Abstract
Alcoholic liver disease (ALD) is characterized by steatosis or fat deposition in the liver and inflammation, which leads to cirrhosis and hepatocellular carcinoma. Induction of target genes without involving changes in DNA sequence seems to contribute greatly to liver injury. Chromatin modifications including alterations in histones and DNA, as well as post-transcriptional changes collectively referred to as epigenetic effects are altered by alcohol. Recent studies have pointed to a significant role for epigenetic mechanisms at the nucleosomal level influencing gene expression and disease outcome in ALD. Specifically, epigenetic alterations by alcohol include histone modifications such as changes in acetylation and phosphorylation, hypomethylation of DNA, and alterations in miRNAs. These modifications can be induced by alcohol-induced oxidative stress that results in altered recruitment of transcriptional machinery and abnormal gene expression. Delineating these mechanisms in initiation and progression of ALD is becoming a major area of interest. This review summarizes key epigenetic mechanisms that are dysregulated by alcohol in the liver. Alterations by alcohol in histone and DNA modifications, enzymes related to histone acetylation such as histone acetyltransferases, histone deacetylases and sirtuins, and methylation enzymes such as DNA methyltransferases are discussed. Chromatin modifications and miRNA alterations that result in immune cell dysfunction contributing to inflammatory cytokine production in ALD is reviewed. Finally, the role of alcohol-mediated oxidative stress in epigenetic regulation in ALD is described. A better understanding of these mechanisms is crucial for designing novel epigenetic based therapies to ameliorate ALD.
Keywords: Alcohol, Epigenetics, Histones, Acetylation, DNA methylation, miRNA, Genes
INTRODUCTION
Chronic alcohol consumption has a significant impact on human health and is identified as a major risk factor for development of liver disease. Alcoholic liver disease (ALD) is one of the leading causes of liver disease mortality worldwide. Alcohol-related liver injury includes a spectrum of pathological conditions in the liver including steatosis, steatohepatitis, cirrhosis and hepatocellular carcinoma. Of the molecular mechanisms studied, epigenetic mechanisms altered by alcohol appear to play a significant role in development and progression of disease. These mechanisms have been identified in parenchymal and non-parenchymal cells in the liver and contribute to induction of fatty liver, inflammation, as well as hepatocyte apoptosis and necrosis. In the past decade, a number of studies have reported epigenetic alterations by alcohol in the liver including histone modification, DNA and histone methylation derived from the methyl group donating system, S-adenosyl methionine (SAMe), miRNA as post-transcriptional modifiers, and chromatin remodeling enzymes responsible for epigenetic regulation such as histone acetyltransferases, histone deacetylases and DNA methyltransferases[1,2]. Defining an epigenetic imprint in alcohol-induced liver injury will provide new insights into pathophysiological mechanisms and open avenues for potential novel epigenetics-based therapeutics.
The goal of this concise article is to review alcohol-mediated epigenetic alterations, in parenchymal and non-parenchymal cells of the liver. Specifically, alcohol-mediated alterations in epigenetic enzymes, miRNAs as epigenetic modifiers, chromatin modifications affecting immune cell function in alcoholic liver, and the role of oxidative stress in chromatin remodeling that regulates alcohol-mediated gene expression in the liver are described.
THE EPIGENETIC CODE
The classical view defines epigenetics as heritable changes that affect gene expression without altering the DNA sequence. Epigenetic regulation of gene expression is facilitated through different mechanisms such as DNA methylation, histone modifications, and RNA-associated silencing by small non-coding RNAs. All these mechanisms are crucial for normal development, differentiation and tissue-specific gene expression. These three systems interact and stabilize one another and can initiate and sustain epigenetic silencing, thus determining heritable changes in gene expression. Alterations in one or more of these systems leads to inappropriate target gene expression or silencing that results in epigenetic regulation of human diseases such as cancer, autoimmune diseases, and age-related as well as neurological disorders[3,4]. Epigenetic abnormalities are diverse, tissue-specific and can occur due to various environmental factors such as toxins and drugs, including alcohol.
Epigenetic regulation of gene expression primarily works through modifying the secondary or tertiary structures of DNA (chromatin), which makes it more or less accessible to transcription. Chromatin is made up of repetitive structural units called nucleosomes. Nucleosomes are comprised of a histone octamer and the DNA that wraps around it. Histones are globular basic proteins that are subject to various covalent modifications that occur primarily on the N-terminal tail[5]. The histone octamer contains two molecules of each of the histones H2A, H2B, H3 and H4, around which the DNA wraps. Histone H1 the “linker histone” along with “linker DNA” physically connects the adjacent nucleosome core particles. Covalent histone modifications appear to act sequentially or in combination to form a recognizable code that is identified by specific proteins to regulate distinct downstream events such as transcriptional activation or repression[5]. Histones are subject to various post-translational modifications such as acetylation, methylation, phosphorylation, ubiquitinylation and sumoylation, all having an impact on gene transcription[5]. Histone acetylation is a transcription-activating modification that is achieved by addition of acetyl groups to lysine residues by enzymes called histone acetyltransferases (HATs). The major sites of acetylation in histone H3 are Lys4, Lys9, Lys14 and Lys28. Acetyl groups are removed by histone deacetylases (HDACs) and this is generally associated with loss of gene expression or silencing[6]. Mammalian HDACs have been classified into four classes. Class I HDACs (1, 2, 3 and 8) are found predominantly in the nucleus, whereas Class II HDACs (4 5, 6, 7, 9 and 10) shuttle between the nucleus and cytoplasm. Class I and Class II HDACs have tissue-specific expression profiles. HDACs 1, 2 and 3 are expressed in various immune cells[7]. Class III HDACs (SIRT1-SIRT7) form a distinct class of NAD-dependent enzymes, can be inhibited by nicotinamide, and are important in DNA repair and anti-apoptotic functions[8]. HDAC 11 possesses properties of Class I and II HDACs and is classified as Class IV. Table 1 illustrates the histone modifications and enzymes linked to the changes.
Table 1.
Histone modifications, enzymes and genes
| Modifications | Enzymes | Target gene transcription |
| Acetylation | ||
| H3 | GNAT | Activating |
| H4 | MYST | Activating |
| H3 and H4 | CBP/p300 | Activating |
| Deacetylation | ||
| H3 and H4 | HDACs 1-11 | Silencing |
| Methylation | ||
| H3K4 | Set1 | Activating |
| H3K36 | Set2 | Activating |
| H3K79 | DoT1L | Activating |
| H3K27 | EZH2 | Silencing |
| H3K9 | SUV39H1 | Silencing |
| H4K20 | SUV4-20H1 | Silencing |
| Phosphorylation | ||
| H3S10 | RSK2 | Silencing |
| Methylated CpG | DNMT1, DNMT3a-3b | Silencing |
Histone methylation is catalyzed by histone methyltransferases at lysine and arginine residues on histone H3 and H4 and can be mono-, di- and tri-methylated. The major sites on histone H3 are Lys4, Lys9, Lys27, Lys36 and Lys79, whereas histone H4 is methylated on Lys20[5]. The methyl group donor is S-adenosyl methionine. The effects of alcohol exposure on SAMe-mediated epigenetic changes have been under investigation[9]. Arginine methylation is transcription-activating, and lysine methylation can cause either transcriptional activation or repression, depending on the lysine residue methylated. For instance, H3K9 can be acetylated as well as methylated and have opposite transcriptional consequences; H3AcK9 causes transcriptional activation whereas H3K9me (mono-, di- and tri-) leads to transcriptional repression. Thus, a balance in H3K9 acetylation and methylation may be important in determining chromatin architecture and gene silencing or activation[5].
Histone phosphorylation is a transcription-activating modification achieved by kinases that catalyze the transfer of a phosphate group from ATP or GTP to the serine or threonine residue of histone H3. Besides phosphorylation, histone H1, H2A, H2B and H3 can be ubiquitinated at lysine residues that activate transcription. Sumoylation on the other hand occurs on lysine residues and is a transcriptionally repressive modification.
DNA methylation involves transfer of a methyl group to cytosine bases at the C5 position of CG dinucleotides, referred to as CpG dinucleotides, and may occur in clusters, known as CpG islands. By definition, CpG islands are genomic regions that are at least 200 base pairs long, with ≥ 50% GC content and ≥ 60% expected CpG ratio[10]. The methyl donor is SAMe and the enzyme involved is DNA methyltransferase (DNMT). Two groups of mammalian DNMTs, one that de novo methylates DNA, and the other that maintains the methylation status, are classified as four different types: DNMT 1, 2, 3A and 3B. Although DNMT 3A and 3B are de novo methylation enzymes[11], DNMT 1 maintains methylation status, whereas the function of DNMT 2 is not yet clear and it has weak methyltransferase activity. DNA methylation leads to transcriptional silencing due to chromatin condensation, increased recruitment of methylated CpG binding transcriptional repressor, and inhibition of DNA binding of transcriptional activators[11]. The unmethylated CpG islands are associated with transcriptionally active promoters, and how CpG islands remain unmethylated is still unclear[12].
Non-coding RNA (ncRNA) is another mechanism of epigenetic regulation and is driven by long or small ncRNAs. Long ncRNAs such as Air[13], Kcnq1ot1[14] and H19[15] exert their epigenetic effect by genomic imprinting, which involves DNA methylation. On the other hand, small ncRNAs such as miRNA affect translational repression of mRNA, mRNA degradation, DNA methylation, and chromatin modification[16]. miRNAs are short [~22 nucleotides(nt)] ncRNAs that regulate gene expression by binding to their cognate binding sites at the 3’-end of the target mRNA, and inhibiting their translation. miRNAs are transcribed from miRNA genes mostly by RNA polymerase II into long primary miRNA (pri-miRNA) transcripts that often contain thousands of nucleotides and form hairpins (stem loops). The pri-miRNA is processed in the nucleus by Drosha-DGCR8 complex to produce 70-80-nt precursor miRNA (pre-miRNA)[16]. Pre-miRNAs are transported from the nucleus to the cytoplasm by exportin-5 and Ran-GTP complex, where they are further processed into ~22-nt long miRNA/miRNA duplex by Dicer, a RNAse type III enzyme. One of the miRNA strands bind to the cognate binding site on the target mRNA with a ~2-nt mismatch, and it is binding of the RNA silencing complex at the 3’ UTR of the target mRNA that represses its translation and results in gene silencing[16].
The crosstalk between various epigenetic mechanisms described above can determine downstream chromatin remodeling and gene expression. Mechanisms such as histone acetylation and methylation, DNA methylation and ncRNA-mediated modifications, are acquired throughout life, and persist, and influence the ability to deal with environmental factors such as nutritional factors, toxicants and lifestyle-related factors including tobacco smoke, alcohol, chemical carcinogens, infectious agents and UV radiation, thus influencing the clinical outcomes of health and disease[17].
EPIGENETIC DYSREGULATION IN ALD
The emerging role of epigenetic regulation of gene expression by alcohol and its effect on organ injury comes from studies in the past decade[1,2]. Here, we review mechanisms related to histone modifications and DNA methylation induced by alcohol exposure in liver cells, which contribute to ALD.
Histone modifications: acetylation, phosphorylation and methylation
Multiple lines of evidence from in vitro and in vivo studies have established that alcohol induces epigenetic modifications in various organs including the gastrointestinal system, brain and liver[2]. In the liver, alcohol alters histone acetylation, methylation as well as phosphorylation. Selective acetylation of histone H3 at lys 9 (H3AcK9) has been observed in primary rat hepatocytes exposed to alcohol in vitro[18]. On the other hand, other lysine residues H3 lys14, lys18 and lys23 were not acetylated. In the liver, H3 acetylation was modulated by alcohol via increased HAT activity and HDAC inhibition[19].
The status of histone acetylation depends on the activity of HAT and HDAC[20]. In some instances, the balance of HAT/HDAC ratio determines the acetylation of histone residues and influences gene expression[21]. Alcohol exposure appears to alter HAT and HDAC activity in hepatocytes[19]. In vitro alcohol exposure of hepatic cells impairs HDAC6 function, which directly affects microtubule dynamics[22]. The mRNA expression of class III HDAC, sirtuin 1 (SIRT1) is reduced in alcohol-exposed hepatocytes[23]. Furthermore, an essential role for SIRT1 in mediating effects of alcohol on SREBP-1 and hepatic lipid metabolism in alcoholic fatty liver has been reported[24]. These results indicate that SIRT1 can be developed as a therapeutic target in ALD. Overall, alcohol seems to influence HATs and HDACs in hepatocytes. Studies are needed to determine the precise role of these altered enzyme activities in the context of ALD in vivo.
Similar to acetylation, phosphorylation of histones is crucial to chromatin modifications, and activates gene transcription downstream of cell signaling events[25]. Acute alcohol exposure modulates H3 phosphorylation at serine 10 and serine 28 in rat hepatocytes, and this is dependent on p38 mitogen-activated protein kinase activity but not extracellular signal-regulated kinase and C-Jun N-terminal kinase[26]. Recent studies also have indicated that in vivo acute alcohol exposure induces H3 serine-10 and serine-28 phosphorylation which was transiently increased at 1 h, but decreased at 4 h after alcohol administration[27]. On the other hand, persistent H3K9 acetylation in the liver was observed at 4 h after acute alcohol exposure in vivo[27]. A relationship between acetylation and phosphorylation in context with gene activation has been examined[25]. For instance, cytokine-induced gene expression mediated by nuclear IKKα leading to nuclear factor (NF)-κB activation involves coupling of H3 phosphorylation at serine 10 and acetylation at lysine 14[28,29]. On the other hand, retinoic acid receptor-β and c-jun gene regulation is linked to phosphorylation of histone H3 and not acetylation, which indicates that these two epigenetic changes can occur independently[30-32]. Whether alcohol induces histone phosphorylation and acetylation synergistically or as independent pathways to regulate target gene expression in ALD remains to be investigated.
Specific methylated lysine residues on histones function as stable epigenetic marks that direct particular biological functions, ranging from transcriptional regulation to heterochromatin assembly[33]. Histone methyltransferases catalyze transfer of a methyl residue predominantly to H3 and H4 histones that impart biological functions such as transcription or epigenetic silencing[33]. Shukla et al[34] have shown differential methylation of H3 and H4 in rat hepatocytes exposed to alcohol in vitro. These studies have indicated that methylation at H3meLys9 is decreased and is associated with downregulation of genes such as Lsdh, whereas increased methylation of H3meLys4 is associated with upregulation of alcohol dehydrogenases (ADH1)[34]. Whether acute or chronic alcohol-mediated histone methylation serves as a stable genomic imprint that determines the transcriptional state of a gene contributing to disease is still unknown.
DNA methylation in ALD
Methylation of cytosine at C5 in the CpG dinucleotides silences transcription, whereas absence of methylation activates transcription. Although 80% of CpG dinucleotides are methylated, unmethylated CpG residues in promoter regions of constitutively active genes are referred to as CpG islands[35]. The predominant methyl donor, SAMe, which is important in DNA methylation, is depleted in alcoholic livers, which results in hyperhomocysteinemia that is commonly observed in patients with ALD[36]. Decreased SAMe in alcoholic livers also seems to affect DNA methylation. Rats fed an intragastric alcohol diet for 9 wk exhibited decreased methionine, SAMe, glutathione and loss of DNA methylation by 40%[37]. This DNA hypomethylation can lead to alteration in gene expression and chromatin structure, results in increased DNA damage and strand breaks[37,38], which predisposes cells to malignant degeneration[39]. An association between alcohol intake and hypomethylation of the O6-methylguanine DNA methyltransferase gene has been noted in context with hepatocellular carcinoma[40]. Studies also have shown that alcohol-metabolizing enzyme, ADH1, is regulated by epigenetic mechanisms in hepatoma cells involving DNA hypomethylation[41]. In addition to direct effects of alcohol on DNA hypomethylation, direct effects of acetaldehyde on DNA methyltranferase[42] and methionine synthase[43,44] have been reported. Whether alcohol directly alters DNA methyltranferase activity and methionine synthase is yet unknown. Chronic alcohol consumption induces global DNA hypomethylation[45], whereas hypermethylation of DNA is observed in human peripheral blood cells after alcohol consumption in humans[46,47]. Alcohol-associated hypomethylation thus far is linked to decrease in SAMe, the methyl donor.
An interplay between histone acetylation and DNA methylation is involved in the process of gene transcription and/or silencing in diseased states[17]. For instance, hypermethylation of CpG islands in target gene promoters triggers deacetylation of local histones, whereas lower levels of histone acetylation seem to sensitize DNA to methylation. Although there is an intimate communication between these two epigenetic phenomena, it is still not clear whether there is any hierarchical order of these events. In chronic alcohol exposed livers, whether an interconnection exists between hyperacetylation of H3K9, loss of methylation of H3K9, and increased methylation of H3K4, along with global hypomethylation of DNA is unknown. Studies undertaken to identify the interplay of epigenetic events will provide new insights into mechanisms of ALD.
miRNAs AS EPIGENETIC MODULATORS IN ALD
The role of miRNAs as epigenetic modulators is apparent from its regulation of gene expression at the post-transcriptional level. miRNAs elicit degradation of target mRNA or hinder translational mechanisms such as initiation, elongation, degradation or segregation of mRNA into P bodies for translational inhibition[48-50]. The importance of miRNAs in liver diseases such as viral hepatitis, alcoholic fatty liver disease and non-alcoholic fatty liver disease (NAFLD), fibrosis, and hepatocellular carcinoma has recently been recognized[51]. Studies have shown that alcohol exposure regulates miRNAs that control post-transcriptional events and influence gene expression that are important in ALD[52]. miRNAs have been linked to lipid metabolism and inflammation in steatohepatitis[53-56]. Dolganiuc et al[52] have shown that alcohol feeding increases and decreases ~1% of known miRNA, whereas methionine/choline-deficient diet upregulates 3% and reduces 1% of known miRNAs. Common to ALD and NAFLD, miR-705 and miR-122 are increased with both diets. However, miR-182, miR-183 and miR-199a-3p are increased in NAFLD and decreased in ALD mouse models. The functional relevance of miRNAs in ALD and NAFLD remains to be determined. Using hepatocyte cultures, recent studies have shown that ~11 miRNAs are altered in hepatic lipid droplets[57]. MiR-181d is the most efficacious inhibitor and reduces lipid droplets by 60%, which confirms its role in cellular synthesis of triglycerides and cholesterol esters[57]. More recent studies have shown that chronic alcohol induces miRNA-155 in liver macrophages[58]. Chronic alcohol exposure also alters miRNAs that affect intestinal permeability[59]. Alcohol intake results in induction of miR-212, which in turn downregulates tight junction protein zonula occludens 1[59]. miRNA-212 is also higher in colon biopsy samples of patients with ALD, which confirms the pathophysiological significance of miRNA-212 in altering intestinal permeability during ALD. The role of miRNAs as epigenetic modulators of gene expression and silencing is becoming evident in ALD. An improved understanding of miRNAs and subsequent post-transcriptional regulatory mechanisms will be of importance in advancing their application as diagnostic or prognostic markers, as well as therapeutic targets in ALD.
EPIGENETICS AND INFLAMMATION IN ALD
The first report on aberrations in the epigenetic code in an inflammatory disease condition such as rheumatoid arthritis was reported in 1990[60]. Anti-inflammatory effects of HDAC inhibitors used in a number of experimental models of inflammatory diseases have confirmed a role for epigenetics in immune function[61-63]. Epigenetic regulatory mechanisms are central to the immune response, allowing an appropriate gene expression pattern in immune cells[64,65]. Environmentally regulated or endogenously mediated epigenetic alterations contribute to environment-host interactions in various forms of inflammatory diseases[66].
Innate immune responses and macrophage function play a central role in ALD[67,68]. Epigenetic modifications that influence inflammatory responses have been reported in macrophages[69] during lipopolysaccharide (LPS) tolerance. This phenomenon is dependent on histone acetylation and H3K4 methylation, as well as chromatin remodeling enzymes such as SW1/SNF[70]. Acute alcohol exposure decreases LPS induced proinflammatory responses in human monocytes[71,72]. Whether acute alcohol mediates histone modifications and recruitment of nucleosome remodeling enzymes at the promoters of the cytokine genes is yet to be determined.
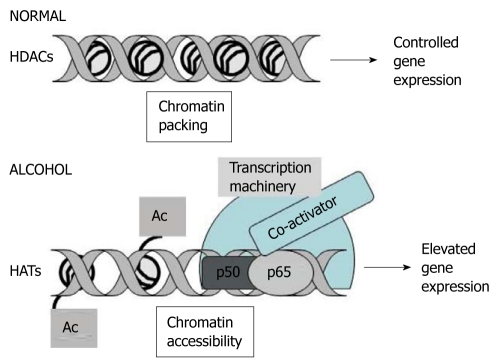
The transcription factor NF-κB is a master regulator of proinflammatory responses in macrophages and monocytes[73,74]. It is the organization of the chromatin structure that controls the kinetics of NF-κB recruitment to target gene promoters that represents a focal point in mediation of transcription cooperativity (Figure 1). Acute and chronic alcohol exposure modulates NF-κB DNA binding that regulates expression of proinflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β and IL-6[68,75]. A tailored immune response that is regulated by NF-κB via interaction with HATs and HDACs elicits epigenetic modifications[76]. For instance, HDAC-1 is known to form a complex with NF-κBp50 homodimers to repress gene transcription[77]. On the other hand, inflammatory stimuli such as LPS can induce phospho-acetylation of histone H3 (K9/S10) at a subset of cytokine and chemokine gene promoters for increased NF-κB recruitment[29,78,79]. Reductions in H3 Lys9 methylation, along with increased H3/H4 acetylation are strongly correlated with RNA polymerase II recruitment, which results in transcription of NF-κB-inducible inflammatory genes[79]. Whether alcohol exposure affects epigenetic mechanisms to prolong or reduce DNA binding of NF-κB to the cytokine gene promoters in monocytes and macrophages is not yet known. Another environmental agent, cigarette smoke induces post-translational modification of phospho-deacetylases, HDAC2 and SIRT1, acetylation of p65-NF-κB and phosphoacetylation of histone H3 that leads to chromatin rearrangement and sustained proinflammatory gene transcription[80,81]. It is likely that chronic alcohol exposure modulates HDAC2 and SIRT1 in immune cells to regulate epigenetic events that promote prolonged proinflammatory responses in ALD.
Figure 1.
Epigenetic regulation of gene expression via histone modification. Under normal conditions, activation of histone deacetylases (HDACs) inhibits acetylation (Ac) of histones, which results in chromatin packaging, restriction of DNA accessibility for transcription, and controlled target gene expression. During alcohol exposure, histone modifications mediated by histone acetyltransferases (HATs) influence elevated transcription of target genes, due to chromatin accessibility, which leads to increased DNA occupancy of transcription factors such as nuclear factor-κB (p50/p65), co-activators and transcriptional complexes, and elevated target gene expression.
In macrophages stimulated with viruses or bacteria, miRNA-155 is the only increased miRNA induced by both stimulants[82,83]. miRNA-155 regulates TNF-α production positively by enhancing its translation[84]. Bala et al[58] recently reported that chronic alcohol induces miRNA-155 in an NF-κB-dependent manner that increases mRNA stability and TNF-α expression in ALD. miR-146 is predominantly expressed in T regulatory and Th1 cells[85] and is upregulated in toll-like-receptor-stimulated macrophages in an NF-κB-dependent manner. miR-146 targets IRAK-1 and TRAF6 genes and is unaffected by chronic alcohol exposure in liver immune cells[58]. Overall, these findings suggest that miRNAs are capable of regulating alcohol-induced innate immune cell function and thus determining cellular memory via miRNA-mediated epigenetic modulation.
ALCOHOL, OXIDATIVE STRESS AND CHROMATIN REMODELING
Oxidative stress regulates chromatin remodeling by alteration of histone acetylation and deacetylation events via HAT/HDAC activity[86,87]. Acute and chronic alcohol exposure increases reactive oxygen species (ROS) production and lowers antioxidant levels that enhance oxidative stress in the liver[88]. Metabolism of alcohol through alcohol dehydrogenase and microsomal cytochrome P450 2E1 leads to enhanced production of ROS in the liver[88]. Acetylation of histone H3 by alcohol in rat hepatocytes is mediated by ROS[89]. Inhibition of NADPH-oxidase-mediated ROS results in decreased H3AcK9, whereas ROS inducers directly increase alcohol-induced acetylation of H3K9, along with induction of ADH1 mRNA expression[89]. A redox-sensitive class III HDAC molecule, SIRT1, is also decreased in alcohol-exposed rat hepatocytes and livers of alcohol-fed rats[24]. Whether alcohol-induced ROS[90] play an important role in modulation of SIRT1 to regulate steatosis remains to be determined. In addition to alcohol, acetaldehyde and acetate, which are products of alcohol metabolism, cause acetylation of H3K9. Pyrazole, an inhibitor of alcohol dehydrogenase and methyl cyanamide, an inhibitor of aldehyde dehydrogenase, both reduce H3K9 acetylation, which indicates that alcohol and its metabolites can trigger acetylation of histone residues. Similar to in vitro observations, in vivo acute alcohol exposure in rats also shows that H3K9 acetylation is significantly increased in the liver[91]. Studies have shown that H3K9 acetylation in hepatocytes due to alcohol exposure correlates with transcriptional increase in alcohol dehydrogenase (ADH1)[19]. It is likely that alcohol-induced acetylation is required for activation of alcohol-metabolizing enzymes, which induce oxidative stress. This, in turn, induces acetylation that creates an amplifying “autocrine loop” between alcohol metabolism and epigenetic events. Future studies to determine the precise role of alcohol-mediated oxidative stress in chromatin modifications in hepatocytes and liver macrophages will identify new pathophysiological mechanisms and epigenetic targets of gene expression in ALD.
Stress-induced heat shock transcription factor (HSF)1 plays an important role as a transcriptional repressor of proinflammatory cytokine genes[92,93]. Recent studies have suggested that HSF1 serves as a master regulator of global acetylation in normal cells, whereas, in stressed cells, HSF1 interacts with HDAC1 and HDAC2, which induce histone deacetylation and chromatin remodeling[94]. Studies from our laboratory have shown that alcohol exposure induces HSF1 DNA binding activity in monocytes and macrophages[95]. Whether alcohol-induced HSF1 induces chromatin reorganization that affects proinflammatory cytokine production is being investigated. Another key stress-induced molecule, heat shock protein (hsp)90, is characterized as an epigenetic “gatekeeper” that interfaces with the environment and may finally determine whether certain epigenetic markers succeed in downstream phenotypic expression[96-98]. Chronic alcohol exposure upregulates hsp90 expression in liver macrophages[95]. It is likely that hsp90 facilitates an alcohol-mediated “epigenetic code” in the liver. Studies to delineate epigenetic effects of alcohol-induced hsp90 on gene transcription in liver macrophages and hepatocytes are awaited.
Hydroxyl radicals generated by oxidative stress interfere with the ability of DNA to function as a substrate for DNMT, which results in global hypomethylation[99,100]. Recent studies have provided evidence for the role of endoplasmic reticulum stress pathways and epigenetic gene regulation[101]. It is likely that alcohol-mediated oxidative stress regulates epigenetic markers in ALD.
CONCLUSION
The interplay of epigenetic mechanisms and their influence on gene transcription in ALD is evolving. Epigenetic alterations associated with acute and chronic alcohol exposure of hepatocytes and immune cells in relation to ALD is discussed in this review. Studies thus far have shown that alcohol exposure, probably via oxidative stress, exhibits differential regulation of acetylation, phosphorylation and methylation of histones that regulate chromatin remodeling and gene expression. The effects of alcohol on DNA methylation in hepatocytes and miRNA regulation have been elucidated. An integrative approach of the various mechanisms that lead to genomic imprinting during alcohol exposure will identify novel pathways in the alcoholic liver and support epigenetic therapeutic interventions.
Footnotes
Supported by PHS Grant # AA017545 (to Mandrekar P) and AA017986 (to Mandrekar P) from the National Institute of Alcohol Abuse and Alcoholism, National Institutes of Health
Peer reviewer: Carlos J Pirola, PhD, FAHA, Medical Research Institute A Lanari, Combatientes de Malvinas 3150, Buenos Aires-1427, Argentina
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
References
- 1.Shukla SD, Aroor AR. Epigenetic effects of ethanol on liver and gastrointestinal injury. World J Gastroenterol. 2006;12:5265–5271. doi: 10.3748/wjg.v12.i33.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shukla SD, Velazquez J, French SW, Lu SC, Ticku MK, Zakhari S. Emerging role of epigenetics in the actions of alcohol. Alcohol Clin Exp Res. 2008;32:1525–1534. doi: 10.1111/j.1530-0277.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- 3.Moss TJ, Wallrath LL. Connections between epigenetic gene silencing and human disease. Mutat Res. 2007;618:163–174. doi: 10.1016/j.mrfmmm.2006.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodenhiser D, Mann M. Epigenetics and human disease: translating basic biology into clinical applications. CMAJ. 2006;174:341–348. doi: 10.1503/cmaj.050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 6.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 7.Dangond F, Hafler DA, Tong JK, Randall J, Kojima R, Utku N, Gullans SR. Differential display cloning of a novel human histone deacetylase (HDAC3) cDNA from PHA-activated immune cells. Biochem Biophys Res Commun. 1998;242:648–652. doi: 10.1006/bbrc.1997.8033. [DOI] [PubMed] [Google Scholar]
- 8.Kruszewski M, Szumiel I. Sirtuins (histone deacetylases III) in the cellular response to DNA damage--facts and hypotheses. DNA Repair (Amst) 2005;4:1306–1313. doi: 10.1016/j.dnarep.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Lieber CS. S-Adenosyl-L-methionine and alcoholic liver disease in animal models: implications for early intervention in human beings. Alcohol. 2002;27:173–177. doi: 10.1016/s0741-8329(02)00230-6. [DOI] [PubMed] [Google Scholar]
- 10.Illingworth RS, Bird AP. CpG islands--’a rough guide’. FEBS Lett. 2009;583:1713–1720. doi: 10.1016/j.febslet.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Jones PA, Liang G. Rethinking how DNA methylation patterns are maintained. Nat Rev Genet. 2009;10:805–811. doi: 10.1038/nrg2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li E, Bird AP. DNA Methylation in mammals. In: Allis CD, Jenuwein T, Reinberg D, editors. Epigenetics. Cold Spring Harbor, NY: CSHL Press; 2007. pp. 341–356. [Google Scholar]
- 13.Memili E, Hong YK, Kim DH, Ontiveros SD, Strauss WM. Murine Xist RNA isoforms are different at their 3’ ends: a role for differential polyadenylation. Gene. 2001;266:131–137. doi: 10.1016/s0378-1119(01)00353-5. [DOI] [PubMed] [Google Scholar]
- 14.Mancini-Dinardo D, Steele SJ, Levorse JM, Ingram RS, Tilghman SM. Elongation of the Kcnq1ot1 transcript is required for genomic imprinting of neighboring genes. Genes Dev. 2006;20:1268–1282. doi: 10.1101/gad.1416906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai X, Cullen BR. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA. 2007;13:313–316. doi: 10.1261/rna.351707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaissière T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res. 2008;659:40–48. doi: 10.1016/j.mrrev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Park PH, Miller R, Shukla SD. Acetylation of histone H3 at lysine 9 by ethanol in rat hepatocytes. Biochem Biophys Res Commun. 2003;306:501–504. doi: 10.1016/s0006-291x(03)01040-4. [DOI] [PubMed] [Google Scholar]
- 19.Park PH, Lim RW, Shukla SD. Involvement of histone acetyltransferase (HAT) in ethanol-induced acetylation of histone H3 in hepatocytes: potential mechanism for gene expression. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1124–G1136. doi: 10.1152/ajpgi.00091.2005. [DOI] [PubMed] [Google Scholar]
- 20.Ito K, Adcock IM. Histone acetylation and histone deacetylation. Mol Biotechnol. 2002;20:99–106. doi: 10.1385/MB:20:1:099. [DOI] [PubMed] [Google Scholar]
- 21.Huber LC, Brock M, Hemmatazad H, Giger OT, Moritz F, Trenkmann M, Distler JH, Gay RE, Kolling C, Moch H, et al. Histone deacetylase/acetylase activity in total synovial tissue derived from rheumatoid arthritis and osteoarthritis patients. Arthritis Rheum. 2007;56:1087–1093. doi: 10.1002/art.22512. [DOI] [PubMed] [Google Scholar]
- 22.Shepard BD, Joseph RA, Kannarkat GT, Rutledge TM, Tuma DJ, Tuma PL. Alcohol-induced alterations in hepatic microtubule dynamics can be explained by impaired histone deacetylase 6 function. Hepatology. 2008;48:1671–1679. doi: 10.1002/hep.22481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lieber CS, Leo MA, Wang X, Decarli LM. Effect of chronic alcohol consumption on Hepatic SIRT1 and PGC-1alpha in rats. Biochem Biophys Res Commun. 2008;370:44–48. doi: 10.1016/j.bbrc.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 24.You M, Liang X, Ajmo JM, Ness GC. Involvement of mammalian sirtuin 1 in the action of ethanol in the liver. Am J Physiol Gastrointest Liver Physiol. 2008;294:G892–G898. doi: 10.1152/ajpgi.00575.2007. [DOI] [PubMed] [Google Scholar]
- 25.Nowak SJ, Corces VG. Phosphorylation of histone H3: a balancing act between chromosome condensation and transcriptional activation. Trends Genet. 2004;20:214–220. doi: 10.1016/j.tig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 26.Lee YJ, Shukla SD. Histone H3 phosphorylation at serine 10 and serine 28 is mediated by p38 MAPK in rat hepatocytes exposed to ethanol and acetaldehyde. Eur J Pharmacol. 2007;573:29–38. doi: 10.1016/j.ejphar.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aroor AR, James TT, Jackson DE, Shukla SD. Differential changes in MAP kinases, histone modifications, and liver injury in rats acutely treated with ethanol. Alcohol Clin Exp Res. 2010;34:1543–1551. doi: 10.1111/j.1530-0277.2010.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamamoto Y, Verma UN, Prajapati S, Kwak YT, Gaynor RB. Histone H3 phosphorylation by IKK-alpha is critical for cytokine-induced gene expression. Nature. 2003;423:655–659. doi: 10.1038/nature01576. [DOI] [PubMed] [Google Scholar]
- 29.Anest V, Hanson JL, Cogswell PC, Steinbrecher KA, Strahl BD, Baldwin AS. A nucleosomal function for IkappaB kinase-alpha in NF-kappaB-dependent gene expression. Nature. 2003;423:659–663. doi: 10.1038/nature01648. [DOI] [PubMed] [Google Scholar]
- 30.Clayton AL, Rose S, Barratt MJ, Mahadevan LC. Phosphoacetylation of histone H3 on c-fos- and c-jun-associated nucleosomes upon gene activation. EMBO J. 2000;19:3714–3726. doi: 10.1093/emboj/19.14.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefebvre B, Ozato K, Lefebvre P. Phosphorylation of histone H3 is functionally linked to retinoic acid receptor beta promoter activation. EMBO Rep. 2002;3:335–340. doi: 10.1093/embo-reports/kvf066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomson S, Clayton AL, Mahadevan LC. Independent dynamic regulation of histone phosphorylation and acetylation during immediate-early gene induction. Mol Cell. 2001;8:1231–1241. doi: 10.1016/s1097-2765(01)00404-x. [DOI] [PubMed] [Google Scholar]
- 33.Rice JC, Allis CD. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Curr Opin Cell Biol. 2001;13:263–273. doi: 10.1016/s0955-0674(00)00208-8. [DOI] [PubMed] [Google Scholar]
- 34.Pal-Bhadra M, Bhadra U, Jackson DE, Mamatha L, Park PH, Shukla SD. Distinct methylation patterns in histone H3 at Lys-4 and Lys-9 correlate with up- & down-regulation of genes by ethanol in hepatocytes. Life Sci. 2007;81:979–987. doi: 10.1016/j.lfs.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Esteller M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet. 2007;8:286–298. doi: 10.1038/nrg2005. [DOI] [PubMed] [Google Scholar]
- 36.Lu SC, Martínez-Chantar ML, Mato JM. Methionine adenosyltransferase and S-adenosylmethionine in alcoholic liver disease. J Gastroenterol Hepatol. 2006;21 Suppl 3:S61–S64. doi: 10.1111/j.1440-1746.2006.04575.x. [DOI] [PubMed] [Google Scholar]
- 37.Lu SC, Huang ZZ, Yang H, Mato JM, Avila MA, Tsukamoto H. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in alcoholic rat liver. Am J Physiol Gastrointest Liver Physiol. 2000;279:G178–G185. doi: 10.1152/ajpgi.2000.279.1.G178. [DOI] [PubMed] [Google Scholar]
- 38.Pogribny IP, Basnakian AG, Miller BJ, Lopatina NG, Poirier LA, James SJ. Breaks in genomic DNA and within the p53 gene are associated with hypomethylation in livers of folate/methyl-deficient rats. Cancer Res. 1995;55:1894–1901. [PubMed] [Google Scholar]
- 39.Martínez-Chantar ML, Corrales FJ, Martínez-Cruz LA, García-Trevijano ER, Huang ZZ, Chen L, Kanel G, Avila MA, Mato JM, Lu SC. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16:1292–1294. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- 40.Lambert MP, Paliwal A, Vaissière T, Chemin I, Zoulim F, Tommasino M, Hainaut P, Sylla B, Scoazec JY, Tost J, et al. Aberrant DNA methylation distinguishes hepatocellular carcinoma associated with HBV and HCV infection and alcohol intake. J Hepatol. 2011;54:705–715. doi: 10.1016/j.jhep.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 41.Dannenberg LO, Chen HJ, Tian H, Edenberg HJ. Differential regulation of the alcohol dehydrogenase 1B (ADH1B) and ADH1C genes by DNA methylation and histone deacetylation. Alcohol Clin Exp Res. 2006;30:928–937. doi: 10.1111/j.1530-0277.2006.00107.x. [DOI] [PubMed] [Google Scholar]
- 42.Garro AJ, McBeth DL, Lima V, Lieber CS. Ethanol consumption inhibits fetal DNA methylation in mice: implications for the fetal alcohol syndrome. Alcohol Clin Exp Res. 1991;15:395–398. doi: 10.1111/j.1530-0277.1991.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 43.Jin B, Park DW, Nam KW, Oh GT, Lee YS, Ryu DY. CpG methylation of the mouse CYP1A2 promoter. Toxicol Lett. 2004;152:11–18. doi: 10.1016/j.toxlet.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Barak AJ, Beckenhauer HC, Tuma DJ. Methionine synthase. a possible prime site of the ethanolic lesion in liver. Alcohol. 2002;26:65–67. doi: 10.1016/s0741-8329(01)00201-4. [DOI] [PubMed] [Google Scholar]
- 45.Lu SC, Mato JM. Role of methionine adenosyltransferase and S-adenosylmethionine in alcohol-associated liver cancer. Alcohol. 2005;35:227–234. doi: 10.1016/j.alcohol.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Bönsch D, Lenz B, Reulbach U, Kornhuber J, Bleich S. Homocysteine associated genomic DNA hypermethylation in patients with chronic alcoholism. J Neural Transm. 2004;111:1611–1616. doi: 10.1007/s00702-004-0232-x. [DOI] [PubMed] [Google Scholar]
- 47.Bönsch D, Lenz B, Fiszer R, Frieling H, Kornhuber J, Bleich S. Lowered DNA methyltransferase (DNMT-3b) mRNA expression is associated with genomic DNA hypermethylation in patients with chronic alcoholism. J Neural Transm. 2006;113:1299–1304. doi: 10.1007/s00702-005-0413-2. [DOI] [PubMed] [Google Scholar]
- 48.Nottrott S, Simard MJ, Richter JD. Human let-7a miRNA blocks protein production on actively translating polyribosomes. Nat Struct Mol Biol. 2006;13:1108–1114. doi: 10.1038/nsmb1173. [DOI] [PubMed] [Google Scholar]
- 49.Humphreys DT, Westman BJ, Martin DI, Preiss T. MicroRNAs control translation initiation by inhibiting eukaryotic initiation factor 4E/cap and poly(A) tail function. Proc Natl Acad Sci USA. 2005;102:16961–16966. doi: 10.1073/pnas.0506482102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol. 2005;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bala S, Marcos M, Szabo G. Emerging role of microRNAs in liver diseases. World J Gastroenterol. 2009;15:5633–5640. doi: 10.3748/wjg.15.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dolganiuc A, Petrasek J, Kodys K, Catalano D, Mandrekar P, Velayudham A, Szabo G. MicroRNA expression profile in Lieber-DeCarli diet-induced alcoholic and methionine choline deficient diet-induced nonalcoholic steatohepatitis models in mice. Alcohol Clin Exp Res. 2009;33:1704–1710. doi: 10.1111/j.1530-0277.2009.01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Wilfred BR, Wang WX, Nelson PT. Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways. Mol Genet Metab. 2007;91:209–217. doi: 10.1016/j.ymgme.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chong MM, Rasmussen JP, Rudensky AY, Littman DR. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J Exp Med. 2008;205:2005–2017. doi: 10.1084/jem.20081219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liston A, Lu LF, O’Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med. 2008;205:1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whittaker R, Loy PA, Sisman E, Suyama E, Aza-Blanc P, Ingermanson RS, Price JH, McDonough PM. Identification of MicroRNAs that control lipid droplet formation and growth in hepatocytes via high-content screening. J Biomol Screen. 2010;15:798–805. doi: 10.1177/1087057110374991. [DOI] [PubMed] [Google Scholar]
- 58.Bala S, Marcos M, Kodys K, Csak T, Catalano D, Mandrekar P, Szabo G. Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor {alpha} (TNF{alpha}) production via increased mRNA half-life in alcoholic liver disease. J Biol Chem. 2011;286:1436–1444. doi: 10.1074/jbc.M110.145870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 60.Richardson B, Scheinbart L, Strahler J, Gross L, Hanash S, Johnson M. Evidence for impaired T cell DNA methylation in systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 1990;33:1665–1673. doi: 10.1002/art.1780331109. [DOI] [PubMed] [Google Scholar]
- 61.Kim YI, Logan JW, Mason JB, Roubenoff R. DNA hypomethylation in inflammatory arthritis: reversal with methotrexate. J Lab Clin Med. 1996;128:165–172. doi: 10.1016/s0022-2143(96)90008-6. [DOI] [PubMed] [Google Scholar]
- 62.Nishida K, Komiyama T, Miyazawa S, Shen ZN, Furumatsu T, Doi H, Yoshida A, Yamana J, Yamamura M, Ninomiya Y, et al. Histone deacetylase inhibitor suppression of autoantibody-mediated arthritis in mice via regulation of p16INK4a and p21(WAF1/Cip1) expression. Arthritis Rheum. 2004;50:3365–3376. doi: 10.1002/art.20709. [DOI] [PubMed] [Google Scholar]
- 63.Blanchard F, Chipoy C. Histone deacetylase inhibitors: new drugs for the treatment of inflammatory diseases? Drug Discov Today. 2005;10:197–204. doi: 10.1016/S1359-6446(04)03309-4. [DOI] [PubMed] [Google Scholar]
- 64.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447:433–440. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 65.Natoli G. Maintaining cell identity through global control of genomic organization. Immunity. 2010;33:12–24. doi: 10.1016/j.immuni.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 66.Yung RL, Julius A. Epigenetics, aging, and autoimmunity. Autoimmunity. 2008;41:329–335. doi: 10.1080/08916930802024889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hines IN, Wheeler MD. Recent advances in alcoholic liver disease III. Role of the innate immune response in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G310–G314. doi: 10.1152/ajpgi.00094.2004. [DOI] [PubMed] [Google Scholar]
- 68.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–1266. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 70.Ramirez-Carrozzi VR, Nazarian AA, Li CC, Gore SL, Sridharan R, Imbalzano AN, Smale ST. Selective and antagonistic functions of SWI/SNF and Mi-2beta nucleosome remodeling complexes during an inflammatory response. Genes Dev. 2006;20:282–296. doi: 10.1101/gad.1383206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mandrekar P, Dolganiuc A, Bellerose G, Kodys K, Romics L, Nizamani R, Szabo G. Acute alcohol inhibits the induction of nuclear regulatory factor kappa B activation through CD14/toll-like receptor 4, interleukin-1, and tumor necrosis factor receptors: a common mechanism independent of inhibitory kappa B alpha degradation? Alcohol Clin Exp Res. 2002;26:1609–1614. doi: 10.1097/01.ALC.0000036926.46632.57. [DOI] [PubMed] [Google Scholar]
- 72.Mandrekar P, Catalano D, White B, Szabo G. Moderate alcohol intake in humans attenuates monocyte inflammatory responses: inhibition of nuclear regulatory factor kappa B and induction of interleukin 10. Alcohol Clin Exp Res. 2006;30:135–139. doi: 10.1111/j.1530-0277.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 73.Ghosh S. Regulation of inducible gene expression by the transcription factor NF-kappaB. Immunol Res. 1999;19:183–189. doi: 10.1007/BF02786486. [DOI] [PubMed] [Google Scholar]
- 74.Karin M, Delhase M. The I kappa B kinase (IKK) and NF-kappa B: key elements of proinflammatory signalling. Semin Immunol. 2000;12:85–98. doi: 10.1006/smim.2000.0210. [DOI] [PubMed] [Google Scholar]
- 75.Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFkappaB activation by ethanol in human monocytes. Int Immunol. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- 76.Vanden Berghe W, Ndlovu MN, Hoya-Arias R, Dijsselbloem N, Gerlo S, Haegeman G. Keeping up NF-kappaB appearances: epigenetic control of immunity or inflammation-triggered epigenetics. Biochem Pharmacol. 2006;72:1114–1131. doi: 10.1016/j.bcp.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 77.Zhong H, May MJ, Jimi E, Ghosh S. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell. 2002;9:625–636. doi: 10.1016/s1097-2765(02)00477-x. [DOI] [PubMed] [Google Scholar]
- 78.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1) EMBO J. 2003;22:1313–1324. doi: 10.1093/emboj/cdg139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saccani S, Pantano S, Natoli G. p38-Dependent marking of inflammatory genes for increased NF-kappa B recruitment. Nat Immunol. 2002;3:69–75. doi: 10.1038/ni748. [DOI] [PubMed] [Google Scholar]
- 80.Rajendrasozhan S, Yao H, Rahman I. Current perspectives on role of chromatin modifications and deacetylases in lung inflammation in COPD. COPD. 2009;6:291–297. doi: 10.1080/15412550903049132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang SR, Chida AS, Bauter MR, Shafiq N, Seweryniak K, Maggirwar SB, Kilty I, Rahman I. Cigarette smoke induces proinflammatory cytokine release by activation of NF-kappaB and posttranslational modifications of histone deacetylase in macrophages. Am J Physiol Lung Cell Mol Physiol. 2006;291:L46–L57. doi: 10.1152/ajplung.00241.2005. [DOI] [PubMed] [Google Scholar]
- 82.Shen Z, Ajmo JM, Rogers CQ, Liang X, Le L, Murr MM, Peng Y, You M. Role of SIRT1 in regulation of LPS- or two ethanol metabolites-induced TNF-alpha production in cultured macrophage cell lines. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1047–G1053. doi: 10.1152/ajpgi.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, Lund E, Dahlberg JE. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc Natl Acad Sci USA. 2005;102:3627–3632. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, et al. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol. 2007;179:5082–5089. doi: 10.4049/jimmunol.179.8.5082. [DOI] [PubMed] [Google Scholar]
- 85.Monticelli S, Ansel KM, Xiao C, Socci ND, Krichevsky AM, Thai TH, Rajewsky N, Marks DS, Sander C, Rajewsky K, et al. MicroRNA profiling of the murine hematopoietic system. Genome Biol. 2005;6:R71. doi: 10.1186/gb-2005-6-8-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ito K, Hanazawa T, Tomita K, Barnes PJ, Adcock IM. Oxidative stress reduces histone deacetylase 2 activity and enhances IL-8 gene expression: role of tyrosine nitration. Biochem Biophys Res Commun. 2004;315:240–245. doi: 10.1016/j.bbrc.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 87.Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol. 2004;68:1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 88.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 89.Choudhury M, Park PH, Jackson D, Shukla SD. Evidence for the role of oxidative stress in the acetylation of histone H3 by ethanol in rat hepatocytes. Alcohol. 2010;44:531–540. doi: 10.1016/j.alcohol.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thakur V, Pritchard MT, McMullen MR, Wang Q, Nagy LE. Chronic ethanol feeding increases activation of NADPH oxidase by lipopolysaccharide in rat Kupffer cells: role of increased reactive oxygen in LPS-stimulated ERK1/2 activation and TNF-alpha production. J Leukoc Biol. 2006;79:1348–1356. doi: 10.1189/jlb.1005613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim JS, Shukla SD. Acute in vivo effect of ethanol (binge drinking) on histone H3 modifications in rat tissues. Alcohol Alcohol. 2006;41:126–132. doi: 10.1093/alcalc/agh248. [DOI] [PubMed] [Google Scholar]
- 92.Singh IS, He JR, Calderwood S, Hasday JD. A high affinity HSF-1 binding site in the 5’-untranslated region of the murine tumor necrosis factor-alpha gene is a transcriptional repressor. J Biol Chem. 2002;277:4981–4988. doi: 10.1074/jbc.M108154200. [DOI] [PubMed] [Google Scholar]
- 93.Xie Y, Chen C, Stevenson MA, Auron PE, Calderwood SK. Heat shock factor 1 represses transcription of the IL-1beta gene through physical interaction with the nuclear factor of interleukin 6. J Biol Chem. 2002;277:11802–11810. doi: 10.1074/jbc.M109296200. [DOI] [PubMed] [Google Scholar]
- 94.Fritah S, Col E, Boyault C, Govin J, Sadoul K, Chiocca S, Christians E, Khochbin S, Jolly C, Vourc’h C. Heat-shock factor 1 controls genome-wide acetylation in heat-shocked cells. Mol Biol Cell. 2009;20:4976–4984. doi: 10.1091/mbc.E09-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mandrekar P, Catalano D, Jeliazkova V, Kodys K. Alcohol exposure regulates heat shock transcription factor binding and heat shock proteins 70 and 90 in monocytes and macrophages: implication for TNF-alpha regulation. J Leukoc Biol. 2008;84:1335–1345. doi: 10.1189/jlb.0407256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- 97.Sangster TA, Queitsch C, Lindquist S. Hsp90 and chromatin: where is the link? Cell Cycle. 2003;2:166–168. [PubMed] [Google Scholar]
- 98.Pigliucci M. Epigenetics is back! Hsp90 and phenotypic variation. Cell Cycle. 2003;2:34–35. doi: 10.4161/cc.2.1.274. [DOI] [PubMed] [Google Scholar]
- 99.Lim SO, Gu JM, Kim MS, Kim HS, Park YN, Park CK, Cho JW, Park YM, Jung G. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: methylation of the E-cadherin promoter. Gastroenterology. 2008;135:2128–2240. doi: 10.1053/j.gastro.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 100.Wachsman JT. DNA methylation and the association between genetic and epigenetic changes: relation to carcinogenesis. Mutat Res. 1997;375:1–8. doi: 10.1016/s0027-5107(97)00003-1. [DOI] [PubMed] [Google Scholar]
- 101.Esfandiari F, Medici V, Wong DH, Jose S, Dolatshahi M, Quinlivan E, Dayal S, Lentz SR, Tsukamoto H, Zhang YH, et al. Epigenetic regulation of hepatic endoplasmic reticulum stress pathways in the ethanol-fed cystathionine beta synthase-deficient mouse. Hepatology. 2010;51:932–941. doi: 10.1002/hep.23382. [DOI] [PMC free article] [PubMed] [Google Scholar]