples, unless the person was a smoker or otherwise induced CYP1A levels with diet or drug exposure. However, CYP1A1 is expressed in many non-hepatic tissues and was highly expressed in freshly isolated hAECs and decreased after differentiation on ECMs.
Figure 3. Basement membrane matrix proteins influence hepatic differentiation of hAECs.
Gene expression of hAECs after two week of differentiation. hAECs were culture on Matrigel or liver-derived ECM (L-ECM). mRNA levels expressed as arbitrary numbers normalized to Cyclophilin-A.
Liver-Derived extracellular matrix efficiently promotes differentiation of hAECs into hepatic cells with metabolic activity and inducible enzymes
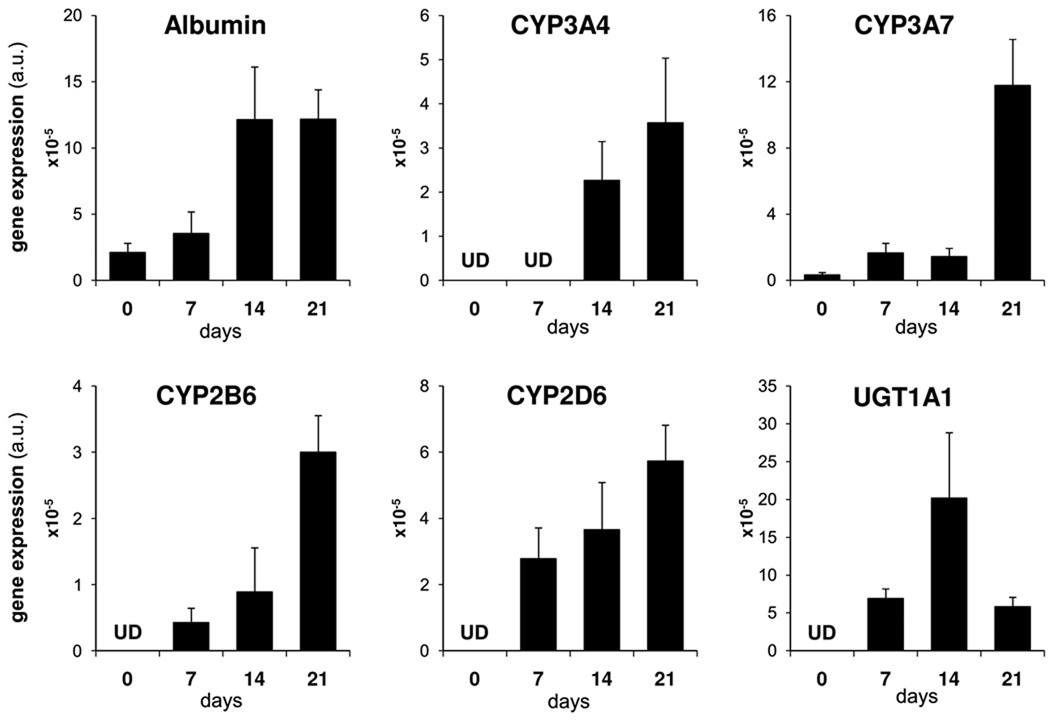
L-ECM was utilized in a second set of experiments to verify the changes in gene expression over a three week period (Figure 4). Albumin, CYP3A4, 3A7, 2B6 and 2D6 mRNA levels increased over time with a peak at day 21.
Figure 4. Liver-Derived extracellular matrix promotes differentiation of hAECs into hepatic cells over time.
Gene expression of hAECs over a three week differentiation protocol on L-ECM. mRNA levels expressed as arbitrary numbers normalized to Cyclophilin-A.
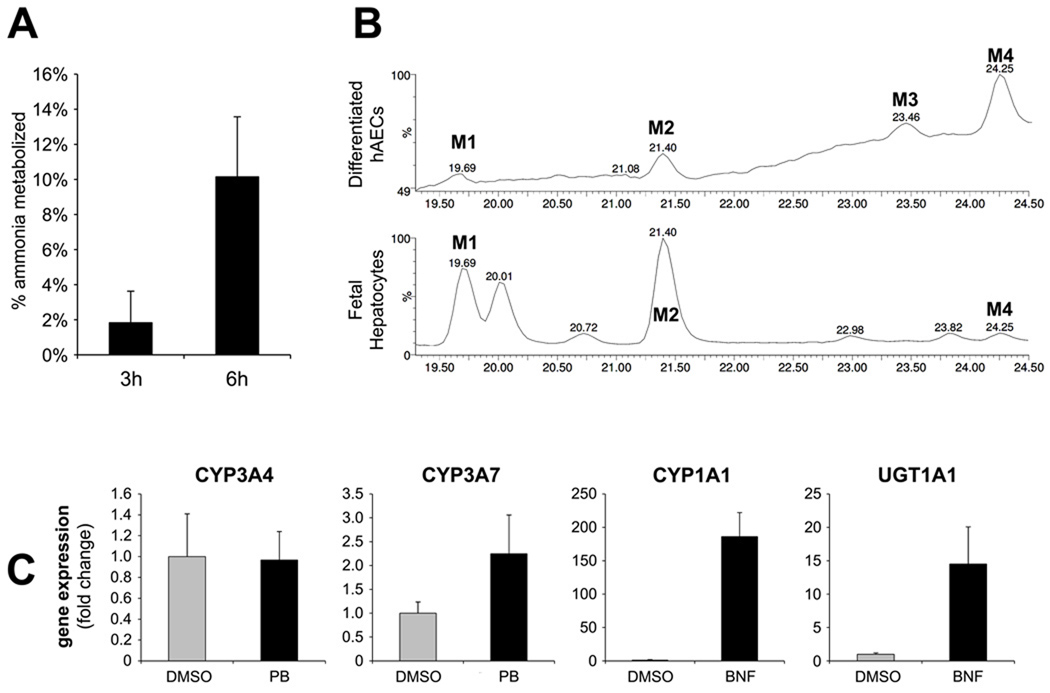
At the end of the three-week differentiation protocol, metabolic activity was measured. The ability to metabolize ammonia is a characteristic of mature hepatocytes. Differentiated hAECs were capable of metabolizing ammonia (1mM initial concentration) by 2% at 3 hours and 10% at 6 hours (Figure 5A).
Figure 5. Liver-Derived extracellular matrix efficiently promotes differentiation of hAECs into hepatic cells with metabolic activity and inducible enzymes.
(A) percent of ammonia metabolized by differentiated hAECs in a time period of 3 and 6 hours; (B) LC-MS chromatograms of 17-OHPC and its metabolites derived from incubation of 17-OHPC with differentiated hAECs and fresh human fetal hepatocytes. Incubation of 17-OHPC with differentiated hAECs generated 4 metabolites of which 2 were the major metabolites (M2 and M4). Incubation of 17-OHPC with human fetal hepatocytes generated numerous metabolites of which M1, M2 and M4 were common with differentiated hAECs. (C) Gene expression levels of hAEC-derived hepatic cells after 3-days induction with Phenobarbital (PB) or β-naphtoflavone (BNF). mRNA levels expressed as arbitrary numbers normalized to Cyclophilin-A and relative to untreated control (DMSO).
Since differentiation of stem cells to hepatocyte-like cells would likely pass through a fetal liver-like stage we investigated the metabolism of a compound known to be metabolized by both fetal and adult liver, but to different metabolites depending on the age of the tissue donor. 17-hydroxyprogesterone caproate (17-OHPC) is metabolized by CYP3A enzymes, in both human adult and fetal hepatocytes (33, 34). The ability of differentiated hAECs to metabolize 17-OHPC was assessed by LC-MS. Incubation with 17-OHPC generated 4 detectable metabolites (M1-M4) (Figure 5B, top). Metabolites at similar retention times were observed in fetal hepatocytes (Figure 5B, bottom).We have previously reported metabolites M1 and M2 to be isoform specific and are produced by CYP3A7, the CYP3A isoform expressed mainly in fetal liver (33), while the M1 and M2 metabolites were not produced in incubations with adult hepatocytes (data not shown). The production of the M1 and M2 metabolites suggests that differentiated hAECs expressed the fetal isoform, CYP3A7. The expression of CYP3A7 was confirmed by qRT-PCR in hAECs. The identity of metabolite of M3 could not be elucidated due to the low amounts produced.
In mature liver CYP3A enzymes are induced by PB, while CYP1A and UGT1A enzymes are induced after treatment with β-naphtoflavone (BNF) (31). In order to invesitigate the inducibility of these enzymes on differentiated hAECs, the cells were treated for 3 days with PB and BNF. No increase in gene expression was measured for CYP3A4, while CYP3A7 was induced by ~2 fold with PB (Figure 5C). A 186-fold induction of CYP1A1 and a ~15-fold induction of UGT1A1 were measured after treatment with BNF.
Naïve hAECs differentiate into mature hepatocytes upon transplantation into SCID/beige mouse liver
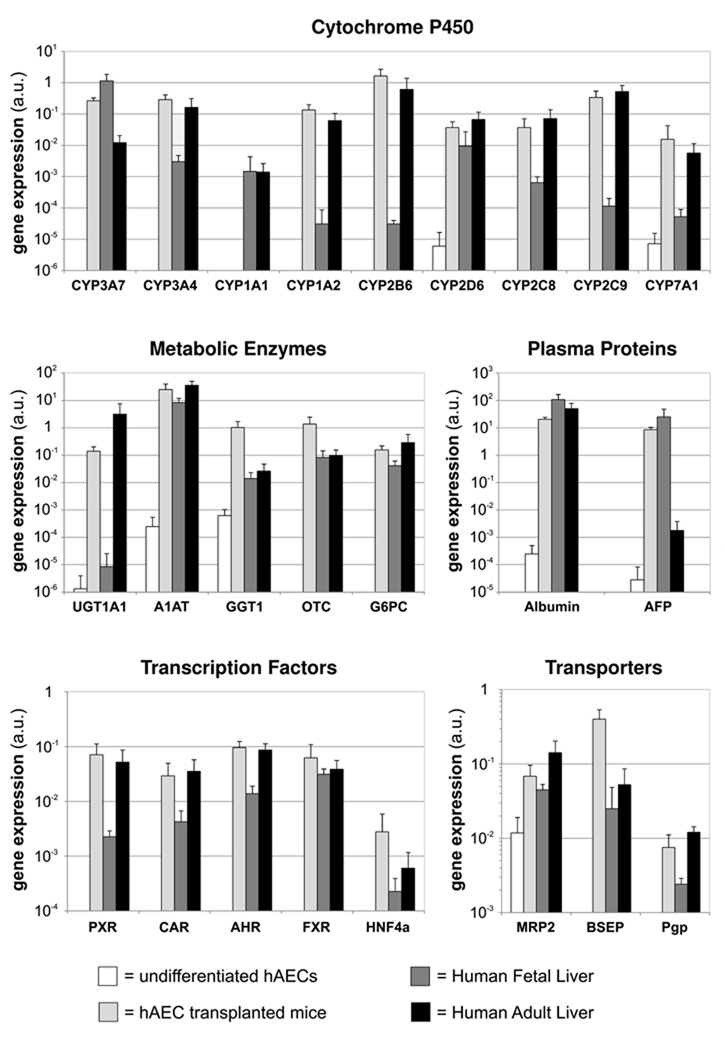
The prior studies showing hepatic induction of hAECs when they were co-cultured with mouse hepatocytes suggested that the close proximity of hepatocytes or the liver microenvironment, in general, could induce hepatic differentiation of hAE cells. To examine the influence of the liver microenvironment in vivo on hAECs differentiation, freshly isolated naïve hAECs were transplanted into the liver of SCID/beige mice pretreated with RS. Six months after transplantation, human DNA was detected in mouse livers, confirming the engraftment of hAECs. Repopulation levels in both hAECs transplanted animals and control animals (receiving human adult hepatocytes) ranged from 0.1 to 1% as assessed by human DNA quantification (data not shown). The differentiation of hAECs to mature hepatocyte-like cells was investigated by qRT-PCR utilizing human specific primer/probes. Most mature liver genes were expressed at levels comparable to those of authentic human adult livers, including the major CYP genes, other metabolic enzymes, plasma proteins, and hepatocyte enriched transcription factors and genes encoding hepatic transported proteins (Figure 6).
Figure 6. Naïve hAECs differentiate into mature hepatocytes upon transplantation into SCID/beige mouse liver.
Gene expression of hAECs 6 months after transplantation into mouse host livers. Comparison with undifferentiatied hAECs, human fetal liver and human adult liver. mRNA levels detected with human specific primer/probes and expressed as arbitrary numbers normalized to Cyclophilin-A.
Naïve rAECs integrate and form clusters of mature hepatocytes upon transplantation into syngenic rats
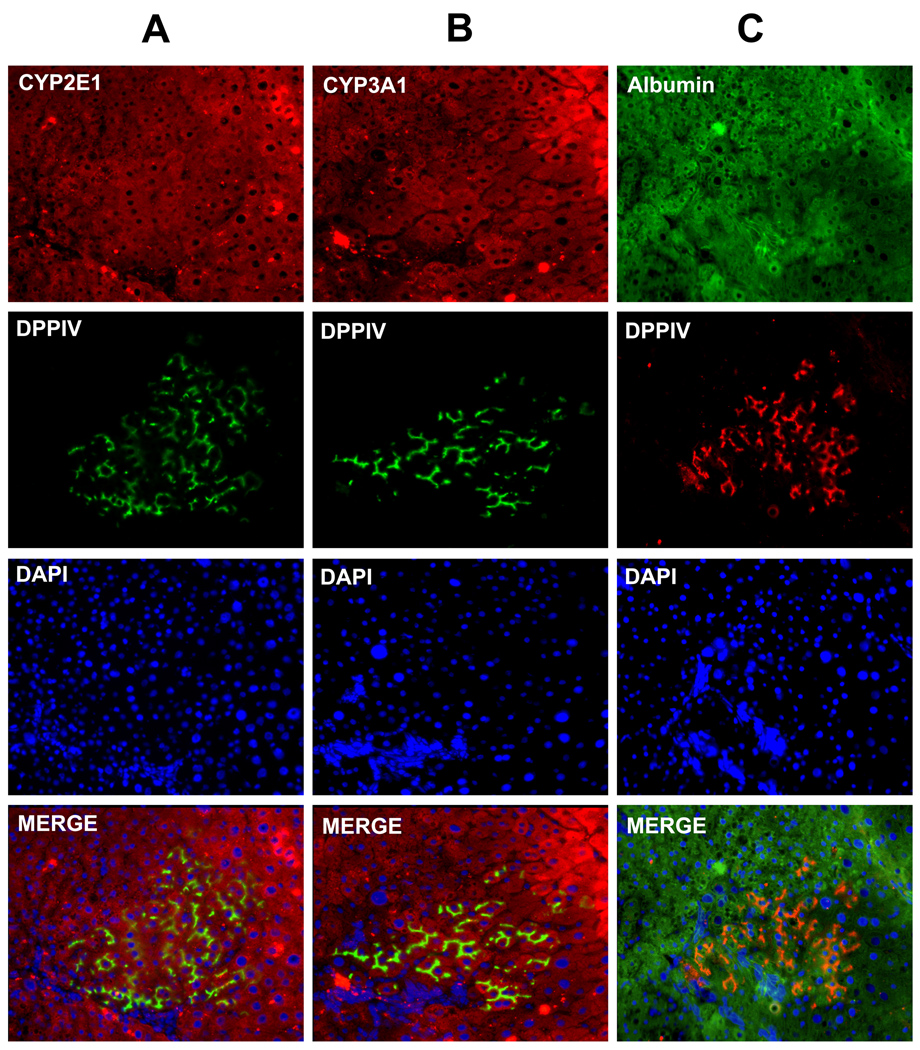
RS pretreatment of the liver is known to be less effective on mice than on rats. In fact, very low levels of repopulation were observed in the mouse transplants. In order to test the ability of amniotic cells to engraft and replicate in the liver, a syngenic rat model was investigated (35). To avoid the xenotransplantation of hAECs, rAECs were isolated from term pregnant rats and immediately transplanted into the liver of a syngenic animal pretreated with RS. Recipient animals were DPPIV− while rAECs were isolated from DPPIV+ tissues. This way, it was possible to observe clusters of donor-derived DPPIV+ cells at 2, 6, and 12 months after transplant. These cells showed a pattern of expression of DPPIV typical of mature hepatocytes (Figure 7). Donor-derived cell clusters comprised up to ~4000 cells at 12 months. These clusters were positive for CYP2E1, 3A1 and Albumin (Figure 7) with a pattern of expression indistinguishable from the surrounding liver.
Figure 7. Naïve rAECs integrate and form clusters of mature hepatocytes upon transplantation into syngenic rats.
Immunofluorescence staining of serial frozen section of rat livers after transplantation of rAECs. Recipient animals were DPPIV− while transplanted rAECs were isolated from DPPIV+ tissues. Clusters of positive differentiated cells can be found into the host liver. (A) Double stain for DPPIV (green) and CYP2E1 (red). (B) Double stain for DPPIV (green) and CYP3A1 (red). (C) Double stain for DPPIV (red) and Albumin (green). Differentiated rAECs expressed hepatocyte markers at levels comparable to the surrounding liver.
DISCUSSION
The use of hepatocyte transplantation as a clinical alternative to whole organ transplant has been limited by the lack of sufficient numbers of functionally proficient cells. Stem cell derived hepatocytes have been proposed as an alternative source of cells for transplantation. Several research groups have established protocols to differentiate various stem cell types into definitive endoderm, and then into cells with hepatocyte characteristics (14, 36). These reports describe varying degrees of success, and researchers are still confronted with ethical issues related to the use of stem cells derived from human embryos or fetuses. Placenta-derived stem cells are isolated from a tissue that is normally discarded after a live birth. Moreover, they retain characteristics of ESCs, thus representing a novel source of cells for clinical application. It is commonly accepted that ESCs need to differentiate to definitive endoderm prior to further differentiation to endoderm-derived cell types (36). Activin-A, a member of the TGFβ family, can have different effects on stem cells, depending on their source and stage of differentiation. Several investigators reported that endodermal differentiation of some ESC lines is enhanced by exposure to Activin-A (29, 37) while recent work from other groups suggests that Activin/Nodal signaling might inhibit the early stages of ESC differentiation in vitro, by playing a key role in maintaining an undifferentiated state (38–40).
In these studies, hAECs did not express endoderm markers after treatment with Activin-A, but rather upregulated the expression of stem cell genes (Figure 1). Also, Activin-A did not improve long term hepatic differentiation of hAECs, suggesting that this regulatory protein is not required for endoderm differentiation of hAECs (Supplemental Figure 1).
The idea that the liver microenvironment may be critical for the induction of hepatic differentiation has been supported by the results obtained co-culturing ESCs with different hepatic cell types (i.e. hepatocytes, stellate cells) (41).. Co-culture with mouse hepatocytes improved hepatic differentiation of hAECs (Figure 2A), which were shown to express markers of mature hepatocytes along with metabolically active and inducible CYP3A enzymes (Figure 2B). Co-culture with mouse hepatocytes is a difficult and inconvenient way to induce hepatic differentiation of hAECs. We surmised that hepatocyte conditioned media might provide the same inductive influence in a protocol more easily standardized. Unfortunately, no strong hepatic inductive effect in gene expression was observed with human hepatocyte conditioned media (data not shown) which suggests that interaction with neighboring cells enhances hepatic commitment of hAECs.
When cell to cell interaction is lost, basement membrane matrix proteins are critical to the maintenance of a differentiated state in primary human hepatocytes (26). Extracellular matrices (ECM) were utilized as a substrate for differentiation of hAECs (Figure 3). Interestingly, matrigel, a commercial matrix preparation which is known to enhance and maintain differentiation of adult hepatocytes, was ineffective at inducing hepatic differentiation of hAE cells. However, L-ECM was shown to strongly induce expression of mature hepatocyte marker genes and activities (Figure 3 and 4). Differentiated hAECs were able to metabolize Ammonia, 17-OHPC and possessed inducible CYP3A and 1A enzymes (Figure 5). The in vitro results suggest that the presence of extracellular matrix proteins together with a cocktail of growth factors, cytokines and hormones are required for proficient differentiation of hAECs into hepatocyte-like cells.
Although these results are promising, the expression levels of hepatocyte genes of in vitro differentiated hAECs were closer to those of fetal cells rather than adult hepatocytes (supplemental table 2). Although expression is low, hAECs expressed genes characteristic of adult human liver. CYP 3A4 and 1A2 are typically expressed in adult hepatocytes, while 3A7 and 1A1 are more highly expressed in fetal cells (42). Hepatocyte-like cells derived from hAECs also metabolized drugs in a manner similar to fetal human hepatocytes, as shown by the metabolism of 17-OHPC (Figure 5B). A characteristic of some of the CYP and phase II enzymes is that their expression can be induced by prior exposure to prototypical inducing agents (31). Differentiated hAECs expressed CYP 3A7, 1A1 and UGT1A1 which were induced by exposure to PB or BNF (Figure 5C); however, in the current studies, CYP 3A4 and 1A2 were not responsive to the treatment unless the hAECs were co-cultured with mouse hepatocytes (Figure 2) suggesting that the liver microenvironment significantly contributes to the hepatic induction of the hAECs. Extremely important in the interpretation of these results with the mouse co-culture experiments is the observation that prior exposure of the hAECs cocultures to Rif induced the metabolism of TE to the 6-β-hydroxy metabolite, a standard assay for human CYP3A4 (31). Since Rif is a specific inducer of human CYP3A4 with little or no activity toward mouse CYP3A genes/activities (32), these results clearly indicate that the CYP3A metabolic activity observed in these experiments results from the human cells present in the cultures. Another compound, PB, which induces both the mouse and human CYP3A genes shows a moderate induction of metabolic activity in the cultures of mouse alone, and a more robust activity when the human cells are present. These results strongly suggest that the difference in metabolic activity between the hAEC/mouse co-cultures and the cultures with only mouse cells can be attributed to the induction of CYP3A4 in the hAECs.
Given only the partial differentiation of hAECs to hepatocyte-like cells, in vitro, and the strong inductive influence of the mouse hepatocyte co-culture experiments, we examined the fate of the cells following transplantation into mouse liver. Profiling of genes normally expressed in adult human liver, with PCR primers that are specific for human transcripts, revealed a mature level of expression of 23 out of 24 genes examined (Figure 6) in the hAECs in mouse liver at 6 months following transplantation. These results suggest that the hAECs can differentiate to mature hepatocyte-like cells following transplantation, in vivo. In support of this hypothesis, hAECs transplants were recently shown to be effective for the correction of the serum amino acid and brain neurochemical imbalances normally observed in a mouse model of Maple Syrup Urine Disease (43).
The well characterized Retrorsine-based model of liver repopulation was used for the in vivo studies described above (35, 44); however only low levels of repopulation with human cells were observed (<3%). RS treatment is known to be less effective on mice than on rats. In order to test the ability of amniotic cells to engraft and replicate in the liver, a syngenic rat model was used (35). RS-treated DPPIV− rats were transplanted with DPPIV+, but otherwise syngenic rAECs. Large clusters of DPPIV+, rAEC-derived hepatocyte-like cells were observed, indicating that rAECs were able to engraft and incorporate into the parenchyma to form cells with morphology typical of mature hepatocytes. These cells were positive for Albumin, CYP2E1 and 3A1 (Figure 7). Based on the results obtained in the in vivo studies, we believe that the liver microenvironment itself strongly induces hepatocyte differentiation of amniotic epithelial cells. This study demonstrates that amniotic epithelial cells differentiate, in vitro, into hepatocyte-like cells with characteristics of fetal hepatocytes while in vivo they mature into cells with a gene expression profile comparable to adult hepatocytes. Genes involved in metabolic liver disease such as OTC, A1AT and UGT1A1 and BSEP, were highly expressed in hAECs after transplantation. We suggest that hAECs represent a non controversial source of cells for liver-based regenerative medicine.
Supplementary Material
Gene expression of hAECs at different time points after hepatic induction with or without Activin-A pretreatment. mRNA levels are expressed as arbitrary numbers normalized to Cyclophilin-A.
Acknowledgments
Grant Support:
This work was supported in part by a grant from Pfizer, Inc. Studies with adult human liver and hepatocytes were supported in part by grants from the National Institutes of Health (NIH). N01-DK-7-0004/HHSN26700700004C and RC1DK086135 (SCS). Fetal human liver tissue was provided as a service from the Laboratory of Developmental Biology at the University of Washington which was supported by NIH Award Number 5R24HD0008836 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. Studies with fetal human liver were supported in part by NIH grant R01-GM081344. The content of this manuscript does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health.
List of Abbreviations
- hAECs
human Amniotic Epithelial Cells
- rAECs
rat Amniotic Epithelial Cells
- RS
Retrorsine
- ESCs
Embryonic Stem Cells
- qRT-PCR
real-time Reverse Trancriptase-PCR
- DMEM
Dulbecco's Modified Eagle Medium
- FBS
Fetal Bovine Serum
- EGF
Epidermal Growth Factor
- IMDM
Iscove's Modified Dulbecco's Medium
- FGF2
basic Fibroblast Growth Factor
- HGF
Hepatocyte Growth Factor
- Dex
Dexamethasone
- mHeps
mouse hepatocytes
- CYP
Cytochrome P450
- ECM
Extracellular Matrix
- L-ECM
liver-derived Extracellular Matrix
- HMM
Hepatocyte Maintenance Medium
- HPLC
high pressure liquid chromatography
- IP
intraperitoneal
- DPPIV
dipeptidyl peptidase type IV
- FOXA2
forkhead box A2
- SOX17
sex determining region Y-box 17
- A1AT
alpha-1 anti-trypsin
- Rif
Rifampicin
- PB
Phenobarbital
- TE
Testosterone
- ASGPR1
Asialoglycoprotein receptor 1
- 17-OHPC
17-hydroxyprogesterone caproate
- UGT1A
Uridine 5'-diphospho-glucuronosyltransferase 1 family type A
- BNF
β-naphtoflavone
- TGFβ
Transforming Growth Factor beta
- OTC
Ornithine Transcarbamylase
- BSEP
Bile Salt Export Pump
Footnotes
Potential conflict of interest: Dr. Stephen C. Strom owns stock in Stemnion, LLC.
REFERENCES
- 1.Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis. 1999;19:39–48. doi: 10.1055/s-2007-1007096. [DOI] [PubMed] [Google Scholar]
- 2.Fisher RA, Bu D, Thompson M, Tisnado J, Prasad U, Sterling R, Posner M, et al. Defining hepatocellular chimerism in a liver failure patient bridged with hepatocyte infusion. Transplantation. 2000;69:303–307. doi: 10.1097/00007890-200001270-00018. [DOI] [PubMed] [Google Scholar]
- 3.Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, Fox IJ. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111:1262–1267. doi: 10.1542/peds.111.6.1262. [DOI] [PubMed] [Google Scholar]
- 4.Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, Meroni M, et al. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317–318. doi: 10.1016/S0140-6736(02)07529-3. [DOI] [PubMed] [Google Scholar]
- 5.Strom S, Fisher R. Hepatocyte transplantation: new possibilities for therapy. Gastroenterology. 2003;124:568–571. doi: 10.1053/gast.2003.50072. [DOI] [PubMed] [Google Scholar]
- 6.Strom SC, Fisher RA, Thompson MT, Sanyal AJ, Cole PE, Ham JM, Posner MP. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63:559–569. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- 7.Nakazawa F, Cai H, Miki T, Dorko K, Abdelmeguid A, Walldorf J, Lehmann T, et al. Proceedings of Falk Symposium, Hepatocyte Transplantation. Volume 126. Lancaster, UK: Kouwer Academic Publishers; 2002. Human hepatocyte isolation from cadaver donor liver; pp. 147–158. [Google Scholar]
- 8.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 9.Nagata H, Ito M, Cai J, Edge AS, Platt JL, Fox IJ. Treatment of cirrhosis and liver failure in rats by hepatocyte xenotransplantation. Gastroenterology. 2003;124:422–431. doi: 10.1053/gast.2003.50065. [DOI] [PubMed] [Google Scholar]
- 10.Cai J, Ito M, Nagata H, Westerman KA, Lafleur D, Chowdhury JR, Leboulch P, et al. Treatment of liver failure in rats with end-stage cirrhosis by transplantation of immortalized hepatocytes. Hepatology. 2002;36:386–394. doi: 10.1053/jhep.2002.34614. [DOI] [PubMed] [Google Scholar]
- 11.Wege H, Chui MS, Le HT, Strom SC, Zern MA. In vitro expansion of human hepatocytes is restricted by telomere-dependent replicative aging. Cell Transplant. 2003;12:897–906. doi: 10.3727/000000003771000138. [DOI] [PubMed] [Google Scholar]
- 12.Miki T, Marongiu F, Ellis ECS, Dorko K, Mitamura K, Ranade A, Gramignoli R, et al. Production of Hepatocyte-Like Cells from Human Amnion. In: Dhawan A, Hughes RD, editors. Hepatocyte Transplantation. Volume 481. Humana Press; 2008. [Google Scholar]
- 13.Iwamuro M, Komaki T, Kubota Y, Seita M, Kawamoto H, Yuasa T, Shahid JM, et al. Hepatic differentiation of mouse iPS cells in vitro. Cell Transplant. 2010;19:841–847. doi: 10.3727/096368910X508960. [DOI] [PubMed] [Google Scholar]
- 14.Basma H, Soto-Gutierrez A, Yannam GR, Liu L, Ito R, Yamamoto T, Ellis E, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833–848. doi: 10.1053/j.gastro.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Davila JC, Cezar GG, Thiede M, Strom S, Miki T, Trosko J. Use and application of stem cells in toxicology. Toxicol Sci. 2004;79:214–223. doi: 10.1093/toxsci/kfh100. [DOI] [PubMed] [Google Scholar]
- 17.Parolini O, Alviano F, Bagnara GP, Bilic G, Buhring HJ, Evangelista M, Hennerbichler S, et al. Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells. 2008;26:300–311. doi: 10.1634/stemcells.2007-0594. [DOI] [PubMed] [Google Scholar]
- 18.Parolini O, Alviano F, Bergwerf I, Boraschi D, De Bari C, De Waele P, Dominici M, et al. Toward cell therapy using placenta-derived cells: disease mechanisms, cell biology, preclinical studies, and regulatory aspects at the round table. Stem Cells Dev. 2010;19:143–154. doi: 10.1089/scd.2009.0404. [DOI] [PubMed] [Google Scholar]
- 19.De Coppi P, Bartsch G, Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100–106. doi: 10.1038/nbt1274. [DOI] [PubMed] [Google Scholar]
- 20.Miki T, Lehmann T, Cai H, Stolz DB, Strom SC. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–1559. doi: 10.1634/stemcells.2004-0357. [DOI] [PubMed] [Google Scholar]
- 21.Miki T, Strom SC. Amnion-derived pluripotent/multipotent stem cells. Stem Cell Rev. 2006;2:133–142. doi: 10.1007/s12015-006-0020-0. [DOI] [PubMed] [Google Scholar]
- 22.Akle CA, Adinolfi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981;2:1003–1005. doi: 10.1016/s0140-6736(81)91212-5. [DOI] [PubMed] [Google Scholar]
- 23.Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Miki T, Marongiu F, Ellis E, S CS. Chapter 1. Isolation of amniotic epithelial stem cells. Curr Protoc Stem Cell Biol. 2007 doi: 10.1002/9780470151808.sc01e03s3. Unit 1E 3. [DOI] [PubMed] [Google Scholar]
- 25.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 26.Sellaro TL, Ranade A, Faulk DM, McCabe GP, Dorko K, Badylak SF, Strom SC. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 2010;16:1075–1082. doi: 10.1089/ten.tea.2008.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker JA, Kilroy GE, Xing J, Shewale J, Sinha SK, Batzer MA. Human DNA quantitation using Alu element-based polymerase chain reaction. Anal Biochem. 2003;315:122–128. doi: 10.1016/s0003-2697(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 28.Thompson NL, Hixson DC, Callanan H, Panzica M, Flanagan D, Faris RA, Hong WJ, et al. A Fischer rat substrain deficient in dipeptidyl peptidase IV activity makes normal steady-state RNA levels and an altered protein. Use as a liver-cell transplantation model. Biochem J. 1991;273(Pt 3):497–502. doi: 10.1042/bj2730497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 30.Asahina K, Fujimori H, Shimizu-Saito K, Kumashiro Y, Okamura K, Tanaka Y, Teramoto K, et al. Expression of the liver-specific gene Cyp7a1 reveals hepatic differentiation in embryoid bodies derived from mouse embryonic stem cells. Genes Cells. 2004;9:1297–1308. doi: 10.1111/j.1365-2443.2004.00809.x. [DOI] [PubMed] [Google Scholar]
- 31.Kostrubsky VE, Ramachandran V, Venkataramanan R, Dorko K, Esplen JE, Zhang S, Sinclair JF, et al. The use of human hepatocyte cultures to study the induction of cytochrome P-450. Drug Metab Dispos. 1999;27:887–894. [PubMed] [Google Scholar]
- 32.Kocarek TA, Schuetz EG, Strom SC, Fisher RA, Guzelian PS. Comparative analysis of cytochrome P4503A induction in primary cultures of rat, rabbit, and human hepatocytes. Drug Metab Dispos. 1995;23:415–421. [PubMed] [Google Scholar]
- 33.Sharma S, Ellis EC, Dorko K, Zhang S, Mattison DR, Caritis SN, Venkataramanan R, et al. Metabolism of 17alpha-hydroxyprogesterone caproate, an agent for preventing preterm birth, by fetal hepatocytes. Drug Metab Dispos. 2010;38:723–727. doi: 10.1124/dmd.109.029918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma S, Ou J, Strom S, Mattison D, Caritis S, Venkataramanan R. Identification of enzymes involved in the metabolism of 17alpha-hydroxyprogesterone caproate: an effective agent for prevention of preterm birth. Drug Metab Dispos. 2008;36:1896–1902. doi: 10.1124/dmd.108.021444. [DOI] [PubMed] [Google Scholar]
- 35.Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, et al. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153:319–329. doi: 10.1016/S0002-9440(10)65574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brolen G, Sivertsson L, Bjorquist P, Eriksson G, Ek M, Semb H, Johansson I, et al. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J Biotechnol. 2010;145:284–294. doi: 10.1016/j.jbiotec.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, Fehling HJ, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131:1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 38.Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- 39.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 40.Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, Hayek A. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- 41.Moore RN, Dasgupta A, Rajaei N, Yarmush ML, Toner M, Larue L, Moghe PV. Enhanced differentiation of embryonic stem cells using co-cultivation with hepatocytes. Biotechnol Bioeng. 2008;101:1332–1343. doi: 10.1002/bit.21987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hines RN, McCarver DG. The ontogeny of human drug-metabolizing enzymes: phase I oxidative enzymes. J Pharmacol Exp Ther. 2002;300:355–360. doi: 10.1124/jpet.300.2.355. [DOI] [PubMed] [Google Scholar]
- 43.Skvorak KJ, Dorko K, Hansel M, Marongiu F, Tahan V, Gibson KM, Sun Q, et al. Human Amnion Epithelial (hAE) Stem Cell Transplant Improves Disease Phenotype and Survival in the Intermediate Maple Syrup Urine Disease (iMSUD) Mouse Model. HEPATOLOGY. 2010;52 suppl:413A. [Abstract] [Google Scholar]
- 44.Laconi S, Curreli F, Diana S, Pasciu D, De Filippo G, Sarma DS, Pani P, et al. Liver regeneration in response to partial hepatectomy in rats treated with retrorsine: a kinetic study. J Hepatol. 1999;31:1069–1074. doi: 10.1016/s0168-8278(99)80320-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene expression of hAECs at different time points after hepatic induction with or without Activin-A pretreatment. mRNA levels are expressed as arbitrary numbers normalized to Cyclophilin-A.