Abstract
The mammalian respiratory lineage, consisting of the trachea and lung, originates from the ventral foregut in an early embryo. Reciprocal signaling interactions between the foregut epithelium and its associated mesenchyme guide development of the respiratory endoderm, from a naive sheet of cells to multiple cell types that line a functional organ. This review synthesizes current understanding of the early events in respiratory system development, focusing on three main topics: 1) specification of the respiratory system as a distinct organ of the endoderm, 2) patterning and differentiation of the nascent respiratory epithelium along its proximal-distal axis, and 3) plasticity of the respiratory cells during the process of development. This review also highlights areas in need of further study, including determining how early endoderm cells rapidly switch their responses to the same signaling cues during development, and how the general proximal-distal pattern of the lung is converted to fine-scale organization of multiple cell types along this axis.
Keywords: endoderm, respiratory system, patterning
Introduction
The image of a newborn being welcomed into the world with a spank from the doctor to jump start breathing serves well to demonstrate the importance of a functional gas-exchange system in postnatal life. The trachea and lungs of the respiratory lineage are composed of an endoderm-derived epithelial cells surrounded by a mesoderm-derived mesenchymal cells. During embryogenesis, reciprocal signaling interactions between these two cell lineages guide the patterning and subsequent development of a functional respiratory system (Shannon and Hyatt, 2004). While many of the molecular players in this process have been identified, significant gaps in our knowledge remain.
General cellular development is often modeled as a sequential process (Slack, 1991). First, cells are specified to a certain fate, but for a certain period of time remain labile to alter their fate in response to cues from their environment. Next, cells become determined, as they commit to a certain fate and are no longer able to alter their developmental trajectory in response to external cues. Third, cells differentiate to assume the morphology and function of the mature cell type. The purpose of this mini-review is to discuss what is known about these processes in respiratory development, both during the separation of the respiratory lineage from other endoderm-derived lineages, and during patterning of the respiratory system along its proximal-distal (P-D) axis. We will start by outlining the timing of these events as well as the signaling molecules involved. We will primarily discuss findings from mouse, but will also refer to results from other model organisms during similar stages of development. This review is not intended to be a comprehensive review of endoderm or lung development, and the interested reader is referred to other excellent reviews on these topics (Cardoso and Lu, 2006, Zorn and Wells, 2009, Morrisey and Hogan, 2010).
Prenatal development of the respiratory system: sequence of events
The embryonic endoderm emerges through the process of gastrulation at approximately embryonic day (E) 6.5-8.0 in the mouse (Zorn and Wells, 2009) (Fig. 1A). A recent study demonstrates that as definitive endoderm cells emerge from the epiblast layer, they intercalate among visceral endoderm cells, and that both populations contribute to the gut tube (Kwon et al., 2008). Subsequently at E8.5, the endoderm folds to produce a gut tube (Fig. 1B).
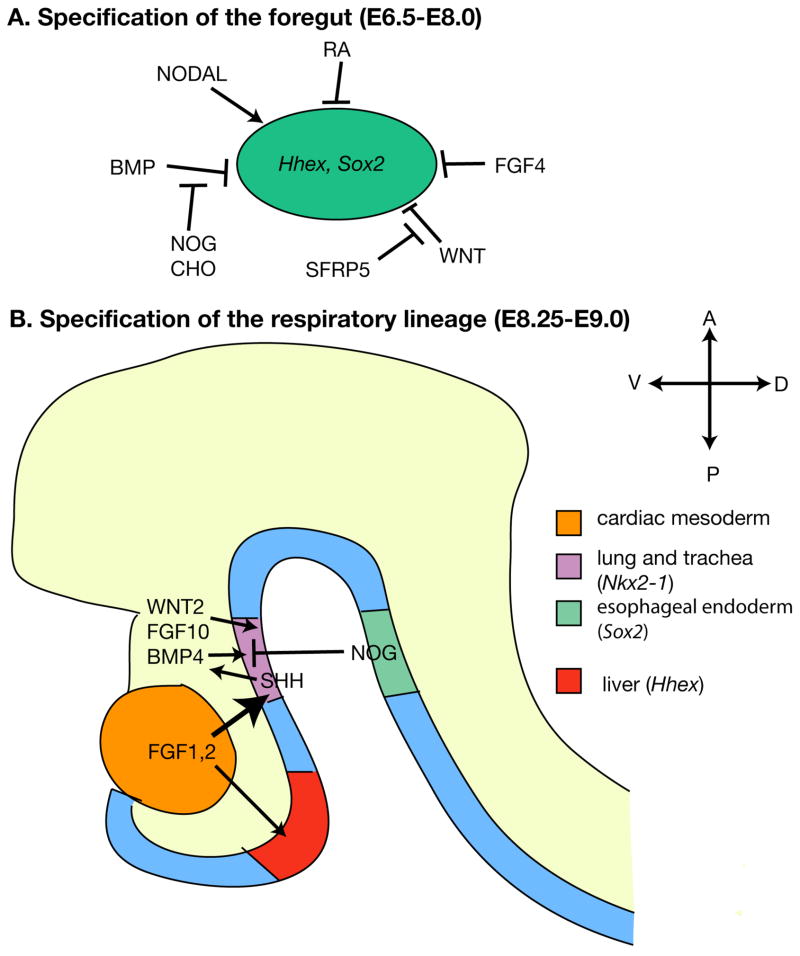
Figure 1. Signaling interactions during step-wise specification of the respiratory lineage.
Schematic depictions of key secreted ligands and transcription factors involved in specifying the respiratory progenitors. (A) During gastrulation, signals secreted from the adjacent mesoderm and ectoderm specify the foregut endoderm, which expresses marker genes Hhex and Sox2. (B) As the gut tube folds, multiple signals interact to induce trachea and lung fate in the ventral endoderm. NOG = Noggin, CHO = Chordin
While the first morphological appearance of the respiratory primordium is not apparent until a pair of lung buds form at E9.5, respiratory specification likely occurs much earlier. The earliest known marker of the respiratory lineage is the transcription factor Nkx2-1 (also known as Thyroid Transcription Factor 1, Ttf1), which is detectable in the ventral foregut by RT-PCR as early as E8.25, at the 8-somite stage (Lazzaro et al., 1991, Minoo et al., 1999, Serls et al., 2005) (Fig. 1B). While it is commonly used as a marker of the respiratory lineage, it is not exclusively expressed there, as it is also expressed in the developing thyroid and brain (Lazzaro et al., 1991). In Nkx2-1 null mutant embryos, although primary lung buds form, the trachea is absent, suggesting that it is essential for the initiation of a subset of the respiratory system (Minoo et al., 1999). While the timing of Nkx2-1 expression suggests that the respiratory lineage is first specified at E8.25, another study using an inducible lineage-labeling system suggests that progenitors of the distal respiratory system (peripheral lung tubules) may be set aside prior to or coincident with gastrulation (Perl et al., 2002). The identification and characterization of additional respiratory markers is needed to more accurately depict the early events of respiratory specification.
At E9.5, two ventral lung evaginations establish the left and right lung buds (Fig. 2A). Concomitantly, the foregut anterior to the buds separates into two parallel tubes, a ventral trachea and dorsal esophagus. Following elongation of the primary lung buds into the main bronchi, multiple branches form following a stereotypical sequence (Metzger et al., 2008). The respiratory system is patterned along its proximal-distal (P-D) axis (Fig. 2). The proximal region gives rise to the trachea and major conductive airways, and the distal region gives rise to the smaller bronchioles and gas exchange units. Differentiation along the P-D axis begins at E14.5, the final result being cells of distinct morphologies and functions. In the mature lung, the proximal airways are lined primarily with ciliated, basal, neuroendocrine, and secretory cell types, while distal gas-exchange units are lined with type I and type II alveolar epithelial cells (AECs, also termed pneumocytes) (Fig. 3) (Cardoso and Lu, 2006, Crystal et al., 2008). At birth, the various cell types fulfill distinct roles including cleaning and moisturizing air, production of surfactant and facilitation of gas exchange.
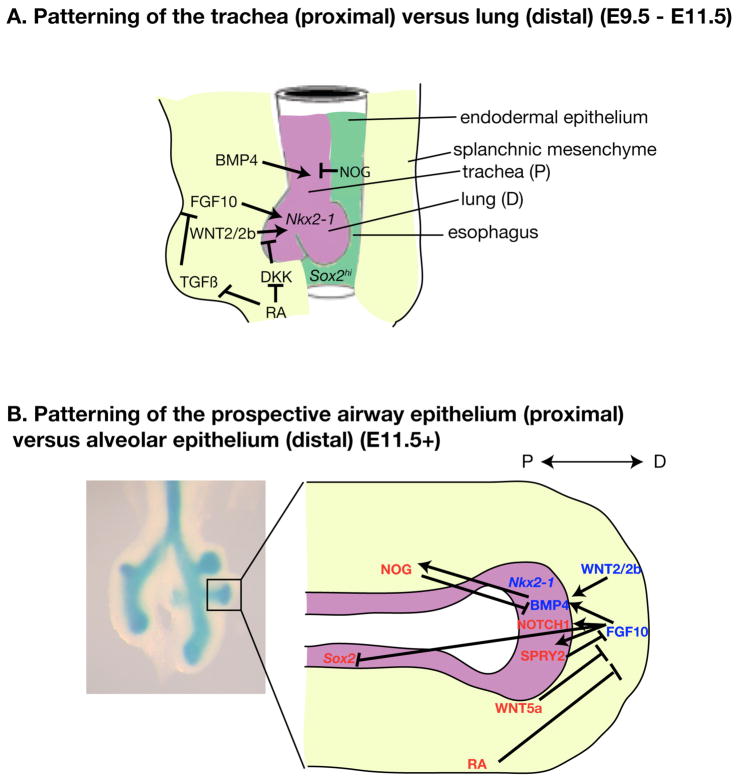
Figure 2. Signaling interactions during P-D patterning of the respiratory epithelium.
Schematic depictions of key secreted ligands and transcription factors involved in patterning the developing respiratory system. (A) During lung budding, multiple ligands are secreted from epithelium and the surrounding mesenchyme to distinguish the trachea and nascent lung buds. (Modified from Que et al., 2006). (B) During lung branching morphogenesis, multiple secreted ligands and transcription factors interact to distinguish the airway epithelium versus alveolar epithelium. Left panel is a wholemount image of lung at E11.5. Boxed area is illustrated in the right panel, with the epithelium depicted in blue. Signals that promote distal fate are labeled in blue. Signals that promote proximal fate are labeled in red.
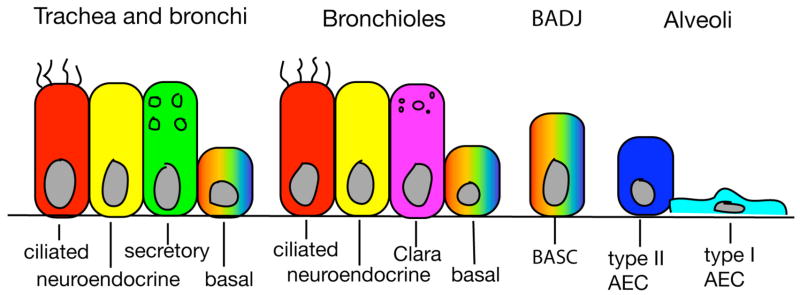
Figure 3. Differentiated cell types within the respiratory epithelium.
Schematic depiction of relative locations of the various cell types in the mature lung. Notch signaling regulates the fate decision between secretory and non-secretory cell fates, while TGFβ specifically promotes the differentiation program of Clara cells. The location of putative multipotent stem cells (basal cells and BASCs) are indicated with rainbow shading. BADJ – bronchioalveolar duct junction. BASC – bronchioalveolar stem cell.
Step-wise specification and dynamic responses to inductive cues
The respiratory system originates from a stereotypical location within the ventral foregut endoderm. In addition to the respiratory system, the foregut gives rise to an impressive array of organs, including the thymus, thyroid, esophagus, liver, pancreas, and stomach. The midgut and hindgut, in contrast, primarily give rise to the small and large intestine (Zorn and Wells, 2009). The first specification step is to distinguish the foregut from that of mid/hindgut. Several transcription factors have been implicated as indicators of endoderm patterning: Hhex and Sox2 are expressed in the foregut, while Cdxge nes are expressed in mid/hindgut (Fig. 1A) (Zorn and Wells, 2009). Multiple studies demonstrate that this foregut versus mid/hindgut pattern is established during gastrulation and shortly thereafter by ectodermal and mesodermal cues including BMP (Tiso et al., 2002, Matsushita et al., 2008), FGF (Davidson et al., 2000, Wells and Melton, 2000, Dessimoz et al., 2006, Li et al., 2008), WNT (McLin et al., 2007, Li et al., 2008), and retinoic acid (RA) (Bayha et al., 2009). For example, explant studies in chick have demonstrated that signals from Hensen’s node or its derivatives specify ingressing endoderm cells as foregut as indicated by Sox2 expression, and that BMP antagonists Noggin or Chordin are sufficient for this effect (Matsushita et al., 2008). Together, these data suggest that endoderm cells adopt an anterior fate unless programmed into more posterior fate through the combined action of secreted factors. In fact, it has been previously noted that the mechanism of endoderm patterning closely mirrors that of neural patterning, in which neural tissue has an anterior fate unless “posteriorized” by multiple signaling pathways (McLin et al., 2007). One exception to this pattern is the finding that Tgfβ family member Nodal, which is highly expressed in the early anterior primitive streak, is necessary for anterior fate (Vincent et al., 2003).
Following specification of the foregut, a second specification step towards respiratory identity is to partition the foregut into distinct organ compartments, including trachea/lung. Along the A-P axis of the developing foregut, studies have focused on cues that lead to differential specification of the respiratory versus liver and pancreas cell fates (Fig. 1B). Strong evidence suggests that FGF signaling plays a dosage-dependent role in this process (Wandzioch and Zaret, 2009, Serls et al., 2005, Jung et al., 1999, Calmont et al., 2006). For example, it has been shown that foregut endoderm cells from 2- to 5-somite stage embryos (E8.0-E8.5) adopt pancreatic fate (Pdx1 expression) when cultured in the absence of FGF, adopthepatic fate ( Hhex expression) when cultured in a low concentration of FGF, and adoptlung fate ( Nkx2-1 expression) when cultured in a high concentration of FGF (Serls et al., 2005). These and data from related studies suggest that increasing levels of FGF signal promote a progressively more anterior organ identity, from pancreas, to liver, to lung (Wandzioch and Zaret, 2009, Serls et al., 2005, Jung et al., 1999, Calmont et al., 2006). Remarkably, this is opposite to the finding during gastrulation where increasing levels of FGF promote posterior (mid/hindgut) identity(Dessimoz et al., 2006, Wells and Melton, 2000).
There are additional examples for distinct endoderm responses to the same signal at consecutive time periods. As mentioned above, at gastrula stage during foregut specification, overexpression of WNT leads to inhibition of foregut markers including Nkx2-1 (McClin and Zorn). A short time later during specification of the respiratory lineage (E8.25-E9.0 in mouse), inactivation of either WNT ligands (Wnt2/2b) or the key WNT effector β-Catenin lead to either loss or reduction of Nkx2-1, while forced activation of WNT/β-Catenin signaling via ectopic expression of a dominant-stable allele of β-Catenin in the ventral stomach promotes Nkx2-1 expression (Goss et al., 2009, Harris-Johnson et al., 2009). In contrast, at later stages of development(E18.5), forced activation of WNT/β-Catenin signaling via expression of a β-Catenin/LEF fusion protein causes lung epithelium to transdifferentiate into intestinal epithelium (Okubo and Hogan, 2004). Therefore, similar to FGF, it appears that WNT/β-Catenin can either promote or inhibit the respiratory fate depending on the context (timing, tissue site and perhaps level) of signaling.
This dynamic endoderm response to signaling is not restricted to the respiratory lineage. In the developing mouse liver and pancreas, BMP promotes liver over pancreas at 3-4 somites, but produces the opposite response at 5-6 somites (Wandzioch and Zaret, 2009). How the transcriptional regulatory circuitry is “rewired” in just a few hours remains an important question to be answered in developmental biology. Interestingly, TGFβ signaling plays an indirect role in permitting pancreatic development, by restraining the specification of cells competent to respond until they move into the BMP-inductive region at 5-6 somites (Wandzioch and Zaret, 2009).
Compared to A-P patterning of the foregut endoderm, relatively little is known regarding dorsal-ventral (D-V) patterning of this tissue. Opposite to respiratory precursors in the ventral foregut endoderm, the cells of the dorsal foregut will give rise to the esophagus (Fig. 1B). It is important to note that folding of the endoderm to form the gut tube brings cells from distinct regions along the A-P axis into close apposition along the D-V axis. Fate mapping studies in chick demonstrate that cells at the anterior end of Hensen’s node of mid-primitive streak stage embryos ultimately contribute to the ventral midline of the foregut, thus giving rise to the respiratory primordia (Matsushita et al., 2008). In contrast, cells located more posteriorly in the mid-primitive streak stage embryos will contribute to the dorsal midline of the foregut, thus giving rise to the esophagus. These data suggest that prospective-dorsal and prospective-ventral endoderm cells ingress at different times, and follow different migratory routes during gastrulation (Rosenquist, 1971, Kimura et al., 2006, Matsushita et al., 2008). These data have led some researchers to propose that separation of the respiratory and digestive tracts should most accurately be viewed as separate morphogenetic programs executed independently by two pre-patterned semi-circular canals, rather than patterning and division of a single tube (Brown and James, 2009). More consideration need to be paid to the morphogenetic history of the dorsal versus ventral endoderm cells to better understand their pre-existing molecular differences leading up to D-V patterning of the foregut.
Different transcription factor genes mark the dorsal versus ventral endoderm cells in a common foregut tube (Fig. 1B, 2A). Sox2, initially expressed throughout the entire foregut, is downregulated in the respiratory domain by E9.0 (Sherwood et al., 2009), coincident with the upregulation of Nkx2-1 in this domain. In addition to being markers, Nkx2-1 and Sox2 are necessary for formation of the ventral trachea and dorsal esophagus, respectively (Minoo et al., 1999, Que et al., 2007). Loss-of-function mutations of one transcription factor causes upregulation of the other along the entire D-V axis (Que et al., 2007), suggesting that they inhibit each other’s expression.
Multiple signaling pathways have been implicated in D-V patterning of the foregut tube, including BMP (Que et al., 2006, Li et al., 2007, Li et al., 2008), SHH (Litingtung et al., 1998) and FGF (Que et al., 2007) (Fig. 1B). For example, Bmp4 is expressed in the ventral mesenchyme surrounding the undivided foregut tube while Noggin, which encodes a BMP antagonist, is expressed in the dorsal foregut endoderm (Que et al., 2006, Li et al., 2007). Loss- and gain-of-BMP pathway mutants show tracheal agenesis and esophageal atresia, respectively, suggesting that proper balance of BMP signaling is essential for the formation of the two distinct lineages (Que et al., 2006, Li et al., 2007, Li et al., 2008). Shh is expressed in the ventral foregut endoderm, and Shh mutant embryos show defects in tracheal-esophageal separation (Litingtung et al., 1998). While it is not currently known whether SHH and BMP interact during D-V patterning of the foregut, at later stages of lung development, SHH secreted by lung epithelium induces Bmp4 in the mesenchyme (Weaver et al., 2003). A similar relationship could then at least partially explain the overlap in Shh and Bmp mutant phenotypes in the foregut. Evidence suggests that cells remain responsive to D-V signaling cues for a significant amount of time after the initial establishment of pattern. At E11.0, which is after the separation of the esophagus from the trachea, culturing the esophagus in the presence of exogenous FGF10 leads to downregulation of digestive markers and upregulation of respiratory markers (Que et al., 2007).
As morphogenesis proceeds, the nascent lung buds elongate and branch. At the same time, they are patterned along the P-D axis, resulting in the differentiation of distinct cell types (Fig. 2,3). We next turn our attention to what is known about how this process occurs.
P-D patterning of the respiratory system
Compared to respiratory specification, more is known about how the respiratory system is patterned along its P-D axis. We will discuss two stages of P-D patterning: distinction between the trachea (proximal) versus lung (distal); and within the lung, distinction between the airway epithelium (proximal) versus alveolar epithelium (distal). Perl et al. found that by using an inducible SpC-driven Cre system, they could genetically label distal lung epithelium without labeling tracheal and bronchial cells before E8.5 (Perl et al., 2002). Consistent with this, a more recent study using an inducible Id2-CreERT2 has shown that cells in the distal tip of the elongating lung branch at E11.5 ultimately contribute to all lung epithelium, but not tracheal epithelium cell types. These data suggest that precursors for the trachea versus lung are separated perhaps as early as when the respiratory lineage is set aside from most other foregut-derived organ progenitors. Data from knockout mice show that a number of genes are necessary for trachea but not initial lung bud formation, including Nkx2-1 (Minoo et al., 1999), Shh (Litingtung et al., 1998), and Bmp4 (Li et al., 2008). Conversely, Fgf10 and its cognate receptor Fgfr2 are essential for lung but not trachea formation (Min et al., 1998, Arman et al., 1999, Sekine et al., 1999, De Moerlooze et al., 2000). These results support the concept that distinct genetic programs promote trachea versus lung development.
For P-D patterning within the lung, a large number of genes have been identified that are preferentially expressed in the distal epithelium, with a smaller number preferentially expressed in the proximal epithelium (Liu and Hogan, 2002, Lu et al., 2004). Many of the same factors that regulate patterning of foregut endoderm are redeployed for the growing respiratory system (Fig. 2B). Interestingly, the opposing gradients of Sox2 and Nkx2-1 along the D-V axis of the separating esophagus and trachea at E10.0 are subsequently observed along the P-D axis of the elongating lung at E11.5 (Gontan et al., 2008, Que et al., 2009). During lung branching morphogenesis Sox2 inhibits branching and promotes proximal cell fate, while Nkx2-1 is required for branching and distal cell fate (Minoo et al., 1999, Yuan et al., 2000, Gontan et al., 2008, Que et al., 2009, Tompkins et al., 2009). Familiar signaling pathways are critical for P-D patterning of the branching lung as compared to earlier lung developmental events, although distinct ligands and receptors may be deployed at different stages. During respiratory specification (E8.25-E9.0), FGF1 and FGF2 from the cardiac mesenchyme signal to FGFR1 and FGFR4 in the endoderm (Serls et al., 2005). Starting from the initiation of lung budding however (E9.5 onwards), FGF10 from the mesenchyme surrounding the nascent lung buds signals to FGFR2 in the endoderm to drive outgrowth of lung buds and elongation of epithelial branches (Bellusci et al., 1997, Sekine et al., 1999, Weaver et al., 2000) (Figure 2A, C). Whether the employment of distinct FGF ligands and receptors in specification versus budding/branching is functionally relevant deserves further study.
In addition to acting as a chemoattractant for lung epithelial branching, FGF signaling plays a critical role in lung P-D patterning. Loss of FGF signaling through conditional deletion of Fgf10 or Fgfr2 after lung budding results in expansion of proximal marker Sox2 and downregulation of distal marker Sox9 (Abler et al., 2009). Overexpression of Fgf10 promotes distal fate while inhibiting terminal differentiation of lung epithelium (Nyeng et al., 2008). A similar paradigm for FGF signaling exists in P-D patterning of the limb, suggesting that this may be an evolutionarily-conserved function of FGF (Tabin and Wolpert, 2007, Mariani et al., 2008).
Upstream of Fgf10, while the comprehensive mechanism remains to be uncovered, several genes have been identified that control the dynamic and spatially-restricted pattern of Fgf10 expression. During lung branching morphogenesis, RA inhibits the expression of Fgf10 (Malpel et al., 2000) (Fig. 2B). Interestingly, the effects of RA on Fgf10 expression are stage-dependent. Opposite to its impact on Fgf10 expression at branching stage, RA promotes the initial expression of Fgf10 at budding stage, likely through repressing TGFβ signaling (Desai et al., 2004, Chen et al., 2007, Chen et al., 2010) (Fig. 2A). The mechanism by which the relationship between RA and FGF signaling changes in such a short period of time is unknown. The transcription factors Tbx4 and Tbx5 also promote Fgf10 expression, but since they are expressed ubiquitously in the mesenchyme, they likely act with additional factors to achieve the spatially restricted Fgf10 expression (Cebra-Thomas et al., 2003, Sakiyama et al., 2003).
Downstream of Fgf10, it induces additional signaling pathways that promote distal cell fate (Fig. 2B). For example, Bmp4, which is induced by FGF10 in the distal epithelium starting from E11.0, acts to promote distal and restrict proximal fate (Weaver et al., 1999, Hyatt et al., 2002, Hyatt et al., 2004, Eblaghie et al., 2006, Sun et al., 2008). This epithelial expression of Bmp4 is in addition to its earlier-initiated expression in the mesenchyme (Que et al., 2006, Li et al., 2007), although the significance of this additional site of expression is not known. Reduction of BMP signaling through overexpression of a dominant-negative receptor dnBmpr1b or secreted antagonist Xnoggin results in expansion of proximal cell markers and downregulation of distal markers (Weaver et al., 1999), and loss of BMP receptor Bmpr1a results in defects in distal lung development (Eblaghie et al., 2006, Sun et al., 2008).
Following their critical role in respiratory lineage specification mentioned above, Wnt2 and Wnt2b, two canonical Wnt genes, continue to be expressed in distal lung mesenchyme during lung branching morphogenesis (Yin et al., 2008, Yi et al., 2009) (Fig. 2B). Similar to FGF, loss-of-function studies demonstrate that endogenous levels of WNT/β-Catenin signaling are essential to promote distal lung fate (Mucenski et al., 2003, Shu et al., 2005), while gain-of-function studies suggest that WNT/β-Catenin signaling are sufficient to prevent differentiation (Reynolds et al., 2008). Upstream of WNT, its activity is promoted by RA through repression of WNT antagonist Dickkopf (Dkk) (Chen et al., 2010) (Fig. 2A). Downstream of WNT, its ability to promote distal fate and inhibit differentiation is mediated, at least in part, though its regulation of Fgfr2, Bmp4 and Mycn expression in the distal epithelium (Okubo et al., 2005, Shu et al., 2005, Cox et al., 2007) (Fig. 2B).
In addition to canonical WNT/β-Catenin pathway, there is evidence that non-canonical WNT, such as WNT5a signaling also plays a role in lung P-D patterning. Wnt5a is expressed most highly in the distal epithelium and mesenchyme (Li et al., 2002) (Fig. 2B). Loss of Wnt5a results in expansion of the distal epithelium and inhibition of differentiation, as well as increased expression of Bmp4 and Fgf10. This raises the possibility that WNT5a may control P-D patterning by inhibiting the expression of these distal-promoting factors.
Similar to Wnt5a, additional genes implicated in promoting proximal cell fate do so through antagonism of one or more distalizing signals (Fig. 2B). Spry2, which is induced by FGF10 in the distal epithelium, feeds back to limit FGF activity, thereby reducing the expression of distal markers (Tefft et al., 1999, Mailleux et al., 2001). A negative feedback loop also limits the amount of BMP signaling, as BMP4 induces expression of its antagonist Noggin in the proximal mesenchyme (Weaver et al., 2003). Overexpression of Noggin in the distal lung epithelium promotes proximal cell fate, suggesting that Nogginrestricts the domain of distal cell fate promoted by BMP4 (Weaver et al., 1999).
Unique among signals important for P-D patterning of the respiratory system, Notch and its transmembrane ligands act through cell-cell contacts, at a finer scale compared to the secreted signals mentioned above. A number of Notch ligands are expressed in the distal epithelium of the lung during early development (Post et al., 2000), and appear to be downstream of FGF10 signaling (Tsao et al., 2008). Chemical inhibition of Notch signaling results in overexpansion of distal fate and loss of proximal fate in cultured lung explants, suggesting that Notch functions to restrict distal fate (Tsao et al., 2008). Notch signaling pathway also plays a role in lung epithelial cell differentiation as discussed below.
Differentiation of the respiratory epithelium
Following broad-stroke patterning of the respiratory system, fine-scale differentiation of the respiratory epithelium proceeds in a P-D direction, starting with the appearance of neuroendocrine cells in the proximal airway at E14.5, and ending with the differentiation of type I and type II AECs in the distal epithelium shortly before birth (Fig. 3) (Morrisey and Hogan, 2010). Although a number of transcription factors necessary for formation of particular cell types in the lung have been identified (Chen et al., 1998, Wan et al., 2004, Wan et al., 2008, Chen et al., 2009, Que et al., 2009), few signaling pathways have been implicated in differentiation of cells within either the proximal or distal compartments of the respiratory system.
Notch signaling has recently been shown to be involved in the fate choice among cells in the proximal lung (Fig. 3). The Notch target Hes1 is expressed in the trachea, bronchi, and bronchiolar airways, but is absent from the distal saccules at E18.5 (Guseh et al., 2009). Loss-of-function Notch pathway mutants show an absence of Clara cells and overabundance of ciliated and neuroendocrine cells in proximal airways (Tsao et al., 2009, Morimoto et al., 2010), while gain-of-function mutants have fewer ciliated cells and overabundance of goblet cells (Guseh et al., 2009). Together, these data suggest that Notch signaling functions in the proximal lung to promote Clara and goblet cell fate at the expense of ciliated and neuroendocrine fate. The TGFβ type I receptor Activin like kinase 5 (Alk5) is also expressed in the bronchiolar epithelium, and inactivation of Alk5 results in loss of Clara cells while leaving differentiation of other cell types relatively unperturbed (Xing et al., 2010). This raises the possibility that TGFβ may operate after Notch to promote differentiation of Clara cells once their fate has been determined. Subsequent experiments demonstrate that TGFβ promotes Clara cell differentiation in a SMAD-independent fashion, through upregulation of pERK and pAKT. It remains possible that other signaling pathways function in lung epithelial cell differentiation, and their roles in this late process may be obscured by the requirements for these signals in earlier lung developmental events. Late and cell type-specific inactivation of these pathway members may reveal additional signaling components essential for epithelial cell differentiation.
Plasticity of the respiratory lineage
The issue of respiratory epithelium plasticity can be considered on several levels: commitment to respiratory versus other (e.g. digestive) cell fate, commitment to trachea versus lung cell fate, and commitment to one versus another terminally differentiated cell fate. Lineage analysis and Nkx2-1 expression data suggest that the respiratory progenitors are specified by E8.25, if not earlier (Perl et al., 2002, Lazzaro et al., 1991, Minoo et al., 1999, Serls et al., 2005). However, determination of respiratory cell fate occurs surprisingly late in development. At lung branching stage (E11.0 and later), ectopic activation of WNT can cause lung epithelium to transdifferentiate into intestine (Okubo and Hogan, 2004), while exogenous FGF can cause esophageal epithelium to transdifferentiate into respiratory epithelium (Que et al., 2007). These results suggest that cells remain competent to switch between the respiratory and digestive fates even after the morphological appearance of distinct structures.
On trachea versus lung determination, in spite of the evidence suggesting early separation of trachea versus lung progenitors, tracheal epithelium from E13 rat lungs (similar to E11 in mouse) can form buds and express markers of distal lung fates when grafted adjacent to mesenchyme from the distal lung, while distal lung epithelium will fail to branch and will express markers of proximal respiratory fate when grafted adjacent to proximal lung epithelium (Shannon, 1994, Shannon et al., 1998). Interestingly, lung mesenchyme from near full-term embryos shows similar inducing capability as mesenchyme from E13 rat embryos (Deimling et al., 2007). At the molecular level, explant culture studies indicate that FGF, often in combination with other factors in the medium, is capable of reprogramming tracheal epithelium into a distal lung fate (Shannon et al., 1999, Ohtsuka et al., 2001, Hyatt et al., 2004).
Within the developing lung during cell differentiation, several recent studies suggest that proximal and distal lung epithelial cells in late-gestation lungs show different levels of phenotypic stability (Deimling et al., 2007). Tissue recombination experiments indicate that when recombined with mesenchyme from skin or intestine, lung epithelium from near-term rat embryos continues to express Clara cell marker Clara cell secretory protein (also known as Scgb1a1), but downregulates type II AEC markers surfactant protein A, B and C (SpA, SpB, and SpC). These data suggest that Clara cells maintain their characteristics in the absence of promoting signals from lung mesenchyme, but type II AECs still require factors from distal lung mesenchyme to maintain their characteristics. An additional study demonstrates that ectopic expression of Sox17 in the distal airways of adult mice reprograms a subset of AECs into Clara and ciliated cells (Lange et al., 2009) suggesting that plasticity may be retained in at least a subset of respiratory cells into adulthood.
Despite evidence for a proximal-to-distal sequence of cell fate commitment(Rawlins et al., 2009a), there appear to be pockets of cells in the adult lung epithelium that remain labile. For example, basal cells within the trachea and bronchial epithelium remain capable of generating multiple cell types during homeostatic or repair conditions (Rock et al., 2009) (Fig. 3). More distally at the bronchioalveolar duct junction, bronchioalveolar stem cells (BASCs) have been identified to exhibit multipotency and the ability to self-renewal (Kim et al., 2005, Nolen-Walston et al., 2008, Zhang et al., 2008). Determining the identity, location, and hierarchical organization of adult pulmonary stem/progenitor cells is an area of active research. For a more comprehensive review on this topic, see Rawlins in this issue.
Perspectives: from phenomenology to hypothesis
The formation of a functional respiratory epithelium from ventral foregut endoderm consists of a series of intricately organized events, of which we are only beginning to understand. We have identified many of the signaling pathways and transcription factors involved in respiratory development, but we know little about how they are integrated with each other, and what cis-regulatory modules mediate this integration. These are questions that pertain to each of the developmental events discussed here. For example, during respiratory specification, the earliest known marker of the respiratory lineage, Nkx2-1, is not strictly essential for respiratory specification (Kimura et al., 1999), suggesting that it is only one of several factors involved in this process. Tracing upstream, the transcription factor genes currently known to directly activate the expression of Nkx2-1: Foxa2 (Ikeda et al., 1996), Gata6 (Shaw-White et al., 1999), and Sp1 and Sp3 (Li et al., 2000), all show expression patterns broader than Nkx2-1, suggesting that either these factors function together to drive Nkx2-1 expression in their shared domain, or that additional factors are involved. Whether and how known signaling pathways may participate in regulating the expression or activity of these transcription factors are questions that require more investigation (Vincent et al., 2003). For the goal of elucidating the gene regulatory networks that govern the various stages of respiratory development, we can perhaps learn from prior successful studies whereby combining gene expression/perturbation analyses and identification of relevant cis-regulatory modules, researchers have successfully developed explanatory models for endomesoderm formation in the sea urchin embryo (Ben-Tabou de-Leon and Davidson, 2009, Peter and Davidson, 2010). Similar approaches, while likely more challenging in the complex mammalian respiratory system, would prove fruitful in advancing our understanding of lung/trachea development.
Two additional open questions are: how do cells alter their responses to the same signaling cues in periods as short as a few hours (Davidson et al., 2000, Serls et al., 2005, Wandzioch and Zaret, 2009); and how do cells maintain their plasticity to respond to inducing signals for a long period of time after organ establishment (Okubo and Hogan, 2004, Que et al., 2007)? These seemingly divergent behaviors may in fact be dictated by similar epigenetic regulation of the responsible transcriptome. Whether and how the known genetic network is integrated with potential epigenetic mechanisms is a fruitful direction to explore. Given its vital importance starting at first breath, reconstructing the formation and maintenance of a healthy respiratory system has been and will continue to be a rewarding theme of study.
Acknowledgments
We would like to thank John Fallon and Elke Ober for critical reading of this manuscript, and for their valuable insights.
References
- Abler LL, Mansour SL, Sun X. Conditional gene inactivation reveals roles for Fgf10 and Fgfr2 in establishing a normal pattern of epithelial branching in the mouse lung. Dev Dyn. 2009;238:1999–2013. doi: 10.1002/dvdy.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arman E, Haffner-Krausz R, Gorivodsky M, Lonai P. Fgfr2 is required for limb outgrowth and lung-branching morphogenesis. Proc Natl Acad Sci U S A. 1999;96:11895–11899. doi: 10.1073/pnas.96.21.11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayha E, Jorgensen MC, Serup P, Grapin-Botton A. Retinoic acid signaling organizes endodermal organ specification along the entire antero-posterior axis. PLoS One. 2009;4:e5845. doi: 10.1371/journal.pone.0005845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellusci S, Grindley J, Emoto H, Itoh N, Hogan BL. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124:4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- Ben-Tabou de-Leon S, Davidson EH. Experimentally based sea urchin gene regulatory network and the causal explanation of developmental phenomenology. Wiley Interdiscip Rev Syst Biol Med. 2009;1:237–246. doi: 10.1002/wsbm.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E, James K. The lung primordium an outpouching from the foregut! evidence-based dogma or myth? J Pediatr Surg. 2009;44:607–615. doi: 10.1016/j.jpedsurg.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: Questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Cebra-Thomas JA, Bromer J, Gardner R, Lam GK, Sheipe H, Gilbert SF. T-box gene products are required for mesenchymal induction of epithelial branching in the embryonic mouse lung. Dev Dyn. 2003;226:82–90. doi: 10.1002/dvdy.10208. [DOI] [PubMed] [Google Scholar]
- Chen F, Cao Y, Qian J, Shao F, Niederreither K, Cardoso WV. A retinoic acid-dependent network in the foregut controls formation of the mouse lung primordium. J Clin Invest. 2010;120:2040–2048. doi: 10.1172/JCI40253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Desai TJ, Qian J, Niederreither K, Lu J, Cardoso WV. Inhibition of tgf beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development. 2007;134:2969–2979. doi: 10.1242/dev.006221. [DOI] [PubMed] [Google Scholar]
- Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Knowles HJ, Hebert JL, Hackett BP. Mutation of the mouse hepatocyte nuclear factor/forkhead homologue 4 gene results in an absence of cilia and random left-right asymmetry. J Clin Invest. 1998;102:1077–1082. doi: 10.1172/JCI4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WS, Shin CH, Stainier DY. Bmp2 signaling regulates the hepatic versus pancreatic fate decision. Dev Cell. 2008;15:738–748. doi: 10.1016/j.devcel.2008.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B, Kislinger T, Wigle DA, Kannan A, Brown K, Okubo T, Hogan B, Jurisica I, Frey B, Rossant J, Emili A. Integrated proteomic and transcriptomic profiling of mouse lung development and nmyc target genes. Mol Syst Biol. 2007;3:109. doi: 10.1038/msb4100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: Current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson BP, Cheng L, Kinder SJ, Tam PP. Exogenous FGF-4 can suppress anterior development in the mouse embryo during neurulation and early organogenesis. Dev Biol. 2000;221:41–52. doi: 10.1006/dbio.2000.9663. [DOI] [PubMed] [Google Scholar]
- De Moerlooze L, Spencer-Dene B, Revest JM, Hajihosseini M, Rosewell I, Dickson C. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- Deimling J, Thompson K, Tseu I, Wang J, Keijzer R, Tanswell AK, Post M. Mesenchymal maintenance of distal epithelial cell phenotype during late fetal lung development. Am J Physiol Lung Cell Mol Physiol. 2007;292:L725–41. doi: 10.1152/ajplung.00221.2006. [DOI] [PubMed] [Google Scholar]
- Desai TJ, Malpel S, Flentke GR, Smith SM, Cardoso WV. Retinoic acid selectively regulates Fgf10 expression and maintains cell identity in the prospective lung field of the developing foregut. Dev Biol. 2004;273:402–415. doi: 10.1016/j.ydbio.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Dessimoz J, Opoka R, Kordich JJ, Grapin-Botton A, Wells JM. FGF signaling is necessary for establishing gut tube domains along the anterior-posterior axis in vivo. Mech Dev. 2006;123:42–55. doi: 10.1016/j.mod.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol. 2006;291:67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci U S A. 2009;106:9286–9291. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontan C, de Munck A, Vermeij M, Grosveld F, Tibboel D, Rottier R. Sox2 is important for two crucial processes in lung development: Branching morphogenesis and epithelial cell differentiation. Dev Biol. 2008;317:296–309. doi: 10.1016/j.ydbio.2008.02.035. [DOI] [PubMed] [Google Scholar]
- Goss AM, Tian Y, Tsukiyama T, Cohen ED, Zhou D, Lu MM, Yamaguchi TP, Morrisey EE. Wnt2/2b and beta-catenin signaling are necessary and sufficient to specify lung progenitors in the foregut. Dev Cell. 2009;17:290–298. doi: 10.1016/j.devcel.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guseh JS, Bores SA, Stanger BZ, Zhou Q, Anderson WJ, Melton DA, Rajagopal J. Notch signaling promotes airway mucous metaplasia and inhibits alveolar development. Development. 2009;136:1751–1759. doi: 10.1242/dev.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Johnson KS, Domyan ET, Vezina CM, Sun X. Beta-catenin promotes respiratory progenitor identity in mouse foregut. Proc Natl Acad Sci U S A. 2009;106:16287–16292. doi: 10.1073/pnas.0902274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt BA, Shangguan X, Shannon JM. BMP4 modulates fibroblast growth factor-mediated induction of proximal and distal lung differentiation in mouse embryonic tracheal epithelium in mesenchyme-free culture. Dev Dyn. 2002;225:153–165. doi: 10.1002/dvdy.10145. [DOI] [PubMed] [Google Scholar]
- Hyatt BA, Shangguan X, Shannon JM. FGF-10 induces SP-C and Bmp4 and regulates proximal-distal patterning in embryonic tracheal epithelium. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1116–26. doi: 10.1152/ajplung.00033.2004. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Shaw-White JR, Wert SE, Whitsett JA. Hepatocyte nuclear factor 3 activates transcription of thyroid transcription factor 1 in respiratory epithelial cells. Mol Cell Biol. 1996;16:3626–3636. doi: 10.1128/mcb.16.7.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kimura S, Ward JM, Minoo P. Thyroid-specific enhancer-binding protein/thyroid transcription factor 1 is not required for the initial specification of the thyroid and lung primordia. Biochimie. 1999;81:321–327. doi: 10.1016/s0300-9084(99)80077-7. [DOI] [PubMed] [Google Scholar]
- Kimura W, Yasugi S, Stern CD, Fukuda K. Fate and plasticity of the endoderm in the early chick embryo. Dev Biol. 2006;289:283–295. doi: 10.1016/j.ydbio.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kwon GS, Viotti M, Hadjantonakis AK. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev Cell. 2008;15:509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange AW, Keiser AR, Wells JM, Zorn AM, Whitsett JA. Sox17 promotes cell cycle progression and inhibits TGF-beta/Smad3 signaling to initiate progenitor cell behavior in the respiratory epithelium. PLoS One. 2009;4:e5711. doi: 10.1371/journal.pone.0005711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113:1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- Li C, Ling X, Yuan B, Minoo P. A novel DNA element mediates transcription of Nkx2.1 by Sp1 and Sp3 in pulmonary epithelial cells. Biochim Biophys Acta. 2000;1490:213–224. doi: 10.1016/s0167-4781(99)00183-9. [DOI] [PubMed] [Google Scholar]
- Li C, Xiao J, Hormi K, Borok Z, Minoo P. Wnt5a participates in distal lung morphogenesis. Dev Biol. 2002;248:68–81. doi: 10.1006/dbio.2002.0729. [DOI] [PubMed] [Google Scholar]
- Li Y, Gordon J, Manley NR, Litingtung Y, Chiang C. Bmp4 is required for tracheal formation: A novel mouse model for tracheal agenesis. Dev Biol. 2008;322:145–155. doi: 10.1016/j.ydbio.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Litingtung Y, Ten Dijke P, Chiang C. Aberrant bmp signaling and notochord delamination in the pathogenesis of esophageal atresia. Dev Dyn. 2007;236:746–754. doi: 10.1002/dvdy.21075. [DOI] [PubMed] [Google Scholar]
- Li Y, Rankin SA, Sinner D, Kenny AP, Krieg PA, Zorn AM. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008;22:3050–3063. doi: 10.1101/gad.1687308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hogan BL. Differential gene expression in the distal tip endoderm of the embryonic mouse lung. Gene Expr Patterns. 2002;2:229–233. doi: 10.1016/s1567-133x(02)00057-1. [DOI] [PubMed] [Google Scholar]
- Lu J, Qian J, Izvolsky KI, Cardoso WV. Global analysis of genes differentially expressed in branching and non-branching regions of the mouse embryonic lung. Dev Biol. 2004;273:418–435. doi: 10.1016/j.ydbio.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Tefft D, Ndiaye D, Itoh N, Thiery JP, Warburton D, Bellusci S. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech Dev. 2001;102:81–94. doi: 10.1016/s0925-4773(01)00286-6. [DOI] [PubMed] [Google Scholar]
- Malpel S, Mendelsohn C, Cardoso WV. Regulation of retinoic acid signaling during lung morphogenesis. Development. 2000;127:3057–3067. doi: 10.1242/dev.127.14.3057. [DOI] [PubMed] [Google Scholar]
- Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008;453:401–405. doi: 10.1038/nature06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita S, Urase K, Komatsu A, Scotting PJ, Kuroiwa A, Yasugi S. Foregut endoderm is specified early in avian development through signal(s) emanating from Hensen’s node or its derivatives. Mech Dev. 2008;125:377–395. doi: 10.1016/j.mod.2008.02.003. [DOI] [PubMed] [Google Scholar]
- McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1(−/−) mouse embryos. Dev Biol. 1999;209:60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, Kopan R. Canonical notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of clara versus ciliated cell fate. J Cell Sci. 2010;123:213–224. doi: 10.1242/jcs.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: Genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. Beta-catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem. 2003;278:40231–40238. doi: 10.1074/jbc.M305892200. [DOI] [PubMed] [Google Scholar]
- Nolen-Walston RD, Kim CF, Mazan MR, Ingenito EP, Gruntman AM, Tsai L, Boston R, Woolfenden AE, Jacks T, Hoffman AM. Cellular kinetics and modeling of bronchioalveolar stem cell response during lung regeneration. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1158–65. doi: 10.1152/ajplung.00298.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyeng P, Norgaard GA, Kobberup S, Jensen J. FGF10 maintains distal lung bud epithelium and excessive signaling leads to progenitor state arrest, distalization, and goblet cell metaplasia. BMC Dev Biol. 2008;8:2. doi: 10.1186/1471-213X-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka N, Urase K, Momoi T, Nogawa H. Induction of bud formation of embryonic mouse tracheal epithelium by fibroblast growth factor plus transferrin in mesenchyme-free culture. Dev Dyn. 2001;222:263–272. doi: 10.1002/dvdy.1206. [DOI] [PubMed] [Google Scholar]
- Okubo T, Hogan BL. Hyperactive wnt signaling changes the developmental potential of embryonic lung endoderm. J Biol. 2004;3:11. doi: 10.1186/jbiol3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci U S A. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter IS, Davidson EH. The endoderm gene regulatory network in sea urchin embryos up to mid-blastula stage. Dev Biol. 2010;340:188–199. doi: 10.1016/j.ydbio.2009.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post LC, Ternet M, Hogan BL. Notch/Delta expression in the developing mouse lung. Mech Dev. 2000;98:95–98. doi: 10.1016/s0925-4773(00)00432-9. [DOI] [PubMed] [Google Scholar]
- Que J, Choi M, Ziel JW, Klingensmith J, Hogan BL. Morphogenesis of the trachea and esophagus: Current players and new roles for noggin and bmps. Differentiation. 2006;74:422–437. doi: 10.1111/j.1432-0436.2006.00096.x. [DOI] [PubMed] [Google Scholar]
- Que J, Luo X, Schwartz RJ, Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J, Okubo T, Goldenring JR, Nam KT, Kurotani R, Morrisey EE, Taranova O, Pevny LH, Hogan BL. Multiple dose-dependent roles for Sox2 in the patterning and differentiation of anterior foregut endoderm. Development. 2007;134:2521–2531. doi: 10.1242/dev.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009a;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009b;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Hogan BL. Epithelial stem cells of the lung: Privileged few or opportunities for many? Development. 2006;133:2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, Mariani T, Di PY, Taketo MM, Stripp BR. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells. 2008;26:1337–1346. doi: 10.1634/stemcells.2008-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist GC. The location of the pregut endoderm in the chick embryo at the primitive streak stage as determined by radioautographic mapping. Dev Biol. 1971;26:323–335. doi: 10.1016/0012-1606(71)90131-x. [DOI] [PubMed] [Google Scholar]
- Sakiyama J, Yamagishi A, Kuroiwa A. Tbx4-Fgf10 system controls lung bud formation during chicken embryonic development. Development. 2003;130:1225–1234. doi: 10.1242/dev.00345. [DOI] [PubMed] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, Yagishita N, Matsui D, Koga Y, Itoh N, Kato S. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Serls AE, Doherty S, Parvatiyar P, Wells JM, Deutsch GH. Different thresholds of fibroblast growth factors pattern the ventral foregut into liver and lung. Development. 2005;132:35–47. doi: 10.1242/dev.01570. [DOI] [PubMed] [Google Scholar]
- Shannon JM. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol. 1994;166:600–614. doi: 10.1006/dbio.1994.1340. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Gebb SA, Nielsen LD. Induction of alveolar type II cell differentiation in embryonic tracheal epithelium in mesenchyme-free culture. Development. 1999;126:1675–1688. doi: 10.1242/dev.126.8.1675. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Shaw-White JR, Bruno MD, Whitsett JA. GATA-6 activates transcription of thyroid transcription factor-1. J Biol Chem. 1999;274:2658–2664. doi: 10.1074/jbc.274.5.2658. [DOI] [PubMed] [Google Scholar]
- Sherwood RI, Chen TY, Melton DA. Transcriptional dynamics of endodermal organ formation. Dev Dyn. 2009;238:29–42. doi: 10.1002/dvdy.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol. 2005;283:226–239. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Slack JMWMW. From egg to embryo : Regional specification in early development. 2. xix. Cambridge [England] New York: Cambridge University Press; 1949–1991. p. 328. [Google Scholar]
- Stripp BR. Hierarchical organization of lung progenitor cells: Is there an adult lung tissue stem cell? Proc Am Thorac Soc. 2008;5:695–698. doi: 10.1513/pats.200801-011AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Chen H, Chen C, Whitsett JA, Mishina Y, Bringas P, Jr, Ma JC, Warburton D, Shi W. Prenatal lung epithelial cell-specific abrogation of Alk3-bone morphogenetic protein signaling causes neonatal respiratory distress by disrupting distal airway formation. Am J Pathol. 2008;172:571–582. doi: 10.2353/ajpath.2008.070286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin C, Wolpert L. Rethinking the proximodistal axis of the vertebrate limb in the molecular era. Genes Dev. 2007;21:1433–1442. doi: 10.1101/gad.1547407. [DOI] [PubMed] [Google Scholar]
- Tefft JD, Lee M, Smith S, Leinwand M, Zhao J, Bringas P, Jr, Crowe DL, Warburton D. Conserved function of mSpry-2, a murine homolog of drosophila sprouty, which negatively modulates respiratory organogenesis. Curr Biol. 1999;9:219–222. doi: 10.1016/s0960-9822(99)80094-3. [DOI] [PubMed] [Google Scholar]
- Tiso N, Filippi A, Pauls S, Bortolussi M, Argenton F. BMP signalling regulates anteroposterior endoderm patterning in zebrafish. Mech Dev. 2002;118:29–37. doi: 10.1016/s0925-4773(02)00252-6. [DOI] [PubMed] [Google Scholar]
- Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, Whitsett JA. Sox2 is required for maintenance and differentiation of bronchiolar clara, ciliated, and goblet cells. PLoS One. 2009;4:e8248. doi: 10.1371/journal.pone.0008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Chen F, Izvolsky KI, Walker J, Kukuruzinska MA, Lu J, Cardoso WV. Gamma-secretase activation of notch signaling regulates the balance of proximal and distal fates in progenitor cells of the developing lung. J Biol Chem. 2008;283:29532–29544. doi: 10.1074/jbc.M801565200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao PN, Vasconcelos M, Izvolsky KI, Qian J, Lu J, Cardoso WV. Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development. 2009;136:2297–2307. doi: 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. Cell fate decisions within the mouse organizer are governed by graded nodal signals. Genes Dev. 2003;17:1646–1662. doi: 10.1101/gad.1100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Luo F, Wert SE, Zhang L, Xu Y, Ikegami M, Maeda Y, Bell SM, Whitsett JA. Kruppel-like factor 5 is required for perinatal lung morphogenesis and function. Development. 2008;135:2563–2572. doi: 10.1242/dev.021964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan H, Xu Y, Ikegami M, Stahlman MT, Kaestner KH, Ang SL, Whitsett JA. Foxa2 is required for transition to air breathing at birth. Proc Natl Acad Sci U S A. 2004;101:14449–14454. doi: 10.1073/pnas.0404424101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandzioch E, Zaret KS. Dynamic signaling network for the specification of embryonic pancreas and liver progenitors. Science. 2009;324:1707–1710. doi: 10.1126/science.1174497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol. 2003;258:169–184. doi: 10.1016/s0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Weaver M, Dunn NR, Hogan BL. Bmp4 and Fgf10 play opposing roles during lung bud morphogenesis. Development. 2000;127:2695–2704. doi: 10.1242/dev.127.12.2695. [DOI] [PubMed] [Google Scholar]
- Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development. 1999;126:4005–4015. doi: 10.1242/dev.126.18.4005. [DOI] [PubMed] [Google Scholar]
- Wells JM, Melton DA. Early mouse endoderm is patterned by soluble factors from adjacent germ layers. Development. 2000;127:1563–1572. doi: 10.1242/dev.127.8.1563. [DOI] [PubMed] [Google Scholar]
- Xing Y, Li C, Li A, Sridurongrit S, Tiozzo C, Bellusci S, Borok Z, Kaartinen V, Minoo P. Signaling via Alk5 controls the ontogeny of lung clara cells. Development. 2010;137:825–833. doi: 10.1242/dev.040535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Domyan ET, Lewandoski M, Sun X. Fibroblast growth factor 9 signaling inhibits airway smooth muscle differentiation in mouse lung. Dev Dyn. 2009;238:123–137. doi: 10.1002/dvdy.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, White AC, Huh SH, Hilton MJ, Kanazawa H, Long F, Ornitz DM. An FGF-WNT gene regulatory network controls lung mesenchyme development. Dev Biol. 2008;319:426–436. doi: 10.1016/j.ydbio.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B, Li C, Kimura S, Engelhardt RT, Smith BR, Minoo P. Inhibition of distal lung morphogenesis in Nkx2.1(−/−) embryos. Dev Dyn. 2000;217:180–190. doi: 10.1002/(SICI)1097-0177(200002)217:2<180::AID-DVDY5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Goss AM, Cohen ED, Kadzik R, Lepore JJ, Muthukumaraswamy K, Yang J, DeMayo FJ, Whitsett JA, Parmacek MS, Morrisey EE. A Gata6-wnt pathway required for epithelial stem cell development and airway regeneration. Nat Genet. 2008;40:862–870. doi: 10.1038/ng.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorn AM, Wells JM. Vertebrate endoderm development and organ formation. Annu Rev Cell Dev Biol. 2009;25:221–251. doi: 10.1146/annurev.cellbio.042308.113344. [DOI] [PMC free article] [PubMed] [Google Scholar]