Abstract
The evaluation of signaling pathways leading to gene induction by VEGF-A and IL-1 in endothelial cells supports the importance of the NF-κB pathway for the IL-1-induced gene repertoire, whereas VEGF-A is a strong and preferential trigger of signals via PLC-γ. This leads (i) via Ca++ to the activation of calcincurin and NFAT and (ii) via PKC and the MEK/ERK MAPK pathway to the upregulation of EGR-1. Part of the VEGF-triggered gene induction depends on a cooperation of the transcription factors NFAT and EGR-1. Gene activation via PLC-γ provides VEGF with the potency to induce a wide spectrum of genes including many also upregulated by IL-1. A gene upregulated by VEGF and IL-I is the DSCR-1 gene, which encodes an inhibitor of calcincurin. DSCR1 is induced by NFAT or NF-κB and limits Ca++ signaling in a negative feed-back loop. Similarly, NAB2, a corepressor of EGR-1, is induced by EGR-1 and limits EGR-1 effects. Adenoviral overexpression of DSCRI or NAB2 inhibited part of VEGF-induced gene expression and reduced sprouting in angiogenesis models.
1. Introduction
The endothelium-specific growth factors important for vasculogenesis and angiogenesis include five members of the VEGF family and four members of the angiopoietin family [23]. They exert their specific activities through binding to three forms of VEGF receptors and two forms of Tie receptors. VEGF-A signaling mainly via VEGFR-2 (VEGF receptor-2) mediates the major growth and differentiation activities for vascular endothelial and progenitor cells and is the primary factor initiating physiological sprouting angiogenesis [4]. VEGF-C, based on its ability to bind to the lymphatic-specific VEGFR-3, is important for the formation of the lymphatic system [1]. PlGF (placenta growth factor) and VEGF-B, which bind to VEGFR-1, and VEGF-D binding to VEGFR-2 and -3 are the remaining isoforms of the VEGF family. They appear to have more subtile roles in adult vascular remodeling, coronary vascularization or the lymphatic system, respectively. Ang-1 by binding to the Tie-2 receptor and potentially co-activating Tie-1 [19] has a role in vascular remodeling events, probably optimizing the manner in which endothelial cells integrate with supporting cells. Ang-2 on the other hand appears to antagonize Ang-1 action by blocking its binding to Tie-2 [5,23].
The VEGF and Tie receptors are transmembrane proteins with intrinsic tyrosine kinase activity in their cytoplasmic domains. It is still largely unclear to what extent the multitude of different tyrosines in the cytoplasmic tails (the VEGFR-2 contains 19 tyrosine residues) are differentially phosphorylated, bind different SH-2 domain-containing proteins and thus lead to the activation of receptor-specific intracellular signaling and gene induction pattern. It seems, that in addition to differences in the temporal and spatial expression of the various receptors, a receptor-specific signaling pattern is responsible for the differential effects of the various growth factors and receptors which govern the differentiation, growth, tube formation and maturation aspects involved in new vessel formation.
Endothelial cells express further a number of receptors found on many different cell types, which can either synergize or interfere with signals of the endothclial-specific receptors. Among those are the bFGF receptor and members of the PDGF and EGF receptor families [2,24]. There is further considerable evidence that receptors for inflammatory cytokines play important roles and participate in the cross-regulation of inflammation and angiogenesis in many pathologies [10,18]. Whereas the main angiogenic factors as well as PDGF and EGF mediate their effects via tyrosine kinase receptors, binding of inflammatory cytokines to their receptors generates signals by recruiting cytoplasmic adaptor molecules which link to the IκB-kinase complex. The IL-1 receptor recruits first the adaptor protein MyD88 to the cytoplasmic pait of the IL-1 receptor [16] and MyD88 bridges via IRAK and TRAF6 to the protein kinases TAK-1 and NIK and these activate the 1κB-kinase complex. When IKK-2 (IκB kinase-2) in the complex is activated it phosphorylates IκB leading to ubiquitinylation and proteasomal degradation of IκB which releases the NFκB subunits for nuclear transfer [6].
In an attempt to define the specificities in signaling and gene induction of the different receptors present on endothelial cells, which contribute to the angiogenic and inflammatory response, we have so far investigated in detail the signal transduction pathways initiating at VEGFR-2 and compared them to the signals of the EGFR and the IL-1 receptor. This was done by directly studying induced signaling pathways and how they interact to trigger the induction of selected genes. Initially, we have used the tissue factor (TF) gene as a model of a gene induced by VEGF and IL-1. Then we have investigated by microarray analyses the complete gene repertoires induced by the same receptors and to what extent they overlap or lead to the induction of distinct genes. Furthermore, we have tested whether the adenoviral overexpression of natural feed-back inhibitors detected in our screens, such as DSCR-1 and NAB2, could be used to inhibit VEGF-triggered gene expression and angiogenesis models.
2. Tissue factor as a model for an IL-1 and VEGF-regulated gene
To determine specificities of VEGF-mediated signaling and gene induction we have investigated the downstream signaling of VEGFR-2 and the gene repertoire induced in comparison to another, non-endothelial specific growth factor receptor, the EGFR, and the inflammatory cytokines TNF-α and IL-1 [11–13]. Our initial data had shown that EGR-1 is an important transcription factor for VEGF-mediated gene induction in endothelial cells [11]. We have then analyzed signals at the MAP kinase level originating from the VEGFR-2 and leading to EGR-1. The obtained results showed that in endothelial cells VEGF-A mainly activates ERK1/2 and p38, but only trace amounts of JNK. The upregulation of EGR-1 by VEGF could be completely blocked by a specific MEK inhibitor, but not by a p38 inhibitor, demonstrating the upregulation of EGR-1 via the MEK/ERK MAP-kinase cascade [13]. Furthermore, EGR-1 upregulation was blocked by a PKC inhibitor suggesting that PKC was involved in VEGF-mediated MEK/ERK and EGR-1 induction. This is in contrast to EGF-induced EGR-1, which cannot be inhibited by PKC inhibitors.
The TF gene promoter was used in these studies as we have shown that TF is an example of a gene/protein upregulated not only by inflammatory cytokines [15], but also by VEGF-A in endothelial cells [11]. Therefore TF seems to be a valid model to delineate principal signaling pathways leading to gene activation during the angiogenic and inflammatory activation of endothelial cells. Whereas we had previously shown that NFκB activation is essential for TF gene induction by TNF-α [22] and a specific p65/c-Rel heterodimer regulates the TF promoter [15], we found NFκB not activated by VEGF-A [11,13]. However, the obtained data displayed that VFGF-A is a strong and preferential trigger of signals via PLC-γ, PKC and Ca++ On the one hand PKC-α and PKC-ε are activated and lead to the induction of the MFK/ERK MAP-kinase pathway and consecutively to the upregulation of EGR-1. The Ca++ signals, on the other hand, activate calcineurin and the transcription factor NFAT (see Fig. 1). NFAT cooperatively with EGR-1 activates the TF gene in endothelial cells [25]. NFAT has been previously shown to bind to a site closely overlapping with the NFκB binding site in the TF promoter [3]. Based on these data it appeared that full transcriptional response of the TF and potentially other genes in response to VEGF requires EGR-1 and NFAT.
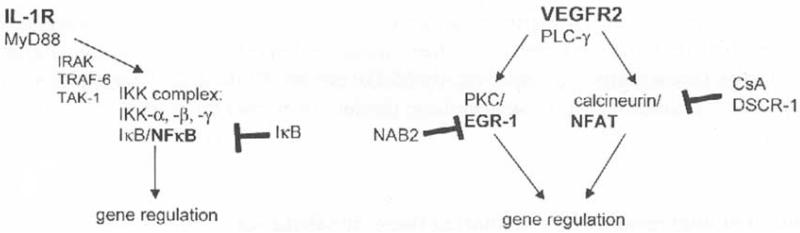
Fig. 1.
Signal tranduction by VEGF-A/VEGFR2 in comparison to IL-1/IL-1R. Major characteristic pathways activated by VEGF-A/VEGFR2 and the IL-1 receptor in endothelial cells arc shown. VHGF-A couples via Tyr1175 to PLC-γ [20,21] activating the Ca++/calcineurin and PKC/MAPK pathways. This leads to the induction of the transcription factors NFAT and EGR-L which are important for part of the VEGF-induced gene repertoire and the angiogenic/proliferative response [8,11,13,14,25]. The IL-1 receptor induces genes to a large extent via NFκB [17,22]. A significant fraction of the NF-κB-induced genes appears to be also upregulated by VEGF-A-induced NFAT VEGF-A does not trigger NF-κR activation to a significant extent [13]. Adenoviral overexpression of natural feed-back inhibitors inhibits gene induction via these pathways, e.g. IκB interferes with NFκB, NAB2 with EGR-1 and DSCR1 with calcincurin/NFAT activation. Alternatively, calcineurin/NFAT induction can be blocked by cyclosporin A (CsA).
3. VEGF-regulated gene repertoire in comparison to EGF and IL1
In the next step we have investigated this more closely by Affymetrix microarray analysis of the genes induced by VEGF-A in endothelial cells in comparison to the genes induced by EGF and IL-1 (B. Schweighofer and E. Hofer, in preparation). The obtained data show that VEGF-A induces a large spectrum of more than 100 genes. It is a characteristic feature of the VEGF-A-induced gene repenoire that about 40% of these genes are also upregulated by IL-1. In contrast, this fraction of genes cannot be upregulated by EGF. It seems that gene activation via PLC-γ, PKC and calcineurin provides VEGF-A with the competence to regulate many genes in common with IL-1. The EGFR, which can not induce the same genes, does not trigger PLC-γ and NFAT in endothelial cells, but rather induces the MAPK pathway solely via Ras [25]. Based on these data it is possible that VEGFR-2 signaling and gene regulation includes an inflammatory component, which at least in part is caused by the upregulation of a group of genes with NFAT and NF-κB binding sites in their promoters, IL-1 uses NF-κB and VEGF NFAT for their upregulation. Furthermore, about 10% of the genes are specifically regulated by VFGF and not by EGF or IL-1 and we propose that these might be important for the strong angiogenic properties of the factor. One example of a specifically VEGF and not EGF or IL-1 regulated gene is HLX1, a diverged human homeobox gene, which we have chosen for further analysis (J. Schultes and E. Hofer, in preparation).
4. Inhibition of angiogenesis by modulating these signals/genes
Another aspect of our work deals with the identification of natural feed-back inhibitors of endothelial cell activation, which could be used to inhibit angiogenic gene induction. As a matter of fact the groups of Aird and Gerber [7,14] and our laboratory (B. Schweighofer and E. Hofer, in preparation) find that among the genes most strongly upregulated by VEGF is the DSCR1 gene, which encodes an inhibitor of calcineurin. It is induced by NFAT likely to shut down calcineurin and consecutive NFAT activation in a negative feed-back loop. Another example is NAB2, a specific corepressor of EGR-1, which is induced by EGR-1 and limits EGR-1 effects [8]. As suggested by the cooperative gene regulation observed for EGR-1 and NFAT, adenoviral overexpression of DSCR1 and NAB2 inhibited VEGF-induced expression of genes with potential importance for angiogenesis reflected in reduced sprouting and tubule formation in angiogenesis models [8,14].
In contrast to the positive effects on proliferation and angiogenesis of VEGF-A induced transient expression of EGR-1, sustained adenoviral overexpression of EGR-1 itself led to preferential strong induction of negative feed-back mechanisms including upregulation of transcriptional inhibitors such as NAB2 as well as anti-angiogenic, anti-proliferative and pro-apoptotic genes. EGR-1 expressing adenoviruses applied to endothelial cells inhibited not only angiogenesis in cellular and animal models, but furthermore inhibited tumor growth in a murine fibrosarcoma model [9]. This may serve as an example, that the modulation of natural inhibitive feed-back pathways could be exploited for inhibition of angiogenesis.
5. Conclusions
Whereas NF-κB is the essential transcription factor in the inflammatory response mediated by IL-1, EGR-1 and NFAT are important transcription factors in VFGF-A/VEGFR2-mediatcd gene regulation. Both factors seem to cooperatively activate transcription of part of the VEGF-induced gene repertoire. EGR-1 is upregulated by VEGF via PLC-γ, PKCα/ε and the MEK/ERK MAP kinase pathway, NFAT is activated via PLC-γ, Ca++ and calcineurin. In contrast to VEGFR-2, some tyrosine kinase receptors, such as the EGFR, can not activate NFAT. VEGF via VEGFR-2 is competent to induce a large gene repertoire, 40% of which overlaps with IL1 induced genes. It appears that at least part of the genes induced by VEGF in common with IL-1 are regulated by VEGF via NFAT and by IL-1 via NF-κB. This property of VEGF to induce a large spectrum of genes including genes also regulated by inflammatory mediators and a smaller number of VEGF-specific genes could be important for the strong angiogenic properties of the factor. Modulation of natural inhibitory feed-back loops leading via EGR-1 to NAB2 and via calcineurin/NFAT to DSCR1 by adenoviral overexpression of the respective inhibitors leads to inhibition of in vitro and in vivo angiogenesis models.
Acknowledgements
We are grateful to our colleagues of the Department of Vascular Biology for important help and discussions. The work of the authors was supported by grants of the Austrian Science bund (NFN-S94-3) and the European Commission (LSHC-CT-2005-518178).
References
- [1].Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- [2].Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) bur not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer Res. 2006;66:2173–2180. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- [3].Armesilla AL, Lorenzo E, Gomez del Arco P, Martinez-Martinez S, Alfranca A, Redondo JM. Vascular endothelial growth factor activates nuclear factor of activated T cells in human endothelial cells: a role for tissue factor gene expression. Mol. Cell. Biol. 1999;19:2032–2043. doi: 10.1128/mcb.19.3.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- [5].Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, Gale NW, Witzenrath M, Rosseau S, Suttorp N, Sobke A, Herrmann M, Preissner KT, Vajkoczy P, Augustin HG. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat. Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- [6].Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- [7].Hesser BA, Liang XH, Camenisch G, Yang S, Lewin DA, Scheller R, Ferrara N, Gerber HP. Down syndrome critical region protein 1 (DSCR1), a novel VEGF target gene that regulates expression of inflammatory maikers on activated endothelial cells. Blood. 2004;104:149–158. doi: 10.1182/blood-2004-01-0273. [DOI] [PubMed] [Google Scholar]
- [8].Lucerna M, Mechtcheriakova D, Kadl A, Schabbauer G, Schafer R, Gruber F, Koshelnick Y, Muller HD, Issbrucker K, Clauss M, Binder BR, Hofer E. NAB2, a corepressor of EGR-1, inhibits vascular endothelial growth factor-mediated gene induction and angiogenic responses of endothelial cells. J. Biol. Chem. 2003;278:11433–11440. doi: 10.1074/jbc.M204937200. [DOI] [PubMed] [Google Scholar]
- [9].Lucerna M, Pomyje J, Mechtcheriakova D, Kadl A, Gruber F, Bilban M, Sobanov Y, Schabbauer G, Breuss J, Wagner O, Bischoff M, Clauss M, Binder BR, Hofer E. Sustained expression of early growth response protein-1 blocks angiogenesis and tumor growth. Cancer Res. 2006;66:6708–6713. doi: 10.1158/0008-5472.CAN-05-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Majno G. Chronic inflammation: links with angiogenesis and wound healing. Am. J. Pathol. 1998;153:1035–1039. doi: 10.1016/S0002-9440(10)65648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mechtcheriakova D, Wlachos A, Holzmuller H, Binder BR, Hofer E. Vascular endothelial cell growth factor-induced tissue factor expression in endothelial cells is mediated by EGR-1. Blood. 1999;93:3811–3823. [PubMed] [Google Scholar]
- [12].Mechtcheriakova D, Clauss M, Hofer E. Specificity, diversity and convergence in angiogenic and inflammatory signaling in endothelial cells. In: Catravas JD, Callow AD, Ryan US, Simionescu M, editors. Vascular Endothelium: Source and Target of Inflammatory mediators. IOS Press; Amsterdam: 2001. pp. 211–226. [Google Scholar]
- [13].Mechtcheriakova D, Schabbauer G, Lucerna M, Clauss M, De Martin R, Binder BR, Hofer E. Specificity, diversity, and convergence in VEGF and TNF-alpha signaling events leading to tissue factor up-regulation via EGR-1 in endothelial cells. Faseb J. 2001;15:230–242. doi: 10.1096/fj.00-0247com. [DOI] [PubMed] [Google Scholar]
- [14].Minami T, Horiuchi K, Miura M, Abid MR, Takabe W, Noguchi N, Kohro T, Ge X, Aburatani H, Hamakubo T, Kodama T, Aird WC. Vascular endothelial growth factor- and thrombin-induced termination factor, Down syndrome critical region-1, attenuates endothelial cell proliferation and angiogenesis. J. Biol. Chem. 2004;279:50537–50554. doi: 10.1074/jbc.M406454200. [DOI] [PubMed] [Google Scholar]
- [15].Moll T, Czyz M, Holzmuller H, Hofer-Warbinek R, Wagner E, Winkler H, Bach FH, Hofer E. Regulation of the tissue Factor promoter in endothelial cells. Binding of NF kappa B-, AP-1-, and Spl-like transcription factors. J. Biol. Chem. 1995;270:3849–3857. doi: 10.1074/jbc.270.8.3849. [DOI] [PubMed] [Google Scholar]
- [16].O’Neill LA. Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr. Top. Microbiol. Immunol. 2002;270:47–61. [PubMed] [Google Scholar]
- [17].Oitzinger W, Hofer-Warbinek R, Schmid JA, Koshelnick Y, Binder BR, de Martin R. Adenovirus-mediated expression of a mutant IkappaB kinase 2 inhibits the response of endothelial cells to inflammatory stimuli. Blood. 2001;97:1611–1617. doi: 10.1182/blood.v97.6.1611. [DOI] [PubMed] [Google Scholar]
- [18].Rajashekhar G, Willuweit A, Patterson CE, Sun P, Hilbig A, Breier G, Helisch A, Clauss M. Continuous endothelial cell activation increases angiogenesis: evidence for the direct role of endothelium linking angiogenesis and inflammation. J. Vase. Res. 2006;43:193–204. doi: 10.1159/000090949. [DOI] [PubMed] [Google Scholar]
- [19].Saharinen P, Kerkela K, Ekman N, Marron M, Brindle N, Lee GM, Augustin H, Koh GY, Alitalo K. Multiple angiopoietin recombinant proteins activate the Tiel receptor tyrosine kinase and promote its interaction with Tie2. J. Cell. Biol. 2005;169:239–243. doi: 10.1083/jcb.200411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sakurai Y, Ohgimoto K, Kataoka Y, Yoshida N, Shibuya M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. USA. 2005;102:1076–1081. doi: 10.1073/pnas.0404984102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. Embo J. 2001;20:2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wrighton CJ, Hofer-Warbinek R, Moll T, Eytner R, Bach FH, de Martin R. Inhibition of endothelial cell activation by adenovirus-mediated expression of I kappa B alpha, an inhibitor of the transcription factor NF-kappa B. J. Exp. Med. 1996;183:1013–1022. doi: 10.1084/jem.183.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specille growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- [24].Zeller PJ, Skalak TC, Ponce AM, Price RJ. In vivo chemotactic properties and spatial expression of PDGF in developing mesenteric microvascular networks. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2116–H2125. doi: 10.1152/ajpheart.2001.280.5.H2116. [DOI] [PubMed] [Google Scholar]
- [25].Schabbauer G, Schweighofer B, Mechtcheriakova D, Lucerna M, Binder BR, Hofer E. Thromb. Haemost. 2007 doi: 10.1160/th07-01-0037. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]