SUMMARY
Cell polarization is essential throughout development for proliferation, migration and differentiation. However, it is not known how extracellular cues correctly orient cell polarity at distinct stages of development. Here we show that the endocytic adapter protein, Numb, previously characterized for its role in cell proliferation, subsequently plays an important role in cell migration. In neural precursors stimulated with the chemotactic factor BDNF, Numb binds to activated TrkB, the BDNF receptor, and functions both as an endocytic regulator for TrkB and as a scaffold for atypical PKC (aPKC). Thus Numb promotes BDNF-dependent aPKC activation. Interestingly, Numb is also a substrate of aPKC. When phosphorylated, Numb exhibits increased efficacy in binding TrkB and in promoting a chemotactic response to BDNF. Therefore, Numb functions in a feed-forward loop to promote chemotaxis of neural precursors, linking BDNF, an extracellular cue, to aPKC, a critical component of the intrinsic polarity machinery.
INTRODUCTION
Cell polarity is an essential process during development. Regulation of cell polarization is achieved by asymmetric distribution of key intracellular signaling molecules in response to extracellular cues (Affolter and Weijer, 2005; Bryant and Mostov, 2008; Huttenlocher, 2005; Mellman and Nelson, 2008). The intracellular regulatory molecules are highly conserved and have largely been identified by studies in genetically tractable organisms (Goldstein and Macara, 2007; Solecki et al., 2006). In contrast, a wide variety of extracellular cues can regulate cell polarity at different stages of development and in different organisms (Arimura and Kaibuchi, 2007; Barnes et al., 2008). However, the mechanisms by which extrinsic cues promote cell polarization during cellular processes such as proliferation and migration are not yet fully understood.
Among the extracellular cues that promote polarization are chemotactic factors that stimulate directed migration, a process that can readily be studied in the developing nervous system. Neural precursors are generated in specialized proliferative zones, and then migrate in a directed fashion to the location where they will become mature neurons (Hatten, 1999; Kriegstein and Noctor, 2004). During cerebellar development, granule cell precursors (GCPs) originate in the upper rhombic lip, and then migrate tangentially to form a second proliferative region, the external granule cell layer (EGL). GCPs proliferate in the EGL, then exit cell cycle and migrate radially along Bergmann glia; they traverse the molecular layer (ML) and Purkinje cell layer (PCL), and finally reach the internal granule cell layer (IGL) (Hatten, 1999, 2002). These migratory paths are guided by extracellular cues (Komuro and Yacubova, 2003), including brain-derived neurotrophic factor (BDNF), which is distributed in a gradient that increases from the EGL to the IGL (Borghesani et al., 2002; Zhou et al., 2007a). Cues such as BDNF trigger cell polarization as distinguished by correct positioning of the centrosome and asymmetric distribution of signaling modules (Barnes et al., 2008; Higginbotham and Gleeson, 2007).
Emerging evidence indicates that endocytic trafficking plays a key role in cell polarization and migration (Ulrich and Heisenberg, 2009). In migrating neural precursor cells, endosomal vesicles accumulate at the leading process (Schaar and McConnell, 2005). Real-time imaging shows that endocytic markers clathrin and dynamin are polarized toward, and localized at the leading edge of migrating cells (Rappoport and Simon, 2003). Integrin receptors colocalize with the endocytic protein dynamin at focal contacts of migrating cells, and perturbation of integrin endocytosis inhibits cell polarization and migration (Caswell and Norman, 2008; Ezratty et al., 2005; Proux-Gillardeaux et al., 2005). In carcinoma cells, a gradient of epidermal growth factor (EGF) stimulates EGF receptor (EGFR) internalization such that internalized EGFR preferentially accumulates at the side of the cell facing the EGF source (Bailly et al., 2000). Impaired endocytosis of EGFR decreases migration of Drosophila oocyte border cells (Jekely et al., 2005). Endocytic trafficking of Rac is critical for cytoskeleton changes during cell migration (Palamidessi et al., 2008). Studies from our laboratory indicate that a BDNF gradient stimulates the localization of TrkB-containing signaling endosomes in or adjacent to the leading process of migrating GCPs. Thus asymmetric distribution of activated TrkBs polarizes the cell for directional migration (Zhou et al., 2007a). However, the molecular mechanisms by which TrkB receptor endocytosis results in cell polarization and migration are not yet understood.
Here we demonstrate that Numb, an endocytic adapter protein, plays a critical role in granule cell polarization during migration, in addition to its well-described roles in asymmetric cell division and cell fate determination. Early genetic studies in Drosophila indicated that Numb functions as a cell fate determinant during mitosis, such that asymmetric segregation of Numb enables daughter cells to assume different fates (Guo et al., 1996; Rhyu et al., 1994). Similarly, in mice, Numb is expressed in dividing precursors, where it has been implicated in cell fate decision (Petersen et al., 2004; Zhong et al., 1996; Zhou et al., 2007b). However, Numb continues to be expressed in the brain long after most proliferation has ended. Studies thus far have documented a role for Numb in neuronal differentiation and maturation (Huang et al., 2005; Klein et al., 2004; Nishimura et al., 2003). Here we show that Numb promotes radial migration of cerebellar GCPs after cell fate commitment. The transcription factor Math1 (Atoh1) is required for specification of GCPs (Ben-Arie et al., 1997). Using math1-Cre to carry out conditional ablation of Numb in cerebellar GCPs, we identified a role for Numb in BDNF-induced GCP migration both in vitro and in vivo. We demonstrate that Numb binds activated TrkB and promotes TrkB endocytosis and polarization. Numb also functions as a scaffold for the polarity component aPKC, and is required for BDNF-dependent activation of this kinase. Thus, Numb links the chemotactic cue BDNF to intrinsic cellular polarity machinery, thereby enabling a chemotactic gradient to regulate cell polarization and directional movement.
RESULTS
Numb Is Required for GCP Migration
Previous studies demonstrated that genetic elimination of Numb in the early hindbrain using an engrailed2 promoter-driven Cre results in altered cerebellar development (Klein et al., 2004). To decipher the exact role of Numb in cerebellar development, we first asked which cells in developing cerebellum express Numb. As shown in Figure S1, Numb is expressed in several distinct cerebellar neurons: immature GCPs in the EGL, and mature granule cells in the IGL, visualized using an antibody to the granule cell marker Zic (Figure S1, A1-A5); Purkinje cells visualized by Calbindin staining (Figure S1, B1-B5); cerebellar Golgi cells in the IGL, detected by staining with anti-neurogranin (Figure S1, C1-C5) and Basket/Stellate cells in the ML, marked by parvalbumin staining (Figure S1, D1-D5). Numb expression was not detected in Bergmann glia cells (Figure S1, E1-E5). Given the broad expression of Numb in cerebellar neurons, it is difficult to determine the role that Numb plays in individual cell types during cerebellar development by analyzing the published mutants (Klein et al., 2004). Therefore we used math1-Cre to selectively abrogate Numb expression in cerebellar GCPs. Since Numbl, a homologue of Numb, shares a similar structure, expression pattern and biological function with Numb, we conditionally ablated both the Numb and Numbl genes to eliminate any potential function compensation between two molecules. Homozygous Numbflx/flx/Numblflx/flx mice were crossed with math1-Cre transgenic mice to generate math1-Cre/Numbflx/flx/Numblflx/flx animals. In the absence of Cre recombinase, Numbflx/flx/Numblflx/flx mice are indistinguishable from the wild type; similarly, math1-Cre transgenic mice show no defects in GCP proliferation and migration (Figure S2). To verify that Numb proteins were ablated specifically in granule cells, we immunostained sections from postnatal day 7 (P7) with a specific antibody to Numb. As shown in Figure S3A, in the presence of math1-Cre Numb expression was efficiently and selectively ablated in committed granule cells (Figure S3, A1-8), and Math1-Cre-mediated Numb/Numbl gene disruption in developing granule cells occurs postnatally. In conditional Numb/Numbl knockout mice, Numb continues to be expressed at P1 when GCPs are predominantly undergoing proliferation. Numb expression is largely eliminated at P7, when precursors are actively exiting cell cycle and beginning radial migration (Figure S3B-D). Math1-Cre/Numbflx/flx/Numblflx/flx mice are viable and fertile. However, examination of developing cerebellum revealed that the EGL is thicker in these mice than in wild-type littermates or Numbflx/flx/Numblflx/flx mice (Figure 1A and Figure S3E). This resembles a phenotypic change observed in engrailed2-cre/Numbflx/flx/Numblflx/flx mice (Klein et al., 2004), indicating that Numb has a role in GCPs even after they have committed to a granule cell lineage through the actions of Math1. As this phenotype could result from impairment of granule cell migration, proliferation, survival, and/or differentiation, we examined each of these possibilities.
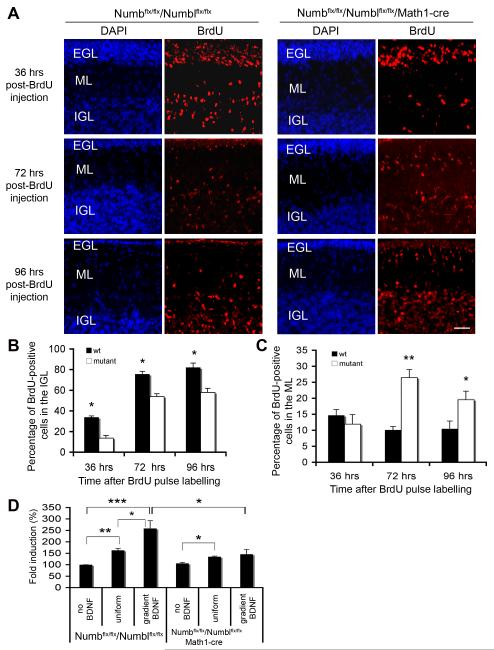
Figure 1. Numb Is Required For GCP Migration.
(A) GCP migration is impaired in conditional mutant mice. Dividing GCPs in P10 conditional mutants (Math1-Cre/Numbflx/flx/Numblflx/flx) and control littermates (Numb flx/flx/Numblflx/flx) were labeled with BrdU. Cerebellar sections were stained with anti-BrdU (red) and DAPI (blue) at indicated times post-injection. EGL, external germinal layer; ML, molecular layer; PCL, Purkinje cell layer; IGL, internal granule cell layer. Scale bar: 50 μm.
(B) Migrated granule cells in IGL. At 36, 72 and 96 hours post-BrdU labeling, the number of migrated granule cells in the IGL is reduced in conditional mutant mice (*P<0.05).
(C) Migrating granule cells in the ML. At 72 and 96 hours post-BrdU labeling, more migrating granule cells remain in the ML of conditional mutant mice (*, P<0.05).
(D) Conditional deletion of Numb/Numbl impairs BDNF-induced GCP chemotaxis in vitro. GCPs from P6 cerebella of conditional mutant mice (Math1-Cre/Numbflx/flx/Numblflx/flx) or control littermates (Numbflx/flx/Numblflx/flx) were placed in the upper chamber of Transwell apparatus; cells that migrated to the lower side of the membrane after 18 hours were counted. For each experiment, the number of migrating cells was normalized to that of migrating GCPs from wild-type mice in the absence of BDNF (*, P< 0.05; **, P< 0.05 and ***, P< 0.001; n=4).
To determine if conditional ablation of Numb/Numbl affects GCP migration we labeled a cohort of granule cells on P10 with a pulse of bromodeoxyuridine (BrdU), then examined migration of labeled cells over the next four days (Figure 1A). The percentage of BrdU-labeled GCPs that have migrated from the EGL to the IGL was lower in math1-Cre/Numbflx/flx/Numblflx/flx than in control wild-type or Numbflx/flx/Numblflx/flx littermates at all time points post-labeling, suggesting that conditional deletion of Numb/Numbl impairs GCP migration from the EGL to the IGL (Figure 1A-1C, Figure S4). Similar defects in migration were observed in math1-Cre/Numbflx/flx mice, whereas conditional Numbl mutant mice show no obvious phenotypic changes (Figure S5), indicating that Numb plays the predominant role in GCP migration.
As dysregulation of GCP proliferation and apoptosis could also contribute to the observed defects in EGL thickness, we asked if conditional deletion of Numb/Numbl alters GCP proliferation and survival. We examined proliferation of GCPs in the EGL of conditional mutants and control littermates at P7 and P12 by S-phase labeling of BrdU (Figure S6, A1-A2) and by immunostaining with anti-phosphohistone H3, a M-phase marker for proliferating cells (Figure S6, A3-A4). There is no significant difference in GCP proliferation between math1-Cre/Numbflx/flx/Numblflx/flx mice and control littermates (Figure S6, B-C). To determine whether conditional Numb/Numbl ablation affects GCP apoptosis, we examined cell death in the EGL at P7 and P12 by TUNEL staining (Figure S6, D-E). No significant difference in apoptosis is detected between conditional mutants and control littermates. Together, these results indicate that accumulation of GCPs in the EGL of math1-Cre/Numbflx/flx/Numblflx/flx mutants results from impaired GCP migration rather than dysregulation of GCP proliferation or survival.
Granule Cell Differentiation is Not Altered in Conditional Numb/Numbl Mutants
Previous studies examining the role of Numb in the developing hindbrain demonstrated that Numb has a role in neuronal maturation (Klein et al., 2004). To determine whether Numb plays a role in neuronal maturation of GCPs even after cells have committed to this lineage by expressing Math1, we analyzed GCP differentiation. As shown, there is no difference in expression of granule cell transcription factors Zic1 and Pax6 in Math1-Cre/Numbflx/flx/Numblflx/flx mutants and control littermates (Figure S6, F-I). Expression of L1, a cell adhesion molecule involved in GCP radial migration, is also similar in conditional mutants and controls (Figure S6, J-K), as is expression of GABA receptor α6 subunit, a marker for fully differentiated granule cells in the IGL (Figure S6, L-M). Morphologic development of granule cell axons and dendrites as visualized by Golgi staining did not differ between conditional mutants and control mice (Figure S6, N-Q). These findings indicate that Numb has a specialized role in migration after the granule cell lineage has been specified by Math1 expression. Migration defects of GCPs in conditional Numb/Numbl mutants could be due to indirect effects of mutant GCPs on Bergmann glia development such that the Bergmann glia no longer function as propitious substrates for migration. However, immunostaining of the P10 cerebellum with antibody to glial fibrillary acidic protein (GFAP) revealed that the organization of Bergmann glial fibers in math1-Cre/ Numbflx/flx/Numblflx/flx mice is similar to that in control mice (Figure S6, R-S).
Numb Is Required for BDNF-induced GCP Chemotaxis
The phenotypic deficits of conditional Numb or Numb/Numbl mutant in cerebellar development resemble those seen in bdnf−/− mice, as both exhibit a thicker EGL and impaired GCP migration (Borghesani et al., 2002; Schwartz et al., 1997). As BDNF functions as a chemoattractant factor for GCPs (Borghesani et al., 2002; Zhou et al., 2007a), we used a Boyden chamber assay to determine whether Numb is required for BDNF-induced chemotaxis. Consistent with prior studies, BDNF in the lower chamber promotes chemotaxis of GCPs isolated from Numbflx/flx/Numblflx/flx mice, and is more efficacious at promoting migration than uniform BDNF (same concentration in both chambers). While math1-Cre/Numbflx/flx/Numblflx/flx GCPs exhibit normal migration in the absence of chemotactic factor or in uniform BDNF, migration of these mutant GCPs in response to a BDNF gradient is significantly impaired (Figure 1D). These data indicate that Numb in GCPs plays a role in BDNF-dependent migration, and in intracellular pathways that link BDNF to a resultant chemotactic response.
Numb Interacts With Activated TrkB
Previous studies have shown that BDNF activation of its cognate receptor TrkB mediates GCP migration (Zhou et al., 2007a). TrkB, a tyrosine kinase receptor highly expressed in developing GCPs, contains a NPXY515 motif in the cytoplasmic tail that conforms to the optimal binding site for the phosphotyrosine-binding (PTB) domain of Numb (Li et al., 1998; Zwahlen et al., 2000). We therefore asked whether interactions between TrkB and Numb might explain the defect in migration shown here. To determine whether TrkB and Numb interact, we asked if these proteins can co-precipitate when expressed in a heterologous system. As indicated, Numb co-precipitates with TrkB receptors, and BDNF stimulation increases this interaction (Figure 2A).
Figure 2. Numb Interacts With TrkB.
(A) pcDNA3-TrkB-Flag was co-transfected with empty vector or pcDNA3-myc-Numb into HEK 293T cells. Lysates were precipitated with anti-Flag or anti-Myc, and immunoprecipitates (IPs) were blotted with anti-Myc or anti-Flag. Lysates were blotted for expression control.
(B) Numb interacts with TrkB in GCPs. GCPs were stimulated with BDNF or vehicle for 10 minutes; lysates were precipitated with anti-Numb, then blotted with anti-TrkB. Membrane was stripped and re-probed with anti-Numb.
(C) Schematic of Numb structure and truncated Numb proteins.
(D) Numb interacts with TrkB in developing cerebellum. Immobilized full-length (FL) or truncated Numb on sepharose beads was incubated with cerebellar lysates (P7) and blotted with anti-TrkB.
(E) TrkB kinase activity is required for interaction with Numb. pcDNA3-Myc-Numb was co-transfected with empty vector or pcDNA3-TrkB-Flag wild-type (wt), Y515F or K571M mutant into HEK 293T cells. Lysates were precipitated with anti-Myc or anti-Flag, and blotted with anti-Flag or anti-phosphotyrosine (pY). Membrane was re-probed with anti-Myc or anti-Flag.
(F) Numb directly binds TrkB in vitro. Bacteria-derived GST-Numb (FL) immobilized on sepharose beads was incubated with purified TrkB (FL) or His-TrkB cytoplasmic tail (CT) and blotted with anti-TrkB and anti-His.
(G) ATP enhances TrkB interaction with Numb. GST or GST-TrkB cytoplasmic tail (CT) immobilized on sepharose beads was incubated with purified Numb (FL) in the presence or absence of ATP, and blotted with anti-Numb.
To determine whether this interaction is physiologically significant, we analyzed the interaction of endogenous Numb and endogenous TrkB in GCPs. In primary cultures BDNF promotes co-precipitation of Numb and TrkB (Figure 2B). To understand the molecular basis for this ligand-stimulated interaction, we analyzed the domains of Numb and TrkB required for binding. As shown, GST-Numb fusion protein immobilized on sepharose beads precipitates endogenous TrkB from cerebellar lysates; either the Numb PTB or the PRR domain alone can precipitate TrkB, albeit at lower levels than the full-length construct (Figure 2C, D). Thus both domains contribute to the ability of Numb to interact with activated TrkB. A PTB domain usually interacts with a NPXY motif in a kinase-dependent manner, and the nature of the TrkB NPXY motif is ideal for interacting with Numb PTB (Yaich et al., 1998). Myc-tagged Numb was co-transfected with Flag-tagged wild-type TrkB (wt), or TrkB (Y515F) or kinase-dead TrkB (K571M) into HEK 293T cells. As shown in Figure 2E, Numb interacts with wild-type TrkB and this interaction was augmented by BDNF stimulation, whereas mutation at Y515 reduced, but did not eliminate, the ability of TrkB to interact with Numb. Interestingly, a mutation at K571 of TrkB, which leads to loss of kinase activity as confirmed by immunoblot with anti-pY, completely abrogates interaction with Numb (Figure 2E). These data indicate that NPXY515 motif contributes to TrkB interaction with Numb and that TrkB kinase activity is essential for this interaction.
Numb may interact directly with transmembrane receptors, or may require an intermediary protein (Guo et al., 1996; Hutterer and Knoblich, 2005). To determine if Numb directly interacts with TrkB, an in vitro binding assay was carried out, in which recombinant, purified GST or GST-Numb prepared from bacteria was immobilized on sepharose beads and incubated with purified full-length TrkB or TrkB cytoplasmic tail (CT). As shown, Numb binds directly to full length TrkB or to TrkB CT (Figure 2F). Moreover, addition of ATP in the reaction promotes TrkB interaction with Numb in vitro (Figure 2G), consistent with data that activation of TrkB kinase promotes its interaction with Numb.
Numb Is Required for TrkB Endocytosis and Polarization
Numb binds to α-adaptin, and thereby functions as an adaptor protein regulating endocytosis of membrane receptors (Berdnik et al., 2002; Hutterer and Knoblich, 2005). To determine whether Numb is appropriately localized to mediate TrkB endocytosis, we examined Numb, α-adaptin and TrkB in migrating GCPs. As shown, Numb colocalizes with α-adaptin in the leading processes of GCPs in the inner EGL (EGLb) (Figure 3A1-A4). Colocalization of Numb and α-adaptin can also be visualized in individual GCPs migrating along Bergmann glia in culture (Figure 3A5-A8). Similarly, Numb colocalizes with TrkB in the leading processes of GCPs in the inner EGL (Figure 3B1-B4) and can be seen near the plasma membrane and at intracellular sites in the leading processes of GCPs migrating along Bergmann glia (Figure 3B5-B8). As BDNF stimulates TrkB internalization and polarization of TrkB-containing signaling endosomes in migrating GCPs (Figure 3C, D) (Zhou et al., 2007a), Numb is appropriately positioned to work with α-adaptin to mediate TrkB endocytosis in migrating GCPs. Therefore, we asked whether Numb interaction with TrkB plays a role in BDNF-induced TrkB endocytosis, polarization and GCP migration. First, we measured the relative level of surface TrkB on GCPs in response to BDNF by immunostaining with an antibody to the TrkB extracellular domain. Immunostaining without permeabilization assesses surface TrkB, while immunostaining after permeabilization assesses total TrkB; surface TrkB relative to total TrkB was then calculated for each condition at each time point. Relative surface TrkB at time 0 was set as 100%. As shown in Figure 4A, in wild-type GCPs surface TrkB decreased in response to BDNF treatment. In contrast, surface TrkB levels on GCPs from math1-Cre/Numbflx/flx/Numblflx/flx mutant mice showed little change with BDNF treatment (Figure 4A). The impact of Numb mutation on surface TrkB level could reflect changes in TrkB internalization and recycling or TrkB synthesis or degradation. Therefore, to measure internalized TrkB more directly we carried out biotin-labeling of surface molecules, then analyzed subsequent internalization of biotinylated TrkB. Biotin moieties that remain on the cell surface were removed with a reduced glutathione solution, then cells were lysed, and lysates were precipitated with streptavidin-conjugated sepharose beads. Internalized TrkB in immunoprecipitates was detected by western blot with anti-TrkB. Consistent with previous reports (Du et al., 2003), BDNF stimulation causes rapid TrkB internalization in cultured GCPs and GCPs infected with control shRNA lentivirus, and this internalization process continues through 2 hours of BDNF treatment (Figure 4B, C). In contrast, BDNF-induced TrkB internalization was attenuated in GCPs infected with Numb shRNA lentivirus (Figure 4C, lanes 5, 6 top panel). Together, these studies demonstrate that Numb is required for BDNF-induced TrkB endocytosis in GCPs.
Figure 3. Numb Colocalizes With α-adaptin and TrkB in Leading Processes of Migrating GCPs.
Phase images (Phase) of GCPs co-cultured with Bergmann glia in vitro (A5, B5, C1, C5 and D1). Broken lines: Bergmann glia. DAPI stain (blue). Scale bar: 5 μm.
(A) Numb colocalizes with α-adaptin in the leading processes of GCPs in the inner EGL in vivo (A1-A4) and GCPs migrating along Begmann glia fibers in response to BDNF in vitro (A5-A8). Arrows indicate colocalized Numb and α-adaptin.
(B) Numb colocalizes with TrkB in the leading processes of GCPs in the inner EGL in vivo (B1-B4) and in GCPs migrating along Bergmann glia in response to BDNF in vitro (B5-B8). Arrows indicate the leading process and the front of the cell where Numb and α-adaptin colocalize.
(C) TrkB (C1-C4) or activated TrkB (p-Trk) (C5-C8) colocalizes with α-adaptin (green) in the leading processes of GCPs migrating along Bergmann glia in response to BDNF. Arrows indicate the leading process and the front of the cell where TrkB (red) and α-adaptin colocalize.
(D) Activated TrkB (red) receptors are localized to the leading process, not the Golgi apparatus (green) of GCPs migrating along Bergmann glia in response to BDNF in vitro. GCPs stained with anti-phospho-Trk (Tyr490) (p-Trk). Arrows indicate the leading process and the front of the cell where activated TrkB was detected.
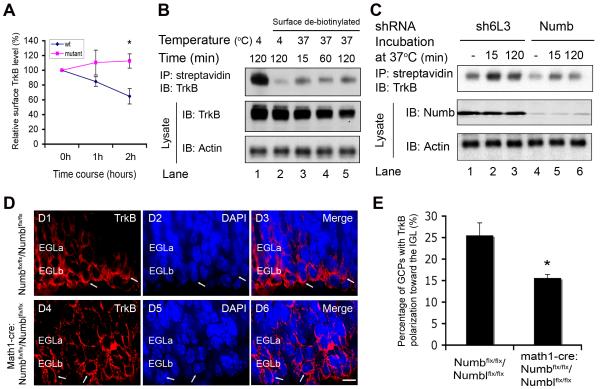
Figure 4. Numb Deletion Impairs BDNF-induced TrkB Endocytosis and Polarization.
(A) Conditional deletion of Numb/Numbl inhibits BDNF-induced TrkB endocytosis in GCPs. GCPs from conditional Numb/Numbl or control littermate mice were treated with BDNF as indicated. Surface or total TrkB level was measured by staining with anti-TrkB as described in Methods. Relative surface TrkB for each time point was calculated by dividing the value of surface TrkB signal by that of total TrkB signal, and normalized to relative surface TrkB in that experiment at time 0 (*, P<0.05; n=4).
(B) Time course of BDNF-stimulated TrkB internalization measured by biotinylation of cell surface molecules and detection of internalized TrkB by blot with anti-TrkB. BDNF induces rapid TrkB internalization and this continues through 2 hours.
(C) Knockdown of Numb attenuates BDNF-induced TrkB internalization. GCPs infected with Numb-specific shRNA or control shRNA lentivirus were cultured and treated with BDNF for 30 minutes on ice, and labeled with biotin for 30 minutes. Biotinylated cells were incubated at 37 °C or on ice for 2 hours. Biotin moieties on the cell surface were removed with reduced glutathione. Cells were lysed and precipitated with streptavidin sepharose. Internalized biotinylated-surface TrkBs were blotted with anti-TrkB. Aliquots of lysates were blotted with anti-Numb. Data shown represent one of three independent experiments.
(D) Conditional deletion of Numb/Numbl impairs TrkB polarization. Cerebellar sections from P7 conditional mice (Math1-Cre/ Numbflx/flx/Numblflx/flx) (D4-D6) and control littermates (Numbflx/flx/Numblflx/flx) (D1-D3) were stained with anti-TrkB (red). DAPI stain (blue). Scale bar: 5 μm.
(E) Analysis of TrkB polarization in the inner EGL (EGLb) of Math1-Cre/Numbflx/flx/Numblflx/flx mutant and control littermate mice as described in Figure S7B (*, P<0.05; n = 8).
See also Figure S7.
Our previous studies indicate that both endocytosis and TrkB polarization at the front of the cell are critical for BDNF-induced GCP migration (Zhou et al., 2007a). Therefore we asked if mutation of Numb/Numbl affects TrkB polarization in cerebellar GCPs. Cerebellar sections from math1-Cre/Numbflx/flx/Numblflx/flx mutant mice or control mice at P7 were immunostained with anti-TrkB. Consistent with previous observations, TrkB receptors are polarized at the front of migrating GCPs in wild-type cerebellum (Figure 4D1-D3). In contrast, TrkB polarization is significantly impaired in math1-Cre/Numbflx/flx/Numblflx/flx mutants (Figure 4, D4-D6; 4E). These data indicate that Numb regulates endocytosis and polarization of TrkB to leading processes of migrating GCPs.
BDNF Activation of TrkB Promotes Numb Polarization
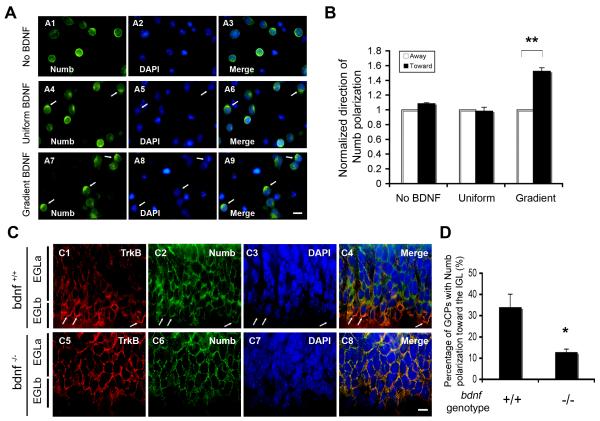
Numb colocalizes with TrkB in the leading processes of migrating GCPs in culture or in developing cerebellum (Figure 3). Moreover, BDNF stimulates association of TrkB with Numb (Figure 2) and promotes TrkB polarization (Zhou et al., 2007a). These observations led us to ask if BDNF stimulation also promotes Numb polarization. Purified GCPs were exposed to vehicle control, uniform BDNF or a BDNF gradient, then immunostained with anti-Numb. Numb localization to one side of GCPs was counted as “polarization”. For each cell, Numb localization at the side of the cell toward the BDNF source was marked as “toward” and Numb localization opposite to the BDNF source was considered “away” (Figure S7). A gradient of BDNF, unlike uniform BDNF, promotes Numb localization toward the BDNF source (Figure 5A, B). Similarly, examination of Numb localization in vivo revealed that Numb is polarized to the front of GCPs in the inner EGL of wild-type mice, whereas Numb polarization at the front of GCPs was significantly decreased in bdnf −/− mice (Figure 5C, D). Thus, BDNF induces Numb polarization to leading processes of migratory GCPs in vitro and in vivo.
Figure 5. BDNF Gradient Promotes Numb Polarization.
(A) Purified GCPs were exposed to vehicle (A1-A3), uniform (A4-A6) or gradient BDNF (A7-A9) for 12 hours in vitro, then stained with anti-Numb (green). BDNF source is at the top. DAPI stain (blue). Arrows indicate representative GCPs with Numb polarization. Scale bar: 5 μm.
(B) Analysis of Numb polarization in GCPs in response to BDNF as shown in (A). For each condition (no BDNF, uniform and gradient BDNF), the number of GCPs with Numb polarization toward the BDNF source was normalized to that of GCPs with Numb polarization away from the BDNF source (**, P<0.001; n = 4).
(C) Representative images of Numb polarization in vivo. Cerebellar sections from P7 control (C1-C4) and bdnf−/− mutant mice (C5-C8) were stained with anti-TrkB (red), anti-Numb (green). DAPI stain (blue). Scale bar: 5 μm.
(D) Analysis of Numb polarization in EGLb of control and bdnf−/− mice in (C) as described in Figure S7 (*, P<0.05; n = 4).
Numb Recruits aPKC to Promote GCP Migration
Chemotaxis requires correct orientation of the cell polarity machinery in response to extracellular cues. Our data has shown that Numb, an endocytic adaptor, mediates redistribution of TrkB receptor to the leading process, and that Numb itself is also polarized to the front of GCPs. These observations suggest that Numb may be an important player connecting the orientation signal to intracellular polarity machinery. To determine whether Numb also recruits signaling molecules involved in cell polarization, we focused on atypical PKC (aPKC), a component of the conserved Par polarity complex (Goldstein and Macara, 2007). In previous studies, PKCζ, a member of aPKC subfamily, has been shown to localize at the leading edge of migrating cerebellar GCPs (Solecki et al., 2004). As shown, Numb binds PKCζ in a heterologous system (Figure 6A) and this interaction is also clearly evident in cerebellar GCPs (Figure 6B). Moreover, BDNF stimulation of cultured GCPs potentiates the interaction of PKCζ and Numb (Figure 6C). Further evidence that BDNF promotes the interaction between Numb and aPKC in GCPs in vivo was obtained from genetic mutant mice; while Numb interacts with PKCζ in lysates from cerebellar tissue of wild-type animals, co-precipitation of Numb and PKCζ is dramatically reduced in bdnf−/− cerebellar tissue (Figure 6D). Thus, both in vivo and in cultured GCPs, BDNF promotes the interaction of Numb with aPKC. To determine whether Numb directly interacts with PKCζ, we purified bacterially expressed Numb and active PKCζ. As shown, purified Numb can indeed bind to active PKCζ immobilized on the beads, indicating that there is a direct interaction of the two proteins (Figure 6E).
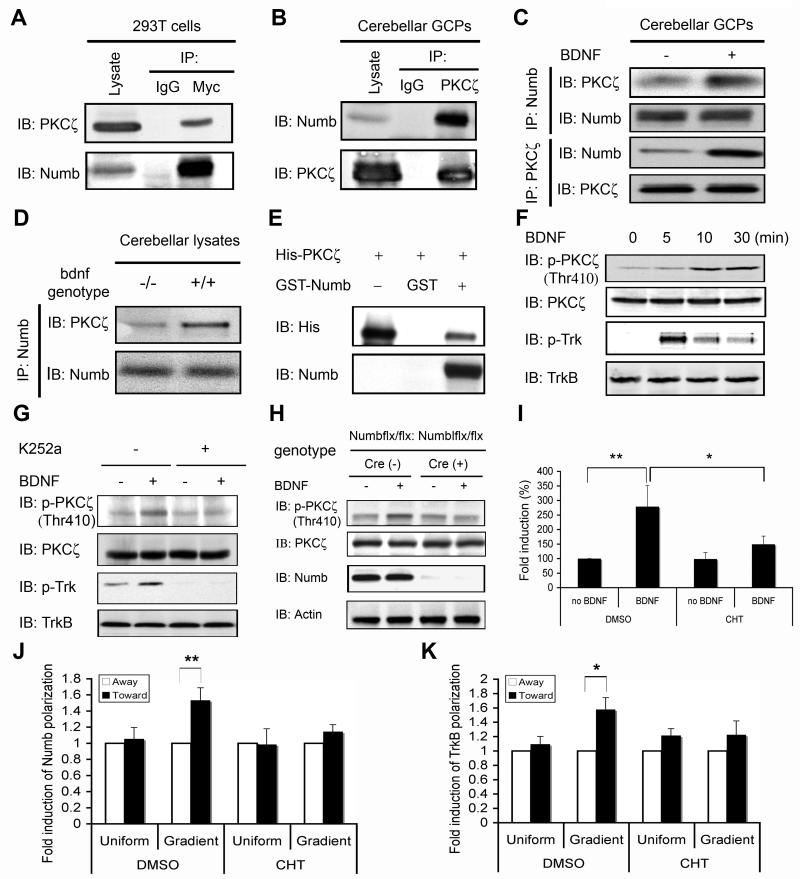
Figure 6. Numb Interacts With aPKC to Promote GCP Chemotaxis.
(A) PKCζ interacts with Numb. 293T cells were transfected with pcDNA3-myc-Numb plasmids, and lysates were precipitated with control IgG or anti-Myc then blotted with anti-PKCζ. Membrane was reprobed with anti-Numb.
(B) PKCζ interacts with Numb in cerebellar GCPs. GCP lysates (P7) were immunoprecipitated with anti-PKCζ or control IgG then blotted with anti-Numb. Membrane was reprobed with anti-PKCζ.
(C) BDNF stimulation promotes PKCζ interaction with Numb in GCPs. GCPs from P7 mice were treated with vehicle or BDNF for 10 minutes; lysates were precipitated and blotted with indicated antibodies.
(D) BDNF deletion impairs PKCζ interaction with Numb. Lysates from P7 wild type and bdnf−/− cerebellum were precipitated with anti-Numb followed by blot with anti-PKCζ and anti-Numb.
(E) Numb binds active PKCζ. GST-Numb or GST immobilized on sepharose beads was incubated with purified His-tagged active PKCζ at 30 °C for 1 hour. Bound protein complex was washed and analyzed by blot with anti-His and anti-Numb, respectively. First lane, His-PKCζ alone.
(F) Time course of PKCζ activation by BDNF. GCPs from P7 were stimulated with BDNF for indicated times; lysates were blotted as indicated.
(G) Inhibition of TrkB kinase blocks BDNF-induced PKCζ activation. GCPs were treated with K252a for 30 minutes prior to BDNF stimulation; lysates blotted as indicated.
(H) Numb is required for BDNF-induced aPKC activation. GCPs from P7 Math1-Cre/Numbflx/flx/Numblflx/flx or control littermates (Numbflx/flx/Numblflx/flx) were treated with BDNF or vehicle. Lysates were blotted with indicated antibodies.
(I) Inhibition of PKCζ activity impairs BDNF-induced GCP chemotaxis. GCPs from P6 were treated with 10 μM chelerythrine (CHT) or vehicle and a BDNF chemotaxis assay was performed using Boyden chamber (*, P<0.05; **, P< 0.001; n=3).
(J) Inhibition of aPKC activity by CHT prevents BDNF-induced Numb polarization in GCPs. For each condition (uniform and gradient BDNF), the number of GCPs with Numb polarization toward the plug (BDNF source) was normalized to that of GCPs with Numb polarization away from the plug (**, P<0.001; n = 3).
(K) Inhibition of aPKC activity by CHT prevents BDNF-induced TrkB polarization in GCPs. For each condition (uniform and gradient BDNF), the number of GCPs with TrkB polarization toward the agarose plug (BDNF source) was normalized to that of GCPs with TrkB polarization away from the agarose plug (**, P<0.01; n = 3).
BDNF stimulation of GCPs leads to PKCζ phosphorylation at Thr410, a site in the activation loop critical for activation (Figure 6F) (Newton, 2001). Moreover, BDNF-induced PKCζ phosphorylation is prevented by Trk kinase inhibitor K252a (Figure 6G), indicating that BDNF activation of PKCζ acts through TrkB. We next asked if Numb functions as a linker protein that is required for BDNF-induced PKCζ activation. As shown, genetic deletion of Numb/Numbl dramatically impaired BDNF-induced PKCζ activation in GCPs (Figure 6H). Together these data indicate that BDNF stimulation of TrkB recruits Numb to the activated receptor, and that Numb functions both as an endocytic adapter and a scaffold protein to recruit and activate aPKC.
Previous studies have demonstrated that aPKC localizes at the leading edge of migrating GCPs (Solecki et al., 2004). To determine whether aPKC functions in BDNF-induced GCP chemotaxis, we asked whether chelerythrine, a pharmacologic inhibitor of aPKC, alters BDNF-dependent migration in a Boyden chamber assay. As shown in Figure 6I, chelerythrine prevents the chemotactic response of GCPs to BDNF, without altering basal migration. Moreover, inhibition of aPKC kinase activity by chelerythrine significantly prevents BDNF-induced Numb (Figure 6J) and TrkB polarization (Figure 6K). Thus, BDNF-dependent activation of aPKC is needed for Numb and TrkB polarization as well as the chemotactic response of GCPs to BDNF.
Numb Is A Substrate of aPKC
Analysis of Numb protein sequence using Scansite Software (http://scansite.mit.edu/) identifies that there are three potential PKCζ phosphorylation sites (S7, S265 and S284) all of which are conserved across species (Nishimura and Kaibuchi, 2007). We generated a phospho-specific antibody to pS284 of Numb (Figure 7 A, B), and verified that this site can be phosphorylated by activated aPKC. As shown, BDNF stimulation of GCPs induces pS284 Numb phosphorylation (Figure 7C). In these experiments, we included Calyculin A, a phosphatase inhibitor that prevents Numb dephosphorylation, and so allows us to detect BDNF-dependent changes in phosphorylation state. Phosphorylated Numb colocalizates with TrkB in the leading processes of migrating GCP (Figure 7D). To explore the consequences of Numb phosphorylation for BDNF-dependent chemotaxis, we asked whether phosphorylation affects the interaction between TrkB and Numb. A mutant form of Numb, in which three serine phosphorylation sites are replaced by alanines so phosphorylation by aPKC cannot occur, still retains the ability to interact with TrkB. However, the strength of this association can no longer be modified in response to BDNF (Figure 7E). Together these data suggest that Numb both potentiates receptor endocytosis and functions as a scaffold to recruit and activate aPKC. Once activated, aPKC phosphorylates Numb at critical sites, and thereby promotes formation or stability of TrkB/Numb interactions. In this way phosphorylation of Numb provides a feed-forward loop to facilitate a chemotactic response to BDNF (Figure 7F).
Figure 7. Numb Is A Substrate of aPKC.
(A) PKCζ phosphorylates Numb in a dose-dependent manner. Purified GST-Numb immobilized on glutathione sepharose beads was incubated with purified His-PKCζ. Bound protein complex was detected by blot with a monoclonal antibody phospho-Ser/Thr (MPM2) or antibody against phospho-Numb (p-Numb (S284)).
(B) Specificity of anti-phospho-Numb (S284). HEK 293T cells were transfected with plasmids expressing wt Numb or Numb mutant (S284A). Lysates were precipitated with anti-Numb. Numb IPs were incubated with or without active PKCζ, and blotted with anti-p-Numb (S284) and anti-Numb, respectively.
(C) BDNF stimulates Numb phosphorylation by PKCζ in vivo. GCPs from P7 mice were treated with 50 ng/ml BDNF for 5 minutes in the presence or absence of phosphatase inhibitor calyculin-A and/or 10 μM PKC inhibitor chelerythrine. Lysates were blotted with anti-p-Numb (S284) and anti-Numb, respectively.
(D) Phosphorylated Numb (green) colocalizes with TrkB (red) in the leading processes of GCPs along Bergmann glia in response to BDNF. Staining with p-Numb (S284) antibody and TrkB antibody. DAPI stains nuclei (blue). Scale bar: 5 μm
(E) Numb mutations at PKCζ phosphorylation sites reduce Numb interaction with TrkB. HEK 293T cells co-transfected with plasmid expressing TrkB-GFP and plasmid expressing wild type Numb (wt) or Numb mutant for all three aPKC phosphorylation sites (mut), and treated with vehicle or BDNF. Lysates precipitated with anti-GFP and blotted with anti-Numb and anti-GFP, respectively.
(F) Model for Numb function in cell migration. In response to a BDNF gradient, Numb associates with activated TrkB to regulate receptor endocytosis and recruit aPKC. Activated aPKC then phosphorylates Numb. Phosphorylation of Numb serves in a feed-forward loop to potentiate binding of Numb to TrkB and thereby fosters formation of TrkB/Numb endosomal complexes. Endocytosis is required for Tiam1-mediated Rac activation and localization of signaling endosomes to leading processes and/or for recycling TrkB to plasma membrane in leading processes.
DISCUSSION
Extracellular chemotactic cues orient a cell so it can migrate in the appropriate direction. The data presented here indicate that BDNF regulates intracellular localization of Numb and Numb-dependent endocytic trafficking to induce cell polarity. We demonstrate that conditional ablation of Numb/Numbl in cerebellar GCPs after lineage commitment impairs GCP migration both in vivo and in vitro. In response to a BDNF gradient, Numb associates with activated TrkB to regulate receptor endocytosis and recruit aPKC for activation. Activated aPKC then phosphorylates Numb at critical sites. Phosphorylation of Numb serves in a feed-forward loop to potentiate binding of Numb to TrkB and thereby fosters formation of TrkB/Numb endosomal complexes (Figure 7F). Previous studies demonstrated that endocytosis is needed for activation of Rac by Tiam1, a signaling pathway critical for migration (Palamidessi et al., 2008; Zhou et al., 2007a). Thus regulated interactions between endocytic protein Numb and the BDNF receptor TrkB link an extracellular chemotactic cue to the cellular polarity machinery and so enable directed migration in response to a shallow gradient.
Endocytic trafficking has long been recognized as a general mechanism for attenuating extracellular signals (Disanza et al., 2009; Polo and Di Fiore, 2006). However, accumulating evidence suggests that endocytic trafficking may play an important role in potentiating cell migration (Le Roy and Wrana, 2005; Ulrich and Heisenberg, 2009). The first link of endocytosis to cell migration came from the observation that integrin receptors colocalize with the endocytic regulator dynamin at focal contacts of migrating cells (Bretscher, 1989; Caswell and Norman, 2008; Ezratty et al., 2005). Disruption of integrin internalization and recycling dramatically impairs integrin polarization and cell migration, indicating that endocytosis modulates turnover of integrin receptors during cell migration (Caswell and Norman, 2008). In cortical neural precursors, endocytosis of cadherin receptor promotes radial migration (Kawauchi et al., 2010). Similarly, blocking endocytosis of platelet-derived growth factor receptor, EGFR or TrkB prevents polarization of these receptor tyrosine kinases (RTKs) to the leading processes of migrating cells and thus impairs directional migration in response to a growth factor gradient (Assaker et al., 2010; Jekely et al., 2005; Kawada et al., 2009; Zhou et al., 2007a). In migrating border cells, a plasma membrane-endosome trafficking cycle is responsible for maintaining active RTK at the leading edge of migrating border cells (Assaker et al., 2010). Endocytic trafficking has also been implicated in sharpening the gradient of SDF-1, an extracellular cue for germ cell migration (Boldajipour et al., 2008; Valentin et al., 2007), thereby promoting directional migration. Thus endocytic trafficking is important for multiple aspects of chemotactic migration, including maintenance of the gradient of extracellular guidance cues, formation and polarization of signaling endosomes to amplify the gradient of intracellular signals, recycling of receptor kinases, and elimination of adhesive contacts (Fletcher and Rappoport, 2010).
In this study, we demonstrate that Numb, a component of the endocytic complex, plays an important role in cell polarization and is required for directional migration of neural precursors during brain development. Numb function was first implicated in cell migration when genetic studies in Drosophila indicated that Numb mutants exhibit defective migration of glia cells along axonal tracts (Edenfeld et al., 2007). Studies in Hela cells indicate that Numb can promote cell migration due to its interaction with integrin β1 receptor (Nishimura and Kaibuchi, 2007). During epithelial-mesenchymal transition, Numb functions as an endocytic protein for E-cadherin to regulate cell migration (Wang et al., 2009). The studies presented here provide a mechanism for the action of Numb in cell migration: Numb binds the receptor for a chemotactic factor and functions as a scaffold that recruits an additional polarity component, aPKC, to the receptor complex. Furthermore, Numb induces endocytosis and relocalization of the receptor to the front of the cell. The Numb-receptor complex may remain in signaling endosomes within the leading process, and/or be recycled to the front of the cell to promote directional migration in response to receptor activation.
Models of chemotactic responses have highlighted the requirement for localized, positive feedback loops within intracellular signaling pathways (Charest and Firtel, 2006). Positive feedback amplifies the initial response to an extracellular gradient and results in the formation of a stable leading process at the front of a migrating cell (Sasaki et al., 2007). Here we demonstrate that BDNF-dependent activation of TrkB stimulates atypical PKC, and this process requires Numb. In turn, activated aPKC phosphorylates Numb and phosphorylated Numb displays enhanced binding to TrkB; this facilitates the ability of Numb to promote endocytosis and polarized localization of TrkB. In this way, aPKC and Numb stabilize a signaling complex at the leading process, and so function as a positive feedback to promote chemotaxis (Figure 7F).
Numb was originally identified as a cell lineage determinant in Drosophila. Asymmetric segregation of Numb during mitosis enables the daughter cells to assume distinct fates (Guo et al., 1996; Rhyu et al., 1994). In mice, Numb has been reported to maintain a pool of neural stem/progenitor cells during early development; thus Numb/Numbl −/− mice exhibit malformation of the forebrain with precocious neuron production and depletion of neural progenitors (Petersen et al., 2002; Petersen et al., 2004; Rasin et al., 2007; Zhou et al., 2007b). A previous study in which engrailed2-Cre was used to delete Numb/Numbl in the early hindbrain suggested that Numb has multiple roles in cerebellar development, including a role in neuronal maturation (Klein et al., 2004). However, when we use math1-Cre to delete Numb/Numbl in GCPs at a stage subsequent to cell fate commitment, we identified a distinctive role for Numb in precursor cell migration, independent of functions in proliferation, survival or differentiation.
While Numb is best known for its early role in fate choice, and is now shown to regulate migration, Numb has also been implicated in neurite growth after migration has occurred (Huang et al., 2005; Nishimura et al., 2003; Nishimura et al., 2006). In hippocampal neurons, Numb localizes at axonal growth cones where it mediates internalization of the L1 adhesion molecule, thereby regulating outgrowth (Nishimura et al., 2003), and also localizes to dendritic spines where it interacts with EphB2 receptor (Nishimura et al., 2006). Together, these data indicate that Numb plays multiple roles at different stages of neuronal development. We propose that there is a common molecular mechanism underlying Numb functions at different stages of neuronal development. Numb binds directly to several endocytic proteins including Eps15 and α-adaptin (Salcini et al., 1997; Santolini et al., 2000). In addition, Numb contains a phosphotyrosine-binding domain (PTB) that promotes interactions with several transmembrane proteins (Guo et al., 1996; Hutterer and Knoblich, 2005; Jafar-Nejad et al., 2002; Nishimura et al., 2003; Nishimura and Kaibuchi, 2007); we now show this list also includes TrkB. Finally, Numb interacts with other conserved molecules such as aPKC, and the Par complex (Nishimura and Kaibuchi, 2007). Hence, Numb functions as an adaptor protein that targets transmembrane cargo molecules for polarized endocytosis and provides a scaffold to interact with other polarity components. These functions enable Numb to play an important role in mitosis, in cell fate, in migration and in neurite outgrowth (Pece et al., 2011). While BDNF stimulates polarized location of Numb and TrkB, at other developmental stages Numb polarization is likely to be regulated by distinct extracellular cues, such as Notch ligands during mitosis, or EphrinB during spine formation (Pece et al., 2011). In this way, molecular links between extrinsic cues and Numb relocalization may allow the microenvironment to regulate cell polarity at key points in development.
EXPERIMENTAL PROCEDURES
Animals
Breeding pairs of BALB/c, brain-derived neurotrophic factor bdnf +/−, conditional Numb and Numb-like (Numbl) and Math1-Cre transgenic mice were described previously (Ernfors et al., 1994; Helms et al., 2000; Klein et al., 2004; Matei et al., 2005; Schuller et al., 2007).
Cell Culture, Transfection and Lentivirus Production
Cell culture, transient transfection and lentivirus production was described previously (Zhou et al., 2007a).
GCP and Bergmann glia Cultures and Lentiviral Infection
Culture of GCPs, co-culture of GCPs with Bergmann glia and lentiviral infection of GCPs were carried out as described previously (Choi et al., 2005; Klein et al., 2004; Zhou et al., 2007a).
TrkB Internalization
TrkB internalization was evaluated by two approaches: measuring relative surface TrkB level by immunostaining with anti-TrkB (Chemicon) and directing internalized TrkB by biotinylation of surface TrkB with EZ-Link® Sulfo-NHS-biotin (Pierce) as described previously (Du et al., 2003).
In vivo BrdU labeling, Migration, Proliferation and Apoptosis Assay
Mice at P6, P10, P11 and P12 were injected intraperitoneally with BrdU (100 μg/g animal weight), perfused with 4% PFA and sacrificed after 4 hours for proliferation and survival assay, and sacrificed after 36, 72 and 96 hours for in vivo migration assay. Matched 10 μm cryosections from Math1-Cre/Numbflx/flx/Numblflx/flx mutants and control littermates (Numbflx/flx/Numblflx/flx) were used for assay.
In vitro Chemotaxis Assay
In vitro chemotaxis assay was performed using a 24-well Boyden chamber system (Costar) as described previously (Chiaramello et al., 2007; Lu et al., 2001).
Purification of Recombinant Proteins
Purification of recombinant proteins was carried out as described previously (Miyamoto et al., 2006). TrkB-Flag was purified from HEK 293T cells transfected with pcDNA3-TrkB-Flag and GST fusion proteins were purified from bacteria.
Evaluation and Quantification of Protein Polarization
In vitro protein polarization in response to BDNF was carried out using purified GCPs exposed to exogenous BDNF (no BDNF, BDNF gradient or uniform) as described previously (Zhou et al., 2007a). GCPs were then fixed in 4% PFA and immunostained with anti-Numb or TrkB and DAPI counterstaining. Evaluation and quantification of protein polarization was performed as described in Figure S7.
Statistics
Means ± SEM are shown. Statistical analysis by Student’s t-test unless otherwise indicated. See Supplement for additional procedures.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Maria Pazyra-Murphy and Jennifer Kalscheuer for technical assistance, Louis Reichardt (University of Califonia, San Francisco, USA) for the generous gift of TrkB antibody, Michel Cayouette (Institut de recherches cliniques de Montréal, Canada) for mouse full-length Numb cDNA, Salvatore Piece (European Institute of Oncology, Italy) for GST-Numb construct, Jeng-shin Lee (Harvard Medical School) for pHAGE-CMV-MCS-IZsGreen vector system and David Rowitch (University of California, San Francisco) for Math1-Cre transgenic mice. We thank members of the Segal lab for thoughtful comments. This work was supported by grants from NIH (NS377057 RAS, the Lefler Foundation (MP and RAS), the DFCI Low-grade Astrocytoma Program (PZ) and Autism Speaks Foundation (XZ).
Footnotes
SUPPLEMENTAL INFORMATION Supplemental Information includes Supplemental Experimental Procedures and seven figures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Affolter M, Weijer CJ. Signaling to cytoskeletal dynamics during chemotaxis. Dev Cell. 2005;9:19–34. doi: 10.1016/j.devcel.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci. 2007;8:194–205. doi: 10.1038/nrn2056. [DOI] [PubMed] [Google Scholar]
- Assaker G, Ramel D, Wculek SK, Gonzalez-Gaitan M, Emery G. Spatial restriction of receptor tyrosine kinase activity through a polarized endocytic cycle controls border cell migration. Proc Natl Acad Sci U S A. 2010;107:22558–22563. doi: 10.1073/pnas.1010795108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly M, Wyckoff J, Bouzahzah B, Hammerman R, Sylvestre V, Cammer M, Pestell R, Segall JE. Epidermal growth factor receptor distribution during chemotactic responses. Mol Biol Cell. 2000;11:3873–3883. doi: 10.1091/mbc.11.11.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes AP, Solecki D, Polleux F. New insights into the molecular mechanisms specifying neuronal polarity in vivo. Curr Opin Neurobiol. 2008;18:44–52. doi: 10.1016/j.conb.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, Matzuk MM, Zoghbi HY. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- Berdnik D, Torok T, Gonzalez-Gaitan M, Knoblich JA. The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev Cell. 2002;3:221–231. doi: 10.1016/s1534-5807(02)00215-0. [DOI] [PubMed] [Google Scholar]
- Boldajipour B, Mahabaleshwar H, Kardash E, Reichman-Fried M, Blaser H, Minina S, Wilson D, Xu Q, Raz E. Control of chemokine-guided cell migration by ligand sequestration. Cell. 2008;132:463–473. doi: 10.1016/j.cell.2007.12.034. [DOI] [PubMed] [Google Scholar]
- Borghesani PR, Peyrin JM, Klein R, Rubin J, Carter AR, Schwartz PM, Luster A, Corfas G, Segal RA. BDNF stimulates migration of cerebellar granule cells. Development. 2002;129:1435–1442. doi: 10.1242/dev.129.6.1435. [DOI] [PubMed] [Google Scholar]
- Bretscher MS. Endocytosis and recycling of the fibronectin receptor in CHO cells. Embo J. 1989;8:1341–1348. doi: 10.1002/j.1460-2075.1989.tb03514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell P, Norman J. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 2008;18:257–263. doi: 10.1016/j.tcb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Charest PG, Firtel RA. Feedback signaling controls leading-edge formation during chemotaxis. Curr Opin Genet Dev. 2006;16:339–347. doi: 10.1016/j.gde.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Chiaramello S, Dalmasso G, Bezin L, Marcel D, Jourdan F, Peretto P, Fasolo A, De Marchis S. BDNF/ TrkB interaction regulates migration of SVZ precursor cells via PI3-K and MAP-K signalling pathways. Eur J Neurosci. 2007;26:1780–1790. doi: 10.1111/j.1460-9568.2007.05818.x. [DOI] [PubMed] [Google Scholar]
- Choi Y, Borghesani PR, Chan JA, Segal RA. Migration from a mitogenic niche promotes cell-cycle exit. J Neurosci. 2005;25:10437–10445. doi: 10.1523/JNEUROSCI.1559-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disanza A, Frittoli E, Palamidessi A, Scita G. Endocytosis and spatial restriction of cell signaling. Mol Oncol. 2009;3:280–296. doi: 10.1016/j.molonc.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Feng L, Zaitsev E, Je HS, Liu XW, Lu B. Regulation of TrkB receptor tyrosine kinase and its internalization by neuronal activity and Ca2+ influx. J Cell Biol. 2003;163:385–395. doi: 10.1083/jcb.200305134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenfeld G, Altenhein B, Zierau A, Cleppien D, Krukkert K, Technau G, Klambt C. Notch and Numb are required for normal migration of peripheral glia in Drosophila. Dev Biol. 2007;301:27–37. doi: 10.1016/j.ydbio.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368:147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- Fletcher SJ, Rappoport JZ. Moving forward: polarised trafficking in cell migration. Trends Cell Biol. 2010;20:71–78. doi: 10.1016/j.tcb.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17:27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- Hatten ME. New directions in neuronal migration. Science. 2002;297:1660–1663. doi: 10.1126/science.1074572. [DOI] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Higginbotham HR, Gleeson JG. The centrosome in neuronal development. Trends Neurosci. 2007;30:276–283. doi: 10.1016/j.tins.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Li H, Tang AA, Wiggins AK, Neve RL, Zhong W, Jan LY, Jan YN. Targeted deletion of numb and numblike in sensory neurons reveals their essential functions in axon arborization. Genes Dev. 2005;19:138–151. doi: 10.1101/gad.1246005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher A. Cell polarization mechanisms during directed cell migration. Nat Cell Biol. 2005;7:336–337. doi: 10.1038/ncb0405-336. [DOI] [PubMed] [Google Scholar]
- Hutterer A, Knoblich JA. Numb and alpha-Adaptin regulate Sanpodo endocytosis to specify cell fate in Drosophila external sensory organs. EMBO Rep. 2005;6:836–842. doi: 10.1038/sj.embor.7400500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafar-Nejad H, Norga K, Bellen H. Numb: “Adapting” notch for endocytosis. Dev Cell. 2002;3:155–156. doi: 10.1016/s1534-5807(02)00228-9. [DOI] [PubMed] [Google Scholar]
- Jekely G, Sung HH, Luque CM, Rorth P. Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev Cell. 2005;9:197–207. doi: 10.1016/j.devcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Kawada K, Upadhyay G, Ferandon S, Janarthanan S, Hall M, Vilardaga JP, Yajnik V. Cell migration is regulated by platelet-derived growth factor receptor endocytosis. Mol Cell Biol. 2009;29:4508–4518. doi: 10.1128/MCB.00015-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, Kubo K, Nakajima K, Nabeshima Y, Hoshino M. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron. 2010;67:588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Klein AL, Zilian O, Suter U, Taylor V. Murine numb regulates granule cell maturation in the cerebellum. Dev Biol. 2004;266:161–177. doi: 10.1016/j.ydbio.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Komuro H, Yacubova E. Recent advances in cerebellar granule cell migration. Cell Mol Life Sci. 2003;60:1084–1098. doi: 10.1007/s00018-003-2248-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein AR, Noctor SC. Patterns of neuronal migration in the embryonic cortex. Trends Neurosci. 2004;27:392–399. doi: 10.1016/j.tins.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Signaling and endocytosis: a team effort for cell migration. Dev Cell. 2005;9:167–168. doi: 10.1016/j.devcel.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Li SC, Zwahlen C, Vincent SJ, McGlade CJ, Kay LE, Pawson T, Forman-Kay JD. Structure of a Numb PTB domain-peptide complex suggests a basis for diverse binding specificity. Nat Struct Biol. 1998;5:1075–1083. doi: 10.1038/4185. [DOI] [PubMed] [Google Scholar]
- Lu Q, Sun EE, Klein RS, Flanagan JG. Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell. 2001;105:69–79. doi: 10.1016/s0092-8674(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, Morris K, Feng F, Jones K, Lee J, Fritzsch B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn. 2005;234:633–650. doi: 10.1002/dvdy.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Tanoue A, Wu C, Mobley WC. TrkB binds and tyrosine-phosphorylates Tiam1, leading to activation of Rac1 and induction of changes in cellular morphology. Proc Natl Acad Sci U S A. 2006;103:10444–10449. doi: 10.1073/pnas.0603914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Fukata Y, Kato K, Yamaguchi T, Matsuura Y, Kamiguchi H, Kaibuchi K. CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nat Cell Biol. 2003;5:819–826. doi: 10.1038/ncb1039. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell. 2007;13:15–28. doi: 10.1016/j.devcel.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Yamaguchi T, Tokunaga A, Hara A, Hamaguchi T, Kato K, Iwamatsu A, Okano H, Kaibuchi K. Role of numb in dendritic spine development with a Cdc42 GEF intersectin and EphB2. Mol Biol Cell. 2006;17:1273–1285. doi: 10.1091/mbc.E05-07-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamidessi A, Frittoli E, Garre M, Faretta M, Mione M, Testa I, Diaspro A, Lanzetti L, Scita G, Di Fiore PP. Endocytic trafficking of Rac is required for the spatial restriction of signaling in cell migration. Cell. 2008;134:135–147. doi: 10.1016/j.cell.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Pece S, Confalonieri S, P RR, Di Fiore PP. NUMB-ing down cancer by more than just a NOTCH. Biochim Biophys Acta. 2011;1815:26–43. doi: 10.1016/j.bbcan.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419:929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- Petersen PH, Zou K, Krauss S, Zhong W. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nat Neurosci. 2004;7:803–811. doi: 10.1038/nn1289. [DOI] [PubMed] [Google Scholar]
- Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Proux-Gillardeaux V, Gavard J, Irinopoulou T, Mege RM, Galli T. Tetanus neurotoxin-mediated cleavage of cellubrevin impairs epithelial cell migration and integrin-dependent cell adhesion. Proc Natl Acad Sci U S A. 2005;102:6362–6367. doi: 10.1073/pnas.0409613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappoport JZ, Simon SM. Real-time analysis of clathrin-mediated endocytosis during cell migration. J Cell Sci. 2003;116:847–855. doi: 10.1242/jcs.00289. [DOI] [PubMed] [Google Scholar]
- Rasin MR, Gazula VR, Breunig JJ, Kwan KY, Johnson MB, Liu-Chen S, Li HS, Jan LY, Jan YN, Rakic P, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–491. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Salcini AE, Confalonieri S, Doria M, Santolini E, Tassi E, Minenkova O, Cesareni G, Pelicci PG, Di Fiore PP. Binding specificity and in vivo targets of the EH domain, a novel protein-protein interaction module. Genes Dev. 1997;11:2239–2249. doi: 10.1101/gad.11.17.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolini E, Puri C, Salcini AE, Gagliani MC, Pelicci PG, Tacchetti C, Di Fiore PP. Numb is an endocytic protein. J Cell Biol. 2000;151:1345–1352. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki AT, Janetopoulos C, Lee S, Charest PG, Takeda K, Sundheimer LW, Meili R, Devreotes PN, Firtel RA. G protein-independent Ras/PI3K/F-actin circuit regulates basic cell motility. J Cell Biol. 2007;178:185–191. doi: 10.1083/jcb.200611138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaar BT, McConnell SK. Cytoskeletal coordination during neuronal migration. Proc Natl Acad Sci U S A. 2005;102:13652–13657. doi: 10.1073/pnas.0506008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller U, Zhao Q, Godinho SA, Heine VM, Medema RH, Pellman D, Rowitch DH. Forkhead transcription factor FoxM1 regulates mitotic entry and prevents spindle defects in cerebellar granule neuron precursors. Mol Cell Biol. 2007;27:8259–8270. doi: 10.1128/MCB.00707-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA. Abnormal cerebellar development and foliation in BDNF−/− mice reveals a role for neurotrophins in CNS patterning. Neuron. 1997;19:269–281. doi: 10.1016/s0896-6273(00)80938-1. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Govek EE, Tomoda T, Hatten ME. Neuronal polarity in CNS development. Genes Dev. 2006;20:2639–2647. doi: 10.1101/gad.1462506. [DOI] [PubMed] [Google Scholar]
- Solecki DJ, Model L, Gaetz J, Kapoor TM, Hatten ME. Par6alpha signaling controls glial-guided neuronal migration. Nat Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- Ulrich F, Heisenberg CP. Trafficking and cell migration. Traffic. 2009;10:811–818. doi: 10.1111/j.1600-0854.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Valentin G, Haas P, Gilmour D. The chemokine SDF1a coordinates tissue migration through the spatially restricted activation of Cxcr7 and Cxcr4b. Curr Biol. 2007;17:1026–1031. doi: 10.1016/j.cub.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Wang Z, Sandiford S, Wu C, Li SS. Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. Embo J. 2009;28:2360–2373. doi: 10.1038/emboj.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaich L, Ooi J, Park M, Borg JP, Landry C, Bodmer R, Margolis B. Functional analysis of the Numb phosphotyrosine-binding domain using site-directed mutagenesis. J Biol Chem. 1998;273:10381–10388. doi: 10.1074/jbc.273.17.10381. [DOI] [PubMed] [Google Scholar]
- Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron. 1996;17:43–53. doi: 10.1016/s0896-6273(00)80279-2. [DOI] [PubMed] [Google Scholar]
- Zhou P, Porcionatto M, Pilapil M, Chen Y, Choi Y, Tolias KF, Bikoff JB, Hong EJ, Greenberg ME, Segal RA. Polarized signaling endosomes coordinate BDNF-induced chemotaxis of cerebellar precursors. Neuron. 2007a;55:53–68. doi: 10.1016/j.neuron.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Atkins JB, Rompani SB, Bancescu DL, Petersen PH, Tang H, Zou K, Stewart SB, Zhong W. The mammalian Golgi regulates numb signaling in asymmetric cell division by releasing ACBD3 during mitosis. Cell. 2007b;129:163–178. doi: 10.1016/j.cell.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Zwahlen C, Li SC, Kay LE, Pawson T, Forman-Kay JD. Multiple modes of peptide recognition by the PTB domain of the cell fate determinant Numb. Embo J. 2000;19:1505–1515. doi: 10.1093/emboj/19.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.