Abstract
The objective of this pre-formulation study was to systematically investigate the effects of two surfactants (Brij 58® and Tween 80®) and change in solution pH on in vitro permeation of naltrexone HCl (NTX-HCl) across tissue engineered human buccal mucosa. For the study, 10 mg/mL solutions of Tween 80® (0.1 and 1 % w/v) and Brij 58® (1 % w/v) were prepared in standard artificial saliva buffer solution (pH 6.8). For studying pH effects, solution pH was adjusted to either 7.5 or 8.2. As controls, three concentrations of NTX-HCl (2.5, 10 and 25 mg/mL) were prepared. Using NTX standard solution (10mg/ml; pH 6.8), the permeation was observed between in vitro human and ex vivo porcine mucosa. It was observed that Brij 58® increased the permeation rates of NTX significantly. The flux of 10mg/ml solution (pH 6.8) increased from 1.9 ± 0.6 (×102) to 13.9 ± 2.2 (×102) μg/cm2/h (approximately 6 fold) in presence of 1% Brij 58®. Increasing pH of NTX-HCl solution was found to increase the drug flux from 1.9 ± 0.6 (×102) (pH 6.8) to 3.0 ± 0.6 (×102) (pH 7.4) and 8.0 ± 3.5 (×102) (pH 8.2) μg/cm2/h respectively. Histological analyses exhibited no tissue damage due to exposure of buccal tissue to Brij 58®. The mean permeability coefficients (Kp) for 2.5, 10 and 25 mg/mL solutions of NTX-HCl (pH 6.8) were 5.0 (×10−2), 1.8 (×10−2) and 3.2 (×10−2) cm/h respectively, consistent with data from published literature sources. Increase of NTX flux observed with 1% Brij 58® solution may be due to the effects of ATP. Increase in flux and the shortening of lag time observed by increasing in solution pH confirmed earlier finding that distribution coefficient (log D) of NTX is significantly affected by small increments in pH value and therefore plays an important role in NTX permeation by allowing faster diffusion across tissue engineered human buccal membranes.
Keywords: Naltrexone hydrochloride, pre-formulation study, penetration enhancers, Tween 80®, Brij 58®, pH effect, concentration effect, permeability coefficients (Kp), Enhancement Ratio (ER)
1. Introduction
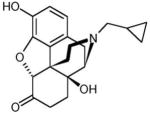
Naltrexone (NTX) is considered to be an important therapeutic agent for the prevention of alcoholism. NTX, due to its similarity in structure to morphine, acts as an opioid antagonist with high affinity for the μ- and κ-opioid receptor sites in human brain (Metcalf and Coop, 2005) (Table 1). This drug is believed to interfere with the process of ‘ethanol reward’ in the mesolimbic dopamine pathway (Lee et al., 2005) by making alcohol less rewarding following blockage of opiate receptors (Sinclair, 2001; Kaufer and DeKosky, 2005).
Table 1.
Chemical structure and physiochemical properties of NTX-HCl
The values were taken from ChemIDplusAdvanced (National Library of Medicine)
Currently, NTX is primarily delivered through the oral route however, following oral ingestion, the compound is observed to have a low plasma half-life (~4 hr), and to undergo high first pass metabolism (>98%) leading to low bioavailability - the target organ being the brain. In addition, metabolic products of NTX-HCl formed by hepatic breakdown of the drug can lead to a range of gastrointestinal and neuropsychiatric adverse reactions (Oncken et al., 2001; Comer et al., 2002; Valiveti et al., 2005). It is evident from previous NTX permeation studies that significant improvements in NTX delivery can be achieved by the use of alternate routes of administration. Therefore, several studies describing the delivery of NTX using the buccal route have been reported (Hussain et al., 1987; Hussain et al., 1988; Rathbone et al., 1994; Giannola et al., 2007a; Giannola et al., 2007b). The buccal route has been observed to offer distinct advantages over the oral route through a) the lack of first pass metabolism increasing bioavailability and b) reduction of the risk of adverse effects by preventing the formation of metabolic products due to hepatic enzymatic degradation. Compared to the transdermal route, the buccal route has the potential to be comparatively better for a hydrophilic molecule like NTX. This is due to the lower buccal membrane content of non-polar lipids, ceramides and glycosylceramides compared to skin, lipids which are believed to form the majority of permeation barrier (Squier and Hall, 1984).
Giannola et al. reported a pre-formulation study using different concentrations of NTX (15, 30 and 60 mg/ml) prepared in artificial saliva and natural human saliva. The permeation of the drug was tested across tissue-engineered buccal mucosa with/without application of iontophoresis (applied current of 0.5, 1 and 2 mA). Effect of different concentrations (0.1, 0.5 and 1%) of bile salts e.g. sodium dehydrocholate (NaDHC), EDTA disodium salt (NaEDTA) and trisodium citrate dihydrate (TNaC) (as permeation enhancers) on NTX permeation were also evaluated. The study provided permeation parameters (Kp, flux and enhancement) for NTX across tissue-engineered buccal mucosa. Iontophoresis was found to show enhancement ratios (ER) of 1.5 and 3.1 (solution prepared in artificial mucosa); and 2.8 and 4.9 (solution prepared in natural saliva) at a current density of 1 and 2 mA respectively (Giannola et al., 2007a). In a follow-up formulation study, sublingual tablets of NTX were prepared by direct compression of drug loaded (56%) poly-octylcyanoacrylate (poly-OCA) matrices and similar observations were made using tissue-engineered buccal mucosa and ex vivo porcine buccal mucosa (Giannola et al., 2007b). These studies clarified that NTX formulations can effectively deliver the drug across buccal mucosa in vitro however, NaDHC, NaEDTA and TNaC did not affect NTX permeation at three concentrations (0.1%, 0.5 and 1%). In other studies, anionic NaDHC (MW= 424.51 Da; CMC = 140–170 mM at 298K) has been shown to exhibit poor solubilization properties compared to related cholate or deoxycholate salts (McBain et al., 1948; Lairon et al., 1978). Na EDTA (MW= 372.24 Da), an anionic chelating agent in solution, has been shown to exhibit slight improvement in solubilization of norfloxacin in the past but the effect could not be explained by the results obtained in the study (Dos Santos et al., 2003). TNaC (MW= 258.06 Da), also anionic, is used as an anticoagulant and its mechanism as a permeation enhancer has not been explained (Rama Prasad et al., 2004).
It has been shown that the critical micelle concentration (CMC) and the hydrophilic-lipophilic balance (HLB) are the two main parameters of enhancers that relate to the disruption of biological membranes leading to an increase in permeation (Egan, 1976). It has also been observed that non-ionic compounds, in general, are less irritant compared to ionic compounds (Davis et al., 1970; Volkering et al., 1995). Based on this, two non-ionic surfactants - Brij 58® (polyoxyethylene (20) cetyl ether) (MW= 1309.68; CMC=0.010 mM at 298K) and Tween 80® (polyoxyethylene (20) sorbitan monooleate) (MW= 1120; CMC=0.007 mM at 298K) were selected for the current study (Lairon et al., 1978; Hait and Moulik, 2001; Miraglia et al., 2010). According to directive of the EEC, both surfactants are being considered as “non-hazardous” and exhibiting acceptable LD50 values (Directive67/548/EEC, 2010).
Effect of slight changes in pH microenvironment on NTX permeation was also studied. Since the effect of Brij 58® has not been studied on buccal tissue morphology, Brij 58® treated and untreated porcine buccal mucosa was observed by sectioning and hematoxylin and eosin (H & E) staining. In addition, the effect of drug concentration (2.5, 10 and 25 mg/ml) on NTX permeation across buccal mucosa was observed and compared with a previous study (Giannola et al., 2007a). Correlation of in vitro permeation of NTX across tissue engineered human and ex vivo porcine buccal mucosa was also performed using standard NTX solution of 10 mg/ml (pH 6.8).
2. Material and Methods
2.1. Materials
Naltrexone hydrochloride (NTX-HCl), urea, potassium chloride (KCl), monobasic potassium phosphate (KH2PO4), potassium thiocyanate (KSCN) and ferric chloride (FeCl3) were purchased from Spectrum Chemicals (New Brunswick, NJ). Tween® 80, Brij® 58, endotoxin-free water, NaCl, were purchased from Sigma Aldrich (St. Louis, MO). Permount® mounting reagent and NaOH were purchased from Fisher Scientific (Pittsburgh, PA). All HPLC solvents and tissue processing solvents (xylene, ethanol, paraffin) for sectioning were analytical grade and were purchased from Fisher Scientific. The tissue-engineered human buccal mucosa EpiOral™ 606 was ordered from MatTek Corporation (Ashland, MA). Porcine cheek samples were obtained from Barton’s Farms and Biologicals (Great Meadows, NJ).
2.2. Preparation of Solutions
Buffer mimicking artificial saliva (pH 6.8) was prepared using appropriate amounts of NaCl, KCl, KSCN, KH2PO4 and urea (Gal et al., 2001; Giannola et al., 2007b). Different concentrations of NTX-HCl (2.5, 10 and 25 mg/ml) were prepared in artificial saliva (pH 6.8) to observe the effect of concentration. For studying pH effect, a 10 mg/ml (pH 6.8) solution was adjusted to 7.4 or 8.2 (Corning pH-meter 430) using 0.1 N NaOH. For studying the surfactant effects, a 10 mg/ml solution of NTX-HCl was supplemented with either Tween® 80 (0.1 and 1 % w/v) or Brij 58® (1 % w/v).
2.3. Preparation of buccal tissue (tissue engineered human and pig buccal mucosa) for permeation studies
Tissue engineered human buccal mucosa EpiOral™ 606 was ordered from MatTek Corporation (Ashland, MA). After arrival, the tissues were stored at 4°C and were used within 24–36 hrs of arrival. The tissues were cut from the inserts and placed in vertical Franz diffusion cells (Permegear Inc., Bethlehem, PA). Porcine buccal tissue was stored under − 30 °C. Before the experiment, the porcine cheeks were completely thawed at room temperature and the underlying connective tissue was removed using a scalpel blade and carefully trimmed to a thickness of 300 – 400 μm. Prior to the experiment, the tissues were allowed to equilibrate in PBS for 30 mins and were then mounted on the Franz cell. Buffer was added to both sides of the tissue and this was left to equilibrate for 15–20 mins before adding the test solution.
2.4. Franz cell permeation studies
Buccal tissues were kept hydrated after mounting on vertical Franz diffusion cells. The donor compartment was filled with 300 μl of NTX-HCl solution. The Franz cell receptor compartment was filled with 5.1 ml phosphate buffer, pH 7.4, maintained at 37°C with the help of a thermostatic water pump Haake DC10 (Karlsruhe, Germany) and stirred continuously at 600 rpm. Samples (300 μl) were withdrawn from the receptor at a time periods of 0, 0.5, 1, 1.5, 2, 2.5, 3, 3.5, 4 and 6 hrs and replaced with an equivalent volume of the buffer and stored at 4°C before analysis. The progressive dilutions in drug concentration were corrected using the equation:
| (Eq.1) |
where Mt(n) is the current cumulative mass of drug transported across the membrane at time t, Cn represents the concentration of drug in receiver medium before collection, ΣCm represents the summed total of the previous measured concentrations [m=1 to (n=1)]; Vr and Vs are volume of the receiver medium and volume of sample removed for analysis respectively. The passive diffusion data was analyzed using Fick’s first law and the values of flux, lag time, permeability coefficient and cumulative amount permeated at 6 hr (Q6) were recorded (Siegel, 1984).
| (Eq.2) |
Equation taken from (Benson, 2005).
The numbers of replicates for the permeation studies were 3 or 4.
2.5. Data collection and analysis
Drug concentrations were determined using Agilent HPLC 1100 consisting of a standard quaternary pump, diode array detector, an autosampler and vacuum degasser (Model G1311A), run by Chemstation software version B.03.01. The stationary phase was an Agilent XDB C-8 reversed-phase 4.5 mm × 5 μ column maintained at 25 °C. The mobile phase was 65:35 methanol: NH4H2PO4 buffer (0.3 % w/v) with flow rate of 1.5 mL/min and injection volume 10 μl. The retention time for NTX was 1.65 ± 0.1 min at 283 nm (Tambwekar et al., 2003). Using external standard method, the linearity (R2 = 0.999) and limit of detection (1 μg/ml) were determined. The external standards prepared were 0.5, 1, 2.5, 5, 10, 25, 50 and 100 μg/ml in the mobile phase.
2.6. Statistical Analysis
The results are expressed as mean ± S.D (standard deviation) and statistically analyzed by performing one-way ANOVA using Minitab® software followed by a post-hoc Tukey’s range test.
2.7. Histology of tissues (evaluation of Brij 58® effects)
Porcine buccal tissue mounted in Franz cells was treated with 300μl of 1% Brij 58® solution prepared in artificial saliva (donor) and phosphate buffer maintained at 37°C (receptor). Untreated controls were also included. After 6 hr, the tissues were fixed in 10 % formalin solution overnight and then transferred to 70% ethanol for at least 24 hrs. The fixed tissues were then processed in a tissue processor (Leica TP 1020) followed by preparation of paraffin blocks (Leica EG1160). The paraffin sections were then cut (10 μM) (Riechert Jung 2030 Biocut Microtome) and kept on slide warmer for 24 hrs. H & E staining was performed on the sections using standard protocols (Ihcworld, 2010). The slides were then observed under a microscope (Nikon Eclipse E600) and digital pictures were taken.
3. Results and discussion
From this study, it was found that Tween 80® showed a slight concentration dependent retardation effect and Brij 58® exhibited a strong enhancing effect on NTX permeation across tissue engineered human buccal mucosa. For 10 mg/ml NTX solution (pH 6.8) supplemented with 0.1 % w/v and 1 % w/v Tween 80®, the flux decreased from 1.9 ± 0.6 (×102) (control) to 1.4 ± 0.9 (×102) and 0.5 ± 0.1 (×102) μg/cm2/hr respectively however, this decrease was found to be statistically not significant (p < 0.05). The Kp values showed a statistically non-significant (p < 0.05) decrease for solutions containing Tween 80® from 1.8 ± 0.6 (×10−2) (control) to 1.4 ± 0.8 (×10−2) (0.1% w/v) and 0.5 ± 0.1 cm/h (1 % w/v). The Q6 values for control [8.4 ± 2.6 (×102) μg/cm2] showed no statistical difference (p < 0.01) from solution containing 0.1% Tween 80® [5.8 ± 1.4 (×10−2)μg/cm2] but was found to be statistically different (p < 0.01) compared to the solution containing 1% w/v Tween 80® [3.0 ± 0.5 μg/cm2 (×102)]. For 10 mg/ml NTX solution (pH 6.8) supplemented with 1% w/v Brij 58® solution, flux increased significantly from 1.9 ± 0.6 (×102) to 13.9 ± 2.2 (×102) μg/cm2/hr (p < 0.05). However, a significant (p < 0.05) increase compared to controls was observed with solution containing 1 % w/v Brij 58® and Kp was found to be 14.0 ± 2.2 (×10−2) cm/h (ER=7.7). No difference in lag times was observed following application of enhancers and it remained relatively constant at ~1.3–1.5 hr (p < 0.05). The use of 1% w/v Brij 58® increased the Q6 values from 8.4 ± 2.6 (×102) μg/cm2 to 61.4 ± 2.1 (×102) μg/cm2 –an increase of 7 fold (Table 2, Figure 1).
Table 2.
Permeation parameters for NTX-HCl (10 mg/ml; pH 6.8) solutions with surfactants - Tween 80® and Brij 58® for tissue-engineered buccal mucosa
| Conc. (mg/ml) | Control (no enhancer) | 0.1% Tween 80® | 1% Tween 80® | 1% Brij 58® |
|---|---|---|---|---|
| Parameter | Mean ± SD (N) | Mean ± SD (N) | Mean ± SD (N) | Mean ± SD (N) |
| Kp(×10−2) (cm/h) | 1.8 ± 0.6 (4) | 1.4 ± 0.8 (3) | 0.5 ± 0.1 (3) | 14.0 ± 2.0 (4) |
| Tlag (h) | 1.5 ± 0.5 (4) | 1.4 ± 0.6 (3) | 1.5 ± 0.2 (3) | 1.3 ± 0.3 (4) |
| Flux (×102) (μg/cm2/h) | 1.9 ± 0.6 (4) | 1.4 ± 0.9 (3) | 0.5 ± 0.1 (3) | 13.9 ± 2.2 (4) |
| Q6 (×102) (μg/cm2) | 8.4 ± 2.6 (4) | 5.8 ± 1.4 (3) | 3.0 ± 0.5 (3) | 61.4 ± 2.1 (4) |
| ER | Control | 0.7 | 0.3 | 7.7 |
Kp = Permeability coefficient; Tlag = Lag time; Q6 = cumulative amount permeated after 6 hours), ER = Enhancement Ratio, SD = Standard deviation
Figure 1.
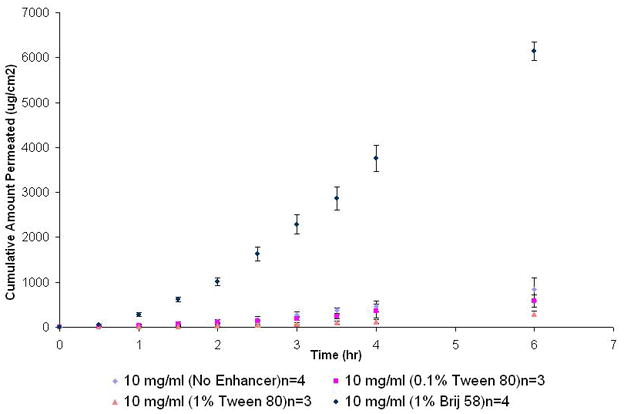
Permeation profile of NTX-HCl (10 mg/ml; pH 6.8) with two enhancers (Tween 80® and Brij 58®).
There is a large library of compounds available as permeation enhancers for both buccal as well as transdermal use (Osborne and Henke, 1997). The surfactants - Brij 58® and Tween 80® - were selected for this study based on their non-ionic nature, high HLB values and low critical micelle concentrations (CMC) (Helenius and Simons, 1975). In general, surfactants act by causing disaggregation of lipids in the biological membrane leading to loosening of membrane barrier structure and ultimately causing an increase in the permeation of the compounds (Buyukozturk et al., 2009). Based on a general understanding of enhancer mechanisms, it is very difficult to provide a rationale for the enhancement of a given permeant using a specific enhancer (Williams and Barry, 2004). Brij58® used in this investigation increased in vitro permeation of NTX across tissue-engineered buccal mucosa by 6–7 folds. It was found following an extensive literature search that the enhancement of NTX by Brij58® may be partially explained by its effects on biological membranes. A study has been reported where 42 detergents were tested and Brij58® was found to increase the permeability of the plasma membrane to adenosine triphosphate (ATP) without inhibiting or activating ATPase and also increasing H+ transport across the membrane (Palmgren et al., 1990; Johansson et al., 1995). It is possible that NTX permeation could be a process facilitated by ATP however, in order to confirm this hypothesis, more experiments have to be performed. This could also explain the increase in flux of NTX during in vivo permeation comparative to in vitro permeation due to the presence of readily available ATP to carry the molecule across the membrane (Giannola et al., 2007b; Campisi et al., 2010).
Naltrexone HCl is a weak acid and at 32°C, it exhibits calculated pKa values of 8.20 and 9.63. The first value corresponds to the dissociation of proton on aliphatic nitrogen and the second value corresponds to dissociation of the phenolic proton (Table 1) (Kaufman et al., 1975b; Milewski and Stinchcomb, 2011). The standard pH of NTX used in the experiment was 6.8, which corresponds to the physiological pH of the buccal cavity (Bardow et al., 2000). In order to observe the pH effect on permeation, pH was varied from 6.8 to 7.5 and 8.2 and therefore in all the solutions, majority of NTX molecules were expected to be positively charged however, the increase in pH facilitated the conversion of NTX-HCl salt to its base thus allowing the unionized form the drug to permeate more readily through lipid-containing mucous membranes, consistent with the pH-partition theory (Shore et al., 1957).
In NTX solutions, pH has been shown to have a marked effect on the partition coefficient (log D) of the compound. It has been observed that at 37°C, a pH increase from 7.1 to 7.7 resulted in a three-fold increase in log D values of NTX from 7.11 to 22.57 (Kaufman et al., 1975a). In this study, it was observed that the flux values of 10 mg/ml NTX increased with pH- 1.9 ± 0.6 (×102) (pH 6.8) to 3.0 ± 0.6 (×102) (pH 7.5) and 8.0 ± 3.5 (×102) μg/cm2/h (pH 8.2) respectively – an approximately 4 fold increase from pH 6.8 to pH 8.2. The Kp values also increased with pH from 1.8 ± 0.6 (×10−2) (pH 6.8) to 3.0 ± 0.6 (×10−2) (pH 7.5, ER=1.6) and 7.9 ± 3.5 (×10−2) cm/h (pH 6.8), an enhancement of 4.4 over the control solution. The Q6 values increased from 8.4 ± 2.6 (×102) (pH 6.8) to 16.2 ± 3.9 (×102) (pH 7.5) and 37.4 ± 11.0 (×102) μg/cm2 (p< 0.005) (Table 3, Figure 2). It is apparent from the data that the increase in Kp values at different pH correlated proportionately to increasing log D values of NTX (Kaufman et al., 1975a). Due to increased distribution coefficient values, a decrease in lag time for 10 mg/ml NTX solution was observed with increasing pH, from 1.5 ± 0.5 hr (pH 6.8) to 0.5 ± 0.1 (pH 7.5) and 0.9 ± 0.4 hr (pH 8.2).
Table 3.
Permeation parameters for NTX-HCl (10 mg/ml, no surfactants) solutions at different pH (6.8, 7.5 and 8.2) for tissue-engineered buccal mucosa
| pH | 6.8 | 7.5 | 8.2 |
|---|---|---|---|
| Parameter | Mean ± SD (N) | Mean ± SD (N) | Mean ± SD (N) |
| Kp(×10−2) (cm/h) | 1.8 ± 0.6 (4) | 3.0 ± 0.6 (4) | 7.9 ± 3.5 (4) |
| Tlag (h) | 1.5 ± 0.5 (4) | 0.5 ± 0.1 (4) | 0.9 ± 0.4 (4) |
| Flux (×102) (μg/cm2/h) | 1.9 ± 0.6 (4) | 3.0 ± 0.6 (4) | 8.0 ± 3.5 (4) |
| Q6 (×102) (μg/cm2) | 8.4 ± 2.6 (4) | 16.2 ± 3.9 (4) | 37.4 ± 11.0 (4) |
| ER | Control | 1.6 | 4.4 |
Kp = Permeability coefficient; Tlag = Lag time; Q6 = cumulative amount permeated after 6 hours), SD = Standard deviation.
Figure 2.
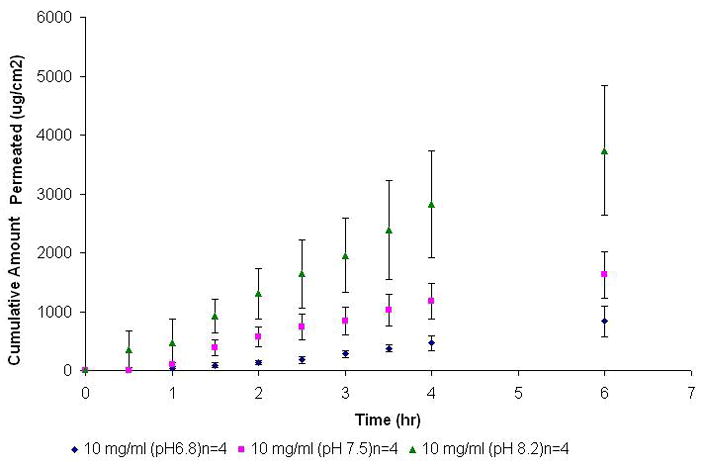
Permeation profile of NTX-HCl (10 mg/ml, no surfactants) at different pH (6.8, 7.5 and 8.2) across tissue engineered human buccal mucosa.
The microscopic evaluation of stained tissue, the treated samples showed no significant difference compared to the untreated tissues (Figures 3a and 3b) which confirms that Brij 58® can be effectively used in the formulations without causing any obvious damage to the buccal mucosa.
Figure 3.
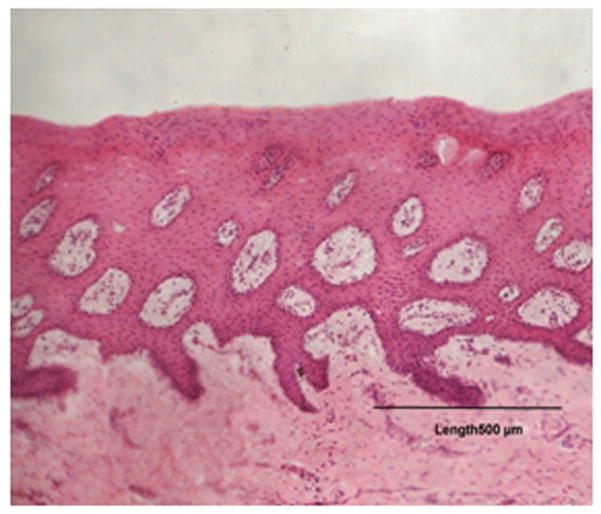
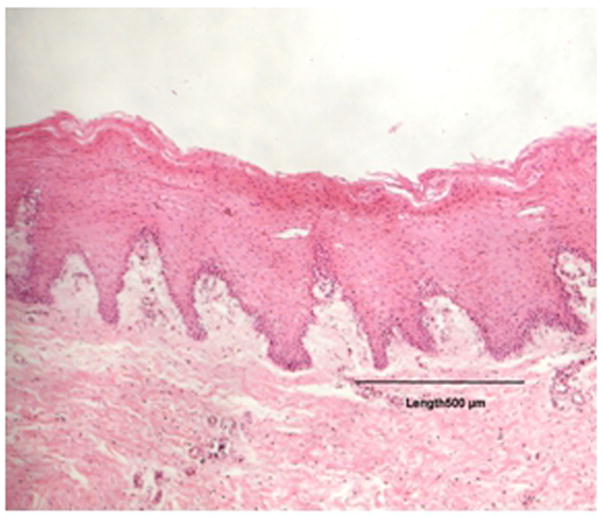
Figure 3 (a): Control porcine buccal tissue.
Figure 3 (b): Treated porcine buccal tissue (1% Brij 58®).
The Kp values for 2.5, 10 and 25 mg/ml NTX solutions were found to be 5.0 ± 1.5 (×10−2), 1.8 ± 0.7 (×10−2), 3.2 ± 1.0 (×10−2) cm/h respectively (p ≤ 0.01) (Table 4). These Kp values obtained from these experiments agree with previously published values of 4.4 (×10−2), 4.0 (×10−2) and 3.0 (×10−2) for 15, 40 and 60 mg/ml solutions of NTX respectively (Giannola et al., 2007a). The flux value for the 2.5 and 10 mg/ml NTX solutions (pH 6.8) was found to be 1.3 ± 0.4 (×102) and 1.9 ± 0.6 (×102) μg/cm2/hr respectively and differed statistically (p< 0.01) with flux value of 8.0 ± 2.6 (×102) μg/cm2/hr obtained for the 25 mg/ml NTX solution. The cumulative amount permeated at 6 hr (Q6) showed an increase from 5.9 ± 2.2 (×102) to 8.4 ± 2.6 (×102) and 30.9 ± 9.0 (×102) with increasing concentrations of 2.5, 10 and 25 mg/ml respectively (Figure 4, Table 4).
Table 4.
Permeation parameters of NTX-HCl (pH 6.8, no surfactants) solutions at different concentrations (2.5, 10 and 25 mg/ml) for tissue-engineered buccal mucosa
| Conc. (mg/ml) | 2.5 mg/ml | 10 mg/ml | 25 mg/ml |
|---|---|---|---|
| Parameter | Mean ± SD (N) | Mean ± SD (N) | Mean ± SD (N) |
| Kp(×10−2) (cm/h) | 5.0 ± 1.5 (3) | 1.8 ± 0.7 (4) | 3.2 ± 1.0 (4) |
| Tlag (h) | 1.0 ± 0.4 (3) | 1.5 ± 0.5 (4) | 1.5 ± 0.04 (4) |
| Flux(×102) (μg/cm2/h) | 1.3 ± 0.7 (3) | 1.9 ± 0.6 (4) | 8.0 ± 2.6 (4) |
| Q6 (×102) (μg/cm2) | 5.9 ± 2.2 (3) | 8.4 ± 2.6 (4) | 30.9 ± 9.0 (4) |
Kp = Permeability coefficient; Tlag = Lag time; Q6 = cumulative amount permeated after 6 hours), SD = Standard deviation
Figure 4.
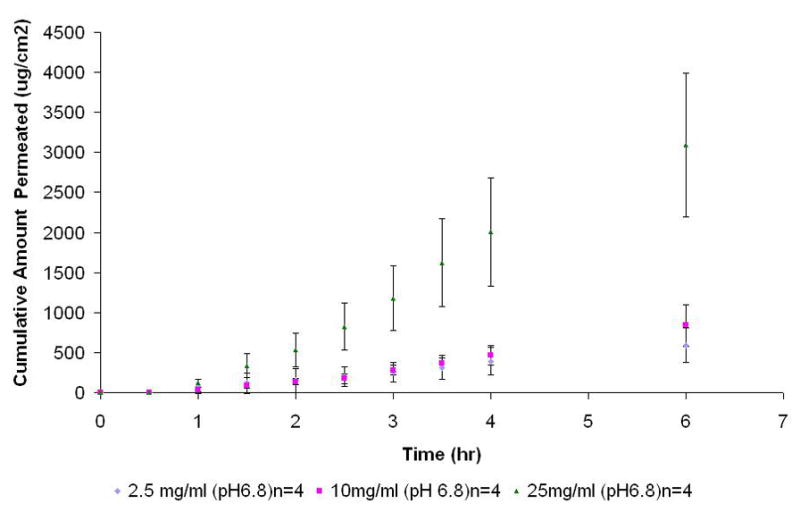
Permeation profile of NTX-HCl (pH 6.8, no surfactants) at different concentrations (2.5, 10 and 25 mg/ml) across tissue engineered human buccal mucosa.
When comparing the in vitro permeation of tissue-engineered human and porcine buccal mucosa, it was observed that the standard solution of NTX (10 mg/ml; pH 6.8) showed no difference (p≤ 0.01) in Kp values between tissue-engineered human buccal mucosa [1.8 ± 0.7 (×10−2) cm/h] and porcine tissues [1.9 ± 0.8 cm/h (×10−2)]. The lag time across the tissue engineered buccal mucosa was found to be less than 2 hrs and showed no statistical difference between the two mucosal types (p<0.05) (Figure 5, Table 5). The Kp for porcine buccal and lag time values also agree with previously reported data (Giannola et al., 2007a; Giannola et al., 2007b). The flux for standard solution (10 mg/ml; pH 6.8) across porcine buccal mucosa [2.0 ± 0.9 (×102) μg/cm2/hr] was found to very similar to that for tissue-engineered buccal tissue [2.0 ± 0.6 (×102) μg/cm2/hr] (p< 0.01). No statistical difference was found between the Q6 values between the two mucosal types used which is also consistent with previous findings on NTX-HCl permeation (p<0.05) (Giannola et al., 2007b).
Figure 5.
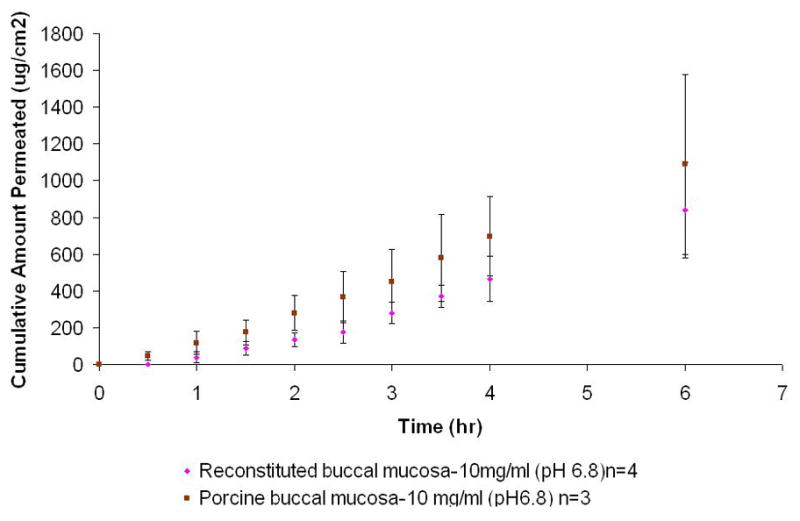
Comparison of permeation profile of NTX-HCl (10 mg/ml; pH 6.8, no surfactant) across tissue engineered human buccal mucosa and porcine buccal mucosa.
Table 5.
Permeation parameters of NTX-HCl (10 mg/ml; pH 6.8, no surfactants) solutions between tissue-engineered human buccal mucosa and ex vivo porcine buccal mucosa.
| Buccal type | Tissue engineered buccal mucosa | Porcine mucosa buccal | |
|---|---|---|---|
| Parameter | Mean ± SD (N) | Mean ± SD (N) | |
| Kp(×10−2) (cm/h) | 1.8 ± 0.7 (4) | 1.9 ± 0.8 (3) | |
| Tlag (h) | 1.5 ± 0.5 (4) | 1.6 ± 0.2 (3) | |
| Flux (×102) (μg/cm2/h) | 1.9 ± 0.6 (4) | 2.0 ± 0.9 (3) | |
| Q6 (×102) (μg/cm2) | 8.4 ± 2.6 (4) | 10.9 ± 4.9 (3) | |
Kp = Permeability coefficient; Tlag = Lag time; Q6 = cumulative amount permeated after 6 hours), SD = Standard deviation.
Based on this study and previously published studies (Giannola et al., 2007b), it is evident that NTX has shown good correlation during in vitro permeation between tissue-engineered and ex vivo porcine buccal mucosa. However, we find that in the literature, in vitro results are not consistent with those obtained from in vivo studies. Studies performed on pigs suggest that bioavailability of NTX increases significantly during in vivo conditions and the difference in permeation with/without iontophoresis is diminished (Campisi et al., 2010). The buccal bioavailability of NTX in rats has also been found to be relatively high (~70%) (Hussain et al., 1987). This is in contrast to results observed in in vitro permeation of NTX using an ex vivo porcine buccal model (Giannola et al., 2007b) and results obtained from this study but it supports the hypothesis that ATP might be playing an active role in NTX permeation in vivo. From this discussion, it is can also be cautiously asserted that in vitro experimentations might not be able to predict correct in vivo experimental trends for NTX.
4. Conclusions
This study systematically evaluated the effect of surfactants (Brij 58® and Tween 80®) and pH on the buccal delivery of NTX in an in vitro permeation study. It was found that permeation of NTX across reconstituted human buccal mucosa produced an enhancement of 7.7 with the use of Brij 58® (at 1% w/v). The mechanism of enhancement is unclear however, based on literature it is possible that at least in vivo the NTX permeation may be facilitated by ATP. Slightly increasing the pH of NTX solution from 6.8 to pH 7.5 and pH 8.5 increased the permeation by a factor of 1.6 and 4.4 respectively. This increase in permeation appears to be a direct result of increase in drug partition caused by increase in pH, consistent with the pH-partition hypothesis. An increase in pH caused an increased NTX flux and shorter lag times suggesting that NTX permeation is influenced by its distribution coefficient. The porcine buccal tissue showed no damage when exposed to Brij 58® under physiological conditions.
Acknowledgments
The authors would like to acknowledge NIH grant #1R43AA018894-01 from National Institute on Alcohol Abuse and Alcoholism (NIAAA) for completion of this study. We are also grateful to Lawrence Hu for the processing of porcine buccal tissue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardow A, Moe D, Nyvad B, Nauntofte B. The buffer capacity and buffer systems of human whole saliva measured without loss of CO2. Archives of Oral Biology. 2000;45:1–12. doi: 10.1016/s0003-9969(99)00119-3. [DOI] [PubMed] [Google Scholar]
- Benson HAE. Transdermal drug delivery: Penetration enhancement techniques. Current Drug Delivery. 2005;2:23–33. doi: 10.2174/1567201052772915. [DOI] [PubMed] [Google Scholar]
- Buyukozturk F, Benneyan J, Carrier RL. Effect of emulsion based drug delivery systems on rate of drug release and intestinal permeability enhancement. Bioengineering Conference, 2009 IEEE 35th Annual Northeast; 2009. pp. 1–2. [Google Scholar]
- Campisi G, Giannola LI, Florena AM, De Caro V, Schumacher A, Göttsche T, Paderni C, Wolff A. Bioavailability in vivo of naltrexone following transbuccal administration by an electronically-controlled intraoral device: A trial on pigs. Journal of Controlled Release. 2010;145:214–220. doi: 10.1016/j.jconrel.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Comer S, Collins E, Kleber H, Nuwayser E, Kerrigan J, Fischman M. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology. 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WW, Pfeiffer RR, Quay JF. Normal and promoted gastrointestinal absorption of water-soluble substances I: Induced rapidly reversible hyperabsorptive state in the canine fundic stomach pouch. Journal of Pharmaceutical Sciences. 1970;59:960–963. doi: 10.1002/jps.2600590708. [DOI] [PubMed] [Google Scholar]
- Directive67/548/EEC. European Commission Environment - Dangerous Substances Directive. 2010. [Google Scholar]
- Dos Santos I, Fawaz F, Lagueny AM, Bonini F. Improvement of norfloxacin oral bioavailability by EDTA and sodium caprate. International Journal of Pharmaceutics. 2003;260:1–4. doi: 10.1016/s0378-5173(03)00257-6. [DOI] [PubMed] [Google Scholar]
- Egan RW. Hydrophile-lipophile balance and critical micelle concentration as key factors influencing surfactant disruption of mitochondrial membranes. The Journal of Biological Chemistry. 1976;251:4442–4447. [PubMed] [Google Scholar]
- Gal JY, Fovet Y, Adib-Yadzi M. About a synthetic saliva for in vitro studies. Talanta. 2001;53:1103–1115. doi: 10.1016/s0039-9140(00)00618-4. [DOI] [PubMed] [Google Scholar]
- Giannola LI, De Caro V, Giandalia G, Siragusa MG, Campisi G, Florena AM, Ciach T. Diffusion of naltrexone across reconstituted human oral epithelium and histomorphological features. European Journal of Pharmaceutics and Biopharmaceutics. 2007a;65:238–246. doi: 10.1016/j.ejpb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Giannola LI, De Caro V, Giandalia G, Siragusa MG, Tripodo C, Florena AM, Campisi G. Release of naltrexone on buccal mucosa: Permeation studies, histological aspects and matrix system design. European Journal of Pharmaceutics and Biopharmaceutics. 2007b;67:425–433. doi: 10.1016/j.ejpb.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Hait S, Moulik S. Determination of critical micelle concentration (CMC) of nonionic surfactants by donor-acceptor interaction with lodine and correlation of CMC with hydrophile-lipophile balance and other parameters of the surfactants. Journal of Surfactants and Detergents. 2001;4:303–309. [Google Scholar]
- Helenius A, Simons K. Solubilization of membranes by detergents. Biochimica et Biophysica Acta. 1975;415:29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Hussain M, Aungst B, Koval C, Shefter E. Improved buccal delivery of opioid analgesics and antagonists with bitterless prodrugs. Pharmaceutical Research. 1988;5:615–618. doi: 10.1023/a:1015958417047. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Aungst BJ, Kearney A, Shefter E. Buccal and oral bioavailability of naloxone and naltrexone in rats. International Journal of Pharmaceutics. 1987;36:127–130. [Google Scholar]
- Ihcworld. H&E Staining Method and Protocol - Harris. 2010. [Google Scholar]
- Johansson F, Olbe M, Sommarin M, Larsson C. Brij 58, a polyoxyethylene acyl ether, creates membrane vesicles of uniform sidedness. A new tool to obtain inside-out (cytoplasmic side-out) plasma membrane vesicles. The Plant Journal. 1995;7:165–173. doi: 10.1046/j.1365-313x.1995.07010165.x. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, DeKosky ST. Principal of the pharmacotherapy of substances abuse disorders. Neurobiology of Mental Illness. 2005:1272. [Google Scholar]
- Kaufman JJ, Koski WS, Benson DW, Semo NM. Narcotic and narcotic antagonist pKa’s and partition coefficients and their significance in clinical practice. Drug and Alcohol Dependence. 1975a;1:103–114. doi: 10.1016/0376-8716(75)90012-5. [DOI] [PubMed] [Google Scholar]
- Kaufman JJ, Semo NM, Koski WS. Microelectrometric titration measurement of the pKa’s and partition and drug distribution coefficients of narcotics and narcotic antagonists and their pH and temperature dependence. Journal of Medicinal Chemistry. 1975b;18:647–655. doi: 10.1021/jm00241a001. [DOI] [PubMed] [Google Scholar]
- Lairon D, Nalbone G, Lafont H, Leonardi J, Domingo N, Hauton JC, Verger R. Inhibition of lipase adsorption at interfaces. Role of bile salt micelles and colipase. Biochemistry. 1978;17:205–208. doi: 10.1021/bi00595a001. [DOI] [PubMed] [Google Scholar]
- Lee YK, Park SW, Kim YK, Kim DJ, Jeong J, Myrick H, Kim YH. Effects of naltrexone on the ethanol-induced changes in the rat central dopaminergic system. Alcohol and Alcoholism. 2005;40:297–301. doi: 10.1093/alcalc/agh163. [DOI] [PubMed] [Google Scholar]
- McBain JW, Wilder AG, Merrill RC. Solubilization of water-insoluble dye by colloidal electrolytes and non-ionizing detergents. The Journal of Physical and Colloid Chemistry. 1948;52:12–22. doi: 10.1021/j150457a002. [DOI] [PubMed] [Google Scholar]
- Metcalf MD, Coop A. Kappa Opioid Antagonists: Past Successes and Future Prospects. AAPS Journal. 2005;7:E704–E722. doi: 10.1208/aapsj070371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewski M, Stinchcomb A. Vehicle composition influence on the microneedle-enhanced transdermal flux of naltrexone hydrochloride. Pharmaceutical Research. 2011;28:124–134. doi: 10.1007/s11095-010-0191-x. [DOI] [PubMed] [Google Scholar]
- Miraglia D, Rodríguez J, Minardi R, Schulz P. Critical micelle concentration and HLB of the sodium oleate–hexadecyltrimethylammonium bromide mixed system. Journal of Surfactants and Detergents. 2010:1–8. [Google Scholar]
- Oncken C, Van Kirk J, Kranzler HR. Adverse effects of oral naltrexone: analysis of data from two clinical trials. Psychopharmacology. 2001;154:397–402. doi: 10.1007/s002130000666. [DOI] [PubMed] [Google Scholar]
- Osborne DW, Henke JJ. Skin permeation enhancers cited in technical literature. Pharmaceutical Technology. 1997 November;:58–66. [Google Scholar]
- Palmgren MG, Sommarin M, Ulvskov P, Larsson C. Effect of detergents on the H+-ATPase activity of inside-out and right-side-out plant plasma membrane vesicles. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1990;1021:133–140. doi: 10.1016/0005-2736(90)90025-j. [DOI] [PubMed] [Google Scholar]
- Rama Prasad YV, Minamimoto T, Yoshikawa Y, Shibata N, Mori S, Matsuura A, Takada K. In situ intestinal absorption studies on low molecular weight heparin in rats using Labrasol as absorption enhancer. International Journal of Pharmaceutics. 2004;271:225–232. doi: 10.1016/j.ijpharm.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Rathbone MJ, Drummond BK, Tucker IG. The oral cavity as a site for systemic drug delivery. Advanced Drug Delivery Reviews. 1994;13:1–22. [Google Scholar]
- Shore PA, Brodie BB, Hogben CAM. The gastric secretion of drugs: A pH partition hypothesis. Journal of Pharmacology and Experimental Therapeutics. 1957;119:361–369. [PubMed] [Google Scholar]
- Siegel A. The structure and function of oral mucosa. In: Meyer J, Squier CA, Gerson SJ, editors. Permeability of the oral mucosa. Pergamon Press; Oxford: 1984. pp. 98–104. [Google Scholar]
- Sinclair JD. Evidence about the use of naltrexone and for different ways of using it in the treatment of alcoholism. Alcohol and Alcoholism. 2001;36:2–10. doi: 10.1093/alcalc/36.1.2. [DOI] [PubMed] [Google Scholar]
- Squier CA, Hall BK. The permeability of mammalian nonkeratinized oral epithelia to horseradish peroxidase applied in vivo and in vitro. Archives of Oral Biology. 1984;29:45–50. doi: 10.1016/0003-9969(84)90041-4. [DOI] [PubMed] [Google Scholar]
- Tambwekar KR, Kakariya RB, Garg S. A validated high performance liquid chromatographic method for analysis of nicotine in pure form and from formulations. Journal of Pharmaceutical and Biomedical Analysis. 2003;32:441–450. doi: 10.1016/s0731-7085(03)00236-x. [DOI] [PubMed] [Google Scholar]
- Valiveti S, Paudel KS, Hammell DC, Hamad MO, Chen J, Crooks PA, Stinchcomb AL. In vitro in vivo correlation of transdermal naltrexone prodrugs in hairless guinea pigs. Pharmaceutical Research. 2005;22:981–989. doi: 10.1007/s11095-005-4593-0. [DOI] [PubMed] [Google Scholar]
- Volkering F, Breure AM, van Andel JG, Rulkens WH. Influence of nonionic surfactants on bioavailability and biodegradation of polycyclic aromatic hydrocarbons. Applied and Environmental Microbiology. 1995;61:1699–1705. doi: 10.1128/aem.61.5.1699-1705.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AC, Barry BW. Penetration enhancers. Advanced Drug Delivery Reviews. 2004;56:603–618. doi: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]