Abstract
Conjugated linoleic acid (CLA) has been shown to positively influence calcium and bone metabolism. Earlier, we showed that CLA (equal mixture of c9t11-CLA and t10c12-CLA) could protect age-associated bone loss by modulating inflammatory markers and osteoclastogenesis. Since, c9t11-CLA and t10c12-CLA isomers differentially regulate functional parameters and gene expression in different cell types, we examined the efficacy of individual CLA isomers against age-associated bone loss using 12 months old C57BL/6 female mice fed for 6 months with 10% corn oil (CO), 9.5% CO + 0.5% c9t11-CLA, 9.5% CO + 0.5% t10c12-CLA or 9.5% CO + 0.25% c9t11-CLA + 0.25% t10c12-CLA. Mice fed a t10c12-CLA diet maintained a significantly higher bone mineral density (BMD) in femoral, tibial and lumbar regions than those fed CO and c9t11-CLA diets as measured by dual-energy-x-ray absorptiometry (DXA). The increased BMD was accompanied by a decreased production of osteoclastogenic factors i.e. RANKL, TRAP5b, TNF-alpha and IL-6 in serum. Moreover, a significant reduction of high fat diet-induced bone marrow adiposity was observed in t10c12-CLA fed mice as compared to that of CO and c9t11-CLA fed mice, as measured by Oil-Red-O staining of bone marrow sections. In addition, a significant reduction of osteoclast differentiation and bone resorbing pit formation was observed in t10c12-CLA treated RAW 264.7 cell culture stimulated with RANKL as compared to that of c9t11-CLA and linoleic acid treated cultures. In conclusion, these findings suggest that t10c12-CLA is the most potent CLA isomer and it exerts its anti-osteoporotic effect by modulating osteoclastogenesis and bone marrow adiposity.
Keywords: Conjugated linoleic acids, Aging, Inflammation, bone resorption, Cytokines, Cell differentiation
Introduction
Osteoporosis constitutes a major worldwide public health burden, characterized by enhanced skeletal fragility. Nearly 200 million people worldwide, including approximately 25 million Americans, suffer from osteoporosis, a thinning of the bones. This leads to osteoporotic fracture, which is largely a problem of the elderly, particularly women [May et al., 1994]. Osteoporosis related costs are a major economic concern, expected to increase to $131 billion by year 2050 [Johnell, 1997]. After attaining peak bone mass between the ages of 20–30, both men and women start losing bone at a rate of about 0.5% to 1% yearly [McGarry and Kiel, 2000]. Bone mineral density (BMD) appears to decline with increasing age [Chevalley et al., 1991]. The decline in BMD and increased fracture risk are an inevitable part of the aging process, requiring new strategies for its prevention. However, osteoporosis is easier to prevent than to treat. Dietary therapy and/or lifestyle changes are considered as viable alternatives to minimize bone loss and to decrease the necessity for drug therapy to prevent osteoporosis. It is well established that dietary patterns modulate BMD in the elderly [Tucker et al., 2002]. Our recent study with CLA showed that it increases bone mass in both cancellous and cortical bones in young male Balb/C and aging female C57BL/6 mice [Banu et al., 2006a; Rahman et al., 2007].
Bone metabolism is the combination of bone resorption by osteoclasts and bone formation by osteoblasts. Increase in bone resorption is considered as the main contributor of bone loss, leading to osteoporosis; this loss is accompanied by increased bone marrow adiposity. The type of dietary fat intake and lifestyle choices are important determinants of age-related osteoporosis. The fats present in Western diets consist predominantly of saturated fatty acids and also n-6 polyunsaturated fatty acids (PUFA’s), derived from sources like corn, safflower, sunflower and soybean oils [Simopoulos, 1991]. The predominant n-6 fatty acid in these oils is linoleic acid (18:2n-6, LA), which is found to act as a pro-inflammatory fatty acid [Rahman et al., 2007].
CLA refers to a mixture of positionally and geometrically conjugated dienoic isomers of linoleic acid (LA). The c9t11-CLA isomer represents approximately 80% of the total isomers in dairy and ruminant fats; whereas, c9t11- and t10c12-CLA are equally abundant (usually 30–40% of each isomer) in commercial mixtures [Andreoli et al., 2009]. In recent decades, interest in CLA has increased due to its many bioactive properties related to health. In fact, very recently CLA has received FDA approval as GRAS (Generally Recognized As Safe) category for use in various food supplements. The benefits seem to be very clear, especially in some experimental animal models [Andreoli et al., 2009; Banu et al., 2006b; Bhattacharya et al., 2006b; Bhattacharya et al., 2005; Rahman et al., 2007]. In some animal models, dietary CLA reduce carcinogenesis, decrease body fat, increase lean body mass, enhance feed efficiency, protect against oxidative stress, modulate circulating lipids and prevent impaired glucose tolerance in diabetes [Bhattacharya et al., 2006a]. Several of these effects are controversial. In addition, some adverse results in some animal models: hepatomegaly [Tsuboyama-Kasaoka et al., 2000] and hepatic steatosis [Belury and Kempa-Steczko, 1997] have been noted. However, because of its approval by FDA as a GRAS category, it can be used in different food products.
Currently, the focus has been on the effects of CLA on skeletal health. Recently, it has been reported that dietary CLA may positively benefit BMD in postmenopausal women [Brownbill et al., 2005]. Increased whole body-ash in young mice fed diet with CLA supplementation suggests that CLA may enhance bone mineralization and protect against bone loss [Park et al., 1997]. Anhydrous butterfat, a rich natural source of CLA, also stimulated the rate of bone formation in young growing chicks by modulating prostaglandin (PG) E2, which plays an important role in the local regulation of bone formation and bone resorption [Watkins et al., 1997]. However, while CLA has been shown to increase bone mass, ash and/or mineral content in some studies with young growing experimental animals (mice [Park et al., 1997] and pigs [Thiel-Cooper et al., 2001]), others have reported a lack of effect in rats [Kelly, 2001; Li et al., 1999] and in pigs [Demaree et al., 2002]. We have demonstrated the beneficial effect of CLA against aging associated BMD loss in C57BL/6 mice [Rahman et al., 2007]. However, the effect of individual CLA isomers on age associated bone loss has not been investigated thoroughly. Hence, the present study was designed to examine the effect of corn oil (n-6 fatty acid), as a control for a westernized diet, and CLA isomers (c9t11-CLA and t10c12-CLA) on BMD in 12 month old female C57BL/6 mice fed for 6 months. The primary objective of the present investigation is to unravel the isomer specific effect of CLA on age associated bone loss in female C57BL/6 mice.
Materials and Methods
Animals
Eleven month-old female C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine 04609 USA). The age and weight matched animals were housed in a standard controlled animal care facility in cages (5 mice/cage) and fed a standard diet (Harlan Teklad LM-485) for one month. The animals were maintained in temperature controlled room (22–25°C, 45% humidity) on a 12:12-h dark-light cycle. National Institutes of Health guidelines were strictly followed, and all the studies were approved by the Institutional Laboratory Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio (San Antonio, TX). At completion of twelve month of age, the mice were divided into four groups, containing 15 mice in each. The mice were fed the American Institute of Nutrition (AIN)-93 diet, supplemented with CLA isomers and corn oil (CO) ad libitum for 6 months. Body weights were recorded weekly.
Diet preparation
The AIN-93 diets were supplemented with 10% CO as a fat diet. Four types of diets were (1) AIN-93 diet supplemented with 10% CO (CO) (2) AIN-93 diet supplemented with 9.5% CO and 0.5% c9t11-CLA (c9t11) (3) AIN-93 diet supplemented with 9.5% CO and 0.5% t10c12-CLA (t10c12) and (4) AIN-93 diet supplemented with 9.5% CO, 0.25% c9t11-CLA and 0.25% t10c12-CLA (CLA mix). The CLA isomers were supplied by Lipid Nutrition, Channahon, IL, USA. The c9t11-CLA enriched diet contained approximately 61% of c9t11-CLA isomer and t10c12-CLA contained 71 % of t10c12-CLA. The composition of the semi-purified diet per 100 g of diet was described earlier [Halade et al., 2009a; Halade et al., 2009b]. Fresh diet was provided everyday in the afternoon (between 3–4pm) and leftover food was removed daily to prevent rancidity.
Measurement of bone mineral density (BMD)
Bone mineral density was measured using dual-energy X-ray absorptiometry (DXA) Lunar PIXImus (GE, Madison, WI) and data was analyzed with PIXImus software, as described previously [Bhattacharya et al., 2006b; Bhattacharya et al., 2005; Rahman et al., 2007]. Scanning was performed first at baseline (12 month of age) and again at the end of 6 months of experimental diet’s feeding, as a final value.
Serum cytokines measurement
Serum cytokine levels for TNF-α and IL-6 were measured by standard ELISA techniques, using commercially available BD OptEIA ELISA kits for TNF-α and IL-6 (BD Biosciences, San Diego, CA), as described previously [Bhattacharya et al., 2006c].
Serum RANKL and TRAP5b measurement
Serum receptor activator of NF-κB ligand (RANKL) and tartrate resistant acid phosphatase (TRAP) were measured using mouse free sRANKL and mouse TRAP5b ELISA assay kits from Immunodiagnostic System (IDS) Inc. (Fountain Hills, AZ), according to the manufacturer’s instructions.
Tissue collection for biochemical analysis and bone histology
After completion of experimental diet feeding, mice were sacrificed. Blood was obtained by intraorbital capillary plexus and serum was collected and stored at −80°C. Bones were collected, and fixed in 10% neutral buffered formalin (NBF). Sections of bone were processed and embedded in paraffin and stained for Oil Red O.
Oil Red O staining of bone sections
Rear leg bone specimens (10 mice/group) from each dietary group were collected and trimmed of excess tissue and fixed in 10% NBF for 48 hrs at room temperature (RT). Bones were then placed in solution 1 (500ml 70% ethylene glycol, 5 grams linoleic acid, mixed for 1 hr at RT, allowed to stand several hours at RT in a separatory funnel, and the bottom layer drawn off) for 3 days at 56°C. Bones were then rinsed for at least 8 hrs in several changes of 70% ETOH at RT; then rinsed with several changes of water for at least 8 hrs, then decalcified with 2% chromic acid for 40 hrs at 4°C, and rinsed with water for 8 hrs, followed by incubation with 5% sodium bicarbonate/water for 24 hrs at RT. The bones were then rinsed with several changes of water for 24–72 hrs at RT. Bones were then placed in 70% ETOH, processed and embedded in paraffin, following standard protocol. Paraffin blocks were microtomed at 5μm sections on glass slides and allowed to dry overnight. Sections were then deparaffinized through xylene and graded alcohols. Sections were incubated in Oil red solution (Oil red O 0.7g and 200ml isopropanol, mixed, left overnight at RT, then filtered, and diluted in 120ml water, left overnight at 4°C, and filtered) for 1 hr at RT. Sections were then rinsed with water until clear, and counterstained with hematoxylin and crystal mounted.
Osteoclast like cell formation assay
RAW 264.7 cells were suspended in phenol free α-MEM containing 10% fetal bovine serum (FBS) and plated at a concentration of 2 × 104 cells/well in a 48-well culture dish. Cells were cultured in the presence of 50 ng/ml RANKL and incubated for 24 hrs. Various concentrations of LA, c9t11-CLA, t10c12-CLA or CLA-mix were added to the cultures. The medium and factors were replaced every 3 days. After 5 days of culture in the presence of RANKL and isomers, the cells were fixed and stained for TRAP, using TRAP staining kit from Sigma (St Louis, MO), according to the manufacturer’s instruction [Rahman, 2006]. TRAP+ cells with more than 3 nuclei were counted as TRAP +ve multinucleated cells (MNCs).
Pit formation assay
RAW 264.7 cells were suspended in phenol free α-MEM containing 10% FBS and plated at a concentration of 1 × 104 cells/well in an Osteoclast Activity Assay Substrate 24-well plate (OAAS™) (OCT USA Inc, CA 90501), in the presence or absence of 50 ng/ml RANKL and incubated for 24 hrs. Subsequently, 50 μM of LA, c9t11-CLA, t10c12-CLA or CLA-mix were added to the cultures. Half of the medium with fresh factors were replaced every 2 days. After 7 days of culture, the plates were washed in 6% sodium hypochlorite solution to remove the cells. The resorbed areas on the plates were captured with a digital camera attached to a microscope and analyzed by Metaview Image Analysis System [Rahman, 2006]. Results were expressed in % resorption area.
Statistical analysis
Data are presented as mean values ± S.E.M. Differences among the groups (CO, c9,t11-CLA, t10,c12-CLA and CLA mix) were tested by one-way analysis of variance and student’s t-test. Newman-Keuls post hoc test were used followed by ANOVA if multiple correlations were made. A p value < 0.05 was considered statistically significant. The analyses were performed with Graphpad prism for Windows (La Jolla, CA).
Results
Effect of dietary fat on BMD
BMD was measured at 12 month of age (baseline) and after 6 months of experimental diets (final value). Data were shown in Table 1. A notable reduction of BMD in lumbar regions of bone with age was observed in CO fed mice. However, a significant protection of BMD loss in lumbar regions of bone was observed with t10c12-CLA. With c9t11-CLA and CLA-mix no statistically significant protection was observed in these regions as some slight although insignificant protection is indicated. Slight but insignificant increase in BMD value in distal femoral metaphysis and tibial diaphysis regions was observed in t10c12-CLA and CLA-mix supplemented mice as compared to CO and c9t11-CLA supplemented mice. However, there was significant increase in femoral diaphysis region in t10c12-CLA supplemented mice as compared to CO and c9t11-CLA supplemented mice. Some slight although insignificant increase in BMD value in this region was noted in CLA-mix supplemented mice. A similar kind of protection of BMD loss in different regions of bones was also observed in a separate experiment with 18 month old C57BL/6 female mice fed for 3 months with CO and CLA isomers diets (data not shown). These data suggest that t10c12-CLA and CLA-mix fed aging mice maintain higher cancellous and cortical BMD compared to CO and c9t11-CLA fed mice. Therefore, t10c12-CLA isomer might be the active component of CLA-mix in maintaining higher BMD during aging.
Table 1.
Effect of CLA isomers on bone mineral density (BMD) (g/cm2) in C57BL/6 mice.
| Parameter | Diet | ||||
|---|---|---|---|---|---|
| CO | c9t11-CLA | t10c12-CLA | CLA-mix | ||
| L2 (g/cm2) | Baseline | 0.061±0.001 | 0.059±0.001 | 0.064±0.001 | 0.067±0.003 |
| Final | 0.059±0.003 | 0.060±0.002 | 0.076±0.002a | 0.070±0.003 | |
| % Difference | −2.20±5.33 | 2.50±4.80 | 20.77±4.00 a | 9.08±4.25 | |
| L3 (g/cm2) | Baseline | 0.058±0.001 | 0.061±0.001 | 0.061±0.001 | 0.064±0.001 |
| Final | 0.057±0.003 | 0.064±0.003 | 0.072±0.002a | 0.076±0.003c | |
| % Difference | −1.13±7.52 | 4.30±4.34 | 20.15±5.83c | 20.44±10.47 | |
| L4 (g/cm2) | Baseline | 0.059±0.002 | 0.056±0.002 | 0.059±0.001 | 0.058±0.003 |
| Final | 0.058±0.002 | 0.059±0.003 | 0.074±0.001a | 0.063±0.003 | |
| % Difference | −1.193±6.00 | 10.50±5.25 | 26.60±5.16a | 15.23±13.98 | |
| DFM (g/cm2) | Baseline | 0.092±0.001 | 0.091±0.001 | 0.092±0.001 | 0.096±0.002 |
| Final | 0.098±0.002 | 0.098±0.001 | 0.104±0.001c | 0.106±0.004 | |
| % Difference | 7.12±3.44 | 8.51±1.82 | 11.82±2.30 | 10.14±2.07 | |
| PTM (g/cm2) | Baseline | 0.096±0.001 | 0.093±0.001 | 0.099±0.001 | 0.096±0.003 |
| Final | 0.102±0.003 | 0.104±0.002 | 0.106±0.001 | 0.110±0.003 | |
| % Difference | 7.07±3.54 | 6.97±1.44 | 11.04±1.40 | 15.02±2.23 | |
| FD (g/cm2) | Baseline | 0.056±0.001 | 0.056±0.001 | 0.057±0.001 | 0.058±0.001 |
| Final | 0.065±0.001 | 0.065±0.001 | 0.070±0.002b | 0.067±0.001 | |
| % Difference | 15.05±2.85 | 15.86±3.97 | 22.70±2.80c | 16.65±3.24 | |
| TD (g/cm2) | Baseline | 0.044±0.002 | 0.045±0.001 | 0.044±0.001 | 0.045±0.002 |
| Final | 0.045±0.001 | 0.046±0.002 | 0.047±0.001 | 0.048±0.002 | |
| % Difference | 3.26±1.56 | 3.64±1.50 | 9.12±1.68 | 9.05±1.49 | |
12 month old mice were fed for 6 months with experimental diets. Values are means ± SEM, n = 15. Each group was compared to CO control group by Student’s t-test. p<0.05 was considered significant.
p<0.001,
p<0.01 and
p<0.05.
DFM: Distal femoral metaphysis; PTM: Proximal tibial metaphysis; FD: Femoral diaphysis; TD: Tibial diaphysis; L2: Lumbar vertebra 2; L3: Lumbar vertebra 3; L4: Lumbar vertebra 4.
Effect of CLA on serum cytokines
To examine if CLA isomers differentially affect proinflammatory cytokines, we determined the serum IL-6 and TNF-α levels in c9t11-CLA, t10c12-CLA, CLA-mix and CO fed mice. Serum TNF-α and IL-6 levels were decreased significantly in both c9t11-CLA and t10c12-CLA fed mice, including CLA-mix fed mice, compared to CO fed mice (Figure 1A & B). The results indicate that both CLA isomers equally down-regulate the osteoclastogenic proinflammatory cytokines, IL-6 and TNF-α. CLA-mix fed mice exhibited little bit more reduction of these cytokines in serum than individual isomers do. Since both the CLA isomers independently reduce the pro-inflammatory cytokines production in C57BL/6 mice, CLA-mix fed mice exhibit more reduction of these cytokines may be because of the combined effects of these isomers.
Figure 1. Effect of CLA isomers on the production of inflammatory cytokines.
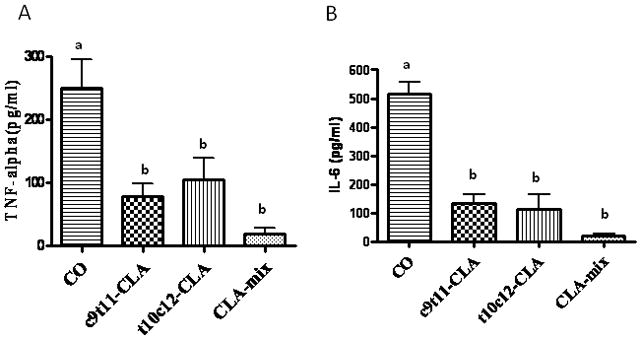
Twelve month old C57BL/6 mice were fed with experimental diets for 12 months and serum was collected after sacrifice. Serum was analyzed for TNF-α (A) and IL-6 (B) using standard ELISA kits. Each bar represents the mean ± S.E.M. of 5 samples. Value with different superscripts are significantly different at p<0.05 by Newman-Keuls’ one way ANOVA with multiple comparison test. A. CO vs. c9t11-CLA (p<0.05), CO vs. t10c12-CLA (p<0.05), CO vs. CLA-mix (p<0.01). B. CO vs. c9t11-CLA (p<0.001), CO vs. t10c12-CLA (p<0.001), CO vs. CLA-mix (p<0.001).
Effect of CLA on serum sRANKL and TRAP5b
To examine if CLA isomers differentially affect the pivotal osteoclastogenic factor, RANKL, we examined the level of sRANKL by ELISA. Interestingly, RANKL level was significantly less in t10c12-CLA and CLA-mix fed mice than in CO and c9t11-CLA fed mice (Figure 2A). As serum TRAP5b levels indicate the current status of bone resorbing osteoclast’s function, i.e. TRAP activity, we measured the serum TRAP5b level as a bone resorption marker, by means of a mouse TRAP5b ELISA kit. Similarly, we found a significant reduction of TRAP5b activity in t10c12-CLA and CLA-mix fed mice (Figure 2B). These results support the notion that t10c12-CLA isomer might be the active component of CLA-mix, modulating osteoclastogenesis.
Figure 2. Effect of CLA isomers on osteoclastogenic factor and bone resorption marker.
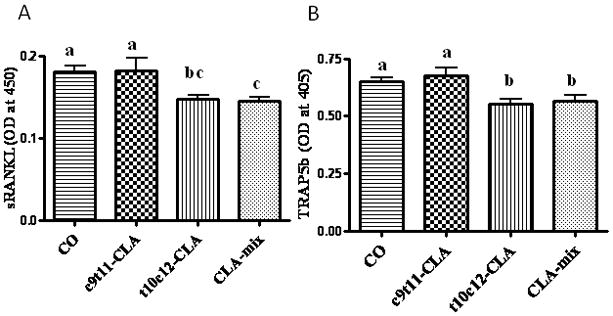
Twelve month old C57BL/6 mice were fed with experimental diets for 12 months and serum was collected after sacrifice. Serum was analyzed for RANKL (A), as an osteoclastogenic factor, and for TRAP5b (B), as a bone resorbing factor. Each bar represents the mean ± S.E.M. of 8 samples. Value with different superscripts are significantly different at p<0.05 by Newman-Keuls’ one way ANOVA with multiple comparison test. A. CO vs. t10c12-CLA (p<0.01), CO vs. CLA-mix (p<0.05), c9t11-CLA vs. CLA-mix (p<0.05), c9t11-CLA vs. t10c12-CLA (p<0.05). B. CO vs. t10c12-CLA (p<0.05), CO vs. CLA-mix (p<0.05), c9t11-CLA vs. CLA-mix (p<0.05), c9t11-CLA vs. t10c12-CLA (p<0.01).
Effect of CLA isomers on bone marrow adiposity
Bone marrow adipogenesis is a normal physiologic process in all mammals. Adipocyte in bone marrow regions increases with age. As both osteoblasts and adipocytes differentiate from a common progenitor cell, the more adipocytes mean less osteoblasts. Age associated increase in adipocytes is considered as one of the vital factors for less bone formation during aging. CLA is a well known dietary fatty acid capable of reducing adipogenesis. Therefore, we examined if CLA has any effect in reducing age associated adipocytes generation in bone marrow regions. We also examined if CLA isomers differentially affect this parameter. Interestingly, we found that only t10c12-CLA and CLA-mix are capable of reducing bone marrow adipocytes, compared to CO fed control (Figure 3). However, c9t11-CLA alone did not show any notable reduction of bone marrow adipocytes (Figure 3). These results indicate that t10c12-CLA is the active component of CLA-mix, exerting its effect by inhibiting adipogenesis; thereby, helping osteogenesis.
Figure 3. Effect of CLA isomers on bone marrow adipocytes.
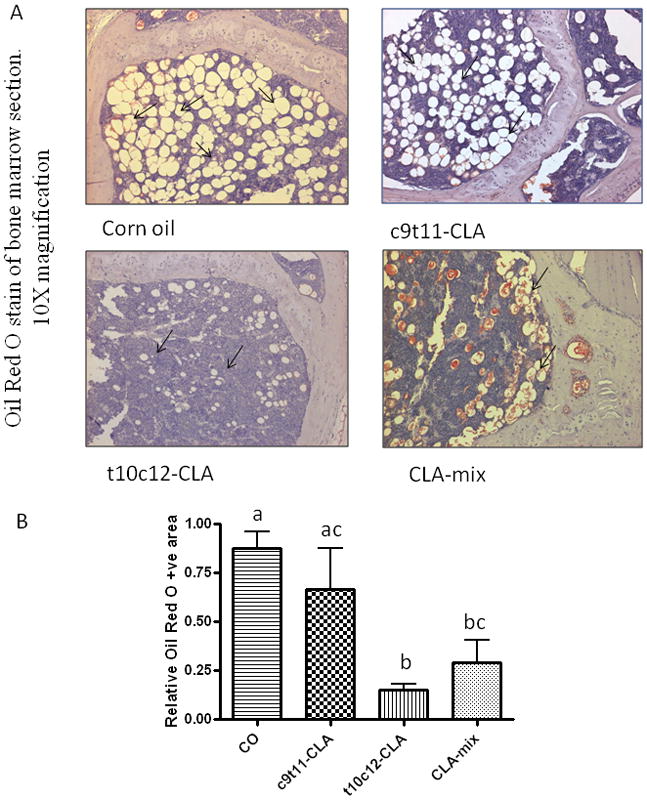
Twelve month old C57BL/6 mice were fed with experimental diets for 12 months. Bones (femur and tibia) were collected after sacrifice and fixed in 10% neutral buffered saline (NBF). Sections of bone were processed and embedded in paraffin and stained for Oil Red O. Arrow shows the adipocytes in the bone marrow cavity (A). The data represent the mean ± S.E.M. of adipocyte area of the upper half of the diaphysis of femur from 3 mice per group (B). Value with different superscripts are significantly different at p<0.05 by Newman-Keuls’ one way ANOVA with multiple comparison test. 10X magnification. CO vs. t10c12-CLA (p<0.05), CO vs. CLA-mix (p<0.05), c9t11-CLA vs. t10c12-CLA (p<0.05).
Effect of CLA isomers on RANKL-stimulated TRAP-positive osteoclast like cells formation in RAW 264.7 cells
As RAW 264.7 cells differentiate into osteoclasts in the presence of RANKL, we examined the effect of CLA isomers on the osteoclast differentiation of RAW 264.7 cells, in the presence of RANKL, using TRAP staining. Previously, we showed that CLA-mix dose dependently inhibited total TRAP activity and multinucleated osteoclast like cells formation in RANKL stimulated RAW 264.7 cells. But it was not known whether c9t11-CLA and t10c12-CLA differentially affect osteoclast differentiation in RAW264.7 cells. In this study, we examined the effect of individual isomers on RANKL stimulated osteoclast differentiation of RAW264.7 cells. As seen in the in vivo experiments, only t10c12-CLA and CLA mix are capable of inhibiting osteoclast differentiation in RAW264.7 cells, as compared to the linoleic acid control (Figure 4). There was no inhibitory effect observed in c9t11-CLA treated cultures. These results indicate, for the first time, that t10c12-CLA is the active component of CLA-mix, exerting its effect by modulating bone resorbing osteoclast formation.
Figure 4. Effect of CLA isomers on TRAP-positive osteoclast formation.
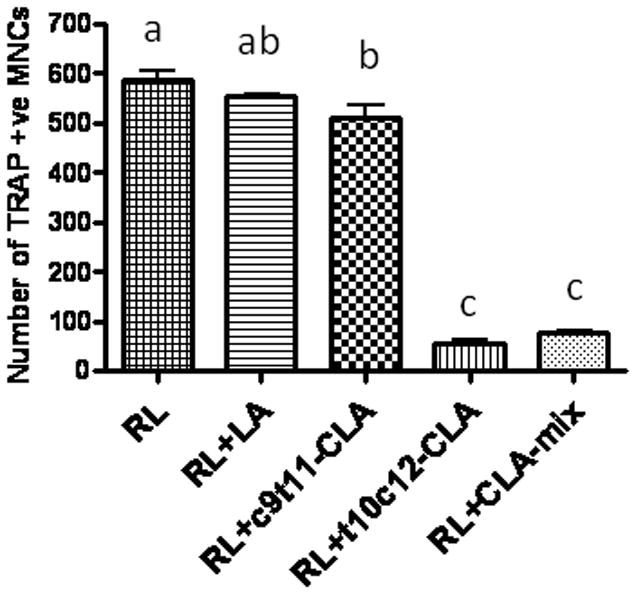
RAW 264.7 cells were suspended in phenol free α-MEM containing 10% FBS and plated at a concentration of 2 × 104 cells/well into a 48-well culture dish (Corning, NY), with different concentrations of linoleic acid (LA), c9t11-CLA, or t10c12-CLA at a concentration of 50 μM, in the presence of 50 ng/ml RANKL. The medium and factors were replaced every 3 days. After 5 days of culture with factors, the cells were fixed and stained for TRAP using TRAP staining kit, according to the manufacturer’s instruction. TRAP+ cells, with more than 3 nuclei, were counted as TRAP +ve multinucleated cells (MNCs). Each bar represents the mean ± S.E.M. of 3 independent triplicate cultures. Value with different superscripts are significantly different at p<0.05 by Newman-Keuls’ one way ANOVA with multiple comparison test. RL, Receptor activator of NF-κB. LA vs. t10c12-CLA (p<0.001), LA vs. CLA-mix (p<0.001), c9t11-CLA vs. t10c12-CLA (p<0.001), c9t11-CLA vs. CLA-mix (p<0.001).
Effect of CLA isomers on bone resorption in RANKL-stimulated RAW 264.7 cells
As we have already seen that t10c12-CLA dramatically inhibits the formation of osteoclast like multinucleated cells, which are believed to be responsible for bone resorption, we further examined if CLA isomers differentially affect these mature osteoclasts to resorb bone. We used calcium phosphate coated culture plates to stimulate RAW264.7 cells, with RANKL, to differentiate into mature osteoclasts, having bone resorbing capacity. RANKL stimulated RAW 264.7 cells showed a number of resorption areas (Figure 5A). Cultures treated with t10c12-CLA and CLA-mix showed significantly reduced numbers and areas of resorption pits, compared to cultures treated with either RANKL alone or together with LA (Figure 5B). There was no notable reduction of bone resorbing pit formation in c9t11-CLA treated cultures. These results indicate that t10c12-CLA is the active component of CLA-mix, exerting its effect by inhibiting osteoclastic bone resorption.
Figure 5. Effect of CLA isomers on bone resorbing pit formation.
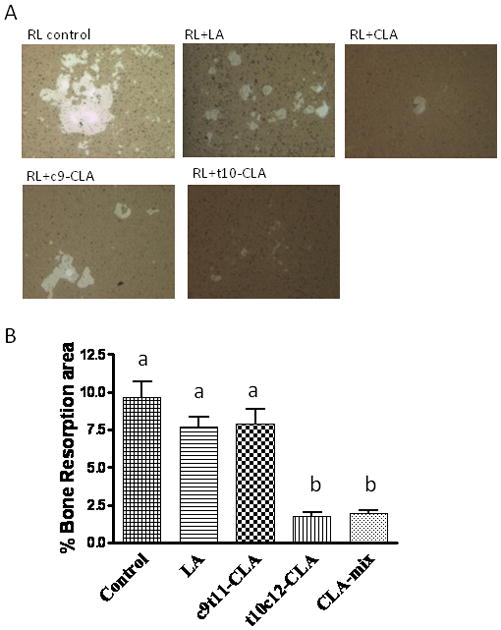
RAW 264.7 cells were suspended in phenol free α-MEM containing 10% FBS and plated at a concentration of 1 × 104 cells/well on an Osteoclast Activity Assay Substrate (OAAS™) plate (OCT USA Inc, CA 90501) in the presence of LA (50μM), c9t11-CLA (50μM), or t10c12-CLA (50μM), with 50 ng/ml RANKL. These plates were coated with artificial bone (calcium phosphate coating). Half of the medium with factors was replaced every 2 days. After 7 days of culture in the presence of factors, the plates were washed in 6% sodium hypochlorite solution to remove the cells. The resorbed areas on the plates were captured with a digital camera attached to the microscope and analyzed by Metaview Image Analysis System (A). Each bar represents the mean ± S.E.M. of 3 independent triplicate cultures (B). Value with different superscripts are significantly different at p<0.05 by Newman-Keuls’ one way ANOVA with multiple comparison test. RL, Receptor activator of NF-κB. 10X magnification. LA vs. t10c12-CLA (p<0.001), LA vs. CLA-mix (p<0.001), c9t11-CLA vs. t10c12-CLA (p<0.001), c9t11-CLA vs. CLA-mix (p<0.001).
Discussion
The progressive loss of bone mass is believed to play a major role in the pathogenesis of frailty and functional impairment that occurs with advancing age. Our hypothesis is that a high fat diet, already prevalent in the American population when supplemented with CLA, counteracts inflammation and related excessive osteoclastic bone resorption, and as a result, improves bone mass and strength. Accordingly, we first investigated the isomer specific effect of CLA on the prevention of age associated bone loss in aging C57BL/6 mice since previous studies showed that CLA protects bone mineral density loss [Bhattacharya et al., 2006a; Bhattacharya et al., 2005; Rahman et al., 2007]. These earlier studies however, were conducted with an equal mixture of c9t11-CLA and t10c12-CLA isomers. Mixed CLA isomers variably affect bone resorption in animals and decrease osteoclast formation and activity in murine osteoclasts. Such variable effects may be due to the different isomers present in commercial preparations of CLA, and the specific effects of the predominant individual isomers, c9t11-CLA and t10c12-CLA are not clear. Here we show for the first time effect of individual CLA isomers in attenuating age-related bone loss. In earlier study, we showed that C57BL/6 mice when fed with 10% CO exhibit increased levels of inflammation, obesity and osteoporosis [Halade et al.; Halade et al.]. In this study, we observed that inclusion of CLA isomers, especially t10c12-CLA in the high-fat-diet (10% CO), maintained a higher BMD during aging, most likely by modulating inflammatory mediators, bone marrow adiposity and osteoclastogenesis. In a previous study, both c9t11-CLA and t10c12-CLA isomers are reported to inhibit osteoclast formation and activity in human CD14+ monocytes [Platt and El-Sohemy, 2009a]. In our study, we found that only the t10c12-CLA isomer exhibits anti-osteoclastogenic effects in RANKL stimulated murine macrophage cell line RAW264.7. These variations could be due to the different cell types used. However, in both studies CLA-mix showed inhibition of osteoclastogenesis.
In this study we have chosen 12 month old female C57BL/6 mice. After attaining peak bone mass between the ages of 20–30, both men and women start losing bone at a rate of about 0.5% to 1% yearly [McGarry and Kiel, 2000]. Based on a comparative biology calculation, 12 months of mouse age is equivalent to 35 years of human age, which is full adulthood. Similarly 18 months of mouse age corresponds to 52 year of human age. Women at the age of menopause at around 50–55 year old start losing BMD at an accelerated rate. To protect this accelerated BMD loss at the age of menopause preventive medicine/supplement should be taken from the adult age to at least premenopausal age. Therefore, in this study, we chose to use 12 month female C57BL/6 mice to feed for 6 months with experimental diets supplemented with CLA isomers in order to compare these data directly to human studies.
In this study, we focused primarily on the protective effect of CLA isomers against bone resorption; however, CLA has been reported to enhance immunity and bone formation [Brownbill et al., 2005]. Current evidence suggests that CLA may help decrease bone loss, by reducing prostaglandins in bone tissue [Watkins and Seifert, 2000; Watkins et al., 1997] or by enhancing calcium absorption [Kelly et al., 2003]. CLA was also reported to increase the expression of the bone formation markers, osteocalcin and alkaline phosphatase, in murine osteoblastic cell lines [Watkins et al., 2001]. Very recently, Park et al reported that in male mice, t10c12-CLA but not c9t11-CLA is capable of enhancing bone formation in the presence of 1% calcium supplementation which is consistent with our findings that the t10c12-CLA may be the useful CLA isomer capable of improving BMD [Park and Terk]. We analyzed the effects of individual isomers c9t11-CLA and t10c12-CLA on BMD using DEXA in the cancellous and cortical bones of the femur, tibia and lumbar spine in aging C57BL/6 female mice. When 12-month-old mice were fed either CO or CO+c9t11-CLA or CO+t10c12-CLA or CO+CLA-mix for 6 months, t10c12-CLA and CLA-mix fed mice were found to maintain a higher BMD in pure cortical and cancellous bones. These findings correlated with decreased levels of pro-inflammatory cytokines TNF-α and IL-6 in the serum of t10c12-CLA and CLA-mix fed mice, as compared to CO control mice. However, serum TNF-α and IL-6 levels were significantly lower in c9t11-CLA fed mice, without affecting osteoclastogenesis. Slightly enhanced but non-significant reduction of both TNF-α and IL-6 levels was noted in CLA-mix group as compared to individual isomers groups. This effect could be due to combined effect of c9t11-CLA and t10c12-CLA. Anti-inflammatory effect of both c9t11-CLA and t10c12-CLA isomers have been reported [Platt and El-Sohemy, 2009a]. There are evidences that antiosteoclastogenic drugs protect bone loss by blunting the production of IL-1, IL-6, and TNF-α [Manolagas and Jilka, 1995; Poli et al., 1994], which stimulate stromal cell production of RANKL and macrophage colony stimulating factor (M-CSF), the sole regulators of osteoclastogenesis [Cappellen et al., 2002; Hofbauer et al., 1999; Kimble et al., 1996]. Cheng et al reported that CLA inhibits LPS-induced inflammatory events in the macrophage cell line RAW 264.7, an osteoclast precursor cell, by negatively regulating inflammatory mediators and NF-κB activation [Cheng et al., 2004] [Iotsova et al., 1997]. Differentiation of osteoclasts is regulated by the osteoclastogenic factor RANKL [Suda et al., 1992; Suda, 1999]. We therefore examined the level of RANKL in the serum of CLA isomers fed mice. Significantly lower levels of serum RANKL was observed in t10c12-CLA and CLA-mix fed animals compared to CO and c9t11-CLA fed animals. This result indicates that t10c12-CLA is likely the active component in CLA, mediating protection against age-associated BMD loss by modulating osteoclastogenesis. However, further study should be undertaken to determine the effect of individual isomers on bone formation parameters.
Here we also determined the anti-bone resorbing capacity of individual isomers by measuring the level of serum TRAP5b. TRAP is primarily a cytochemical marker of macrophages, osteoclasts and dendritic cells [Lamp and Drexler, 2000]. Although osteoclasts contain abundant TRAP and are responsible for bone resorption, the total TRAP activity in the serum as measured by colorimetric methods, little reflects bone turnover [Igarashi et al., 2002]. TRAP 5 can be further separated into 5a and 5b by electrophoresis. TRAP 5b is considered a prominent product of osteoclasts. Thus serum TRAP 5b levels might reflect the status of bone resorption more closely [Igarashi et al., 2002]. Therefore, the reduction of TRAP5b activity in the t10c12-CLA and CLA-mix fed mice suggests a reduction of bone resorbing activity. These findings indicate the possible mechanisms of age associated bone loss protection by t10c12-CLA and CLA-mix may be the modulation of osteoclastogenic bone resorption by altering osteoclastogenic factors. To determine the effect of individual isomers on osteoclastogenesis, we stimulated RAW 264.7 cells with RANKL in the presence of individual isomers, and found a dramatic inhibition of TRAP +ve osteoclast cells formation in t10c12-CLA and CLA-mix treated cultures, as compared to linoleic acid and c9t11-CLA treated cultures. However, Platt et al found that both c9t11-CLA and t10c12-CLA inhibit osteoclast formation from CD14+ monocytes, with c9t11-CLA showing a greater effect. We did not find an inhibitory effect with c9t11-CLA in the RAW264.7 cells. These variations could be due to different cell types used although both are of monocytic lineage. Furthermore, using RAW264.7 cells, we analyzed the bone resorbing pit formation in CLA isomers treated cultures and found a dramatic reduction of bone resorbing pit formation in t10c12-CLA and CLA-mix treated cells, as compared to CO and c9t11-CLA treated cells. However, Platt and colleagues found a similar level of inhibition of osteoclast activity with both c9t11-CLA and t10c12-CLA in CD14+ monocytes. Our results indicate that t10c12-CLA isomer is the active isomer in the CLA-mix, in inhibiting RANKL stimulated osteoclast differentiation and function in RAW264.7 cells.
Bone marrow adipogenesis is a normal physiologic process in all mammals, although its full function is unknown. With aging, the composition of bone marrow shifts to favor the presence of adipocytes, osteoclast activity increases, and osteoblast function declines, resulting in osteoporosis [Rosen et al., 2009; Rosen and Klibanski, 2009]. The mesenchymal stem cell is the marrow precursor for adipocytes, as well as osteoblasts. There is an inverse relationship between adipocytes and osteoblasts within the bone marrow cavity. Increased bone marrow adiposity is believed to be the reason for less bone formation during aging. Decreased bone mass is observed in age-related osteoporosis, due to increased marrow adipose tissue [Pei and Tontonoz, 2004]. Due to CLA’s capability to reduce adipogenesis, it is possible that it may reduce adipocytes in bone marrow regions. Interestingly, we found that both t10c12-CLA and CLA mix fed mice exhibited fewer adipocytes in bone marrow regions, whereas c9t11-CLA did not appear to have any effect. It is well established that only t10c12-CLA is capable of reducing the abdominal adipocytes [Halade et al., 2009a; Halade et al., 2009b; Wang and Jones, 2004]. However, here we show for the first time that t10c12-CLA, but not c9t11-CLA, is able to reduce bone marrow adiposity during aging. This may be one of the mechanisms by which t10c12-CLA exerts its effect against age-associated BMD loss. Thus, t10c12-CLA will negatively modulate adipogenesis, while promoting osteogenesis. Unraveling the interface between bone and fat at a molecular and cellular level is likely to lead to a better understanding of several diseases and to the development of drugs, for both osteoporosis and obesity [Rosen et al., 2009; Rosen and Klibanski, 2009]. However, more studies are needed to understand the interrelationship among hematopoietic, osteoblastic, and adipogenic cells within the marrow niche [Rosen et al., 2009; Rosen and Klibanski, 2009].
In our study shows that t10c12-CLA and CLA mix can prevent age-associated BMD loss by inhibiting the production of pro-inflammatory and pro-osteoclastogenic factors, osteoclastic bone resorption and bone marrow adiposity. It is very likely that these agents may inhibit bone resorption by suppressing NF-κB and MAPK signaling pathways. Since these agents also reduce bone marrow adiposity, it is also likely that they may up-regulate Wnt signaling to promote osteoblastogenesis as osteoblasts and adipocytes arise from the same progenitor cells, mesenchymal stem cells. Recently, Platt et al showed that t10c12-CLA isomer is capable of escalating osteoblast differentiation in human mesenchymal stem cells by up-regulating Wnt signaling [Platt and El-Sohemy, 2009b]. However, isomer specific effects have to be analyzed in future studies.
In conclusion, our study shows that the t10c12-CLA isomer is the active component of the CLA-mix that exerts an anti-osteoporotic effect. However, the t10c12-CLA isomer alone is known to have some adverse effects, such as: fatty liver formation, insulin resistance, etc., which can be corrected by combining with the c9t11-CLA isomer, which is known to improve insulin sensitivity, as well as fatty liver formation [Moloney et al., 2007]. As CLA-mix also showed an equal efficacy as of t10c12-CLA isomer alone; therefore, the CLA-mix could be an ideal dietary supplement to protect/delay age-associated BMD loss. Moreover, the majority of currently available anti-osteoporotic drugs exert its effect by inhibiting osteoclastogenesis, meaning less bone resorption, without stimulating bone formation. Old dense bone may not represent strong bone. Therefore, agents that modulate both bone resorption and bone formation might provide a better option for maintaining the quality and strength of bone during aging. We and others have shown that CLA inhibits osteoclastogenesis [Platt and El-Sohemy, 2009a; Rahman et al., 2007; Rahman et al., 2006] and others have shown its positive effects on bone formation [Platt and El-Sohemy, 2009b; Watkins and Seifert, 2000; Watkins et al., 1997]. We recently showed that c9t11-CLA and t10c12-CLA also act differentially in vivo. c9t11-CLA had no effect in reducing fat mass and improving BMD, whereas t10c12-CLA was able to reduce fat mass and improve lean mass in young and middle aged mice [Halade et al., 2009a; Halade et al., 2009b]. Furthermore, our very recent findings that CLA-mix can modulate bone marrow adiposity also support the notion that CLA may help bone formation. Thus, CLA-mix could be a promising dietary supplement to prevent age associated BMD loss by modulating both bone resorption and bone formation. More studies with different ratios of c9t11-CLA and t10c12-CLA are warranted to establish their mechanisms of action in modulating bone mass, muscle mass and fat mass during aging.
Acknowledgments
We acknowledge Kazi Nishu for her technical help. We also acknowledge Dr. Anthony J. Valente for his kind review of our manuscript for scientific and grammatical corrections.
Grants: Contract grant sponsor, NIH; Contract grant number, AG027562 and AG034233-01A1.
References
- Andreoli MF, Gonzalez MA, Martinelli MI, Mocchiutti NO, Bernal CA. Effects of dietary conjugated linoleic acid at high-fat levels on triacylglycerol regulation in mice. Nutrition. 2009;25:445–52. doi: 10.1016/j.nut.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Banu J, Bhattacharya A, Rahman M, O’Shea M, Fernandes G. Effects of conjugated linoleic acid and exercise on bone mass in young male Balb/C mice. Lipids Health Dis. 2006a;5:7. doi: 10.1186/1476-511X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu J, Bhattacharya A, Rahman M, O’Shea M, Fernandes G. Effects of conjugated linoleic acid and exercise on bone mass in young male Balb/C mice. Lipids Health Dis. 2006b;5:7. doi: 10.1186/1476-511X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belury MA, Kempa-Steczko A. Conjugated linoleic acid modulates hepatic lipid composition in mice. Lipids. 1997;32:199–204. doi: 10.1007/s11745-997-0025-0. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Banu J, Rahman M, Causey J, Fernandes G. Biological effects of conjugated linoleic acids in health and disease. J Nutr Biochem. 2006a;17:789–810. doi: 10.1016/j.jnutbio.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Rahman MM, McCarter R, O’Shea M, Fernandes G. Conjugated linoleic acid and chromium lower body weight and visceral fat mass in high-fat-diet-fed mice. Lipids. 2006b;41:437–44. doi: 10.1007/s11745-006-5117-3. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Rahman MM, Sun D, Lawrence R, Mejia W, McCarter R, O’Shea M, Fernandes G. The combination of dietary conjugated linoleic acid and treadmill exercise lowers gain in body fat mass and enhances lean body mass in high fat-fed male Balb/C mice. J Nutr. 2005;135:1124–30. doi: 10.1093/jn/135.5.1124. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Sun D, Rahman M, Fernandes G. Different ratios of eicosapentaenoic and docosahexaenoic omega-3 fatty acids in commercial fish oils differentially alter pro-inflammatory cytokines in peritoneal macrophages from C57BL/6 female mice. J Nutr Biochem. 2006c doi: 10.1016/j.jnutbio.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Brownbill RA, Petrosian M, Ilich JZ. Association between dietary conjugated linoleic acid and bone mineral density in postmenopausal women. J Am Coll Nutr. 2005;24:177–81. doi: 10.1080/07315724.2005.10719463. [DOI] [PubMed] [Google Scholar]
- Cappellen D, Luong-Nguyen NH, Bongiovanni S, Grenet O, Wanke C, Susa M. Transcriptional program of mouse osteoclast differentiation governed by the macrophage colony-stimulating factor and the ligand for the receptor activator of NFkappa B. J Biol Chem. 2002;277:21971–82. doi: 10.1074/jbc.M200434200. [DOI] [PubMed] [Google Scholar]
- Cheng WL, Lii CK, Chen HW, Lin TH, Liu KL. Contribution of conjugated linoleic acid to the suppression of inflammatory responses through the regulation of the NF-kappaB pathway. J Agric Food Chem. 2004;52:71–8. doi: 10.1021/jf0348626. [DOI] [PubMed] [Google Scholar]
- Chevalley T, Rizzoli R, Nydegger V, Slosman D, Tkatch L, Rapin CH, Vasey H, Bonjour JP. Preferential low bone mineral density of the femoral neck in patients with a recent fracture of the proximal femur. Osteoporos Int. 1991;1:147–54. doi: 10.1007/BF01625444. [DOI] [PubMed] [Google Scholar]
- Demaree SR, Gilbert CD, Mersmann HJ, Smith SB. Conjugated linoleic acid differentially modifies fatty acid composition in subcellular fractions of muscle and adipose tissue but not adiposity of postweaning pigs. J Nutr. 2002;132:3272–9. doi: 10.1093/jn/132.11.3272. [DOI] [PubMed] [Google Scholar]
- Halade GV, Rahman MM, Fernandes G. Differential effects of conjugated linoleic acid isomers in insulin-resistant female C57Bl/6J mice. J Nutr Biochem. 2009a doi: 10.1016/j.jnutbio.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Halade GV, Rahman MM, Fernandes G. Effect of CLA isomers and their mixture on aging C57Bl/6J mice. Eur J Nutr. 2009b;48:409–18. doi: 10.1007/s00394-009-0029-7. [DOI] [PubMed] [Google Scholar]
- Halade GV, Rahman MM, Williams PJ, Fernandes G. Combination of conjugated linoleic acid with fish oil prevents age-associated bone marrow adiposity in C57Bl/6J mice. J Nutr Biochem. doi: 10.1016/j.jnutbio.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halade GV, Rahman MM, Williams PJ, Fernandes G. High fat diet-induced animal model of age-associated obesity and osteoporosis. J Nutr Biochem. doi: 10.1016/j.jnutbio.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255–9. doi: 10.1016/s8756-3282(99)00162-3. [DOI] [PubMed] [Google Scholar]
- Igarashi Y, Lee MY, Matsuzaki S. Acid phosphatases as markers of bone metabolism. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:345–58. doi: 10.1016/s1570-0232(02)00431-2. [DOI] [PubMed] [Google Scholar]
- Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med. 1997;3:1285–9. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- Johnell O. The socioeconomic burden of fractures: today and in the 21st century. Am J Med. 1997;103:20S–25S. doi: 10.1016/s0002-9343(97)90023-1. discussion 25S–26S. [DOI] [PubMed] [Google Scholar]
- Kelly GS. Conjugated linoleic acid: a review. Altern Med Rev. 2001;6:367–82. [PubMed] [Google Scholar]
- Kelly O, Cusack S, Jewell C, Cashman KD. The effect of polyunsaturated fatty acids, including conjugated linoleic acid, on calcium absorption and bone metabolism and composition in young growing rats. Br J Nutr. 2003;90:743–50. doi: 10.1079/bjn2003951. [DOI] [PubMed] [Google Scholar]
- Kimble RB, Srivastava S, Ross FP, Matayoshi A, Pacifici R. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1 and tumor necrosis factor-mediated stimulation of macrophage colony-stimulating factor production. J Biol Chem. 1996;271:28890–7. doi: 10.1074/jbc.271.46.28890. [DOI] [PubMed] [Google Scholar]
- Lamp EC, Drexler HG. Biology of tartrate-resistant acid phosphatase. Leuk Lymphoma. 2000;39:477–84. doi: 10.3109/10428190009113378. [DOI] [PubMed] [Google Scholar]
- Li Y, Seifert MF, Ney DM, Grahn M, Grant AL, Allen KG, Watkins BA. Dietary conjugated linoleic acids alter serum IGF-I and IGF binding protein concentrations and reduce bone formation in rats fed (n-6) or (n-3) fatty acids. J Bone Miner Res. 1999;14:1153–62. doi: 10.1359/jbmr.1999.14.7.1153. [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332:305–11. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- May H, Murphy S, Khaw KT. Age-associated bone loss in men and women and its relationship to weight. Age Ageing. 1994;23:235–40. doi: 10.1093/ageing/23.3.235. [DOI] [PubMed] [Google Scholar]
- McGarry KA, Kiel DP. Postmenopausal osteoporosis. Strategies for preventing bone loss, avoiding fracture. Postgrad Med. 2000;108:79–82. 85–8, 91. doi: 10.3810/pgm.2000.09.1.1206. [DOI] [PubMed] [Google Scholar]
- Moloney F, Toomey S, Noone E, Nugent A, Allan B, Loscher CE, Roche HM. Antidiabetic effects of cis-9, trans-11-conjugated linoleic acid may be mediated via anti-inflammatory effects in white adipose tissue. Diabetes. 2007;56:574–82. doi: 10.2337/db06-0384. [DOI] [PubMed] [Google Scholar]
- Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW. Effect of conjugated linoleic acid on body composition in mice. Lipids. 1997;32:853–8. doi: 10.1007/s11745-997-0109-x. [DOI] [PubMed] [Google Scholar]
- Park Y, Terk M. Interaction between dietary conjugated linoleic acid and calcium supplementation affecting bone and fat mass. J Bone Miner Metab. doi: 10.1007/s00774-010-0212-1. [DOI] [PubMed] [Google Scholar]
- Pei L, Tontonoz P. Fat’s loss is bone’s gain. J Clin Invest. 2004;113:805–6. doi: 10.1172/JCI21311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt I, El-Sohemy A. Effects of 9cis,11trans and 10trans,12cis CLA on osteoclast formation and activity from human CD14+ monocytes. Lipids Health Dis. 2009a;8:15. doi: 10.1186/1476-511X-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt ID, El-Sohemy A. Regulation of osteoblast and adipocyte differentiation from human mesenchymal stem cells by conjugated linoleic acid. J Nutr Biochem. 2009b;20:956–64. doi: 10.1016/j.jnutbio.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Poli V, Balena R, Fattori E, Markatos A, Yamamoto M, Tanaka H, Ciliberto G, Rodan GA, Costantini F. Interleukin-6 deficient mice are protected from bone loss caused by estrogen depletion. Embo J. 1994;13:1189–96. doi: 10.1002/j.1460-2075.1994.tb06368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Bhattacharya A, Fernandes G. Conjugated linoleic acid inhibits osteoclast differentiation of RAW264.7 cells by modulating RANKL signaling. J Lipid Res. 2006;47:1739–1748. doi: 10.1194/jlr.M600151-JLR200. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Bhattacharya A, Banu J, Fernandes G. Conjugated linoleic acid protects against age-associated bone loss in C57BL/6 female mice. J Nutr Biochem. 2007;18:467–74. doi: 10.1016/j.jnutbio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Bhattacharya A, Fernandes G. Conjugated linoleic acid inhibits osteoclast differentiation of RAW264.7 cells by modulating RANKL signaling. J Lipid Res. 2006;47:1739–48. doi: 10.1194/jlr.M600151-JLR200. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–24. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–14. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–63. doi: 10.1093/ajcn/54.3.438. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13:66–80. doi: 10.1210/edrv-13-1-66. [DOI] [PubMed] [Google Scholar]
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- Thiel-Cooper RL, Parrish FC, Jr, Sparks JC, Wiegand BR, Ewan RC. Conjugated linoleic acid changes swine performance and carcass composition. J Anim Sci. 2001;79:1821–8. doi: 10.2527/2001.7971821x. [DOI] [PubMed] [Google Scholar]
- Tsuboyama-Kasaoka N, Takahashi M, Tanemura K, Kim HJ, Tange T, Okuyama H, Kasai M, Ikemoto S, Ezaki O. Conjugated linoleic acid supplementation reduces adipose tissue by apoptosis and develops lipodystrophy in mice. Diabetes. 2000;49:1534–42. doi: 10.2337/diabetes.49.9.1534. [DOI] [PubMed] [Google Scholar]
- Tucker KL, Chen H, Hannan MT, Cupples LA, Wilson PW, Felson D, Kiel DP. Bone mineral density and dietary patterns in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr. 2002;76:245–52. doi: 10.1093/ajcn/76.1.245. [DOI] [PubMed] [Google Scholar]
- Wang YW, Jones PJ. Conjugated linoleic acid and obesity control: efficacy and mechanisms. Int J Obes Relat Metab Disord. 2004;28:941–55. doi: 10.1038/sj.ijo.0802641. [DOI] [PubMed] [Google Scholar]
- Watkins BA, Lippman HE, Le Bouteiller L, Li Y, Seifert MF. Bioactive fatty acids: role in bone biology and bone cell function. Prog Lipid Res. 2001;40:125–48. doi: 10.1016/s0163-7827(00)00016-3. [DOI] [PubMed] [Google Scholar]
- Watkins BA, Seifert MF. Conjugated linoleic acid and bone biology. J Am Coll Nutr. 2000;19:478S–486S. doi: 10.1080/07315724.2000.10718951. [DOI] [PubMed] [Google Scholar]
- Watkins BA, Shen CL, McMurtry JP, Xu H, Bain SD, Allen KG, Seifert MF. Dietary lipids modulate bone prostaglandin E2 production, insulin-like growth factor-I concentration and formation rate in chicks. J Nutr. 1997;127:1084–91. doi: 10.1093/jn/127.6.1084. [DOI] [PubMed] [Google Scholar]


