Abstract
Objective
To provide an estimate of the incidence of herpes simplex virus (HSV) eye disease in a community-based cohort, and to investigate the impact of prophylactic oral antiviral therapy on HSV recurrences and outcomes.
Methods
All Olmsted County, Minnesota residents diagnosed with ocular HSV from 1976 through 2007 were retrospectively reviewed. The frequency of recurrences and adverse outcomes, such as vision loss or need for surgery, were compared between untreated patients and those treated prophylactically with oral antiviral medication.
Results
394 patients with ocular HSV were identified, yielding an annual incidence of new cases of 11.8 (95% C.I.: 10.6 to 13.0) per 100,000 population. No trends in incidence or adverse outcomes were identified over the 32 year period. Oral antiviral therapy was prescribed in 175 patients. Patients were 9.4 (95% C.I.: 5.0 to 17.9) times more likely to have a recurrence of epithelial keratitis, 8.4 (95% C.I.: 5.2 to 13.7) times more likely to have a recurrence of stromal keratitis, and 34.5 (95% C.I.: 10.8 to 111.1) times more likely to have a recurrence of blepharitis or conjunctivitis if not being treated prophylactically at the time of the recurrence. Twenty patients experienced adverse outcomes, and 17 (85%) were not being treated with oral antiviral medications immediately preceding the adverse event.
Conclusions
Oral antiviral prophylaxis was associated with a decreased risk of recurrence of epithelial keratitis, stromal keratitis, conjunctivitis and blepharitis due to HSV. Patients with adverse outcomes due to ocular HSV were usually not being treated with oral antiviral prophylaxis.
Introduction
Herpes simplex virus (HSV) is a common cause of corneal disease and is the leading infectious cause of corneal blindness among developed nations.1 Following the initial exposure and primary systemic infection, HSV establishes a latent infection in the trigeminal or other sensory ganglia. Reactivation of latent HSV in the sensory ganglia may lead to initial or recurrent disease in one or occasionally both eyes.2 Common ocular manifestations of HSV include blepharitis, conjunctivitis, keratitis, and uveitis. Posterior segment disease, such as acute retinal necrosis, is rare. Recurrence rates of ocular HSV after an initial episode have been estimated at 10% at one year, 23% at two years, 36% at 5 years, and over 60% at 20 years.3 Periodic reactivations in the cornea are particularly important, because the cumulative effect of re-infection may lead to stromal inflammation or neurotrophic keratitis, resulting in scar or perforation.4
Studies outside the U.S have estimated incidence rates of HSV eye disease ranging from approximately 4 to 13 new cases per 100,000 population.5,6,7 A previous Rochester, Minnesota study estimated an incidence of 8.4 new cases per 100,000 and 20.7 total episodes per 100,000 population per year.1 Extrapolating these data to the United States population in 2000, the last year for which accurate census data are available, yields an estimated incidence of approximately 24,000 new cases and 58,000 total episodes per year.
Introduced in the United States in 1983, acyclovir is a potent antiviral that has been shown to be effective in the treatment of and prophylaxis against HSV, significantly reducing the recurrence rates of genital8,9,10 and orofacial11 infections. Subsequently, the related drugs valacyclovir and famciclovir were introduced in 1994 and 1995. The Herpetic Eye Disease Study (HEDS) demonstrated that the use of prophylactic oral acyclovir in patients with a recent history of ocular HSV decreased the incidence of ocular recurrences by 45% during a 12-month treatment period.12 A separate retrospective analysis found that the beneficial effect of oral acyclovir persisted even when the drug was taken for 18 months or longer.13 While it has been suggested that oral antiviral prophylaxis may have contributed to the decrease in penetrating keratoplasties necessitated by ocular HSV,14 the impact of prophylaxis on the long-term outcomes of ocular HSV has not been well investigated.
The Rochester Epidemiology Project (REP) is a medical records linkage system established in 1976 to facilitate epidemiologic study of disease by tracking all diagnostic and surgical procedure codes for the residents of Olmsted County, Minnesota.15 The REP includes patients treated at Mayo Clinic, Olmsted Medical Center, and their affiliated hospitals and clinics and has been used in a multitude of previous studies to determine trends in incidence and outcomes of eye disease.16
We used the REP resources to re-examine the incidence of HSV eye disease in a community-based cohort and to study the effect of the introduction of oral antiviral therapy on the natural history of HSV eye disease. Our purpose was to establish whether long-term prophylactic oral antiviral therapy had an impact on the frequency of recurrences and adverse outcomes due to ocular HSV.
Methods
After approval by the institutional review boards of Mayo Clinic and Olmsted Medical Center, the resources of the REP were used to identify all potential incident cases of HSV eye disease in Olmsted County, Minnesota between January 1, 1976 and December 31, 2007. Medical records were retrieved for all patients with diagnostic codes related to HSV eye disease. Assigned diagnostic codes were based on Mayo Clinic modifications of the International Classification of Diseases, Ninth Revision, Clinical Modification and included codes 054.40 (herpes simplex, ophthalmic), 054.41 (blepharitis, herpes simplex), 054.42 (dendritic keratitis, herpes simplex), 054.43 (herpes keratitis, simplex), 054.44 (herpes iritis, simplex), 054.79 (ophthalmic herpes simplex, NEC). Cases coded as having non-ophthalmic herpes simplex and herpes zoster were not retrieved. Residency status was confirmed using methods developed by the REP. The coauthors reviewed all medical records to identify all cases of HSV eye disease during the study period. Incident cases included only those patients whose initial episode occurred during the study period. Patients who reported initial episodes prior to establishing residency in Olmsted County were excluded from the study. The complete medical records for all incident cases were then abstracted to include information on demographics, diagnoses, medications, therapeutic interventions, recurrences, and adverse outcomes. Data were collected for every clinic visit during the study period for all incident cases. If not specifically recorded in the medical record for a given visit, the date on which a medication was started or stopped was estimated to be at the mid-point between two visits. Cases in which the clinical findings and/or laboratory evidence were inadequate for or inconsistent with a diagnosis of ocular HSV were excluded after review by the senior clinician-investigator (K.H.B.) HSV-related “events” were defined as ocular disease, such as blepharitis, conjunctivitis, keratitis, uveitis, and/or posterior segment disease deemed by the examining clinician to be due to HSV greater than one month after quiescence, as described in Table 1. Adverse outcomes were defined as irreversible loss of vision to 20/200 or worse, corneal perforation or any surgical intervention necessitated by HSV-related complications (ie. trabeculectomy, keratoplasty, conjunctival flap, permanent tarsorrhaphy). Cataract, cataract surgery or glaucoma surgery for a patient diagnosed with glaucoma prior to the diagnosis of HSV were not considered an adverse outcome.
Table 1.
Definitions of ocular HSV “events” established prior to performing chart review.
| Blepharitis | Ulcerative lid lesions |
|---|---|
| Conjunctivitis | Conjunctival inflammation associated with a positive microbiological examination and/or conjunctival epithelial defect |
| Epithelial Keratitis | Dendritic or geographic epithelial irregularity or ulceration |
| Neurotrophic Keratitis | Indolent intrapalpebral epithelial irregularity or ulceration |
| Subepithelial Stromal Keratitis | Subepithelial nummular, patchy, sectoral infiltrate or opacity |
| Deep Stromal Keratitis | Infiltrate, haze, opacity not limited to the subepithelial stroma |
| Neovascularization | Corneal vessels at any depth extending more than 2 mm from the limbus |
| Lipid Infiltrate | Any “hard infiltrate” associated with neovascularization |
| Necrotizing Keratitis | An epithelial defect associated with stromal loss and infiltrate |
| Endotheliitis | Sectoral or nummular stromal edema associated spatially with keratoprecipitates and a lack of stromal infiltrate |
| Uveitis | Anterior chamber cell and flare in a patient with other prior or simultaneous known manifestations of HSV ocular disease |
Incidence rates were calculated based on US census data with linear interpolation for intervening years for which data were not available. Rates were age- and sex-adjusted to census data for the 2000 U.S. white population. Rates were compared by using Poisson distribution and trends in incidence were evaluated by using the Poisson regression model.
The time to an event or adverse outcome was estimated by using the Kaplan-Meier method. For patients having more than one adverse outcome, only the time to the first recorded adverse outcome was considered. The association between prophylactic oral antiviral therapy and recurrence was investigated by using Cox proportional hazards models. Prophylaxis was used as a time-dependent covariate.
Results
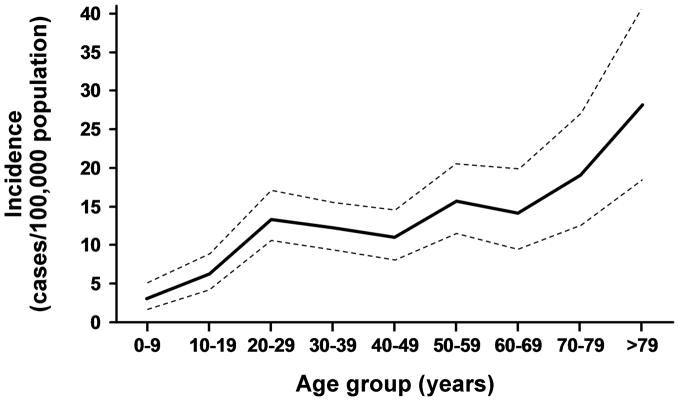
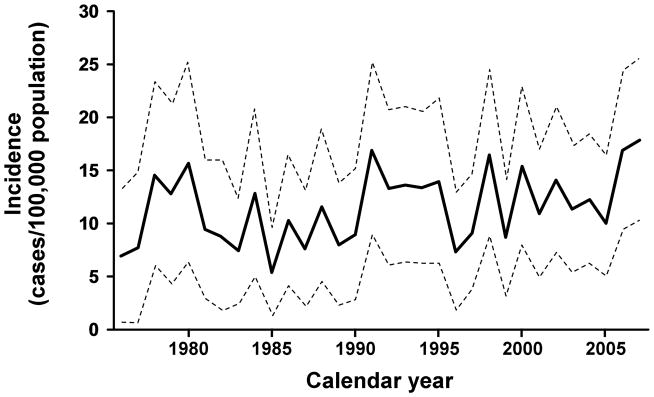
A manual review of 644 medical histories with diagnostic codes consistent with potential ocular HSV disclosed 394 confirmed cases. The mean age at onset was 43 years (range 1 to 91 yrs). There were 181 (46%) male and 213 female (54%) patients. The mean follow-up in this study was 7.7 years. Nine cases, 3 of whom were female, were identified as non-Olmsted County residents and therefore were excluded from the incidence rate calculations. The age- and sex- adjusted incidence of new cases of HSV keratitis during the entire study period was 9.2 (95% C.I.: 8.1 to 10.3) per 100,000 population per year, and the incidence of any new cases of ocular HSV was 11.8 (95% C.I.: 10.6 to 13.0) per 100,000 population per year. Annual rates for ocular HSV per 100,000 among men [11.9 (10.0 to 13.7)] and women [12.0 (10.3 to 13.6)] were similar (p=0.26). The incidence per 100,000 population per year increased with age, from 3.1 (1.8 to 5.0) during the first decade of life to 28.1 (18.7 to 40.7) after the ninth decade (p<0.001). (Figure 1) There was no trend in the age- and sex- adjusted incidence during the 32 year period. (Figure 2) The rate was 10.4 (7.8 to 13.0)/100,000 during the first 8 years and 13.6 (11.3 to 15.9)/100,000 during the final 8 yrs. (p=0.36). Extrapolating these data to prevalence among age groups, the likelihood of developing ocular HSV was 0.23% by 30 years, 0.49% by 50 years and 0.94% by 80 years.
Figure 1.
Age-specific incidence rates of initial HSV ocular disease during the study period, 1976 through 2007. Incidence rates are sex-adjusted to the 2000 U.S. white population.
Figure 2.
The incidence of initial HSV ocular disease, 1976 through 2007. Rates are age- and sex-adjusted to the 2000 U.S. white population.
Among the 394 cases, dendritic epithelial keratitis was the most common initial presentation and included 233, or 59% of patients. Sixty four (16%) patients presented with other forms of keratitis, 79 (20%) with blepharitis or blepharoconjunctivitis, 16 (4%) with conjunctivitis alone, and 2 (0.5%) with uveitis. There were 16 (4%) patients with simultaneous, bilateral involvement at initial presentation, and 4 additional patients (1%) with simultaneous bilateral involvement at the time of recurrence. No cases of retinitis, optic neuritis or other posterior segment disease were identified either at initial presentation or as a recurrent event.
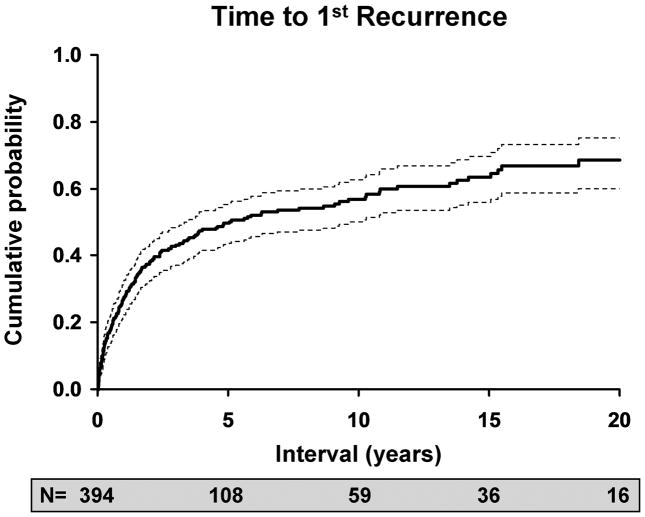
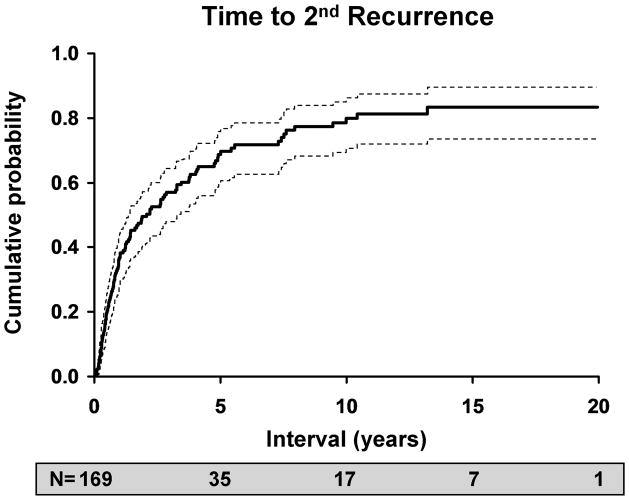
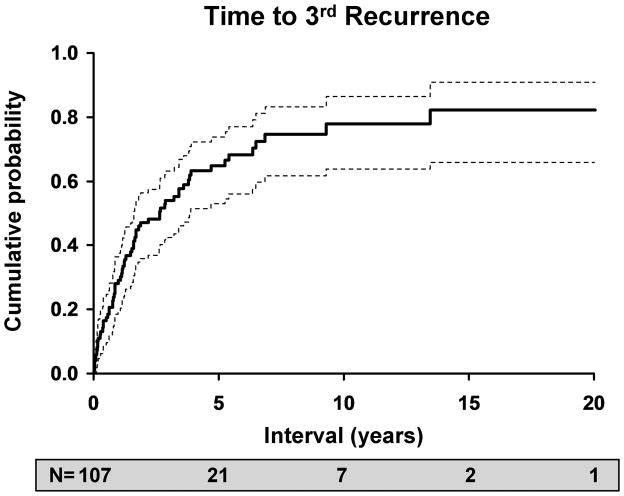
The likelihood of an inflammatory or infectious episode of ocular HSV after an initial episode was estimated to be 27% at 1 year (95% C.I: 22–32%), 50% at 5 years (95% C.I.: 43–55%), 57% at 10 years (95% C.I.: 50-63%) and 63% at 20 years (95% C.I.: 60–75%). Of 169 patients who experienced a first recurrence, 108 had a second recurrence, for a rate of 38% at 1 year (95% C.I.: 30–46%), 67% at 5 years (95% C.I.: 58–74%), 78% at 10 years (95% C.I.: 69–85%) and 83% at 20 years (95% C.I.: 73–89%). 65 patients experienced a third recurrence, and the rates were similar to those for a second recurrence, with 29% at 1 year (95% C.I.: 19–38%), 65% at 5 years (95% C.I.: 53–75%), 78% at 10 years (95% C.I.: 64–86%), and 82% at 20 years (95% C.I.: 66–91%).
Overall, 175 patients (44%) had been treated with oral antiviral medication and were on therapy for a mean of 2.8 years, or 36% of the duration of their follow-up. While on oral prophylactic therapy, 4 patients experienced a first recurrence, 10 developed an epithelial recurrence, 20 had a stromal recurrence, and 3 developed blepharitis or conjunctivitis. Using Cox proportional hazards models, we investigated the relationship of ocular HSV recurrences with oral antiviral prophylaxis. Treatment with oral antiviral prophylaxis decreased the relative risk of first recurrence by a factor of 2.9 (95% C.I.: 1.0–7.7). When we conducted a subgroup analysis of recurrences, we found patients were 9.4 (95% C.I. 5.0 to 17.9) times more likely to have a recurrence of epithelial keratitis, 8.4 (95% C.I.: 5.2 –13.7) times more likely to have a recurrence of stromal (subepithelial, deep or necrotizing) keratitis, and 34.5 (95% C.I.: 10.8 to 111.1) times more likely to have a recurrence of blepharitis or conjunctivitis if not being treated with oral antiviral prophylaxis at the time of the recurrence. (Table 2) The proportional hazards assumption was verified for each subgroup and none of the models violated that assumption.
Table 2.
Relative risk of recurrent ocular HSV for the patients not being treated with oral antiviral medication.
| Recurrent HSV event | Relative risk (95% C.I.) |
|---|---|
| Epithelial keratitis | 9.4 (5.0–17.9) |
| Stromal keratitis (subepithelial, deep, or necrotizing) | 8.4 (5.2 –13.7) |
| Conjunctivitis or blepharitis | 34.5 (10.9–111.1) |
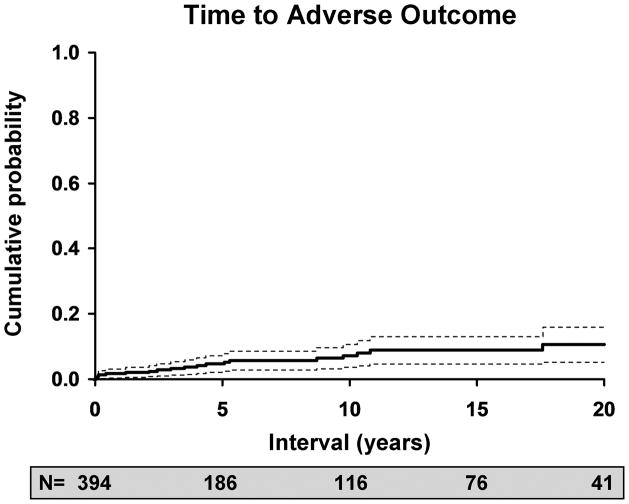
Twenty patients experienced adverse outcomes during this study. (Table 3) The risk of adverse outcomes was 5% at 5 years (95% C.I.: 2–7%), 7% at 10 years (95% C.I.: 3–11%), and 11% at 20 years (95% C.I.: 5–16%). (Figure 4) These patients had an average of 4.1 recurrences of ocular HSV prior to their adverse outcome. When we investigated trends in the incidence of adverse outcomes, we found that the risk of adverse outcomes did not decrease during the latter portion of the study period during which antiviral prophylaxis was available. However, 17 of 20 (85%) patients were not on prophylactic oral antiviral medication at the time of the last recurrence immediately preceding the adverse event, and 14 of 20 (65%) had never been treated with prophylactic oral antiviral therapy.
Table 3.
Adverse outcomes due to HSV eye disease, 1976 through 2007.*
| Adverse Outcome | N |
|---|---|
| Visual Loss (20/200 or worse) | 11 |
| Corneal Perforation | 1 |
| Enucleation | 1 |
| Glaucoma Surgery | 1 |
| Keratoplasty | 5 |
| Conjunctival Flap | 1 |
| Permanent Tarsorraphy | 0 |
| Total | 20 |
For patients experiencing more than one adverse outcome, only the first adverse outcome is listed.
Figure 4.
Kaplan-Meier estimate of the risk of an adverse outcome due to ocular HSV over time. N is the number of patients at risk at any point in time.
Discussion
The purpose of our study was two-fold; to provide a recent estimate of the incidence of HSV eye disease in a community-based cohort, and to investigate the impact of prophylactic oral antiviral therapy on recurrence and outcomes of HSV eye disease. We estimated an age and sex-adjusted annual incidence rate of 9.2 per 100,000 population new cases of keratitis and 11.8 per 100,000 of any ocular HSV. Liesegang and colleagues demonstrated an age-adjusted incidence of 8.4 new cases of ocular HSV per 100,000 in a previous Rochester, Minnesota study which included cases from 1950 through 1982,1 and overlaps with our current study. Our estimated incidence would be 12.0 per 100,000 population including only 1983 and beyond. Labetoulle and co-investigators prospectively surveyed eye care providers in France and found an incidence of 13.2 per 100,000 person-years for new cases of herpes keratitis.5 Ribaric estimated the incidence of HSV keratitis at 4.2 to 12.5 per 100,000 in 1976,6 while Mortensen and Sjolie found an incidence of keratitis of 12 per 100,000 in 1979.7 Despite the differences in technique of ascertaining incident cases, our incidence rates are approximately similar to other studies.
In contrast to the previous Rochester, Minnesota study, we did observe an increase in the incidence of new cases with aging. This peaked in the ninth decade of life at 28 (95% C.I.: 8.7 to 40.7) cases per 100,000 population, whereas the previous study reported a peak of about 13 cases per 100,000 population from age 55 to 74. The study from France also reported a peak incidence of 32 (15.3 to 48.9) cases per 100,000 after age 80.5 The cause for an increase in new cases with age perhaps may be due to decreased immunity against the latent virus in the elderly, analogous to the age-related increase in incidence among patients with herpes zoster.17,18 However, the confidence intervals for our study and that of the French study are wide, questioning whether the high incidence among the very elderly is certain or artefactual.
Our calculated recurrence of ocular HSV disease following an initial episode was higher than previous estimates, even though our data include patients who were on prophylactic therapy as well as those who were not. Our recurrence rates of ocular HSV were 27% at 1 year, 50% at 5 years, 57% at 10 years, and 63% at 20 years, in comparison to Liesegang and colleagues’ calculated recurrence rates of 9.6% at 1 year, 36% at 5 years, and 63.2% at 20 years3. Patients with one recurrence following an initial episode had an even higher risk of additional recurrences, with up to 38% at one year and 67% by five years. The Herpetic Eye Disease Study (HEDS) estimated a recurrence rate of any type of ocular HSV of 32% at one year among patients in the control group.12 Recurrences are a substantial problem, as multiple recurrences increase the risk of corneal scarring and visual loss, and these data suggest that recurrence rates are even higher than previously suspected.
Oral antiviral prophylaxis was associated with a decrease in the rates of recurrence of epithelial keratitis, stromal keratitis, and conjunctivitis and blepharitis due to HSV while the antiviral was being administered. Our data suggest a more profound effect than previous studies that have established the beneficial effect of oral acyclovir therapy.
The HEDS trial entailed randomization of 703 patients with a history of HSV eye disease to either acyclovir 400 mg twice daily or placebo for a treatment period of 12 months, followed by 6 months of observation. Investigators found recurrence rates of 19% in the acyclovir group and 32% in the placebo group, demonstrating a 45% decrease in all ocular HSV recurrences among patients treated prophylactically with oral acyclovir for 12 months.12 The most pronounced effect was seen among patients with a history of multiple recurrences or prior stromal keratitis.19
In a retrospective analysis of antiviral prophylaxis among patients with prior recurrent disease, Uchoa and colleagues demonstrated higher rates of recurrence and shorter time to recurrence in patients who were treated with oral antivirals for only 12 months versus those treated longer than 12 months, suggesting a benefit of treatment with oral acyclovir beyond one year.13
Rezende and colleagues conducted a retrospective study of oral antiviral prophylaxis in patients with and without atopy, and demonstrated a more substantial treatment effect than previous studies, with a 76% decrease in infectious recurrences in atopics and a 69% decrease in inflammatory episodes in non-atopics.20
We can not explain our more dramatic results with certainty, other than to note that our community-based study reflects an unbiased population that may have less severe disease than those patients studied at referral centers. We also recorded whether patients reported antiviral medication use at the time of their recurrence or adverse event, which is different than studying patients who were prescribed antiviral prophylaxis. The HEDS investigators attempted to evaluate whether compliance was a factor in their results; 89% of participants in the treatment group were at least 80% compliant and 72% were at least 90% compliant. However, compliance rates were similar among those patients who developed a recurrence and those who did not.11 Cole and Chu subsequently re-analyzed the HEDS data using a structural nested model to generate compliance-corrected data. These investigators concluded that the original intent-to-treat analysis may have underestimated the treatment effect by 34%, thereby resulting in an actual reduction of recurrences by 59% rather than 45%.21
We were not able to demonstrate a change in the incidence of adverse outcomes during the latter part of the study after the publication of the HEDS data in 1998. This was likely due to the small number of patients with adverse outcomes. However, we did find that of the 20 patients who experienced an adverse outcome, 17 (85%) were not being treated with oral antiviral medications either at the time of their last recurrence immediately preceding or at the time of their adverse outcome. Considering that these patients had on average 4 recurrences prior to their adverse event, future providers and patients should have ample opportunity to begin oral prophylactic treatment. We did not attempt to quantify the reasons the clinician prescribed prophylaxis versus observation (a limitation of the study). However, it is our personal experience that patients discontinue or are not prescribed prophylactic medication for several reasons, including medication cost, patients’ perceived lack of need for or lack of effect of the medication, failure to refill prescriptions (either on the part of the patient or provider), lack of or long intervals between follow-up visits, and the providers’ reluctance to prescribe chronic medication.
Previous studies established the beneficial effect of prophylactic oral antiviral medication on ocular HSV only during the period the antiviral was being administered, and no studies have evaluated the outcomes in patients treated for many years or whether recurrence rates return to baseline values if prophylaxis is discontinued after many years of therapy and quiescence. Although patients in the current study were treated for an average of 2.8 years, our study design and power do not allow us to address these questions with certainty. Additionally, the analysis done by Lairson and colleagues brought into question the cost-effectiveness of prophylactic oral acyclovir therapy. By using the HEDS data as a premise for their study, these investigators cited a high cost in preventing each HSV recurrence.22 However, if the HEDS data under-estimated the true efficacy of prophylactic oral therapy, then the cost of disease prevention may have been over-estimated.
The strengths of this study included the large number of subjects and the long-term follow-up, with a mean of 7.7 yrs. The community-based cohort helped to eliminate referral bias, and perhaps more accurately represents real-world care of ocular HSV. The inclusion of cases from 1976 through 1982 from a previously published study1 allowed an analysis of trends over a 32-year period and increased the statistical power of the study. Cases before 1976 were not included, because REP databases are inaccurate prior to this date. If we had limited our analysis to cases after the introduction of acyclovir prophylaxis, we may have introduced bias. The characteristics of prophylactically treated patients, such as disease severity, may have been different from those for whom the treating physician elected no prophylaxis.
A potential weakness of the study is that we did not perform a direct comparison between all cases examined prior to the introduction of prophylactic oral therapy to all cases examined after its’ introduction. Instead, untreated patients studied after the availability of prophylaxis were included with those presenting earlier, and all were treated statistically as a single group. Again, this may have introduced bias due to differences between treated and untreated groups. It should also be noted that many patients are included in both groups: in the treated group during the time period in which they were taking antiviral medication and in the untreated group when they were not taking medication.
Several other limitations should be addressed. This was a retrospective study, which relied on the accuracy and completeness of clinicians’ records. Laboratory confirmation of HSV was not available in most cases. It is also likely that our study did not capture all incident cases and all episodes of disease, as patients may not have sought care or may have sought care outside of Olmsted County. Cases may have been misdiagnosed or miscoded, causing an inherent underestimation of our number of cases. We also did not examine the effect of different antiviral drugs or dosage regimens on recurrences or adverse outcomes. Because of the challenges of identifying and defining comorbid disease in a retrospective study averaging almost 8 years of follow-up per subject, we did not collect data or perform subgroup analyses that could have identified risk factors for HSV occurrence or recurrence, such as atopy, diabetes or immunosuppressive therapy. Lastly, factors affecting the decision by providers or patients to use oral antiviral medications could bias our results.
Overall, this community-based retrospective study demonstrated a stable incidence of herpes simplex virus eye disease during a recent 32-year period. We found a more dramatic protective effect of oral antiviral prophylaxis on recurrences of ocular HSV than had been described previously. While we were not able to show that availability of antiviral prophylaxis was associated with a decrease in the incidence of adverse outcomes, we did find that at the time of their adverse outcome, patients were likely to have had an average of 4 prior episodes of recurrent disease and were unlikely to have been on oral antiviral prophylaxis. The results of this study suggest that oral antiviral prophylaxis should be considered for those patients with frequent recurrences of corneal disease. Additionally, we recommend an evaluation of the possible barriers preventing compliance with antiviral prophylaxis and a reassessment of the cost-effectiveness of long-term oral antiviral therapy.
Figure 3.
Kaplan-Meier estimates of risk versus: A. time to first recurrence of HSV after an initial episode of disease; B. time to second recurrence after a first recurrence of ocular HSV; C. time to third recurrence after a second recurrence of ocular HSV.
Acknowledgments
This study was supported by an unrestricted grant from Research to Prevent Blindness, Inc, New York, NY and the Mayo Foundation.
Footnotes
Presented in part at the Annual Meeting of the Association for Research in Vision and Ophthalmology at Fort Lauderdale, FL, in May, 2009.
None of the authors have any financial interests.
References
- 1.Liesegang TJ, Melton LJ, Daly PJ, et al. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–9. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 2.Liesegang TJ. The biology of herpes simplex and varicella zoster virus infections. Ophthalmology. 1992;99:781–99. doi: 10.1016/s0161-6420(92)31921-9. [DOI] [PubMed] [Google Scholar]
- 3.Liesegang TJ, Melton LJ, Daly PJ, et al. Epidemiology of ocular herpes simplex. Natural history in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1160–5. doi: 10.1001/archopht.1989.01070020226030. [DOI] [PubMed] [Google Scholar]
- 4.Liesegang TJ. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea. 1999;18:127–43. doi: 10.1097/00003226-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Labetoulle M, Auquier P, Conrad H, et al. Incidence of herpes simplex virus keratitis in France. Ophthalmology. 2005;112:888–95. doi: 10.1016/j.ophtha.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 6.Ribaric V. The incidence of herpetic keratitis among population. Ophthalmologica. 1976;173:19–22. doi: 10.1159/000307814. [DOI] [PubMed] [Google Scholar]
- 7.Mortensen KK, Sjolie AK. Keratitis dendritica. An epidemiological investigation. Acta Ophthalmologica. 1979;57:750–4. doi: 10.1111/j.1755-3768.1979.tb01840.x. [DOI] [PubMed] [Google Scholar]
- 8.Mertz GJ, Jones CC, Mills J, et al. Long-term acyclovir suppression of frequently recurring genital herpes simplex virus infection: a multicenter double-blind trial. JAMA. 1988;260:201–6. [PubMed] [Google Scholar]
- 9.Kaplowitz LG, Baker D, Gelb L, et al. Prolonged continuous acyclovir treatment of normal adults with frequently recurring genital herpes simplex virus infection. JAMA. 1991;265:747–51. [PubMed] [Google Scholar]
- 10.Mattison HR, Reichman RC, Benedetti J, et al. Double-blind, placebo-controlled trial comparing long-term suppressive with short-term oral acyclovir therapy for management of recurrent genital herpes. Am J Med. 1988;85:20–25. [PubMed] [Google Scholar]
- 11.Rooney JF, Strauss SE, Mannix ML, et al. Oral acyclovir to suppress frequently recurrent herpes labialis: a double-blind, placebo-controlled trial. Ann Intern Med. 1993;118:268–72. doi: 10.7326/0003-4819-118-4-199302150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. NEJM. 1998;339:300–6. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 13.Uchoa UBC, Rezende RA, Carrasco MA, et al. Long-term acyclovir use to prevent recurrent ocular herpes simplex virus infection. Arch Ophthalmol. 2003;121:1702–4. doi: 10.1001/archopht.121.12.1702. [DOI] [PubMed] [Google Scholar]
- 14.Cosar CB, Sridhar MS, Cohen EJ. Indications for penetrating keratoplasty and associated procedures, 1996-2000. Cornea. 2002;21:148–51. doi: 10.1097/00003226-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 16.Severson EA, Baratz KH, Hodge DO, Burke JP. Herpes zoster ophthalmicus in Olmsted County, Minnesota: Have systemic antivirals made a difference? Arch Ophthalmol. 2003;121:386–90. doi: 10.1001/archopht.121.3.386. [DOI] [PubMed] [Google Scholar]
- 17.Ragozzino MW, Melton LJ, 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine. 1982;61:310–6. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Burke BL, Steele RW, Beard OW, Wood JS, Cain TD, Marmer DJ. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–3. [PubMed] [Google Scholar]
- 19.Herpetic Eye Disease Study Group. Oral acyclovir for herpes simplex virus eye disease: effect on prevention of epithelial keratitis and stromal keratitis. Arch Ophthalmol. 2000;118:1030–6. [PubMed] [Google Scholar]
- 20.Rezende RA, Bisol T, Hammersmith K, et al. Efficacy of oral antiviral prophylaxis in preventing ocular herpes simplex virus recurrences in patients with and without self-reported atopy. Am J Ophthalmol. 2006;142:563–7. doi: 10.1016/j.ajo.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Cole SR, Chu H. Effect of acyclovir on herpetic ocular recurrences using a structural nested model. Contemp Clin Trials. 2005;26:300–310. doi: 10.1016/j.cct.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Lairson DR, Begley CE, Reynolds, Wilhelmus KR. Prevention of herpes simplex virus eye disease. A cost-effectiveness analysis. Arch Ophthalmol. 2003;121:108–12. doi: 10.1001/archopht.121.1.108. [DOI] [PubMed] [Google Scholar]