Abstract
The effect of intermittently occurring, non-reservoir host species on pathogen transmission and prevalence in a reservoir population is poorly understood. We investigated whether voles, Microtus spp., which occur intermittently, influenced estimated standing antibody prevalence (ESAP) to Sin Nombre hantavirus (SNV, Bunyaviridae: Hantavirus) among deer mice, Peromyscus maniculatus, whose populations are persistent. We used 14 years of data from central Montana to investigate whether ESAP among deer mice was related to vole presence or abundance while controlling for the relationship between deer mouse abundance and ESAP. We found a reduction in deer mouse ESAP associated with the presence of voles, independent of vole abundance. A number of studies have documented that geographic locations which support a higher host diversity can be associated with reductions in pathogen prevalence by a hypothesized dilution effect. We suggest a dilution effect may also occur in a temporal dimension at sites where host richness fluctuates. Preservation of host diversity and optimization of environmental conditions which promote occurrence of ephemeral species, such as voles, may result in a decreased ESAP to hantaviruses among reservoir hosts. Our results may extend to other zoonotic infectious diseases.
Keywords: Sin Nombre virus, Dilution effect, Transmission, Temporal, Density independence
Introduction
As with many organisms, population abundance of small mammals fluctuates greatly both temporally and spatially (Krebs 1996). For example, some species of small mammals may be present in a location year round, but exhibit fluctuations in population abundance over time, whereas other species may appear and disappear with varying frequency (Wilson et al. 1986; Singleton 1989; Krebs 1996; Dickman et al. 1999; Korpimaki et al. 2004). Furthermore, fluctuations in the occurrence and abundance of hosts may have consequences for the dynamics of their pathogens (Singleton 1985; Cavanagh et al. 2004; Altizer et al. 2006; Telfer et al. 2007; Carver et al. 2008). Several studies have examined the role of host species diversity on the dynamics of pathogen transmission for vector-borne and directly transmitted diseases (Matuschka and Spielman 1992; Van Buskirk and Ostfeld 1995; Norman et al. 1999; Ostfeld and Keesing 2000a; Calisher et al. 2002; LoGiudice et al. 2003; Mills 2005, 2006; Telfer et al. 2005; Ezenwa et al. 2006; Tersago et al. 2008). For example, reduced infection prevalence of Ixodes scapularis ticks with Borrelia burgdorferi (the bacterium responsible for Lyme disease) and deer mice, Peromyscus maniculatus, with Sin Nombre virus (SNV, Bunyaviridae: Hantavirus; the agent of hantavirus pulmonary syndrome) is associated with increasing richness and diversity of small mammals (LoGiudice et al. 2003; Mills 2005, 2006). One hypothesis to explain this reduction is the dilution effect (Matuschka and Spielman 1992; Van Buskirk and Ostfeld 1995; Norman et al. 1999), which we define after Keesing et al. (2006) as the net effect whereby species diversity reduces pathogen prevalence in host or vector populations by any of a variety of mechanisms, for both vector-borne and directly transmitted pathogens (see also Ostfeld and Keesing 2000a, b). Previous investigations of the dilution effect focused on spatial variability in host diversity and associated pathogen prevalence. It is possible, however, that changes in pathogen prevalence might also be associated with temporal changes in diversity of a host assemblage at a given location.
Sin Nombre virus is a rodent-borne zoonotic pathogen that can spill over to humans causing severe morbidity and mortality (Nichol et al. 1993). The primary rodent reservoir for SNV is the deer mouse, P. maniculatus. Infection with SNV in deer mice appears chronic, and transmission between deer mice appears to occur most frequently via intraspecific aggression (Mills et al. 1999; Douglass et al. 2001; Calisher et al. 2007). Other rodent species, such as voles, Microtus spp., occur intermittently in the environment, and their interactions with deer mice may have a role in the transmission dynamics of SNV. Hantavirus antibodies have frequently been documented in voles in North America, and likely are associated with arvicoline hantaviruses, such as Prospect Hill virus (PHV), Bloodland Lake virus, or Isla Vista virus (Yanagihara et al. 1987; Hjelle et al. 1995; Rowe et al. 1995; Song et al. 1995; Bennett et al. 1999; Scharninghausen et al. 1999).
The potential for voles to influence transmission of SNV among deer mice is limited to three hypothesized outcomes: (1) no effect, if voles have a similar host competence or do not interact with deer mice; (2) an amplification effect (see Keesing et al. 2006), if voles are more competent hosts and interact with deer mice or if voles cause a behavioral shift in deer mice that enhances intraspecific contact and transmission among deer mice; or (3) a dilution effect, if voles are less competent hosts and interact with deer mice or cause a behavioral shift in deer mice which leads to a reduction in intraspecific transmission.
Hantavirus–host relationships, however, are highly specific: only the specific co-adapted host species is likely to develop a chronic infection and shed large quantities of virus into the environment for extended periods (Rowe et al. 1995; Bennett et al. 1999; Yates et al. 2002; Mills 2005). Most rodent species, and especially those distantly related to the co-adapted host, are thought to be dead-end hosts for hantaviruses with which they have not evolved a specific association (Yates et al. 2002; Mills 2005), and accordingly voles are likely to be dead-end hosts for SNV.
Numerous studies of vole and mouse interactions demonstrate competition in nature, with voles influencing contact rates and excluding mice from some habitats (e.g., Morris and Grant 1972; Bowker and Pearson 1975; Kozakiewicz and Boniecki 1994; Schulte-Hostedde and Brooks 1997). For example, Clay et al. (2009b) found increased diversity of rodent species (of which one vole species, Lemiscus curtatus, was the third most numerous species) resulted in a reduced number of intraspecific encounters among deer mice. These studies strongly suggest that the presence of Microtus spp. can influence intraspecific interactions among deer mice (and consequently transmission of SNV), directly by occupying a proportion of contacts deer mice would have with one another, and indirectly by modifying deer mouse behavior and intraspecific interactions within habitats. Accordingly, the influence of voles on transmission of SNV among deer mice may be proportional to vole abundance, or largely independent of vole abundance, if even a small number of voles cause a shift in mouse behavior or distribution. Because the influence of voles on transmission of SNV among deer mice has not been considered previously, we examine the relationship between voles and the prevalence of infection, as determined by the presence of antibody to SNV (estimated standing antibody prevalence, ESAP), in deer mice under abundance-dependent (likely reflective of a density-dependent relationship) and abundance-independent (presence vs absence) processes. We hypothesize that temporal fluctuations in vole presence and abundance will be associated with a reduction in prevalence of infection with SNV, as measured by ESAP, in deer mice.
In this study, we use monthly data on rodent dynamics from three live trapping grids from a 14-year continuous dataset from Cascade County, central Montana. We use hantavirus ESAP from two of the three grids over the entire study period, and over a 4-year period for the third grid. Prior to examining relationships between voles and ESAP in deer mice, we examine possible factors which may result in erroneous interpretations at each trapping grid: coincidental correlation between voles and ESAP in deer mice, correlation between vole and deer mouse abundance, and if ESAP in deer mice is influenced by deer mouse abundance (density-dependent transmission; see Anderson and May 1992; Keeling and Rohani 2008). Where appropriate, we control for confounding factors in our analyses.
Materials and methods
This investigation was undertaken on three live-trapping grids (grid numbers 10, 11 and 12) located near Cascade, Montana (46°59.3′N, 111°35.3′W, 1,408 m asl). The three trapping grids were in grassland habitat supporting an active cattle ranch (Douglass et al. 1996). Voles and deer mice were live trapped for three consecutive nights each month on all three grids for 170 consecutive months between June 1994 and July 2008. Trapping grids were 1 ha in area and consisted of 100 equally spaced Sherman live-capture traps (H.B. Sherman Traps. Tallahassee, FL, USA), baited with rolled oats and peanut butter and provisioned with polyester Fiberfil bedding. Upon capture, each rodent was given a uniquely numbered model #1005-1 ear-tag (National Band and Tag, Newport, KY, USA) and its species, sex, body mass, reproductive condition and presence of scars or wounds were recorded. The distance between grids 10 and 11 was approximately 550 m, while it was >2,000 m from grids 10 and 11 to grid 12. No deer mice or voles, trapped on one grid, have been detected on any of the other grids over the course of this study.
Blood samples, which were later tested for antibodies to SNV, were routinely collected each month from grids 11 and 12. Blood samples from grid 10 were collected less frequently (May 1997–March 1998 and June 2004–October 2007), and so subsequent analyses of antibodies to SNV were restricted to these months. Antibody in deer mice was detected using an enzyme immunoassay that has been previously described (Feldmann et al. 1993; Mills et al. 1997). The assay detects antibodies to all known North American hantaviruses, but does not distinguish among those viruses. We followed animal handling, blood collection and safety precautions described by Mills et al. (1995) and approved by the University of Montana IACUC.
We used the minimum number of individuals known to be alive (MNA) during a 3-day trapping session each month as an index of population abundance for both deer mice and voles (cf. Krebs 1966). The minimum number of SNV antibody-positive (infected) deer mice (MNI) during each trapping session was calculated in the same way. More complex population estimates, such as Jolly–Seber, were not used because these population models are unreliable when abundance is low and numbers of recaptures are few, as was the case for calculating the number of infected deer mice. We acknowledge MNA and MNI are conservative measures of abundance and infection (Luis et al. 2010). In some situations, deer mice determined to be SNV antibody-negative during one trapping session would not be trapped in the following session, but then recorded as SNV antibody-positive in the next session. In such cases, it was not possible to know if these mice were positive or negative in the month they were not trapped. We adopted the conservative approach of considering deer mice as positive from the date antibodies were first detected. Estimated standing antibody prevalence (ESAP) of deer mice for a given month was calculated by dividing MNI by MNA (Mills et al. 1999). Because hantaviruses cause chronic (likely life-time) infection in their hosts, we consider antibody-positive deer mice to be actively infected and likely shedding infectious virus (Mills et al. 1999).
Two species of vole occur at Cascade, Microtus pennsylvanicus and Microtus montanus. The only reliable morphological character distinguishing the two species is the presence of an extra cusp on the upper middle molar in M. pennsylvanicus (Hall and Kelson 1959), making it difficult to identify the species in the field. Accordingly, we combined both species for analysis. Of those voles that died in traps (allowing careful examination) approximately 75% were M. pennsylvanicus and 25% M. montanus (n = 20; Douglass, unpublished data). Four other species of mammals were trapped across the three grids (Neotoma cinerea, Spermophilus richardsonii, Thomomys talpoides and Zapus princeps). However, because the total number trapped over the 14-year period for these four species combined was only 65 (1.34% of all captures), they were not common enough for an examination of their influence on hantavirus dynamics in deer mice.
Analyses
To evaluate if the frequency of vole occurrence [absence (MNA = 0) and presence (MNA ≥1)] differed among months over the 14 years of sampling at each grid, a Chi-square goodness of fit test was used, assuming equal probability of occurrence each month. A Monte Carlo simulation of the multinomial sampling distribution (100,000 permutations) was used to estimate the relative probability for grids where the expected frequency was less than five during any month. General linear models (GLM) were used to determine if vole MNA and deer mouse MNA and ESAP differed among months. The relationships of deer mouse MNA with vole MNA and occurrence were evaluated using Spearman correlation and GLM, respectively.
To evaluate the role of vole MNA and occurrence on deer mouse ESAP, we first identified an optimal model to explain the relationship between deer mouse MNA and ESAP, a logarithmic relationship. We then determined if that model could be improved by vole MNA and occurrence, by examining the residual variation in deer mouse ESAP. The relationships of residual deer mouse ESAP with vole MNA and occurrence were explored using linear regression and GLM, respectively.
Chi-square analyses and Monte Carlo simulations were undertaken using R (http://www.r-project.org/). The remaining analyses were performed using SPSS 15.0 (SPSS, Chicago, IL, USA). Data in GLM analyses were checked for normality and equal variances using Shapiro–Wilk’s and Levene’s tests accordingly, prior to analyses, and non-normal data were normalized by log transformation.
Results
The total numbers of individuals trapped at all three trapping grids combined for this 14-year period were 3,700 deer mice and 1,082 voles. Voles were captured in 43, 31 and 49% of the 170 months which rodents were trapped on grids 10, 11 and 12, respectively.
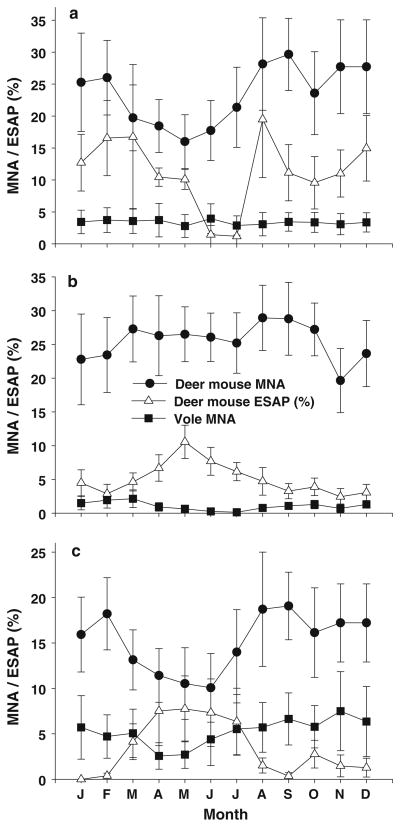
Abundance of voles did not differ among months (GLM: grid 10, F11,158 = 0.037, P = 0.999; grid 11, F11,158 = 0.765, P = 0.675; grid 12, F11,158 = 0.253, P = 0.993; Fig. 1a–c), and neither did occurrence of voles among months (Table 1). There was considerable within-month variation in deer mouse abundance (Fig. 1a–c). Consequently the differences in mean deer mouse abundance among months were not statistically significant (GLM: grid 10, F11,158 = 1.068, P = 0.390; grid 11, F11,158 = 0.898, P = 0.544; grid 12, F11,158 = 1.237, P = 0.267; Fig. 1a–c), although there was a tendency for reduced abundance of deer mice during summer months at grids 10 and 12. Monthly ESAP among deer mice was highest in May at grid 11 (GLM: F11,158 = 1.975, P = 0.034 Fig. 1b), with a similar trend at grid 12 (GLM: F11, 158 = 1.690, P = 0.080, Fig. 1c). ESAP at grid 10, where mice were tested for SNV less frequently, did not differ among months (GLM: F11, 158 = 1.200, P = 0.318, Fig. 1a). Deer mouse MNA was positively correlated with vole MNA and occurrence at all three grids (Table 2, Fig. 2a).
Fig. 1.
Mean (±1 SE) vole, Microtus spp., and deer mouse, Peromyscus maniculatus, the minimum number of individuals known to be alive (MNA) (square and circle, respectively) and deer mouse estimated standing antibody prevalence (ESAP) (triangle) at live trapping grids 10 (a), 11 (b) and 12 (c). Note different scales on y-axis of graphs
Table 1.
Chi-square goodness of fit test for the occurrence of voles at each live trapping grid between June 1994 and July 2008 for each given month (number of months trapped), assuming equal probability of occurrence on a given month
| Month | Observed/expected frequency |
||
|---|---|---|---|
| Grid 10 | Grid 11 | Grid 12 | |
| January (14) | 6/6 | 5/4.3 | 7/6.9 |
| February (14) | 5/6 | 5/4.3 | 7/6.9 |
| March (14) | 5/6 | 5/4.3 | 7/6.9 |
| April (14) | 6/6 | 5/4.3 | 6/6.9 |
| May (14) | 5/6 | 4/4.3 | 7/6.9 |
| June (15) | 6/6.4 | 3/4.6 | 6/7.4 |
| July (15) | 6/6.4 | 2/4.6 | 7/7.4 |
| August (14) | 7/6 | 4/4.3 | 8/6.9 |
| September (14) | 7/6 | 5/4.3 | 6/6.9 |
| October (14) | 6/6 | 5/4.3 | 8/6.9 |
| November (14) | 7/6 | 2/4.3 | 7/6.9 |
| December (14) | 7/6 | 7/4.3 | 8/6.9 |
| χ2 | 1.221 | 1.326 | 1.048 |
| df | 11 | 11 | 11 |
| P | 0.999 | 0.676 | 0.999 |
Monte Carlo simulations (100,000 permutations of the multinomial sampling distribution) were used to estimate significance for grid 11, because the expected frequency was fewer than 5
Table 2.
Spearman correlations of overall relationships between vole, Microtus spp., and deer mouse, Peromyscus maniculatus, minimum number of individuals known to be alive (MNA) for each live trapping grid (upper section of table)
| Grid | 10 | 11 | 12 |
|---|---|---|---|
| Vole and deer mouse MNA | |||
| ρ | 0.475 | 0.474 | 0.378 |
| n | 170 | 170 | 170 |
| P | <0.001 | <0.001 | <0.001 |
| Voles occurrence and deer mouse MNA | |||
| Absent | 15.619 (1.195) | 19.551 (0.998) | 9.756 (0.855) |
| Present | 32.575 (3.296) | 38.942 (3.346) | 20.131 (2.174) |
| F | 28.952 | 53.235 | 20.305 |
| df | 1,168 | 1,168 | 1,168 |
| P | <0.001 | <0.001 | <0.001 |
Mean (±1 SE) minimum number of individuals known to be alive (MNA) of deer mice when voles were absent and present for each live trapping grid (lower section of table), with general linear model of comparisons between absent and present data Significant results shown in bold
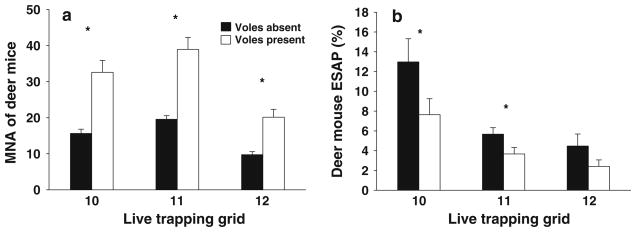
Fig. 2.
Mean (±1 SE) dear mouse MNA (a) and ESAP (b) when voles were absent (black bars) and present (white bars) for each live trapping grid. Asterisk represents significant comparisons between presence and absence data (Tables 2 and 3). Note different scales on y-axis of graphs
After controlling for significant relationships between deer mouse MNA and ESAP (logarithmic regressions: grid 10, R2 = 0.482, F1,50 = 47.534, P < 0.001; grid 11, R2 = 0.362, F1,168 = 95.92, P < 0.001; grid 12, R2 = 0.067, F1,168 = 12.104, P < 0.001), deer mouse ESAP was found to be unrelated to vole MNA (Table 3). Mean ESAP of deer mice, however, was significantly lower when voles were present at grids 10 and 11 (mean reduction of 59 and 64%, respectively; Table 3, Fig. 2b). There was also a reduction in mean ESAP of deer mice, when voles were present, at grid 12 (mean reduction of 54%; Table 3, Fig. 2b), though this was a non-significant trend likely reflecting low ESAP levels at this grid.
Table 3.
Relationships (linear regression) between vole, Microtus spp. MNA and ESAP of deer mice, P. maniculatus, for each live trapping grid (upper section of table)
| Grid | 10 | 11 | 12 |
|---|---|---|---|
| Vole MNA and deer mouse ESAP | |||
| R2 | 0.030 | 0.004 | 0.010 |
| F | 1.578 | 0.641 | 1.750 |
| df | 1,50 | 1,168 | 1,168 |
| P | 0.215 | 0.425 | 0.188 |
| Vole occurrence and deer mouse ESAP | |||
| Absent | 12.975 (2.342) | 5.672 (0.656) | 4.467 (1.219) |
| Present | 7.638 (1.610) | 3.677 (0.652) | 2.406 (0.662) |
| F | 4.634 | 8.029 | 3.309 |
| df | 1,50 | 1,168 | 1,168 |
| P | 0.036 | 0.005 | 0.071 |
Mean (±1 SE) estimated standing antibody prevalence (ESAP) of deer mice, when voles were absent and present for each live trapping grid (lower section of table), with general linear model of comparisons. Relationship between deer mouse minimum number of individuals known to be alive (MNA) and ESAP was controlled for in all cases
Significant results shown in bold
Discussion
Although the effect of host abundance on prevalence of infectious disease has been the subject of much research (density- and frequency-dependent transmission; see Anderson and May 1992; Keeling and Rohani 2008), the impact of simple presence or absence of other host species and temporal dynamics on the occurrence of these species has received little attention in the scientific literature. Yet the presence of other species may have significant impacts on pathogen dynamics. Deer mice are persistent at our three trapping grids in Cascade, Montana, whereas voles are ephemeral in their occurrence. Here, we investigated if voles at our study sites influenced the temporal dynamics of hantavirus infection in deer mice by processes dependent or independent of vole MNA. We found that the presence of voles was associated with a reduction in hantavirus ESAP among deer mice.
At all three trapping grids, there was a consistent negative relationship between the presence of voles and prevalence of antibody to SNV in deer mice, independent of vole MNA (a density-independent relationship). This result suggests that, during periods when voles are present, transmission of SNV among deer mice is reduced. The percentage reduction in ESAP among deer mice when voles were present was profound (mean range 54–64%). Accordingly, voles may be responsible for decreasing transmission of SNV in deer mouse populations by processes independent of their abundance (a density-independent relationship). In this study, we dealt with a small-mammal assemblage of only two to three species. Nevertheless, using species richness as a surrogate for diversity (Ostfeld and Keesing 2000b), our results are consistent with a dilution effect as defined in its broadest sense as “the net effect of species diversity reducing disease risk by any of a variety of mechanisms” (Keesing et al. 2006).
For vector-borne pathogens (such as Lyme Disease or West Nile virus), mechanisms which underpin the dilution effect are relatively intuitive; a generalist vector which feeds upon a host population with variable reservoir competence. For directly transmitted pathogens, such as SNV, identifying mechanisms by which host diversity negatively influence pathogen prevalence are more challenging. The most probable mechanisms by which voles may cause a reduction in the prevalence of infection with SNV in deer mice are if (1) voles interact with deer mice, but are less competent hosts, and the number of daily encounter events among these rodents (intraspecific and interspecific) are relatively fixed, or (2) voles are dominant competitors causing behavioral or distributional shifts in deer mice which leads to reductions in intraspecific transmission (Morris and Grant 1972; Bowker and Pearson 1975; Kozakiewicz and Boniecki 1994; Schulte-Hostedde and Brooks 1997).
It would seem probable that the relative effect of a dilution host, or agent (cf., Clay et al. 2009a), on infection prevalence within a reservoir host population would be a density-dependent process, and such has been documented for vector-borne diseases, e.g., Bartonella sp. (Telfer et al. 2005). Accordingly, a density-independent effect of vole abundance on deer mouse ESAP may appear counterintuitive. This study suggests that even a small number of voles influences transmission among deer mice. In support of our density-independent finding, Clay et al. (2009a, b) documented reductions in SNV antibody prevalence among deer mice in the presence of non-reservoir competitors, and concluded that this was related to reduced intraspecific encounters, survival or dispersal of deer mice in the presence of other rodent species. The intermittent presence of voles at our three grids suggests that voles we trapped were dispersers (from local refuges following increased population abundance in these areas), which are known to be aggressive in their intraspecific and interspecific interactions (see Krebs and Myers 1974 and references therein). Voles and deer mice create and utilize passageways (runs) through vegetation at our live-trapping grids (Douglass, personal observation). Accordingly, the presence of aggressive, competitively dominant, voles dispersing into our grids and utilizing these passageways would likely disrupt movement (use of passageways) and intraspecific contact patterns among deer mice resulting in fewer SNV transmission events. Further, evidence of a dominant competitor influencing interactions among an inferior competitor was found in a non-disease study. Ovadia et al. (2005) found Allenby’s gerbils (Gerbillus andersoni allenbyi) engaged in fewer intraspecific aggressive interactions when in the presence of a larger-bodied competitor, G. pyramidum, independent of G. pyramidum population density. Body mass, population densities and interspecific relationships between G. pyramidum and G. allenbyi (Ovadia et al. 2005) were comparable to those of voles and deer mice observed among our grids. Taken together, our study and those of Clay et al. (2009a, b) and Ovadia et al. (2005) mechanistically suggest that a non-reservoir species can incite a reduction in pathogen transmission (and prevalence of infection) among a reservoir host by density-independent processes, when this dilution agent is competitively dominant. These studies provide stronger support for the second of the proposed mechanisms in the previous paragraph, but do not exclude the possibility of both mechanisms occurring simultaneously.
The transmission of directly transmitted zoonotic pathogens is often considered a function of host abundance (density-dependent and frequency-dependent transmission; see Keeling and Rohani 2008). Indeed, there was a positive logarithmic relationship between deer mouse MNA and ESAP in this study (although the relationship at grid 12 was weak, reflecting low ESAP). The relationship between deer mouse MNA and ESAP was characteristic of frequency-dependent transmission, because ESAP was a saturating function of MNA, whereas a linear relationship between MNA and ESAP would be more indicative of density-dependant transmission (Keeling and Rohani 2008). We also found a positive relationship between vole MNA and deer mouse MNA, which likely reflects favorable environmental conditions for both species (Ostfeld 1985; Krebs 1996; Pearce and Venier 2005). It was beyond the scope of this investigation to determine the causal nature of these population fluctuations, but further investigations would be valuable (see Luis et al. 2010).
When the relationship between deer mouse MNA and ESAP was controlled for, we found ESAP among deer mice was reduced when voles were present, independent of vole MNA. It is possible this relationship is due to an effect of environmental factors on ESAP among deer mice, rather than voles. Environmental factors undoubtedly influence deer mouse abundance directly (Luis et al. 2010), and collectively deer mouse abundance and intraspecific encounters influence transmission of SNV (Mills et al. 1999; Douglass et al. 2001; Calisher et al. 2007; Clay et al. 2009c). The relationship between environmental factors and deer mouse ESAP is likely indirect in most cases. We anticipate that, having controlled for the effect of deer mouse MNA on ESAP, indirect influences of environmental factors on deer mouse ESAP are also minimized. Moreover, widespread evidence of interactions between mice and voles in nature (Morris and Grant 1972; Bowker and Pearson 1975; Kozakiewicz and Boniecki 1994; Schulte-Hostedde and Brooks 1997) suggests that voles are a more parsimonious explanation for reductions of ESAP among deer mice, as observed here.
Though the antibody test we used to detect SNV in deer mice is broadly cross-reactive with all known New World hantaviruses, we assume deer mice are predominantly infected with SNV, an assumption supported by numerous studies (Rowe et al. 1995; Rawlings et al. 1996; Bennett et al. 1999; Calisher et al. 2005; Schountz et al. 2007; Safronetz et al. 2008). Rowe et al. (1995), and Bennett et al. (1999) used RT-PCR to test antibody-positive M. montanus (1 of 8 individuals tested) and M. californicus (5 of 40 individuals tested) for SNV, but did not detect SNV in voles. RT-PCR tests of antibody-positive voles from Cascade for SNV have also not detected the virus (Kuenzi, unpublished data). It is possible that voles may on occasion become infected with SNV, but do not develop chronic infections involving shedding of large quantities of the virus. It is also possible that voles may not be susceptible to SNV infection. Voles in Montana host Prospect Hill virus (PHV) or Prospect Hill-like viruses (overall 11% of Microtus spp. (n = 643) for western Montana, tested between 1994 and 1999, were antibody positive for a hantavirus; Douglass et al. 2001), and virus from one M. pennsylvanicus that was examined from grid 12 was identified as PHV (Calisher, unpublished data). It is important to acknowledge the possible existence of multiple hantaviruses, but we consider it unlikely that PHV, or any other hantavirus in voles, influences prevalence of SNV among deer mice.
Communities of small mammals, at this study site and across Montana, are dominated by deer mice, and prevalence of infection with SNV is higher than in many studies elsewhere in the western USA (Douglass et al. 2001). Nevertheless, some variation in small-mammal species richness, such as the occurrence of two vole species (M. pennsylvanicus and M. montanus) at some grids, but not others, may contribute to observed differences in ESAP among grids. If such is the case, a reduction in hantavirus infection among deer mice may be due to the presence of more than one putative dilution host species. Further studies examining if the temporal relationship described in this study is also observed at other sites (see potential sites described by Douglass et al. 2001) would be valuable.
This study has specifically focused on how interspecific relationships influence prevalence of SNV on a temporal scale. Our focus, however, does not preclude intraspecific processes that contribute to variation in SNV prevalence, in addition to interspecific interactions. Prevalence of SNV is influenced by various population demographic factors, such as population size, age structure and sex (e.g., Douglass et al. 1996, 2001; Mills et al. 1997, 1999; Calisher et al. 2007), which can vary seasonally (e.g., Carver et al. 2010; Luis et al. 2010). We consider it unlikely, however, that any of these factors confound the findings of this study, because the occurrence and abundance of voles did not exhibit seasonality. As such, deer mouse population demography, age and sex specific infection rates and seasonality in ESAP among grids was holistically encompassed within our analyses. Disentangling the simultaneous influences of intraspecific and interspecific determinants of SNV prevalence among deer mice is an important future direction in understanding the natural dynamics of this pathogen, and will likely contribute to further improvements in predicting risk of human exposure.
Conclusions
Our results are consistent with a dilution effect hypothesis, suggesting the presence of voles is associated with a reduction in prevalence of a hantavirus infection among deer mice by processes independent of vole abundance. Observations here are consistent with other studies of diversity and pathogen prevalence (e.g., Ostfeld and Keesing 2000a; Calisher et al. 2002; LoGiudice et al. 2003; Mills 2005; Ezenwa et al. 2006). Future studies in this area are needed to confirm the mechanisms by which the dilution effect is suggested to occur. Nevertheless, our study expands upon previous investigations by suggesting the hypothesized dilution effect may be viewed not only on a spatial scale but may also apply to temporal processes, where fluctuations in host species occurrence shape within-site variability in pathogen prevalence and, by extension, possible transmission risk to humans. We suggest preservation of species diversity and optimization of environmental conditions which promote occurrence of ephemeral species, such as voles, may result in decreased risk of human HPS, particularly when deer mouse abundance is low. Our results may extend to other zoonotic infectious diseases.
Acknowledgments
We thank the private ranch owner at Cascade for allowing us access to his property. Numerous individuals provided valuable assistance in the field including K. Coffin, R. Van Horn, C. Rognli, T. Wilson, W. Semmens, K. Hughes, A. Skypala, D. Waltee, B. Lonner, J. Wilson, A. Leary, A. Alvarado, J. Bertoglio and F. Arneson. K. Wagoner provided database support, encouragement and general advice. R. Ostfeld and two anonymous reviewers provided constructive feedback, which greatly improved this paper. Financial support was provided by NIH grant P20 RR16455-06-07,08 from the INBRE-BRIN program of the National Center for Research Resources and the U.S. Centers for Disease Control and Prevention, Atlanta, GA, through cooperative agreement. The authors declare that the study described herein complies with the laws of the United States of America. The findings and conclusions presented here are those of the author(s) and do not necessarily represent the views of the funding agencies.
Contributor Information
Scott Carver, Email: scott.carver@colostate.edu, Department of Biology, Montana Tech of the University of Montana, 1300 Park St, Butte, MT 59701, USA. Department of Microbiology, Immunology and Pathology, Colorado State University, 1619 Campus Delivery, Fort Collins, CO 80523, USA.
Amy Kuenzi, Department of Biology, Montana Tech of the University of Montana, 1300 Park St, Butte, MT 59701, USA.
Karoun H. Bagamian, Population Biology, Ecology and Evolution Program, Emory University, 1510 Clifton Rd, Atlanta, GA 30322, USA
James N. Mills, Special Pathogens Branch, Division of Viral and Rickettsial Diseases, Centers for Disease Control and Prevention, 1600 Clifton Road, Atlanta, GA 30333, USA
Pierre E. Rollin, Special Pathogens Branch, Division of Viral and Rickettsial Diseases, Centers for Disease Control and Prevention, 1600 Clifton Road, Atlanta, GA 30333, USA
Susanne N. Zanto, Public Health Laboratory, Montana Department of Public Health and Human Services, Helena, MT 59604, USA
Richard Douglass, Department of Biology, Montana Tech of the University of Montana, 1300 Park St, Butte, MT 59701, USA.
References
- Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M, Rohani P. Seasonality and the dynamics of infectious diseases. Ecol Lett. 2006;9:467–484. doi: 10.1111/j.1461-0248.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- Anderson RM, May RM. Infectious diseases of humans: dynamics and control. Oxford University Press; New York: 1992. [Google Scholar]
- Bennett SG, Webb JP, Madon MB, Childs JE, Ksiazek TG, Torrez-Martinez N, Hjelle B. Hantavirus (Bunyaviridae) infections in rodents from orange and San Diego Counties, California. Am J Trop Med Hyg. 1999;60:75–84. doi: 10.4269/ajtmh.1999.60.75. [DOI] [PubMed] [Google Scholar]
- Bowker LS, Pearson PG. Habitat orientation and interspecific interaction of Microtus pennsylvanicus and Peromyscus leucopus. Am Midl Nat. 1975;94:491–496. [Google Scholar]
- Calisher CH, Root JJ, Mills JN, Beaty BJ. Assessment of ecologic and biologic factors leading to hantavirus pulmonary syndrome, Colorado, USA. Croat Med J. 2002;43:330–337. [PubMed] [Google Scholar]
- Calisher CH, Root JJ, Mills JN, Rowe JE, Reeder SA, Jentes ES, Wagoner K, Beaty BJ. Epizootiology of Sin Nombre and El Moro Canyon hantaviruses, southeastern Colorado, 1995–2000. J Wildl Dis. 2005;41:1–11. doi: 10.7589/0090-3558-41.1.1. [DOI] [PubMed] [Google Scholar]
- Calisher CH, Wagoner KD, Amman BR, Root JJ, Douglass RJ, Kuenzi AJ, Abbott KD, Parmenter C, Yates TL, Ksiazek TG, Beaty BJ, Mills JN. Demographic factors associated with prevalence of antibody to Sin Nombre Virus in deer mice in the Western United States. J Wildl Dis. 2007;43:1–11. doi: 10.7589/0090-3558-43.1.1. [DOI] [PubMed] [Google Scholar]
- Carver S, Sakalidis V, Weinstein P. House mouse abundance and Ross River virus notifications in Victoria, Australia. Int J Infect Dis. 2008;12:528–533. doi: 10.1016/j.ijid.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Carver S, Mills JN, Kuenzi A, Flietstra T, Douglass R. Sampling frequency differentially influences interpretation of zoonotic pathogen and host dynamics: Sin Nombre virus and deer mice. Vector-Borne Zoonot Dis. 2010;10:575–583. doi: 10.1089/vbz.2009.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh RD, Lambin X, Ergon T, Bennett M, Graham IM, van Soolingen D, Begon M. Disease dynamics in cyclic populations of field voles (Microtus agrestis): cowpox virus and vole tuberculosis (Mycobacterium microti) Proc R Soc Lond B. 2004;271:859–867. doi: 10.1098/rspb.2003.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay CA, Lehmer EM, Jeor SS, Dearing MD. Sin Nombre virus and rodent species diversity: a test of the dilution and amplification hypotheses. PLoS One. 2009a;4:e6467. doi: 10.1371/journal.pone.0006467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay CA, Lehmer EM, Jeor SS, Dearing MD. Testing mechanisms of the dilution effect: deer mice encounter rates, Sin Nombre virus prevalence and species diversity. Ecohealth. 2009b;6:250–259. doi: 10.1007/s10393-009-0240-2. [DOI] [PubMed] [Google Scholar]
- Clay CA, Lehmer EM, Previtali A, St Jeor S, Dearing MD. Contact heterogeneity in deer mice: implications for Sin Nombre virus transmission. Proc R Soc Lond B. 2009c;276:1305–1312. doi: 10.1098/rspb.2008.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman CR, Mahon PS, Masters P, Gibson DF. Long-term dynamics of rodent populations in arid Australia: the influence of rainfall. Wildl Res. 1999;26:389–403. [Google Scholar]
- Douglass RJ, VanHorn R, Coffin KW, Zanto SN. Hantavirus in Montana deer mouse populations: preliminary results. J Wildl Dis. 1996;32:527–530. doi: 10.7589/0090-3558-32.3.527. [DOI] [PubMed] [Google Scholar]
- Douglass RJ, Wilson T, Semmens WJ, Zanto SN, Bond CW, Van Horn RC, Mills JN. Longitudinal studies of Sin Nombre virus in deer mouse-dominated ecosystems of Montana. Am J Trop Med Hyg. 2001;65:33–41. doi: 10.4269/ajtmh.2001.65.33. [DOI] [PubMed] [Google Scholar]
- Ezenwa VO, Godsey MS, King RJ, Guptill SC. Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk. Proc R Soc Lond B. 2006;273:109–117. doi: 10.1098/rspb.2005.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann H, Sanchez A, Morzunov S, Spiropoulou CF, Rollin PE, Ksiazek TG, Peters CJ, Nichol ST. Utilization of autopsy RNA for the synthesis of the nucleocapsid antigen of a newly recognized virus associated with hantavirus pulmonary syndrome. Virus Res. 1993;30:351–367. doi: 10.1016/0168-1702(93)90101-r. [DOI] [PubMed] [Google Scholar]
- Hall ER, Kelson KR. Mammals of North America. Ronald; New York: 1959. [Google Scholar]
- Hjelle B, Jenison SA, Goade DE, Green WB, Feddersen RM, Scott AA. Hantaviruses: clinical, microbiologic, and epidemiologic aspects. Crit Rev Clin Lab Sci. 1995;32:469–508. doi: 10.3109/10408369509082592. [DOI] [PubMed] [Google Scholar]
- Keeling MJ, Rohani P. Modeling infectious diseases in humans and animals. Princeton University Press; Princeton: 2008. [Google Scholar]
- Keesing F, Holt RD, Ostfeld R. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Korpimaki E, Brown PR, Jacob J, Pech RP. The puzzles of population cycles and outbreaks of small mammals solved? Bioscience. 2004;54:1071–1079. [Google Scholar]
- Kozakiewicz A, Boniecki P. Intra-specific and interspecific behaviors in bank vole and striped field-mouse under enclosure conditions. Acta Theriol. 1994;39:29–36. [Google Scholar]
- Krebs CJ. Demographic changes in fluctuating populations of Microtus californicus. Ecol Monogr. 1966;36:239–273. [Google Scholar]
- Krebs CJ. Population cycles revisited. J Mammal. 1996;77:8–24. [Google Scholar]
- Krebs CJ, Myers JM. Population cycles in small mammals. Adv Ecol Res. 1974;8:267–399. [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis AD, Douglass RJ, Mills JN, Bjørnstad ON. The effect of seasonality, density and climate on the population dynamics of Montana deer mice, important reservoir hosts for Sin Nombre hantavirus. J Anim Ecol. 2010;79:462–470. doi: 10.1111/j.1365-2656.2009.01646.x. [DOI] [PubMed] [Google Scholar]
- Matuschka FR, Spielman A. Loss of Lyme disease spirochetes from Ixodes ricinus ticks feeding on European blackbirds. Exp Parasitol. 1992;74:151–158. doi: 10.1016/0014-4894(92)90042-9. [DOI] [PubMed] [Google Scholar]
- Mills JN. Regulation of rodent-borne viruses in the natural host: implications for human disease. Arch Virol Suppl. 2005;19:45–57. doi: 10.1007/3-211-29981-5_5. [DOI] [PubMed] [Google Scholar]
- Mills JN. Biodiversity loss and emerging infectious disease: an example from the rodent-borne hemorrhagic fevers. Biodiversity. 2006;7:9–17. [Google Scholar]
- Mills JN, Yates TL, Childs JE, Parmenter RR, Ksiazek TG, Rollin PE, Peters CJ. Guidelines for working with rodents potentially infected with hantavirus. J Mammal. 1995;76:716–722. [Google Scholar]
- Mills JN, Ksiazek TG, Ellis BA, Rollin PE, Nichol ST, Yates TL, Gannon WL, Levy CE, Engelthaler DM, Davis T, Tanda DT, Frampton JW, Nichols CR, Peters CJ, Childs JE. Patterns of association with host and habitat: antibody reactive with Sin Nombre virus in small mammals in the major biotic communities of the southwestern United States. Am J Trop Med Hyg. 1997;56:273–284. doi: 10.4269/ajtmh.1997.56.273. [DOI] [PubMed] [Google Scholar]
- Mills JN, Ksiazek TG, Peters CJ, Childs JE. Long-term studies of hantavirus reservoir populations in the southwestern United States: a synthesis. Emerg Infect Dis. 1999;5:135–142. doi: 10.3201/eid0501.990116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RD, Grant PR. Experimental studies of competitive interaction in a two-species system. J Anim Ecol. 1972;41:275–290. [Google Scholar]
- Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, Sanchez A, Childs J, Zaki S, Peters CJ. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Norman R, Bowers RG, Begon M, Hudson PJ. Persistence of tick-horne virus in the presence of multiple host species: tick reservoirs and parasite mediated competition. J Theor Biol. 1999;200:111–118. doi: 10.1006/jtbi.1999.0982. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS. Limiting resources and territoriality in microtine rodents. Am Nat. 1985;126:1–15. [Google Scholar]
- Ostfeld RS, Keesing F. Biodiversity and disease risk: the case of Lyme disease. Conserv Biol. 2000a;14:722–728. [Google Scholar]
- Ostfeld RS, Keesing F. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can J Zool. 2000b;78:2061–2078. [Google Scholar]
- Ovadia O, Abramsky Z, Kotler BP, Pinshow B. Inter-specific competitors reduce inter-gender competition in Negev Desert gerbils. Oecologia. 2005;142:480–488. doi: 10.1007/s00442-004-1726-9. [DOI] [PubMed] [Google Scholar]
- Pearce J, Venier L. Small mammals as bioindicators of sustainable boreal forest management. For Ecol Manag. 2005;208:153–175. [Google Scholar]
- Rawlings JA, TorrezMartinez N, Neill SU, Moore GM, Hicks BN, Pichuantes S, Nguyen A, Bharadwaj M, Hjelle B. Cocirculation of multiple hantaviruses in Texas, with characterization of the small (S) genome of a previously undescribed virus of cotton rats (Sigmodon hispidus) Am J Trop Med Hyg. 1996;55:672–679. doi: 10.4269/ajtmh.1996.55.672. [DOI] [PubMed] [Google Scholar]
- Rowe JE, Stjeor SC, Riolo J, Otteson EW, Monroe MC, Henderson WW, Ksiazek TG, Rollin PE, Nichol ST. Coexistence of several novel hantaviruses in rodents indigenous to North America. Virology. 1995;213:122–130. doi: 10.1006/viro.1995.1552. [DOI] [PubMed] [Google Scholar]
- Safronetz D, Drebot MA, Artsob H, Cote T, Makowski K, Lindsay LR. Sin Nombre virus shedding patterns in naturally infected deer mice (Peromyscus maniculatus) in relation to duration of infection. Vector-Borne Zoonotic Dis. 2008;8:97–100. doi: 10.1089/vbz.2007.0113. [DOI] [PubMed] [Google Scholar]
- Scharninghausen JJ, Pitts RM, Bickham JW, Davis DS, Mills JN. Evidence of hantavirus infection in Microtus orchrogaster. St Louis County, Missouri Trans Mo Acad Sci. 1999;33–34:23–25. [Google Scholar]
- Schountz T, Calisher CH, Richens TR, Rich AA, Doty JB, Hughes MT, Beaty BJ. Rapid field immunoassay for detecting antibody to Sin Nombre virus in deer mice. Emerg Infect Dis. 2007;13:1604–1607. doi: 10.3201/eid1310.070383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Hostedde AI, Brooks RJ. An experimental test of habitat selection by rodents of Algonquin Park. Can J Zool. 1997;75:1989–1993. [Google Scholar]
- Singleton GR. Population dynamics of Mus musculus and its parasites in Mallee wheatlands in Victoria Australia during and after a drought. Aust Wildl Res. 1985;12:437–446. [Google Scholar]
- Singleton G. Population dynamics of an outbreak of house mouse (Mus domesticus) in the Mallee wheatlands of Australia—hypothesis of plague formation. J Zool. 1989;219:495–515. [Google Scholar]
- Song WM, Torrezmartinez N, Irwin W, Harrison FJ, Davis R, Ascher M, Jay M, Hjelle B. Isla Vista virus—a genetically novel hantavirus of the California vole Microtus californicus. J Gen Virol. 1995;76:3195–3199. doi: 10.1099/0022-1317-76-12-3195. [DOI] [PubMed] [Google Scholar]
- Telfer S, Bown KJ, Sekules R, Begon I, Hayden T, Birtles R. Disruption of a host-parasite system following the introduction of an exotic host species. Parasitology. 2005;130:661–668. doi: 10.1017/s0031182005007250. [DOI] [PubMed] [Google Scholar]
- Telfer S, Begon M, Bennett M, Bown KJ, Burthe S, Lambin X, Telford G, Birtles R. Contrasting dynamics of Bartonella spp. in cyclic field vole populations: the impact of vector and host dynamics. Parasitology. 2007;134:413–425. doi: 10.1017/S0031182006001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersago K, Schreurs A, Linard C, Verhagen R, van Dongen S, Leirs H. Population, environmental, and community effects on local bank vole (Myodes glareolus) Puumala virus infection in an area with low human incidence. Vector-Borne Zoonotic Dis. 2008;8:235–244. doi: 10.1089/vbz.2007.0160. [DOI] [PubMed] [Google Scholar]
- Van Buskirk J, Ostfeld RS. Controlling Lyme disease by modifying the density and species composition of tick hosts. Ecol Appl. 1995;5:1133–1140. [Google Scholar]
- Wilson BA, Bourne AR, Jessop RE. Ecology of small mammals in coastal heath at Anglesea, Victoria. Aust Wildl Res. 1986;13:397–406. [Google Scholar]
- Yanagihara R, Daum CA, Lee PW, Baek LJ, Amyx HL, Gajdusek DC, Gibbs CJ. Serological survey of Prospect Hill virus infection in indigenous wild rodents in the USA. Trans R Soc Trop Med Hyg. 1987;81:42–45. doi: 10.1016/0035-9203(87)90275-6. [DOI] [PubMed] [Google Scholar]
- Yates TL, Mills JN, Parmenter CA, Ksiazek TG, Parmenter RR, Vande Castle JR, Calisher CH, Nichol ST, Abbott KD, Young JC, Morrison ML, Beaty BJ, Dunnum JL, Baker RJ, Salazar-Bravo J, Peters CJ. The ecology and evolutionary history of an emergent disease: Hantavirus pulmonary syndrome. Bioscience. 2002;52:989–998. [Google Scholar]