Abstract
Fibronectins are adhesive glycoproteins that can be found in tissue matrices and circulating in various fluids of the body. The variable composition of fibronectin molecules facilitates a diversity of interactions with cell surface receptors that suggest a role for these proteins beyond the structural considerations of the extracellular matrix. These interactions implicate fibronectin in the regulation of mechanisms that also determine cell behavior and activity. The two major forms, plasma fibronectin (pFn) and cellular fibronectin (cFn), exist as balanced amounts under normal physiological conditions. However, during injury and/or disease, tissue and circulating levels of cFn become disproportionately elevated. The accumulating cFn, in addition to being a consequence of prolonged tissue damage, may in fact stimulate cellular events that promote further damage. In this review, we summarize what is known regarding such interactions between fibronectin and cells that may influence the biological response to injury. We elaborate on the effects of cFn in the liver, specifically under a condition of chronic alcohol-induced injury. Studies have revealed that chronic alcohol consumption stimulates excess production of cFn by sinusoidal endothelial cells and hepatic stellate cells while impairing its clearance by other cell types resulting in the build up of this glycoprotein throughout the liver and its consequent increased availability to influence cellular activity that could promote the development of alcoholic liver disease. We describe recent findings by our laboratory that support a plausible role for cFn in the promotion of liver injury under a condition of chronic alcohol abuse and the implications of cFn stimulation on the pathogenesis of alcoholic liver disease. These findings suggest an effect of cFn in regulating cell behavior in the alcohol-injured liver that is worth further characterizing not only to gain a more comprehensive understanding of the role this reactive glycoprotein plays in the progression of injury but also for the insight further studies could provide towards the development of novel therapies for alcoholic liver disease.
Keywords: Fibronectin, Liver disease, Alcoholic liver disease, Endocytosis, Cellular fibronectin
INTRODUCTION
Fibronectins are ubiquitous, multifunctional, high-molecular weight glycoproteins that have been implicated in a wide array of fundamental biological processes specific to their structure and distribution in the body. These proteins have been the subject of extensive study for over 60 years yet their physiological roles remain to be completely defined. Most reports emphasize their critical participation in biological phenomena involving the modulation of components in the extracellular environment. However, there is a growing body of evidence that reveals fibronectins may also be directly involved in regulating cellular behavior, particularly in injured tissue and under pathological circumstances.
HISTORY
Two classes of fibronectin exist in vivo, each discovered through widely different research initiatives. Plasma fibronectin (pFn), which is found primarily as a soluble dimer circulating in various body fluids, was first identified as “cold-insoluble globulin” during post World War II studies on the fractionation of human blood plasma[1]. Twenty-five years later, the search for tumor markers led to the discovery of cellular fibronectin (cFn) described then as the “large external transformation sensitive (LETS) protein” or “galactoprotein”, and later determined to also be the “surface fibroblast antigen”[2-4]. This fibronectin is found predominantly as an insoluble, multimeric, fibrillar constituent of extracellular matrices. Separate biochemical and cell biological analyses of these two glycoprotein types drew similar conclusions that eventually led to the convergence of such studies and the realization that these molecules are, in fact, related[5-7]. The common term of “fibronectin” was agreed upon to define these similar proteins, but it was only after detailed genetic and structural analyses could be made that this similarity was truly understood[8].
During a marked collaborative period, several other research groups became aware that their glycoproteins of interest, originating from sources other than plasma and fibroblasts, resembled fibronectin in character. It was revealed that these proteins were, in fact, variants of fibronectin, as it appeared they all derive from the same complex gene. This gene consists of more than 45 distinct coding (exons) and non-coding (introns) nucleotide sequences, which can be transcribed from a single promoter into alternatively spliced messenger RNAs that account for the multiple isoforms of fibronectin found in human tissue[9-11].
STRUCTURE
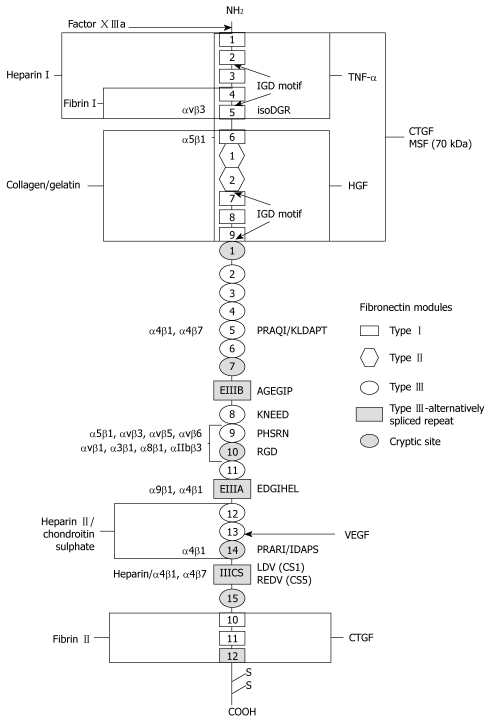
Generally, the functional protein is composed of two similar, but not always identical subunits of 220 to 250 kDa that are joined by disulphide bonds at the carboxyl-termini to create the characteristic fibronectin dimer. Greater than 90% of the structure of each fibronectin monomer is defined by variable combinations of three different types of homologous repeating domains termed Types I, II and III that are linked by short peptide segments (Figure 1)[8]. There are twelve Type I (~40 amino acid residues) and two Type II (~60 amino acid residues) homologous repeats in each fibronectin sequence that are individually folded to form sheets of β-strands stabilized by disulphide bonds. Type III repeats (~90 amino acid residues) of which there are fifteen to seventeen per sequence flanked by Type I and Type II regions, are similarly organized into overlapping β-sheets. However, these modules lack disulphide bridges, thus have greater conformational latitude. Type I and Type II modules are each encoded by a single exon, while Type III modules are coded for by 2 exons each with the exception of extra domains A and B (denoted EIIIA, EDA or EDI and EIIIB, EDB or EDII respectively) and the ninth Type III domain (III-9). Rather, these particular domains are each encoded by single exons of somewhat extended lengths[8-11].
Figure 1.
Domain structure and interaction sites of fibronectin. Fibronectin is a dimer comprised of two subunits which are covalently joined by two disulfide bridges near the COOH-terminus. Each subunit consists of three types of homologous structural domains called I, II, and III. Recognition sequences, integrin binding sites, cryptic sites and interactive regions of the molecule are labeled.
Considerable molecular subunit diversity results from the complex splicing of the fibronectin primary transcript at three specific sites that code for the Type III domains, EIIIA, EIIIB, and a region of the molecule towards the carboxyl-terminus that links Type III units, III-14 and III-15, referred to as the Type III connecting segment (IIICS) or variable (V) domain[8]. Patterns of inclusion or exclusion of the sequences of these three alternatively spliced domains confer variability among species that express fibronectin[8,11]. In fact, variations between the two biological forms of this glycoprotein are also largely attributed to differential splicing of pre-mRNA. Neither subunit of the pFn dimer contains the EIIIA and EIIIB sequences and only one of these subunits has a V-region. Alternatively, cFn contains variable proportions of all three domains[8,11]. Furthermore, the V-domain undergoes a more intricate tissue-specific splicing mechanism that results in its sequence being either entirely included or excluded, or only variable parts of it being present in the molecule. In humans, such splicing patterns generate five V-region variants. Altogether, these mechanisms can potentially produce greater than twenty human subtypes, which can be assembled to form a diverse array of fibronectin heterodimers. Such varied composition, particularly for cFn, is likely associated with a diverse array of functions.
Further structural complexity is established through post-translational modifications of the fibronectin molecule. Though both phosphorylation and sulfation have been observed, these modifications do not appear to account for significant differences among isoforms[12]. However, analyses have revealed considerable variation in the glycosylation profiles of fibronectin molecules derived from different sources[8,13]. All forms of fibronectin contain significant amounts of carbohydrate (5%-10%) that are predominantly in the form of biantennary asparagine-linked side chains that reside mostly among Type III repeats. Some heterogeneity, with regard to the number and size of these carbohydrate side chains, is present among individual fibronectin polypeptide units. Studies have shown that domains with carbohydrate moieties are resistant to proteolysis and that glycosylation contributes to the conformational stability of the fibronectin molecule[14]. Interestingly, cFn variants have greater carbohydrate content than pFn which may serve to protect the function of cFn molecules in areas of active proteolysis and tissue remodeling where they are normally found. Additional evidence suggests that glycosylation may be involved in modulating the binding affinity of fibronectin to other matrix, as well as, cell-surface proteins[15]. Rotundo et al[16] revealed that the composition of the carbohydrate side chain determines whether fibronectin associates with a receptor on the surface of liver parenchymal cells that is involved in the clearance of endogenous glycoproteins from circulation. The presence of terminal galactose residues on the carbohydrate side chains of cFn make it a natural ligand for this receptor, termed the Ashwell or asialoglycoprotein receptor (ASGP-R), however, the fully sialylated chains of pFn are not recognized[16,17]. Approximately 80%-85% of the terminal carbohydrates of cFn are not capped by sialic acid[8].
Typically, the absence of sialic acid caps on its carbohydrate chains suggests that a protein is defective either as a result of normal catabolic mechanisms or pathogen induced sialidase activity[18]. These proteins may be harmful and induce a defensive response from the body if they are not rapidly removed. Perhaps cFn, as a naturally occurring desialylated glycoprotein, is intended to provoke a similar response from tissues that generate it in excess locally particularly during conditions of disease and disrepair.
FUNCTIONAL INTERACTIONS AND CHARACTER
The complex structure of fibronectins, their extensive presence in various tissues and fluids of the body, and their conserved expression across species, suggest that these molecules are important to fundamental biological processes. This is conclusively demonstrated by the lethality of FN gene inactivation during early murine embryonic development[19]. A greater understanding of this fundamental position can be obtained through a closer examination of its molecular architecture.
Fibronectin’s functional properties are mapped by specific domains of modular repeats along the molecule itself[8,20,21]. Polypeptide regions linking these globular domains are particularly susceptible to proteolysis, thus are readily cleaved to form fibronectin fragments of defined structural and functional character. Analyses of these fragments have led to the identification of distinct interacting sites along the fibronectin molecule that provide some insight into the physiological role of this glycoprotein (Figure 1)[8,20,21].
The domain represented by the amino-terminal fragment of fibronectin is composed of type I homologous repeats that can bind to a variety of substrates including matrix heparin and cell-surface heparan sulfate proteoglycans, glycosphingolipids found in membranes of central nervous system tissues, as well as to bacteria[22]. Of particular relevance is this domain’s strong affinity for fibrin, an insoluble plasma protein essential to blood clotting, to which fibronectin can be covalently stabilized via factor XIIIa transglutaminase-catalyzed cross-linking[23]. This cross-linking mechanism can also facilitate other fibronectin interactions with asymmetric acetylcholinesterase and Staphylococcus aureus[24,25]. Thrombospondin, present in tissue matrices and implicated in platelet aggregation, also binds to fibronectin at its amino-terminal domain[26]. These interactions suggest the participation of fibronectin in such events as cell adhesion, blood clotting, as well as pathogen recognition and/or clearance.
Immediately adjacent to the amino-terminal domain is the highly glycosylated collagen/gelatin binding site of the fibronectin molecule[8,20,21]. Fibronectin has demonstrated variable affinity for the various types of collagen in their native forms, however, it also adheres quite effectively to the unfolded regions of the denatured collagen triple helix[27]. Under physiological conditions, it appears that Type I collagen, which is found at elevated levels in the matrices of injured tissue, is in an unfolded state thus could readily interact with fibronectin[28]. The same domain that adheres to collagen can also bind to the C1q component of the complement system, facilitating fibronectin’s involvement in the clearance of immune complexes and cellular debris during the body’s defense response[29].
Situated at the carboxyl-terminal region of fibronectin is the molecule’s major heparin binding domain[30]. It comprises Type III repeats along with a variable segment that is determined by the tissue of origin. Nearest to the interchain disulphide bonds is a region consisting entirely of Type I modules that contains the molecule’s second fibrin binding site. However, this domain does not exhibit the diverse interactions of its amino-terminal counterpart and plays a more minor role in fibronectin-fibrin binding[30].
Clearly, the structure-function relationships of the terminal regions of fibronectin are well defined and reflect similar substrate affinities and functional character. Conversely, the central, more variable region of the fibronectin molecule remains more obscure. This central region is made up entirely of Type III homologous repeats that include the alternatively spliced EIIIA and EIIIB sequences positioned between repeats 11 and 12, and 7 and 8, respectively[8-10]. The heightened susceptibility of this section of the molecule to protease activity precludes it from the extensive fragmentation analysis that has been used to characterize the amino- and carboxyl-termini. Rather, the functional character of this large area of fibronectin must be determined through alternative analytical means. Accordingly, primary sequence data analysis has revealed the presence of a plausible DNA and heparin binding site adjacent to the collagen binding domain at the amino end of the central region; while the extra domains, EIIIA and EIIIB, have been implicated in a variety of roles based largely on in vitro analyses of their increased presence in fibronectin, particularly, under certain conditions of injury and disease[31-34].
Unlike the isoforms found in embryonic tissue, fibronectin molecules from healthy adult tissues include very low levels of EIIIA and EIIIB[35,36]. However, the variants with EIIIA and EIIIB will re-appear in abundance during such processes as wound repair and tissue regeneration[32,37]. Elevated levels of EIIIA and EIIIB fibronectin isoforms are also present under the pathological conditions of fibrosis and tumorigenesis[32,34,38]. Studies on malignant and benign remodeling activity in bone, as well as in human gingival tissue, show an increased presence of these extra domain containing forms of fibronectin[39,40]. In addition, these variants are also considered to be important mediators of the extensive interactions between participating cells and their environment during vascular morphogenesis[31]. Apparently, the EIIIA and EIIIB domains confer a role for fibronectin molecules containing them in processes that involve elaborate tissue modification and re-organization.
Interestingly, each fibronectin splice variant appears to be expressed in a tissue- and cell-specific manner, triggered by different stimuli at variable times. These distinctions have been demonstrated in studies on bone fracture repair that reveal a diffused expression pattern for EIIIA-containing fibronectin throughout the connective tissue that accumulates in the fracture gap during the granulation phase of healing, while the EIIIB-containing isoform remains localized in osteoblastic cells at the periphery of the newly differentiating tissue. Similarly, during the early stages of hepatic fibrosis, sinusoidal cells are the predominant source of EIIIA enriched fibronectin, which may be involved in the activation of hepatic stellate cells that subsequently produce the EIIIB inclusive fibronectin protein[32]. Additional evidence of such temporally and spatially distinct functions for EIIIA and EIIIB fibronectin splice variants can also be found in studies on chondrogenesis, renal fibrosis and various forms of lung cancer[41-43]. These observations suggest that the expression and function of fibronectin molecules with EIIIA and EIIIB segments may be regulated by specifically coordinated independent mechanisms that facilitate the transformative systems in various adult tissues.
More defined roles for the EIIIA and EIIIB domains have been identified in such events as matrix assembly, cell adhesion, migration and differentiation, as well as in cell cycle progression and mitogenic signal transduction, which are all relevant for tissue alteration, proliferation and development[42-45]. According to these in vitro studies both the EIIIA and the EIIIB domains appear to have equally essential, though different, roles in the aforementioned processes.
However, recent studies using genetically engineered mice seem to suggest a more critical function for EIIIA than EIIIB in vivo. Strains incapable of expressing EIIIA-containing fibronectin proteins have significantly shorter lifespans than their control counterparts[46]. Though these EIIIA knock-out mice exhibit wound healing defects, altered behavior and impaired motor coordination, they also develop fewer and smaller atherosclerotic lesions and appear to be protected from progressive fibrosis after bleomycin-induced lung tissue damage[34,46-48]. However, the in vivo function of the EIIIB domain remains obscure. No distinct phenotype has been observed in EIIIB knock-out mouse models aside from the impaired ability of extracted fibroblasts to form a significant pericellular matrix[44,49]. Nevertheless, this domain is highly conserved among vertebrates, thus it must have some biological importance. Perhaps the EIIIB domain plays a compensatory role in the absence of EIIIA during certain developmental processes, as mice devoid of EIIIA grow normally while the EIIIA and EIIIB double knock-out mice have lethal defects[19,46]. The EIIIB domain may have a significant function during the body’s response to stress brought on by injury and disease, as these are the conditions under which the extra domain-inclusive fibronectin isoforms are upregulated.
Located in the tenth Type III module (III-10), on an exposed loop in the central region of the fibronectin molecule is a three-amino acid consensus sequence, Arg-Gly-Asp (RGD), that has been identified as the main site of cellular attachment to fibronectin[8,50]. Adjacent to this site, in the ninth Type III module (III-9) exists a Pro-His-Ser-Arg-Asn (PHSRN) sequence that acts synergistically to enhance the binding affinity of cells to the III-10 RGD sequence[51]. These repeats, critical to cell-fibronectin contact, lie in a region between the two alternatively spliced EIIIA and EIIIB domains. Several studies suggest that the inclusion or exclusion of these extra domains may affect the conformation of the fibronectin molecule in that region which, in turn, determines the interactions of the RGD and PHSRN sites with specific receptors on the surfaces of nearby cells[52,53]. These interactions can trigger a cascade of distinct intracellular signals that translate into a multitude of different responses.
The receptors largely responsible for mediating these fibronectin-induced effects belong to a family of heterodimeric transmembrane glycoprotein complexes known as integrins[54]. Each integrin is comprised of two non-covalently associated α- and β-subunits that link the fibronectin-rich extracellular matrix with the cytoskeleton of the cell[55]. Structural studies have revealed that residues along the fibronectin molecule, external to specific integrin binding sites, play a critical role in optimizing the specificity and stability of this receptor-ligand assemblage[56]. Moreover, binding affinity is determined not only by the external configuration of the fibronectin ligand, but also by internal mechanisms that modulate the condition of the receptors themselves. Certain intracellular events can affect the association of the integrin α and β cytoplasmic tails, thus also, the activation state and the affinity of the receptor for specific ligands[57]. As such, integrins can function both as sensors (inside-out signaling) determining the presence of fibronectin, then mediating cell attachment and matrix assembly; and upon ligation with fibronectin, they can function as effectors (outside-in signaling) promoting ligand-induced biochemical processes[58].
To date, a dozen members of the integrin family have been shown to interact with fibronectin[50,59]. Not surprisingly, the major cell-binding III-10 RGD sequence on the fibronectin molecule is a key integrin-recognition motif and critical binding site for several of these cell surface receptors[8,50]. Of these integrin heterodimers, the prototype fibronectin receptor, α5β1, binds with greatest specificity. It is widely expressed, and likely serves as the major mediator of fibronectin-cell interactions in most tissues. Binding of α5β1 to the RGD motif is optimized through its association with the PHSRN synergy sequence on the adjacent III-9 repeat[51]. This sequence is also recognized by the platelet integrin αIIbβ3[60].
Other regions of fibronectin, besides repeats III-9 and III-10, have also been reported to interact with integrins. The α5β1 receptor exhibits a low affinity attachment to the N-terminal region of fibronectin, specifically to an Asn-Gly-Arg (NGR) sequence in the fifth Type I module (I-5) that has been converted through deamidation and isomerization to an isoAsp-Gly-Arg (isoDGR) sequence[61]. Though deamidated proteins typically undergo loss of function, this modification on the fibronectin molecule may bring about a gain of function. Studies have shown that the isoDGR sequence is also a high affinity binding site for the αvβ3 integrin, which is involved in regulating endothelial adhesion and blood vessel formation[61]. Protein deamidation is also linked to aggregate formation through a process resembling matrix assembly, thus the isoDGR sequence may be involved in fibronectin fibrillogenesis[62]. Tissue accumulation of proteins with atypical aspartyl residues is often observed during injury and disease[63,64]. Though unconfirmed, it seems likely that the prevalent form of fibronectin under such conditions would also contain a modified I-5 repeat.
Alternative splicing of the fibronectin primary transcript results in the production of structurally diverse molecules with very specific configurations and binding capabilities. The arrangement of alternatively spliced domains determines the level of exposure and accessibility of binding sites in involved regions of the protein[52,53,65]. However, the existence of internal integrin recognition sequences suggests that, in addition to structural considerations, these domains can also directly influence fibronectin’s effect on cell behavior. The alternatively spliced Type III connecting segment (IIICS) of the fibronectin carboxyl-terminal region contains two active binding sequences, Leu-Asp-Val (LDV, residues1-25) and Arg-Glu-Asp-Val (REDV, residues 90-109), that interact with the leukocyte integrin receptors, α4β1 and α4β7[66-68]. The sequence, Glu-Asp-Gly-Ile-His-Glu-Leu (EDGIHEL) in the EIIIA domain is recognized by integrins α9β1 and α4β1 during cell adhesion and wound healing[69]. Structural analyses have revealed the presence of a conserved Ala-Gly-Glu-Gly-Ile-Pro (AGEGIP) sequence on the EIIIB beta strand CC’ loop (links beta strands C and C’) that is part of an acidic groove created by the interface of the EIIIB domain with the adjacent eighth type III module (III-8)[52]. However, the specific binding partners for this site are yet to be identified. It is apparent that each variation in the splicing pattern of these alternate domains would produce distinct combinations of binding sites that would have a differential influence on cell behavior when engaged by the appropriate receptor.
Proteoglycan receptors, such as the integral membrane syndecan-1,-2 and -4, as well as, glycosyl-phosphatidylinositol (GPI)-anchored proteoglycans such as glypican-1, have been linked to the fibronectin mediated processes of cell adhesion, cytoskeletal organization and matrix assembly[70,71]. These proteins can link directly to fibronectin through their covalently attached flexible glycosaminoglycan (GAG) chains of heparan sulfate (HS) or chondroitin sulfate (CS). Although proteoglycans are capable of affecting cell behavior directly by independently engaging relevant intracellular pathways, most reports suggest that these receptors are more likely to be involved in complementary functions that support the activities of other fibronectin receptors, specifically integrins, that are considered to have a more significant role in certain cellular events[72,73]. The spatial arrangement of the respective binding sites for each receptor along the fibronectin molecule facilitates their cooperative regulation of the adhesive functions of the cell that influence movement and morphology, as well as, pericellular fibronectin fibril assembly[72-75]. Alternatively, the proximity of these binding sites could also allow for direct regulatory interactions between the different receptors themselves. Additional receptor collaboration may involve the strategic recruitment of distant fibronectin molecules, detected by the extended ectodomains of the proteoglycan receptors, for closer positioning to the cell surface where more efficient integrin binding can occur. Cooperative mechanisms may also include the transduction of signals by activated proteoglycans to effect an appropriate distribution of integrins and influence their consequent function. A synergistic convergence of such signals could occur, which would reinforce a particular effect, as illustrated by the fibronectin-induced activation of both α5β1 and syndecan-4 to promote cell adhesion and matrix contraction in the unstable environment of a wound during tissue repair[73]. Evidently, the functional regulation of fibronectin-mediated cellular processes entails some degree of coordinated activity between participating proteoglycan and integrin receptors, however, the actual mechanisms behind these interactions remain obscure.
Considering the number of receptors from the integrin family itself, that are reported to interact with fibronectin and the diversity of such interactions, it is expected that cross-regulation between activated integrins must also occur to ensure appropriate receptor cooperation or antagonism. Examination of the complex sequence of events that lead to the directional migration of a cell along a fibronectin fibrillar matrix, has revealed a pattern of spatially and temporally-regulated binding and alternate signaling by the α5β1 and αvβ3 integrin receptors, both of which recognize the same fibronectin domain[76]. Though not considered to be a fibronectin-specific receptor under normal conditions due to its indiscriminate adhesion to a variety of ECM molecules, the neutrophil receptor, αMβ2, during the cellular response to inflammation, binds with greater than normal avidity to fibronectin to effectively hinder directed migration[77]. Coordinated communication between α5β1 that facilitates chemotaxis, and αMβ2 must take place to ensure the effective translocation of the neutrophil to the site of injury where its presence is secured by additional interactions between αMβ2 and the fibronectin-enriched matrix so that it may effect the appropriate defensive response. The adhesive properties of the cell are further enhanced by the collaborative signaling of α5β1 and α4β1, each of which binds to different sites along the fibronectin molecule and interacts differentially with the provisional matrix that forms with granulation tissue during wound healing and repair[73]. Clearly, effective regulation of these fibronectin-integrin mediated physiological processes involves some coordinated crosstalk between the respective signaling pathways.
Each event induced by a particular set or sequence of interactions with fibronectin is not only dependent upon cooperative receptor activity but also upon synchronous availability and accessibility of respective ligand binding sites. When fibronectin is first secreted into the interstitial space of tissues, it exists as a soluble dimer whose compact form, stabilized by intramolecular forces, conceals many of these ligand binding sites and regions of significant adhesive character[78]. Though this closed conformation under physiological conditions is programmed by fibronectin’s inherent structure, environmental factors can effect changes to its shape that expose these otherwise embedded sites, to potential binding partners[79]. It is thought that upon ligation to integrins and other cell surface receptors, particularly α5β1, which recognizes the already accessible RGD loop, a cooperative unfolding and elongation of the respective arms of the dimer is initiated which then expose these sites to binding by key receptors involved in fibronectin fibrillogenesis[80]. Gradual extension of this originally tightly-folded molecule is largely attributed to cell-traction forces that are induced by cytoskeleton-dependent events and transmitted through adherent receptors[81,82]. The globular structure of individual Type III modules is disrupted, revealing intradomain binding sites that readily interact with their counterparts on other dissociated fibronectin molecules to launch progressive self-association that can lead to fibrillar matrix assembly[83,84]. These uncharacterized hidden or cryptic sites, as they are termed, are only activated when exposed by conformational changes that counter the native configuration of fibronectin. This suggests that these sites are involved in functional interactions, other than self recognition, particular to modifications in the extracellular environment that challenge normal physiological conditions.
As previously mentioned, the native fibronectin molecule is vulnerable to proteolysis, particularly along the unfolded polypeptide links between the compact domains. Cell-derived tensile forces increase the incidence of unfolding, thus create more unprotected regions that can be acted upon by endogenous proteases. Fibronectin is a known substrate of numerous different proteases, particularly the aggrecanases such as ADAM (A Disintegrin And Metalloproteinase)-8 and ADAM-TS (with Thrombospondin Motifs)-4, as well as matrix metalloproteases (MMPs) such as the gelatinases, MMP-2 and -9, the metalloelastase, MMP-12 and the membrane-type (MT)1-MMP. Interestingly, studies reveal that fibronectin itself induces the release and activity of several of these proteases, likely as a homeostatic response during ECM maintenance, that is regulated in part by its association with integrins and membrane-anchored MMPs[85,86]. Ongoing studies have revealed the presence of numerous specific cleavage sites or neoepitopes along the molecule that suggests the proteolytic degradation of fibronectin is not an arbitrary process and may have some functional value[87]. Random mechanical fragmentation, however, does also occur, especially among the less resilient Type I and Type II domains that have more restricted conformations.
The fibronectin fragments that result from these proteolytic events would have distinct folding patterns from their intact forms on the native molecule, therefore variably exposed binding sites. These peptides are, consequently, able to interface with dissimilar binding partners than the intact molecule thus they may act quite differently. In fact, certain fibronectin fragments have been associated with bioactivities that are quite disparate from those of the parent molecule and likely serve a regulatory role[73]. Many studies report a similar competence between fragments and native fibronectin to modulate protease activity, while other reports reveal that such proteolytic potential exists in the fibronectin fragments themselves[88,89]. These fibronectin-derived proteases or fibronectinases are capable of autodigestion but otherwise remain cryptic in nature, as no associated physiological role nor other mechanism has yet been identified. Other fragments can exhibit chemotactic activity, promote apoptosis, regulate anabolic and catabolic processes or induce the release of nitric oxide and cytokines[90-92]. Analyses of these fibronectin fragments and their specific functions can reveal distinct interactions that may provide further insight into the physiological role of the native protein itself. Studies of endogenous fragments that contain the EIIIA domain, for example, have revealed a unique interaction with Toll-like receptor (TLR)-4 that stimulates the release of proinflammatory cytokines[92]. These data suggest that fibronectin isoforms containing this alternatively-spliced domain may have a role in the physiological response to inflammation in injured tissue.
Fibronectin also adheres to a variety of different signaling molecules and regulates their distribution and access to other binding partners and cells[93,94]. Tumor necrosis factor (TNF)-α, upon release from activated cells at the site of inflammation, can complex with the amino terminal domains of fibronectin in the surrounding matrix[93]. This interaction confines this proinflammatory cytokine near to its source thus ensuring its availability to further stimulate cells in the region to release proteases as part of the defense response[95]. Fibronectin may also be involved in presenting a growth factor to its cognate receptor in a manner that will enhance a desired physiological effect. This is demonstrated when hepatocyte growth factor (HGF) or vascular endothelial growth factor (VEGF) attaches to fibronectin with a specific juxtapositioning that promotes the coordinated interaction and costimulation of the respective growth factor receptor and the fibronectin-binding α5β1 integrin, to amplify the proliferation and migration of endothelial cells[94]. Though fibronectin does not interact directly with transforming growth factor (TGF)-β, but with the latent TGF-β binding proteins (LTBPs) to which the TGF-β-confined small latent complex (SLC) is covalently bound, it is able to sequester TGF-β, and regulate its activation by proteases and matrix remodeling forces[96,97].
The detection of additional binding partners is ongoing, as is the recognition, with each associated function, that fibronectin is more than a mere component of biological scaffolds and conduits of cellular activity. It is a repository for both intrinsic ligands that can be proteolytically transformed into soluble signaling peptides, and extrinsic ligands that can be regulated through complex formation. Moreover, its pliable constitution hints at mechanotransducing capabilities. This glycoprotein has the potential to affect cell behavior in a myriad of different ways.
SIGNALING PROCESSES
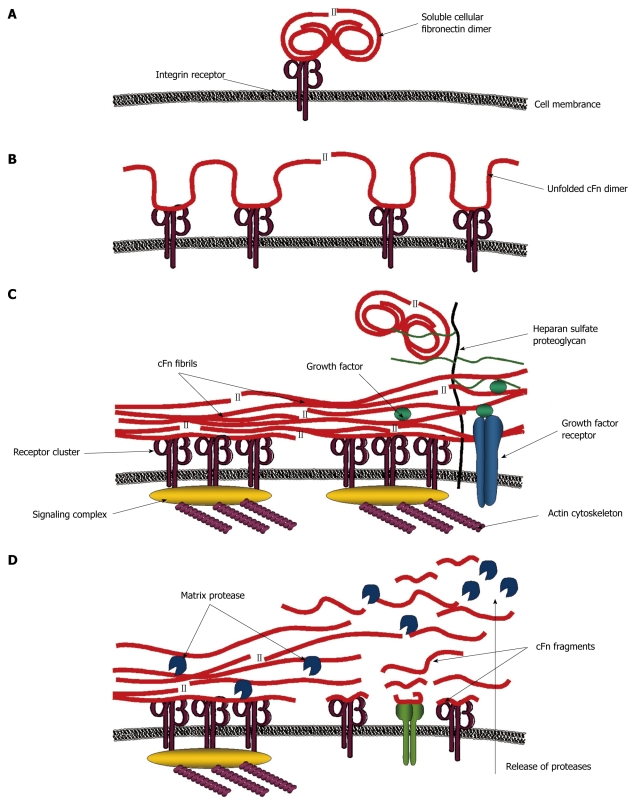
The mechanisms by which fibronectin may affect signaling events, as indicated by the diversity in its interactions, are expected to be quite involved (Figure 2). The initiating influence on cell behavior may be conceived when the soluble fibronectin molecule is ligated by a cognate receptor, likely an integrin heterodimer, and activates the first recognition-dependent sequence of molecular signals within the cell. Cytoskeletal restructuring and the consequent stimulation of certain intracellular complexes promote cell contractility which causes conformational changes in the attached molecule[80]. Intramolecular dissociation ensues and the compact dimer unfolds to expose additional binding sites and recognition sequences, which upon engagement, can trigger simultaneous cascades of signals that further influence cell behavior. Fibronectin self-association between attached and extended molecules, modulated again by cellular events such as receptor clustering, takes place to form an adhesive template upon which additional fibronectin molecules can also unfold. The progressive layering and interweaving of these elongated and stretched out fibronectin fibrils eventually results in the formation of a connective web between neighboring cells. Other matricellular components, recognizable to fibronectin can also be incorporated into this structure. The diversity and distribution of these elements can affect the character and signaling propensity of this fibronectin matrix that may now behave as a cohesive unit or solid-phase ligand. Nevertheless, individual interactions can impose changes to matrix ligand architecture that may create a flow of signals through interconnected molecules. Mechanical stress brought about by such extracellular perturbations can also alter the connectivity between ligand and receptor, and change the composition of signals being relayed[98]. Mechanotransduction is further regulated by the composition and density of this matrix ligand. A dense and correspondingly rigid matrix creates more exogenous tension which can affect such cellular activity as spreading and directed motility[99]. Protease release is also up-regulated in response to matrix rigidity. The resulting cleavage of fibronectin fibrils produces fragments with signaling properties that differ from the intact molecule. New interactions are formed that stimulate a different array of signal transduction pathways to evoke a differential cellular response. Other molecules, whose association with fibronectin have been compromised by proteolytic remodeling, are now also accessible to responsive cells[100]. All of these mechanisms by which fibronectin may influence cell behavior comprise a dynamic system that has complex spatially and temporally regulated components. Signaling pathways, though individually engaged, are part of a collective communication network between fibronectin and the cell.
Figure 2.
Model for signaling processes mediated by fibronectin. A: Initial signals are mediated when soluble compact cellular fibronectin (cFn) binds a cognate receptor; B: This causes cFn to unfold and interact with other receptors inducing further signals; C: Receptor clustering and the formation of signaling complexes lead to the reorganization of the actin cytoskeleton which creates tensile forces, conveyed through the integrin receptors to further stretch cFn into fibrillar form. Exposed cryptic sites interact with other cFn fibrils in matrix assembly. Access to growth factors and other molecules is regulated by cFn binding. Heparan sulfate proteoglycans also bind to cFn and recruit distant molecules closer to the cell surface; D: All of these interactions create a cascade of different signals, some of which promote matrix protease release. Resulting cFn fragments activate additional intracellular signaling pathways. Thus, cFn can regulate cell behavior via numerous different mechanisms.
Most of these pathways are thought to be driven by integrin-mediated signaling processes. Focal adhesion, stress fiber formation, and cell translocation are determined by integrin recruitment of focal adhesion kinases (FAK) with subsequent activation of the phosphatidylinositol 3-kinase (PI3K) signal transduction pathways[101,102]. However, recent studies have also implicated other receptors and associated pathways in the regulation of these events. It has been shown that cell adhesion and migration, as well as cytoskeleton reorganization are also determined by syndecan-2 and -4 mediate mechanisms that involve protein kinase C (PKC)-dependent activation of the small GTPase molecules, Rac, Cdc42 and Rho[71,72,103]. Fibronectin-induced cell survival and proliferation are also regulated via the integrin-mediated FAK/PI3K pathway[104]. Other coordinated signals originating from a different set of fibronectin receptors may exist but have yet to be identified. Studies suggest that cytokine release and protease production that result from nuclear factor (NF)-κB activation or mitogen-activated protein kinases (MAPKs) signaling may also be mediated by toll-like receptors (TLRs) and MT-MMPs in addition to integrins, however, this needs to be further clarified[92,105]. Continued examination of the numerous interactions between fibronectin and cells, may reveal the identity of additional pathways and signaling mechanisms which would further our understanding of this multifaceted protein in its regulation of various cellular processes.
Clearly, the involvement of multiple ligand-receptor systems in fibronectin signaling requires intricate regulation and the appropriate integration of respective pathways to ensure optimal cellular activity. This elaborate network calls attention to the important role this glycoprotein plays in numerous biological processes. Disruption of these coordinated events could certainly have severe and deleterious consequences.
ROLE IN DISEASE
The aforementioned interactions and associated functions can generally be ascribed to both classes of fibronectin except where the alternatively spliced EIIIA and EIIIB domains are involved, as only the cellular forms of fibronectin contain these extra structures. Both pFn and cFn are secreted by cells as soluble globular proteins. Hepatocytes are the primary source of pFn, which is readily secreted into the bloodstream for distribution throughout the body. Cellular fibronectin, however, is produced locally in tissues, predominantly by resident fibroblasts and endothelial cells, to be deposited in the pericellular matrix. Nevertheless, it can also be taken up into the circulation. Conventionally, cFn is thought to be the main form of fibronectin found in the extracellular matrix of tissues, however, recent studies have determined that an almost equivalent fraction of matrix fibronectin is plasma-derived[106]. It appears that under normal physiological conditions, there is a balance of both types of fibronectin in intact tissues. It is not surprising then, to discover that unusually elevated levels of cFn are indicative of some underlying disturbance in the tissue of origin, which could very well have some pathological consequence.
Cellular fibronectin plays a critical role in tissue-specific morphogenesis and cellular differentiation during embryonic development[19]. These events recur in adult tissues during conditions that require regeneration or repair. Therefore, the accumulation of cFn at sites of injury and tissue perturbation where morphogenetic processes are again active is normal. Studies show that cFn regulates cell migration in damaged tissue, where it also stimulates fibroblast transitioning to its activated phenotype[73,107]. Other reports highlight the chemotactic activity of cFn and its regulation of growth factors during active wound repair[108]. Particularly convincing are data from knockout animal studies that reveal defective wound healing in cFn-deficient mice[46]. Clearly, this isoform is essential to the processes of tissue repair. Therefore, conditions involving any form of tissue damage would be marked by an increase in cFn production and release, particularly by cells near the site of injury. Under normal regulation, these events would culminate in the restoration of tissue function and integrity and a reduction in cFn to physiological levels.
However, under certain circumstances cFn levels remain elevated. Fibronectin-mediated cellular activity persists and may even be amplified. Otherwise uninvolved or down-regulated signaling mechanisms, eventually, become activated. Accordingly, cellular behavior adjusts and the maintenance of normal physiological processes changes to the promotion of pathological ones.
A prominent feature of many disorders associated with elevated levels of cFn is the persistent production and deposition of extracellular matrix proteins in affected tissue. This build-up of scar tissue may have started innocently, as a regulated wound healing response to chronic injury. However, it eventually becomes a fibroproliferative process that progressively destroys tissue integrity. Such fibrotic damage has been observed in many organ systems, particularly hepatic, pulmonary and renal systems[109-111]. Though it has been suggested that fibrosis may be reversible, most conditions do not improve but gradually progress to organ failure.
The pathological implications of cFn accumulation are considerably complex and widespread, reflecting the multifunctional capacity of this protein to influence cell behavior. The effects of cFn manifest in a tissue specific manner that may be exacerbated by other underlying factors unique to each disorder. For example, the cFn-induced fibrotic response may be complicated by factors involving the source of chronic injury. These parallel yet interdependent effects need to be recognized in order to achieve a deeper understanding of the molecular basis for these conditions and the specific role that cFn may play in their progression.
ROLE IN LIVER DISEASE
In the normal liver, the most abundant matrix protein is plasma fibronectin. This is not surprising, considering it is originally synthesized by hepatocytes. It can be detected in the subendothelial space of Disse where it comprises a major part of the low-density matrix that connects hepatocytes with the endothelial cells that line the sinusoids[112]. Cellular fibronectin, however, is present at very low levels throughout the liver. It is localized primarily in the pericellular matrix that surrounds the cells but also exists as bundles connected to the microvilli of hepatocytes in the space of Disse[113].
Naturally, most studies on fibronectin that reference the liver focus on the plasma isoform and of these, only a small percentage deal with disease. Most of those reports deal with the effects of hepatic insufficiency on pFn production and physiological function. Very few address a potential role for fibronectin itself in the incidence of liver damage that creates conditions of insufficiency. Such liver damage may result from a range of potential pathogenic mechanisms accounted for by autoimmune diseases (autoimmune hepatitis), genetic disorders (Alagille syndrome, alpha-1 antitrypsin deficiency, hemachromatosis, Wilson’s disease), viral infection (hepatitis A, hepatitis B, hepatitis C), disorders of uncertain etiology (cancer, primary sclerosing cholangitis, non-alcoholic fatty liver disease) and those attributed to systemic disease (Reye’s syndrome, Budd-Chiari syndrome) and toxic insult (alcoholic liver disease).
Many of these conditions have acute and chronic presentation. Under conditions of acute liver damage, wound healing would not be a prolonged process. It would entail a quick remodeling of the ECM to create a cFn-rich provisional matrix, which would be involved in modulating repair activity to restore liver integrity. The onset of this response would be marked by a sudden surge in fibronectin production, which would just as suddenly diminish once the repair is complete. However, under conditions of chronic liver damage, wound healing would no longer be a finite process. The initial surge in cFn production may likely persist.
Accordingly, studies concerning such conditions of sustained and chronic liver damage report a considerable increase in patient blood plasma levels of cFn[114]. Immunohistochemical and RT-PCR analyses also reveal elevated amounts of cFn and its mRNA in the tissue of diseased livers[115,116]. These findings confirm that cFn production does persist as a likely response to mechanisms perpetuating liver injury under such conditions of chronic disease. The only role cFn has been considered to have in the course of these events is as an indicator of the onset of progressive damage. As such, it has potential utility as a biomarker for chronic liver disease.
Currently, a liver biopsy is the only means by which clinicians can accurately determine the extent of liver damage in patients suffering from chronic disease. However, it is an invasive, painful and inherently risky procedure that does not always provide information that would lead to significant alterations in treatment, especially if severe liver damage was already a concern[117]. Therefore, the development of additional biomarker tests that could reduce the prevalence of unnecessary biopsies is of great interest. Much consideration has been given towards developing a means to incorporate cFn as a marker in this system. However, elevated levels of cFn are not specific to hepatic injury, as similar concentrations have also been detected in the plasma of patients with no known hepatic pathologies, but who suffer from some other tissue-related chronic disease[114]. This lack of specificity diminishes the utility of cFn as a diagnostic indicator of liver disease. Nevertheless, increasing cFn levels remain a reliable indicator of sustained tissue damage. Perhaps tests for cFn could be incorporated in a panel that includes tests for the more specific markers of hepatic damage, alanine aminotransferase (ALT) and gamma glutamyl transpeptidase (GGT), thus providing a means to determine whether a detected increase in cFn levels is, in fact, related to liver disease[118]. A ratio of ALT or GGT levels to cFn could also be instructive.
These efforts to determine a clinical application for cFn have led to further investigations of the pathophysiological events in chronic liver disease that result in its up-regulated levels. It was previously believed that such up-regulation was a response to injury and had no relevance to the progression of disease itself. However, recent studies suggest that cFn may participate in, and may even promote, the progression of injury that marks these chronic conditions.
Chronic injury of the liver may initially manifest as altered lipid metabolism that leads to the accumulation of fat deposits in hepatic cells. There is no evidence to date to suggest any cFn involvement in this process. However, as the injury persists, an inflammatory response is induced that could involve cFn-regulated wound healing activity. Studies that currently report a regulatory role for cFn during inflammation in the liver address acute rather than chronic injury conditions. Although studies from other systems suggest that cFn does affect the behavior of immune and inflammatory cells, thus may also be a potent mediator of the inflammatory response to chronic injury, further investigation is still required to confirm such a role for cFn in chronic liver disease[119,120].
In response to injury, hepatic sinusoidal endothelial cells (SECs) become activated and increase their production of cFn[32]. It has been suggested that this event occurs in the very early stages of damage, and could, in fact, be among the initial reactions to the detection of harmful stimuli. For example, the hepatitis B virus x antigen (HbxAg) has been shown to activate fibronectin gene expression in liver cells via an NF-κB-dependent mechanism[121]. The direct detection of HbxAg by SECs could, therefore, be an initiating event in the progression of hepatitis B-induced liver injury. The resulting upsurge in cFn production dramatically increases the total concentration of cFn in the liver to a level that is several-fold above normal[32].
Of particular interest, Jarnagin et al[32] showed that greater than 80% of the cFn produced by SECs during injury, 12-24 h after stimulation, contain the alternatively spliced EIIIA domain. As mentioned earlier in this text, the fibronectin EIIIIA domain has been implicated in cell adhesion and pro-inflammatory cytokine production through its interaction with cell surface receptors, α9β1 and α4β1 integrins, and TLR-4[69,92]. Moreover, studies have also shown that cFn activates α5β1 signaling via the RGD motif in its major cell binding domain to upregulate the production of MMPs. These MMPs are involved in ECM remodeling events that can also produce cFn fragments, which can further stimulate cell behavior. Thus, it would not be too bold to suggest that cFn may be involved in regulating many of the cellular responses to injury in the liver.
In fact, activation of hepatic stellate cells (HSCs) during injury is mediated by cFn[32]. Studies reveal that HSCs will transition to their myofibroblastic phenotype via a TGF-β1 regulated mechanism induced by EIIIA cFn[122]. Further studies show that TGF-β1 up-regulates the expression of the fibronectin receptor, α5β1 in HSCs, making them more responsive to cFn thus reinforcing its effect[123].
Once activated, HSCs are involved in mediating most of the ECM remodeling activity that leads to fibrotic damage in the liver. These cells are also the major source of matrix protein constituents in connective scar tissue that manifests during disease progression. HSCs also produce cFn in continually increasing amounts, but of a different composition than the cFn secreted by SECs. The cFn secreted by HSCs is also predominantly of the EIIIIA variety, constituting 42% of the total, 7 d post-activation[32]. However, there is also a significant increase in the relative amount of cFn produced that contains the EIIIB domain (9% of the total after 7 d). The physiological significance of this increase in EIIIB variant levels and the regulatory relevance of timing its production during the advanced stages of chronic hepatic injury, are yet to be determined.
Each form of chronic liver disease may present with variable distinction between the stages of injury; however, should the incidence of damage continue along this general trajectory, fibrotic scarring will totally compromise organ function. Without a transplant, death is certain. It has become apparent that cFn has a functional role in this progression of liver injury, thus may warrant greater attention for its pathogenic nature than for any biomarker potential.
ALCOHOLIC LIVER DISEASE
As the body’s major detoxifying organ, the liver is the primary site of alcohol metabolism, and is particularly susceptible to the detrimental effects of alcohol abuse. Though the association between liver injury and the excessive consumption of alcohol was established over 200 years ago, we are still unable to fully understand how such damage occurs, nor have we developed any thoroughly effective strategies to counter the progression of alcoholic liver disease (ALD).
ALD initially manifests as fatty liver (steatosis), a reversible condition characterized by increased fat deposition in the liver cells, which leads to hepatomegaly (enlarged liver) and can progress to alcoholic hepatitis, a more serious condition marked by inflammatory changes. Persistent damage prompts the development of scar tissue (fibrosis), which will eventually replace the functional tissue of the liver resulting in alcoholic cirrhosis, hepatic failure and death.
Evidence suggests that alcohol itself and its metabolites are direct hepatoxins that stimulate changes in the cells of the liver which result in a cascade of responses culminating in tissue damage[124-129]. The toxicity of alcohol is linked to its oxidation which is catalyzed mainly by the multi-variant cytosolic enzyme, alcohol dehydrogenase (ADH), to produce acetaldehyde, which is further processed in mitochondria to form acetate, most of which escapes to the blood[126,130]. Acetaldehyde binds reactive amino acid residues in proteins to form acetaldehyde-protein adducts which can impair secretion and enzymatic activity[128]. As the level of alcohol increases with ongoing consumption, microsomal enzymes, predominantly cytochrome p450 isozymes, as well as peroxisomal catalase (minor pathway), become involved in metabolizing alcohol with the additional creation of reactive oxygen species (ROS) and hydroxyl radicals that provoke lipid peroxidation events and the release of further harmful metabolites[131,132].
The conversion of alcohol involves the reduction of a co-enzyme intermediate, nicotinamide adenine dinucleotide (NAD+) to generate NADH which increases the NADH/NAD+ ratio and redox state within cells. A highly reduced intracellular environment sustained by persistent metabolism of alcohol will greatly impair the cell’s ability to function normally. Under these conditions, hepatic cells are rendered more vulnerable to damage from the reactive metabolites of alcohol whose concentrations are correspondingly increased[124,127-129].
Typically, these cells would respond to the increasing levels of such harmful byproducts by releasing factors that stimulate mechanisms of tissue defense and repair. However, such mechanisms are impaired in liver tissue that has been subject to prolonged insult by alcohol. Rather than countering the progression of injury, the cellular response reinforces it.
For example, SECs respond to increasing levels of harmful adduct modified proteins formed during alcohol metabolism by up-regulating their output of the EIIIA variant of cFn that is involved in tissue repair[132]. Under a condition of chronic alcohol metabolism, this process will persist, resulting in an elevation in the levels of cFn in the liver. Restoration of homeostatic levels of this glycoprotein will usually occur towards the end of a wound healing response to injury. In the liver, this turnover of cFn is mediated, in part, by the hepatocyte specific ASGP-R[17]. However, studies have shown that the cellular processes of this receptor, particularly those that involve protein trafficking, are particularly susceptible to the effects of alcohol[133-135]. Several alcohol-induced alterations in ASGP-R activity have been identified that contribute to impaired receptor-mediated uptake of its ligands[136,137], which could include cFn. This coupling of persistent production with ineffectual clearance would lead to a build-up of cFn in the alcohol-injured liver. This has been demonstrated in studies using a rat model of alcohol consumption. Significantly elevated levels of cFn were detected in the livers of animals subject to prolonged alcohol administration which correlated with the inability of the hepatocytes from these animals to adequately internalize and degrade cFn[138,139]. Though these observations have not yet been corroborated in human liver tissue, a blood plasma study found elevated levels of cFn in patients suffering from alcoholic cirrhosis which suggests that the hepatic levels of cFn were also high[114].
The functional character of cFn suggests that its accumulation would exacerbate the deleterious effects of sustained alcohol abuse on the liver. A clearer understanding of how this reactive glycoprotein influences the cellular events that promote injury may reveal new targets for the development of effective treatments for ALD.
To this purpose our lab employed a rat model that is extensively used in alcohol research. Male Wistar rats were pair-fed a nutritionally adequate Lieber-DeCarli liquid diet that contained 6.4% alcohol by volume as 36% of total calories or an isocaloric control diet. Animals maintained on this diet exhibit morning (i.e. 9 am CST) blood alcohol levels of 100 to 150 mg/dL (21.7 to 32.6 mmol/L)[140]. These concentrations correspond to levels found among chronic drinkers in the human population. After twelve weeks of feeding, 60% more cFn was detected in livers of the alcohol-fed animals than from pair-fed controls. Furthermore, we found that the hepatocytes from these alcohol-fed animals exhibited a diminished capacity to degrade cFn that correlates with its accumulation. As the purpose of this study was to ascertain whether cFn contributes to the development of advanced liver injury, animals were fed for a shorter duration of 4-6 wk, sufficient for the development of the early stages of alcoholic liver injury but not prolonged enough for substantial cFn accumulation to have already taken place[138,139]. Moreover, the Lieber-DeCarli rodent model rarely ever sustains injury beyond fatty liver, thus it is an appropriate system to investigate whether cFn could provoke further inflammation and/or a fibrotic response when added exogenously to cultured cells isolated from the livers of alcohol-fed animals.
The pro-fibrogenic propensity of cFn has largely been attributed to its observed effects on HSC activation and proliferation[32]. Moreover, studies have shown that fibronectin fibrils have a particular affinity for collagen Type I molecules that are synthesized by activated HSCs and are major constituents of the connective tissue that forms during fibrosis. Formation of the matrix during the fibrotic response to injury requires a stable ECM layer of cFn[141]. These reports imply that the build-up of cFn in the liver of chronic consumers of alcohol would be sufficient to initiate fibrotic damage.
However, cFn has also been implicated in the recruitment and activation of other cell types besides HSCs during the wound healing response. These cells may be involved in the pro-inflammatory activity that precedes HSC activation and may even prime the conditions in the liver for an HSC response. Kupffer cells (KCs), as the resident macrophages of the liver, are the primary mediators of such inflammatory activity in response to alcohol-induced injury that occurs during the early onset of damage. Anchored at strategic intervals throughout the hepatic sinusoid, these macrophages sentinel portal flow entering the liver lobule for incongruous and harmful substances. Accordingly, they can detect early changes in the hepatic environment arising from alcohol-induced injury such as the increasing levels of cFn. In healthy tissue, Kupffer cells orchestrate defensive and reparative processes through their phagocytic activity and production of soluble signaling molecules. However, under a condition of chronic alcohol administration, excess cFn provokes a response in Kupffer cells that actually promotes rather than protects against further tissue damage[142].
Kupffer cells are the major source of TNF-α and IL-6 during the liver’s homeostatic response to tissue damage. Elevated levels of these pro-inflammatory cytokines are characteristically detected in the serum of patients with alcohol-induced liver injury. These cytokines stimulate autocrine and paracrine effects that result in the activation of other liver cell types during the injury process[143]. For example, both TNF-α and IL-6 are involved in the transition of HSCs to their myofibroblast-like phenotype. These cells in turn, accelerate the production of ECM proteins that heralds the fibrogenic response to injury[143].
Regions of the liver that first respond to the toxic effects of alcohol contain both cFn-enriched matrices and elevated numbers of KCs[144]. The behavior of KCs in these regions is likely influenced by the high concentration of cFn present. In fact, studies from our lab have shown that cFn has a profound effect on the KC secretion of TNF-α and IL-6, and therefore may be involved in promoting the KC-mediated activation of HSCs[142].
ECM remodeling poses a homeostatic challenge to cells that prompts the production of agents that can restore and maintain normal tissue architecture. MMPs and their inhibitors (TIMPs) are key agents that regulate this process. An imbalance in the relation of MMPs to TIMPs can lead to profound changes in the composition of the ECM such as is found in various pathological conditions including alcoholic fibrosis[113,145]. We believe that increasing levels of cFn, itself a constituent of the ECM, can prompt events leading to such imbalance in susceptible tissue.
Though HSCs are the most prolific source of factors that regulate the deposition of matrix components in the liver, during the early stages of fibrotic injury and prior to HSC activation KCs assume this role. Our studies have revealed that in response to increasing levels of cFn, cultured KCs from both control and alcohol-fed animals secrete significantly higher amounts of MMP-2 protein than their untreated counterparts. This increase in protease levels may be a regulatory response to excess cFn, a known substrate of MMP-2. However, we also found that the cells from alcohol-fed animals released significantly more of the associated inhibitor, TIMP-2, than matched control and untreated cells. Correspondingly, though the total MMP-2 secreted by the KCs from alcohol-fed animals was elevated, most of the enzyme detected was still in the less operational precursor form (pro-MMP-2)[142]. These results suggest that a higher degree of MMP-2 inhibition exists under a condition of alcohol administration. The consequent reduced degradative capacity of this protease could, in turn, contribute to the eventual build-up of ECM proteins characteristic of fibrotic injury.
These findings also imply that chronic alcohol consumption alters the homeostatic response of KCs to the build-up of proteins in the ECM. This may be another regulatory mechanism compromised by excessive alcohol metabolism that may contribute to the accumulation of cFn in the liver. The inhibition of matrix proteases is reinforced with each increase in cFn, creating a cycle that may later also facilitate the deposition of other matrix proteins involved in the fibrogenic process. Collectively, these studies suggest a role for cFn in Kupffer cell activation that contributes to the progression of alcohol-induced liver injury that may lead to fibrogenesis (Figure 3).
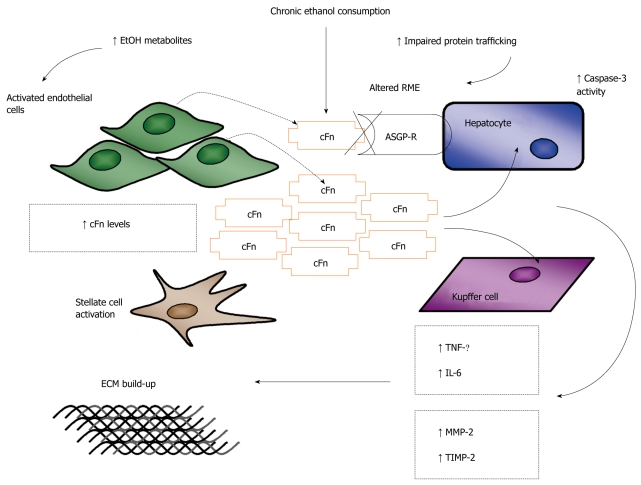
Figure 3.
Schematic representation of the proposed model of ethanol-induced liver injury linking altered asialoglycoprotein receptor clearance of cellular fibronectin with hepatocyte and kupffer cell activation by the accumulating protein. The alcohol induced up regulation of cellular fibronectin (cFn) production by sinusoidal endothelial cells (SECs) and its impaired clearance by the hepatocyte-specific asialoglycoprotein receptor (ASGP-R) leads to the accumulation of cFn in the liver. Hepatocytes (HCs) and kupffer cells (KCs) are stimulated by cFn to produce the pro-inflammatory/pro-fibrogenic cytokines, tumor necrosis factor (TNF)-α and interleukin (IL)-6, which further activate hepatic stellate cell (HSCs) stimulating their transformation to the pro-fibrogenic phenotype. HCs and KCs are also stimulated to produce the matrix degrading enzyme, matrix metalloproteinase (MMP)-2 and its corresponding inhibitor, tissue inhibitor of metalloproteinase (TIMP)-2. Greater levels of TIMP-2 are secreted resulting in the inhibition of MMP-2 activity and subsequent build-up of the extracellular matrix (ECM), characteristic of the early onset of fibrotic liver damage. RME: Receptor mediated endocytosis.
Hepatocytes are the chief functioning cells of the liver and key targets for mediators of injury. Accordingly, studies on the effects of various hepatoxins focus on disruptions to hepatocytic processes, such as alcohol-induced impairments to ASGP-RME as previously determined by our lab. As an extension of this work, we examined whether the consequent accumulated cFn would itself also be toxic to hepatocytes[138]. We found that elevated concentrations of cFn induced a significant increase in caspase-3 activity, a marker of apoptosis (programmed cell-death), in hepatocytes from alcohol-fed animals after a 20-h incubation.
It was also observed, in these same cells, a corresponding increase in the secretion of TNF-α and IL-6. This treatment with elevated, pathology-associated, concentrations of soluble cFn also stimulated cultured hepatocytes from both control and ethanol-fed animals to secrete significantly higher amounts of MMP-2. As previously mentioned, the activity of MMPs is dependent upon their balanced relationship with corresponding TIMPs. We also found that in the presence of elevated levels of cFn, cultured HCs from ethanol-fed animals secreted more TIMP-2 protein than their control and untreated counterparts. These cells, much like the KCs from ethanol fed animals, also release TIMP-2 in excess of MMP-2. Again, this disparity in the relative levels of these proteins would lead to an inhibition of MMP-2 activity which contributes to a reduction in matrix protein degradation and the subsequent build-up of the ECM.
The response by the hepatocytes to treatment with high concentrations of cFn was, however, not particularly robust relative to KCs, suggesting that the secretion of these factors may have a more localized purpose. For example, TNF-α, in particular, has been implicated in both the inflammatory and apoptotic responses of cells[146,147]. It is thus plausible that the observed increase in both the release of IL-6, and in the activity of caspase-3, may be attributed to autocrine TNF-α signaling after cFn induction. However, as demonstrated by other ECM molecules, cFn may also influence cell death directly[148,149]. Moreover, MMP-2 and TIMP-2 produced by these hepatocytes in response to cFn treatment may be involved in the immediate degradation of the surrounding matrix, producing reactive fragments of cFn which would have only a localized effect on hepatocyte behavior[150]. It is plausible that the hepatocyte response to excess cFn affects hepatocytes alone and is not to be included as part of the collective signaling pool. Thus these findings also suggest that hepatocytes may be more involved in reinforcing their own demise during a condition of alcohol-induced injury than previously assumed (Figure 3). The specific mechanisms underlying these observed responses will, however, require further examination.
This seemingly unconventional response observed in hepatocytes could be explained by recent reports that suggest that these cells possess an inherent plasticity that makes them more susceptible to changes in the tissue microenvironment that could compromise pure epithelial character[151,152]. This plasticity is essential to the regenerative capacity of the liver that allows for recovery from sustained damage, which is an inevitable consequence of its function as a detoxifying organ. However, this poses a unique challenge for the researcher studying a specific aspect of hepatocyte behavior, as current isolation procedures and culture techniques can also alter the character of these cells. These findings should be further explored using new techniques involving 3D cultures and liver slices that are more representative of in vivo conditions[153,154].
CONCLUSION
Under normal conditions, cFn is an ostensibly innocuous and minor component of the ECM that is produced locally by tissues where it concentrates in the pericellular matrix that surrounds cells. It debuts as a critical factor for embryonic development, after which its levels greatly diminish, and are only re-established in adult tissue during events that involve regeneration or repair. However, its elevated presence has also been associated with various chronic disorders that are characterized by extensive tissue damage. These conditions create doubts as to whether cFn is truly a mediator of healing processes or an instigator of disrepair.
In healthy tissue, where physiological processes are appropriately regulated, cFn production is stimulated in response to signs of injury. Its upregulation provokes extreme activity that is essential for the repair of damaged tissue. Once tissue integrity has been restored cFn production is reduced to homeostatic levels and normal cFn turnover is restored. However, during a condition of relentless attack by agents that compromise tissue function, the regulatory mechanisms that restrict cFn activity become impaired. A condition of unbridled ‘wound healing’ develops that actually causes more tissue damage rather than repair.
In the liver, chronic metabolism of alcohol leads to the build-up of harmful byproducts which cause the increased production of cFn by hepatic SECs, as well as its impaired clearance by the hepatocyte specific ASGP-R. Although the physiological relevance of the resulting accumulation of cFn in the liver parenchyma remains debatable, evidence suggests cFn is not a static component of the hepatic scaffold, but a dynamic mediator of cellular events that may promote the progression of liver damage associated with chronic alcohol abuse. This reactive glycoprotein stimulates specific cells in the liver to release pro-inflammatory and pro-fibrogenic factors which create further tissue damage and disrepair that lead to alcoholic fibrogenesis (Figure 3).
Despite the medical community’s initiatives to educate and intervene, alcohol consumption rates world-wide continue to rise and with it the development of alcoholic liver disease with often fatal outcome. Currently, there are no effective measures to counter this epidemic. Therefore, the effects and underlying mechanisms of cFn-induced cell behavior in the alcohol-injured liver are worth further characterizing, not only to gain a more comprehensive understanding of the role this glycoprotein plays in the progression of alcohol-induced liver injury but also for any insight such investigation could provide towards the development of desperately needed novel therapies for alcoholic liver disease.
Footnotes
Supported by The National Institute on Alcohol Abuse and Alcoholism and the US Department of Veterans Affairs
Peer reviewer: Fernando J Corrales, Associate Professor of Biochemistry, Division of Hepatology and Gene Therapy, Proteomics Laboratory, CIMA, University of Navarra, Avd. Pío XII, 55, Pamplona, 31008, Spain
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH
References
- 1.Morrison PR, Edsall JT, Miller SG. Preparation and properties of serum and plasma proteins; the separation of purified fibrinogen from fraction I of human plasma. J Am Chem Soc. 1948;70:3103–3108. doi: 10.1021/ja01189a080. [DOI] [PubMed] [Google Scholar]
- 2.Gahmberg CG, Hakomori SI. Altered growth behavior of malignant cells associated with changes in externally labeled glycoprotein and glycolipid. Proc Natl Acad Sci USA. 1973;70:3329–3333. doi: 10.1073/pnas.70.12.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaheri A, Ruoslahti E. Disappearance of a major cell-type specific surface glycoprotein antigen (SF) after transformation of fibroblasts by Rous sarcoma virus. Int J Cancer. 1974;13:579–586. doi: 10.1002/ijc.2910130502. [DOI] [PubMed] [Google Scholar]
- 4.Hynes RO. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci USA. 1973;70:3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada KM, Kennedy DW. Fibroblast cellular and plasma fibronectins are similar but not identical. J Cell Biol. 1979;80:492–498. doi: 10.1083/jcb.80.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamada KM, Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978;275:179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]
- 7.Vuento M, Wrann M, Ruoslahti E. Similarity of fibronectins isolated from human plasma and spent fibroblast culture medium. FEBS Lett. 1977;82:227–231. doi: 10.1016/0014-5793(77)80590-5. [DOI] [PubMed] [Google Scholar]
- 8.Hynes RO. Fibronectins. In: Rich A, editor. New York: Springer-Verlag; 1990. pp. 113–175. [Google Scholar]
- 9.Owens RJ, Kornblihtt AR, Baralle FE. Fibronectin, the generation of multiple polypeptides from a single gene. Oxf Surv Eukaryot Genes. 1986;3:141–160. [PubMed] [Google Scholar]
- 10.Schwarzbauer JE. Fibronectin: from gene to protein. Curr Opin Cell Biol. 1991;3:786–791. doi: 10.1016/0955-0674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzbauer JE, Spencer CS, Wilson CL. Selective secretion of alternatively spliced fibronectin variants. J Cell Biol. 1989;109:3445–3453. doi: 10.1083/jcb.109.6.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali IU. Phosphorylation of fibronectin in quiescent and growing cell cultures. FEBS Lett. 1983;151:45–48. doi: 10.1016/0014-5793(83)80339-1. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda M, Levery SB, Hakomori S. Carbohydrate structure of hamster plasma fibronectin. Evidence for chemical diversity between cellular and plasma fibronectins. J Biol Chem. 1982;257:6856–6860. [PubMed] [Google Scholar]
- 14.Olden K, Parent JB, White SL. Carbohydrate moieties of glycoproteins. A re-evaluation of their function. Biochim Biophys Acta. 1982;650:209–232. doi: 10.1016/0304-4157(82)90017-x. [DOI] [PubMed] [Google Scholar]
- 15.Jones GE, Arumugham RG, Tanzer ML. Fibronectin glycosylation modulates fibroblast adhesion and spreading. J Cell Biol. 1986;103:1663–1670. doi: 10.1083/jcb.103.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotundo RF, Rebres RA, Mckeown-Longo PJ, Blumenstock FA, Saba TM. Circulating cellular fibronectin may be a natural ligand for the hepatic asialoglycoprotein receptor: possible pathway for fibronectin deposition and turnover in the rat liver. Hepatology. 1998;28:475–485. doi: 10.1002/hep.510280227. [DOI] [PubMed] [Google Scholar]
- 17.Rotundo RF, Vincent PA, McKeown-Longo PJ, Blumenstock FA, Saba TM. Hepatic fibronectin matrix turnover in rats: involvement of the asialoglycoprotein receptor. Am J Physiol. 1999;277:G1189–G1199. doi: 10.1152/ajpgi.1999.277.6.G1189. [DOI] [PubMed] [Google Scholar]
- 18.Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971;246:1461–1467. [PubMed] [Google Scholar]
- 19.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 20.Sekiguchi K, Hakomori S. Functional domain structure of fibronectin. Proc Natl Acad Sci USA. 1980;77:2661–2665. doi: 10.1073/pnas.77.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner DD, Hynes RO. Topological arrangement of the major structural features of fibronectin. J Biol Chem. 1980;255:4304–4312. [PubMed] [Google Scholar]
- 22.Hayashi M, Schlesinger DH, Kennedy DW, Yamada KM. Isolation and characterization of a heparin-binding domain of cellular fibronectin. J Biol Chem. 1980;255:10017–10020. [PubMed] [Google Scholar]
- 23.Hörmann H, Seidl M. Affinity chromatography on immobilized fibrin monomer, III. The fibrin affinity center of fibronectin. Hoppe Seylers Z Physiol Chem. 1980;361:1449–1452. [PubMed] [Google Scholar]
- 24.Emmerling MR, Johnson CD, Mosher DF, Lipton BH, Lilien JE. Cross-linking and binding of fibronectin with asymmetric acetylcholinesterase. Biochemistry. 1981;20:3242–3247. doi: 10.1021/bi00514a040. [DOI] [PubMed] [Google Scholar]
- 25.Mosher DF, Proctor RA. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980;209:927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- 26.Homandberg GA, Kramer-Bjerke J. Thrombospondin binds to amino-terminal fragments of plasma fibronectin. Thromb Res. 1987;48:329–335. doi: 10.1016/0049-3848(87)90445-2. [DOI] [PubMed] [Google Scholar]
- 27.Yamada KM. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- 28.Leikina E, Mertts MV, Kuznetsova N, Leikin S. Type I collagen is thermally unstable at body temperature. Proc Natl Acad Sci USA. 2002;99:1314–1318. doi: 10.1073/pnas.032307099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rimoldi MT, Tenner AJ, Bobak DA, Joiner KA. Complement component C1q enhances invasion of human mononuclear phagocytes and fibroblasts by Trypanosoma cruzi trypomastigotes. J Clin Invest. 1989;84:1982–1989. doi: 10.1172/JCI114388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi M, Yamada KM. Domain structure of the carboxyl-terminal half of human plasma fibronectin. J Biol Chem. 1983;258:3332–3340. [PubMed] [Google Scholar]
- 31.Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogenesis. 2009;12:165–175. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: cellular source and biological activity of the EIIIA segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kriegsmann J, Berndt A, Hansen T, Borsi L, Zardi L, Bräuer R, Petrow PK, Otto M, Kirkpatrick CJ, Gay S, et al. Expression of fibronectin splice variants and oncofetal glycosylated fibronectin in the synovial membranes of patients with rheumatoid arthritis and osteoarthritis. Rheumatol Int. 2004;24:25–33. doi: 10.1007/s00296-003-0316-1. [DOI] [PubMed] [Google Scholar]
- 34.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, et al. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vartio T, Laitinen L, Närvänen O, Cutolo M, Thornell LE, Zardi L, Virtanen I. Differential expression of the ED sequence-containing form of cellular fibronectin in embryonic and adult human tissues. J Cell Sci. 1987;88(Pt 4):419–430. doi: 10.1242/jcs.88.4.419. [DOI] [PubMed] [Google Scholar]
- 36.Pagani F, Zagato L, Vergani C, Casari G, Sidoli A, Baralle FE. Tissue-specific splicing pattern of fibronectin messenger RNA precursor during development and aging in rat. J Cell Biol. 1991;113:1223–1229. doi: 10.1083/jcb.113.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caputi M, Melo CA, Baralle FE. Regulation of fibronectin expression in rat regenerating liver. Nucleic Acids Res. 1995;23:238–243. doi: 10.1093/nar/23.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oyama F, Hirohashi S, Sakamoto M, Titani K, Sekiguchi K. Coordinate oncodevelopmental modulation of alternative splicing of fibronectin pre-messenger RNA at ED-A, ED-B, and CS1 regions in human liver tumors. Cancer Res. 1993;53:2005–2011. [PubMed] [Google Scholar]
- 39.Csiszar A, Wiebe C, Larjava H, Häkkinen L. Distinctive molecular composition of human gingival interdental papilla. J Periodontol. 2007;78:304–314. doi: 10.1902/jop.2007.060165. [DOI] [PubMed] [Google Scholar]
- 40.Kilian O, Dahse R, Alt V, Zardi L, Rosenhahn J, Exner U, Battmann A, Schnettler R, Kosmehl H. Expression of EDA and EDB fibronectin splice variants in bone. Bone. 2004;35:1334–1345. doi: 10.1016/j.bone.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Van Vliet A, Baelde HJ, Vleming LJ, de Heer E, Bruijn JA. Distribution of fibronectin isoforms in human renal disease. J Pathol. 2001;193:256–262. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH783>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 42.Oyama F, Hirohashi S, Shimosato Y, Titani K, Sekiguchi K. Oncodevelopmental regulation of the alternative splicing of fibronectin pre-messenger RNA in human lung tissues. Cancer Res. 1990;50:1075–1078. [PubMed] [Google Scholar]
- 43.Han F, Adams CS, Tao Z, Williams CJ, Zaka R, Tuan RS, Norton PA, Hickok NJ. Transforming growth factor-beta1 (TGF-beta1) regulates ATDC5 chondrogenic differentiation and fibronectin isoform expression. J Cell Biochem. 2005;95:750–762. doi: 10.1002/jcb.20427. [DOI] [PubMed] [Google Scholar]
- 44.Fukuda T, Yoshida N, Kataoka Y, Manabe R, Mizuno-Horikawa Y, Sato M, Kuriyama K, Yasui N, Sekiguchi K. Mice lacking the EDB segment of fibronectin develop normally but exhibit reduced cell growth and fibronectin matrix assembly in vitro. Cancer Res. 2002;62:5603–5610. [PubMed] [Google Scholar]
- 45.Chen W, Culp LA. Adhesion mediated by fibronectin’s alternatively spliced EDb (EIIIB) and its neighboring type III repeats. Exp Cell Res. 1996;223:9–19. doi: 10.1006/excr.1996.0053. [DOI] [PubMed] [Google Scholar]
- 46.Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE. Regulated splicing of the fibronectin EDA exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149–160. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004;104:11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- 48.Chauhan AK, Moretti FA, Iaconcig A, Baralle FE, Muro AF. Impaired motor coordination in mice lacking the EDA exon of the fibronectin gene. Behav Brain Res. 2005;161:31–38. doi: 10.1016/j.bbr.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Matuskova J, Chauhan AK, Cambien B, Astrof S, Dole VS, Piffath CL, Hynes RO, Wagner DD. Decreased plasma fibronectin leads to delayed thrombus growth in injured arterioles. Arterioscler Thromb Vasc Biol. 2006;26:1391–1396. doi: 10.1161/01.ATV.0000216282.58291.c6. [DOI] [PubMed] [Google Scholar]
- 50.Main AL, Harvey TS, Baron M, Boyd J, Campbell ID. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell. 1992;71:671–678. doi: 10.1016/0092-8674(92)90600-h. [DOI] [PubMed] [Google Scholar]
- 51.Aota S, Nagai T, Yamada KM. Characterization of regions of fibronectin besides the arginine-glycine-aspartic acid sequence required for adhesive function of the cell-binding domain using site-directed mutagenesis. J Biol Chem. 1991;266:15938–15943. [PubMed] [Google Scholar]
- 52.Bencharit S, Cui CB, Siddiqui A, Howard-Williams EL, Sondek J, Zuobi-Hasona K, Aukhil I. Structural insights into fibronectin type III domain-mediated signaling. J Mol Biol. 2007;367:303–309. doi: 10.1016/j.jmb.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carnemolla B, Leprini A, Allemanni G, Saginati M, Zardi L. The inclusion of the type III repeat ED-B in the fibronectin molecule generates conformational modifications that unmask a cryptic sequence. J Biol Chem. 1992;267:24689–24692. [PubMed] [Google Scholar]
- 54.Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 55.Tamkun JW, DeSimone DW, Fonda D, Patel RS, Buck C, Horwitz AF, Hynes RO. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986;46:271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- 56.Takagi J, Strokovich K, Springer TA, Walz T. Structure of integrin alpha5beta1 in complex with fibronectin. EMBO J. 2003;22:4607–4615. doi: 10.1093/emboj/cdg445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arnaout MA, Mahalingam B, Xiong JP. Integrin structure, allostery, and bidirectional signaling. Annu Rev Cell Dev Biol. 2005;21:381–410. doi: 10.1146/annurev.cellbio.21.090704.151217. [DOI] [PubMed] [Google Scholar]
- 58.Dedhar S, Hannigan GE. Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Curr Opin Cell Biol. 1996;8:657–669. doi: 10.1016/s0955-0674(96)80107-4. [DOI] [PubMed] [Google Scholar]
- 59.Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275:21785–21788. doi: 10.1074/jbc.R000003200. [DOI] [PubMed] [Google Scholar]
- 60.Bowditch RD, Hariharan M, Tominna EF, Smith JW, Yamada KM, Getzoff ED, Ginsberg MH. Identification of a novel integrin binding site in fibronectin. Differential utilization by beta 3 integrins. J Biol Chem. 1994;269:10856–10863. [PubMed] [Google Scholar]
- 61.Curnis F, Longhi R, Crippa L, Cattaneo A, Dondossola E, Bachi A, Corti A. Spontaneous formation of L-isoaspartate and gain of function in fibronectin. J Biol Chem. 2006;281:36466–36476. doi: 10.1074/jbc.M604812200. [DOI] [PubMed] [Google Scholar]
- 62.Shimizu T, Matsuoka Y, Shirasawa T. Biological significance of isoaspartate and its repair system. Biol Pharm Bull. 2005;28:1590–1596. doi: 10.1248/bpb.28.1590. [DOI] [PubMed] [Google Scholar]
- 63.Shimizu T, Fukuda H, Murayama S, Izumiyama N, Shirasawa T. Isoaspartate formation at position 23 of amyloid beta peptide enhanced fibril formation and deposited onto senile plaques and vascular amyloids in Alzheimer’s disease. J Neurosci Res. 2002;70:451–461. doi: 10.1002/jnr.10350. [DOI] [PubMed] [Google Scholar]
- 64.Lanthier J, Bouthillier A, Lapointe M, Demeule M, Béliveau R, Desrosiers RR. Down-regulation of protein L-isoaspartyl methyltransferase in human epileptic hippocampus contributes to generation of damaged tubulin. J Neurochem. 2002;83:581–591. doi: 10.1046/j.1471-4159.2002.01150.x. [DOI] [PubMed] [Google Scholar]
- 65.Santas AJ, Peterson JA, Halbleib JL, Craig SE, Humphries MJ, Peters DM. Alternative splicing of the IIICS domain in fibronectin governs the role of the heparin II domain in fibrillogenesis and cell spreading. J Biol Chem. 2002;277:13650–13658. doi: 10.1074/jbc.M111361200. [DOI] [PubMed] [Google Scholar]
- 66.Humphries MJ, Komoriya A, Akiyama SK, Olden K, Yamada KM. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987;262:6886–6892. [PubMed] [Google Scholar]
- 67.Komoriya A, Green LJ, Mervic M, Yamada SS, Yamada KM, Humphries MJ. The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J Biol Chem. 1991;266:15075–15079. [PubMed] [Google Scholar]
- 68.Mould AP, Humphries MJ. Identification of a novel recognition sequence for the integrin alpha 4 beta 1 in the COOH-terminal heparin-binding domain of fibronectin. EMBO J. 1991;10:4089–4095. doi: 10.1002/j.1460-2075.1991.tb04985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shinde AV, Bystroff C, Wang C, Vogelezang MG, Vincent PA, Hynes RO, Van De Water L. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities. J Biol Chem. 2008;283:2858–2870. doi: 10.1074/jbc.M708306200. [DOI] [PubMed] [Google Scholar]
- 70.Tumova S, Woods A, Couchman JR. Heparan sulfate chains from glypican and syndecans bind the Hep II domain of fibronectin similarly despite minor structural differences. J Biol Chem. 2000;275:9410–9417. doi: 10.1074/jbc.275.13.9410. [DOI] [PubMed] [Google Scholar]
- 71.Midwood KS, Mao Y, Hsia HC, Valenick LV, Schwarzbauer JE. Modulation of cell-fibronectin matrix interactions during tissue repair. J Investig Dermatol Symp Proc. 2006;11:73–78. doi: 10.1038/sj.jidsymp.5650005. [DOI] [PubMed] [Google Scholar]
- 72.Kusano Y, Oguri K, Nagayasu Y, Munesue S, Ishihara M, Saiki I, Yonekura H, Yamamoto H, Okayama M. Participation of syndecan 2 in the induction of stress fiber formation in cooperation with integrin alpha5beta1: structural characteristics of heparan sulfate chains with avidity to COOH-terminal heparin-binding domain of fibronectin. Exp Cell Res. 2000;256:434–444. doi: 10.1006/excr.2000.4802. [DOI] [PubMed] [Google Scholar]
- 73.Echtermeyer F, Streit M, Wilcox-Adelman S, Saoncella S, Denhez F, Detmar M, Goetinck P. Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J Clin Invest. 2001;107:R9–R14. doi: 10.1172/JCI10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Integrins and syndecan-4 make distinct, but critical, contributions to adhesion contact formation. Soft Matter. 2007;3:372–376. doi: 10.1039/b614610d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Humphries MJ, Mostafavi-Pour Z, Morgan MR, Deakin NO, Messent AJ, Bass MD. Integrin-syndecan cooperation governs the assembly of signalling complexes during cell spreading. Novartis Found Symp. 2005;269:178–188; discussion 188-192, 223-230. [PubMed] [Google Scholar]
- 76.Morgan MR, Byron A, Humphries MJ, Bass MD. Giving off mixed signals--distinct functions of alpha5beta1 and alphavbeta3 integrins in regulating cell behaviour. IUBMB Life. 2009;61:731–738. doi: 10.1002/iub.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lishko VK, Yakubenko VP, Ugarova TP. The interplay between integrins alphaMbeta2 and alpha5beta1 during cell migration to fibronectin. Exp Cell Res. 2003;283:116–126. doi: 10.1016/s0014-4827(02)00024-1. [DOI] [PubMed] [Google Scholar]
- 78.Johnson KJ, Sage H, Briscoe G, Erickson HP. The compact conformation of fibronectin is determined by intramolecular ionic interactions. J Biol Chem. 1999;274:15473–15479. doi: 10.1074/jbc.274.22.15473. [DOI] [PubMed] [Google Scholar]
- 79.Ugarova TP, Zamarron C, Veklich Y, Bowditch RD, Ginsberg MH, Weisel JW, Plow EF. Conformational transitions in the cell binding domain of fibronectin. Biochemistry. 1995;34:4457–4466. doi: 10.1021/bi00013a039. [DOI] [PubMed] [Google Scholar]
- 80.Wierzbicka-Patynowski I, Schwarzbauer JE. The ins and outs of fibronectin matrix assembly. J Cell Sci. 2003;116:3269–3276. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- 81.Lemmon CA, Chen CS, Romer LH. Cell traction forces direct fibronectin matrix assembly. Biophys J. 2009;96:729–738. doi: 10.1016/j.bpj.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci USA. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aguirre KM, McCormick RJ, Schwarzbauer JE. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J Biol Chem. 1994;269:27863–27868. [PubMed] [Google Scholar]
- 84.Vakonakis I, Staunton D, Rooney LM, Campbell ID. Interdomain association in fibronectin: insight into cryptic sites and fibrillogenesis. EMBO J. 2007;26:2575–2583. doi: 10.1038/sj.emboj.7601694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Das S, Banerji A, Frei E, Chatterjee A. Rapid expression and activation of MMP-2 and MMP-9 upon exposure of human breast cancer cells (MCF-7) to fibronectin in serum free medium. Life Sci. 2008;82:467–476. doi: 10.1016/j.lfs.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 86.Saad S, Gottlieb DJ, Bradstock KF, Overall CM, Bendall LJ. Cancer cell-associated fibronectin induces release of matrix metalloproteinase-2 from normal fibroblasts. Cancer Res. 2002;62:283–289. [PubMed] [Google Scholar]
- 87.Zack MD, Arner EC, Anglin CP, Alston JT, Malfait AM, Tortorella MD. Identification of fibronectin neoepitopes present in human osteoarthritic cartilage. Arthritis Rheum. 2006;54:2912–2922. doi: 10.1002/art.22045. [DOI] [PubMed] [Google Scholar]
- 88.Liz MA, Sousa MM. Deciphering cryptic proteases. Cell Mol Life Sci. 2005;62:989–1002. doi: 10.1007/s00018-005-4544-2. [DOI] [PubMed] [Google Scholar]
- 89.Ding L, Guo D, Homandberg GA. Fibronectin fragments mediate matrix metalloproteinase upregulation and cartilage damage through proline rich tyrosine kinase 2, c-src, NF-kappaB and protein kinase Cdelta. Osteoarthritis Cartilage. 2009;17:1385–1392. doi: 10.1016/j.joca.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 90.Jee SW, Wang S, Kapila YL. Specific pro-apoptotic fibronectin fragments modulate proteinase expression in periodontal ligament cells. J Periodontol. 2004;75:523–530. doi: 10.1902/jop.2004.75.4.523. [DOI] [PubMed] [Google Scholar]
- 91.Pichika R, Homandberg GA. Fibronectin fragments elevate nitric oxide (NO) and inducible NO synthetase (iNOS) levels in bovine cartilage and iNOS inhibitors block fibronectin fragment mediated damage and promote repair. Inflamm Res. 2004;53:405–412. doi: 10.1007/s00011-004-1279-8. [DOI] [PubMed] [Google Scholar]
- 92.Gondokaryono SP, Ushio H, Niyonsaba F, Hara M, Takenaka H, Jayawardana ST, Ikeda S, Okumura K, Ogawa H. The extra domain A of fibronectin stimulates murine mast cells via toll-like receptor 4. J Leukoc Biol. 2007;82:657–665. doi: 10.1189/jlb.1206730. [DOI] [PubMed] [Google Scholar]
- 93.Alon R, Cahalon L, Hershkoviz R, Elbaz D, Reizis B, Wallach D, Akiyama SK, Yamada KM, Lider O. TNF-alpha binds to the N-terminal domain of fibronectin and augments the beta 1-integrin-mediated adhesion of CD4 T lymphocytes to the glycoprotein. J Immunol. 1994;152:1304–1313. [PubMed] [Google Scholar]
- 94.Wijelath ES, Rahman S, Namekata M, Murray J, Nishimura T, Mostafavi-Pour Z, Patel Y, Suda Y, Humphries MJ, Sobel M. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res. 2006;99:853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vaday GG, Hershkoviz R, Rahat MA, Lahat N, Cahalon L, Lider O. Fibronectin-bound TNF-alpha stimulates monocyte matrix metalloproteinase-9 expression and regulates chemotaxis. J Leukoc Biol. 2000;68:737–747. [PubMed] [Google Scholar]
- 96.Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 98.Schwartz MA. Cell biology. The force is with us. Science. 2009;323:588–589. doi: 10.1126/science.1169414. [DOI] [PubMed] [Google Scholar]
- 99.Li S, Guan JL, Chien S. Biochemistry and biomechanics of cell motility. Annu Rev Biomed Eng. 2005;7:105–150. doi: 10.1146/annurev.bioeng.7.060804.100340. [DOI] [PubMed] [Google Scholar]
- 100.Mott JD, Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr Opin Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reiske HR, Kao SC, Cary LA, Guan JL, Lai JF, Chen HC. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J Biol Chem. 1999;274:12361–12366. doi: 10.1074/jbc.274.18.12361. [DOI] [PubMed] [Google Scholar]
- 102.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 103.Dovas A, Yoneda A, Couchman JR. PKCbeta-dependent activation of RhoA by syndecan-4 during focal adhesion formation. J Cell Sci. 2006;119:2837–2846. doi: 10.1242/jcs.03020. [DOI] [PubMed] [Google Scholar]
- 104.Rodríguez-Juan C, de la Torre P, García-Ruiz I, Díaz-Sanjuán T, Muñoz-Yagüe T, Gómez-Izquierdo E, Solís-Muñoz P, Solís-Herruzo JA. Fibronectin increases survival of rat hepatic stellate cells--a novel profibrogenic mechanism of fibronectin. Cell Physiol Biochem. 2009;24:271–282. doi: 10.1159/000233252. [DOI] [PubMed] [Google Scholar]
- 105.Pulai JI, Chen H, Im HJ, Kumar S, Hanning C, Hegde PS, Loeser RF. NF-kappa B mediates the stimulation of cytokine and chemokine expression by human articular chondrocytes in response to fibronectin fragments. J Immunol. 2005;174:5781–5788. doi: 10.4049/jimmunol.174.9.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moretti FA, Chauhan AK, Iaconcig A, Porro F, Baralle FE, Muro AF. A major fraction of fibronectin present in the extracellular matrix of tissues is plasma-derived. J Biol Chem. 2007;282:28057–28062. doi: 10.1074/jbc.M611315200. [DOI] [PubMed] [Google Scholar]
- 107.Briggs SL. The role of fibronectin in fibroblast migration during tissue repair. J Wound Care. 2005;14:284–287. doi: 10.12968/jowc.2005.14.6.26789. [DOI] [PubMed] [Google Scholar]
- 108.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 109.Jiao J, Friedman SL, Aloman C. Hepatic fibrosis. Curr Opin Gastroenterol. 2009;25:223–229. doi: 10.1097/mog.0b013e3283279668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Friedman SL. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 111.Reynolds BC, Paton JY, Howatson AG, Ramage IJ. Reversible chronic pulmonary fibrosis associated with MMF in a pediatric patient: a case report. Pediatr Transplant. 2008;12:228–231. doi: 10.1111/j.1399-3046.2007.00707.x. [DOI] [PubMed] [Google Scholar]
- 112.Hahn E, Wick G, Pencev D, Timpl R. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin. Gut. 1980;21:63–71. doi: 10.1136/gut.21.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Odenthal M, Neubauer K, Meyer zum Büschenfelde KH, Ramadori G. Localization and mRNA steady-state level of cellular fibronectin in rat liver undergoing a CCl4-induced acute damage or fibrosis. Biochim Biophys Acta. 1993;1181:266–272. doi: 10.1016/0925-4439(93)90031-u. [DOI] [PubMed] [Google Scholar]
- 114.Haglund C, Ylätupa S, Mertaniemi P, Partanen P. Cellular fibronectin concentration in the plasma of patients with malignant and benign diseases: a comparison with CA 19-9 and CEA. Br J Cancer. 1997;76:777–783. doi: 10.1038/bjc.1997.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martinez-Hernandez A. The hepatic extracellular matrix. I. Electron immunohistochemical studies in normal rat liver. Lab Invest. 1984;51:57–74. [PubMed] [Google Scholar]
- 116.Tavian D, De Petro G, Colombi M, Portolani N, Giulini SM, Gardella R, Barlati S. RT-PCR detection of fibronectin EDA and EDB mRNA isoforms: molecular markers for hepatocellular carcinoma. Int J Cancer. 1994;56:820–825. doi: 10.1002/ijc.2910560611. [DOI] [PubMed] [Google Scholar]
- 117.Joy D, Scott BB. To perform or not to perform liver biopsy: an alternative view. Gut. 2003;52:610. doi: 10.1136/gut.52.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sheehan M, Haythorn P. Predictive values of various liver function tests with respect to the diagnosis of liver disease. Clin Biochem. 1979;12:262–263. doi: 10.1016/s0009-9120(79)80122-8. [DOI] [PubMed] [Google Scholar]
- 119.Scanzello CR, Plaas A, Crow MK. Innate immune system activation in osteoarthritis: is osteoarthritis a chronic wound? Curr Opin Rheumatol. 2008;20:565–572. doi: 10.1097/BOR.0b013e32830aba34. [DOI] [PubMed] [Google Scholar]
- 120.Yasuda T. Cartilage destruction by matrix degradation products. Mod Rheumatol. 2006;16:197–205. doi: 10.1007/s10165-006-0490-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Norton PA, Reis HM, Prince S, Larkin J, Pan J, Liu J, Gong Q, Zhu M, Feitelson MA. Activation of fibronectin gene expression by hepatitis B virus x antigen. J Viral Hepat. 2004;11:332–341. doi: 10.1111/j.1365-2893.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 122.Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou X, Zhang Y, Zhang J, Zhu H, Zhou X, Du W, Zhang X, Chen Q. Expression of fibronectin receptor, integrin alpha 5 beta 1 of hepatic stellate cells in rat liver fibrosis. Chin Med J (Engl) 2000;113:272–276. [PubMed] [Google Scholar]
- 124.Duryee MJ, Willis MS, Freeman TL, Kuszynski CA, Tuma DJ, Klassen LW, Thiele GM. Mechanisms of alcohol liver damage: aldehydes, scavenger receptors, and autoimmunity. Front Biosci. 2004;9:3145–3155. doi: 10.2741/1467. [DOI] [PubMed] [Google Scholar]
- 125.Goldin R. The pathogenesis of alcoholic liver disease. Int J Exp Pathol. 1994;75:71–78. [PMC free article] [PubMed] [Google Scholar]
- 126.Lieber CS. Metabolism of alcohol. Clin Liver Dis. 2005;9:1–35. doi: 10.1016/j.cld.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 127.Schaffert CS, Duryee MJ, Hunter CD, Hamilton BC, DeVeney AL, Huerter MM, Klassen LW, Thiele GM. Alcohol metabolites and lipopolysaccharide: roles in the development and/or progression of alcoholic liver disease. World J Gastroenterol. 2009;15:1209–1218. doi: 10.3748/wjg.15.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tuma DJ, Casey CA. Dangerous byproducts of alcohol breakdown--focus on adducts. Alcohol Res Health. 2003;27:285–290. [PMC free article] [PubMed] [Google Scholar]
- 129.You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1–G6. doi: 10.1152/ajpgi.00056.2004. [DOI] [PubMed] [Google Scholar]
- 130.Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- 131.Cederbaum AI. Role of lipid peroxidation and oxidative stress in alcohol toxicity. Free Radic Biol Med. 1989;7:537–539. doi: 10.1016/0891-5849(89)90029-4. [DOI] [PubMed] [Google Scholar]
- 132.Thiele GM, Duryee MJ, Freeman TL, Sorrell MF, Willis MS, Tuma DJ, Klassen LW. Rat sinusoidal liver endothelial cells (SECs) produce pro-fibrotic factors in response to adducts formed from the metabolites of ethanol. Biochem Pharmacol. 2005;70:1593–1600. doi: 10.1016/j.bcp.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 133.Tworek BL, Wiegert RL, Jeanette JP, Tuma DJ, Casey CA. Differential effects of monensin on asialoglycoprotein receptor function after short-term ethanol administration. Biochem Pharmacol. 1998;55:1603–1609. doi: 10.1016/s0006-2952(98)00005-7. [DOI] [PubMed] [Google Scholar]
- 134.Tworek BL, Tuma DJ, Casey CA. Decreased binding of asialoglycoproteins to hepatocytes from ethanol-fed rats. Consequence of both impaired synthesis and inactivation of the asialoglycoprotein receptor. J Biol Chem. 1996;271:2531–2538. doi: 10.1074/jbc.271.5.2531. [DOI] [PubMed] [Google Scholar]
- 135.McCashland TM, Tuma DJ, Sorrell MF, Casey CA. Zonal differences in ethanol-induced impairments in hepatic receptor binding. Alcohol. 1993;10:549–554. doi: 10.1016/0741-8329(93)90081-x. [DOI] [PubMed] [Google Scholar]
- 136.McVicker BL, Casey CA. Effects of ethanol on receptor-mediated endocytosis in the liver. Alcohol. 1999;19:255–260. doi: 10.1016/s0741-8329(99)00043-9. [DOI] [PubMed] [Google Scholar]
- 137.McVicker BL, Tuma DJ, Kubik JA, Hindemith AM, Baldwin CR, Casey CA. The effect of ethanol on asialoglycoprotein receptor-mediated phagocytosis of apoptotic cells by rat hepatocytes. Hepatology. 2002;36:1478–1487. doi: 10.1053/jhep.2002.37137. [DOI] [PubMed] [Google Scholar]
- 138.Aziz-Seible RS, McVicker BL, Kharbanda KK, Casey CA. Cellular fibronectin stimulates hepatocytes to produce factors that promote alcohol-induced liver injury. World J Hepatol. 2011;3:45–55. doi: 10.4254/wjh.v3.i2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gillis SE, Nagy LE. Deposition of cellular fibronectin increases before stellate cell activation in rat liver during ethanol feeding. Alcohol Clin Exp Res. 1997;21:857–861. [PubMed] [Google Scholar]
- 140.de la M Hall P, Lieber CS, DeCarli LM, French SW, Lindros KO, Järveläinen H, Bode C, Parlesak A, Bode JC. Models of alcoholic liver disease in rodents: a critical evaluation. Alcohol Clin Exp Res. 2001;25:254S–261S. doi: 10.1097/00000374-200105051-00041. [DOI] [PubMed] [Google Scholar]
- 141.Sottile J, Shi F, Rublyevska I, Chiang HY, Lust J, Chandler J. Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. Am J Physiol Cell Physiol. 2007;293:C1934–C1946. doi: 10.1152/ajpcell.00130.2007. [DOI] [PubMed] [Google Scholar]
- 142.Aziz-Seible RS, Lee SM, Kharbanda KK, McVicker BL, Casey CA. Ethanol feeding potentiates the pro-inflammatory response of kupffer cells to cellular fibronectin. Alcohol Clin Exp Res. 2011;35:717–725. doi: 10.1111/j.1530-0277.2010.01389.x. [DOI] [PubMed] [Google Scholar]
- 143.Tsukamoto H. Cytokine regulation of hepatic stellate cells in liver fibrosis. Alcohol Clin Exp Res. 1999;23:911–916. [PubMed] [Google Scholar]
- 144.Naito M, Hasegawa G, Ebe Y, Yamamoto T. Differentiation and function of Kupffer cells. Med Electron Microsc. 2004;37:16–28. doi: 10.1007/s00795-003-0228-x. [DOI] [PubMed] [Google Scholar]
- 145.Martin J, Eynstone L, Davies M, Steadman R. Induction of metalloproteinases by glomerular mesangial cells stimulated by proteins of the extracellular matrix. J Am Soc Nephrol. 2001;12:88–96. doi: 10.1681/ASN.V12188. [DOI] [PubMed] [Google Scholar]
- 146.Rath PC, Aggarwal BB. TNF-induced signaling in apoptosis. J Clin Immunol. 1999;19:350–364. doi: 10.1023/a:1020546615229. [DOI] [PubMed] [Google Scholar]
- 147.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 148.Kapila YL, Wang S, Dazin P, Tafolla E, Mass MJ. The heparin-binding domain and V region of fibronectin regulate apoptosis by suppression of p53 and c-myc in human primary cells. J Biol Chem. 2002;277:8482–8491. doi: 10.1074/jbc.M108932200. [DOI] [PubMed] [Google Scholar]
- 149.Marastoni S, Ligresti G, Lorenzon E, Colombatti A, Mongiat M. Extracellular matrix: a matter of life and death. Connect Tissue Res. 2008;49:203–206. doi: 10.1080/03008200802143190. [DOI] [PubMed] [Google Scholar]
- 150.Kapila YL, Kapila S, Johnson PW. Fibronectin and fibronectin fragments modulate the expression of proteinases and proteinase inhibitors in human periodontal ligament cells. Matrix Biol. 1996;15:251–261. doi: 10.1016/s0945-053x(96)90116-x. [DOI] [PubMed] [Google Scholar]
- 151.Wells RG. Cellular sources of extracellular matrix in hepatic fibrosis. Clin Liver Dis. 2008;12:759–768, viii. doi: 10.1016/j.cld.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zeisberg M, Yang C, Martino M, Duncan MB, Rieder F, Tanjore H, Kalluri R. Fibroblasts derive from hepatocytes in liver fibrosis via epithelial to mesenchymal transition. J Biol Chem. 2007;282:23337–23347. doi: 10.1074/jbc.M700194200. [DOI] [PubMed] [Google Scholar]
- 153.Klassen LW, Thiele GM, Duryee MJ, Schaffert CS, DeVeney AL, Hunter CD, Olinga P, Tuma DJ. An in vitro method of alcoholic liver injury using precision-cut liver slices from rats. Biochem Pharmacol. 2008;76:426–436. doi: 10.1016/j.bcp.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Meng Q. Three-dimensional culture of hepatocytes for prediction of drug-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2010;6:733–746. doi: 10.1517/17425251003674356. [DOI] [PubMed] [Google Scholar]