Abstract
We have previously reported that local tumor irradiation, without inducing cell death, can augment the therapeutic efficacy of intratumoral (i.t.) dendritic cell (DC) vaccination. This study examined potential mechanisms underlying radiation enhancement of i.t. DC therapy in this setting. Even though ionizing radiation did not mediate tumor cell kill, bone marrow-derived DCs acquired in vitro tumor antigens from irradiated D5 murine melanoma cells more efficiently than from untreated cells. This radiation-enhanced loading of DCs did not induce DC maturation, but was associated with improved cross-priming of T cells both in vitro and in vivo. Furthermore, in vivo pulsing of DCs with irradiated versus untreated tumor cells resulted in superior presentation of tumor antigens to T cells. In addition, tumor irradiation facilitated homing of i.t. administered DCs to the draining lymph node, possibly by down-regulating CCL21 expression within the tumor mass. Studies of the tumor microenvironment in irradiated versus untreated tumors did not reveal significant inflammatory changes. Moreover, radiation did not promote accumulation of CD4+ or CD8+ effector T cells within solid tumors. Our results indicate that, without inducing cytotoxicity, tumor irradiation can enhance the ability of DCs to capture tumor antigens, migrate to the draining lymph node, and present processed antigens to T cells. These findings may prove useful in designing future strategies for human cancer immunotherapy.
Keywords: Cancer vaccines, Cellular immunotherapy, Dendritic cells, Radiation, Tumor immunology
INTRODUCTION
In recent years, much attention has focused on employing DCs for induction of anti-tumor immune responses (1). Direct injection of unpulsed DCs into solid tumors constitutes a particularly attractive approach for immunotherapy of cancer. While most strategies for DC tumor vaccines require in vitro priming of DCs with tumor antigens provided in various forms, this approach is based on in vivo – in situ pulsing of DCs, and utilizes the readily available host’s tumor mass as the source of tumor antigens. Additional potential advantages of this approach include ensuring delivery of functional DCs into the desired site for antigen uptake, and eliciting immunity against multiple relevant host-specific tumor antigens without MHC allele restrictions. I.t. administration of unpulsed-DCs has been shown to mediate tumor regression in animal models (2, 3), as well as in patients with metastatic melanoma and breast carcinoma (4). However, the anti-tumor efficacy is limited and some tumors are resistant to this mode of therapy (2, 3).
We and others have reported that local tumor irradiation enhances the therapeutic efficacy of i.t. DC administration in various murine tumor models (3, 5, 6). Furthermore, even tumors that are resistant to DC immunization alone can respond to combined treatment with DCs plus radiation. The combined treatment has been shown to elicit a potent local and systemic anti-tumor response. A recent phase I clinical trial conducted in patients with advanced hepatoma demonstrated that combination of radiotherapy and i.t. DC injection is feasible, safe, and capable of inducing tumor-specific immune responses as well as clinical responses (7).
However, the mechanisms involved in radiation enhancement of i.t. DC therapy have not yet been fully defined. Eliciting an anti-tumor immune response via this approach mandates that exogenously administered DCs capture antigens from surrounding tumor cells, and then migrate to the draining lymph node to present processed antigens to T cells (8). Moreover, in order to mediate tumor regression, effector T cells elicited by DC immunization, must traffic to the tumor site and infiltrate the solid mass (9). In this study, we examined potential pathways by which exposure of tumor cells to radiation might potentiate DC or DC-elicited effector T cell function.
Radiation is known to induce cell death in various tumors (10). Since DCs are capable of acquiring antigens from apoptotic and necrotic cells and eliciting an effective immune response (11, 12), radiation enhancement of i.t. DC therapy has been attributed mostly to its cytotoxic effect (5, 6, 13). However, in the tumor models we had utilized to demonstrate that radiotherapy augments i.t. DC administration, radiation modestly suppressed tumor growth by inhibiting cell proliferation rather than inducing cell death (3). Therefore, in this study we tested whether radiation could modulate the immunogenicity of live tumor cells. As some solid human malignancies fail to undergo significant apoptosis or necrosis in response to radiotherapy, elucidation of this matter might have considerable clinical implications.
Exposure of normal tissues to radiation has been reported to trigger inflammatory responses (14). Induction of a proinflammatory microenvironment within solid tumors has the potential to augment DCs function by providing maturation stimuli thought to be important for generating cross-priming rather than cross-tolerance (15), and has the potential to enhance recruitment of effector T cells into the tumor mass (16, 17). Therefore, we investigated whether characteristics of an inflammatory microenvironment could be detected within irradiated tumors.
MATERIALS AND METHODS
Mice
Female C57BL/6 mice with a CD45.2 or a CD45.1 phenotype were purchased from Harlan Laboratories (Indianapolis, IN) or the Jackson Laboratory (Bar Harbor, ME), respectively. Experiments were conducted using CD45.2 mice unless otherwise specified. Female homozygous OT-1 T-cell receptor (TCR) transgenic mice on a C57BL/6 CD45.2 background were a generous gift from Dr. Lloyd Stoolman (University of Michigan, Ann Arbor, MI). These mice express a TCR that recognizes the dominant H-2Kb-restricted ovalbumin (OVA) epitope (SIINFEKL). All mice were housed in specific pathogen-free conditions at the Animal Maintenance Facility of the University of Michigan Medical Center and used for experiments at 8 weeks of age or older. The University of Michigan Committee on Use and Care of Animals reviewed and approved all animal protocols.
Tumors
D5 melanoma is a poorly immunogenic subclone of the B16-BL6 tumor of spontaneous origin in the C57BL/6 strain (18). D5-G6 is a D5 clone, transduced to express murine granulocyte macrophage colony-stimulating factor (GM-CSF) (18). B16-OVA is a B16-F10 melanoma cell line transduced to express ovalbumin (OVA). This line was obtained from Dr. Kenneth Rock (University of Massachusetts, Amherst, MA). EL-4 is a T-cell thymoma syngeneic to C57BL/6 mice. Tumor cells were maintained by in vitro culture in complete medium (CM) (3). Excluding EL-4, cells were harvested using trypsin-Versene Mixture (BioWhittaker, Walkersville, MD).
Ionizing Radiation
In vitro radiation was administered in a single dose of 60 Gy using the Gammacell 3000 Elan MDS Nordion. For in vivo radiation, mice were placed in plastic constrainers to ensure immobilization. Local tumor irradiation was delivered in 5 consecutive daily fractions of 8.5 Gy using the PANTAK Therapax DXT 300 Model X-Ray Unit (Cast Haven, CT). S.c. tumors were irradiated on days 7 to 11 after tumor cell inoculation.
Detection of Apoptotic and Necrotic Cells
Immediately and 18 hours after exposure of D5 cells to radiation, tumor cell suspensions were analyzed using the TACS annexin V-FITC apoptosis detection kit (R&D Systems, Inc., Minneapolis, MN) according to the manufacturer’s protocol.
Generation of Bone Marrow-Derived DCs and in Vitro Antigen Pulsing
DCs and D5 tumor lysate were prepared as previously described (3). DC preparations contained less than 1% CD19+ or CD14+ cells by flow cytometry analysis. For in vitro antigen pulsing, DCs were suspended in CM supplemented with 10 ng/ml GM-CSF and 10 ng/ml interleukin (IL)-4 (both from Pepro Tech, Inc., Rocky Hill, NJ) at 1×106 cells/ml, and cultured for 18 h with either irradiated D5, untreated D5, or D5 tumor lysate at a ratio of 3 tumor cells:1 DC. DCs pulsed with irradiated tumor cells and DCs pulsed with non-irradiated tumor cells were harvested by collecting non-adherent cells. Unpulsed-DCs and tumor lysate pulsed-DCs were harvested using a cell scraper.
5-(and 6-) Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE) Staining
CFSE staining of cells was performed as previously described (3). A concentration of 1 μM was used for flow cytometry analysis, and 5 μM was used for immunocytochemical assessment.
Flow Cytometric Analysis
All monoclonal antibodies (mAbs) were purchased from BD Pharmingen (San Diego, CA). Fc receptors were blocked by incubating cells for 10 minutes at 4°C with purified anti-CD16/CD32 mAb. Cells were stained for 30 minutes at 4°C with the fluorochrome-conjugated mAbs of interest, and with matched isotype control mAbs recommended by the manufacturer in 50 μl PBS supplemented with 5% fetal bovine serum (BioWhittaker). The cells were then washed twice, and fixed in 2%-paraformaldehyde (Sigma, St. Louis, MO) in PBS. Analysis of stained cells was performed using a FACSCalibur cytometer with CellQuest software obtained from Becton Dickinson (Mountain View, CA).
In some experiments, immediately before analysis on the FACSCalibur, a known quantity of 15 μm polystyrene microbeads (Bangs Laboratories, Fishers, IN) was added to each sample. The absolute number of cells of interest in each sample was calculated using the following formula: (number of cells of interest analyzed in sample) × (number of beads added to sample)/(number of beads analyzed in sample). For each sample, 5×105 or 1×106 events were acquired and analyzed. Data acquired from samples stained with isotype control mAbs were subtracted from the data obtained from corresponding samples stained with the mAbs of interest. Results were normalized for tumor weight.
Analysis of DC Antigen Loading
Irradiated and untreated D5 cells were labeled with CFSE, and used for in vitro DC pulsing. Then, pulsed-DCs were harvested, washed, and stained using R-phycoerythrin (R-PE)-conjugated anti-I-Ab mAb.
In Vivo Tumor Models
To induce s.c. tumors, mice were inoculated s.c. in the right flank with 2×106 tumor cells/0.1 ml PBS. Bilateral s.c. tumors were established by inoculating mice in bilateral flanks with 1×106 tumor cells/0.1 ml PBS. To induce experimental lung metastases, mice were inoculated i.v. via the lateral tail vein with 1×105 tumor cells/0.5 ml PBS. Tumor draining lymph nodes (TDLNs) were induced by establishing bilateral s.c. tumors. Inguinal lymph nodes were harvested after 9 days, mechanically disrupted using the blunt end of a 5-ml syringe in an etched Petri dish, and filtered through a 70 μm cell strainer to yield a single cell suspension.
Measurement of IFNγ Secretion of D5-G6 TDLN Cells
IFNγ ELISPOT assays were performed as previously described (3). D5-G6 TDLN cells (105 in 200 μl CM) were placed into pre-coated wells, and cultured for 24 hours in the absence or presence of various ratios of DCs pulsed with irradiated or non-irradiated tumor cells.
For cytokine release assays, D5-G6 TDLN cells (2×106) were cultured at various ratios with DCs pulsed with irradiated or non-irradiated tumor cells in 24-well culture plates (2 ml/well). After 48 hours, supernatants were collected and quantified for IFNγ content using an ELISA set (BD Biosciences, San Diego, CA).
Assessment of In Vivo Responses to Immunization with DCs pulsed with irradiated tumor cells
D5 pulmonary metastases were induced on day 0. DCs pulsed with irradiated tumor cells (106/0.1 ml PBS) were administered i.d. on days 3, 7, and 11. On day 15, mice were euthanized for collection of spleens and lungs. In IFNγ ELISPOT assays, erythrocyte-depleted splenocytes (5×104 in 100 μl CM) were placed into pre-coated wells and cultured for 24 hours in the absence or presence of irradiated D5 or EL-4 tumor cells (2.5×104 in 100 μl CM). Lungs were insufflated and fixed in Fekette’s solution. Pulmonary metastases were enumerated in a blinded, coded fashion under a magnifying glass.
Measurement of Cytokine Release of D5 TDLN Cells
S.c. D5 tumors were established and irradiated. Unpulsed-DCs (1×106/0.05 ml PBS) were administered i.t. on day 11, after the last dose of radiation was delivered. After 48 hours, TDLNs from treated and control groups of mice were pooled together (5 mice per group), and homogenized to single cell suspensions. In 24-well culture plates (2 ml CM/well), TDLN cells (1×106) were co-cultured with irradiated D5 tumor cells (1×105), or activated with 1μg/ml immobilized anti-CD3 mAb. After 24 hours, supernatants were collected and analyzed for IFNγ, GM-CSF, and IL-2 content using ELISA sets (BD Biosciences).
Immunocytochemical Assessment of DC migration
S.c. D5 tumors were established and irradiated. CFSE-labeled unpulsed-DCs (5×106/0.05 ml PBS) were administered i.t. either on day 12, 14, or 18. Tumors and TDLNs were harvested 24, 48, and 72 hours after each i.t. injection. Harvested tissues were fixed in 10% formalin, embedded in paraffin, and sectioned for staining. Immunocytochemical staining was performed as described by Skitzki et al. (19) using anti-FITC, horseradish peroxidase-conjugated Fab′ fragments (DakoUSA, Carpinteria, CA) or an isotype matched anti-digoxin horseradish peroxidase conjugate (DakoUSA). Tumor and lymph node sections were developed using vector VIP (Vector Laboratories Inc., Burlingame, CA) or a diaminobenzidine reagent (Sigma), respectively, according to the manufacturer’s protocol. Sections were counterstained with methyl green or hematoxylin, respectively, and mounted in permount.
Quantitative real time RT-PCR
Irradiated and untreated s.c. D5 tumors, the tumor beds comprised of the connective tissue containing blood and lymph vessels that surrounds the tumor, and TDLNs were harvested 4 hours after the last dose of radiation. RNA was extracted using TRIzol (Invitrogen Life Technologies, Carlsbad, CA), and reverse transcribed with oligo (dT) and SuperScript II (Invitrogen). PCR reactions (Cepheid Smart Cycler, Sunnyvale, CA) were conducted in quadruplicates for each individual sample using 250 nM primers, 4 mM MgCl2, and 1/20,000 final dilution of Sybr Green I. PCR amplifications were performed using: 1 cycle of 95°C for 4 minutes, 40 cycles of 95°C for 15 seconds, t58°C for 15 seconds, and 72°C for 30 seconds. Hypoxanthine-guanine phosphoribosyl transferase (HPGRT) was used as an endogenous reference. Primers for CCL21 (20) (5′-AGACTCAGGAGCCCAAAGCA-3′ forward and 5′-GTTGAAGCAGGGCAAGGGT-3′ reverse) and for HPGRT (5′-CCTAAGATGAGCGCAAGTTGAA-3′ forward and 5′-CCACAGGACTAGAACACCTGCTAA-3′ reverse) were synthesized by Invitrogen. PCR products were resolved on 1% agarose gels. Data were analyzed using the 2−ΔΔCT method (21).
Characterization of Immune Infiltrates and Cytokines within Irradiated D5 Tumors
Bilateral s.c. D5 tumors were established. Right tumors only were locally irradiated. Tumors were harvested 1, 8, and 15 days after delivery of the last dose of radiation. At least 4 mice were evaluated at each time point. Tumors were processed as previously described (19). Briefly, individual whole tumors were minced with scissors and placed into a 35-μm medicon (DakoUSA) with 1 ml of PBS, and processed twice for 45 seconds in a Medimachine (DakoUSA) to create single cell suspensions. Samples were filtered through a 70 μm cell strainer. Cells were stained using PE-Cy5-conjugated anti-CD45 mAb and R-PE-conjugated anti-CD4, -CD8, -NK1.1, -CD14, -CD11c or –Ly6G mAbs.
To quantify levels of extra-cellular cytokines, tumors were homogenized in PBS containing protease inhibitors (Sigma). Bilateral tumors from 6 mice were analyzed individually. The amount of cytokines in the collected supernatants was determined using a cytometric bead array (CBA) mouse inflammation kit (BD Biosciences) on a FACSCaliber cytometer equipped with CBA software according to the manufacture’s instructions. Results were normalized for tumor weight and volume of supernatant.
Trafficking of Effector Cells in the D5 Tumor Model
Effector cells were generated as previously described (3). Briefly, s.c. D5 tumors were induced in CD45.1 mice. Unpulsed-DCs (1×106) were administered i.t. on days 6, 11, 14, and 18, and tumors were locally irradiated on days 7 to 11. Erythrocyte-depleted splenocytes, harvested on day 21 from treated mice, were pooled and used for adoptive transfer.
Bilateral s.c. D5 tumors were established in CD45.2 mice. After 14 days, CD45.1 effector cells (2×107) were infused i.v. Tumors were harvested from 5 mice 48 hours after adoptive transfer. In additional mice, right tumors only were irradiated prior to adoptive transfer. Tumors were harvested from 5 mice at 24, 48, and 72 hours after adoptive transfer. Tumor-derived single cell suspensions were stained using biotin-conjugated anti-CD45.1 mAb followed by streptavidin-PE-Cy5 and R-PE-conjugated anti-CD4, -CD8 or -NK1.1 mAbs.
Trafficking of Effector Cells in the B16-OVA Tumor Model
B16-OVA TDLNs were induced in OT-1 CD45.2 mice. TDLN and spleen cells (1×106/ml CM) were activated with immobilized anti-CD3 mAb for 2 days in 24-well plates (2 ml/well), and then expanded in CM supplemented with 60 IU/ml IL-2 (Chiron Therapeutics, Emeryville, CA) for 3 days. Activated and expanded cells were labeled with CFSE and used for adoptive transfer. Ninety eight percent of the cells were CD8+ by FACS analysis (data not shown).
Bilateral s.c. B16-OVA tumors were established in C57BL/6 CD45.1 mice. Right tumors only were irradiated. On day 11, after the last dose of radiation was administered, effector cells (1×107) were injected i.v. Tumors were harvested from at least 5 mice at 24, 48, and 72 hours after adoptive transfer. Samples were stained using biotin-conjugated anti-CD45.2 mAb followed by streptavidin-PE-Cy5, and R-PE-conjugated anti-CD8 mAb. To determine the proliferation state of adoptively transferred cells within tumors, the geometrical mean of CFSE intensity of CD45.2+CD8+ was recorded. In addition, at each time point evaluated, bilateral tumors harvested from one mouse were used for immunocytochemical assessment of infused CFSE-labeled cells.
Statistical Analysis
Data were evaluated by paired or unpaired t test (2 cohorts) and one-way analysis of variance (ANOVA) followed by Bonferroni test for multiple comparisons (>2 cohorts). P values < 0.05 were considered statistically significant.
RESULTS
D5 irradiation promotes in vitro antigen uptake by DCs without inducing cell death
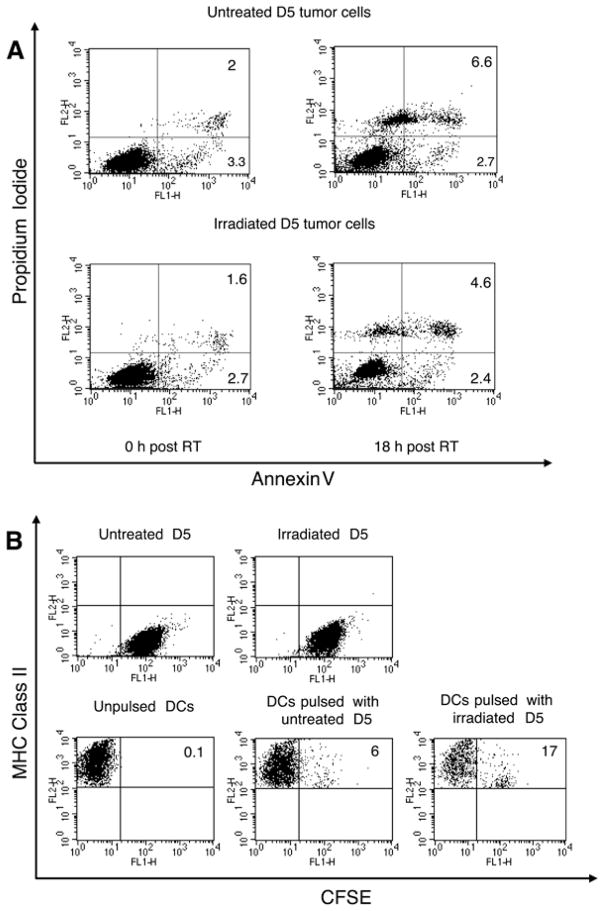
Ionizing radiation (either a single dose of 10 Gy delivered to cells in culture, or 5 consecutive daily fractions of 8.5 Gy delivered to s.c. tumors) does not induce significant apoptosis or necrosis of D5 cells (3). Nevertheless, we hypothesized that DCs can acquire antigens from irradiated cells more efficiently than from untreated cells. To test this hypothesis, D5 cells were subjected to a single radiation dose of 60 Gy or left untreated. Immediately after irradiation, live cells within each population were labeled with CFSE, and then co-cultured with DCs for 18 hours. After DC pulsing, non-adherent cells were stained with R-PE-conjugated anti-I-Ab mAb and analyzed by flow cytometry. In addition, immediately and 18 hours after irradiation, samples of irradiated and untreated D5 cells were stained with annexin V-fluorescein and propidium iodide and analyzed for viability. In this context, viability refers to the capacity to maintain cell membrane integrity; it does not imply a capacity to proliferate. Immediately after irradiation, 4.3% of irradiated cells and 5.3% of untreated cells were non-viable (Figure 1A). After 18 hours of culture, irradiated and untreated cells contained 7% and 9.3% non-viable cells, respectively. Therefore, in both cell populations, 3–4% of cells died during culture. In contrast, exposure of D5 cells to UVB light (200Mj/cm2), followed by 4-hour culture, induced 85% cell death (data not shown, reference 3). This data confirms that throughout the co-culture period radiation did not mediate tumor cell kill. Both untreated and irradiated D5 cells did not express MHC class II molecules (Figure 1B, upper panels). Whereas only 6% of DCs pulsed with untreated tumor cells stained double positive, 17% of DCs acquired CFSE-labeled proteins from irradiated tumor cells. As the level of MHC class II expression on DCs pulsed with irradiated tumor cells was not down-regulated compared with unpulsed-DCs, acquisition of antigen was probably mediated by the most immature DCs within the preparation, those expressing a relatively low-level of MHC class II. This experiment was repeated 5 times with consistent and statistically significant results (p<0.01). The source of CFSE-labeled proteins captured by DCs may have been non-viable tumor cells, as 3–4% of cells did die during the co-culture period. However, labeled antigen could have also been derived from viable tumor cells since DCs possess a unique capacity to uptake antigen from live cells (22). Either way, exposure of tumor cells to radiation enhanced in vitro loading of DCs with tumor antigens despite the lack of cytotoxicity induction.
FIGURE 1.
A, Radiation does not induce apoptosis or necrosis in D5 cell cultures. Immediately and 18 hours after a single radiation dose of 60 Gy, D5 cells were analyzed for apoptotic and necrotic cells using the Annexin V-FITC/PI FACS assay. Untreated cells served as a negative control. The lower left quadrant represents viable cells, the lower right quadrant depicts cells in early apoptosis, and the upper right quadrant shows necrotic and late apoptotic cells. B, DCs acquire antigens from irradiated D5 cells more efficiently than from untreated tumor cells. DCs were cultured with CFSE-stained irradiated versus untreated D5 cells for 18 hours. After pulsing, cells were labeled with R-PE-conjugated anti-MHC class II mAb and analyzed by flow cytometry. Bottom dot plots were gated on FL2 positive cells. Some of the control groups, namely CFSE-labeled irradiated and untreated D5 cells, and unpulsed-DCs are shown. The numbers inside the dot plots shown in A and B represent the percentage of cells within the corresponding quadrant. Experiments were repeated at least three times with similar results.
The maturation status of DCs after co-culture with irradiated versus untreated D5 cells was examined by analyzing expression of co-stimulatory cell surface markers using flow cytometry. While culturing DCs in the presence of LPS (500 ng/ml) up-regulated MHC class II, CD80, CD86, and CD40, exposure of DCs to irradiated tumor cells had no detectable effect on the expression of these markers (data not shown). The level of expression remained similar to that seen on unpulsed-DCs and DCs pulsed with non-irradiated tumor cells. Furthermore, ELISA assays performed on supernatants of the above mentioned DC cultures detected IL-12p70 only in the presence of LPS (data not shown).
Radiation-enhanced DC loading is associated with improved antigen presentation to T cells both in vitro and in vivo
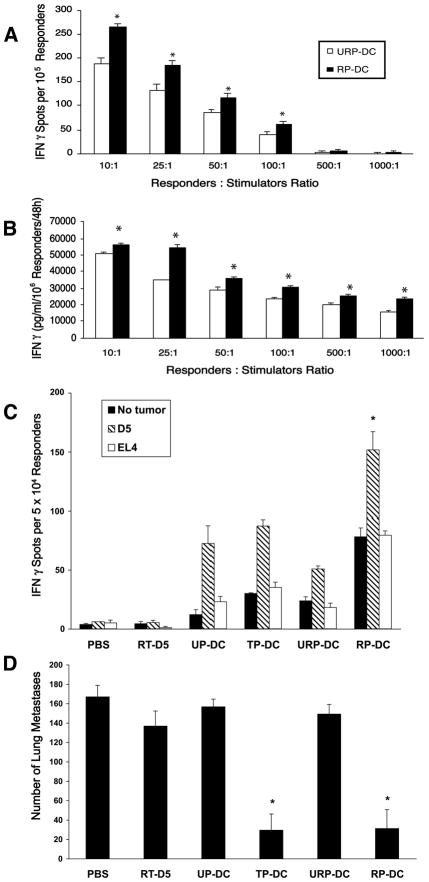
Next we evaluated whether radiation-enhanced loading of DCs correlates with superior antigen presentation in vitro. To this end, we measured IFNγ secretion of D5-G6 TDLN cells in response to stimulation with DCs pulsed with irradiated versus non-irradiated tumor cells. Since D5 is a poorly immunogenic tumor, ex vivo activated D5 TDLN cells do not secrete IFNγ in response to D5 stimulation, and can not mediate tumor regression upon adoptive transfer to D5 tumor bearing hosts (18). Ex vivo activated D5-G6 (D5 tumor cells that express GM-CSF) TDLN cells, however, have been shown to produce in vitro D5 tumor-specific IFNγ, that correlates with in vivo anti-D5 tumor reactivity. An ELISPOT assay (Figure 2A) demonstrated that stimulation with DCs pulsed with irradiated tumor cells yielded a significantly higher incidence of IFNγ secreting D5-G6 TDLN cells compared with stimulation with DCs pulsed with non-irradiated tumor cells (p<0.05). Moreover, a cytokine release assay (Figure 2B) showed that D5-G6 TDLN cells released higher levels of IFNγ upon stimulation with DCs pulsed with irradiated versus non-irradiated tumor cells (p<0.05).
FIGURE 2.
Tumor irradiation improves T cell cross-priming in vitro and in vivo. A and B, D5-G6 TDLN cells were stimulated in vitro by irradiated versus non-irradiated tumor cells pulsed-DCs (RP-DC versus URP-DC, respectively) at various ratios. The incidence of IFN-γ-secreting cells (A) and the amount of IFN-γ released to the supernatant (B) were determined via ELISPOT and ELISA assays, respectively. In control samples containing D5-G6 TDLN cells alone, RP-DCs alone, URP-DCs alone, and D5-G6 TDLN cells co-cultured with unpulsed-DCs no IFNγ spots or IFNγ secretion were detected. (A), Data are reported as the average number of spots per 1×105 responders ± SE of triplicate samples. (B), Data are reported as the average concentration of IFNγ (pg/ml) per 1×106 responders per 48 h ± SE of triplicate samples. *, P<0.05. C and D, DCs pulsed with irradiated tumor cells (RP-DCs) are more effective than DCs pulsed with non-irradiated tumor cells (URP-DCs) in eliciting tumor-specific IFN-γ secretion of splenocytes (C) and mediating lung metastases regression (D). Mice were inoculated i.v. with D5 tumor cells on day 0. RP-DCs were administered i.d. on days 3, 7, and 11. Control groups received either no treatment (PBS), irradiated D5 cells (RT-D5), unpulsed-DCs (UP-DCs), tumor lysate pulsed-DCs (TP-DCs) or DCs pulsed with non-irradiated tumor cells (URP-DCs). Spleens and lungs were harvested 15 days after tumor inoculation. C, Splenocytes from treated and control mice were cultured with or without specific or irrelevant irradiated tumor cells in an IFN-γ ELISPOT assay. Data are reported as the average number of spots per 5 × 104 responders ± SE of triplicate samples. *, P<0.01 versus all other groups. D, Pulmonary metastases were enumerated. Data are reported as the mean number of metastases ± SE of six mice per group. * P<0.001 versus PBS, RT-D5, UP-DC and URP-DC. Experiments were repeated at least two times with similar results.
To examine whether radiation-enhanced loading of DCs is also associated with improved antigen presentation in vivo, mice bearing D5 pulmonary metastases were immunized i.d. with DCs pulsed with irradiated versus non-irradiated tumor cells. Induction of anti-tumor responses was monitored by IFN-γ ELISPOT assays of splenocytes (Figure 2C) and by enumerating lung metastases (Figure 2D). Immunization with DCs pulsed with irradiated tumor cells induced significantly more IFN-γ secreting cells compared with control groups (p<0.01 versus all other groups). This response was tumor-specific as a reduced incidence of spots was detected spontaneously or in response to EL-4 cells, a non-relevant tumor syngeneic to C57BL/6 mice. After subtracting the background from the incidence of D5-specific IFNγ-secreting cells, the difference between DCs pulsed with irradiated versus non-irradiated tumor cells remained statistically significant (p<0.004). Immunization with irradiated tumor cells pulsed-DCs mediated significant regression of lung tumors (p<0.001 versus all other groups except tumor lysate pulsed-DCs). The anti-tumor efficacy of vaccination with DCs pulsed with irradiated tumor cells was comparable to that obtained utilizing our positive control, D5 tumor lysate pulsed-DCs. In contrast, vaccination with DCs pulsed with non-irradiated tumor cells failed to induce a significant response.
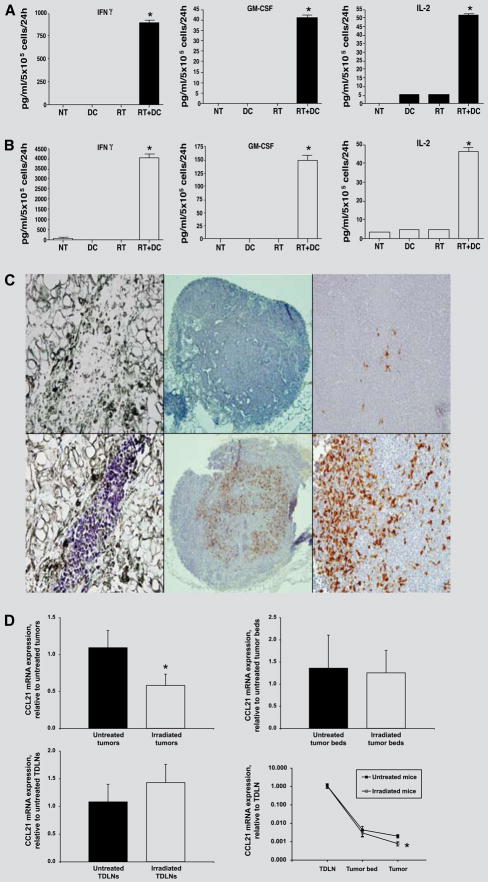
To explore whether radiation-enhanced DC loading and antigen presentation might occur during DC pulsing in vivo, unpulsed-DCs were administered i.t. to irradiated D5 tumors. Two days later, TDLN cells were analyzed for cytokine secretion in response to D5 tumor cell stimulation (Figure 3A). Lymph node cells draining untreated tumors; non-irradiated tumors injected with DCs; and, irradiated tumors secreted low or undetectable amounts of IFNγ, GM-CSF, and IL-2. On the other hand, lymph node cells draining irradiated tumors that had been injected with DCs produced significantly higher levels of these cytokines (p<0.001 versus all other groups). Furthermore, ex vivo activation of D5 TDLN cells with anti-CD3 mAb resulted in significant release of cytokines only when tumors were treated with radiation combined with i.t. DC administration (Figure 3B, p<0.001 versus all other groups). Collectively, these findings suggest that tumor irradiation augments T cell cross-priming by exogenous DCs.
FIGURE 3.
A and B, Lymph node cells draining D5 tumors subjected to combined treatment with radiation plus DCs, but not to monotherapies, undergo effective in vivo priming to tumor antigens. Unpulsed-DCs were injected into irradiated D5 tumors. Control groups of mice received either no treatment (NT), DC only, or radiation only (RT). After 2 days, TDLN cells were harvested and analyzed for IFN-γ, GM-CSF, and IL-2 secretion in response to either D5 cells (A) or anti-CD3 mAb activation (B). Data are reported as the average concentration of cytokine (pg/ml) per 5 × 105 responders per 24 h ± SE of triplicate samples. *, P<0.001 versus all other groups. C, Tumor irradiation enhances DC migration to the draining lymph node. CFSE-labeled unpulsed-DCs were injected into irradiated versus untreated s.c. D5 tumors either 1, 3, or 7 days after radiation. Tumors and TDLNs were harvested 24, 48, and 72 hours after each i.t. injection. Fluorescein-labeled cells were detected using an anti-FITC, horseradish peroxidase-conjugated antibody. Positive cells stained purple in tumor sections, and brown in lymph node sections. Representative fields from two independent experiments are shown [original magnification, X400 (left and right) or X100 (middle)]. Upper left, Tumor section stained with an isotype matched control antibody. Lower left, Tumor section stained with anti-FITC antibody. Upper middle and right, Section of a lymph node draining an untreated tumor. Lower middle and right, Section of a lymph node draining an irradiated tumor. D Tumor irradiation decreases CCL21 gene expression within the tumor mass by one-half compared with untreated tumors (upper left). But CCL21 gene expression within the tumor beds (upper right), and the tumor draining lymph nodes (lower left) does not change significantly between irradiated and untreated tumors. As a result, CCL21 concentration gradient between the tumor and the draining lymph node is increased (lower right). Expression of CCL21 mRNA within irradiated and untreated s.c. D5 tumors, tumor beds, and TDLNs was measured by quantitative real time RT-PCR. CCL21 mRNA expression was first normalized to the expression of HPGRT, and was then normalized to the average expression of CCL21 mRNA in untreated tumors (upper left), in untreated tumor beds (upper left), in untreated TDLNs (lower left) or in corresponding TDLNs (lower right). RNA was extracted from five tumors, three tumor beds, and three TDLNs per group, and gene expression was examined in individual samples. * p < 0.05. In upper right and lower left panels p is non-significant.
Tumor irradiation promotes DC migration to the draining lymph node
We explored whether tumor irradiation facilitates DC homing to the draining lymph node. CFSE-labeled unpulsed-DCs were injected into irradiated versus untreated s.c. D5 tumors either on day 1, 3, or 7 after the last dose of radiation. Using immunocytochemical staining for FITC, exogenously administered DCs could be visualized within both irradiated and untreated tumors for at least 72 hours after i.t. injection (Fig. 3C). The staining was specific for fluorescein-labeled cells, as sections stained with an isotype matched control antibody showed no reactivity. At 24, 48, and 72 hours after each i.t. DC administration and in two independent experiments, most lymph nodes (11/14, 79%) draining untreated tumors contained few CFSE-labeled DCs. In contrast, the majority of lymph nodes (12/14, 86%) draining irradiated tumors contained a vast number of positive cells. These findings suggest that DCs injected into irradiated tumors traffic to the draining lymph node more efficiently than DCs injected into untreated tumors.
We investigated whether the increased DC migration observed after tumor irradiation could be related to modulation of the CCR7-CCL21 migratory pathway (23). CCR7 is a chemokine receptor that drives DC migration to the lymphatics. A concentration gradient of CCL21, one of CCR7 ligands expressed by endothelial lymphatic and stromal cells, regulates DC trafficking to the draining lymph node. In vitro pulsing of DCs with irradiated versus untreated D5 cells did not up-regulate CCR7 expression on the cell surface (data not shown). However, quantitative real time RT-PCR analysis showed that tumor irradiation reduced CCL21 mRNA expression within the tumor mass by 50% compared with untreated tumors (Fig. 3D upper left panel, p<0.05). Importantly, CCL21 gene expression within the tumor beds and the TDLNs did not differ significantly between irradiated and untreated tumors (Fig. 3D upper right panel, p>0.9; and lower left panel, p>0.4)). These results suggest that an increase in CCL21 concentration gradient between the tumor and the draining lymph node (Fig. 3D lower right panel) might be one mechanism by which tumor irradiation facilitates exit of DCs from the tumor mass.
Irradiated D5 tumors do not constitute a pro-inflammatory microenvironment
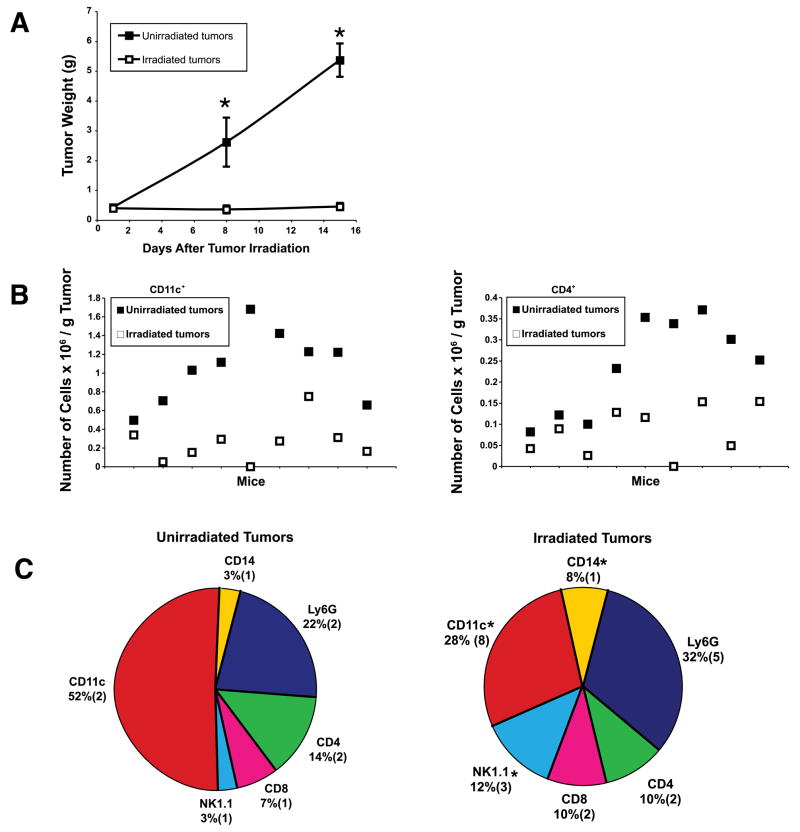
Normal tissues and certain solid tumors, on exposure to ionizing radiation, have been reported to become infiltrated with white blood cells (14, 16, 17). Therefore, using flow cytometry, we characterized the immune infiltrate within irradiated versus untreated s.c. D5 tumors. Analysis was performed 1, 8, and 15 days after tumor irradiation. D5 growth was significantly inhibited in irradiated versus untreated tumors (Figure 4A). Nevertheless, one day after irradiation, the absolute number of CD11c+ and CD4+ cells per gram of tumor was significantly higher in untreated versus irradiated tumors (Figure 4B, CD11c+ p=0.0006, CD4+ p=0.0027). Even though the absolute number of NK1.1+ and CD14+ cells (macrophages) did not increase in irradiated tumors, their relative percentage within the immune infiltrate did increase significantly (Figure 4C, p<0.03). No other significant and consistent differences in the content of tumor infiltrating cells (CD11c+, CD4+, CD8+, NK1.1+, CD14+ and Ly6G+) were detected at the time points evaluated (data not shown).
FIGURE 4.
Characterization of the immune infiltrate within irradiated versus untreated s.c. D5 tumors. A, Mice were inoculated s.c. in bilateral flanks with D5 tumor cells on day 0. Right flank tumors only were locally irradiated on days 7 to 11. Tumors were harvested and weighed on days 12, 19, and 26. Data are reported as the mean tumor weight in grams ± SE of five mice per group. This experiment was repeated two times with similar results. *, P<0.04. B, Twelve day tumors described in A were mechanically disaggregated to single cell suspensions, stained for CD45 and CD11c (left) or CD4 (right) and analyzed by flow cytometry. The absolute number of cells of interest × 106 per gram tumor for each individual tumor was calculated using polystyrene microbeads. Bilateral tumors harvested from the same mouse are represented by the same X axis value. Data represents cumulative results of two experiments. P=0.0006 (left), P=0.0027 (right). C, Tumors described in B were stained for CD45 and CD11c, CD4, CD8, NK1.1, CD14 or Ly6G and analyzed by flow cytometry. Data are reported as the mean percentage (SE) of nine tumors from two independent experiments. *, P<0.03.
We also assessed the levels of some inflammatory cytokines within the extra-cellular microenvironment of irradiated versus untreated D5 tumors. The amount of IL-12p70, TNFα, IFNγ, monocyte chemoattractant protein-1 (MCP-1), IL-6, and IL-10 in the supernatants of tumor-derived single cell suspensions was quantified using a CBA kit. No statistically significant differences between irradiated versus untreated D5 tumors were detected (data not shown).
Based on these results, we conclude that during the first 2 weeks after radiation, which represents the relevant time frame for i.t. DC injection in our tumor model, irradiated D5 tumors do not appear to constitute a pro-inflammatory microenvironment.
Radiation does not promote accumulation of effector T cells within tumors
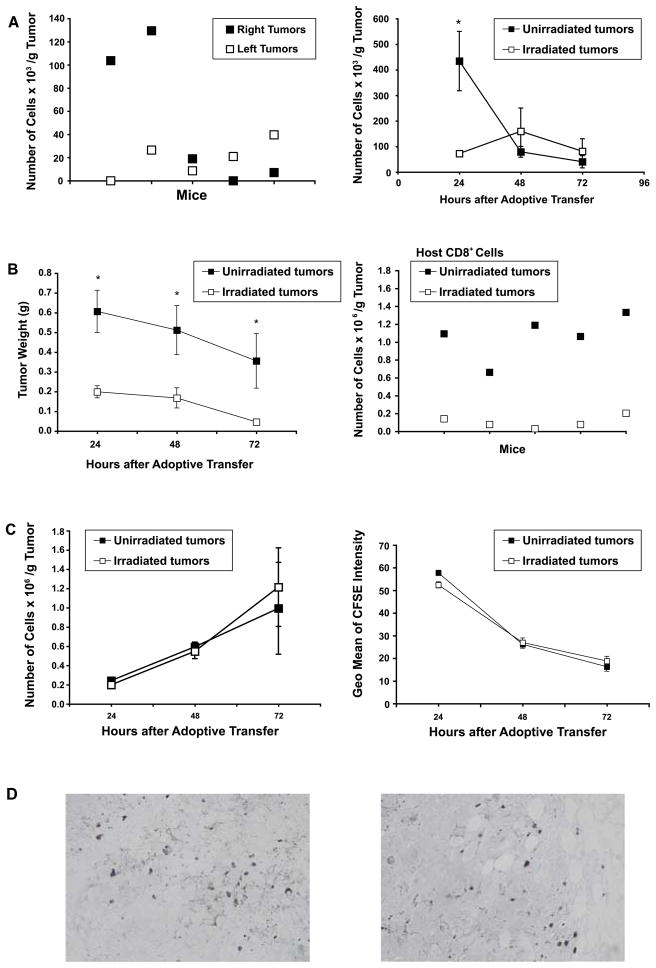
We hypothesized that radiotherapy might facilitate trafficking of effector T cells, elicited by DC immunization, into solid tumors. To test this hypothesis, we adoptively transferred CD45.1 effector cells i.v. to CD45.2 mice bearing bilateral s.c. D5 tumors. Effector cells consisted of splenocytes harvested from s.c. D5 tumor bearing mice treated with local tumor irradiation plus i.t. DC administration. These splenocytes have been shown to mediate tumor destruction in vivo (3). FACS analysis, performed 48 hours after adoptive transfer, demonstrated that CD4+ effector cells localized randomly to right versus left flank tumors (Figure 5A, left panel). This data validated our experimental dual tumor model. In additional mice in the same study, prior to adoptive transfer, tumors on the right flank only were subjected to irradiation. At 24 hours after adoptive transfer, untreated tumors contained significantly more CD4+ effector cells per gram of tumor compared with irradiated tumors (p=0.034). No significant differences were observed at 48 and 72 hours. CD8+ or NK1.1+ effector cells could not be detected within both irradiated and untreated tumors (data not shown).
FIGURE 5.
A (left), Adoptively transferred CD4+ effector cells localize randomly between dual s.c. solid tumors. CD45.2 mice were inoculated s.c. in bilateral flanks with D5 cells on day 0. CD45.1 effector cells (2×107) were injected i.v. on day 14. Tumors were harvested 48 hours after adoptive transfer, mechanically disaggregated to single cell suspensions, stained for CD45.1 and CD4 and analyzed by FACS. Each data point represents an individual tumor, and bilateral tumors harvested from the same mouse are represented by the same X axis value. P=0.3361, not significant. (right), CD4+ effector cells do not localize preferentially to irradiated s.c. tumors. In additional mice in the study detailed above, right tumors only were locally irradiated on days 7 to 11. Tumors were harvested 24, 48, and 72 hours after adoptive transfer and processed as described above. Data are reported as the mean number of cells × 103 per gram of tumor ± SE of five tumors per group. *, P=0.034. B, C, and D, CD8+ effector cells do not localize preferentially to irradiated s.c. tumors. CD45.1 mice were inoculated s.c. in bilateral flanks with B16-OVA cells on day 0. Right tumors only were locally irradiated on days 7 to 11. CFSE-labeled CD45.2 CD8+ effector cells (1×107) derived from OT-1 mice were injected i.v. on day 11, after the last dose of radiation was administered. Twenty four, 48, and 72 hours after adoptive transfer, tumors were harvested, weighed, and mechanically disaggregated to single cell suspensions. Samples were stained for CD45.2 and CD8 and analyzed by FACS. B (left) Tumor irradiation inhibits B16-OVA tumor growth. Data are reported as the mean tumor weight ± SE of at least five tumors per group. *, P<0.05. (right), Twenty four hours after adoptive transfer, non-irradiated tumors contained significantly more host CD8+ cells per gram of tumor compared with irradiated tumors. Each data point represents an individual tumor, and bilateral tumors harvested from the same mouse are represented by the same X axis value. P<0.001. C (left), Data are reported as the mean number of adoptively transferred CD8+ cells × 106 per gram of tumor ± SE of at least five tumors per group. (right), Transferred CD8+ effector cells detected within irradiated versus non-irradiated tumors proliferated to the same extent. The geometrical mean of CFSE intensity of CD45.2+CD8+ effector cells within irradiated and non-irradiated tumors was recorded at 24, 48, and 72 hours after infusion. Data are reported as the mean of the geometrical mean of CFSE intensity ± SE of at least five tumors per group. D, B16-OVA tumors harvested 24, 48 and 72 hours after adoptive transfer were fixed in formalin and embedded in paraffin. Fluorescein-labeled cells were detected using an anti-FITC, horseradish peroxidase-conjugated polyclonal antibody. Representative fields from non-irradiated (left) and irradiated (right) tumors are shown (magnification, X400).
Since tumor regression due to local administration of unpulsed-DCs has been shown to be dependent on CD8+ cells (2, 6), we investigated whether tumor antigen-specific CD8+ cells migrate preferentially to irradiated versus non-irradiated tumors. To this end, we utilized transgenic OT-1 mice as a source of effector cells in conjunction with the B16-OVA tumor model. CFSE-labeled CD45.2 effector cells were transferred i.v. to CD45.1 mice bearing bilateral s.c. B16-OVA tumors. Prior to adoptive transfer, radiation was delivered to right flank tumors only. B16-OVA tumor growth was significantly inhibited in irradiated versus non-irradiated tumors (Figure 5B left panel, p<0.05). Analysis of the host immune infiltrate showed that 24 hours after adoptive transfer non-irradiated B16-OVA tumors contained significantly more endogenous CD8+ cells per gram of tumor compared with irradiated B16-OVA tumors (Figure 5B, right panel, p<0.001). No statistically significant differences were detected at later time points (data not shown). When splenocytes from naïve C57BL/6 CD45.2 mice were transferred i.v., they could infiltrate neither irradiated nor untreated tumors (data not shown). However, when ex vivo anti-CD3 activated and IL-2 expanded tumor antigen-specific CD8+ T cells generated from TDLNs and spleens of B16-OVA sensitized OT-1 mice were infused, they were readily detected within all s.c. tumors. These effector cells have been shown to mediate regression of established s.c. B16-OVA tumors (Figure 5B left panel, and unpublished data). At 24, 48, and 72 hours after adoptive transfer, irradiated and non-irradiated B16-OVA tumors contained comparable numbers of adoptively transferred CD8+ effector cells per g of tumor (Figure 5C, left panel). Immunocytochemical staining for fluorescein labeled cells confirmed these results (Figure 5D). Based on dye dilution, the proliferation state of adoptively transferred effector cells in irradiated versus non-irradiated tumors was similar (Figure 5C, right panel). Collectively, our data suggests that at early time points (<72 hours) local irradiation does not enhance accumulation of effector T cells within the tumor mass.
DISCUSSION
Clinical trials of DC-based tumor vaccines have shown limited therapeutic efficacy to date (1, 24–26). Therefore, exploration of novel strategies aiming to enhance the potency of DC-based immunotherapy of cancer is pertinent. Characterization of factors capable of modulating DC function might serve as a key to designing effective new treatment protocols for human cancer.
Failure of the immune system to mediate tumor regression in cancer patients has been attributed to various factors including the existence of an immunosuppressive microenvironment within solid tumors (27). This immunosuppressive microenvironment is believed to be actively induced by cancer cells. As a result, many tumors contain few endogenous DCs. DCs that do manage to infiltrate the tumor mass have been shown to be dysfunctional due to secretion of vascular endothelial growth factor (VEGF), IL-6, macrophage colony-stimulating factor, prostaglandin E2, IL-10, and transforming growth factor –β (TGFβ) by tumor or tumor infiltrating cells. Moreover, some tumors have been reported to actively recruit plasmacytoid rather than myeloid DCs by releasing CXCL12 (28). Within the tumor mass, plasmacytoid DCs induce regulatory CD8+ cells that suppress tumor antigen-specific T cell effector function (29).
In vitro priming of DCs with tumor antigens mandates either autologous tumor tissue that may be difficult to obtain, or using peptides of defined shared tumor associated antigens (TAAs) which compromises the potential repertoire of the elicited immune response and limits treatment to certain malignancies and specific patients expressing the corresponding MHC allele. While in vivo pulsing of DCs with tumor antigens provided by the host tumor mass alleviates these impediments, it introduces new obstacles that seem to stem from low efficiency of antigen uptake by exogenously administered DCs. Since human tumors are considered to be poorly immunogenic, the use of non-altered host tumor cells as the source of tumor antigens for cross-priming T cells might prove counterproductive.
Combining i.t. DC administration with local tumor irradiation attempts to overcome these obstacles by delivering large numbers of functional myeloid DCs into a tumor mass whose immunogenicity has been enhanced. In tumors that undergo significant apoptosis or necrosis in response to radiotherapy, radiation-enhanced tumor immunogenicity is well documented (5, 6, 13) and readily explained (30). In this report, however, we show that radiation-enhanced tumor immunogenicity can occur without induction of cell death. This could be mediated through augmented uptake of antigens by DCs from irradiated yet viable tumor cells. It is widely assumed that cell death is required before cell associated antigens can be captured and cross-presented by DCs. However, it has been reported that DCs are the only antigen presenting cell also capable of acquiring antigens from live cells via a process termed nibbling (22, 31). Further studies are needed in order to investigate whether tumor irradiation can enhance DC nibbling, and to confirm that tumor irradiation enhances antigen uptake by DCs in vivo. Radiation-enhanced tumor antigen capture and presentation by DCs may also be partially accounted for by reports demonstrating that exposure of tumor cells to radiation up-regulates expression of TAAs (32–34). In addition, radiation might suppress the release of inhibitory factors such as TGFβ and VEGF from tumor cells, thus enabling DCs to function at their full capacity (35, 36). In our tumor model, a significant difference in IL-10 levels between irradiated and untreated tumors could not be detected.
The number of antigen-carrying DCs that reach the draining lymph node has been shown to correlate with the potency of the elicited T cell response (37). Active retention of i.t. administered DCs by cancer cell-derived IL-8 has been reported (38). Hence, strategies capable of facilitating DC homing to the draining lymph node might enhance the therapeutic efficacy of DC vaccines. Transduction of DCs with CCR7 gene has been shown to enhance DC migration from the skin to the draining lymph node (39). Martin-Fontecha et al. (37) have demonstrated that pre-injection of the vaccine site with TNF or IL-1α increases the efficiency of DC migration by up-regulating CCL21 expression within dermal lymphatic vessels. Here we report that DCs injected into irradiated versus untreated tumors home to the TDLN with a striking efficiency independent of an inflammatory microenvironment. Acquisition of antigens from apoptotic tumor cells has been reported to induce CCR7 expression on DCs (40). However, we did not observe up-regulated expression of CCR7 on DCs after co-culture with irradiated D5 cells. This finding is in accordance with the lack of maturation induction observed after pulsing DCs with irradiated tumor cells. Down-regulation of CCL21 gene expression within irradiated versus untreated tumors was identified in this study. This radiation-induced reduction in the expression of CCL21 within the tumor microenvironment might facilitate migration of exogenously i.t. administered DCs to the draining lymph node along an increased CCL21 concentration gradient. Furthermore, this finding might account for the reduction in the number of endogenous DCs, CD4+, and CD8+ cells detected within irradiated versus untreated tumors. The early preferential localization of CD4+ effector cells to non-irradiated versus irradiated tumors might be attributed at least partially to this finding as well. Further studies are needed in order to establish that the down-regulation of CCL21 expression observed within irradiated tumors plays a role in promoting migration of immune cells out of the tumor mass. Use of CCR7−/− mice may shed light on whether CCR7-independent mechanisms are involved in radiation-enhanced immune cell migration. The data presented in this study shows that local tumor irradiation augments cross-priming of TDLN cells by exogenously administered DCs. This finding may be due to enhanced antigen uptake by DCs at the tumor site, facilitated DC migration to the draining lymph node, improved antigen presentation by DCs at the draining lymph node, or most likely a combination of these factors.
In our tumor model, local radiation enhanced i.t. DC therapy without triggering an inflammatory response and without facilitating accumulation of effector T cells within the tumor. However, this might not be the case for all tumors. Ganns et al. (16) reported that administration of radiation (10 Gy) three weeks prior to adoptive transfer of activated tumor-specific T cells increased the accumulation of donor CD4+ and CD8+ T cells within solid pancreatic insulinomas. Besides the difference in tumor type, location, and time point used for evaluation, that study utilized whole body rather than local irradiation. Utilizing the B16-F0/OVA tumor model, Lugade et al. (17) reported that intramuscular induced tumors subjected to local fractionated irradiation (5 Gy × 3) contained significantly more host CD45, CD11c, and CD11b, but not CD4 or CD8 cells, compared with untreated tumors. They also demonstrated, using adoptive transfer studies, that tumor-antigen specific T cells accumulated in increased numbers within single dose irradiated (15 Gy) tumors versus untreated tumors. Gattinoni et al. (41) reported, utilizing the B16-F10 tumor model, that total body irradiation (5 Gy), but not local tumor irradiation, enhanced the anti-tumor activity of adoptively transferred tumor-specific CD8+ cells. The enhanced therapeutic efficacy was shown not to be mediated by direct effect of radiation on tumor growth, or by increased number of adoptively transferred effector cells within the tumor, but rather by enhanced effector function of the CD8+ cells caused by systemic depletion of host cellular immune elements leading to an increased availability of homeostatic cytokines. In our study, even though local radiation did not promote accumulation of effector CD8+ cells within B16-OVA tumors, local radiation did enhance the anti-tumor efficacy of the adoptively transferred cells. Direct effect of a higher dose of ionizing radiation (42.5 Gy) on tumor growth, and increased effector function of adoptively transferred cells within locally irradiated tumors constitute potential explanations for this finding. The later notion is supported by Chakraborty et al. (42) who have shown that exposure of tumor cells to local radiation can up-regulate cell surface expression of Fas and thus render the cells more susceptible to T cell killing. Therefore, tumor irradiation may enhance DC-based immunotherapy of cancer via both afferent and efferent mechanisms.
Damage-associated molecular patterns (DAMPs) are comprised of exogenous pathogen-associated molecular patterns (PAMPs) and endogenous alarmins (43). Alarmins are a group of unique intracellular proteins that are released by dying cells following trauma-mediated tissue damage, and are able to both recruit and activate antigen presenting cells (44). The danger signals conveyed by DAMPs are detected via receptors expressed on host immune cells, and ultimately elicit inflammatory and immune responses. Recently, irradiated dying tumor cells have been shown to secrete the high-mobility-group box 1 (HMGB1) alarmin (45). Binding of HMGB1 to Toll-like receptor 4 (TLR4) expressed on DCs was found to induce efficient processing and cross-presentation of tumor-derived antigens. In addition, extracellular HMGB1 has been reported to act as a chemoattractant and activator of DCs (46), and as a mediator of inflammation (47). In light of these findings, release of DAMPs from irradiated dying tumor cells may play a potential role in radiation enhancement of i.t. DC therapy, and in induction of inflammation within irradiated solid tumors. Ionizing radiation kills target cells primarily by inducing apoptosis. Tumor cells, however, can acquire resistance to apoptosis by the expression of anti-apoptotic proteins, by the down-regulation or mutation of pro-apoptotic proteins, or by alterations of the p53 pathway (48). In our tumor model, as is unfortunately the case in some solid human malignancies, exposure to radiation did not mediate tumor cell kill. Therefore, it is likely that DAMPs release did not occur as well. Lack of DAMPs may provide a potential explanation for the absence of inflammation detected within irradiated tumors in this study.
This report shows that tumor irradiation augments the ability of DCs to capture tumor antigens, home to the draining lymph node, and mediate effective antigen presentation. Surprisingly, this enhanced DC function is independent of radiation-induced cell death and radiation-induced local inflammation. It appears to be mediated, at least partially, by the capacity of ionizing radiation to enhance the immunogenicity of live tumor cells. Currently radiation is widely used as a cytotoxic therapy. Employing this modality for its immunomodulatory properties might expand its future role in the treatment of cancer patients.
Acknowledgments
This work was supported by NIH grant CA59327 and the Gillson Longenbaugh Foundation.
Footnotes
All authors have declared there are no financial conflicts of interest in regards to this work
References
- 1.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17:163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Candido KA, Shimizu K, McLaughlin JC, et al. Local administration of dendritic cells inhibits established breast tumor growth: implications for apoptosis-inducing agents. Cancer Res. 2001;61:228–236. [PubMed] [Google Scholar]
- 3.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63:8466–8475. [PubMed] [Google Scholar]
- 4.Triozzi PL, Khurram R, Aldrich WA, et al. Intratumoral injection of dendritic cells derived in vitro in patients with metastatic cancer. Cancer. 2000;89:2646–2654. doi: 10.1002/1097-0142(20001215)89:12<2646::aid-cncr18>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 5.Kim KW, Kim SH, Shin JG, et al. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int J Cancer. 2004;109:685–690. doi: 10.1002/ijc.20036. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Xia D, Bi X, et al. Combined radiation therapy and dendritic cell vaccine for treating solid tumors with liver micro-metastasis. J Gene Med. 2005;7:506–517. doi: 10.1002/jgm.692. [DOI] [PubMed] [Google Scholar]
- 7.Chi KH, Liu SJ, Li CP, et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005;28:129–135. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 8.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 9.Mukai S, Kjaergaard J, Shu S, et al. Infiltration of tumors by systemically transferred tumor-reactive T lymphocytes is required for antitumor efficacy. Cancer Res. 1999;59:5245–5249. [PubMed] [Google Scholar]
- 10.Jonathan EC, Bernhard EJ, McKenna WG. How does radiation kill cells? Curr Opin Chem Biol. 1999;3:77–83. doi: 10.1016/s1367-5931(99)80014-3. [DOI] [PubMed] [Google Scholar]
- 11.Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 12.Sauter B, Albert ML, Francisco L, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nikitina EY, Gabrilovich DI. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001;94:825–833. doi: 10.1002/1097-0215(20011215)94:6<825::aid-ijc1545>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Quarmby S, Kumar P, Kumar S. Radiation-induced normal tissue injury: role of adhesion molecules in leukocyte-endothelial cell interactions. Int J Cancer. 1999;82:385–395. doi: 10.1002/(sici)1097-0215(19990730)82:3<385::aid-ijc12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 15.Demaria S, Bhardwaj N, McBride WH, et al. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63:655–666. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 17.Lugade AA, Moran JP, Gerber SA, et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 18.Arca MJ, Krauss JC, Aruga A, et al. Therapeutic efficacy of T cells derived from lymph nodes draining a poorly immunogenic tumor transduced to secrete granulocyte-macrophage colony-stimulating factor. Cancer Gene Ther. 1996;3:39–47. [PubMed] [Google Scholar]
- 19.Skitzki J, Craig RA, Okuyama R, et al. Donor cell cycling, trafficking, and accumulation during adoptive immunotherapy for murine lung metastases. Cancer Res. 2004;64:2183–2191. doi: 10.1158/0008-5472.can-03-2799. [DOI] [PubMed] [Google Scholar]
- 20.Rangel-Moreno J, Moyron-Quiroz J, Kusser K, et al. Role of CXC chemokine ligand 13, CC chemokine ligand (CCL) 19, and CCL21 in the organization and function of nasal-associated lymphoid tissue. J Immunol. 2005;175:4904–4913. doi: 10.4049/jimmunol.175.8.4904. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Harshyne LA, Watkins SC, Gambotto A, et al. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–3723. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 23.Saeki H, Moore AM, Brown MJ, et al. Cutting edge: secondary lymphoid-tissue chemokine (SLC) and CC chemokine receptor 7 (CCR7) participate in the emigration pathway of mature dendritic cells from the skin to regional lymph nodes. J Immunol. 1999;162:2472–2475. [PubMed] [Google Scholar]
- 24.Chang AE, Redman BG, Whitfield JR, et al. A phase I trial of tumor lysate-pulsed dendritic cells in the treatment of advanced cancer. Clin Cancer Res. 2002;8:1021–1032. [PubMed] [Google Scholar]
- 25.Geiger JD, Hutchinson RJ, Hohenkirk LF, et al. Vaccination of pediatric solid tumor patients with tumor lysate-pulsed dendritic cells can expand specific T cells and mediate tumor regression. Cancer Res. 2001;61:8513–8519. [PubMed] [Google Scholar]
- 26.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 27.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 28.Zou W, Machelon V, Coulomb-L’Hermin A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 29.Wei S, Kryczek I, Zou L, et al. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 30.Nouri-Shirazi M, Banchereau J, Bell D, et al. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses. J Immunol. 2000;165:3797–3803. doi: 10.4049/jimmunol.165.7.3797. [DOI] [PubMed] [Google Scholar]
- 31.Harshyne LA, Zimmer MI, Watkins SC, et al. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J Immunol. 2003;170:2302–2309. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- 32.Hareyama M, Imai K, Kubo K, et al. Effect of radiation on the expression of carcinoembryonic antigen of human gastric adenocarcinoma cells. Cancer. 1991;67:2269–2274. doi: 10.1002/1097-0142(19910501)67:9<2269::aid-cncr2820670910>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto H, Takahashi T, Mitsuhashi N, et al. Modification of tumor-associated antigen (CEA) expression of human lung cancer cells by irradiation, either alone or in combination with interferon-gamma. Anticancer Res. 1999;19:307–311. [PubMed] [Google Scholar]
- 34.Kunala S, Macklis RM. Ionizing radiation induces CD20 surface expression on human B cells. Int J Cancer. 2001;96:178–181. doi: 10.1002/ijc.1018. [DOI] [PubMed] [Google Scholar]
- 35.Kobie JJ, Wu RS, Kurt RA, et al. Transforming growth factor beta inhibits the antigen-presenting functions and antitumor activity of dendritic cell vaccines. Cancer Res. 2003;63:1860–1864. [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 37.MartIn-Fontecha A, Sebastiani S, Hopken UE, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feijoo E, Alfaro C, Mazzolini G, et al. Dendritic cells delivered inside human carcinomas are sequestered by interleukin-8. Int J Cancer. 2005;116:275–281. doi: 10.1002/ijc.21046. [DOI] [PubMed] [Google Scholar]
- 39.Okada N, Mori N, Koretomo R, et al. Augmentation of the migratory ability of DC-based vaccine into regional lymph nodes by efficient CCR7 gene transduction. Gene Ther. 2005;12:129–139. doi: 10.1038/sj.gt.3302358. [DOI] [PubMed] [Google Scholar]
- 40.Hirao M, Onai N, Hiroishi K, et al. CC chemokine receptor-7 on dendritic cells is induced after interaction with apoptotic tumor cells: critical role in migration from the tumor site to draining lymph nodes. Cancer Res. 2000;60:2209–2217. [PubMed] [Google Scholar]
- 41.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chakraborty M, Abrams SI, Camphausen K, et al. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003;170:6338–6347. doi: 10.4049/jimmunol.170.12.6338. [DOI] [PubMed] [Google Scholar]
- 43.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 44.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 46.Yang D, Chen Q, Yang H, et al. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 48.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]