Abstract
Background
The cytochrome P450 enzyme CYP26 (retinoic acid 4-hydroxylase) initiates the catabolism of all-trans retinoic acid (tRA) and limits the effects of tRA. The CYP26 enzyme acts on tRA, but not 13-cis RA (isotretinoin), a retinoid used to treat severe acne. However, 13-cis RA can isomerize to tRA, which can then be metabolized by CYP26.
Objective
In healthy subjects, we assessed the variability of CYP26 enzymatic activity. We then investigated whether response to oral 13-cis RA among acne patients correlates with variability in CYP26 expression.
Methods
In healthy subjects, we isolated microsomal fractions from the epidermis of keratome biopsies and measured CYP26 enzymatic activity in untreated skin and skin treated with tRA. Enzymatic activity was determined based on rate of formation of 4-hydroxy RA (pg/min) per mg microsomal protein. Using real-time PCR we quantified CYP26 mRNA induction after tRA application in acne patients who responded or did not respond to one course of 13-cis RA.
Results
In normal skin (N=118), CYP26 enzymatic activity was widely variable (1–180 pg/min per mg microsomal fraction; mean 42.7 ± 3.5). Furthermore, CYP26 enzymatic activity was inducible in a dose-dependent manner in normal skin following tRA application, but not correlated with age or sex (N=29). In acne patients, CYP26 mRNA induction following 0.1% tRA application did not differ (P>0.05) between subjects who responded (N=8, 587±325 fold) or did not respond (N=8, 657±227 fold) to one course of 13-cis RA.
Limitations
The small number of acne patients treated with 13-cis RA was a major limitation.
Conclusion
Factors other than CYP26 activity may determine response to isotretinoin in acne.
Keywords: cytochrome, retinoic acid 4-hydroxylase, isotretinoin
INTRODUCTION
Retinoids influence various cellular processes in the skin, such as keratinocyte and sebocyte differentiation. Some well-known retinoids include 13-cis retinoic acid (isotretinoin, 13-cis RA) and its stereoisomers, all-trans retinoic acid (tretinoin, tRA) and 9-cis retinoic acid (alitretinoin, 9-cis RA). Many of the biological effects of retinoids are mediated by two families of receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs). These receptors function as dimeric, ligand-dependent transcription factors, indicating that retinoids elicit biological effects in part by altering gene expression. Whereas tRA and 9-cis RA bind to RARs and RXRs, respectively, 13-cis RA binds with little or no affinity to either type of receptor.1,2 Instead, 13-cis RA appears to exert many of its effects by first isomerizing to tRA.3 Thus, 13-cis RA acts as a prodrug that modulates cellular function via tRA-RAR interactions.
In humans, the level of tRA is regulated by a feedback system. Cytochrome P450 (CYP) enzymes are primarily responsible for tRA catabolism. One of these enzymes, known as CYP26 or RA 4-hydroxylase, initiates the process of tRA catabolism and is thus crucial for limiting the biological activity of tRA.2 CYP26 metabolizes tRA to less active derivatives, including 4-hydroxy RA and 4-oxo RA4, but does not directly metabolize other retinoids, such as 9-cis RA or 13-cis RA.2 Rather, these cis-retinoids are thought to isomerize to tRA before being acted upon by CYP26.5 Furthermore, CYP26 has a retinoic acid response element (RARE) and is selectively induced by tRA.2 Following exposure of human skin to 0.1% tRA cream, enzymatic activity of CYP26 is induced substantially.5 Thus, CYP26 is induced by tRA and also targets tRA for inactivation. Additionally, we have previously found that gene expression of CYP26 correlates with its enzymatic activity.2
Clinically, oral 13-cis RA (isotretinoin) is the most effective treatment for severe nodulocystic acne. While the exact biological mechanism of this drug remains unclear, 13-cis RA is effective in most (up to 89%) acne patients.6 However, approximately 16% of treated subjects require a second course.7 One analysis demonstrated that 70% of patients under 12 years treated with 13-cis RA relapsed within 1 year and required a second course of treatment, as compared to 34.8% of patients between 14 to 16 years old.8
Since genetic polymorphism exists for CYP26,9 we hypothesized that patients whose acne did not clear after one course of 13-cis RA (defined as “non-responders”) may have higher baseline levels of CYP26 or greater ability to induce CYP26 than patients who responded to one treatment course (defined as “responders”). Our reasoning was that upregulated CYP26 expression in non-responders may increase the catabolism of retinoids, diminishing the levels of active tRA derived from 13-cis RA isomerization. To investigate this, we first measured the range of CYP26 enzymatic activity in normal human skin and confirmed the inducibility of this enzyme by tRA. To investigate mechanisms underlying the improvement of acne with isotretinoin treatment, we then measured the inducibility of CYP26 in the skin of acne patients who were previously treated with this drug.
MATERIALS & METHODS
Subjects
This study was approved by the University of Michigan Medical School Institutional Review Board and conducted in accordance with the Helsinki Declaration. The study was conducted in the Program for Clinical Research in Dermatology (PCRID). Written informed consent was obtained from subjects prior to enrollment. Healthy subjects were enrolled to investigate normal CYP26 expression (N=118) and CYP26 induction by tRA (N=29). “Healthy” subjects were defined as not having any skin disease. In addition, we recruited acne patients (N=16) who had previously completed one course of 13-cis RA. Exclusion criteria included age less than 18 at the time of enrollment, application of topical corticosteroids or retinoids within 14 days, or use of systemic corticosteroids within 28 days or systemic retinoids within 3 months. Pregnancy and lactation were also exclusionary.
Retinoic Acid Application and Biopsies
Trans-RA was dissolved in a vehicle consisting of 95% ethanol-propylene glycol (7 parts ethanol to 3 parts propylene glycol by volume) containing 0.5 mg butylated hydroxytoluene per ml of solvent (Sigma, St. Louis, MO). All work with tRA was conducted under subdued yellow light. In healthy subjects, no treatment was given, or vehicle alone (100 μL) and up to six concentrations of tRA (0.001%, 0.005%, 0.01%, 0.025%, 0.05%, and 0.1%, each 100 μL) were applied under occlusion to different areas of buttock skin for 24 hours. Keratome biopsies (1 inch by 3 inches) were obtained as previously described.10
In acne patients previously treated with 13-cis RA, vehicle (35 μL) and 0.1% tRA (35 μL) were applied to different areas (each 1 in2) of buttock skin. Skin was occluded with plastic wrap and shielded from light with gauze. After 24 hours, punch biopsies (4 mm) of treated and control skin were obtained after injecting local anesthesia (lidocaine). Samples were snap frozen in liquid nitrogen and stored at -70° C for later analysis.
CYP26 Enzymatic Activity
CYP26 enzymatic activity in keratome samples was determined by measuring the formation of 4-hydroxy RA (pg/min per mg microsomal protein) in microsomal fractions that were isolated and prepared from human epidermis, as previously described.5,10 Briefly, CYP26 activity was determined in an ex vivo assay by incubating 100 μg microsomal protein in 0.01 M phosphate buffer (pH 7.4) containing tritiated tRA as substrate (DuPont NEN, Boston, MA) and an NADPH-regenerating system (Sigma, St. Louis, MO).10 Samples were incubated for 30 minutes at 35°C. The reaction was then terminated by adding 100 μL methanol containing 100 μg/mL of butylated hydroxytoluene cooled to -20°C. After centrifugation at 1000 g for 10 minutes, the supernatant fractions were analyzed for tritiated 4-hydroxy RA by reverse phase HPLC and liquid scintillation spectrometry. Due to the limited amount of microsomal protein available, this technique was performed once per biopsy.
Measurement of CYP26 mRNA Levels
In biopsies of skin from acne patients, total RNA was extracted using a commercial RNeasy Mini kit (Qiagen, Chatworth, CA), and reverse transcriptase, real-time polymerase chain reaction (RT-PCR) was performed, as previously described.11 CYP26 mRNA levels were normalized to an internal control, the housekeeping gene 36B4. Primers and probes for CYP26 and 36B4 were produced by a custom oligonucleotide synthesis service (Applied Biosystems, Foster City, CA).
Statistical Analysis
Comparison of CYP26 induction levels following application of varying concentrations of tRA was performed with a repeated measures analysis of variance and Dunnett's multiple comparison procedure. This method was performed twice, as described below. The strength of the linear relationship between CYP26 inductions and other variables was assessed with Pearson's product-moment correlation. To see whether certain variables (age and sex) were significantly correlated with CYP26 induction when tRA concentration was controlled for, multiple linear regression modeling was used with the forward stepwise selection procedure. Inclusion into the model was determined by the significance of each variable's partial correlation coefficient. Age, weight, cumulative dose, and CYP26 mRNA levels of 13-cis RA responders and non-responders were compared using the two-sample Student t-test. All data are summarized as means ± SEM. Significance was attained with P<0.05 for a two-tailed hypothesis. The data were analyzed with SAS statistical software version 9.1 (SAS Institute, Inc. Cary, NC).
RESULTS
CYP26 enzymatic activity is variable in normal human skin
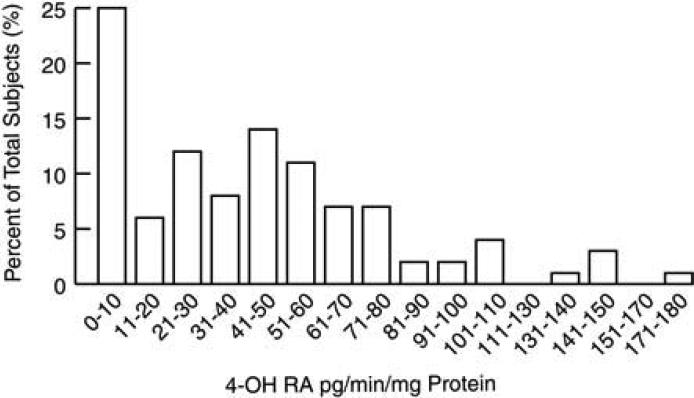
We first assessed basal (constitutive) CYP26 enzymatic activity in normal human skin (82 males and 36 females). The age range of subjects was 19 to 62 years, with a mean age of 35.9 ± 0.9 years. CYP26 enzymatic activity, as determined by formation of 4-hydroxy RA, ranged from 1 to 180 pg/min per mg total epidermal microsomal protein, with a mean level of 42.7 ± 3.5 pg/min per mg total epidermal microsomal protein (Fig. 1). There was no significant difference (P=0.29) in enzymatic activity between females and males (43.8 ± 6.9 and 42.1 ± 4.0 pg/min per mg, respectively). We also found no significant correlation between CYP26 activity and age when comparing all subjects (r=0.05; P=0.59), only female subjects (age range 20-62, mean age 37.3 ± 1.8; r=0.25; P=0.15), and only male subjects (age range 19-60, mean age 35.2 ± 1.1; r=0.06; P=0.58).
Figure 1.
Basal CYP26 enzymatic activity in normal human skin. After isolating microsomal fractions from the epidermis of keratome biopsies obtained from healthy subjects (N=118), basal CYP26 enzymatic activity (expressed as formation of 4-hydroxy RA [pg/min] per mg of total isolated protein) was determined.
CYP26 enzymatic activity is induced in a dose-dependent manner by tRA in normal human skin in vivo
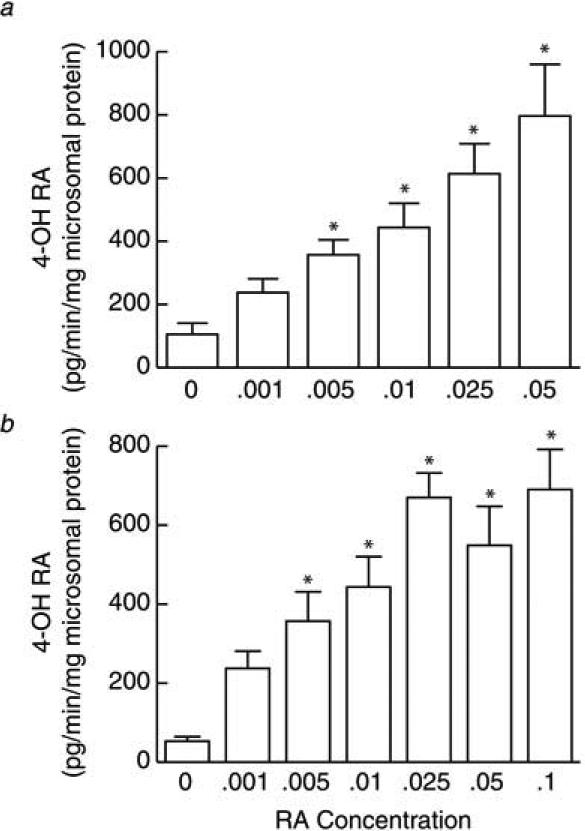
To investigate the characteristics of CYP26 induction, we applied vehicle and six differing concentrations of tRA (0.001%, 0.005%, 0.01%, 0.025%, 0.05% and 0.1%) to buttock skin of healthy subjects (N=29) for 24 hours. No adverse events were experienced by any subject. After obtaining keratome samples of treated and control skin, we measured the inducibility (expression) of CYP26 enzymatic activity. We found a clear dose response, in which subjects expressed higher CYP26 induction with higher concentrations of applied tRA. The data were first analyzed in purest statistical form with six individuals treated with five tRA concentrations (Fig. 2A). To obtain data from additional patients, a second analysis was performed that included the same cohort of six individuals, in addition to 23 patients that were each exposed to only a single tRA concentration, including 0.025% (N=12), 0.05% (N=7), or 0.1% (N=4). Compared with vehicle treatment, both types of analyses revealed significant induction (P<0.05) of CYP26 enzymatic activity at every dose, except 0.001% (Fig. 2B). Additionally, using multivariate regression analysis, we did not observe any significant correlation between CYP26 activity and subject age or sex (data not shown).
Figure 2.
Dose dependence of CYP26 enzymatic activity with tretinoin concentration. Following topical treatment of normal human skin with tretinoin (tRA) for 24 hours, keratome biopsies were obtained. After isolating microsomal fractions from the epidermis, CYP26 enzymatic activity was determined based on formation of 4-hydroxy RA [pg/min] per mg of total isolated protein. (a) Induction of CYP26 enzyme in six individuals treated with vehicle and the indicated concentrations of tRA is shown. (b) This panel displays data obtained from the same cohort as in “a” plus 23 patients that were treated with vehicle and only a single concentration of tRA, including 0.025% (N=12), 0.05% (N=7), or 0.1% (N=4). Fold changes are expressed as means + SEM, relative to untreated skin (normalized to 1, not shown). *P<0.05, compared with vehicle-treated skin.
Isotretinoin responders and non-responders demonstrate similar CYP26 mRNA induction in vivo
Acne patients who had been previously treated with one course of 13-cis RA were divided into responders and non-responders based on physician interview and retrospective chart review (Table 1). Responders (N=8; 3 males and 5 females; mean age 21.9 ± 2.2 years; age range 16 to 32 years; mean weight 67.5 ± 5.2 kg) were defined as subjects in whom acne clinically resolved after one course of treatment. Non-responders (N=8; 4 males and 4 females; mean age 17.9 ± 1.9 years; range 13 to 30 years; mean weight 61.4 ± 2.7) were defined as patients who did not experience substantial or sustained improvement of acne after a single course of 13-cis RA, as judged by the physician, patient, or both. There was no significant difference in the mean age (P=0.19) or weight (P=0.32) of responders versus non-responders at the time of 13-cis RA initiation. After completing their initial treatment course, patients were followed by their primary dermatologist or family physician an average of 7.4±1.4 years (range 10 months to 21 years). Cumulative dosage data were available for 7 responders and 7 non-responders. Comparing responders with non-responders, there was no significant difference (P=0.90) in cumulative dose of 13-cis RA (127.8 ± 14.1 mg/kg versus 131.1 ± 20.9 mg/kg, respectively).
TABLE 1.
Clinical information for acne patients who responded or did not respond to one course of isotretinon.
| Responder | Age at Drug Initiation | Gender | Ethnicity | Weight (kg) | Cumulative Dose (mg/kg) |
|---|---|---|---|---|---|
| 1 | 25 | female | Caucasian | 61.2 | 117.6 |
| 2 | 16 | male | Caucasian | 68 | 52.9 |
| 3 | 32 | female | Caucasian | 54.5 | 157.9 |
| 4 | 17 | male | Caucasian | 61.3 | 132.1 |
| 5 | 18 | female | Caucasian | 56.7 | 141.1 |
| 6 | 29 | female | Caucasian | 77.1 | 125.6 |
| 7 | 19 | female | Caucasian | 68 | 167.6 |
| 8 | 18 | male | Caucasian | 97.7 | not available |
| Mean±SE | 21.9±2.2 | 67.5±5.2 | 127.8±14.1 |
| Non-Responder | Age at Drug Initiation | Gender | Ethnicity | Weight (kg) | Cumulative Dose (mg/kg) |
|---|---|---|---|---|---|
| 1 | 16 | male | Caucasian | 65 | 166.15 |
| 2 | 19 | male | Black | 74.8 | 88.2 |
| 3 | 19 | female | Caucasian | 54.5 | 231 |
| 4 | 16 | male | Caucasian | 63.5 | not available |
| 5 | 14 | female | Caucasian | 49.9 | 136.3 |
| 6 | 30 | male | Caucasian | 61.2 | 116.2 |
| 7 | 16 | female | Caucasian | 56.7 | 84.6 |
| 8 | 13 | female | Asian/Caucasian | 65.8 | 95.4 |
| Mean±SE | 17.9±1.9 | 61.4±2.7 | 131.12±20.9 |
In a previous study, we have shown that CYP26 is transcriptionally regulated and that tRA application elicits increased CYP26 mRNA synthesis and enzymatic activity.2 In that study, CYP26 enzymatic activity correlated with mRNA expression. Therefore, we measured CYP26 mRNA (as opposed to enzymatic activity) in the skin of 13-cis RA responders and non-responders. The use of punch biopsies, rather than keratome biopsies, in these patients also limited our laboratory assay to mRNA quantification. All subjects were treated with 0.1% tRA and vehicle in a paired manner for 24 hours. Side effects occurred in 2 patients (both non-responders) and consisted of mild redness, scaling, and/or itching that readily resolved with emollients or no treatment.
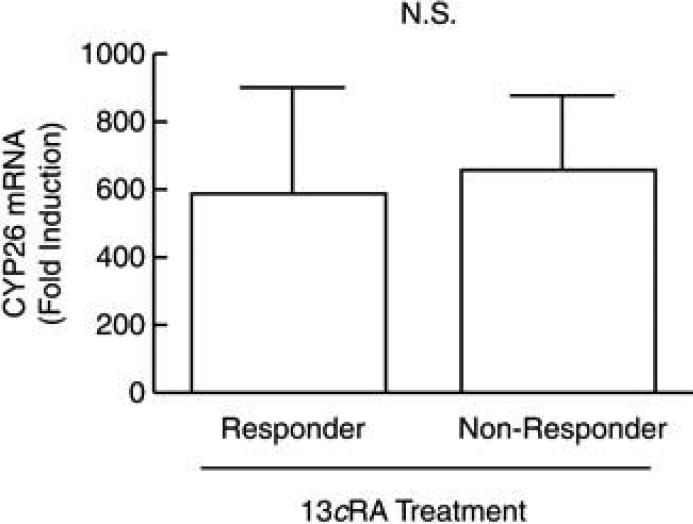
Based on RT-PCR, we found no significant differences (P>0.05) in CYP26 mRNA expression in vehicle-treated skin from responders versus non-responders (data not shown), suggesting that constitutive levels of CYP26 did not differ between the two groups. In tRA-treated skin, responders demonstrated a 587±325 fold induction of CYP26 mRNA compared with vehicle-treated skin (Fig. 3). Non-responders demonstrated a 657±227 fold induction compared with vehicle-treated skin. Comparing fold induction between responders and non-responders, there were no statistically significant differences (P=0.84, Fig. 3). Additionally, no statistically significant differences were found when comparing male (N=3) versus female (N=5) responders (P=0.43), or male (N=4) versus female (N=4) non-responders (P=0.35, data not shown).
Figure 3.
CYP26 mRNA induction in isotretinoin responders and non-responders. Following topical application of 0.1% tRA and vehicle under occlusion for 24 hours, punch biopsies were taken from acne patients who had previously responded (N=8) or did not respond (N=8) to one course of isotretinoin. Total RNA was extracted from these biopsies, and CYP26 mRNA was quantified by real-time polymerase chain reaction. Fold changes are expressed as means + SEM, relative to untreated skin (normalized to 1, not shown). NS, not significant (P>0.05)
DISCUSSION
Isotretinoin, or 13-cis RA, is the most effective medication for severe acne. Despite its use for decades, the mechanism of this drug is not fully understood. Current evidence suggests that isotretinoin may affect many of the factors involved in acne pathogenesis, such as androgenic activity, sebum production, bacteria, and inflammation. Indeed, 13-cis RA has been shown in vitro to decrease androgen production.12 Isotretinoin also decreases sebocyte proliferation and differentiation, and more recently, investigators have found that 13-cis RA induces cell cycle arrest and apoptosis in human sebocyte cell lines.3,13,14 Additionally, neutrophil migration is inhibited by 13-cis RA, and this may be one way 13-cis RA targets inflammation.15
In humans, 13 cis-RA elicits many of its effects by first isomerizing to tRA, a retinoid with important and diverse effects on skin homeostasis. CYP26, a member of the cytochrome P450 system, is responsible for initiating the process of tRA catabolism, regardless of whether tRA is endogenous or derived from exogenous sources (e.g., 13-cis RA isomerization or topical tRA). CYP26 is highly expressed by epidermal keratinocytes in the basal layer of the epidermis, as well as epithelial cells of the eccrine sweat gland and sebaceous gland.16 In addition to acting principally on tRA, CYP26 is also specifically and directly induced by tRA, but not other retinoids.5 Thus, tRA levels in the body are regulated by an orchestrated feedback system.
Based on these observations, we postulated that differences in CYP26 activity may explain some of the variable clinical responses to 13-cis RA in acne patients. We hypothesized that non-responders might be “fast-metabolizers” who have greater CYP26 expression or inducibility. We hypothesized that this might result in decreased levels of isotretinoin-derived tRA, which in turn leads to decreased therapeutic response.
To investigate this possibility, we first measured CYP26 levels in normal skin. We found wide variability in basal CYP26 enzymatic activity. We interpreted this as a reflection of genetic polymorphism for CYP26; however, it is worthwhile to note that expression and activity of CYP26 is complex and likely affected by environmental and endogenous factors (e.g., dietary vitamin A, medications, or hormone levels).17 After application of topical tRA, we found that CYP26 induction in healthy subjects was dose-dependent, but not correlated with age or sex.
Next, we examined skin samples taken from 13-cis RA responders and non-responders. Of note, responders and non-responders did not exhibit significant differences in sex, age, or cumulative dose of the first course of 13-cis RA. Based on our biochemical studies, we found that 13-cis RA responders and non-responders had similar constitutive levels of CYP26 mRNA expression in vehicle-treated skin. Following topical tRA treatment, both groups also had similar levels of CYP26 mRNA induction, although non-responders had a slightly increased, but not statistically significant, mean induction level. Other factors such as gender did not affect CYP26 induction in responders and non-responders. These results suggest that variation in CYP26 basal expression and/or induction may play only a minor role in determining therapeutic response to isotretinoin.
Thus, additional factors may impact the clinical efficacy of oral 13-cis RA. For instance, another cytochrome P450 enzyme, known as CYP2S1, may participate in the hydroxylation of tRA. Possibly, acne patients may exhibit differences in the expression of this enzyme. Moreover, little is known about the mechanisms which regulate the rate of isomerization of 13-cis RA to tRA. Indeed, it is unclear whether isomerization is an active, enzymatically mediated process or a spontaneous phenomenon, or both. Adding to the complexity of this is recent evidence that 13-cis RA exerts some biological effects without first converting to tRA. Isotretinoin and its metabolites, such as 4-oxo-isotretinoin and 4-hydroxy-isotretinoin, appear to directly alter sebaceous gland function in acne patients.13,18,19 It remains unclear if these effects are mediated by retinoid receptors or a different pathway.
Our study was limited by the small number of acne patients. Furthermore, it is likely that our attempt to classify patients strictly as responders or non-responders represents another limiting factor. Although numerous clinical scales for grading acne severity exist (e.g., the Leeds acne grading system), these are subjective. Objective or quantitative methods of evaluation, analogous to Breslow depth for melanoma, are still lacking for acne. In this study, we classified subjects as responders or non-responders based on patient history and retrospective chart review. However, in clinical practice response to therapy often depends on the subjective perception of acne severity as judged by individual physicians and patients. Strict criteria for determining which patients necessitate a second course of 13-cis RA do not exist.
Overall, a great deal of progress has been made in investigating the mechanisms of isotretinoin in acne, but comparatively much less is known about what governs therapeutic response to this drug. This translational study is one of the first attempting to link biochemical factors (i.e., CYP26 activity) to 13-cis RA response. Of note, oral 13-cis RA has a number of serious side effects, such as teratogenicity, increased liver enzymes, hypercholesterolemia, and hypertriglyceridemia, as well as less severe effects, such as mucocutaneous dryness and alopecia.20 Additionally, 13-cis RA has been linked to suicidal ideation, although prospective, randomized data which clearly substantiate this remain elusive. Therefore, anticipating which acne patients may require more than one course of 13-cis RA for therapeutic response could help practitioners consider other treatment options. In this retrospective study, we attempted to determine whether CYP26 induction could be used as a predictive factor for this purpose. Our data suggest that future studies correlating basal (pre-therapy) CYP26 expression to 13-cis RA response in acne patients, as well as studies comparing CYP26 expression in normal versus acne patients, may be more informative in this regard. Clearly, further research is necessary to determine the mechanisms that are involved in treatment response to isotretinoin.
ACKNOWLEDGEMENTS
The authors thank Elizabeth A. Duell, PhD, for performing the enzymatic activity assays; Suzan Rehbine, LPN, for assistance in procuring tissue samples; Craig Hammerberg, PhD, for preparation of tRA; Thy Thy Do, MD, for assistance with data collection; and Laura VanGoor, BFA, for graphic illustrations.
Funding sources: This study was supported by grants 5T32 AR007197 (F.W.) and 1K24 AR02159-01 (S.K.) from the National Institutes of Health, Bethesda, Md.; and the Babcock Research Endowment, University of Michigan, Ann Arbor.
ABBREVIATIONS
- CYP26
Retinoic acid 4-hydroxylase
- tRA
All-trans retinoic acid
- 13-cis RA
13-cis retinoic acid (isotretinoin)
- RAR
Retinoic acid receptor
- RXR
Retinoid X receptor
- RARE
Retinoic acid response element
Footnotes
Conflicts of interest: The authors have no conflict of interest to declare. All listed authors have seen and approved of the manuscript and will sign off on nay subsequent manuscript revisions.
REFERENCES
- 1.Fisher GJ, Voorhees JJ. Molecular mechanisms of retinoid actions in skin. Faseb J. 1996;10:1002–13. doi: 10.1096/fasebj.10.9.8801161. [DOI] [PubMed] [Google Scholar]
- 2.Marikar Y, Wang Z, Duell EA, Petkovich M, Voorhees JJ, Fisher GJ. Retinoic acid receptors regulate expression of retinoic acid 4-hydroxylase that specifically inactivates all-trans retinoic acid in human keratinocyte HaCaT cells. J Invest Dermatol. 1998;111:434–9. doi: 10.1046/j.1523-1747.1998.00297.x. [DOI] [PubMed] [Google Scholar]
- 3.Tsukada M, Schroder M, Roos TC, Chandraratna RA, Reichert U, Merk HF, et al. 13-cis retinoic acid exerts its specific activity on human sebocytes through selective intracellular isomerization to all-trans retinoic acid and binding to retinoid acid receptors. J Invest Dermatol. 2000;115:321–7. doi: 10.1046/j.1523-1747.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 4.Petkovich PM. Retinoic acid metabolism. J Am Acad Dermatol. 2001;45:S136–42. doi: 10.1067/mjd.2001.113715. [DOI] [PubMed] [Google Scholar]
- 5.Duell EA, Kang S, Voorhees JJ. Retinoic acid isomers applied to human skin in vivo each induce a 4-hydroxylase that inactivates only trans retinoic acid. J Invest Dermatol. 1996;106:316–20. doi: 10.1111/1523-1747.ep12342972. [DOI] [PubMed] [Google Scholar]
- 6.Lehucher-Ceyrac D, Weber-Buisset MJ. Isotretinoin and acne in practice: a prospective analysis of 188 cases over 9 years. Dermatology. 1993;186:123–8. doi: 10.1159/000247322. [DOI] [PubMed] [Google Scholar]
- 7.Layton AM, Cunliffe WJ. Guidelines for optimal use of isotretinoin in acne. J Am Acad Dermatol. 1992;27:S2–7. doi: 10.1016/s0190-9622(08)80252-6. [DOI] [PubMed] [Google Scholar]
- 8.Leyden JJ. Oral isotretinoin. How can we treat difficult acne patients? Dermatology. 1997;195(Suppl 1):29–33. doi: 10.1159/000246017. discussion 8-40. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ, Perera L, Coulter SJ, Mohrenweiser HW, Jetten A, Goldstein JA. The discovery of new coding alleles of human CYP26A1 that are potentially defective in the metabolism of all-trans retinoic acid and their assessment in a recombinant cDNA expression system. Pharmacogenet Genomics. 2007;17:169–80. doi: 10.1097/FPC.0b013e32801152d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duell EA, Astrom A, Griffiths CE, Chambon P, Voorhees JJ. Human skin levels of retinoic acid and cytochrome P-450-derived 4-hydroxyretinoic acid after topical application of retinoic acid in vivo compared to concentrations required to stimulate retinoic acid receptor-mediated transcription in vitro. J Clin Invest. 1992;90:1269–74. doi: 10.1172/JCI115990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691–9. doi: 10.1016/s0002-9440(10)62479-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson T, Vahlquist A, Kedishvili N, Torma H. 13-cis-retinoic acid competitively inhibits 3 alpha-hydroxysteroid oxidation by retinol dehydrogenase RoDH-4: a mechanism for its anti-androgenic effects in sebaceous glands? Biochem Biophys Res Commun. 2003;303:273–8. doi: 10.1016/s0006-291x(03)00332-2. [DOI] [PubMed] [Google Scholar]
- 13.Nelson AM, Gilliland KL, Cong Z, Thiboutot DM. 13-cis Retinoic acid induces apoptosis and cell cycle arrest in human SEB-1 sebocytes. J Invest Dermatol. 2006;126:2178–89. doi: 10.1038/sj.jid.5700289. [DOI] [PubMed] [Google Scholar]
- 14.Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Neutrophil gelatinase-associated lipocalin mediates 13-cis retinoic acid-induced apoptosis of human sebaceous gland cells. J Clin Invest. 2008;118:1468–78. doi: 10.1172/JCI33869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wozel G, Chang A, Zultak M, Czarnetzki BM, Happle R, Barth J, et al. The effect of topical retinoids on the leukotriene-B4-induced migration of polymorphonuclear leukocytes into human skin. Arch Dermatol Res. 1991;283:158–61. doi: 10.1007/BF00372055. [DOI] [PubMed] [Google Scholar]
- 16.Heise R, Mey J, Neis MM, Marquardt Y, Joussen S, Ott H, et al. Skin retinoid concentrations are modulated by CYP26AI expression restricted to basal keratinocytes in normal human skin and differentiated 3D skin models. J Invest Dermatol. 2006;126:2473–80. doi: 10.1038/sj.jid.5700432. [DOI] [PubMed] [Google Scholar]
- 17.Ross AC. Retinoid production and catabolism: role of diet in regulating retinol esterification and retinoic Acid oxidation. J Nutr. 2003;133:291S–6S. doi: 10.1093/jn/133.1.291S. [DOI] [PubMed] [Google Scholar]
- 18.Zouboulis CC. Isotretinoin revisited: pluripotent effects on human sebaceous gland cells. J Invest Dermatol. 2006;126:2154–6. doi: 10.1038/sj.jid.5700418. [DOI] [PubMed] [Google Scholar]
- 19.Baron JM, Heise R, Blaner WS, Neis M, Joussen S, Dreuw A, et al. Retinoic acid and its 4-oxo metabolites are functionally active in human skin cells in vitro. J Invest Dermatol. 2005;125:143–53. doi: 10.1111/j.0022-202X.2005.23791.x. [DOI] [PubMed] [Google Scholar]
- 20.Ellis CN, Krach KJ. Uses and complications of isotretinoin therapy. J Am Acad Dermatol. 2001;45:S150–7. doi: 10.1067/mjd.2001.113717. [DOI] [PubMed] [Google Scholar]