Abstract
We report the large-scale synthesis of 1,3-cyclooctanedione in five steps with 29% yield. This molecule is a synthetic precurser to difluorinated cyclooctyne, which participates in a bioorthogonal copper-free click reaction with azides. The final step demonstrates the first successful application of the Wacker-Tsuji oxidation to form a cyclic 1,3-dione.
Keywords: copper-free click chemistry, Wacker-Tsuji oxidation, cyclooctanedione, difluorinated cyclooctyne
In the past decade, bioorthogonal conjugation methods have emerged as powerful and increasingly indispensable tools for performing chemistry in biological contexts.1–3 Notably, the strain-promoted azide-alkyne cycloaddition (SPAAC) has proven especially promising as a cytocompatible ligation method. The reaction boasts nearly all the advantages of the canonical copper(I)-catalyzed Huisgen [2+3] cycloaddition between an azide and a terminal alkyne, but requires no copper catalyst, which is cytotoxic at >0.1 mM concentrations.4,5 Thus, the chemistry can be performed readily (k ~ 10−1 M−1 s−1) in the presence of individual cells as well as full living organisms.6 Bertozzi and coworkers have pioneered the synthesis of several cyclooctyne reagents and have successfully applied their use as chemical probes to perform in vivo imaging to study glycosylation in zebrafish and mice.7,8 In addition, other molecules for strain-promoted ractions with azides have recently been developed including (aza-)dibenzocyclooctynes,9,10 biarylazacyclooctynone,11 and oxanorbornadienes12 which exhibit similar utility.
More recently, the use of Cu-free click chemistry has expanded from simple labeling procedures to include the formation of complex materials.13–15 By end-functionalizing synthetic polypeptides with a difluorinated cyclooctyne reagent (DIFO3)16 and reacting with a four-arm poly(ethylene glycol) tetraazide in an aqueous medium, hydrogels are formed that allow for the direct encapsulation of cells.13,15 Despite this preliminary success, the difficulty of the cyclooctyne synthetic preparation has ultimately hampered the widespread implementation of SPAAC in material formation to date. Additionally, utilizing these reagents in material fabrication requires gram-quantities of the reagents as opposed to the µg- to mg-scale required for labeling experiments. Thus, we sought to develop an improved synthetic route that enables DIFO3 to be formed on the multi-gram scale.
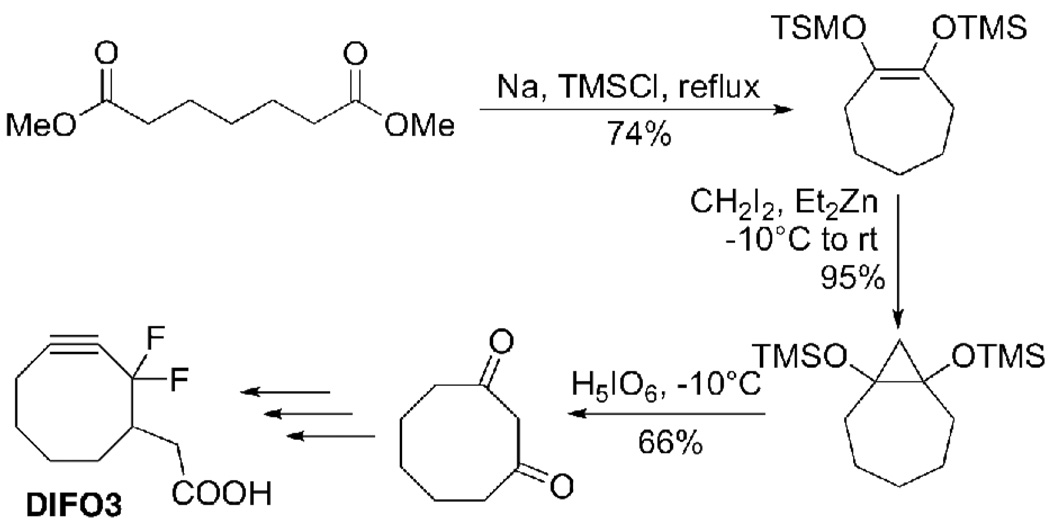
We found the main difficulty in preparing DIFO3 in significant quantity lies in producing large amounts of the key synthetic intermediate 1,3-cyclooctanedione. The published route of Pirrung and Webster17 (Scheme 1) for preparation of this compound has several undesirable characteristics, including the use of large molar equivalents of water-reactive sodium metal and diethylzinc.18 Pyrophoric organometallic reagents are not ideal for larger scale reactions and have in some cases been implicated in laboratory accidents.19 Moreover, the acyloin condensation (Scheme 1, first step) exhibited highly-variable yields in our hands. This is in part due to the nature of cyclizing larger rings: significant oligomerization/polymerization likely occured, as evidenced by the formation of a non-volatile, colored byproduct and reduced product recovery, even with high dilution and slow addition of diester. Thus, we sought to develop a mild, high-yielding synthesis of 1,3-cyclooctanedione starting from an inexpensive, readily-available substrate.
Scheme 1.
Traditional synthesis of DIFO3 from 1,3-cyclooctanedione intermediate.
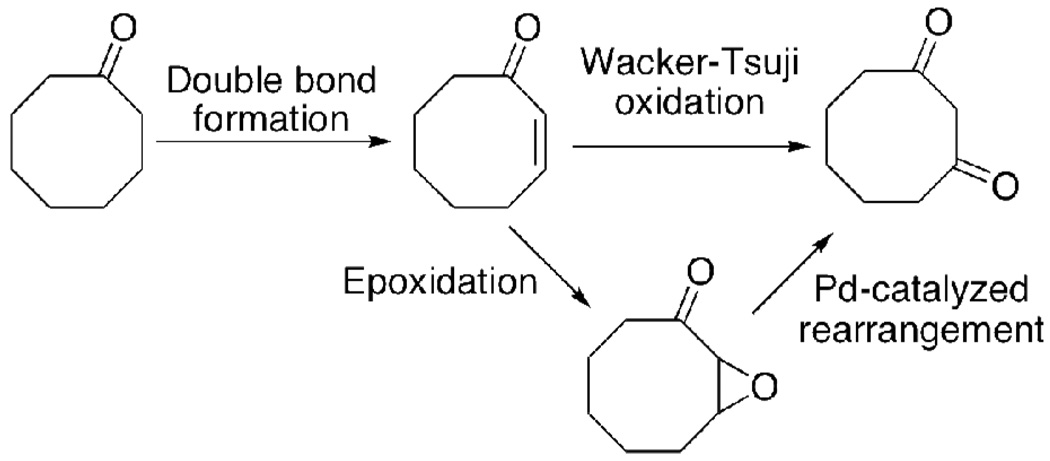
Initially, we were inspired by a kg-scale preparation of 1,3-cycloheptanedione,20,21 whose key step relies on the cycloaddition between dichloroacetyl chloride and 1-trimethylsilyloxycyclopentene. We hypothesized that this synthetic approach could be easily adapted to prepare 1,3-cyclooctanedione. However, we abandoned this route after experiencing difficulty obtaining significant coupling between 1-trimethylsilyloxycyclohexene and dichloroacetyl chloride. Ultimately, cyclooctanone was chosen as a starting point as it is inexpensive ($77 per 100 g via Aldrich), readily available, and several potential methods for installing the ketone functionality at the β-position were identified, including epoxidation and subsequent Pd-catalyzed rearrangement22,23 as well as the direct Wacker-Tsuji oxidation24 (Scheme 2).
Scheme 2.
Strategy for synthesis of 1,3-cyclooctanedione.
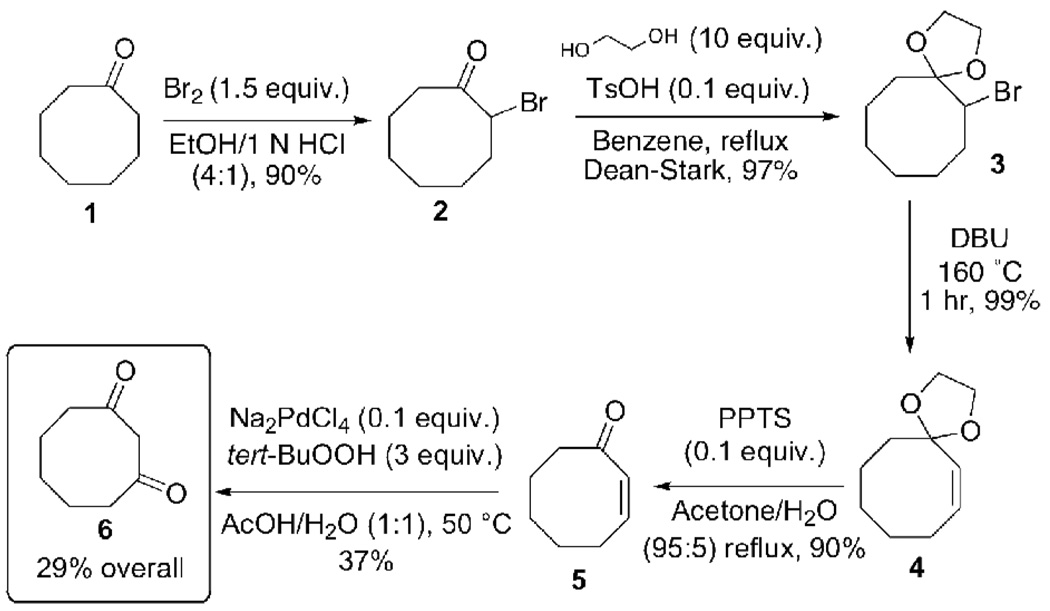
From the starting cyclooctanone (1), enone 5 was synthesized in four steps and 78% yield via α-bromination followed by elimination, an established method to introduce an α,β C=C double bond.25 Bromination of ketone (1) was accomplished in excellent yield (90%, Scheme 3) with 1.5 equiv of Br2 in ethanolic hydrochloric acid and the product was pure enough after standard aqueous workup to obviate chromatographic purification.
Scheme 3.
Complete synthesis of 1,3-cyclooctanedione (6) from cyclooctanone (1) with reaction conditions and obtained yields. Full reaction conditions, as well as 1H, 13C, and COSY NMR and HR-MAS characterization for all compounds can be found in the supporting information.
Many attempts to effect direct dehydrobromination on 2 with various bases and solvent systems were made (KOH in isopropanol, DBU in toluene or dichloromethane,26 and LiBr/Li2CO3 in DMF27). All such attempts, however, failed to generate significant quantity of enone 5. Thus, a two-step protection/deprotection of the ketone moiety was adopted.28 Formation of bromo ketal 3 under Dean-Stark conditions was achieved in near-quantitative yield and reasonable purity. Quantitative dehydrobromination of crude ketal bromide 3 was accomplished after reacting for 1 hr in neat 1,8-diazabicycloundec-7-ene (DBU) at 160 °C. We note that the direct conversion of cyclooctanone 1 to the bromo ketal 3 has been reported elsewhere29,30. Crude product 4 was deprotected via trans-ketalization in PPTS/acetone/water, and resultant enone 5 was recovered in high yield after purification by flash chromatography. Note that while Plumet23 reports a method to synthesize enone 5 in two steps from cyclooctanedione, we feel the extra two steps for the present scheme are justified: The overall yield is higher (78% vs 49%), both require just one chromatographic purification (for the final enone), and the palladium reagent used in the Plumet method (73 mol%) would add considerable cost to a large scale preparation.
Two potential methods to convert enone 5 to 1, 3-cyclooctanedione (6) were identified involving epoxidation then palladium-catalyzed rearrangement.31,23 However, after an initial attempt to form the epoxide from enone (5) under Weitz–Scheffer conditions (aq. H2O2 or TBHP and cat. NaOH)32 failed, we found that Wacker-Tsuji oxidation conditions gave the desired dione in a single step (Scheme 3).24 After briefly screening reaction conditions (Table 1), conversion of enone 5 to dione 6 was realized in 37% yield by treatment with 0.1 equiv Na2PdCl4 and 3 equiv tert-BuOOH in AcOH/H2O (1:1) at 50 °C – undesired formation of unidentified side products resulted in the modest yield of this step. Given the high yields and simple purifications for the preceding steps, even with a yield <40% for the final step, this represents significant improvement over the standard methods for 1,3-cyclooctanedione preparation (Scheme 1)17. Furthermore, to the best of our knowledge, no successful application of the Wacker-Tsuji application to a cyclic substrate has ever been reported.
Table 1.
Isolated yields of dione (6) via the Wacker-Tsuji oxidation of enone (5) for a variety of catalyst and reactant amounts, as well as temperature.
| Na2PdCl4 (mol equiv) |
tert-BuOOH (mol equiv) |
Temperature (°C) | Yield (%) |
|---|---|---|---|
| 0.2 | 1.5 | 50 | 24 |
| 0.2 | 1.5 | 100 | 11 |
| 0.2 | 3 | 50 | 36 |
| 0.2 | 3 | 30 | 36 |
| 0.1 | 1.5 | 50 | 30 |
| 0.1 | 3 | 50 | 37 |
| 0.4 | 1.5 | 50 | 22 |
In summary, we have described a new method to synthesize 1,3-cyclooctanedione in five steps (Scheme 3) using an inexpensive starting material, robust reactions, and with only two steps requiring chromatographic purification. In addition, the first successful application of the Wacker oxidation in the direct synthesis of a cyclic 1,3-dione is shown. Using this method, we were able to generate 15+ g of dione 6 in 29% overall yield which can be used to further synthesize DIFO3 for SPAAC.
Supplementary Material
Acknowledgements
The authors would like to thank C. Bertozzi and J. Baskin for initial communication concerning these efforts. This work was funded by the Howard Hughes Medical Institute. Fellowship assistance to C.A.D. was awarded by the US Department of Education’s Graduate Assistantships in Areas of National Need program.
References
- 1.Prescher JA, Bertozzi CR. Chemistry in living systems. Nat Chem Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 2.Baskin JM, Bertozzi CR. Bioorthogonal Click Chemistry: Covalent Labeling in Living Systems. QSAR Comb. Sci. 2007;26:1211–1219. [Google Scholar]
- 3.Sletten ME, Bertozzi CR. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angewandte Chemie International Edition. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speers AE, Adam GC, Cravatt BF. Activity-based protein profiling in vivo using a copper(i)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 2003;125:4686–4687. doi: 10.1021/ja034490h. [DOI] [PubMed] [Google Scholar]
- 5.Wolbers F, et al. Viability study of HL60 cells in contact with commonly used microchip materials. Electrophoresis. 2006;27:5073–5080. doi: 10.1002/elps.200600203. [DOI] [PubMed] [Google Scholar]
- 6.Baskin JM, et al. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. U. S. A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. In vivo imaging of membrane-associated glycans in developing zebrafish. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang PV, et al. Copper-free click chemistry in living animals. Proc. Natl. Acad. Sci. U. S. A. 2010;107:1821–1826. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ning X, Guo J, Wolfert MA, Boons GJ. Visualizing Metabolically Labeled Glycoconjugates of Living Cells by Copper-Free and Fast Huisgen Cycloadditions13. Angewandte Chemie International Edition. 2008;47:2253–2255. doi: 10.1002/anie.200705456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debets MF, et al. Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3+2) cycloaddition. Chem. Commun. 2009 doi: 10.1039/b917797c. [DOI] [PubMed] [Google Scholar]
- 11.Jewett JC, Sletten EM, Bertozzi CR. Rapid Cu-Free Click Chemistry with Readily Synthesized Biarylazacyclooctynones. J. Am. Chem. Soc. 2010;132:3688–3690. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sander SvB, et al. Metal-Free Triazole Formation as a Tool for Bioconjugation. Chembiochem. 2007;8:1504–1508. doi: 10.1002/cbic.200700278. [DOI] [PubMed] [Google Scholar]
- 13.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat. Mater. 2009;8:659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson JA, Baskin JM, Bertozzi CR, Koberstein JT, Turro NJ. Copper-free click chemistry for the in situ crosslinking of photodegradable star polymers. Chem Commun (Camb) 2008:3064–3066. doi: 10.1039/b803043j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeForest CA, Sims EA, Anseth KS. Peptide-Functionalized Click Hydrogels with Independently Tunable Mechanics and Chemical Functionality for 3D Cell Culture. Chem. Mater. 2010;22:4783–4790. doi: 10.1021/cm101391y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Codelli JA, Baskin JM, Agard NJ, Bertozzi CR. Second-Generation Difluorinated Cyclooctynes for Copper-Free Click Chemistry. J Am Chem Soc. 2008 doi: 10.1021/ja803086r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pirrung MC, Webster NJG. Mechanism of intramolecular photocycloadditions of cyclooctenones. J. Org. Chem. 1987;52:3603–3613. [Google Scholar]
- 18.Bloomfield JJ, Nelke JM, Stojanac Z, Valenta Z. Acyloin Condensation In Which Chlorotrimethylsilane Is Used as a Trapping Agent: 1,2-Bis(Trimethylsilyloxy)Cyclobutene and 2-Hydroxycyclobutanone. Organic Syntheses Coll. 1988;Vol. 6:167. [Google Scholar]
- 19.Kemsley J. Learning from UCLA. Chemical & Engineering News. 2009;87:29–31. [Google Scholar]
- 20.Ragan JA, et al. A Practical Synthesis of Cycloheptane-1,3-dione. Organic Process Research & Development. 1998;2:379–381. [Google Scholar]
- 21.Do NM, Ruth E, Ragan John A. Preparation of cycloheptane-1,3-dione via reductive rign expansion of 1-trimethylsilyloxy-7,7-dichlorobicyclo[3.2.0]heptan-6-one. Organic Syntheses. 2008;85:138–146. [Google Scholar]
- 22.Suzuki M, Watanabe A, Noyori R. Palladium(0)-Catalyzed Reaction of Alpha, Beta-Epoxy Ketones Leading to Beta-Diketones. J. Am. Chem. Soc. 1980;102:2095–2096. [Google Scholar]
- 23.Aljarilla A, et al. Stereochemistry of the Tetrabutylammonium Cyanide-Catalyzed Cyanosylilation of Cyclic α,β-Epoxyketones – Dependence of the Diastereoselectivity on the Ring Size. Eur. J. Org. Chem. 2006;2006:3969–3976. [Google Scholar]
- 24.Tsuji JNH, Hori K. A New Preparative Method for 1,3-Dicarbonyl Compounds by the Regioselective oxidation of alpha,beta-Unsaturated Carbonyl Compounds, Catalyzed by PdCl2 Using Hydroperoxides as the Reoxidant. Chemistry Letters. 1980;1980:257–260. [Google Scholar]
- 25.Larock RC. Comprehensive organic transformations : a guide to functional group preparations, Edn. 2nd. New York ; Chichester [England]: Wiley-VCH; 1999. [Google Scholar]
- 26.Paquette LA, Ross RJ, Springer JP. Stereocontrolled construction of an ingenol prototype having a complete array of oxygenated and unsaturated centers. J. Am. Chem. Soc. 1988;110:6192–6204. doi: 10.1021/ja00226a039. [DOI] [PubMed] [Google Scholar]
- 27.Floyd MB, Weiss MJ. Prostaglandins and congeners. 21. Synthesis of some cyclohexyl analogs (11.alpha.-homoprostaglandins) J. Org. Chem. 1979;44:71–75. [Google Scholar]
- 28.Ruedi G, Hansen H-J. Diradical-Promoted Two-Carbon Ring-Expansion Reactions by Thermal Isomerization: Synthesis of Functionalized Macrocyclic Ketones. Helv. Chim. Acta. 2004;87:1628–1665. [Google Scholar]
- 29.House HO, Sieloff RF, Lee TV, Detar MB. Enones with Strained Double-Bonds .4. The Bicyclo[5.3.1]Undecane System. The Journal of Organic Chemistry. 1980;45:1800–1806. [Google Scholar]
- 30.Meier H, Antony-Mayer C, Schulz-Popitz C, Zerban G. Einführung einer Dreifachbindung in das Bicyclo[6.1.0]nonan-Gerüst. Liebigs Annalen der Chemie. 1987;12:1087–1094. [Google Scholar]
- 31.Lehmann TE, Kuhn O, Kruger J. Process Development and Pilot Plant Scale Synthesis of Spiro[3.5]nonane-6,8-dione. Organic Process Research & Development. 2003;7:913–916. [Google Scholar]
- 32.Weitz E, Scheffer A. The impact of alkali hydrogen superoxyde on unsaturated compounds. Berichte Der Deutschen Chemischen Gesellschaft. 1921;54:2327–2344. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.