Abstract
Objectives
The study was designed to assess the safety, adherence, acceptability, and effect on vaginal microflora of 3% SPL7013 Gel (VivaGel®), a novel dendrimer topical microbicide that inhibits HIV, HSV-2 and HPV in vitro and in animal models.
Design
Phase 1, randomized, double-blind, placebo-controlled study in sexually active women.
Methods
Sixty-one sexually active women aged 18–24 years were recruited from three sites in the United States. Participants were randomized 1:1:1 to receive VivaGel, VivaGel placebo, or a hydroxyethylcellulose (HEC) placebo twice daily for 14 consecutive days. Safety endpoints included genitourinary and/or other adverse events (AE). Changes in vaginal flora were determined from Gram-stained vaginal smears and quantitative vaginal culture.
Results
No serious AEs or withdrawals due to AEs were reported. Genitourinary symptoms were reported as follows: VivaGel (n=17/22; 77.3%), VivaGel placebo (n=14/21; 66.7%) and HEC (n=8/18; 44.4%) (NS, p=0.1). The incidence of abnormal pelvic exam findings was similar across all gel arms of the study. Using pair-wise comparison, women in the VivaGel arm had a significantly higher incidence of related genitourinary AEs compared with women in the HEC gel arm (0.297 versus 0.111 per 100 person years, respectively; p=0.003). Exposure to VivaGel and VivaGel placebo resulted in minor shifts in the vaginal microflora but there was no overall impact on incidence of bacterial vaginosis as assessed by Nugent score.
Conclusions
VivaGel was generally well tolerated and comparable with the VivaGel placebo, although there was a higher incidence of low grade related genital AEs compared to the HEC placebo gel.
Keywords: Phase 1, clinical trial, young women, SPL7013, HIV, microbicide, vaginal gel
Introduction
Despite recent improvements in access to antiretroviral therapy in the developing world, women, and particularly young women continue to get infected with HIV at alarming rates [1] and there is an urgent need for prevention methods that women can initiate and control themselves. Topical microbicides represent one such method [2], and a growing body of data suggests that a safe and effective topical microbicide will be a real option for women in the future. The results of the CAPRISA 004 study of 1% tenofovir gel have demonstrated the proof of concept that an antiretroviral topical vaginal microbicide can prevent HIV acquisition [3].
SPL7013 Gel, or VivaGel®, is a dendrimer-based topical microbicide candidate currently being developed to prevent STIs and as a treatment for bacterial vaginosis. Dendrimers are a relatively new class of macromolecules characterized by multiple layers of subunits branching out from a central core; they are constructed by repeated stepwise addition of branching units to a core [4]. During lead optimization for dendrimer-based microbicides with HIV and herpes simplex virus (HSV) antiviral potential by Starpharma Pty Ltd, SPL7013 emerged as a candidate with significant antiviral properties against HIV and HSV-2 [5].
Four Phase 1 studies of VivaGel have been conducted. Data from the first two studies indicate that 3% VivaGel is safe and well tolerated when administered to the vaginal [6] or penile epithelium [7] for up to seven days. A third study investigated the safety and tolerability of 3.5g of 3% VivaGel when administered vaginally, twice daily for 14 days in healthy, sexually inactive, female volunteers in Kenya and the United States (US). Preliminary data from this study suggested that there was an increased rate of genital AEs in the women receiving VivaGel compared to those receiving the VivaGel placebo [8]. The fourth study investigated the retention and duration of anti-HIV and anti-HSV-2 activity following vaginal administration of VivaGel. The purpose of MTN-004 was to assess the safety and acceptability of VivaGel in young sexually active women in the US. The study was conducted in collaboration with the Microbicide Trials Network (MTN) and the ATN.
Methods
Objectives
The primary objective of the study was to assess the safety of VivaGel on the vulvar and cervicovaginal mucosa of healthy sexually active HIV-negative women aged 18–24 years. Secondary objectives included assessment of product adherence and acceptability as well as the impact of product administration on vaginal microflora. Exploratory objectives included (i) assessment of product administration on a range of immunological parameters including cervicovaginal levels of cytokines, chemokines, and innate immune factors, (ii) systemic absorption of SPL7013, and (iii) the effects of VivaGel on colposcopic findings of the cervix and vagina.
Design
MTN-004 began as a Phase 1, double blind, randomized, placebo controlled comparison of twice daily exposure to VivaGel and VivaGel placebo (1:1) at two clinical sites (San Juan, PR and Tampa, FL). Enrollment began in August 2007 under Version 2.0 of the protocol. The protocol was paused in October 2007 as five of the seven women enrolled in the study had experienced a low grade genital AE. Following an interim data review the protocol was amended to include a third comparison arm (the HEC placebo gel). Enrollment recommenced in October 2008 under Version 3.0 of the protocol. 61 participants were randomized 1:1:1 to the three study arms. In May 2009, a third clinical site (Pittsburgh, PA) was added to accelerate recruitment to the study. Sample size was based on similar Phase I studies of topical microbicide studies. A blinded statistician from the Statistical and Data Management Center (SCHARP) created lists containing randomly generated unique three-digit codes in blocks of six (two per arm) for each clinical site. Prescriptions containing the randomly assigned codes were contained in sealed envelopes, numbered sequentially and assigned by the clinic staff to study participants in sequential order. Site pharmacists dispensed three boxes of product labeled with the same unique three-digit randomization codes and containing ten applicators to each participant. Pharmacists removed tear-off labels from the boxes of product and placed them on pharmacy dispensing records that were contained in concealed envelopes and pre-printed with the randomization codes. Participants, study staff, pharmacists, clinicians and statisticians were blinded to study assignments.
Study Participants
The study population consisted of healthy, non-pregnant, sexually active, HIV-negative women of the ages 18 through 24 years inclusive with a normal genital tract (anatomically normal pelvic exam without evidence of genital infection or deep disruption of the genital epithelium). Participants were required to have regular menstrual cycles with >/= 21 days between menses, to be using adequate contraception (hormonal method, intrauterine device, sterilization, or sexual activity with a vasectomized partner), and to have had a normal pap smear within one year prior to enrollment.
Study Products
The VivaGel formulation used in the study comprised SPL7013 formulated in a water-based gel (methyparaben (0.18%), propylparaben (0.02%), EDTA (0.1%), Carbopol 971P (5.0%), propylene glycol (1.0%), glycerin (1.0%)), with purified water and sodium hydroxide added to adjust the gel pH to approximately 5.0. The VivaGel placebo for this study is the base formulation without SPL7013. The HEC gel contains hydroxyethylcellulose as the gel thickener (2.7%), sodium chloride (0.85%), sorbic acid (0.1%), with purified water and sodium hydroxide added (18). The gel is isotonic and formulated at a pH of 4.4 to avoid disrupting the normal vaginal pH and has minimal buffering capacity in order to avoid the inactivation of sexually transmitted pathogens. For all gels, the dose volume of 3.5 g (equivalent to 3.5 mL) has been selected as the dose that is intended to provide optimum vaginal and cervical coverage while minimizing leakage of product from the vagina [24]. Participants were asked to insert the product in the morning and in the evening, or approximately every 12 hours for fourteen days. All study products had a similar appearance and were provided in identical HTI polypropylene pre-filled applicators (HTI Plastics, Lincoln, NE) (Fig. 1).
Fig. 1.
The HTI vaginal applicator used to deliver the microbicide gel in MTN-004
Study procedures
A total of five scheduled clinic visits were performed. After obtaining informed consent all participants underwent a thorough medical history, a targeted physical examination, and a complete pelvic examination including vaginal swabs for pH, wet prep and Gram stain. A pap smear was obtained when necessary.
Participants who met the inclusion and exclusion criteria during screening proceeded to an enrollment visit. Enrollment was scheduled within 1–2 days of the end of menses to avoid menstrual bleeding during product use and during evaluation of the cervix and vagina. At the enrollment visit participants were randomized, safety labs were repeated and a blood sample was collected for measurement of SPL7013. Pelvic samples were collected for vaginal pH, Gram stain, wet prep, quantitative vaginal cultures and immunological assays (cytokines, chemokines, and innate factors). Colposcopic assessment was performed and photographed. Participants received the first vaginal application of study product in clinic and were provided with 20 applicators for use at home. One and two weeks later participants returned to the clinic for repeat assessment including clinical assessment of AEs, pregnancy testing, safety labs, and gynecologic exam with vaginal pH, wet prep, vaginal microflora, and immunological assays. Used applicators were collected and counted at each visit. Ten additional applicators of study product was distributed at the Week One visit. A colposcopy was performed at the Week Two visit. A final visit was conducted one week after completion of product administration with clinical assessment, pregnancy testing, and collection of safety labs, and vaginal samples. All study visits were timed to avoid menses and included the option to conduct additional laboratory assessment and examination as clinically indicated. Interim contact and unscheduled visits were held as necessary for reported AEs or by request of the participant or investigator.
Clinical Safety and Laboratory Assessment
Emergent AEs were graded using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0, December 2004 as well as Addendum 1 (Female Genital Grading Table for Use in Microbicide Studies (http://rsc.tech-res.com/safetyandpharmacovigilance/). Asymptomatic bacterial vaginosis/candidiasis and colposcopic findings were not reportable AEs. In cases where an AE was covered in both tables, the Female Genital Grading Table for Use in Microbicide Studies was the grading scale utilized. The extent of SPL7013 absorption was determined via assessment of SPL7013 levels in serum samples taken on Day 0 and Day 14, using a validated capillary electrophoresis bioanalytical method conducted at Starpharma Pty Ltd.
Product Acceptability
Overall product like (or dislike) and likelihood of gel use in the future were assessed using an internet based computer assisted self interview.
Analysis of study outcomes
For analyses comparing VivaGel to the VivaGel placebo, data from 43 women were included (4 and 3 participants, respectively, enrolled under Version 2.0 of the protocol, and 18 participants in each of the 2 arms enrolled under Version 3.0 of the protocol) whereas for analyses comparing VivaGel to the HEC gel, data from 36 women were included (18 participants per arm enrolled under Version 3.0).
The primary endpoint of abnormal genital symptoms was defined as those judged by the study investigator to be possibly, probably, or definitely related to product use. Genital symptoms included were as follows: Genital tract pain (pelvic pain, vaginal & vulvar pain); tenderness; dyspareunia; dysmenorrhoea; vulva (itching, edema, erythema, lesions, abrasions, rash); vagina (itching, edema, erythema, dryness, discharge, abrasions, lesions, masses; ruptured cyst); cervix (edema, erythema, discharge, lesions); perianal (erythema, laceration, irritation); urinary tract (urinary frequency, dysuria, hematuria, infection); vulvovaginitis; cervicitis; pelvic inflammatory disease; genital herpes; candida; bacterial vaginosis; menorrhagia; metrorrhagia; unexplained infrequent bleeding; post-coital bleeding. Comparisons were made across the arms of the rates of at least one related abnormal genital symptom using the global Chi-square test for independence. Additionally, to assess the incidence of related abnormal genital symptoms, the number of symptoms was summed for each woman during her follow-up. Due to the short follow-up time each type of symptom was only counted once per woman. A global test for homogeneity of the incidence rates across the arms was conducted based on the Poisson distribution. Similarly, pairwise comparisons of the rates were also made between arms.
The secondary endpoint of product adherence was calculated in two ways, including and excluding the time participants spent on product holds. Product acceptability was defined as the proportion of participants who at their two-week follow-up visit reported via the acceptability questionnaire that they would very likely use the candidate microbicide during sexual intercourse in the future.
Vaginal flora was assessed from Nugent scores defined as follows: Normal, 0 to 3; Intermediate, 4 to 6; Bacterial Vaginosis, 7 to 10. Quantitative measures of >= 1 log change in the dominant members of the microflora included the following:
Lactobacillus (H2O2 positive and negative strains)
anaerobic gram negative rods
Gardnerella vaginalis
Escherichia coli
Staphylococcus aureus
Candida species
Group B Streptococcus, and Enterococcus species
Odds ratios comparing VivaGel placebo or HEC gel versus VivaGel (reference group) for a >= 1 log change in microflora levels were estimated using Generalized Estimating Equations. A logit link function with the exchangeable correlation structure and model-based standard errors were used. All analyses were performed using SAS Version 9.1.3 (SAS Institute, Cary, North Carolina, USA).
Results
Enrollment, retention, and participant disposition
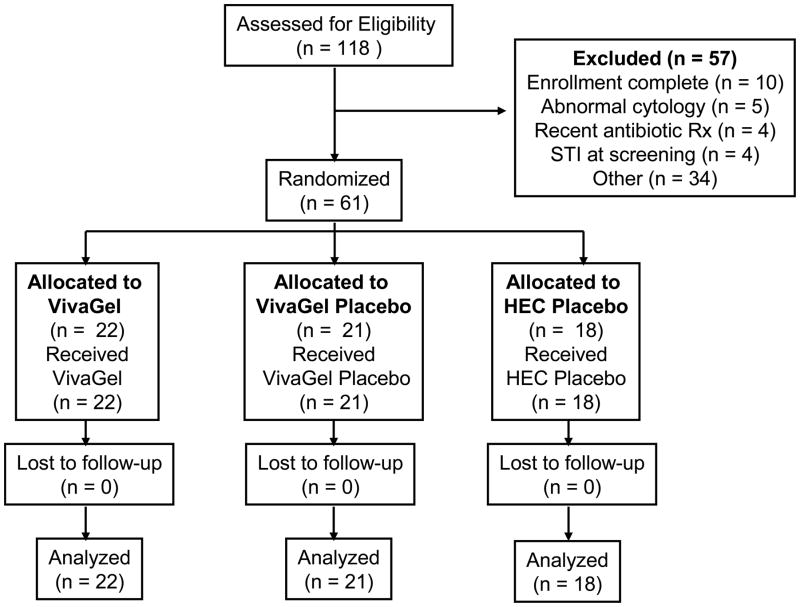
Version 2.0 of the protocol planned to randomize approximately 40 women to either VivaGel or VivaGel placebo. With Version 3.0 of the protocol, a third HEC gel arm was added and the sample size was increased to 61 women who were subsequently randomized to VivaGel (n = 22), VivaGel placebo (n = 21) or the HEC gel (n = 18) (Fig. 2). The average age of participants was 21. All the participants in Puerto Rico (n = 20), 10% of the Tampa participants (n = 3), and 8% of the Pittsburgh participants (n=1) defined themselves as being of Hispanic origin. The majority of the participants defined themselves as white (66%) (Table 1). Each site obtained 100% retention for all study visits.
Fig. 2.
Flow diagram of participant progress through the MTN-004 study
Table 1.
Baseline demographics of each treatment group
| VivaGel | VivaGel Placebo | HEC Gel | All Arms | |
|---|---|---|---|---|
| Participants Enrolled | 22 | 21 | 18 | 61 |
| Age (years) | ||||
| N | 22 | 21 | 18 | 61 |
| Mean (STD) | 20.8 (1.5) | 21.0 (1.6) | 20.8 (1.9) | 20.9 (1.7) |
| Race | ||||
| White | 15 (68%) | 11 (52%) | 14 (78%) | 40 (66%) |
| Mixed | 3 (14%) | 4 (19%) | 2 (11%) | 9 (15%) |
| Black or African | 2 (9%) | 2 (10%) | 1 (6%) | 5 (8%) |
| American | ||||
| Trigueña* | 2 (9%) | 2 (10%) | 1 (6%) | 5 (8%) |
| Asian | 0 | 2 (10%) | 0 | 2 (3%) |
Someone of indigenous, African, and Spanish heritage
Adherence and product acceptability
A total of six participants were placed on product hold during the study; five were in the VivaGel arm (due to: STI (genital herpes, n=1), AEs (UTI, n=2; post coital bleeding, n=1; hymenal ring laceration due to trauma from applicator, n=1)) and one in the VivaGel placebo arm (due to an AE (UTI)). No product holds occurred in the HEC placebo arm. Product adherence was defined as the proportion of women who reported the application of at least 80% of the expected number of study doses over the two weeks of product use. Excluding time on product hold, 95% of the VivaGel recipients were adherent compared to 100% and 94% of the VivaGel placebo and HEC gel arms respectively. In contrast, when time on product hold was included, 77% of the VivaGel recipients were adherent compared to 95% and 94% of the VivaGel placebo and HEC gel arms respectively (Table 2).
Table 2.
Study retention and product adherence by treatment group
| VivaGel | VivaGel Placebo | HEC Gel | All Arms | |
|---|---|---|---|---|
| Participants enrolled | 22 | 21 | 18 | 61 |
| Study retention for all visits | 22 (100%) | 21 (100%) | 18 (100%) | 61 (100%) |
| Percentage of gel use* | ||||
| Excluding time on product hold | ||||
| 0.0% | 0 | 0 | 0 | 0 |
| 0.1% – 49.9% | 0 | 0 | 0 | 0 |
| 50% – 79.9% | 1 (5%) | 0 | 1 (6%) | 2 (3%) |
| ≥ 80.0% | 21 (95%) | 21 (100%) | 17 (94%) | 59 (97%) |
| Including time on product hold | ||||
| 0.0% | 0 | 0 | 0 | 0 |
| 0.1% – 49.9% | 1 (5%) | 0 | 0 | 1 (2%) |
| 50% – 79.9% | 4 (18%) | 1 (5%) | 1 (6%) | 6 (10%) |
| ≥ 80.0% | 17 (77%) | 20 (95%) | 17 (94%) | 54 (89%) |
Up to Week 2 visit
Concerning product acceptability, of the participants assigned to the VivaGel arm, less than half indicated liking it and 41% said they were undecided. In terms of future use, 59% of participants who used VivaGel indicated they were likely to use it in the future, 23% said they were unlikely to use it and 18% were undecided. Overall, the most commonly reported positive aspects of all three gels were ease of use (49%) and possible protection against HIV (38%). The most commonly reported negative aspects of all three gels were messiness (61%) and “other” (43%). Most of the “other” category appeared to be related to gel leakage. Reported differences in acceptability did not appear to be related to any difference in rates of adverse events indicative of genital irritation or inflammation.
Adverse events
There were no Grade 3, 4, or serious AEs during the study. Other adverse events were generally mild (grade 1) and well tolerated. The prevalence of abnormal pelvic exam findings was similar across all arms of the study (Table 3). The percentage of women who were found to have one or more adverse events considered to be possibly, probably or definitely related to product use during the pelvic examination conducted by the investigators was 18.2% in VivaGel, 28.6% in VivaGel placebo, and 22.2% in HEC placebo gel groups. Genital AEs (one or more potentially attributed to product use) were reported as follows: VivaGel (n=17/22; 77.3%), VivaGel placebo (n=14/21; 66.7%) and HEC gel (n=8/18; 44.4%) (NS, p=0.1) (Table 4). Using pair-wise comparison, women in the VivaGel arm had a significantly higher incidence of related genital AEs than women in the HEC gel (0.297 versus 0.111 per 100 person years respectively; p=0.003). Conversely, there were no significant differences observed in incident related genital AEs in the VivaGel arm compared to the VivaGel placebo arm (0.284 vs. 0.196 per 100 person-years, respectively; p=0.2) nor between the VivaGel placebo arm and the HEC gel (0.198 versus 0.111 per 100 person years respectively; p=0.11). The most common genital AEs in the VivaGel arm included dyspareunia (5/22; 22.7%), metrorrhagia (4/22; 18.2%), and vulvovaginal burning (5/22; 22.7%) or pruritis (3/22; 13.6%). The most common genital AEs in the VivaGel placebo arm were metrorrhagia, vaginal discharge, vaginal erythema and vulvar erythema (each in 2/21; 9.5%) and pelvic pain (3/21; 14.3%), and in the HEC gel arm, metrorrhagia (2/18; 11.1%), pelvic pain (3/18; 16.7%), and pruritus (3/18; 16.7%) were most common. There were no grade 3 or higher laboratory values for hematology, liver function, creatinine level and coagulation.
Table 3.
Pelvic exam abnormalities occurring in > 5% of participants by treatment arm
| VivaGel | VivaGel Placebo | HEC Gel | All Arms | |||||
|---|---|---|---|---|---|---|---|---|
| Wk 1 | Wk 2 | Wk 1 | Wk 2 | Wk 1 | Wk 2 | Wk 1 | Wk 2 | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Participants enrolled | 22 | 21 | 18 | 61 | ||||
| Pelvic exam* | ||||||||
| Not done | 0 | 0 | 0 | 1 (5) | 0 | 0 | 0 | 1 (2) |
| No abnormal findings | 14 (64) | 16 (73) | 17 (81) | 11 (52) | 14 (78) | 13 (72) | 45 (74) | 40 (66) |
| Abnormal findings: | 8 (36) | 6 (27) | 4 (19) | 9 (43) | 4 (22) | 5 (28) | 16 (26) | 20 (33) |
| Blood from cervical os | 2 (9) | 3 (14) | 1 (5) | 3 (14) | 0 | 1 (6) | 3 (5) | 7 (11) |
| Erythema | 2 (9) | 2 (9) | 1 (5) | 3 (14) | 0 | 0 | 3 (5) | 5 (8) |
| Abnormal vaginal discharge | 1 (5) | 1 (5) | 3 (14) | 2 (10) | 1 (6) | 1 (6) | 5 (8) | 4 (7) |
| Blood tinged discharge | 3 (14) | 1 (5) | 1 (5) | 0 | 1 (6) | 0 | 5 (8) | 1 (2) |
| Abrasion | 0 | 1 (5) | 0 | 0 | 1 (6) | 1 (6) | 1 (2) | 2 (3) |
| Peeling | 0 | 0 | 0 | 1 (5) | 0 | 1 (6) | 0 | 2 (3) |
| Bleeding from epithelial disruption | 0 | 0 | 0 | 0 | 0 | 1 (6) | 0 | 1(2) |
| Petechiae | 0 | 0 | 0 | 0 | 0 | 1 (6) | 0 | 1 (2) |
| Grossly white finding | 0 | 0 | 0 | 1 (5) | 1 (6) | 0 | 1 (2) | 1(2) |
| Blood in vagina with no identified source | 1 (5) | 0 | 0 | 0 | 1 (6) | 0 | 2 (3) | 0 |
Naked eye, speculum, and bimanual exam assessment
Table 4.
Product related* genital adverse events occurring in > 5% of participants by treatment arm following study enrollment
| VivaGel | VivaGel Placebo | HEC Gel | All Arms | |
|---|---|---|---|---|
| Participants enrolled | 22 | 21 | 18 | 61 |
| Participants with ≥ one adverse event | 17 (77.3%) | 14 (66.7%) | 8 (44.4%) | 39 (63.9%) |
| Infections | 2 (9.1%) | 2 (9.5%) | 0 | 4 (6.6%) |
| Urinary tract infection | 1 (4.5%) | 1 (4.8%) | 0 | 2 (3.3%) |
| Vulvovaginal candidiasis | 0 | 1 (4.8%) | 0 | 1 (1.6%) |
| Vulvovaginitis | 1 (4.5%) | 0 | 0 | 1 (1.6%) |
| Renal and urinary disorders | 3 (13.6%) | 1 (4.8%) | 1 (5.6%) | 5 (8.2%) |
| Dysuria | 2 (9.1%) | 0 | 0 | 2 (3.3%) |
| Hematuria | 1 (4.5%) | 0 | 0 | 1 (1.6%) |
| Urinary incontinence | 0 | 0 | 1 (5.6%) | 1 (1.6%) |
| Reproductive system | 17 (77.3%) | 13 (61.9%) | 7 (38.9%) | 37 (60.7%) |
| Cervical friability | 0 | 0 | 1 (5.6%) | 1 (1.6%) |
| Cervical erythema | 2 (9.1%) | 1 (4.8%) | 0 | 3 (4.9%) |
| Dyspareunia | 5 (22.7%) | 1 (4.8%) | 1 (5.6%) | 7 (11.5%) |
| Metrorrhagia | 4 (18.2%) | 2 (9.5%) | 2 (11.1%) | 8 (13.1%) |
| Pelvic pain | 3 (13.6%) | 3 (14.3%) | 3 (16.7%) | 9 (14.8%) |
| Cervical erosion | 0 | 1 (4.8%) | 1 (5.6%) | 2 (3.3%) |
| Vaginal discharge | 0 | 2 (9.5%) | 0 | 2 (3.3%) |
| Vaginal erythema | 0 | 2 (9.5%) | 0 | 2 (3.3%) |
| Vulvar erythema | 1 (4.5%) | 2 (9.5%) | 0 | 3 (4.9%) |
| Vulvovaginal burning sensation | 5 (22.7%) | 1 (4.8%) | 0 | 6 (9.8%) |
| Vulvovaginal pruritus | 3 (13.6%) | 2 (9.5%) | 3 (16.7%) | 8 (13.1%) |
Adverse experiences considered to be possibly, probably, or definitely related to product use
Pharmacokinetics
There were no detectable levels of SPL7013 in any of the serum samples analysed.
Vaginal microflora
The prevalence of (i.e. number of women with) Enterococcus spp. increased significantly after 1–2 weeks among women using VivaGel (OR 2.0, CI 1.1–3.5, P=0.01) compared to baseline and final visit off product, whereas women using either placebo had no change. The prevalence of the following organisms decreased significantly (P<0.002) among women using VivaGel: Gardnerella vaginalis and pigmented anaerobic gram negative rods (AGNR). With VivaGel Placebo, there was a significant (P<0.03) decrease in prevalence of H2O2 negative Lactobacillus, G. vaginalis, and non-pigmented and pigmented AGNR. Neither VivaGel nor VivaGel Placebo had any effect on the prevalence of H2O2 positive Lactobacillus. Women assigned to HEC Gel had a significant decrease in the prevalence of Group B Streptococcus (GBS) (P=0.001).
Women using VivaGel also had an increase of >1 log in the concentration (CFU/mL) of Enterococcus spp. (1.1 log increase, P=0.002), GBS (1.2 log, P=0.03), and coliforms (1.2 log increase, P=0.005), whereas women using HEC or VivaGel placebo had no change in these organisms. Both VivaGel and VivaGel Placebo caused a >1 log decrease in the concentration of G. vaginalis (1.2 and 1.4 log decrease, respectively, P=0.001).
Overall, women in the VivaGel arm had increased prevalence of Enterococcus spp. only, and the magnitude of changes in concentration of other vaginal microflora was not substantially greater than 1 log. Furthermore, there was no impact on the incidence of BV.
Discussion
This study characterizes the safety and acceptability of VivaGel in young, sexually active women who represent the most at risk population in Sub Saharan Africa [9]. There were no serious AEs and no participants withdrew due to AEs. There was no statistically significant difference in the proportion of women who had one or more genital AE considered to be possibly or probably or definitely related to study product use. In addition, the percentage of women who were found to have one or more AEs considered to be possibly, probably or definitely related to product use during pelvic examination conducted by the investigators was low and equivalent across all arms of the study. However, women in the VivaGel arm had a significantly higher incidence of related genitourinary AEs than women in the HEC gel arm. Genital AE rates were slightly higher in the VivaGel arm compared to women in the VivaGel placebo arm but this difference was not significant.
Exposure to VivaGel resulted in statistically significant shifts in some vaginal microflora. However, there is likely to be little clinical significance of shifts of the magnitude experienced in the current study in organisms such as Enterococcus sp. or coliforms. Importantly, there was little impact on Lactobacillus spp. concentrations or prevalence in women in this study, and no increased incidence of bacterial vaginosis, which is the main clinical outcome of imbalances in vaginal flora.
Despite promising results from CAPRISA 004 [3], there remains a critical need to develop microbicides that provide broad antimicrobial coverage and/or contraceptive efficacy. In this regard, VivaGel has in vitro and preclinical activity against HIV, HSV-2, and HPV [5, 10–13], and has demonstrated contraceptive activity in vivo. In addition, a non-antiretroviral microbicide such as VivaGel might be valuable for women with HIV infection who would like to use microbicides to reduce the risk of HIV transmission to their partners. Unlike antiretroviral microbicides, VivaGel might be able to be provided over the counter rather than linked to voluntary testing and counseling for HIV status.
VivaGel and the VivaGel placebo were generally well tolerated in this study although there was a significant difference in the incidence of Grade 1/2 genital AEs in the VivaGel arm compared with the HEC gel arm. Although the sample size of phase 1 studies is necessarily small, this observation, combined with the shifts in vaginal microflora, and the sense that the participants found the VivaGel product less acceptable than the HEC gel raises the question of whether or not 3% VivaGel used in this study is the optimal formulation of the SPL7013 dendrimer. . There was no significant difference in the incidence of genital AEs between the VivaGel and VivaGel placebo arms of the study suggesting that the excipients in the VivaGel formulation rather than the SPL7013 might be responsible for the higher frequency of genital AEs compared to the HEC gel.
In addition, there was a significant decrease in prevalence of H2O2 negative Lactobacillus, G. vaginalis, non-pigmented and pigmented AGNR with VivaGel placebo, while women assigned to HEC gel had a significant decrease in GBS, suggesting that even the most inert formulations will have some effect on vaginal microflora.
Although the majority of genital AEs in this study were mild or moderate and transient, they might be sufficient to limit the acceptability of VivaGel to healthy sexually active women. Most participants reported issues such as messiness and gel leakage, AEs that might be more related to the intense level of usage in the study rather than specific study products.
Our findings are in keeping with those of Cohen et al. who also demonstrated mild vaginal epithelial irritation and inflammation in their two week study of VivaGel in sexually abstinent US and Kenyan young women [8]. The findings from both of these studies suggest that a two week Phase 1 evaluation of microbicide candidates can potentially identify products that might require reformulation before proceeding to more advanced stages of product development. Indeed, Starpharma Pty Ltd. are now conducting a Phase 1 dose ranging study of a new formulation of VivaGel for the treatment of bacterial vaginosis (www.clinicaltrials.gov: NCT01201057). This is an important observation as others have suggested the need for larger more prolonged Phase 1 studies to evaluate candidate microbicides [14].
Given the urgent need to develop safe and effective microbicides for different populations and with a range of indications, and the inherent uncertainty of drug development, it is critical that the field continues to move new microbicide candidates into and through clinical development.
Acknowledgments
Preliminary findings from this study were presented at Microbicides 2010, Pittsburgh, PA in May 2010. We would like to thank Drs. Roberta Black Jeanna Piper, and Bill Kapogiannis from the NIH for the oversight of this study. The study was conducted by the Microbicide Trials Network with support from the National Institute of Allergy and Infectious Diseases, Division of AIDS (5U01AI068633), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01 HD040533, U01 HD040474), National Institute of Mental Health, Adolescent Medicine Trials Network (ATN) for HIV/AIDS Interventions, and Starpharma Pty Ltd. We would also like to thank the MTN-004 participants for volunteering their time for this study.
The MTN-004 study was registered at www.ClinicalTrials.gov (NCT00442910) and the protocol can be found at http://www.mtnstopshiv.org.
Footnotes
Support from:
National Institute of Allergy and Infectious Diseases, Division of AIDS (5U01AI068633), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01 HD040533, U01 HD040474), National Institute of Mental Health, Adolescent Medicine Trials Network (ATN) for HIV/AIDS Interventions, and Starpharma Pty Ltd.
Authorship contributions
Ian McGowan was the protocol chair, oversaw the implementation of the study, and wrote the manuscript.
Kailazarid Gomez was the operations manager for the study.
Karen Bruder was a Tampa co-investigator and was responsible for implementation of the study at her site.
Irma Febo was the San Juan principal investigator and was responsible for implementation of the study at her site.
Beatrice Chen was the Pittsburgh principal investigator and was responsible for implementation of the study at her site.
Barbra Richardson was the protocol statistician, developed the analysis plan for the study, oversaw all data analyses, and helped interpret analysis results.
Marla Husnik performed data management and data analyses and helped interpret the analysis results.
Edward Livant was the laboratory manager for the study and provided oversight for the site and MTN Core laboratory support of the study.
Clare Price was the clinical manager for Starpharma Pty Ltd, a member of the protocol team, and assisted with provision of VivaGel for the study.
Cindy Jacobson was the study pharmacist for MTN-004.
References
- 1.Pettifor AE, Rees HV, Kleinschmidt I, Steffenson AE, MacPhail C, Hlongwa-Madikizela L, et al. Young people’s sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. AIDS. 2005;19(14):1525–1534. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 2.McGowan I. Microbicides for HIV prevention: reality or hope? Curr Opin Infect Dis. 2010;23(1):26–31. doi: 10.1097/QCO.0b013e328334fe70. [DOI] [PubMed] [Google Scholar]
- 3.Abdool KQ, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCarthy TD, Karellas P, Henderson SA, Giannis M, O’Keefe DF, Heery G, et al. Dendrimers as drugs: discovery and preclinical and clinical development of dendrimer-based microbicides for HIV and STI prevention. Mol Pharm. 2005;2(4):312–318. doi: 10.1021/mp050023q. [DOI] [PubMed] [Google Scholar]
- 5.Tyssen D, Henderson SA, Johnson A, Sterjovski J, Moore K, La J, et al. Structure activity relationship of dendrimer microbicides with dual action antiviral activity. PLoS ONE. 2010;5(8) doi: 10.1371/journal.pone.0012309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Loughlin J, Millwood IY, McDonald HM, Price CF, Kaldor JM, Paull JR. Safety, tolerability, and pharmacokinetics of SPL7013 gel (VivaGel): a dose ranging, phase I study. Sex Transm Dis. 2010;37(2):100–104. doi: 10.1097/OLQ.0b013e3181bc0aac. [DOI] [PubMed] [Google Scholar]
- 7.Chen MY, Millwood IY, Wand H, Poynten M, Law M, Kaldor JM, et al. A randomized controlled trial of the safety of candidate microbicide SPL7013 gel when applied to the penis. J Acquir Immune Defic Syndr. 2009;50(4):375–380. doi: 10.1097/QAI.0b013e318198a7e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen CR, Brown J, Moscicki AB, Bukusi EA, Paull JR, Price CF, et al. A Phase I Randomized Placebo Controlled Trial of the Safety of 3% SPL7013 Gel (VivaGel(R)) in Healthy Young Women Administered Twice Daily for 14 Days. PLoS ONE. 2011;6(1):e16258. doi: 10.1371/journal.pone.0016258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rudy BJ, Kapogiannis BG, Lally MA, Gray GE, Bekker LG, Krogstad P, et al. Youth-specific considerations in the development of preexposure prophylaxis, microbicide, and vaccine research trials. J Acquir Immune Defic Syndr. 2010;54 (Suppl 1):S31–S42. doi: 10.1097/QAI.0b013e3181e3a922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang YH, Emau P, Cairns JS, Flanary L, Morton WR, McCarthy TD, et al. SPL7013 gel as a topical microbicide for prevention of vaginal transmission of SHIV89.6P in macaques. AIDS Res Hum Retroviruses. 2005;21(3):207–213. doi: 10.1089/aid.2005.21.207. [DOI] [PubMed] [Google Scholar]
- 11.Bernstein DI, Stanberry LR, Sacks S, Ayisi NK, Gong YH, Ireland J, et al. Evaluations of unformulated and formulated dendrimer-based microbicide candidates in mouse and guinea pig models of genital herpes. Antimicrob Agents Chemother. 2003;47(12):3784–3788. doi: 10.1128/AAC.47.12.3784-3788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourne N, Stanberry LR, Kern ER, Holan G, Matthews B, Bernstein DI. Dendrimers, a new class of candidate topical microbicides with activity against herpes simplex virus infection. Antimicrob Agents Chemother. 2000;44(9):2471–2474. doi: 10.1128/aac.44.9.2471-2474.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patton DL, Cosgrove Sweeney YT, McCarthy TD, Hillier SL. Preclinical safety and efficacy assessments of dendrimer-based (SPL7013) microbicide gel formulations in a nonhuman primate model. Antimicrob Agents Chemother. 2006;50(5):1696–1700. doi: 10.1128/AAC.50.5.1696-1700.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poynten IM, Millwood IY, Falster MO, Law MG, Andresen DN, Van DL, et al. The safety of candidate vaginal microbicides since nonoxynol-9: a systematic review of published studies. AIDS. 2009;23(10):1245–1254. doi: 10.1097/QAD.0b013e32832b4271. [DOI] [PubMed] [Google Scholar]