Abstract
The results of the CAPRISA 004 and iPrEx HIV prevention studies have demonstrated that topical or systemic use of antiretroviral agents can significantly reduce the risk of HIV acquisition associated with unprotected vaginal or anal sexual intercourse. However, the effect size in these studies was relatively modest and product adherence was generally poor. These observations suggest the need for new approaches to HIV prevention, especially for high risk MSM. Rates of lubricant use are high in MSM practicing receptive anal sex. Consequently, the development of an antiretroviral rectal microbicide gel may provide a safe and effective means of preventing HIV infection with an intervention that is likely to have high acceptability among the target population. The purpose of this article is to describe the challenges and progress in the development of rectal microbicides for HIV prevention.
Keywords: HIV, Microbicides, Rectal, HIV prevention
Introduction
Microbicides are products that are designed to be applied to the vaginal or rectal mucosa with the intent of preventing or at least significantly reducing the acquisition of sexually transmitted infections (STIs) including HIV [1]. The original impetus for vaginal microbicide development was to provide women with options for HIV prevention in settings where their partners were unwilling to use condoms [2]. Years later, the need to also develop a rectal microbicide (RM) became clear, given that a significant proportion of men do not consistently use condoms during anal sex with men or women [3, 4], and that there has consequently been little or no decline in the rates of new HIV infections, particularly in men who have sex with men (MSM) [5]. Unprotected receptive anal intercourse (RAI) is the sexual behavior with the highest per act risk of HIV acquisition, conferring perhaps 10–20 times more risk than unprotected vaginal intercourse [6, 7]. Furthermore, there is increasing epidemiological evidence that women as well as men in both the developed [4, 8, 9] and developing world [10–12] practice RAI, and that a number of men in Sub-Saharan Africa practice RAI while also having sexual relationships with women [13, 14]. Clearly, RMs should be seen as an important HIV prevention technology for all individuals who practice RAI, and not just for MSM.
The development of a safe and effective RM is still in its early stages; however, there are many reasons to believe RMs can be a valuable part of an HIV prevention portfolio. One advantage of RMs is that their use would require only minimal behavioral modification, since sexual lubricant use is already a common component of RAI [15]. Indeed, formative studies have suggested that MSM are willing to participate in RM clinical trials [16, 17] as well as use these products should they become available [18]. Another positive aspect of RMs is that they would offer an alternative to condoms, which some see as a barrier to intimacy, pleasure, and satisfaction [19]. Finally, RMs have the benefit of giving receptive partners the capacity to protect themselves without depending on a partner’s condom use.
Vaginal Microbicide Development
Vaginal microbicide development began approximately 20 years ago as an outgrowth of the topical contraceptive field [2]. The intent was to develop a spermicidal gel that had activity against STIs including HIV. The first vaginal microbicides candidates, such as nonoxynol-9 (N-9) and cellulose sulfate, had broad spectrum in vitro activity against bacterial and viral STIs and some also had contraceptive efficacy. Unfortunately, when these products were evaluated in HIV effectiveness studies, they did not reduce HIV acquisition, and in one trial actually seemed to increase the risk of HIV infection [20].
The microbicide research community has subsequently focused on antiretroviral microbicides such as tenofovir gel. In July 2010, the Centre for the AIDS Programme of Research in South Africa (CAPRISA) 004 study team reported the first proof of concept for tenofovir vaginal gel, which was associated with a significant reduction in HIV acquisition in South African women [21]. In this randomized double-blind, placebo-controlled trial, women using tenofovir gel were overall 39% less likely to become HIV infected and 51% less likely to acquire herpes simplex virus (HSV-2) compared to the placebo group. Product effectiveness appeared to be linked to adherence since women who used the gel 80% or more of the time had a 54% reduction in HIV incidence, while women who used it less than 50% of the time had a 28% rate of protection. Furthermore, the effectiveness of tenofovir gel went from 50% after 12 months down to 39% after 30 months of use, but the difference in effectiveness was associated with lower adherence in the second year. These positive findings raise important questions about adherence and effectiveness, but have also renewed enthusiasm for microbicide development including both vaginal and RMs.
Rectal Microbicide Development
N-9 was the first vaginal microbicide to be evaluated for rectal safety [22]. This study, conducted in HIV positive and negative seroconcordant sexually active male couples, suggested that rectal N-9 was generally well tolerated although there was evidence of minor mucosal damage. The possibility that use of a microbicide, whether vaginal or rectal, might result in mucosal damage continues to be an area of concern within the microbicide field. It is conceivable that these mucosal changes may be asymptomatic but may still increase the risk of HIV infection. Moreover, these mucosal changes might be quite subtle. As one example, increased recruitment and activation of HIV target cells within the genital or rectal mucosa would not be identified through direct observation or microscopic assessment of tissue samples. Consequently, the initial focus of RM development has been to conduct rectal safety assessments of vaginal microbicides using increasingly sophisticated laboratory techniques [23, 24]. These have included collecting intestinal tissue samples from participants exposed to candidate microbicides and determining whether there is evidence of mucosal inflammation using a range of molecular biological and immunological techniques [24].
More recently, there have been attempts to develop microbicides whose properties are better suited for use in the rectal compartment. The majority of sexual lubricants and vaginal microbicides are extremely hyperosmolar, meaning they are potentially more concentrated than body fluids. This property can result in reduced product acceptability and potentially mucosal damage [25]. Rectal specific microbicides will need to be isoosmolar to minimize these problems. Clearly, the rectal compartment is very different from the vaginal compartment. One obvious difference is that the surface area required to be protected using an RM might be much larger than for a vaginal microbicide. Imaging studies of vaginal microbicide distribution have been conducted using radiological techniques such as MRI [26, 27] and similar studies are being conducted in rectal compartments [28]. RMs with varying rheological or flow characteristics are being evaluated in these systems to identify formulations that provide adequate coverage of the intestinal mucosa that might be exposed to infected semen. In addition, rectal specific microbicide applicators are being designed to optimize delivery of the microbicide to the rectal compartment.
Phases of Microbicide Development
Drug development including microbicide development involves a number of different stages. In the preclinical phase, new molecules are evaluated for safety and efficacy in cell lines and animal models. Compounds with an adequate safety profile are then advanced into Phase 1 safety studies where small groups of participants are exposed to the product in very controlled circumstances for relatively short periods of time. The participants in Phase 1 RM studies are usually at very low risk of HIV acquisition and are asked to be sexually abstinent. On completion of these studies, candidate microbicides are then evaluated in Phase 2 studies. Characteristically, Phase 2 microbicide studies are conducted in sexually active populations for three to six months and are designed to identify safety or acceptability issues associated with frequent use of the product. On completion of Phase 2, a candidate microbicide then advances into an effectiveness (Phase 2B/3) study. This is the final phase of testing and seeks to determine whether the product can actually reduce HIV acquisition rates in at risk populations.
Phase 2B/3 evaluation of microbicides is the most arduous phase of assessment. Of necessity, the microbicide intervention has to be evaluated in populations who are already receiving a comprehensive HIV prevention package. The components of this package continue to evolve but would be expected to include diagnosis and treatment of STIs, frequent safer sex counseling, condom provision, and possibly male circumcision [29]. The net effect of these interventions is that the participants enrolled in Phase 2B/3 studies often develop a lower risk of infection than their peers not participating in the study, potentially reducing the overall HIV incidence in the study population and therefore the power to find a statistically significant result. As a consequence, Phase 2B/3 studies are usually large, long, and expensive.
Acceptability of Rectal Microbicides
Rectal microbicides will only play an important role in HIV prevention if the target populations find them acceptable and use them correctly and consistently [30–32]. Although there has been some discussion concerning whether acceptability studies should be postponed until efficacy of a product is demonstrated, others [32–34] have convincingly defended the wisdom of integrating acceptability research in early clinical phases of microbicide development. Morrow and Ruiz [33] state that Phase 1 trial participants “are an invaluable source of information regarding acceptability [for] they constitute the handful of individuals with actual product use experience and, thus, are in the best position to provide feedback on actual product characteristics and how these factors may influence individuals’ willingness to initiate and maintain product use over time”. They suggest that these trials assess a variety of factors, including product scent, color, and texture; clarity of instructions and ease of product preparation and application; qualities of product during and after use; frequency and timing of use; and related covariates, such as history of lubricant use, frequency of anal and vaginal sex, and relationship communication. Rosen et al. [35] and Morrow and Ruiz [33] propose the use of mixed methods (quantitative and qualitative) to assess the different factors. This advice is particularly sound in the case of small trials for which the utility of quantitative findings alone often has been limited [36–38].
Three recent papers have made important contributions to our knowledge of the acceptability of RMs. Importantly, the observations were based on interviews with participants who had actually used experimental rectal products rather than a theoretical discussion of product acceptability. An NICHD funded trial found that a sexually active cohort of middle aged MSM rated volumes up to 35 ml of gel acceptable for use during anal intercourse [39]. In a second study, MSM appeared to prefer microbicide gels rather than rectal suppositories [40]. Acceptability data from a Phase 1 safety study of UC781 gel, an antiretroviral microbicide gel, found the product to be highly acceptable and the majority of participants said that they would use such a product if it was commercially available [41].
Ongoing and Future Rectal Microbicide Trials
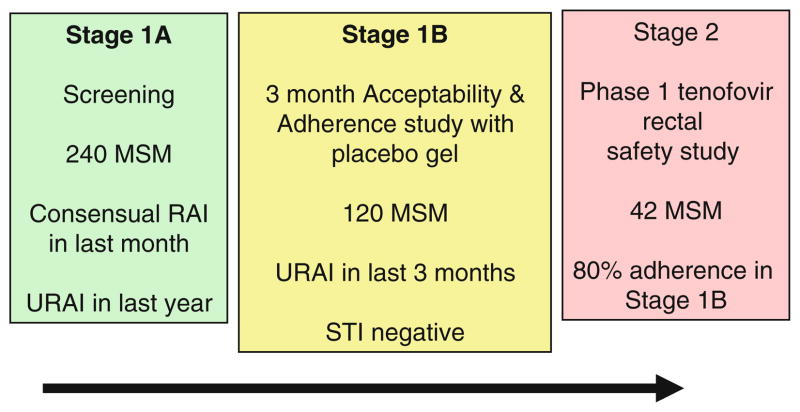
Information is currently lacking concerning microbicide acceptability in younger populations, particularly young adult males from ethnic minority groups and especially MSM with a history of unprotected RAI [42]. To address this issue, the National Institutes of Health (NIH) has recently funded a project entitled “Microbicide safety and acceptability in young men” that attempts to evaluate RM safety, adherence, and acceptability in young ethnic minority MSM in Boston, Pittsburgh, and San Juan. The study design has two stages (Fig. 1): A clinical and behavioral evaluation (Stage 1A) with an acceptability and adherence trial (Stage 1B), followed by a Phase 1 randomized, double-blind, multi-site, placebo-controlled safety trial (Stage 2). The first 120 eligible participants who complete Stage 1A and report unprotected RAI in the previous 3 months will continue on to Stage 1B. During Stage 1B, participants will be given condoms and a placebo gel to use during RAI. Over a 3 month period they will report the frequency of product use and be interviewed about the acceptability of the product. The first 42 participants who complete Stage 1B with ≥80% adherence to product use will be eligible to participate in Stage 2 where they will be randomized to receive an actual microbicide (tenofovir gel) or matched placebo. Each participant will be evaluated for any adverse events after they apply a single dose of the gel in the study clinic, and again after they self-administer once-daily outpatient doses for 7 days. It is hoped that data from this study will provide unique insights into the acceptability, safety, and adherence of RMs in young MSM.
Fig. 1.
Microbicide acceptability and safety in young men [McGowan and Carballo-Dieguez (R01: HD059533-01A1)]: study stages and eligibility criteria
As can be seen in Table 1, RM development has not yet moved beyond Phase 1, although the NIH sponsored Microbicide Trials Network (www.mtnstopsHIV.org) hopes to conduct a Phase 2 rectal safety evaluation of tenofovir gel in 2012. Designing Phase 2B/3 studies to demonstrate the effectiveness of RMs may actually prove less challenging than it has with vaginal microbicides. This is because for effectiveness studies, it is necessary to find at risk populations with annual HIV seroconversion rates in excess of 3%. Vaginal microbicides studies have needed to be conducted in Sub-Saharan Africa, where it is possible to identify populations of at risk women with annual HIV seroconversion rates in excess of 3%. One advantage for the RM development field is that it should be possible to identify high risk MSM populations in North America, Europe, and Asia [5, 43]. This will simplify the operational complexities associated with these large trials and hopefully encourage sponsors to advance RM candidates into Phase 2B/3 evaluation.
Table 1.
Completed or planned RM studies
| Study | Stage and population characteristics | Product | Sponsor | Status |
|---|---|---|---|---|
| RMP-002/MTN-006 | Phase 1 (sexually abstinent) | Vaginal tenofovir gel | NIH/DAIDS/IPCP Program | Completed |
| MTN-007 | Phase 1 (sexually abstinent) | Reduced glycerin formulation of vaginal tenofovir | NIH/DAIDS/MTN | Q4 2010 |
| Project Gel | Phase 1 (sexually active) | Reduced glycerin formulation of vaginal tenofovir | NIH/NICHD | Q2 2011 |
| CHARM Program | Pre-Phase 1 (single dose, sexually abstinent) | Rectal specific tenofovir gel | NIH/DAIDS/IPCP Program | Q2 2011 |
| MTN-017 | Phase 1 (sexually abstinent) | Reduced glycerin formulation of vaginal tenofovir | NIH/DAIDS/MTN | Q4 2011 |
Other HIV Prevention Tools in Development
Rectal microbicide development is not occurring in a vacuum. Several other HIV prevention tools are being developed simultaneously, and these may impact the design of future RM Phase 2B/3 studies. The most relevant study in this regard is the iPrEx study of oral Truvada® pre-exposure prophylaxis (PrEP) currently being conducted in MSM at sites in Peru, Ecuador, Brazil, Thailand, South Africa, and the U.S. (http://globaliprex.com). Data from iPrEx is anticipated to be available late in 2010. Depending on the level of effectiveness seen in the iPrEx study, Truvada PrEP may need to be added to the prevention standard of care package; it would be difficult to contemplate conducting placebo-controlled Phase 2B/3 prevention studies unless participants were also receiving open label Truvada.
Another possible development that could affect the design of future RM studies would be the identification of a partially effective HIV vaccine. Data from the Thai Phase 3 HIV vaccine clinical trial, also known as RV144, tested the “prime-boost” combination of two vaccines: ALVAC® HIV vaccine (the prime) and AIDSVAX® B/E vaccine (the boost) and demonstrated efficacy of 31.2% (95% CI, 1.1–52.1; P = 0.04) [44]. This was encouraging but probably insufficient to warrant roll out of this specific vaccine. Nonetheless, it is safe to say that the HIV prevention research environment is extremely dynamic and that results from ongoing studies have profound implications on the design and feasibility of future RM studies.
Implementation of Rectal Microbicides for HIV Prevention
Assuming that we can develop a safe and effective RM, the next major step would be to determine who would use these products and under what circumstances. The current generation of RMs under development are all antiretroviral gels. They could only be used by HIV negative individuals and would have to be distributed through a health care system. Unintended exposure to an antiretroviral RM by an individual with untreated HIV infection would likely result in the development of HIV resistance. This might generate subsequent challenges in providing an effective antiretroviral regimen for treatment of HIV infection and also present broader public health issues in terms of dissemination of resistant virus throughout at-risk populations. Consequently, provision of antiretroviral RMs will require extensive voluntary counseling and testing for HIV infection as well as ongoing surveillance of individuals using these products. These issues may limit the social desirability of these products unless there is a carefully orchestrated public health campaign that targets RMs to those individuals who could gain the most to benefit from their use. Characterizing this population will be challenging but necessary to focus limited prevention resources to individuals who really need RMs. Parameters may include a history of frequent unprotected RAI and perhaps anorectal STIs. Such individuals may also benefit from using both oral and topical PrEP. Ongoing Phase 1 RM studies will hopefully provide data on the pharmacological consequences of using both oral and topical PrEP as well as preliminary data on the relative efficacy of single or dual therapy using ex vivo explant challenge studies [45]. Successful roll out of RM will also probably require focused marketing and the development of more user friendly delivery devices.
Advocacy for Rectal Microbicides
The increasing momentum of RM development is encouraging and has been driven to a large extent by community advocacy as well as by the reality of the U.S. HIV epidemic that is now clearly concentrated in young ethnic minority MSM [46, 47]. The efforts of groups such as the International Rectal Microbicide Advocates (IRMA) have played a key role in educating the community about advances in RM development. IRMA is composed of a diverse group of community advocates, clinicians, sponsors, and scientists working on RM. Through their website (http://www.rectalmicrobicides.org), frequent interactive teleconferences, and satellite conferences, IRMA plays a critical role in maintaining momentum in RM research.
Conclusion
Rectal microbicide candidates are likely to move into effectiveness studies in the next 5 years. Operational roll out of antiretroviral RMs will be both complex and challenging and will of necessity target the highest risk populations first. Hopefully, RMs will ultimately provide another component of the HIV prevention package that collectively can impact the spread of HIV infection in at risk populations.
Acknowledgments
Dr. McGowan gratefully acknowledges funding from the United States National Institutes of Health to support his research in microbicide development including this review article (5U19AI060614, 5U01AI066734, and 1R01HD059533).
References
- 1.McGowan I. Microbicides for HIV prevention: reality or hope? Curr Opin Infect Dis. 2010;23(1):26–31. doi: 10.1097/QCO.0b013e328334fe70. [DOI] [PubMed] [Google Scholar]
- 2.Stein ZA. HIV prevention: the need for methods women can use. Am J Public Health. 1990;80(4):460–2. doi: 10.2105/ajph.80.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carlos JA, Bingham TA, Stueve A, et al. The role of peer support on condom use among Black and Latino MSM in three urban areas. AIDS Educ Prev. 2010;22(5):430–44. doi: 10.1521/aeap.2010.22.5.430. [DOI] [PubMed] [Google Scholar]
- 4.Gorbach PM, Manhart LE, Hess KL, Stoner BP, Martin DH, Holmes KK. Anal intercourse among young heterosexuals in three sexually transmitted disease clinics in the United States. Sex Transm Dis. 2009;36(4):193–8. doi: 10.1097/OLQ.0b013e3181901ccf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyrer C. Global prevention of HIV infection for neglected populations: men who have sex with men. Clin Infect Dis. 2010;50(Suppl 3):S108–13. doi: 10.1086/651481. [DOI] [PubMed] [Google Scholar]
- 6.Leynaert B, Downs AM, de Vincenzi I. Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. European Study Group on Heterosexual Transmission of HIV. Am J Epidemiol. 1998;148(1):88–96. doi: 10.1093/oxfordjournals.aje.a009564. [DOI] [PubMed] [Google Scholar]
- 7.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999;150(3):306–11. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 8.Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Hyattsville, MD: National Center for Health Statistics; 2005. Sep 15, Report No.: 362. [PubMed] [Google Scholar]
- 9.Misegades L, Page-Shafer K, Halperin D, McFarland W. Anal intercourse among young low-income women in California: an overlooked risk factor for HIV? AIDS. 2001;15(4):534–5. doi: 10.1097/00002030-200103090-00017. [DOI] [PubMed] [Google Scholar]
- 10.Karim SS, Ramjee G. Anal sex and HIV transmission in women. Am J Public Health. 1998;88(8):1265–6. doi: 10.2105/ajph.88.8.1265-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane T, Pettifor A, Pascoe S, Fiamma A, Rees H. Heterosexual anal intercourse increases risk of HIV infection among young South African men. AIDS. 2006;20(1):123–5. doi: 10.1097/01.aids.0000198083.55078.02. [DOI] [PubMed] [Google Scholar]
- 12.Kalichman S, Simbayi L, Cain D, Jooste S. Heterosexual anal intercourse among community and clinical settings in Cape Town, South Africa. Sex Transm Infect. 2009;85(6):411–5. doi: 10.1136/sti.2008.035287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baral S, Trapence G, Motimedi F, et al. HIV prevalence, risks for HIV infection, and human rights among men who have sex with men (MSM) in Malawi, Namibia, and Botswana. PLoS ONE. 2009;4(3):e4997. doi: 10.1371/journal.pone.0004997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low- and middle-income countries 2000–2006: a systematic review. PLoS Med. 2007;4(12):e339. doi: 10.1371/journal.pmed.0040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carballo-Dieguez A, Stein Z, Saez H, Dolezal C, Nieves-Rosa L, Diaz F. Frequent use of lubricants for anal sex among men who have sex with men: the HIV prevention potential of a microbicidal gel. Am J Public Health. 2000;90(7):1117–21. doi: 10.2105/ajph.90.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gross M, Buchbinder SP, Celum C, Heagerty P, Seage GR., III Rectal microbicides for U.S. gay men. Are clinical trials needed? Are they feasible? HIVNET Vaccine Preparedness Study Protocol Team. Sex Transm Dis. 1998;25(6):296–302. doi: 10.1097/00007435-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Carballo-Dieguez A, O’sullivan LF, Lin P, Dolezal C, Pollack L, Catania J. Awareness and attitudes regarding microbicides and nonoxynol-9 use in a probability sample of gay men. AIDS Behav. 2007;11(2):271–6. doi: 10.1007/s10461-006-9128-0. [DOI] [PubMed] [Google Scholar]
- 18.Nodin N, Carballo-Dieguez A, Ventuneac AM, Balan IC, Remien R. Knowledge and acceptability of alternative HIV prevention bio-medical products among MSM who bareback. AIDS Care. 2008;20(1):106–15. doi: 10.1080/09540120701449096. [DOI] [PubMed] [Google Scholar]
- 19.Berg RC. Barebacking: a review of the literature. Arch Sex Behav. 2009;38(5):754–64. doi: 10.1007/s10508-008-9462-6. [DOI] [PubMed] [Google Scholar]
- 20.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360(9338):971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 21.Karim QA, Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tabet SR, Surawicz C, Horton S, et al. Safety and toxicity of nonoxynol-9 gel as a rectal microbicide. Sex Transm Infect. 1999;26(10):564–71. doi: 10.1097/00007435-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 23.McGowan I, Elliott J, Cortina G, et al. Characterization of baseline intestinal mucosal indices of injury and inflammation in men for use in rectal microbicide trials (HIV Prevention Trials Network-056) J Acquir Immune Defic Syndr. 2007;46(4):417–25. doi: 10.1097/QAI.0b013e318156ef16. [DOI] [PubMed] [Google Scholar]
- 24.Anton P, Adler A, Khanukova E, et al. A Phase 1 rectal safety and acceptability study of UC781 microbicide gel. 16th Conference on Retroviruses and opportunistic infections; Montreal. 2009. (abstract 1066) [Google Scholar]
- 25.Fuchs EJ, Lee LA, Torbenson MS, et al. Hyperosmolar sexual lubricant causes epithelial damage in the distal colon: potential implication for HIV transmission. J Infect Dis. 2007;195(5):703–10. doi: 10.1086/511279. [DOI] [PubMed] [Google Scholar]
- 26.Barnhart KT, Pretorius ES, Timbers K, Shera D, Shabbout M, Malamud D. In vivo distribution of a vaginal gel: MRI evaluation of the effects of gel volume, time and simulated intercourse. Contraception. 2004;70(6):498–505. doi: 10.1016/j.contraception.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 27.Barnhart K, Kulp JL, Rosen M, Shera DM. A randomized trial to determine the distribution of four topical gel formulations in the human vagina. Contraception. 2009;79(4):297–303. doi: 10.1016/j.contraception.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Hendrix CW, Fuchs EJ, Macura KJ, Lee LA, Parsons TL, Bakshi RP, et al. Quantitative imaging and sigmoidoscopy to assess distribution of rectal microbicide surrogates. Clin Pharmacol Ther. 2008;83(1):97–105. doi: 10.1038/sj.clpt.6100236. [DOI] [PubMed] [Google Scholar]
- 29.UNAIDS. Ethical considerations in biomedical HIV prevention trials. [Accessed 31 Oct 2010];Joint United Nations Programme on HIV/AIDS (UNAIDS) 2007 http://data.unaids.org/pub/Report/2007/jc1399_ethical_considerations_en.pdf.
- 30.Elias C, Coggins C. Acceptability research on female-controlled barrier methods to prevent heterosexual transmission of HIV: Where have we been? Where are we going? J Womens Health Gend Based Med. 2001;10(2):163–73. doi: 10.1089/152460901300039502. [DOI] [PubMed] [Google Scholar]
- 31.Severy L, Newcomer S. Critical issues in contraceptive and STI acceptability research. J Soc Issues. 2005;61(1):45–65. [Google Scholar]
- 32.Mantell JE, Myer L, Carballo-Dieguez A, Stein Z, Ramjee G, Morar NS, et al. Microbicide acceptability research: current approaches and future directions. Soc Sci Med. 2005;60(2):319–30. doi: 10.1016/j.socscimed.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Morrow KM, Ruiz MS. Assessing microbicide acceptability: a comprehensive and integrated approach. AIDS Behav. 2008;12(2):272–83. doi: 10.1007/s10461-007-9266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tolley EE, Severy LJ. Integrating behavioral and social science research into microbicide clinical trials: challenges and opportunities. Am J Public Health. 2006;96(1):79–83. doi: 10.2105/AJPH.2004.043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen RK, Morrow KM, Carballo-Dieguez A, et al. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: a mixed-methods study. J Womens Health (Larchmt) 2008;17(3):383–92. doi: 10.1089/jwh.2006.0325. [DOI] [PubMed] [Google Scholar]
- 36.Bentley ME, Morrow KM, Fullem A, et al. Acceptability of a novel vaginal microbicide during a safety trial among low-risk women. Fam Plann Perspect. 2000;32(4):184–8. [PubMed] [Google Scholar]
- 37.Morrow K, Rosen R, Richter L, et al. The acceptability of an investigational vaginal microbicide, PRO 2000 Gel, among women in a phase I clinical trial. J Womens Health (Larchmt) 2003;12(7):655–66. doi: 10.1089/154099903322404302. [DOI] [PubMed] [Google Scholar]
- 38.El-Sadr WM, Mayer KH, Maslankowski L, et al. Safety and acceptability of cellulose sulfate as a vaginal microbicide in HIV-infected women. AIDS. 2006;20(8):1109–16. doi: 10.1097/01.aids.0000226950.72223.5f. [DOI] [PubMed] [Google Scholar]
- 39.Carballo-Dieguez A, Exner T, Dolezal C, Pickard R, Lin P, Mayer KH. Rectal microbicide acceptability: results of a volume escalation trial. Sex Transm Dis. 2007;34(4):224–9. doi: 10.1097/01.olq.0000233715.59239.83. [DOI] [PubMed] [Google Scholar]
- 40.Carballo-Dieguez A, Dolezal C, Bauermeister JA, O’Brien W, Ventuneac A, Mayer K. Preference for gel over suppository as delivery vehicle for a rectal microbicide: results of a randomised, crossover acceptability trial among men who have sex with men. Sex Transm Infect. 2008;84(6):483–7. doi: 10.1136/sti.2008.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ventuneac A, Carballo-Dieguez A, McGowan I, et al. Acceptability of UC781 gel as a rectal microbicide among HIV-uninfected women and men. AIDS Behav. 2010;14(3):618–28. doi: 10.1007/s10461-009-9611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rudy BJ, Kapogiannis BG, Lally MA, et al. Youth-specific considerations in the development of preexposure prophylaxis, microbicide, and vaccine research trials. J Acquir Immune Defic Syndr. 2010;54(Suppl 1):S31–42. doi: 10.1097/QAI.0b013e3181e3a922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Griensven F, de Lind van Wijngaarden JW, Baral S, Grulich A. The global epidemic of HIV infection among men who have sex with men. Curr Opin HIV AIDS. 2009 Jul;4(4):300–7. doi: 10.1097/COH.0b013e32832c3bb3. [DOI] [PubMed] [Google Scholar]
- 44.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 45.Elliott J, McGowan I, Adler A, et al. Strong suppression of HIV-1 infection of colorectal explants following in vivo rectal application of UC781 gel: a novel endpoint in a Phase I trial. 16th Conference on Retroviruses and opportunistic infections; Montreal. 2009. (abstract 1067) [Google Scholar]
- 46.Sifakis F, Hylton JB, Flynn C, et al. Racial disparities in HIV incidence among young men who have sex with men: the Baltimore Young Men’s Survey. J Acquir Immune Defic Syndr. 2007;46(3):343–8. doi: 10.1097/QAI.0b013e31815724cc. [DOI] [PubMed] [Google Scholar]
- 47.Sifakis F, Hylton JB, Flynn C, et al. Prevalence of HIV infection and prior HIV testing among young men who have sex with men. The Baltimore young men’s survey. AIDS Behav. 2010;14(4):904–12. doi: 10.1007/s10461-007-9317-5. [DOI] [PubMed] [Google Scholar]