Abstract
Ethnopharmacological relevance
Given the increasing coverage of antiretroviral therapy (ART) for HIV/AIDS treatment as well as the high utilization of herbal medicine, many persons living with HIV/AIDS in sub-Saharan Africa are thought to practice medical pluralism, or the adoption of more than one medical system for their care and treatment. Using a cross-sectional study we sought to document and identify the herbal medicines used by persons living with HIV/AIDS on Mfangano Island, Suba District, Nyanza Province, Kenya.
Materials and Methods
We interviewed herbalists and knowledgeable mothers to obtain information regarding medicinal plants, particularly for HIV/AIDS-related symptoms, HIV/AIDS, and chira (an illness concept with similarities to HIV/AIDS regarding sexual transmission and wasting symptoms). Using systematic sampling, 67 persons living with HIV/AIDS (49 of whom were receiving ART) were selected from an Mfangano Island health clinic and participated in semi-structured interviews.
Results
Interviews with herbalists and mothers identified 40 plant species in 37 genera and 29 families that a person with HIV/AIDS or chira could use for herbal remedies. Overall, 70.1% of persons living with HIV/AIDS had used medicinal plants after HIV diagnosis, most commonly to treat symptoms related to HIV/AIDS. In addition to common vegetables and fruits that can serve medicinal purposes, Azadirachta indica A.Juss. (Meliaceae), Carissa edulis (Forssk.) Vahl (Apocynaceae), and Ximenia americana L. (Olacaceae) were the most frequently cited medicinal plants used by persons living with HIV/AIDS.
Conclusions
Collaboration and communication between biomedical clinicians and herbalists should be encouraged given high rates of concomitant ART-herb usage. Pharmacological, toxicological, and ART-herb interaction studies based on the plants identified in this study and their constituent ingredients should be considered.
Keywords: Medicinal plants, Herbal medicine, Herbalists, Traditional remedies, Ethnobotany, HIV/AIDS, Suba, Luo, Nyanza Province, Kenya, Africa
Introduction
An estimated 22.5 million adults and children live with HIV/AIDS in sub-Saharan Africa (UNAIDS, 2010). Because of the high rates of utilization of traditional healers in sub-Saharan Africa, it is believed that herbal medicines are commonly used for the treatment of HIV and related symptoms (Langois-Klassen et al. 2008; Bodeker 2003; Morris, 2001). Traditional healers and traditional birth attendants provide the first line of care for 70% of the population in Africa (WHO, 2002a). As biomedical systems that often provide antiretroviral therapy (ART) co-exist with traditional medical systems that often provide herbal remedies, a high degree of medical pluralism has been reported among persons living with HIV/AIDS in sub-Saharan Africa (King and Homsy 1997; Langlois-Klassen et al., 2007; Moshabela et al., 2010).
The World Health Organization (WHO) defines herbal medicine as “herbs, herbal materials, herbal preparations, and finished herbal products that contain as active ingredients parts of plants, or plant materials, or combinations thereof” (WHO, 2002b). Although WHO (2002b) has advocated for the integration of traditional medicine into national health systems, there is increasing concern among biomedical health practitioners regarding the potential pharmacokinetic and pharmacodynamic interactions between antiretroviral drugs and medicinal plants (Langois-Klassen et al. 2008).
In North America, for instance, St. John’s wort (Hypericum perforatum) and garlic (Allium sativum) supplements have been shown to reduce the plasma concentrations of the antiretrovirals indinavir and saquinavir, respectively (Piscitelli et al. 2000; Piscitelli et al. 2002). In Africa, some research has raised concern that the African potato (Hypoxis hemerocallidea) or Sutherlandia may inhibit ART drug metabolism and transport (Mills et al., 2005). Despite the lack of rigorous scientific studies specifically examining interactions between other medicinal plants commonly used in sub-Saharan Africa and ART (Homsy 1999), many biomedical centers in sub-Saharan Africa have developed policies that prohibit the use of medicinal plants by individuals receiving ART due to the potential for detrimental ART-herb interactions (Langlois-Klassen et al., 2008). These policies become more salient as ART coverage rates are rising throughout sub-Saharan Africa (UNAIDS, 2010).
Poor communication among health care providers and HIV-infected adults related to herbal medicine has been documented in sub-Saharan Africa (Langlois-Klassen et al., 2008). In one study in western Uganda, 63.5% of respondents reported using herbal medicine after being diagnosed with HIV, but only 16% had informed their biomedical medical practitioners about using these herbs and only 13% of the practitioners had inquired about concomitant ART-herb use (Langlois-Klassen et al., 2007; Langlois-Klassen et al., 2008).
In Kenya, herbal medicines are most commonly consumed in the form of infusions or decoctions prepared from dried plant material (Orwa, 2002). Geissler et al. (2002), Maundu et al., (1999), and Johns and Kokwaro (1990) have explored medicinal plant usage by the Luo of Kenya. Johns and Kokwaro (1990) identified cultivated crops as well as wild fruits and vegetables important for nutritional content, particularly during times of food shortage. Many plants commonly consumed as foods have also been identified to have medicinal value for certain illnesses (Johns and Kokwaro, 1990; Geissler et al., 2002). Luo plant medicines are mainly drunk and most commonly used to treat stomach problems, skin conditions, and coughs or respiratory infections (Geissler et al., 2002).
Chira (Luo) or enkiera (Suba) is a distinct illness concept with a long history in East Africa, yet it shares many similarities with HIV/AIDS regarding sexual transmission and wasting symptoms. Chira, an illness recognized and documented long before the emergence of pandemic HIV/AIDS in Africa, is characterized by extreme thinness, wasting, weakness, and diarrhea, particularly with blood (Parkin, 1978, Whyte and Kariuki, 1991). Chira and AIDS have become inextricably linked in conversations regarding unexplained illnesses, particularly given the high prevalence of HIV/AIDS in the region (Geissler et al., 2002). The causes of chira are all related to the transgression of principles governing sexuality or seniority. For instance, adultery committed during a wife’s pregnancy, contact with an uninherited widow, disregard of seniority rules, or failure to observe the proper separation of sexuality between generations can all bring chira into a family (Parkin, 1978; Whyte and Kariuki, 1991). Although previous studies have explored medicinal plants utilized by the general Luo population, to our knowledge, this is the first study to examine medicinal plant usage among Luo and Suba persons living with HIV/AIDS in Kenya.
The purpose of this study was to assess the use of medicinal plants by persons living with HIV/AIDS, particularly those receiving ART, in Suba District. The specific aims of this investigation included 1) identifying the species, parts, and instructions for use of the medicinal plants most commonly utilized among persons living with HIV/AIDS; 2) understanding herbalists’ perspectives of HIV/AIDS, chira, and related illnesses; and 3) determining the frequency of medicinal plant usage among persons living with HIV/AIDS.
1. Materials and Methods
1.1. Study Community
This study was conducted on Mfangano Island, Suba District, Nyanza Province in Kenya. Mfangano Island is located between longitudes 33’55” E and 34’6” E and latitudes 0’25” S and 0’30” S. Suba District is one of twelve districts in Nyanza Province, bordering Bondo to the north, Homa Bay to the east, Migori to the south, and Lake Victoria to the west. The Lake Victoria basin receives approximately 750–1000 mm of rainfall every year. Two rainy seasons exist; the ‘long rains’ peak in April and the ‘short rains’ peak in October (Survey of Kenya, 1970).
Mfangano Island is one of 16 islands in Suba District located in Lake Victoria. The island’s population is approximately 19,000, and consists of mainly Suba and Luo populations, although Luo is most commonly spoken. In Suba District, 32.9% of the residents are infected with HIV while 80% of hospital beds are occupied with AIDS-related illness (NASCOP, 2005). Food insecurity is a major problem in Suba District, where subsistence farming and fishing are the major occupations for most residents. Wild plants and fruits are important in Luo diets and may be particularly important during periods of food shortage (Johns and Kokwaro, 1991).
The Suba and Luo of Kenya are patrilineal and virilocal, so descent is traced through the male line and women live on the husband’s land (Prince et al., 2001). They practice clan exogamy, meaning that women are born and raised at a distance from where they are married and eventually have children. Residents of Suba District commonly utilize multiple sources for healing and curing (Cohen and Odhiambo, 1989). For instance, uses of medicinal plants are commonly known by Luo mothers and children, as well as recognized healers (Geissler et al., 2002). In particular, 13 year-old children already knew herbs used for the treatment of common illnesses (Prince et al., 2001) and used these herbal medicines without adult consultation (Geissler et al., 2000).
1.2. Study Design
A cross-sectional, mixed method study design was used in this investigation, which included qualitative and quantitative components. Ethnographic qualitative data included participant observation and in-depth open-ended interviews while quantitative data included semi-structured questionnaires conducted in March to April 2009. Ethical approval for this research was provided by the Central University Research Ethics Committee at the University of Oxford and the Suba District Medical Officer of Health.
1.2.1. Qualitative: Medicinal Plants
To explore plant remedies on Mfangano Island, we selected a purposive sample of five herbalists, three female and two male, as well as five mothers with knowledge of herbal medicine, to identify plants that may be used by persons living with HIV/AIDS, for related symptoms, or chira (Geissler et al., 2002). With each of these herbalists, in-depth individual interviews were conducted to elicit information about medicinal plants including the name (in Luo and Suba), part needed, and use. The herbalists went to the locations where they normally gathered their medicines with JN and AJ. Subsequently, all plant specimens identified were collected with the herbalists using guidelines from WHO (2003) and the East African Herbarium in Nairobi. In addition, data on locality, altitude, habitat, description (height, size, branching), frequency, and date collected was recorded to aid in species identification (Agnew and Shirley, 1994; Beentje, 1994; Bridson and Forman, 1992). The plants were gathered as whole specimens or pressed; JK performed the formal identification. Plant identification, medicinal preparation, and usage were cross verified with other reference sources (Geissler et al., 2002; Hirt et al., 2002; Maundu et al., 1999; Johns and Kokwaro, 1991). Voucher specimens were deposited in the East African Herbarium at the National Museums of Kenya.
Content analysis was used to identify major themes about chira, HIV/AIDS, and their treatment (Denzin and Lincoln, 2000). Interviews were coded using a master set of codes determined inductively. Coding and analytic memos were utilized to discern major themes. The themes that emerged were then analyzed for commonalities, variations, and disagreements.
1.2.2. Quantitative: Semi-Structured Questionnaire
1.2.2.1. Participants
Inclusion criteria included being older than 18 years and being HIV-infected. Eligible respondents also had to be a patient at the Family AIDS Care and Education Services (FACES)-supported Kenyan Ministry of Health Sena Health Center on Mfangano Island. FACES is a collaboration between the Kenya Medical Research Institute and the University of California, San Francisco. FACES is the only program that currently provides ART for persons living with HIV/AIDS on Mfangano Island.
Health staff at the Sena clinic conducted systematic sampling by asking every second male and every second female adult patient meeting the inclusion criteria to participate in a research study about HIV/AIDS. Seventy individuals were approached and sixty-seven (96%) consented to the study, 49 receiving ART (TX-ART) and 18 on supportive HIV/AIDS treatment but not receiving ART (TX-SUP). Informed consent was obtained verbally.
1.2.2.2. Data Collection
Interviews were completed with a semi-structured questionnaire consisting of a combination of open and closed-ended questions. Pretesting of the semi-structured interview had been conducted with seven key informants not involved in the study. At the end of each interview respondents were read the list of medicinal plants identified by herbalists and asked if they had consumed the plants or used them in other forms for medicinal purposes while taking ART. The semi-structured interview consisted of 22 questions and lasted an average of 30 minutes. Interviews were conducted between 08:00 and 13:00 Mondays through Thursdays (clinic hours), excluding holidays. All interviews were conducted in private offices on the premises of the Sena clinic, as opposed to in households, so as to minimize stigma associated with identifying an individual as HIV positive. Interviews were conducted in the respondent’s preferred language of either Luo or English; Luo responses were translated into English for analysis purposes. The first author conducted all interviews and a trained research associate, fluent in both English and Luo, assisted in translation of the interviews as necessary.
1.2.2.3. Data Analysis
Descriptive analysis of baseline characteristics of respondents was completed. Tests of univariate association were compiled with the Pearson’s Chi square test for categorical variables. Frequencies of medicinal plants used after HIV diagnosis were calculated. Statistical analyses were conducted with SPSS 12.0 for Windows (SPSS Inc., Chicago, IL) with the level of significance set at p < 0.05.
2. Results
2.1. Medicinal Plants
In total, the five herbalists and five mothers identified 40 plant species in 37 genera and 29 families that they had used to treat chira, HIV/AIDS, or symptoms a person with HIV/AIDS might experience, either directly due to the disease or as a side effect of ART (Table 1).
Table 1.
Medicinal plants used by persons living with HIV/AIDS in Suba District, Kenya
|
Voucher specimen no. |
Luo | Suba | Species | Family | Part useda | Preparation | Use | No (%)b |
Referencesc |
|---|---|---|---|---|---|---|---|---|---|
| JN 7a | Achak | Nyamawere | Launaea cornuta (Hochst. ex Oliv. & Hiern) C.Jeffre | Compositae | LV | Pounded, mixed with water, drunk | Chira | 7.5 | M, G |
| JN 7b | Achak | Nyamawere | Euphorbia heterophyla L. | Euphorbiaceae | LV | Pounded, mixed with water, drunk | Chira | 7.5 | M, G |
| JN 10 | Akech | Ekilulu | Tithonia diversifolia (Hemsl.) A.Gray | Compositae | LV, RT | Decoction drunk, induces vomitting | Stomach ache, headache, bewitching | 13.4 | G |
| JN 31 | Apoth | Itere | Corchorus olitorius L. | Tiliaceae | LV | Pounded, rubbed on tongue and gums, cooked as a vegetable | Wounds, infant’s mouth, growth | 47.8 | M, G, J |
| JN 36 | Arupiny | Commiphora africana (A.Rich.) Engl. | Burseraceae | LV, BK | Pounded, mixed with water, decoction, steambath | Boils, skin rashes | 20.9 | M | |
| JN 23 | Bombwe | Iwombwe | Cyphostemma nr. adenocaule | Vitaceae | RT | Decoction drunk, pounded, rubbed into abdomen | Stomach ache, swollen abdomen | 3.0 | G |
| JN 20 | Chamama | Thevetia peruviana (Pers.) K. Schum. | Apocynaceae | LV | Sap applied to skin | Skin rash, cuts, burns | N/Ae | G | |
| JN 11 | Dek | Isaga | Gynandropsis gynandra (L.) Briq. | Capparaceae | LV | Decoction drunk | Stomach ache | 77.6 | M |
| JN 28 | Dwele | Owu dwele | Melia azedarach L. | Meliaceae | LV | Decoction drunk | Measles, bathing | 13.4 | G |
| JN 8 | Kiandu | Ekiandu | Bersama abyssinica Fresen. | Melianthaceae | BR, RT | Decoction, unprepared, snuffed | Birthing, bleeding | 14.9 | |
| JN 38 | Kitungu | Ekitunguo | Allium sp. [Allium porrum L.] (J)d | Alliaceae | LV | Chewed unprepared, Pounded | Cold, flu, cleaning mouth | 88.1 | J |
| Kitungu saumu | Ekitungu saumu | Allium sativum (H)d | Alliaceae | RT | Crushed, chewed unprepared | Malaria, HIV/AIDS | 23.9 | H | |
| JN 37 | Kuogo | Omusalu | Lannea schweinfurthii | Anacardiaceae | LV, RT | Decoction drunk | Amebas, stomach ache, boils, skin rashes | 10.4 | M, G |
| JN 5 | Machunga | Ichunga | Citrus aurantium L. | Rutaceae | LV, FR | Decoction drunk, chewed unprepared | Replacement milk for nursing mothers, cold, flu | 80.6 | J |
| JN 14 | Majand lum | Owusuwi owa majani | Cymbopogon citratus (stapf) (M)d | Poaceae | LV | Decoction drunk, tea | Headache, purify blood, clean pores | 53.7 | M |
| JN 4 | Mapera | Ipera | Psidium guajava L. | Myrtaceae | LV, FR | Decoction drunk | Malaria, diarrhea | 47.8 | M, G, J |
| JN 30 | Mwarubaine | Arubaine | Azadirachta indica A.Juss. | Meliaceae | LV, BK | Decoction drunk, steambath, washing, rubbed on teeth or gums | Malaria, skin rashes, HIV/AIDS, chira, skin rahes, 40 different illnesses | 40.3 | G, H |
| JN 2 | Naas | Enasi | Cocos nucifera L. | Arecaceae | FR | Applied to skin | Skin rash | 41.8 | M, G |
| JN 6 | Ndim | Endim | Citrus limon (L.) Burm.f. | Rutaceae | LV, FR | Chewed unprepared, decoction drunk | malaria, stomach ache, flu, cold | 67.2 | M, G, J |
| JN 34 | Ngowo, Ng’o | Omukuyu | Ficus sycomorus L. | Moraceae | BK, RT, FR | Decoction drunk | Stomach ache | 37.3 | M, G |
| JN 26 | Oboke | Itu | Ipomoea kituensis Vatke | Convolvulaceae | RT | Decoction drunk | Stomache ache | 1.5 | G |
| JN 16 | Ochol | Omucholi | Euclea divinorum Hiern | Ebenaceae | RT | Decoction | Stomach ache, amebas, boils, skin rashes, constipation | 17.9 | M, J |
| JN 19 | Ochuoga | Omunyore | Carissa edulis (Forssk.) Vahl | Apocynaceae | RT | Decoction drunk | Amebas, gonorrhea, sexually transmitted infections | 29.9 | M, G, J |
| JN 9 | Ogaka | Ikaka | Aloe sp. | Aloaceae | LV | Decoction drunk, applied to affected area | Swelling, boils, burns | 16.4 | M, G |
| JN 29 | Oinglatiang | Inglaatiang | Senna occidentalis (L.) Link | Leguminosae | LV, RT | Decoction drunk, decoction drunk | Stomach ache, diarrhea | 22.4 | G |
| JN 25 | Olemo | Isafu | Ximenia americana L. | Olacaceae | RT, LV, FR | Decoction drunk, steam bath, chewed unprepared | Boils, skin rashes | 26.9 | M, G, J |
| JN 21 | Ombulu | Esiti | Abrus precatorius L. | Leguminosae | LV, RT | Chewed unpreparaed, | Cough, cold, stomach | 14.9 | G |
Medicinal plant remedies most commonly involved leaves (65.0%), roots (45.0%), fruit (22.5%), and bark (12.5%). The bulb, cloves, oil, sap, or cob were used less frequently. The medicines were prepared most frequently by boiling a decoction (72.5%), chewing unprepared (20.0%), pounding or crushing with water (17.5%), or pounding or crushing without water (7.5%).
The most common usages of the remedies were for stomach aches (37.5%), diarrhea (15.0%), malaria (12.5%), chira (12.5%), colds (7.5%), or HIV/AIDS (5.0%).
2.1.1. Causes of Chira
Herbalists identified three themes regarding causes of chira: transgressions of sexual norms causing adult chira (n=5); spousal arguments that cause adult chira (n=3); and causes of chira in children (n=2). The most common theme identified for the cause of chira included the transgressions of sexual norms, involving a husband and a first or second wife. One 80-year old female herbalist said, “You can get chira if you have sex during the harvest, or if you have sex in your brother’s or mother’s bed. Chira can make someone very thin.”
One 68-year old female herbalist said, “Chira comes when you have two wives. When you have two wives it is very important to identify or be strict with the first wife. When you are interested in pursuing a second wife then chira must also just attack you. You must respect your first wife. It is as if your first wife is the key to everything in your family, in your home. When you want to do anything your first wife must know and allow it. If that one is good then you can proceed in doing it. Because if she refuses then you cannot go with your second wife. You’ll have chira if you pursue that.”
Spousal arguments not necessarily involving sexual relations was another cause of chira identified by herbalists.
One 32-year old male herbalist said, “Maybe if you have a fight with your wife and your wife takes a pan and hits you with it then you can also get chira.”
Herbalists also identified ways that children might acquire chira.
One 68-year old female herbalist said, “If you have a dream about having sex with your wife but you don’t have sex, then that is not good. Your baby will get chira, not you or the mother.”
Herbalists noted that although there were multiple causes of adult- and child-onset chira, the symptoms were identical and the illnesses were considered the same.
2.1.2. HIV and Chira
When asked about the relation between HIV/AIDS and chira, a variety of diverse themes emerged. All herbalists reported that they could treat chira, but only one reported that she could treat HIV/AIDS. In general, HIV/AIDS was diagnosed at the health clinic whereas chira could be identified by an herbalist. Herbalists reported that if they saw an HIV positive patient, it was mainly for related gastrointestinal symptoms (n=3). Furthermore, a patient with both HIV and chira was believed to be complicated and carried a poorer prognosis (n=2).
One 68-year old female herbalist said, “Maybe somebody is HIV positive and had diarrhea or vomiting, or is just coming with pain. Maybe the diarrhea and vomiting is too much for someone. Then they come and say they have a problem with diarrhea or vomiting, and ask me to give them an herb to get the diarrhea or vomiting to stop.”
One 62-year old female herbalist said, “I do not know what HIV is or how it works. People just tell me their symptoms, like diarrhea, amebas, or stomach problems and I give them herbs that can help them.”
Herbalists reported difficulty with treating patients with both chira and HIV, co-morbid illnesses that carried poor prognoses.
One 68-year old female herbalist said, “When chira and HIV attacks somebody there is something similar. I ask somebody before treating them if they have gotten a test for HIV. This helps me to understand the disease and treat it. I do not treat any patients for HIV/AIDS, only for chira. But the ones who have both chira and HIV/AIDS, maybe you can treat them, but it will not go well for long because they have both HIV and chira. Being that chira confuses with HIV, what I know is that maybe somebody can come that is having chira and yet is [HIV] positive. Then when she tries to treat chira she will not heal and now she can maybe decide to go for a [HIV] test.”
In contrast to the perspectives of the herbalists, who tended to be older, one 34-year old mother said, “Chira does not exist any more; people know that HIV/AIDS is real and most people are using fewer and fewer herbs.”
2.1.3. Chira Treatment
Discussion about the treatment of chira included three themes: a constant number of patients who sought treatment for chira (n=4), treatment involving drinking a decoction (n=4); treatment involving bathing (n=4); and the secrecy of the ingredients in manyasi, a combination of many plants specifically for the treatment of chira (n=3).
Although HIV/AIDS is increasingly recognized on Mfangano Island, herbalists reported a constant number of patients seeking treatment for chira.
One 68-year old female herbalist said, “I see eight to ten patients for chira every month. I sometimes treat people who have been going to the AIDS clinic.”
Treatment of chira most often involved a drunk decoction, a bathing in water, or both.
One 32-year old male herbalist said, “When a patient comes, the first thing is there is an herb for drinking, othoo. After drinking, then I give them herbs for washing the whole body, ontange tange, and owino. These are the most important ones, but there are many more.”
One 68-year old herbalist said, “The herbs for drinking are only two. And the ones for washing are very many. But nowadays, you can crush the herbs very small and mix them together with Vaseline and apply them as an oil on your body.”
One 27-year old mother said, “Mwarubaine can be used to treat chira. Arubaine means 40 and the tree is named mwarubaine because it can treat 40 diseases. You boil the leaves, then drink the water.”
Unlike most of the other herbal medicines, the ingredients of manyasi were often secret and not generally disclosed to the patient. For instance, the eldest herbalist, an 80-year old female, prepared manyasi and all of the ingredients she described were only in the Suba language, including ekisakra molo, ekigubanyosi, and embatama. These herbs were not mentioned by other herbalists for manyasi.
One 68-year old female herbalist said, “Without doing manyasi you cannot treat chira. You must have so many herbs collected together and then you give the mixture. You are mixing them and there are very many. It is a collective of herbs together. There are some that are not so important. But if you have them and they are close by then you can boil them and mix them. When I give them those medicines they get better from chira.”
One herbalist reported that mwarubaine combined with a manyasi-like mixture could be used to improve the suffering of persons with HIV/AIDS, although the mixture was not necessarily curative.
2.2. Demographics and frequencies of medicinal plant use by persons living with HIV/AIDS
Among the sample of persons living with HIV/AIDS, descriptive variables were stratified and analyzed by treatment subgroup. Within the total 67 person study sample, 49 (73%) patients were receiving ART and 18 (27%) respondents were receiving only supportive treatment (Table 2). Nearly three quarters of the sample (73%) were female, representative of the female majority in the Sena clinic’s patient population. In terms of marital status, 6% were single, 78% were married, and 16% were widowed. In terms of occupation, 9% were unemployed, 25% were farmers, 22% were fishers, 21% were businesspersons, and 22% had other occupations. The mean age, number of children, and years of schooling were 34.7, 2.9, and 6.9 in the overall sample, respectively. For further demographic details, see Table 2.
Table 2.
Respondent characteristics and descriptive statistics
| Treatment subgroup |
|||||||
|---|---|---|---|---|---|---|---|
| Total sample | TX-ART | TX-SUP | P value | ||||
| Variable | n = 67 | % | n = 49 | % | n = 18 | % | |
| Gender | 0.049 | ||||||
| Female | 49 | 73.1% | 39 | 80.0% | 10 | 55.6% | |
| Male | 18 | 26.8% | 10 | 20.0% | 8 | 44.4% | |
| Age | 0.195 | ||||||
| 18 – 29 | 24 | 35.8% | 15 | 30.6% | 9 | 50.0% | |
| 30 – 39 | 26 | 38.8% | 19 | 38.8% | 7 | 38.9% | |
| 40 – 49 | 9 | 13.4% | 9 | 18.4% | 0 | 0.0% | |
| 50 – 60 | 8 | 11.9% | 6 | 12.2% | 2 | 11.1% | |
| Marital status | 0.126 | ||||||
| Single | 4 | 6.0% | 4 | 8.2% | 0 | 0.0% | |
| Married | 52 | 77.6% | 35 | 71.4% | 17 | 94.4% | |
| Widowed | 11 | 16.4% | 10 | 20.4% | 1 | 5.6% | |
| Number of Children | 0.517 | ||||||
| 0 | 7 | 10.4% | 5 | 10.2% | 2 | 11.1% | |
| 1 – 3 | 42 | 62.7% | 29 | 59.2% | 13 | 72.2% | |
| 4 + | 18 | 26.9% | 15 | 30.6% | 3 | 16.7% | |
| Highest education | 0.418 | ||||||
| None | 10 | 14.9% | 6 | 12.2% | 4 | 22.2% | |
| Primary | 38 | 56.7% | 30 | 61.2% | 8 | 44.4% | |
| Secondary and post-secondary | 19 | 28.4% | 13 | 26.5% | 6 | 33.3% | |
| Occupation | 0.579 | ||||||
| Unemployed | 6 | 9.0% | 5 | 10.2% | 1 | 5.6% | |
| Farming | 17 | 25.4% | 14 | 28.6% | 3 | 16.7% | |
| Fishing | 15 | 22.4% | 11 | 22.4% | 4 | 22.2% | |
| Business | 14 | 20.9% | 8 | 16.3% | 6 | 33.3% | |
| Other | 15 | 22.4% | 11 | 22.4% | 4 | 22.2% | |
| Use herbal medicine | 0.045 | ||||||
| Yes | 47 | 70.1% | 31 | 63.3% | 16 | 88.9% | |
| No | 20 | 31.3% | 18 | 36.7% | 2 | 11.1% | |
Persons living with HIV/AIDS receiving only supportive treatment had higher rates of medicinal plant usage and were less often female. Overall, 70% of respondents used herbal medicine, 63% of persons receiving ART used herbal medicine in comparison to 89% of individuals on only supportive treatment used herbal medicine (p = 0.045).
Frequencies of medicinal plants consumed or used for medicinal purposes after HIV/AIDS diagnosis were calculated (Table 1). Persons living with HIV/AIDS most frequently cited consuming or using medicinal plants that were also common vegetables or fruits. The most commonly used vegetables with medicinal value included Allium sp. (n=59) and Gynandropsis gynandra (L.) Briq. (n=52), while the most commonly used fruits with medicinal value included Citrus aurantium (n=54), Carica papaya L. (n=48), and Citrus limon (L.) Burm.f. (n=45). Leaves of Allium sp. are chewed unprepared or pounded for use in colds, the flu, or cleaning the mouth. Leaves of Gynandropsis gynandra (L.) Briq. are used in a decoction and then drunk for stomach aches. Leaves of Citrus aurantium are used in a decoction drunk as replacement milk for nursing mothers, or fruits are chewed unprepared for colds and the flu. The leaves, root, and sap of Carica papaya L. are used in a decoction drunk often with other plants for use in malaria, gonorrhea, or children’s cough. Leaves of Citrus limon (L.) Burm.f. are used in a decoction and then drunk for malaria or stomach aches, while fruits are chewed unprepared for colds and the flu.
The most commonly used plant for purely medicinal purposes was Azadirachta indica A.Juss. (n=27), which corresponded to 40.3% of the sample. Nearly a quarter of the sample reported using Allium sativum (n=16), the only plant in the list that has documented detrimental interactions with some ART regimens. Azadirachta indica A.Juss. is believed to be able to heal 40 different illnesses, and therefore has several different uses. Leaves can be used in a decoction drunk for malaria, or used in a steambath for washing on skin for chira or HIV/AIDS and related skin complications. In addition, the bark can be rubbed on teeth or gums for mouth sores and general oral health. Allium sativum roots are crushed or chewed unprepared for use in malaria or HIV/AIDS.
3. Discussion
Herbalists and mothers reported 40 medicinal plant remedies that might be used for HIV/AIDS, chira, or related symptoms. Treatments for chira, such as the herbs that comprise manyasi, were associated with secrecy and only known by herbalists, rather than mothers or patients. In contrast, medicinal plants used for gastrointestinal symptoms and other common illnesses were frequently known by mothers and patients, confirming the finding that the core of Luo plant medicine is communally shared knowledge (Geissler et al., 2002). Medicinal plants were used most commonly for stomach aches and diarrhea, and not for fevers and headache, similar to findings among Luo mothers and school children (Geissler et al., 2000; Geissler et al., 2002).
Over 70% of persons living with HIV/AIDS in the sample reported using herbal medicines. This study finds the frequency of herbal medicines among persons living with HIV/AIDS to be higher than previously cited estimates in sub-Saharan Africa (WHO, 2002b; Langlois-Klassen et al., 2007).
Persons living with HIV/AIDS may rely on plant medicine because of limited access to or affordability of biomedical care and the availability of traditional medicine (WHO, 2002a). Although a local clinic provides basic health care, including free HIV care and treatment, during the day, residents of Mfangano Island must travel several hours across Lake Victoria by boat to reach the nearest hospital for emergencies or more serious illnesses. Luo and Suba plant medicine is generally communally shared knowledge, so access to medicinal plants may be easier for many residents than the nearest hospital. Persons living with HIV/AIDS may also continue to use medicinal plants due to biomedicine’s inability to provide a definitive cure for HIV infection (Manfredi and Chiodo, 2000).
Many medicinal plants in Suba District also are also commonly consumed wild or cultivated fruits or vegetables. The frequency of usage of some of the individual plants was therefore higher than the overall rate of herbal medicine usage, as some people consumed the fruits or vegetables for non-medicinal purposes. However, we inquired about consumption as well as medicinal plant usage in the frequency charts as any potential interactions with ART might remain whether consumed as medicine or food.
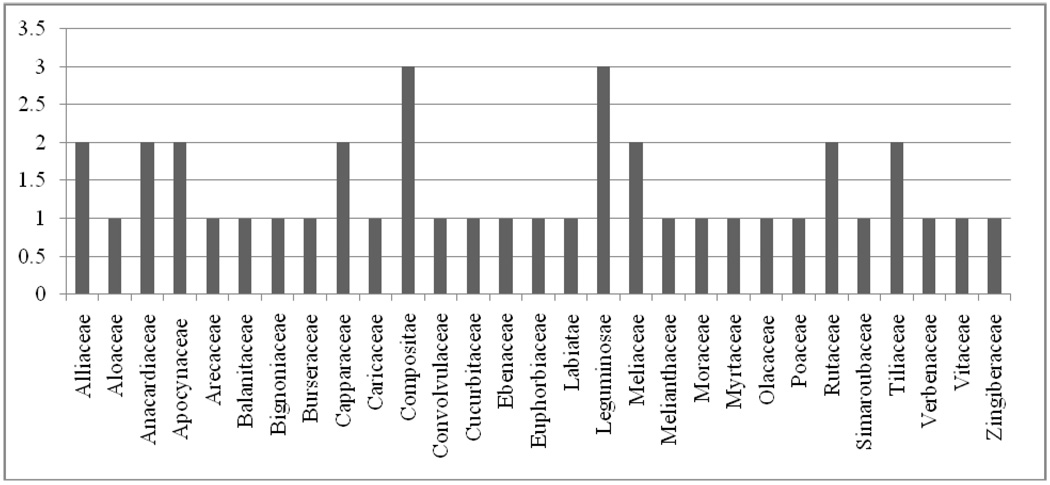
Forty plant species in 37 genera and 29 families were identified for treatment of chira, HIV/AIDS, or related symptoms. The Compositae and Liguminosae families contained the most plant species identified in this study for use by persons living with HIV/AIDS (Figure 1).
Figure 1.
Frequency of plant species used by persons living with HIV/AIDS in the 29 families used in Suba District, Kenya
Plant families
Mwarubaine (Azadirachta indica A.Juss.) was the most frequently cited plant for purely medicinal purposes, used by over 40% of patients in the sample. Many people in Suba District believe it can cure 40 illnesses, including chira, malaria, digestive symptoms, and even HIV/AIDS. As mwarubaine is commonly used throughout Kenya as a medicinal plant, we recommend that future research should examine the potential therapeutic value and herb-antiretroviral drug interactions of Azadirachta indica A.Juss. One study demonstrated that morphine, a plant-based medicine, altered endothelial cell responses to HIV and may prevent viral replication of HIV (Cadet et al., 2001).
Garlic (Allium sativum) is used by nearly a quarter of persons living with HIV/AIDS on Mfangano Island. Because garlic supplements have been shown to lower serum plasma concentration levels of the protease inhibitor saquinavir (Piscitelli et al. 2002), it is possible that there may be interactions with other commonly used protease inhibitors, particularly those metabolized by CYP450. Lopinavir/ritonavir, both protease inhibitors metabolized by CYP450, are part of second line therapy in Kenya and many other resource-limited countries (Kityo et al., 2010).
Patients receiving AIDS supportive care have a higher rate of medicinal plant usage than those on ART. Herbal medicine usage among ART patients was 23% lower than those on supportive therapy alone, a statistically significant difference. Improved access to ART, as well as the frequent recommendations by clinicians that ART and herbal medications should not be mixed, may lead to decreased usage of herbal medicines.
This study had several limitations. First, the small sample size precluded any definitive quantitative conclusions. The cross-sectional study design did not allow for the conclusion of a cause and effect relationship. In addition, the study findings may not be generalizable to persons living with HIV/AIDS outside of rural Kenya, to persons under 18 years of age, and to persons who did not access HIV/AIDS treatment services. Finally, it is possible that patients may have under-reported their use of medicinal plants because they believed them to be forbidden by the clinic (although confidentiality of individual responses was explained and ensured during the consenting process), or over-reported their use of medicinal plants since HIV diagnosis by not distinguishing which plants were used before versus after HIV diagnosis (although the interview questions specified only plant usage after HIV diagnosis).
This investigation’s findings mirror studies in North America and Europe, where herbal medicine is used in addition to ART (Bica et al., 2003; Manfredi and Chiodo, 2000), although at a lower frequency than found in this study. The herbalists and mothers in the study report that herbal medicines are often used to counteract some of the negative side effects of ART, including nausea vomiting, and diarrhea, confirming other studies’ findings (Chang et al., 2003; Langlois-Klassen et al., 2007). Another reason for concurrent herb-ART use includes patients’ increasing awareness of the inability of biomedicine to cure AIDS (Sparber et al, 2000, Langlois-Klassen et al., 2007).
Given the high concomitant use of ART and traditional herbal medicine, biomedical health professionals and herbalists should improve communication and collaboration whenever possible rather than compete for patients. This is reflected by the WHO (2002) recommendation to for better integration of traditional medicine into national health systems. In the absence of clear information regarding several specific drug-herb interactions, collaboration will be necessary to identify any problems should they arise. Communication to avoid the few known detrimental plant-ART interactions is also important.
4. Conclusion
Medicinal plants are frequently used by persons living with HIV/AIDS on ART and supportive care in rural Kenya. Because of the lack of rigorous scientific data examining the interactions between ART and medicinal plants, particularly those used in sub-Saharan Africa, it is difficult for clinicians to make any recommendations regarding the concurrent use of ART and medicinal plants. Nonetheless, biomedical health professionals should inquire about herbal medicine usage as part of history taking and clinical assessments. Health professionals may otherwise unintentionally neglect important herb-drug interactions among HIV-infected patients.
Given the increasing coverage of ART in sub-Saharan Africa (UNAIDS, 2010), further pharmacological investigations are increasingly crucial to identify potential interactions, risks, and benefits of concurrent ART and medicinal plant usage. Collaboration among biomedical clinicians and traditional herbal medicine practitioners should be encouraged to further scientific inquiry, improve communication, and provide patients with the best treatment and supportive care for HIV/AIDS and related symptoms.
Acknowledgements
Thanks to Annika Terrana, Merlin Willcox, Nadine Levin, Elisabeth Hsu, and Caroline Potter for advice and editorial assistance. Insights into Luo and Suba culture and on-site guidance in Mfangano Island were greatly appreciated from Joel Oguta, Eve Oguta, Richard Magarenge, Agnetta Ouma, Samwel Ouma, and the FACES, Ministry of Health, Organic Health Response, and Ekialo Kiona Center staff. This research was made possible by grants from the E.O. James Bequest, All Souls College, University of Oxford, as well as the Dean’s Summer Research Fellowship and the Pathways to Discovery Student Research Fellowship in the Social and Behavioral Sciences, University of California, San Francisco.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agnew ADO, Shirley AC. Upland Kenya wild flowers. Nairobi: East Africa Natural History Society; 1994. [Google Scholar]
- Beentje HJ. Kenya trees, shrubs and liana. Nairobi: National Museums of Kenya; 1994. [Google Scholar]
- Bica I, Tang AM, Skinner S, Spiegelman D, Knox T, Gorbach S, Wilson IB. Use of complementary and alternative therapies by patients with human immunodeficiency virus disease in the era of highly active anti-retroviral therapy. Journal of Alternative and Complementary Medicine. 2003;9:65–76. doi: 10.1089/107555303321222955. [DOI] [PubMed] [Google Scholar]
- Bodeker G. Traditional medicine. In: Cook G, Zumla A, editors. Manson’s Tropical Disease, twenty-first ed. London: WB Saunders; 2003. pp. 33–48. [Google Scholar]
- Bridson D, Forman L. The Herbarium Handbook, revised edition. Kew: Royal Botanic Gardens, Whitstable Litho Printers Ltd.; 1992. [Google Scholar]
- Cadet P, Weeks BS, Bilfinger TV, Mantione KJ, Casares F, Stefano GB. HIV gp120 and morphine alter mu opiate receptor expression in human vascular endothelium. International Journal of Molecular Medicine. 8(2):165–169. doi: 10.3892/ijmm.8.2.165. [DOI] [PubMed] [Google Scholar]
- Chang BL, Van Servellen G, Lombardi E. Factors associated with complementary therapy use in people living with HIV/AIDS receiving antiretroviral therapy. Journal of Alternative and Complementary Medicine. 2003;9:695–710. doi: 10.1089/107555303322524544. [DOI] [PubMed] [Google Scholar]
- Cohen DW, Odhiambo ESA. The Historical Anthropology of An African Landscape. Nairobi: Heineman Kenya; 1989. Siaya. [Google Scholar]
- Denzin N, Lincoln Y. Handbook of Qualitative Research. Thousand Oaks, CA: Sage; 2000. [Google Scholar]
- Geissler PW, Harris SA, Prince RJ, Olsen A, Odhiambo RA, Oketch-Rabah H, Madiega PA, Andersen A, Molgaard P. Medicinal plants used by Luo mothers and children in Bondo district, Kenya. Journal of Ethnopharmacology. 2002;83:39–54. doi: 10.1016/s0378-8741(02)00191-5. [DOI] [PubMed] [Google Scholar]
- Geissler PW, Nokes K, Prince RJ, Odhiambo RA, Aagaard-Hansen J, Ouma JH. Children and medicines: self-treatment of common illnesses among Luo primary school children in western Kenya. Social Science and Medicine. 2000;50(18):1771–1783. doi: 10.1016/s0277-9536(99)00428-1. [DOI] [PubMed] [Google Scholar]
- Hirt HM, Lindsy K. Natural Medicine in the Tropics: Treatments. Germany: Anamed, Winnenden; 2002. [Google Scholar]
- Homsy J. The availability of local and affordable treatment for AIDS in sub-Saharan Africa. Journal of Alternative and Complementary Medicine. 1999;5(6):505–507. doi: 10.1089/acm.1999.5.505. [DOI] [PubMed] [Google Scholar]
- Johns T, Kokwaro JO. Food plants of the Luo of Siaya District, Kenya. Economic Botany. 1991;45(1):103–113. [Google Scholar]
- Johns T, Kokwaro JO, Kimanani EK. Herbal remedies of the Luo of Siaya District, Kenya: Establishing quantitative criteria for consensus. Economic Botany. 1990;44(3):369–381. [Google Scholar]
- King R, Homsy J. Involving traditional healers in AIDS education and counseling in sub-Saharan Africa: a review. AIDS. 1997;11 Suppl A:S217–S225. [PubMed] [Google Scholar]
- Kityo C, Walker AS, Dickinson L, Lutwama F, Kayiwa J, Ssali F, Nalumenya R, Tumukunde D, Munderi P, Reid A, Gilks CF, Gibb DM, Khoo S. Pharmakokinetics of lopinavir-ritonavir with and without nonnucleoside reverse transcriptase inhibitors in Ugandan HIV-infected adults. Antimicrobial Agents and Chemotherapy. 2010;54(7):2965–2973. doi: 10.1128/AAC.01198-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois-Klassen D, Kipp W, Jhangri GS, Rubaale T. Use of traditional herbal medicine by AIDS patients in Kabarole District, Western Uganda. American Journal of Tropical Medicine and Hygiene. 2007;77(4):757–763. [PubMed] [Google Scholar]
- Langlois-Klassen D, Kipp W, Rubaale T. Who’s talking? Communication between health providers and HIV-infected adults related to herbal medicine for AIDS treatment in western Uganda. Social Science & Medicine. 2008;67:165–176. doi: 10.1016/j.socscimed.2008.02.027. [DOI] [PubMed] [Google Scholar]
- Manfredi R, Chiodo F. The effects of alternative treatments for HIV disease on recommended pharmacological regimens. International Journal of Antimicrobial Agents. 2000;13:281–285. doi: 10.1016/s0924-8579(99)00132-6. [DOI] [PubMed] [Google Scholar]
- Maundu P, Ngugi G, Kabuye CH. Traditional Food Plants of Kenya. Kenya Resource Centre for Indigenous Knowledge (KENRIK) Nairobi: National Museums of Kenya; 1999. [Google Scholar]
- Mills E, Foster BC, van Heeswijk R, Phillips E, Wilson K, Leonard B, Kosuge K, Kanfer I. Impact of African herbal medicines on antiretroviral metabolism. AIDS. 2005;19(1):95–97. doi: 10.1097/00002030-200501030-00013. [DOI] [PubMed] [Google Scholar]
- Morris K. Treating HIV in South Africa – a tale of two systems. Lancet. 2001;357:1190. doi: 10.1016/S0140-6736(00)04401-9. [DOI] [PubMed] [Google Scholar]
- Moshabela M, Pronyk P, Williams N, Schneider H, Lurie M. Patterns and implications of medical pluralism among HIV/AIDS patients in rural South Africa. AIDS and Behavior. 2010 doi: 10.1007/s10461-010-9747-3. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National AIDS and STI Control Program (NASCOP) National AIDS and STI Control Program. Nairobi: Ministry of Health; 2005. Sentinel Surveillance of HIV and STDs in Kenya. [Google Scholar]
- Orwa JA. Herbal medicine in Kenya: evidence of safety and efficacy. East African Medical Journal. 2002;79(7):341–342. doi: 10.4314/eamj.v79i7.8835. [DOI] [PubMed] [Google Scholar]
- Parkin DJ. The Cultural Definition of Political Response: Lineal Destiny among the Luo. London: Academic Press; 1978. [Google Scholar]
- Piscitelli SC, Burstein AH, Chaitt D, Alfaro RM, Falloon J. Indinavir concentrations and St. John’s wort. Lancet. 2000;255:547–548. doi: 10.1016/S0140-6736(99)05712-8. [DOI] [PubMed] [Google Scholar]
- Piscitelli SC, Burstein AH, Welden N, Gallicano KD, Falloon J. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clinical Infectious Disease. 2002;34:234–238. doi: 10.1086/324351. [DOI] [PubMed] [Google Scholar]
- Prince RJ, Geissler PW, Nokes K, Maende JO, Okatcha F, Gringorenko E, Sternberg R. Knowledge of herbal and pharmaceutical medicines among Luo children in western Kenya. Anthropology & Medicine. 2001;8(2/3):211–235. [Google Scholar]
- Sparber A, Wootton JC, Bauer L, Curt G, Eisenberg D, Levin T, Steinberg SM. Use of complementary and alternative medicine by adult patients participating in HIV/AIDS clinical trials. Journal of Alternative and Complementary Medicine. 2000;6:415–422. doi: 10.1089/acm.2000.6.415. [DOI] [PubMed] [Google Scholar]
- Survey of Kenya. National Atlas of Kenya. Nairobi: Survey of Kenya; 1970. [Google Scholar]
- UNAIDS. UNAIDS Report on the Global AIDS Epidemic 2010. Geneva: Joint United Nations Programme on HIV/AIDS; 2010. [Google Scholar]
- WHO. WHO Policy Perspectives on Medicines, no. 2, May 2002. Geneva: World Health Organization; 2002a. Traditional medicine – growing needs and potential. [Google Scholar]
- WHO. WHO traditional medicine strategy 2002–2005. Geneva: World Health Organization; 2002b. [Google Scholar]
- WHO. WHO guidelines on good agricultural and collection practices (GACP) for medicinal plants. Geneva: World Health Organization; 2003. [Google Scholar]
- Whyte PR, Kariuki P. Malnutrition and gender relations in western Kenya. Health Transition Review. 1991;1(2):1–16. [PubMed] [Google Scholar]