SUMMARY
The advances in bioorthogonal ligation methods have provided new opportunities for proteomic analysis of newly synthesized proteins, posttranslational modifications and specific enzyme families using azide/alkyne-functionalized chemical reporters and activity-based probes. Efficient enrichment and elution of azide/alkyne-labeled proteins with selectively cleavable affinity tags is essential for protein identification and quantification applications. Here we report the synthesis and comparative analysis of Na2S2O4-cleavable diazobenzene-based affinity tags for bioorthogonal chemical proteomics. We demonstrated that ortho-hydroxyl substituent is required for efficient diazobenzene-bond cleavage and show that these cleavable affinity tags can be used to identify newly synthesized proteins in bacteria targeted by amino acid chemical reporters as well as their sites of modification on endogenously expressed proteins. The diazobenzene-based affinity tags are compatible with in-gel, in-solution and on-bead enrichment strategies and should afford useful tools for diverse bioorthogonal proteomic applications.
INTRODUCTION
Bioorthogonal chemical proteomics has afforded new opportunities to investigate protein function and regulation (Sletten and Bertozzi, 2009; Cravatt et al., 2008). The advent of bioorthogonal ligation methods such as the Staudinger ligation (Jewett and Bertozzi, 2010; Laughlin and Bertozzi, 2009) and click chemistry cycloaddition reactions (Rostovtsev et al., 2002; Tornoe et al., 2002; Best, 2009; Saxon and Bertozzi, 2000) has enabled the introduction of small molecule probes into cells and animals for imaging and proteomic studies of specific proteins that were previously impossible. For example, the administration of azide- or alkyne-functionalized substrates allows metabolic labeling of proteins with bioorthogonal chemical reporters to monitor protein synthesis and/or posttranslational modifications as well as nucleic acid synthesis and lipid metabolism (Dieterich et al., 2007; Baskin et al., 2010; Zhang et al., 2010; Salic and Mitchison, 2008). Alternatively, enzyme/mechanism-based inhibitors (Evans and Cravatt, 2006; Simon and Cravatt, 2010) and natural products (Böttcher et al., 2010) that are modified with azides or alkynes probes can facilitate target identification of small molecules as well as profiling of specific protein families in cells and animals (Cravatt et al., 2008). The pioneering studies by the Cravatt and coworkers demonstrated that alkyne-modified activity-based probes and click chemistry could be used for in-gel fluorescence profiling of enzyme families (Cravatt et al., 2008) as well as characterization of unclassified enzymes using multidimensional protein identification technology (MudPIT) (Simon and Cravatt, 2010) but the robust identification of azide/alkyne-labeled proteins and their subsequent functional analysis is still challenging.
The multiple steps required for bioorthogonal chemical proteomics can significantly limit the identification of proteins and specific amino acids that are targeted by chemical reporters or activity-based probes. In vitro studies suggest that CuI-catalyzed azide-alkyne cycloaddition (CuAAC) is more efficient for bioorthogonal detection of azide/alkyne-modified proteins in cell lysates (Charron et al., 2009; Speers and Cravatt, 2005) although the Staudinger ligation and Cu-free cycloaddition reactions are preferred with live cells and in animals (Jewett and Bertozzi, 2010; Laughlin and Bertozzi, 2009). One important issue is the enrichment and recovery of azide- or alkyne-modified proteins/peptides after bioorthogonal ligation reactions from complex mixtures for mass spectrometry-based protein identification. While functionalized biotinylated affinity tags and streptavidin beads provides an effective approach for enrichment of chemical reporter/probe-labeled proteins/peptides, the high affinity binding (~KD 10−15 M) of biotin to streptavidin makes quantitative elution of captured proteins/peptides from beads challenging and is not ideal for large-scale proteomic studies as well as mapping sites of protein modifications. To address this issue, selectively cleavable affinity tags have been developed for bioorthogonal chemical proteomics. For example, protease- (Speers and Cravatt, 2005; Dieterich et al., 2006), pH- (Veken et al., 2005; Fauq et al., 2005) photo- (Petrotchenko et al., 2009) and redox-cleavable- (Shimkus et al., 1985; Gartner et al., 2007; Verhelst et al., 2007; Nessen et al., 2009) linkers have been incorporated into biotinylated affinity tags to facilitate the elution of proteins and peptides from streptavidin beads for protein identification. The compatibility of cleavable linkers with bioorthogonal ligation conditions and mass spectrometry (MS)-based peptide sequencing is also crucial. CuAAC requires reducing agents such as 1 mM tris(2-carboxyethyl)phosphine (TCEP) or ascorbic acid (Wang et al., 2003; Chan et al., 2004), which is not compatible with disulfide linkers commonly used with biotinylated affinity tags (Shimkus et al., 1985; Gartner et al., 2007; Nessen et al., 2009). However, disulfide cleavable linkers can be used with Cu-free cycloadditions for bioorthogonal proteomic studies, but the synthesis of cyclooctyne reagents is more cumbersome (Nessen et al., 2009). Acylhydrazone linkers are compatible with CuAAC, can be cleaved with mildly acidic conditions (pH ~5.8) for elution of proteins from beads and used to reintroduce detection tags onto recovered proteins, but have not been used for large-scale bioorthogonal proteomics or mapping sites of protein modifications yet (Park et al., 2009). Protease-sensitive cleavable linkers provide mild enzymatic conditions for eluting captured proteins from beads and subsequent protein identification (Speers and Cravatt, 2005; Dieterich et al., 2006). Notably, the development of TEV-protease cleavable affinity tags by the Cravatt laboratory has enabled high content bioorthogonal proteomic studies of activity-based probes and mapping modification sites (Speers and Cravatt, 2005; Weerapana et al., 2009).
Our laboratory has focused on diazobenzene-functionalized biotinylated tags that are stable to reducing conditions of CuAAC and efficiently cleaved by sodium dithionite (Na2S2O4) for bioorthogonal proteomic studies (Figure 1) (Verhelst et al., 2007; Landi et al., 2010; Yang et al., 2010; Yount et al., 2010; Grammel et al., 2010; Rangan et al., 2010). Our first-generation clickable and diazobenzene-functionalized biotinylated tag (azido-diazo-biotin, 1) (Figure 1) enabled the CuAAC-based proteomic analysis of acetylated (Yang et al., 2010) and S-palmitoylated proteins (Yount et al., 2010) in mammalian cells as well as lipoproteins (Rangan et al., 2010) and newly synthesized proteins (Grammel et al., 2010) in bacteria using alkyne-functionalized chemical reporters. Additionally, we generated an alkyne- and diazobenzene-functionalized biotinylated tag (alkynyl-diazo-biotin, 2) (Figure 1) for proteomic analysis of azide-modified proteins and used this cleavable affinity tag to profile fatty-acylated proteins in mammalian cells (unpublished data). Here we describe the modular synthesis, scope and utility of several diazobenzene-functionalized biotinylated tags for bioorthogonal chemical proteomics. We demonstrated that clickable and diazobenzene-based affinity tags require ortho-hydroxy-functionalization of the aromatic ring for efficient Na2S2O4-cleavage and elution of proteins from streptavidin beads for bioorthogonal chemical proteomics. Using amino acid reporters of newly synthesized proteins in bacteria, we showed that these clickable diazobenzene-based affinity tags allow robust protein identification and mapping of modification sites.
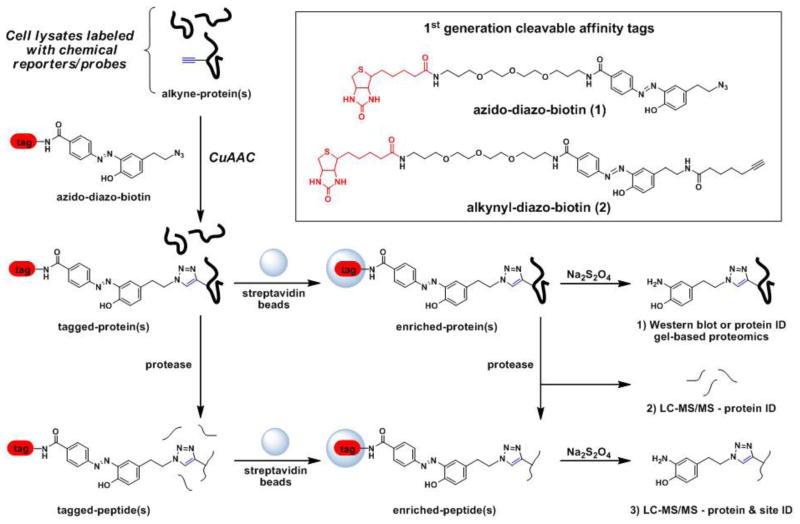
Figure 1. Schematic of selective enrichment and elution of alkyne/azide-labeled proteins or peptides using diazobenzene-based cleavable affinity tags for bioorthogonal proteomics.
In this two-step labeling approach, target proteins labeled with alkyne or azide-functionalized chemical reporters/probes can be selectively reacted with CuAAC reagents for detection and identification. For example, alkyne-modified proteins/peptides can be reacted with azido-diazo-biotin (1) and enriched at the protein level and eluted with Na2S2O4 for gel-based proteomics or subjected to on-bead protease digestion and LC-MS/MS analysis. Alternatively, tagged peptides can be enriched and cleaved with Na2S2O4 for LC-MS/MS analysis. See also Figure S1.
RESULTS
Synthesis and evaluation of second-generation clickable and diazobenzene-based affinity tags
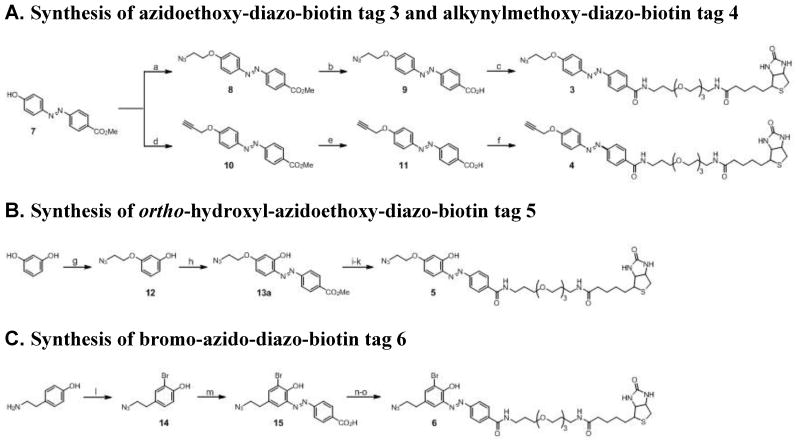
Given the utility of our first-generation clickable diazobenzene-based affinity tags (1 and 2, Figure 1) for bioorthogonal chemical proteomics (Yang et al., 2010; Yount et al., 2010; Grammel et al., 2010; Rangan et al., 2010) we synthesized a second-generation clickable diazobenzene-based affinity tags using a more efficient and modular synthetic route to generate both azide- and alkyne-functionalized diazobenzene affinity tags (Figure 2A). The diazobenzene moiety was generated by coupling of diazonium salt of 4-aminobenzoic acid with phenol as previously described (Bahulayan and Lalithambika, 2003). The resulting diazobenzene acid was esterified and then alkylated with 2-azdioethyl tosylate or propargyl bromide to give compounds 8 and 10, respectively. The methyl esters were then converted into their corresponding acyl chlorides (9 and 11) and reacted with biotin-PEG-NH2 (Huang et al., 2006) to yield the affinity tags 3 and 4, respectively (Figure 2A). Starting from the same intermediate 7, this new synthetic route allows us to generate both azido- and alkyne-derivatized diazobenzene-based affinity tags more efficiently compared to 1 and 2. The overall yields for 1 and 2 were 33% (Yang et al., 2010) and 8% (unpublished data), respectively, whereas 3 and 4 were obtained with 49% and 35% over three steps.
Figure 2. Synthesis of the second-generation diazobenzene-based cleavable affinity tags (3–6).
(a) 2-azidoethyl tosylate, K2CO3, DMF, 0 °C then rt, 85 %; (b) LiOH, THF/H2O (v/v = 1/1), pH = 12.0, 90%; (c) i. oxalyl chloride, cat. DMF, CH2Cl2; ii. biotin-PEG-NH2, Et3N, CH2Cl2, 55 %; (d) propargyl bromide, K2CO3, DMF, rt, 70 %; (e) LiOH, THF/H2O (v/v = 1/1), pH = 12, 90%; (f) i. oxalyl chloride, cat. DMF, CH2Cl2; ii. biotin-PEG-NH2, Et3N, CH2Cl2, 39 %; (g) resorcinol, 2-azidoethyl tosylate, EtOH, KOH(aq), reflux, 60%; (h) i. methyl 4-aminobenzoate, 6 N HCl(aq), NaNO2, 0 °C, 15 min; ii. K2CO3, THF, pH 8.0, 0 °C then rt, 5%; (i) LiOH, THF/H2O (v/v = 1/1), pH = 12.0, >95%; (j) N-hydroxysuccinimide, DCC, THF, 2.5 h; (k) biotin-PEG-NH2, DMF, 4 h, 65% over 2 steps; (l) Br2, AcOH, 84%; (m) i. 4-aminobenzoic acid, 6 N HCl(aq), NaNO2, 0 °C, 25 min; ii. K2CO3, THF, pH 8.0, 0 °C then rt, 8–15%; (n) N-hydroxysuccinimide, DCC, THF, 3 h; (o) biotin-PEG-NH2, DMF, 4 h, 53% over 2 steps. See also Figure S4.
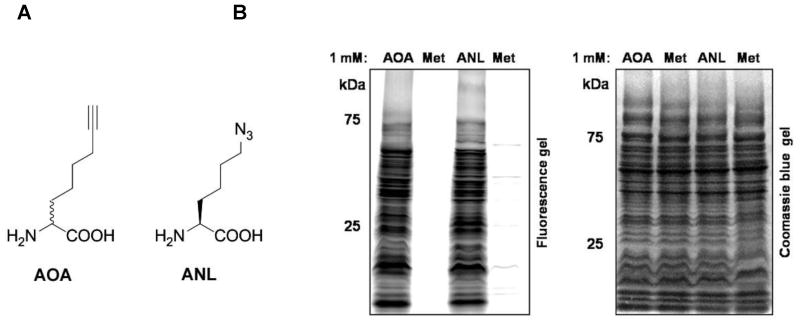
We then evaluated the utility of cleavable affinity tags 3 and 4 for capture and enrichment of azide/alkyne-labeled proteins. As an abundant source of azide/alkyne-labeled proteins, amino-octynoic acid (AOA) and azido-norleucine (ANL)-metabolically labeled S. typhimurium cell lysates were utilized based on our previous studies with these amino acid chemical reporters (Figure 3) (Grammel et al., 2010). AOA (Link et al., 2006; Tanrikulu et al., 2009) and ANL (Grammel et al., 2010) are methionine (Met) surrogates that can be efficiently utilized by mutant Met tRNA synthetases (MetG) when expressed in bacteria such as S. typhimurium, serving as orthogonal chemical reporters of newly synthesized proteins. MetRS-PLL-S. typhimurium was labeled with 1 mM Met (negative control), 1 mM AOA or 1 mM ANL for 3 hours, lyzed with 4% sodium dodecyl sulfate (SDS) buffer to give total cell lysates (Grammel et al., 2010). The bacterial cell lysates were then reacted with clickable fluorophores (Charron et al., 2009) via CuAAC to confirm metabolic labeling of bacterial proteins with AOA or ANL, respectively (Figure 3). Indeed, AOA and ANL enable robust labeling of bacterial proteins in MetRS-PLL-S. typhimurium as previously described (Figure 3) (Grammel et al., 2010). The clickable and cleavable affinity tags 3 and 4 were then evaluated for CuAAC, streptavidin enrichment and Na2S2O4 elution of AOA- and ANL-labeled proteins using our previously reported conditions (Yang et al., 2010; Yount et al., 2010; Grammel et al., 2010; Rangan et al., 2010). Contrary to azido-diazo-biotin 1, cleavable affinity tags 3 and 4 showed relatively poor protein recovery in Na2S2O4-eluants, where most labeled proteins remained bound to the beads that were released after boiling with reducing and denaturing protein loading buffer (Figure S1). Comparative analysis of compounds 1 and 3 with N-ethyl-6-ethynyl-1,8-naphthalimide (Sawa et al., 2006), a model CuAAC substrate, yielded comparable formation of triazole products (Figure S2), demonstrating that click chemistry reactivity was not responsible for the differences in protein elution yields between the first and second generation affinity tags.
Figure 3. (A) Chemical structures of 2-aminooctynoic acid (AOA) and azidonorleucine (ANL). (B) In-gel fluorescent profiling of Met- AOA- and ANL-metabolically-labeled bacterial proteomes.
MetRS-NLL S. typhimurium was labeled with 1 mM Met (negative control), AOA or ANL for 1 hour. The cell lysates were subjected to CuAAC with azido-rhodamine (Charron et al., 2009) and analyzed by in-gel fluorescence scanning. Coomassie blue gel shows proteins were equally loaded.
We analyzed the Na2S2O4-cleavage efficiencies of affinity tags 1, 3 and 4 by HPLC to determine discrepancies in their reactivity (Figure 4A). As expected, the diazobenzene motif of 1 was completely reduced to the corresponding cleaved product after 1 minute with 25 mM Na2S2O4 in phosphate buffer saline (PBS) at pH 7.4 (Figure 4B). The identical conditions resulted in >95% disappearance of 3 and 4, emergence of the diazobenzene cleaved products, but also the accumulation of intermediates that comprised about 40% of the resulting reaction products. (Figures 4C and 4D). These intermediates persisted even after 1 hour (Figures 4C and D). Mass spectrometry analysis of these intermediates revealed partially reduced hydrazine products of 3 and 4 (Figures S3B and S3C). Compounds 3 and 4 both afforded two peaks of equal mass by LC-MS analysis, which likely reflect the cis-trans isomers of diazobenzene moiety presumably due to the lack of ortho-hydroxyl group on their aromatic rings (Figures 4C and 4D). The slower eluting isomers of both 3 and 4 (isomer 2) appear to react with Na2S2O4 more rapidly, as the majority of these compounds are absent after one minute, whereas the less abundant and faster eluting isomers (isomer 1) still persist (Figures 4C and 4D). After 1 hour all starting isomers of 3 and 4 were converted into the Na2S2O4-cleaved product or partially reduced intermediate. We also examined diazobenzene cleavage with 300 mM Na2S2O4, which was reported by Hulme and co-workers for a structurally similar diazobenzene-functionalized affinity tag (Landi et al., 2010). While 300 mM Na2S2O4 improved the cleavage efficiency of 3 and 4, 20–35% of the partially reduced hydrazide products persisted even after 1 hour (Figure 4C and 4D). The different diazobenzene reduction efficiencies observed on our first-generation (1) and second-generation (3 and 4) cleavable affinity tags suggested that the ortho-hydroxyl substituent on the aromatic ring of diazobenzene might be essential for rapid Na2S2O4 reduction.
Figure 4. HPLC analysis of the diazobenzene reduction efficiencies for affinity tags 1 and 3–6.
Each diazobenzene-based affinity tag (2 μL from 5 mM stock solution, final concentration = 0.1 mM) was treated with 100 μL of freshly-made Na2S2O4 (in PBS, pH 7.4) of the indicated concentrations. At the described time points, the reaction solution was immediately injected into analytical reversed-phase HPLC. HPLC analysis was conducted with H2O/CH3CN: 90%/10% to 15%/85% over 20 min. (A) Schematic of reduction of diazobenzene-based affinity tags by Na2S2O4. (B) azido-diazo-biotin (1). (C) azidoethoxy-diazo-biotin (3). (D) alkynylmethoxy-diazo-biotin (4). (E) ortho-hydroxyl-azidoethoxy-diazo-biotin (5). (F) Treatment of tag 4 with 300 mM Na2S2O4 solution of various urea/thiourea concentrations. The cleavage efficiencies enhanced when the amounts of thiourea increased in the cleavage solution. The cleavage efficiency difference between 6 M urea/2 M thiourea and 8M urea indicates that thiourea plays the major role in cleavage efficiency enhancement. (G) bromo-azido-diazo-biotin (6). See also Figure S3.
Synthesis and evaluation of ortho-hydroxylated diazobenzene-functionalized affinity tag
To determine whether ortho-hydroxyl group of the diazobenzene moiety was a key factor for Na2S2O4-cleavage, we generated azidoethoxy ortho-hydroxyl-diazo-biotin affinity tag 5 (Figure 2B). Tag 5 exhibits the same structure as tag 3, except for an ortho hydroxyl group. Compound 5 was synthesized by monoalkylation of resorcinol followed by diazo-coupling of 12 with methyl 4-aminobenzoate to afford 13. Though both the hydroxyl and 2-azidoethoxyl groups of compound 12 can direct diazo-coupling to their ortho positions to yield three possible regioisomers 13a–c (Figure S4), after flash column chromatography, we only isolated one product with the correct mass. 1H-NMR analysis of this product excluded the possibility of regioisomer 13c as it exhibits a different peak splitting pattern from what we observed (Figure S4). To differentiate regioisomer 13a from 13b, compound 13 was subjected to Na2S2O4 reduction to yield product 16, which was then reacted with phenol isothiocyanate followed by cyclization under the treatment with sodium hydroxide and 4-toluenesulfonyl chloride to give compound 17 (Figure S4). Only the 2-aminophenol moiety is capable of forming the compound 17, confirming the diazo-coupling product was the desired regioisomer 13a (Figure S4). Compound 13a was then saponified, converted to N-hydroxysuccinimide ester and coupled with biotin-PEG-NH2 to give cleavable affinity tag 5. The cleavage efficiency of tag 5 was evaluated by treatment with 25 mM Na2S2O4 (in PBS, pH 7.4) for 1 minute. As shown by HPLC analysis (Figure 4E), the reactivity of 5 toward Na2S2O4 is comparable to tag 1, indicating the ortho-hydroxyl group is essential for efficient diazobenzene cleavage.
Comparative analysis of protein enrichment and elution with cleavable affinity tags
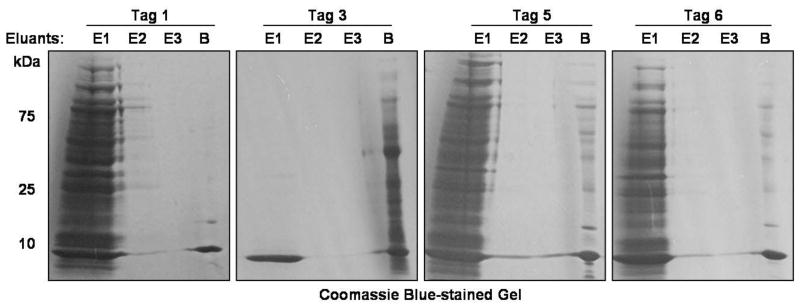
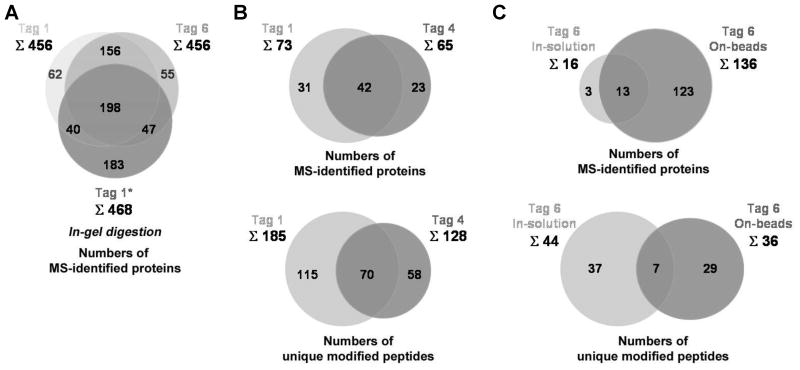
Based on our HPLC analytical results (Figure 4), we reexamined the elution of AOA-labeled proteins, which were clicked to tags 1, 3 and 5, from the streptavidin beads in the gel-based proteomics approach (Figure 1). AOA-labeled S. typhimurium cell lysates were reacted with azide-functionalized affinity tags 1, 3 and 5 via CuAAC and then precipitated to remove excess “click reagents”. Air-dried protein pellets were resuspended in 6 M urea/2 M thiourea/10 mM HEPES pH 8.0 (Choudhary et al., 2009), instead of previously reported 4% SDS (Yang et al., 2010), since this urea buffer is more compatible with the solubility of membrane proteins (Rabilloud et al., 1998) and the conditions used for in-solution protein reduction-alkylation and protease digestion (Smejkal et al., 2006). The protein suspensions were then reduced with 1 mM dithiothreitol (DTT), alkylated with 5.5 mM iodoacetamide and incubated with streptavidin beads. After extensive washing, streptavidin beads-bound 1- and 5-tagged proteins were treated with 25 mM Na2S2O4 in 1% SDS at pH 7.4. In contrast, beads-bound proteins bearing affinity tag 3 were eluted by 300 mM Na2S2O4 in 1% SDS at pH 7.4. Affinity tag 5 exhibited similar protein elution efficiencies as tag 1 (Figure 5). The majority of bound proteins were cleaved from beads within the first 30 minutes. Notably, SDS was essential for solublizing proteins and recovering proteins from the beads during Na2S2O4 elution (Figures S5A and S5B), even though it is not essential for rapid diazobenzene cleavage in vitro (Figure 4). On the other hand, affinity tag 3 still yielded poor protein elution results under the treatment of 300 mM Na2S2O4 (Figure 5), which suggests that the ortho-hydroxyl group is needed for Na2S2O4 elution of proteins from the beads. AOA-metabolically labeled S. typhimurium proteins enriched and recovered by using cleavable affinity tag 1 were then identified by in-gel proteomic protocol (Methods and Figure S5D) and revealed 456 high confident protein hits in which each protein contains at least 2-fold of unique peptides over control samples (Table S1). Unfortunately, the sites of AOA-modification within the recovered proteins were not evident by MS/MS analysis. Nonetheless, in comparison to our previous results with cleavable affinity tag 1 (Grammel et al., 2010), a similar number and profile of AOA-labeled S. typhimurium proteins were identified here using modified protein solubilization conditions (Figure 6A and Table S1).
Figure 5. SDS-PAGE analysis of Na2S2O4-protein elution efficiencies of compounds 1, 3, 5 and 6-tagged proteins.
Coomassie blue gel images show the elution profiles of metabolically-labeled proteins which were reacted with either tag 1, 3, 5 or 6 via CuAAC. Biotinylated proteins were enriched with streptavidin beads from 1.5 mg total cell lysates and then eluted with 25 mM Na2S2O4 (in 1% SDS, pH 7.4, for tags 1, 5 and 6) and 300 mM Na2S2O4 (in 1% SDS, pH 7.4, for tag 3). E1: the first elution fraction, E2: the second elution fraction, E3: the third elution fraction, B: the eluant from boiling the streptavidin beads in 4% SDS buffer/10% β-mercaptoethanol/1×LDS for 10 min. Each elution fraction represents 30 min Na2S2O4-treatment. The beads were washed twice between each elution using the cleavage buffer containing no Na2S2O4. See also Figure S5.
Figure 6. Overview of the proteomic analysis of AOA/ANL-labeled S. typhimurium proteins using diazobenzene-based cleavable affinity tags.
(A) Comparison of tag 1 and tag 6 in the numbers of their MS-identified AOA-labeled proteins via in-gel digestion approach and the comparison of these data to the previously published data (tag 1*) (Grammel et al., 2010). Starting with 4 mg of 1 mM Met/AOA-metabolically labeled cell lysates, the in-gel digestion approach gave total 456 protein hits for either tag 1 or tag 6. (B) Comparison of tag 1 and tag 4 in the numbers of their MS-identified AOA- and ANL-metabolically labeled proteins and unique modified peptides via in-solution digestion approach. Starting with 10 mg of 1 mM Met/AOA and Met/ANL-metabolically labeled cell lysates, the in-solution digestion approach yields total 185 unique modified peptides accounted for 73 proteins for tag 1 and total 128 unique modified peptides accounted for 65 proteins for tag 4. (C) Comparison of on-beads digestion approach and in-solution digestion approach in the numbers of their MS-identified AOA-metabolically labeled proteins and unique modified peptides using 6 as the affinity tag. Starting with 2 mg of 1 mM Met/AOA-metabolically labeled cell lysates, the in-solution digestion approach yields total 44 unique modified peptides accounted for 16 proteins whereas the on-beads digestion approach yields total 136 proteins as well as 36 unique modified peptides. See also Figure S5D and Tables S1–S5.
Analysis of protein modification sites of chemical reporter with cleavable affinity tags
To characterize the protein modification sites of alkyne- or azide-chemical reporters, we adapted the in-solution digestion approach to our diazobenzene-based affinity tags for modified peptide enrichment and elution (Figure 1). For these studies, 10 mg of Met- and AOA-labeled bacterial lysates were reacted with cleavable affinity tag 1 via CuAAC, subjected to protein reduction-alkylation in 6 M urea/2 M thiourea/10 mM HEPES pH 8.0, and then sequentially digested with endoproteinase Lys-C and trypsin. The resulting digested peptide mixtures were then incubated with streptavidin beads for affinity capture. As diazobenzene-based cleavable tags generally exhibit bright yellow color, proteins and peptides that are successfully tagged and captured on the streptavidin beads are readily apparent by yellow beads that after treatment with Na2S2O4 become translucent again. After the yellow-colored beads were extensively washed and reacted with 25 mM Na2S2O4 (in PBS, pH 7.4), the peptide eluants were desalted by C8-reversed phase column, lyophilized to dryness and analyzed by LC-MS/MS using the LTQ-Orbitrap. The search for recovered peptides that contained the AOA-labeled, CuAAC triazole and Na2S2O4 cleaved adduct (M*) of 315 Da revealed 185 unique peptides containing modified amino acid residue in place of Met (Figure 7A and Table S2), which comprised a total of 73 bacterial proteins (Figure 6B and Table S2). We also evaluated alkynyl affinity tag 4 using this protocol with 10 mg of Met- and ANL-labeled bacterial lysates. Surprisingly, 128 unique peptides containing the modified residue in place of Met (Figure 7B and Table S3), which were accounted for a total of 65 MS-identified proteins were recovered using compound 4 (Figure 6B and Table S4). As Na2S2O4-elution of these AOA-modified peptides was conducted in PBS, this result led us to analyze whether the diazobenzene cleavage efficiencies could be affected by detergents, such as SDS that were used in the on-bead protein elution experiments by HPLC. Given the incompatibility of the detergents with HPLC, we thus tested the effects of chaotropic agents on the diazobenzene cleavage instead. Compound 1 showed slightly decreased Na2S2O4-reactivity when 6 M urea/2 M thiourea was included in the reaction buffer (Figures S6A and S6B). However, the diazobenzene reduction yields for both compounds 3 and 4 were improved to ~90% when 2 M thiourea was added to 300 mM Na2S2O4 (Figures 4F and S6C ). These unexpected results led us to revisit the on-bead protein elution of compound 3-tagged proteins using 300 mM Na2S2O4 (in 6 M urea/2 M thiourea/10 mM HEPES pH 8.0). Despite the improved cleavage efficiency observed on HPLC (Figure S6A), compound 3 still yielded poor protein recovery even in the presence of 2 M thiourea (Figure S5C). Overall, the diazobenzene cleavable affinity tags carrying ortho-hydroxyl groups (1 and 5) would allow the most efficient protein identification and mapping of chemical reporter modification sites.
Figure 7. Selected MS/MS spectra of the modified universal stress protein G peptides (aa 70–83).
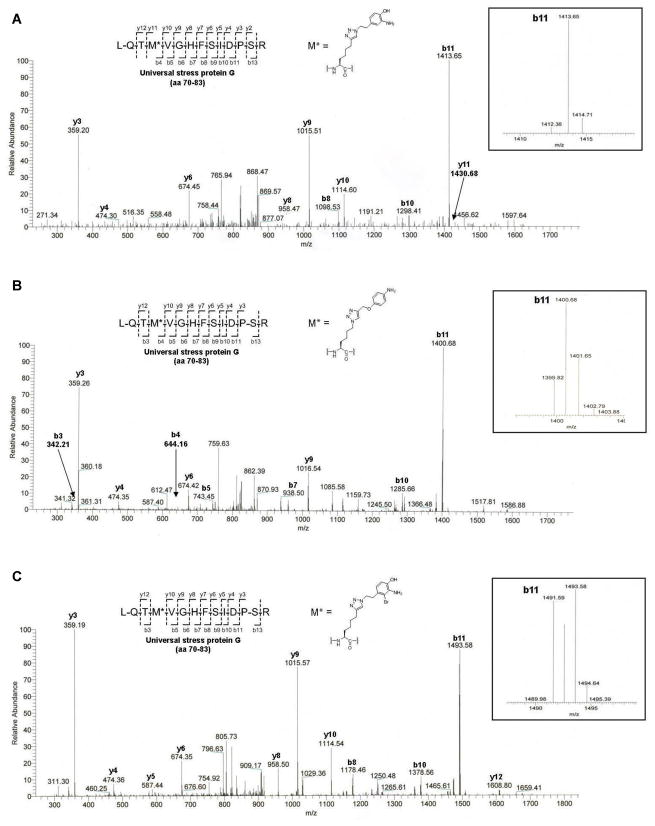
AOA/ANL-metabolically-modified proteins were clicked to tag 1, 4 or 6 via CuAAC and then processed via in-solution proteomic strategy. Selected MS/MS spectra were derived from the results of MS analysis of those streptavidin-enriched and Na2S2O4-eluted modified peptides. (A) MS/MS spectrum of the modified peptide LQTM*VGHFSIDPSR (M* = AOA + tag 1/CuAAC/Na2S2O4-cleavage adduct, the molecular weight of M* = 315). (B) MS/MS spectrum of the modified peptide LQTM*VGHFSIDPSR (M* = ANL + tag 4/CuAAC/Na2S2O4-cleavage adduct, the molecular weight of M* = 301). (C) MS/MS spectrum of the modified peptide LQTM*VGHFSIDPSR (M* = AOA + tag 6/CuAAC/Na2S2O4-cleavage adduct, the molecular weight of M* = 393). See also Tables S2–S4.
Synthesis and evaluation of clickable and cleavable brominated diazobenzene affinity tag
Given the utility of ortho-hydroxylated diazobenzene affinity tags for bioorthogonal chemical proteomic studies, we also synthesized an isotopically-encoded cleavable affinity tag 6 bearing a bromine atom. Naturally occurring bromine has two major isotopes of similar abundance that can facilitate protein identification and analysis of chemical reporter modification sites by mass spectrometry. The brominated diazobenzene-functionalized affinity tag 6 was synthesized as described for compound 1, but starting with 2-bromo-4-(2-azidoethyl)-phenol 14 (Figure 3C). The cleavage efficiency of 6 in the presence of 25 mM Na2S2O4 in PBS, pH 7.4 was then evaluated by HPLC. Within 1 minute, the majority of 6 were reduced to the corresponding cleaved products, while ~10% remained partially-reduced. (Figure 4G). Even though bromine can serve as a deactivating substituent on the benzene ring which might contribute to the observed slightly slower Na2S2O4-reduction efficiency of 6 on HPLC (Figure 4G), cleavable affinity tag 6 exhibited similar performance as tag 1 in CuAAC, streptavidin enrichment and Na2S2O4-elution of AOA-labeled proteins (Figures 5 and S5D). In addition, gel-based bioorthogonal chemical proteomics using affinity tag 6 gave total 456 MS-identified AOA-labeled S. typhimurium proteins with high confidence, 77% of which overlap with those proteins recovered by using affinity tag 1 (Figure 7A and Table S1).
We also carried out site of modification studies with cleavable affinity tag 6. AOA-labeled S. typhimurium lysates (2 mg) were reacted with 6 via CuAAC, subjected to the in-solution protease digestion and peptide enrichment protocol. LC-MS/MS analysis of recovered peptides gave 44 unique peptides containing modified residue in place of Met which were accounted for 16 identified proteins (Table S4 and Figures 6C). As shown in Figure 7C, any peptide ion fragment that bears modified residue (y12, b5–11 and b12) shows a characteristic isotopic pattern of bromine. In addition to gel-based and in-solution proteomic approaches (Figure 1), we also evaluated cleavable affinity tag 6 using on-beads digestion approach. AOA-labeled S. typhimurium lysates (2 mg) were subjected to CuAAC with 6 followed by protein reduction, alkylation and enrichment on the streptavidin beads. After extensive washing, the streptavidin beads-bound proteins were sequentially treated with endoproteinase Lys-C and trypsin (Methods). The protease-digested peptides were then collected, extracted by C8 columns and concentrated for LC-MS/MS analysis. The remaining bound peptides on streptavidin beads were treated with 25 mM Na2S2O4 in PBS, pH 7.4 to elute diazobenzene-linked AOA-labeled peptides for LC-MS/MS analysis. Using this protocol, we identified 136 S. typhimurium proteins together with 36 unique peptides containing the modified residue in place of Met (Table S5 and Figure 6C). All the proteins that were identified in Na2S2O4-eluants were also presented in protease-digested peptide fractions (Table S5B). These results demonstrated that isotopically-encoded cleavable affinity tag 6 allows robust protein identification and mapping of chemical reporter modification sites using different platforms for proteomics.
DISCUSSION
Diazobenzene-functionalized probes have been utilized for diverse chemical biology applications, ranging from photo-induced protein regulation (Banghart et al., 2004; Sadovski et al., 2009; Schierling et al., 2010; Fortin et al., 2008), specific recovery of proteins targeted by activity-based probes (Fonovic et al., 2007), chemical reporters (Yang et al., 2010; Yount et al., 2010; Grammel et al., 2010; Rangan et al., 2010) as well as non-covalent small molecule ligands (Landi et al., 2010; Budin et al., 2009). Here we demonstrate that the clickable diazobenzene-based affinity tags enable robust proteomic analysis and site mapping of proteins targeted by chemical reporters. These biotinylated diazobenzene-based affinity tags are compatible with CuAAC conditions; allow the robust capture of protein/peptides using streptavidin matrices and enable efficient elution of selectively recovered proteins/peptides for proteomics. We show here that the reactivity of diazobenzene towards Na2S2O4 was governed by the substituents on the aromatic ring. Strong electron-donating group, like -OH on ortho position accelerates the Na2S2O4-cleavage, while the presence of weak electron-withdrawing group, like bromine on ortho position slightly impedes this cleavage efficiency. These results are consistent with recent structure-reactivity studies of diazobenzene derivatives by Wagner and coworkers (Leriche et al., 2010). Nevertheless, it is worth to mention that ortho-hydroxyl group may not impose its effect on diazobenzene-bond reactivity toward Na2S2O4 merely through mesomeric effect. As indicated in our studies and Wagner and coworkers (Leriche et al., 2010), para-alkoxylated diazobenzne (3 and 4 in this study) did not possess better cleavage efficiencies, suggesting ortho-hydroxyl group might accelerate the diazobenzene-bond cleavage through other mechanisms such as intramolecular hydrogen bonding with nitrogen atom of diazobenzene (Kuvshinova et al., 2006). Further detailed investigation on Na2S2O4-mediated diazobenzene cleavage mechanism and structure-and-activity studies would help to reveal the roles of ortho-hydroxyl group in this reaction.
Using bacterial cell lysates that were metabolically labeled with amino acid chemical reporters (AOA or ANL), we demonstrated that these cleavable affinity tags (1, 3–6) could be used for proteomic profiling of the newly synthesized proteins in S. typhimurium. These cleavable affinity tags are compatible with diverse platforms for proteomics that also allows mapping sites of protein modifications (Figure 1). The advantage of on-bead protease digestion is that it allows modified peptides to be analyzed separately from non-modified peptides, thus improving protein coverage and detection sensitivity during MS analysis. Notably, the triazole adducts from click chemistry/Na2S2O4-cleavage were relatively small and not readily fragmented during MS/MS experiments, which were useful for mapping sites of AOA/ANL-modification (Figure 7). Our MS/MS analysis of selectively enriched peptides revealed AOA/ANL substituted for Met residues in all peptide sequences (Figure 7). These results confirm that both AOA and ANL are indeed Met surrogates and can be efficiently used by mutant MetGPLL over-expressed in S. typhimurium (Grammel et al., 2010). While ortho-hydroxylated-diazobenzene cleavable affinity tags (1, 2, 5 and 6) are effective for bioorthogonal chemical proteomic applications with alkyne-modified proteins/peptides, the ortho-hydroxylated derivative of tag 4 should be useful for proteomic studies with azide-modified substrates. In addition, the characteristic isotopic pattern of brominated cleavable affinity tag 6 should also aid in distinguishing the modification-bearing peptide ions from other ion peaks. These reagents provide useful tools to circumvent the high affinity binding of biotin to streptavidin reagents for protein enrichment that are complementary to other enzymatic or chemical cleavage methods (Speers and Cravatt, 2005; Dieterich et al., 2006; Nessen et al., 2009; Park et al., 2009; Dirksen et al., 2010).
SIGNIFICANCE
Chemical probes are affording new opportunities to investigate protein and enzyme regulation. Central to these new technologies is the development of bioorthogonal ligation methods for exploring small molecule-protein interactions using specific probes of enzymes or reporters of protein modifications (Sletten and Bertozzi, 2009; Simon and Cravatt, 2010). The identification of the proteins that are targeted by small molecule probes/reporters is essential for these functional studies. We present here the synthesis and characterization of selectively cleavable affinity tags for versatile and robust bioorthogonal chemical proteomic studies. The azide/alkyne-functionalized ortho-hydroxylated-diazobenzene cleavable affinity tags enabled selective enrichment and efficient elution of alkyne- and azide-labeled proteins/peptides from complex mixtures and allowed protein identification and modification-site mapping using diverse proteomic platforms. The advances presented here should facilitate bioorthogonal chemical proteomics studies in diverse biological settings.
EXPERIMENTAL PROCEDURES
Detailed characterization of compound 3–17, metabolic labeling procedure and mass spectrometric analysis method are in supplemental experimental procedures.
CuAAC of cleavable affinity tags with Met, AOA or ANL-labeled cell lysates
Met, AOA or ANL-labeled cell lysates (1–10 mg) were diluted into 4% SDS buffer (4% SDS, 150 mM NaCl, 50 mM triethanolamine, pH 7.4) to give 1mg/mL final protein concentration. Protein mixture was subjected to Cu(I)-catalyzed cycloaddition reaction by adding pre-mixed “click chemistry cocktail” [100 μM cleavable biotin linker, 1 mM TCEP, 100 μM (Tris[(1-benzyl-1H-1, 2, 3-triazole-4-yl)methyl] amine (TBTA) (Chan et al., 2004) and 1 mM CuSO4]. The reaction was allowed to sit at room temperature for 1.5 h. Proteins were then precipitated by adding 10 volume of chilled MeOH, followed by overnight precipitation at −20 °C. Precipitated proteins were pelleted by centrifugation (5,200× g, 30 min, 4 °C), washed trice with chilled MeOH and air-dried.
In-gel trypsin digestion of AOA-labeled proteins for mass spectrometric analysis
Air-dried protein pellets were resuspended in 6 M urea/2 M thiourea/10 mM HEPES pH 8.0 (Choudhary et al., 2009). Proteins were reduced with 1 mM DTT (100 mM stock) for 40 min and then alkylated with 5.5 mM iodoacetamide (550 mM stock) in the dark. Pre-washed streptavidin beads were added and incubated with protein solution at room temperature for 1.5 h on end-over-end rotator. The beads were sequentially washed trice with 6 M urea/2 M thiourea/10 mM HEPES pH 8.0, GIBCO’s PBS and 1% SDS (in 1× GIBCO’s PBS). Centrifugation of the beads between washing steps was carried out (2000× g, 3 min). Bound proteins were cleaved from the beads by treating with the elution buffer (1% SDS, 25 mM Na2S2O4, 1× GIBCO’s PBS) for 30 min trice. Collect and combine the eluants. Removal of the majority of Na2S2O4 from the eluants was achieved by applying the eluants onto the microcon centrifugal filter device (3 kDa NMWL, Millipore) and exchanging the buffer with PBS (the majority of SDS will still retain on the top of the membrane). The concentrated protein mixture was then dried in SpeedVac. Re-solubilize the dried protein pellet with 1×LDS/5% β-mercaptoethanol. Resuspended protein mixtures were boiled at 95 °C for 5 min and then loaded onto SDS-PAGE gel (4–20% Tris-HCl Criterion precast gel, Bio-Rad Laboratories). The profiles of AOA-metabolically labeled proteins together with their negative controls were visualized by Coomassie blue staining. Each lane was sliced into 8 fractions and then each excised gel slice was further cut into more pieces. Gel pieces were washed with 50 mM ammonium bicarbonate (ABC) twice, destained with 50 mM ABC/acetonitrile (50/50) twice, and then dehydrated in 100% acetonitrile. After removing acetonitrile in SpeedVac, gel pieces were rehydrated with trypsin solution (2 μg of trypsin for each vial/gel slice) and incubated in 37 °C water bath for 18 h. The eluted trypsin-digested peptides were then collected and dried in SpeedVac. Re-solubilize the dried peptides in H2O (with 0.1% TFA) and submitted the samples to nano-HPLC/MS/MS analysis (Thermo LTQ-Orbitrap in the Proteomic Resource Center at Rockefeller University).
For comparative SDS-PAGE analysis of different affinity tags (Figure 5), after each Na2S2O4-elution, the beads were washed twice with the elution buffer (without sodium dithionite). The eluant from each cleavage was filtered through Pierce centrifuge column to remove any remaining beads, buffer-exchanged on the microcon centrifugal filter device to reduce the amount of Na2S2O4, and then lyophilized to dryness in SpeedVac. After re-solubilizing the dried protein mixture in 1×LDS/5% β-mercaptoethanol, the protein mixtures were boiled at 95 °C for 5 min and then loaded onto SDS-PAGE gel. On the other hand, after three times of Na2S2O4-elution, the beads were boiled in 4% SDS buffer/10% β-mercaptoethanol/1×LDS for 10 min to elute the remaining un-cleaved proteins.
In-solution protease digestion of AOA/ANL-labeled proteins for MS analysis
Air-dried pellets were resuspended in 6 M urea/2 M thiourea/10 mM HEPES pH 8.0 (Choudhary et al., 2009). Proteins were reduced with 1 mM DTT (100 mM stock) for 40 min and then alkylated with 5.5 mM iodoacetamide (550 mM stock) for 30 min in the dark. Reduce urea concentration to 4.5 M urea/1.5 thiourea by adding 1/3 volume of 10 mM HPEPS pH 8.0. Lysyl endopeptidase (Lys-C, w/w = 1/100) was added and protein digestion was carried out at room temperature for 4 h. Urea concentration was further reduced by adding 3 volumes of 10 mM HEPES pH 8.0 to allow trypsin digestion (w/w = 1/100) being carried out at room temperature for 18 h. The resulting protease-digested peptides were then incubated with pre-washed streptavidin beads at room temperature for 1.5 h on end-over-end rotator. The beads were sequentially washed trice with 1.5 M urea/10 mM HEPES pH 8.0 and 10 mM HEPES pH 8.0. Bound peptides were cleaved from the beads by treating with freshly-made elution buffer (25 mM sodium dithionite, 1× GIBCO’s PBS) for 1 h. Repeat this cleavage step by treating the beads with freshly-made elution buffer for 10 min twice. Collect and combine the eluants. The cleaved peptides were cleaned up by using C8 cartridge (Waters) and eluted with 70% CH3CN/20% H2O/0.1% TFA. Eluted peptides were dried in SpeedVac and then re-solubilize in H2O (with 0.1% TFA) for nano-HPLC/MS/MS analysis.
On-bead protease digestion of AOA-labeled proteins for MS analysis
Air-dried pellets were resuspended in 6 M urea/2 M thiourea/10 mM HEPES pH 8.0 (Choudhary et al., 2009). Proteins were reduced with 1 mM DTT (100 mM stock) for 40 min and then alkylated with 5.5 mM iodoacetamide (550 mM stock) in the dark for 30 min. Pre-washed streptavidin beads were added and incubated with protein mixtures at room temperature for 1.5 h on end-over-end rotator. The beads were sequentially washed trice with 6 M urea/2 M thiourea/10 mM HEPES pH 8.0, 0.2% SDS (in PBS) and PBS. The beads were then re-suspended in 4.5 M urea/1.5 M thiourea/10 mM HEPES pH 8.0 and incubation with Lys-C (w/w = 1/100) at room temperature for 4 h. Urea concentration was further diluted by adding 3 volumes of 10 mM HEPES pH 8.0 to enable trypsin digestion (w/w = 1/100) being carried out at room temperature for 18 h. The beads were then spun down at 2000× g for 1 min. Supernatant (tryptic solution) was collected and the beads were subjected to Na2S2O4-cleavage. Both the tryptic solution and eluant collected from Na2S2O4-cleavage were cleaned-up using C8 cartridge, eluted by 70% CH3CN/20% H2O/0.1% TFA and dried in SpeedVac for mass spectrometric analysis.
Supplementary Material
Acknowledgments
We thank the Rockefeller University Proteomics Resource Center for MS analysis. G. C. is a graduate fellow in the Rockefeller/Sloan-Kettering/Weill-Cornell Tri-Institutional Program in Chemical Biology. H.C.H. acknowledges support from NIH/NIDA (1R21DA025751-01), Northeastern Biodefense Center NIH/NIAID (2 U54 AI057158-06), Irma T. Hirschl/Monique Weill-Caulier Trust and Lerner Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bahulayan DJ, Lalithambika LM. Modified clays as efficient acid-base catalyst systems for diazotization and diazocoupling reactions. Synth Comm. 2003;33:863–869. [Google Scholar]
- Banghart M, Borges K, Isacoff E, Trauner D, Kramer RH. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–1386. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin JM, Dehnert KW, Laughlin ST, Amacher SL, Bertozzi CR. visualizing enveloping layer glycans during zebrafish early embryogenesis. Proc Natl Acad Sci U S A. 2010;107:10360–10365. doi: 10.1073/pnas.0912081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best MD. Click chemistry and bioorthogonal reactions: unprecedented selectivity in the labeling of biological molecules. Biochemistry. 2009;48:6571–6584. doi: 10.1021/bi9007726. [DOI] [PubMed] [Google Scholar]
- Böttcher T, Pitscheider M, Sieber SA. Natural products and their biological targets: proteomic and metabolomic labeling strategies. Angew Chem Int Ed. 2010;49:2680–2698. doi: 10.1002/anie.200905352. [DOI] [PubMed] [Google Scholar]
- Budin G, Dimala MM, Lamour V, Oudet P, Mioskowski C, Meunier S, Brino L, Wagner A. A chemical labeling strategy for proteomics under nondenaturing conditions. ChemBioChem. 2009;11:79–82. doi: 10.1002/cbic.200900641. [DOI] [PubMed] [Google Scholar]
- Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Polytriazoles as copper(I)-stabilizing ligands in catalysis. Org Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- Charron G, Zhang MM, Yount JS, Wilson J, Raghavan AS, Shamir E, Hang HC. Robust fluorescent detection of protein fatty-acylation with chemical reporters. J Am Chem Soc. 2009;131:4967–4975. doi: 10.1021/ja810122f. [DOI] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Wright AT, Kozarich JW. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu Rev Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- Dieterich DC, Link AJ, Graumann J, Tirrell DA, Schuman EM. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT) Proc Natl Acad Sci U S A. 2006;103:9482–9487. doi: 10.1073/pnas.0601637103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich DC, Lee JJ, Link AJ, Graumann J, Tirrell DA, Schuman EM. Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non-canonical amino-acid tagging. Nat Protocols. 2007;2:532–540. doi: 10.1038/nprot.2007.52. [DOI] [PubMed] [Google Scholar]
- Dirksen A, Yegneswaran S, Dawson Philip E. Bisaryl hydrazones as exchangeable biocompatible linkers. Angew Chem Int Ed. 2010;49:2023–2027. doi: 10.1002/anie.200906756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Cravatt BF. Mechanism-based profiling of enzyme families. Chem Rev. 2006;106:3279–3301. doi: 10.1021/cr050288g. [DOI] [PubMed] [Google Scholar]
- Fauq AH, Kache R, Khan MA, Vega IE. Synthesis of acid-cleavable light isotope-coded affinity tags (ICAT-L) for potential use in proteomic expression profiling analysis. Bioconjug Chem. 2005;17:248–254. doi: 10.1021/bc0503059. [DOI] [PubMed] [Google Scholar]
- Fonovic M, Verhelst SHL, Sorum MT, Bogyo M. Proteomics evaluation of chemically cleavable activity-based pProbes. Mol Cell Proteomics. 2007;6:1761–1770. doi: 10.1074/mcp.M700124-MCP200. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Banghart MR, Dunn TW, Borges K, Wagenaar DA, Gaudry Q, Karakossian MH, Otis TS, Kristan WB, Trauner D, Kramer RH. Photochemical control of endogenous ion channels and cellular excitability. Nat Methods. 2008;5:331–338. doi: 10.1038/nmeth.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner CA, Elias JE, Bakalarski CE, Gygi SP. Catch-and-release reagents for broadscale quantitative proteomics analyses. J Proteome Res. 2007;6:1482–1491. doi: 10.1021/pr060605f. [DOI] [PubMed] [Google Scholar]
- Grammel M, Zhang MM, Hang HC. Orthogonal alkynyl amino acid reporter for selective labeling of bacterial proteomes during infection. Angew Chem Int Ed. 2010;49:1–6. doi: 10.1002/anie.201002050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZP, Watson JI, Hwang DSP, Szoka FC., Jr Facile synthesis of multivalent nitrilotriacetic acid (NTA) and NTA conjugates for analytical and drug delivery applications. Bioconjug Chem. 2006;17:1592–1600. doi: 10.1021/bc0602228. [DOI] [PubMed] [Google Scholar]
- Jewett JC, Bertozzi CR. Cu-free click cycloaddition reactions in chemical biology. Chem Soc Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuvshinova SA, Zav’yalov AV, Burmistrov VA, Aleksandriiskii VV, Koifman OI. Mesogenic 4-alkoxy-2-hydroxy-4’-formylaxobenzenes. Russ J Org Chem. 2006;42:393–395. [Google Scholar]
- Landi F, Johansson CM, Campopiano DJ, Hulme AN. Synthesis and application of a new cleavable linker for click-based affinity chromatography. Org Biomol Chem. 2010;8:56–59. doi: 10.1039/b916693a. [DOI] [PubMed] [Google Scholar]
- Laughlin ST, Bertozzi CR. Imaging the glycome. Proc Natl Acad Sci U S A. 2009;106:12–17. doi: 10.1073/pnas.0811481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leriche G, Budin G, Brino L, Wagner A. Optimization of the azobenzene scaffold for reductive cleavage by dithionite; development of an azobenzene cleavable linker for proteomic applications. Eur J Org Chem. 2010 doi: 10.1002/ejoc.201000546. [DOI] [Google Scholar]
- Link AJ, Vink MKS, Agard NJ, Prescher JA, Bertozzi CR, Tirrell DA. Discovery of aminoacyl-tRNA synthetase activity through cell-surface display of noncanonical amino acids. Proc Natl Acad Sci U S A. 2006;103:10180–10185. doi: 10.1073/pnas.0601167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nessen MA, Kramer G, Back J, Baskin JM, Smeenk LEJ, de Koning LJ, van Maarseveen JH, de Jong L, Bertozzi CR, Hiemstra H, de Koster CG. Selective enrichment of azide-containing peptides from complex mixtures. J Proteome Res. 2009;8:3702–3711. doi: 10.1021/pr900257z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KD, Liu R, Kohn H. Useful tools for biomolecule isolation, detection, and identification: acylhydrazone-based cleavable linkers. Chem Biol. 2009;16:763–772. doi: 10.1016/j.chembiol.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Petrotchenko EV, Xiao K, Cable J, Chen Y, Dokholyan NV, Borchers CH. BiPS, a photocleavable, isotopically coded, fluorescent cross-linker for structural proteomics. Mol Cell Proteomics. 2009;8:273–286. doi: 10.1074/mcp.M800265-MCP200. [DOI] [PubMed] [Google Scholar]
- Rabilloud T. Simultaneous reduction and alkylation of protein disulfides in a centrifugal ultrafiltration device prior to two-dimensional gel electrophoresis. Electrophoresis. 1998;19:758–760. doi: 10.1021/pr050439w. [DOI] [PubMed] [Google Scholar]
- Rangan KJ, Yang Y-Y, Charron G, Hang HC. Rapid visualization and large-scale profiling of bacterial lipoproteins with chemical reporters. J Am Chem Soc. 2010 doi: 10.1021/ja101387b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostovtsev VV, Green LK, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective ligation of azides and terminal alkynes. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Sadovski O, Beharry, Andrew A, Zhang F, Woolley GA. Spectral tuning of azobenzene photoswitches for biological applications. Angew Chem Int Ed. 2009;48:1484–1486. doi: 10.1002/anie.200805013. [DOI] [PubMed] [Google Scholar]
- Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong CH. Glycoproteomic probes for fluorescent imaging of fucosylated glycans in vivo. Proc Natl Acad Sci U S A. 2006;103:12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon E, Bertozzi CR. Cell surface engineering by a modified staudinger reaction. Science. 2000;287:2007–2010. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
- Schierling B, Noël AJ, Wende W, Hien LT, Volkov E, Kubareva E, Oretskaya T, Kokkinidis M, Römpp A, Spengler B, Pingoud A. Controlling the enzymatic activity of a restriction enzyme by light. Proc Natl Acad Sci U S A. 2010;107:1361–1366. doi: 10.1073/pnas.0909444107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkus M, Levy J, Herman T. A chemically cleavable biotinylated nucleotide: usefulness in the recovery of protein-DNA complexes from avidin affinity columns. Proc Natl Acad Sci U S A. 1985;82:2593–2597. doi: 10.1073/pnas.82.9.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Activity-based proteomics of enzyme superfamilies: serine hydrolases as a case study. J Biol Chem. 2010;285:11051–11055. doi: 10.1074/jbc.R109.097600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sletten EM, Bertozzi CR. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew Chem Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejkal GB, Li C, Robinson MH, Lazarev AV, Lawrence NP, Chernokalskaya E. Simultaneous reduction and alkylation of protein disulfides in a centrifugal ultrafiltration device prior to two-dimensional gel electrophoresis. J Proteome Res. 2006;5:983–987. doi: 10.1021/pr050439w. [DOI] [PubMed] [Google Scholar]
- Speers AE, Cravatt BF. A tandem orthogonal proteolysis strategy for high-content chemical proteomics. J Am Chem Soc. 2005;127:10018–10019. doi: 10.1021/ja0532842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanrikulu IC, Schmitt E, Mechulam Y, Goddard WA, Tirrell DA. Discovery of Escherichia coli methionyl-tRNA synthetase mutants for efficient labeling of proteins with azidonorleucine in vivo. Proc Natl Acad Sci U S A. 2009;106:15285–15290. doi: 10.1073/pnas.0905735106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornoe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- Veken Pvd, Dirksen EHC, Ruijter E, Elgersma RC, Heck AJR, Rijkers DTS, Slijper M, Liskamp RMJ. Development of a novel chemical probe for the selective enrichment of phosphorylated serine- and threonine-containing peptides. ChemBioChem. 2005;6:2271–2280. doi: 10.1002/cbic.200500209. [DOI] [PubMed] [Google Scholar]
- Verhelst SH, Fonovi M, Bogyo M. A mild chemically cleavable linker system for functional proteomic applications. Angew Chem Int Ed. 2007;46:1284–1286. doi: 10.1002/anie.200603811. [DOI] [PubMed] [Google Scholar]
- Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J Am Chem Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- Weerapana E, Speers AE, Cravatt BF. Tandem orthogonal proteolysis-activity-based protein profiling (TOP-ABPP)-a general method for mapping sites of probe modification in proteomes. Nat Protocols. 2007;2:1414–1425. doi: 10.1038/nprot.2007.194. [DOI] [PubMed] [Google Scholar]
- Yang YY, Ascano JM, Hang HC. Bioorthogonal chemical reporters for monitoring protein acetylation. J Am Chem Soc. 2010;132:3640–3641. doi: 10.1021/ja908871t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount JS, Moltedo B, Yang YY, Charron G, Moran TM, López CB, Hang HC. Palmitoylome profiling reveals S-palmitoylation-dependent antiviral activity of IFITM3. Nat Chem Biol. 2010;6:610–614. doi: 10.1038/nchembio.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, Tsou LK, Charron G, Raghavan AS, Hang HC. Tandem fluorescence imaging of dynamic S-acylation and protein turnover. Proc Natl Acad Sci U S A. 2010;107:8627–8632. doi: 10.1073/pnas.0912306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.