Abstract
Ginseng occupies a prominent position in the list of best-selling natural products in the world. Compared to the long history of use and widespread research on Asian ginseng, the study of American ginseng is relatively limited. In the past decade, some promising advances have been achieved in understanding the chemistry, pharmacology and structure-function relationship of American ginseng. To date, there is no systematic review of American ginseng. In this review, we present the different structures of the ginsenosides in American ginseng, including naturally occurring compounds and those resulting from steaming or biotransformation. Preclinical and clinical studies published in the past decade will also be discussed. We highlight the chemical and pharmacological diversity and potential structural-activity relationship of ginsenosides. Our hope is that this article is a useful reference to chemists and biologists researching American ginseng, and will open the door to novel agents in drug discovery.
Keywords: American ginseng, Panax quinquefolius, Asian ginseng, ginsenosides, diversity, structural-activity relationship
1. Introduction
Ginseng root has been used for thousands of years in the traditional medical system in oriental countries (Ang-Lee et al., 2001; Attele et al., 1999; Wang and Yuan, 2008). It occupies a prominent position on the list of the best-selling medicinal plants in the world (Yun, 2001). Asian ginseng (Panax ginseng C. A. Meer) and American ginseng (Panax quinquefolius L.) are the two most recognized ginseng botanicals around the world (Ang-Lee et al., 2001; Jia and Zhao, 2009). Compared to the long history of use and the copious amounts of research on Asian ginseng (Ang-Lee et al., 2001; Yun, 2001), the study of American ginseng and its constituents is much less extensive (Yuan et al., 2004).
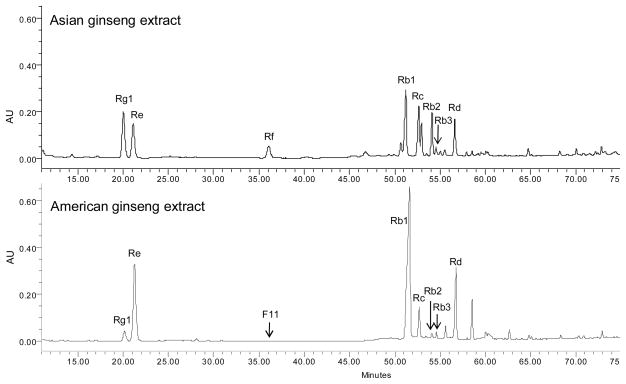
Table 1 and Fig. 1 show the chemical differences of American ginseng and Asian ginseng. As one of the best selling herbs in the U.S., American ginseng is grown in the eastern temperate forest areas of North America, from southern Quebec, Minnesota, and Wisconsin in the north, to Oklahoma, the Ozark Plateau, and Georgia in the south (Assinewe et al., 2003). With the widespread popularity of herbal medicines in the West, the past few decades have witnessed some promising advances in research on American ginseng and its constituents (Li et al., 2010a; Sengupta et al., 2004; Wang and Yuan, 2008). The triterpenoid saponins, called ginseng saponins or ginsenosides, are the major active constituents in American ginseng (Attele et al., 1999; Jia and Zhao, 2009). As shown in Fig. 1 and Table 1, however, American ginseng has a ginsenoside profile different from that of Asian ginseng in terms of total ginsenosides, the ratio of protopanaxadiol (PPD) to protopanaxatriol (PPT), and other marker ginsenosides. In addition, although low Rg1/high Re was reported in most populations of American ginseng, the high Rg1/low Re chemotype was also observed (Schlag and McIntosh, 2006).
Table 1.
Comparison of typical ginsenoside composition of American ginseng (Panax quinquefolius L.) and Asian ginseng (Panax ginseng C. A. Meer).
| Chemical composition | American ginseng | Asian ginseng |
|---|---|---|
| Total ginsenosides | 40–60 gram/kilogram | 20–40 gram/kilogram |
| Major ginsenosides | Rb1, Re, Rd | Rb1, Rg1, Rb2 |
| Pseudoginsenoside F11 | 1.0–2.0 gram/kilogram | 0 |
| Ginsenoside Rf | 0 | 1.0–2.0 gram/kilogram |
| PPD-group to PPT-group | > 2.0 | < 2.0 |
| Rb1: Rg1 | > 5.0 | < 5 .0 |
| Rg1: Rea | < 1.0 | > 1.0 |
| Rb2: Rc | < 0.4 | > 0.4 |
Limited American ginseng population with another phytotype was not involved in this table (Schlag and McIntosh, 2006).
Fig. 1.
Typical chromatograms of Asian ginseng and American ginseng extract by HPLC-UV. Pseudoginsenoside F11 has the same molecular weight and retention times similar to those of ginsenoside Rf. It cannot be detected by UV because there are no chromospheres. Chromatographic and analytical conditions were shown in Sun et al. (2011).
Like Asian ginseng, American ginseng has been reported to have a wide range of pharmacological effects, including effects on the central nervous system, cardiovascular system, endocrine system, immune system and cancer (Court, 2000; Jin et al., 2010; Li et al., 2010a; Yuan and Dey, 2001). The pharmacological activities of American ginseng may be different from those of Asian ginseng (Sievenpiper et al., 2004a). Some structure-activity relationship (SAR) comparisons of homologs have generated interesting evidence supporting the role of specific structural components as requisites in ginsenosides for their activities (Kang et al., 2006; Li et al., 2009c; Popovich and Kitts, 2002; Qi et al., 2010b; Wang et al., 2007c). However, to date, there has been no systematic summary of the chemistry, pharmacology, and structure-function relationships of American ginseng.
In this review, we present the different structures of ginsenosides in American ginseng, including naturally occurring compounds and those resulting from steaming or biotransformation. Preclinical and clinical studies of American ginseng and ginsenosides from the past decade also will be discussed and compared with those of Asian ginseng. We highlight the chemical and pharmacological diversity of ginsenosides and their structure-function relationships. Furthermore, we will attempt to offer perspectives and predict future research trends for this herb.
2. Structural diversity
2.1. Ginsenosides isolated from American ginseng
Ginsenosides share a dammarane-type triterpenoid saponin structure (Fuzzati, 2004). Most ginsenosides belong to a family of steroids with a four trans-ring rigid steroid skeleton (Attele et al., 1999; Wang et al., 2005). More than 60 ginsenosides have been isolated from different parts of Panax quinquefolius (referred to as Panax quinquefolium in some publications), such as the roots, leaves, stems, flower buds and berries (Christensen, 2009; Jia and Zhao, 2009; Jiang et al., 2008; Nakamura et al., 2007; Qu et al., 2009; Yoshikawa et al., 1998). As chemical purification and structural identification techniques are developed, novel ginsenosides continue to be reported (Chen et al., 2009a; Jia et al., 2008; Li et al., 2009a; Nakamura et al., 2007). Differences in sugar types, quantities and attachment positions provide diversity in ginsenoside structures (Fuzzati, 2004; Jia and Zhao, 2009). The changeable C-20 side chain and stereoisomerism further enrich the structural diversity of ginsenosides (Christensen, 2009; Nakamura et al., 2007).
As shown in Fig. 2, ginsenosides isolated from P. quinquefolius can be divided into several groups. PPD and PPT are the two main groups of ginsenosides (Qu et al., 2009). In the PPD group, sugar residues are attached to the β-OH at C-3 and/or C-20. PPD compounds include compounds 1–17 (Chen et al., 2009a; Jiang et al., 2008; Li et al., 2009a; Nakamura et al., 2007; Wang et al., 2001; Yoshikawa et al., 1998). Four malonyl derivatives (18–21, also called “acidic” ginsenosides) have been characterized (Du et al., 2004). In the PPT group, sugar moieties are attached to the α-OH at carbon-6 and/or β-OH at C-20. PPT constituents include compounds 22–32 (Jia et al., 2008; Nakamura et al., 2007; Yoshikawa et al., 1998).
Fig. 2.
Ginsenosides characterized from American ginseng. PPD, protopanaxadiol; PPT, protopanaxatriol; G, ginsenoside; Q, quinquenoside; F, floralquinquenoside; NG, notoginsenoside; QF, quinquefoloside.
Minor ginsenosides isolated from P. quinquefolius include ocotillol-type (compounds 33–36), oleanane-type ginsenosides (compounds 37–38), and dammarane saponins with a modified steroid skeleton (compounds 39–41) (Nakamura et al., 2007; Yoshikawa et al., 1998). Other isolated compounds can be classified as modified C-20 side chain ginsenosides (compounds 42–65). According to C-20 side chain differences, these compounds are subdivided into nine groups (Jiang et al., 2008; Nakamura et al., 2007; Qiu et al., 2009).
2.2. Ginsenosides characterized from steamed American ginseng
The steaming or heating process changed the ginsenoside profile of ginseng products (Chang and Ng, 2009; Wang et al., 2007a). During the steaming process, the notable structural changes are the elimination of sugar moieties and subsequent dehydration at C-20 in ginsenosides (Lau et al., 2004; Ren and Chen, 1999; Wang et al., 2007a). After steaming, the number of original polar ginsenosides decreased, and less polar ginsenosides increased in number correspondingly (Ren and Chen, 1999; Wang et al., 2007a; Wang et al., 2006). The important constituents in steamed American ginseng include several groups of epimers or geometric isomers, namely ginsenosides 20(S)/20(R)-Rg2 (25/66), 20(S)/20(R)-Rh1 (67/68), 20(S)/20(R)-Rg3 (7/69), Rk3 (70) and Rh4 (71), Rk1 (72) and Rg5 (73), accounting for over 90% of total ginsenoside content (Ren and Chen, 1999). Compounds 70–73 belong to the C-20 dehydration ginsenoside group. The 20(S) and 20(R)-ginsenoside compounds represent typical stereoisomers formed by the selective attack of the hydroxyl group after elimination of the glycosyl residue at C-20. Rk1/Rg5 and Rk3/Rh4 represent positional isomers of the double bond at C-20/21 or C-20/22 (Kang et al., 2006).
Under intense conditions, Rg3 may be further transformed to 20(S)-Rh2 (74) and 20(R)-Rh2 (75), and subsequently become the aglycone 20(S)-PPD (76)/20(R)-PPD (77) or even 20-dehydr-PPD (78/79) through chemical degradation. Rk1 and Rg5 may be again transformed to their degradation products like Rk2 (80) and Rh3 (81) (Shin et al., 2006b). Rh1 can be changed to the aglycone 20(S)-PPT (82)/20(R)- PPT (83) or even 20-dehydr-PPT (84/85).
2.3. Ginsenosides identified after biotransformation
As a dietary supplement, American ginseng is usually taken orally. Therefore, the metabolism of ginsenosides has been investigated (Cui et al., 1997). Many experiments tested the degradation of ginsenosides using microbes, enzymes and intestinal bacteria, or animals and humans (Hasegawa, 2004; Tawab et al., 2003). A number of novel ginsenoside structures have been identified after biotransformation. PPD groups like Rb1 and Rd are metabolized to IH-901 (86) (also known as compound K or M1) (Cho et al., 2009; Lee et al., 2000). Rg3 and Rg5, the principal components in steamed American ginseng, are transformed to Rh2 and Rh3, respectively (Shin et al., 2006a). PPT groups like Rg1 and Re are mainly converted to Rh1, F1, and the aglycone PPT (Hasegawa, 2004; Tawab et al., 2003).
As can be seen in Fig. 2, using 20(S)-PPT as the substrate, four new metabolites (87–90) resulted from microbial biotransformation via the fungus Mucor spinosus (Tian et al., 2005). Biotransformation of 20(S)-PPD by the fungus M. spinosus yielded eight metabolites (91–98) (Li et al., 2009b). The results suggest that M. spinosus selectively catalyzed the specific C-12 dehydrogenation of ginsenosides and could catalyze hydroxylation at different positions. Additionally, Mycobacterium sp. selectively catalyzed the specific C-3 dehydrogenation of ginsenosides (Wang et al., 1997). These regiospecific hydroxylation reactions may be important to increase bioactivity (Li et al., 2009b; Tian et al., 2005). Absidia coerulea selectively transformed the PPD group rather than the PPT group of ginsenosides, and produced a series of C-20 side-chain-modified metabolites (Chen et al., 2007).
3. Most studied pharmacological activities
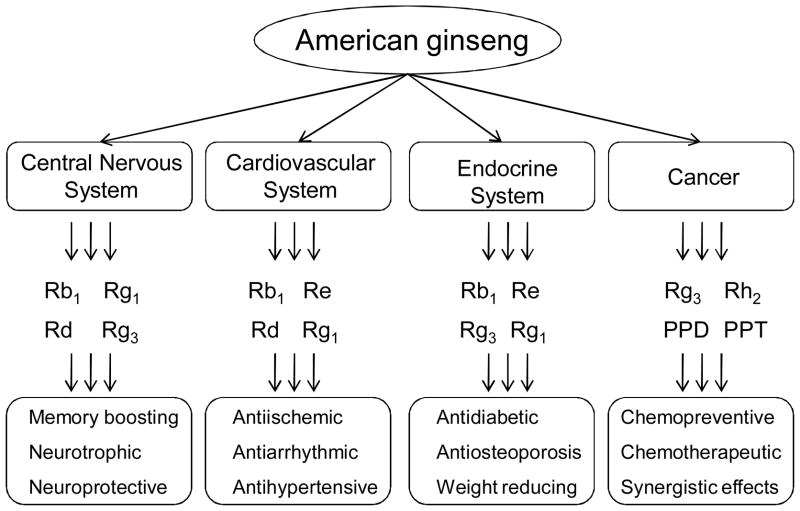
Like Asian ginseng, American ginseng has multiple pharmacological actions. In general, antioxidant, anti-inflammatory, and immunostimulatory activities seem to be related to the possible mechanisms of ginseng. In this section, we report the recent preclinical and clinical advancements in the study of American ginseng and the effects of its ginsenosides on the central nervous system, cardiovascular system, and antidiabetic and anticancer activities (Fig. 3). American ginseng’s immunomodulatory effects and prophylactic effect on acute respiratory illness are not discussed here (McElhaney et al., 2004; Predy et al., 2006; Vohra et al., 2008).
Fig. 3.
Biological and pharmacological activities of American ginseng and ginsenosides.
3.1. Effects on the central nervous system
Ginseng exerted beneficial effects on aging, central nervous system (CNS) disorders, and neurodegenerative diseases (Christensen et al., 2009). Ginsenosides played a major role in these effects (Rausch et al., 2006). The protective effects of ginsenosides Rb1 (Chen et al., 2008d; Yuan et al., 2007), Rg1 (Liu et al., 2010), Rg3 (Tian and Fu, 2006), Rd (Ye et al., 2009), and Re (Chen et al., 2008c) on neurodegeneration have been well studied in animals and in neuronal cell cultures.
American ginseng and ginsenosides enhanced cognitive performance and mood (Bao et al., 2005; Wang et al., 2009b; Zhang et al., 2008; Zhao and Li, 2004). Long-term ginsenoside administration to mice prevented memory loss or impairment (Zhao et al., 2009a; Zhao et al., 2009b). Corsi block and calmness were enhanced after administration of American ginseng to healthy young adults (Scholey et al., 2010).
Ginseng and ginsenosides can rescue neuronal cells by increasing cell survival, extending neurite growth, and rescuing neurons from death either in vivo or in vitro (Radad et al., 2004b; Radad et al., 2006; Rausch et al., 2006). The beneficial effect of ginseng and ginsenosides were shown on neurodegenerative disease models of Parkinson’s and Alzheimer’s diseases (Xu et al., 2005; Xu et al., 2009). Possible mechanisms were inhibition of uptake of MPTP and its active metabolite MPP+ in dopaminergic neurons (Van Kampen et al., 2003), suppression of oxidative stress (Chen et al., 2003), attenuation of MPP+-induced apoptosis (Xu et al., 2005), potentiation of nerve growth factor (Radad et al., 2004a), and activation of the insulin-like growth factor-I receptor signaling pathway (Xu et al., 2009).
Ginsenosides regulated various types of ion channels by interacting with ligand-binding sites or channel pore sites in neuronal and heterologously expressed cells (Nah, et al., 2007). They inhibited voltage-dependent Ca2+, K+, and Na+ channel activities in a stereospecific manner (Liu et al., 2010). Ginsenosides also inhibited ligand-gated ion channels such as N-methyl-D-aspartate, some subtypes of nicotinic acetylcholine, and 5-hydroxytryptamine type 3 receptors (Chen et al., 2010). Ginsenosides also modulated neurotransmission in the brain (Liu et al., 2010; Xue et al., 2006).
When the effects of Rg1 and Rb1 were compared (Joo et al., 2005), both enhanced CNS activities, but there were some differences in pharmacology and mechanism (Cheng et al., 2005b; Liao et al., 2002). For example, Ginsenoside Rb1 promoted neurotransmitter release by a cAMP-dependent protein kinase pathway (Xue et al., 2006); Rg1 produced this effect through a protein kinase II-dependent signaling pathway (Liu et al., 2010). Compared with Rg1, Rb1’s effects were weaker and in some cases even produced an inhibitory effect on the CNS (Chen et al., 2008a). The neuroprotective differences between American and Asian ginseng have not been reported. Since American ginseng has a lower ratio of Rg1/Rb1, it seems to calm the CNS. In contrast, Asian ginseng appears to stimulate the CNS.
3.2. Cardiovascular activities
In the U.S. American ginseng is a popular herbal supplement for patients suffering from cardiovascular disease (Xie et al., 2005a; Wang et al., 2007b). Several anti-ischemic, anti-arrhythmic and anti-hypertensive effects have been observed after the use of American ginseng (Wang et al., 2007b). The pharmacological effects may be produced by the antioxidant properties of the herb (Xie et al., 2005a). The antioxidant activities and the relationship between chemical structure and cardiovascular-protecting functions have been reviewed (Prior and Cao, 2000; Wang et al., 2007b). American ginseng extract had a stronger antioxidant activity than Asian ginseng root (Shao et al., 2004). American ginseng root or berry extract showed antioxidant and protective effects in cultured cardiomyocytes by up-regulating peroxide detoxifying mechanisms (Mehendale et al., 2006; Shao et al., 2004) and activating the Nrf2 pathway (Li et al., 2010b). Ginsenoside Re was one major antioxidant agent that protected cardiomyocytes by scavenging H2O2 and hydroxyl radicals (Xie et al., 2006).
In a randomized, double-blind, placebo-controlled trial of 16 hypertensive individuals given 3 g of American ginseng powder or placebo, American ginseng exerted a neutral acute effect on blood pressure (Stavro et al., 2005). In another clinical trial in 52 hypertensive individuals who took American ginseng 12 weeks, there was no effect on 24-hour blood pressure and renal function (Stavro et al., 2006). Taking American ginseng for 4 weeks (1.6 g/day) before subjects on a treadmill reduced the leakage of creatine kinase during exercise but did not enhance aerobic work capacity (Hsu et al., 2005).
3.3. Antidiabetic effects
Type 2 diabetes, accounting for over 90% of diabetic cases, is a syndrome with disordered metabolism of carbohydrates and lipids because of resistance to insulin action and impaired insulin secretion (Qi et al., 2010a). Both Asian ginseng and American ginseng root showed hypoglycemic effects in diabetic mice models (Attele et al., 2002; Dey et al., 2003; Xie et al., 2002). Using the ob/ob mouse model, we demonstrated that a 12-day treatment of American ginseng leaf and berry extracts decreased fasting blood glucose, improved glucose disposal, and reduced body weight (Attele et al., 2002; Xie et al., 2004a; Xie et al., 2004b). Heated American ginseng had stronger effects than unprocessed ginseng in inhibiting advanced accumulation of glycation endproducts in the diabetic rat kidney (Kim et al., 2007a).
Antidiabetic effects of ginsenosides have been demonstrated in animal models by Rb1 (Shang et al., 2007), Re (Xie et al., 2005b), transformed compounds such as Rb2 (Yokozawa et al., 1993), Rh2 (Lee et al., 2006), compound K (Yoon et al., 2007), and the aglycone 20(S)-PPT (Han et al. 2006). They decreased oxidative stress (Lin et al., 2005; Lin et al., 2008), activated peroxisome proliferator-activated receptor γ, increased GLUT expression, and enhanced PKA-dependent pathways (Park et al., 2008; Shang et al., 2008).
A series of randomized, placebo-controlled acute clinical studies were conducted to evaluate the efficacy of American ginseng in lowering postprandial glycemia in subjects with and without diabetes (Vuksan et al., 2000a; Vuksan et al., 2001a; Vuksan et al., 2000b; Vuksan et al., 2000c). American ginseng demonstrated a good acute safety profile. Escalation of dose and time of administration offered no added benefit in people with diabetes. A time, but not dose-dependent effect was observed in healthy individuals, suggesting that people without diabetes are sensitive to the time of ginseng administration. The effects of 1 g of ginseng extract on glycemic control were tested by a placebo-controlled, crossover trial in subjects with type 2 diabetics (Vuksan and Sievenpiper, 2005; Vuksan et al., 2001b). Fasting glucose and HbA1c were decreased in the extract group compared with the placebo group after 8 weeks. The trials were small, however, longer-term studies are needed.
Evidence indicates that the glycemia-lowering effect of ginseng root may be species dependent. In healthy humans, American ginseng lowered postprandial glycemia, red Asian had no effects, and Asian and wild American ginseng raised glycemia (Sievenpiper et al., 2004b). The part of American ginseng that produced the hypoglycemic effects remains unclear. Some clinical evidence suggested that the ratio of protopanaxadiols to protopanaxatriols is inversely correlated with the glycemia-lowering efficacy of ginseng root. American ginseng with a relatively high ratio has a better effect on acute postprandial glycemic indices in healthy humans than dose Asian ginseng (Sievenpiper et al., 2004b).
3.4. Cancer chemoprevention
Another pharmacological activity of American ginseng and its constituents is cancer chemoprevention and inhibition of tumor growth (Qi et al., 2010b; Wang et al., 2007a; Wang et al., 2009a). American ginseng extract enhanced the chemopreventive effect of 5-fluorouracil (Li et al., 2009d) in human colon cells, suppressed the chromosomal aberration induced by mitomycin C in mice (Pawar et al., 2007), improved cancer-related fatigue in clinic (Barton et al., 2010), and produced radioprotective potential in the lymphocytes of healthy individuals (Lee et al., 2008b; Lee et al., 2010).
Steamed American ginseng has more potent activity than white ginseng on human cancer cells (Wang et al., 2007a; Wang et al., 2006). Steamed ginseng berry extract inhibited colorectal cancer growth both in vitro and in vivo (Xie et al., 2009). Enhanced anticancer potential results from chemical degradation and conversion of the original saponins to new compounds during the steaming process (Wang et al., 2007a; Wang et al., 2009a). Because of higher total ginsenoside concentration, American ginseng had stronger anticancer potential than Asian ginseng (Sun et al., 2010).
The mechanism and cellular/molecular targets of American ginseng against cancer have been studied. Several molecular mechanisms exist and collectively converge on various signaling pathways. These pathways include the regulation of the cell cycle (Wang et al., 2007a), induction of apoptosis (Wang et al., 2006; Wang et al., 2009a), inhibition of angiogenesis (Sengupta et al., 2004; Yue et al., 2006), preventing invasion (Kim et al., 2007b), and reduction of inflammatory response (Jin et al., 2008; Jin et al., 2010). A series of cell cycle proteins, apoptosis-related proteins, growth factors, protein kinases and transcription factors are affected by American ginseng and ginsenosides (King and Murphy, 2010; Kim et al., 2007b; Lee et al., 2000; Peralta et al., 2009; Sengupta et al., 2004; Yue et al., 2006). For example, American ginseng extract can selectively inhibit the expression of the inducible nitric oxide synthase via suppression of signal transducer and activator of transcription cascade in inflamed macrophages (Ichikawa et al., 2009). A lyophilized aqueous extract of American ginseng inhibited induced cyclooxygenase-2 and NF-kappa B activation in breast cancer cells (Peralta et al., 2009). The anticancer effect of steamed American ginseng was enhanced by antioxidants or inhibitors of the NF-kappa B pathway (Li et al., 2010a).
Because tumor malignancy is a complex interaction among genes, cells, and tissues (Aggarwal et al., 2009), there are probably many unknowns in the anticancer mechanisms of ginseng. Because of complex chemical composition and difficulty in reproducibility, most studies focus on individual ginsenosides but not American ginseng extract. Therefore, more scientific clinical trials are needed to test the effects of American ginseng and steamed ginseng against cancer.
4. Potential structural-activity relationship
4.1. Positive relationship of sugar moieties in ginsenosides with antioxidant activity
Oxidative stress contributes to the development of a wide range of diseases: neurodegenerative disorders, cardiovascular diseases, diabetes, cancer, and chronic fatigue syndrome (Giustarini et al., 2009; Heistad et al., 2009). Ameliorating oxidative stress with antioxidants might be an effective strategy for treating various diseases (Giustarini et al., 2009). American ginseng extract exhibited antioxidant activity in lipid and aqueous mediums by both chelation of metal ions and scavenging of free radicals (Kitts et al., 2000). In clinical surveys American ginseng supplementation reduced oxidative stress markers in healthy volunteers (Lee et al., 2008a). Most of the beneficial effects of American ginseng and ginsenosides are partly attributed to their antioxidant and chelating abilities (Liu et al., 2003; Zhao et al., 2009b). The radical scavenging, chelation and oxidant activity of ginsenosides depends upon their sugar moieties and linkage positions, the types of aglycone, and total number of hydroxyl groups. In contrast to flavonoids, ginsenosides are more potent antioxidants than their corresponding glycosides, and sugar moieties are positively correlated to their activities.
Liu et al. (2003) observed that ginsenoside aglycones (i.e., protopanaxadiol and protopanaxatriol), ginsenosides Rg2, Rg3 and Rh2 were prooxidative; ginsenosides Rb1, Rc, R1, Rd, Re, Rb3, Rg1, and Rh1 functioned as antioxidants, and Rc protected human erythrocytes mostly against hemin-induced hemolysis (Li and Liu, 2008). A recent study evaluated the antioxidant activities of ginsenosides on the intracellular reactive oxygen species (ROS) and the radical scavenging activity by a 2′,7′-dichlorodihydrofluorescin diacetate (DCF-DA) method (Chae et al., 2010). Results showed that ginsenosides Rb2 and Rc effectively inhibited intracellular ROS better than ginsenosides Rb1, Rd, Re, Rf, Rg1, Rg2, Rg3, Rh1 and Rh2. The presence of arabinose linked at the glucopyranosyl group may have enhanced the antioxidant activity.
For identification of the active part or point of interaction, some sugar moieties were selected to test hydroxyl scavenging activity. Sugars showed no hydroxyl scavenging activity (Kang et al., 2007). Any sugar substituent that occupies free hydroxyl groups is capable of increasing hydrophilicity and altering access to lipid peroxyl and alkoxyl radicals in membranes (Heim et al., 2002). For antioxidant activity, there might be complicated interactions within one molecule of ginsenoside between sugar moieties and the triterpene dammarane.
4.2. Negative correlation of sugar molecules in ginsenosides to cancer chemoprevention
Sugar molecules within a ginsenoside impact tumor cells. The structure-function relationship of sugar molecules in ginsenosides to anticancer activity has been reviewed (Qi et al., 2010b). In general, anticancer activity is inversely correlated to the number of sugars, i.e., anticancer activities increase with the decrease of sugar number. Ginsenosides Rg3, Rh2, IH-901 or compound K, and ginsenoside aglycones (i.e., PPD and PPT) have remarkable effects on inhibition of various cancer cell growth (Musende et al., 2009). They induce apoptosis (Cheng et al., 2005a), perturb cell cycle events (Choi et al., 2009), prevent invasion and metastasis (Hasegawa et al., 2002), block angiogenesis (Chen et al., 2008b), reverse P-glycoprotein-mediated multidrug resistance (Kim et al., 2003), and produce synergistic effects with conventional chemotherapy agents (Yu et al., 2007). The presence of sugar moieties may reduce the hydrophobic character of the compounds and decrease their permeability to cell membranes. These properties are required to interact with specific membrane proteins or to pass into the nucleus (Ha et al. 2010; Li et al., 2009c).
Sugar linkage positions also affect anticancer activities. The anticancer activity of ginsenosides with a sugar substitute at C-6 is attenuated compared to activity of ginsenosides with sugar linkages at C-3 or C-20 (Li et al., 2009c; Popovich and Kitts, 2002). Any sugar moiety at C-6 may increase steric hindrance and block compounds from extracellular binding to their targets, thus significantly reducing the anticancer activities of ginsenosides (Chen et al., 2009b).
4.3. Stereoselectivity of 20(S) but not 20(R) of ginsenosides in bioactivities
20(S) and 20(R) are stereoisomers of each other that depend on the position of the C-20 hydroxyl in ginsenosides. The different stereochemistries of the 20(S)- and 20(R)-ginsenosides produce different pharmacological effects. 20(S)-Rg3 is more soluble in water than 20(R)-Rg3 (Kang et al., 2007). The hydroxyl radical scavenging activity of 20(S)-Rg3 is higher than that of 20(R)-Rg3 (Lee et al., 2008c). 20(S)-Rg3 is a more efficient regulator of voltage-dependent Ca2+, K+ or Na+ channels (Kang et al., 2005). Rg3 provided neuroprotection against ischemia-induced injury in rat brain by reducing lipid peroxides, scavenging free radicals and improving the energy metabolism (Tian and Fu, 2005). 20(S)-ginsenosides had stronger cytotoxicity effects than their 20(R)-stereoisomers (Popovich and Kitts, 2002; Qi et al., 2010b). In some exceptional examples, 20(R)-Rh2 had a stronger inhibitory effect on osteoclast formation than did 20(S)-Rh2 (Liu et al., 2009). 20(R)-Rg3 inhibited cancer cell invasion and metastasis through angiosuppression (Chen et al., 2008b; Yue et al., 2006).
20(S)-OH is geometrically close to the C-12 hydroxyl of ginsenosides; 20(R)-OH is far from the C-12 hydroxyl (Jeong et al., 2004). 20(S) ginsenosides tend to process the geometrical arrangement of the hydroxyl groups at carbon-12 and -20 (Jeong et al., 2004; Kang et al., 2006). The alkene chain connected to carbon-20 in 20(S)-ginsenosides has a stable, fixed orientation and is packed tightly near the terpenoid. The chain in 20(R)- ginsenosides protrudes further outside and has a flexible structure (Kang et al., 2005; Kang et al., 2007). 20(S)-ginsenosides are thus inaccessible to water molecules because of the alkene chain, which may stabilize hydrogen bonding between these hydroxyl groups and receptors (Kang et al., 2005; Kim et al., 2005). Compact packing around the chiral center of 20(S)-Rg3 aids in hydrophobic interactions with the hydrophobic pocket of the receptor (Kang et al., 2005; Kang et al., 2006). Therefore, the geometrical arrangement of hydroxyl groups at the chiral centers, inaccessibility to water, hydrophobic interactions, and compact structure may be the crucial factors accounting for the stereospecific action of 20(S) ginsenosides.
5. Summary and future perspectives
To date, correlation of various structures to specific pharmacological activity is somewhat limited. The structural heterogeneity of ginsenosides, their multiple mechanisms of action, and the diverse experimental methods used to evaluate their activities pose challenges in assembling a collective hierarchy of SAR. In this article we summarized some views regarding sugar moieties with antioxidant activity, sugar molecules with cancer chemoprevention, and stereoselectivity. SAR comparisons of homologs that differ in a single structural attribute are still needed to generate consistent lines of evidence to support the role of specific structural components as requisites in ginsenosides for their activities. A better understanding of the structure-activity relationships is required for helpful modifications to produce novel agents in medical oncology.
A total of 98 ginsenosides have been identified from American ginseng, including naturally occurring compounds and those resulting from steaming or biotransformation. With the development of chemical and analytical techniques and the characterization of novel compounds, the diversity of ginseng saponins is constantly revealed. The ginsenoside family can also be expanded through the characterization of novel compounds from a closely related genus like Oplopanax (Huang et al., 2010; Li et al., 2010c). Chemical modification further produces a series of novel compounds and expands the targets for the pharmacological activities of ginsenosides. Although many ginsenosides have been characterized from American ginseng, their potential effects have not been quantitatively compared under standard conditions.
Multiple pharmacological actions of American ginseng have been observed on the central nervous, cardiovascular, endocrine, and immune systems. Their neuroprotective, cardioprotective, antidiabetic, antioxidant and anticancer properties have been reviewed above. Reports of the effectiveness of ginseng are sometimes contradictory, perhaps because the chemical content of ginseng root or root extract differs, depending on the method of extraction, subsequent handling, or even the season of its collection. The high variability in ginsenoside composition of ginseng among different species and batches may contribute to equally high variability in efficacy (Vuksan and Sievenpiper, 2005). For example, five batches representative of Ontario-grown American ginseng root produced comparable reductions of postprandial glycemia in healthy individuals; yet 40% of batches may not exert antihyperglycemic activity (Dascalu et al., 2007; Sievenpiper et al., 2004a). American ginseng with a similar profile could have similar efficacy. Unmeasured components such as different peptidoglycans (quinquefolans for American ginseng), various ginsenans, peptides, polysaccharides, fatty acids, and other organic compounds may be active.
Obviously, we must know more to answer the questions about the observed effects of ginseng in complementary and alternative medicine. In the future, widespread interest in American ginseng seems certain to ensure continued research with this herb. With the trend of interdisciplinary research and the development of modern combinatorial techniques, the possibility of gaining novel agents from ginseng seems promising.
Acknowledgments
We thank Sally Kozlik for editing the manuscript. This work was supported in part by NIH/NCCAM grants AT003441, AT004418 and AT005362.
References
- Aggarwal BB, Van Kuiken ME, Iyer LH, Harikumar KB, Sung B. Molecular Targets of Nutraceuticals Derived from Dietary Spices: Potential Role in Suppression of Inflammation and Tumorigenesis. Exp Biol Med. 2009;234:825–849. doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. JAMA. 2001;286:208–216. doi: 10.1001/jama.286.2.208. [DOI] [PubMed] [Google Scholar]
- Assinewe VA, Baum BR, Gagnon D, Arnason JT. Phytochemistry of wild populations of Panax quinquefolius L. (North American ginseng) J Agr Food Chem. 2003;51:4549–4553. doi: 10.1021/jf030042h. [DOI] [PubMed] [Google Scholar]
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- Bao HY, Zhang J, Yeo SJ, Myung CS, Kim HM, Kim JM, Park JH, Cho J, Kang JS. Memory enhancing and neuroprotective effects of selected ginsenosides. Arch Pharm Res. 2005;28:335–342. doi: 10.1007/BF02977802. [DOI] [PubMed] [Google Scholar]
- Barton DL, Soori GS, Bauer BA, Sloan JA, Johnson PA, Figueras C, Duane S, Mattar B, Liu HS, Atherton PJ, Christensen B, Loprinzi CL. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Care Cancer. 2010;18:179–187. doi: 10.1007/s00520-009-0642-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae S, Kang KA, Youn U, Park JS, Hyun JW. A Comparative Study of the Potential Antioxidant Activities of Ginsenosides. J Food Biochem. 2010;34:31–43. [Google Scholar]
- Chang YH, Ng PKW. Effects of extrusion process variables on extractable ginsenosides in wheat-ginseng extrudates. J Agr Food Chem. 2009;57:2356–2362. doi: 10.1021/jf8031827. [DOI] [PubMed] [Google Scholar]
- Chen CF, Chiou WF, Zhang JT. Comparison of the pharmacological effects of Panax ginseng and Panax quinquefolium. Acta Pharmacol Sin. 2008a;29:1103–1108. doi: 10.1111/j.1745-7254.2008.00868.x. [DOI] [PubMed] [Google Scholar]
- Chen GT, Yang M, Lu ZQ, Zhang JQ, Huang HL, Liang Y, Guan SH, Song Y, Wu LJ, Guo DA. Microbial transformation of 20(S)-protopanaxatriol-type saponins by Absidia coerulea. J Nat Prod. 2007;70:1203–1206. doi: 10.1021/np070053v. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhao R, Zeng YM, Meng H, Zuo WJ, Li X, Wang JH. Three new triterpenoid saponins from the leaves and stems of Panax quinquefolium. J Asian Nat Prod Res. 2009a;11:195–201. doi: 10.1080/10286020802682734. [DOI] [PubMed] [Google Scholar]
- Chen JX, Peng HM, Xi OY, He XY. Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma cells. Melanoma Res. 2008b;18:322–329. doi: 10.1097/CMR.0b013e32830b3536. [DOI] [PubMed] [Google Scholar]
- Chen LM, Zhou XM, Cao YL, Hu WX. Neuroprotection of ginsenoside Re in cerebral ischemia-reperfusion injury in rats. J Asian Nat Prod Res. 2008c;10:439–445. doi: 10.1080/10286020801892292. [DOI] [PubMed] [Google Scholar]
- Chen RJY, Chung TY, Li FY, Lin NH, Tzen JTC. Effect of sugar positions in ginsenosides and their inhibitory potency on Na+/K+-ATPase activity. Acta Pharmacol Sin. 2009b;30:61–69. doi: 10.1038/aps.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XC, Huang TW, Zhang J, Song JQ, Chen LM, Zhu YG. Involvement of calpain and p25 of CDK5 pathway in ginsenoside Rb1’s attenuation of beta-amyloid peptide(25–35)-induced tau hyperphosphorylation in cortical neurons. Brain Res. 2008d;1200:99–106. doi: 10.1016/j.brainres.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Chen XC, Zhu YG, Zhu LA, Huang C, Chen Y, Chen LM, Fang F, Zhou YC, Zhao CH. Ginsenoside Rg1 attenuates dopamine-induced apoptosis in PC12 cells by suppressing oxidative stress. Eur J Pharmacol. 2003;473:1–7. doi: 10.1016/s0014-2999(03)01945-9. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Lu TT, Yue XY, Wei N, Jiang YJ, Chen MG, Ni GZ, Liu XF, Xu GL. Neuroprotective effect of ginsenoside Rb1 on glutamate-induced neurotoxicity: With emphasis on autophagy. Neurosci Lett. 2010;482:264–268. doi: 10.1016/j.neulet.2010.07.052. [DOI] [PubMed] [Google Scholar]
- Cheng CC, Yang SM, Huang CY, Chen JC, Chang WM, Hsu SL. Molecular mechanisms of ginsenoside Rh2-mediated G1 growth arrest and apoptosis in human lung adenocarcinoma A549 cells. Cancer Chemother Pharmacol. 2005a;55:531–540. doi: 10.1007/s00280-004-0919-6. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Shen LH, Zhang JT. Anti-amnestic and anti-aging effects of ginsenoside Rg1 and Rb1 and its mechanism of action. Acta Pharmacol Sin. 2005b;26:143–149. doi: 10.1111/j.1745-7254.2005.00034.x. [DOI] [PubMed] [Google Scholar]
- Cho SH, Chung KS, Choi JH, Kim DH, Lee KT. Compound K, a metabolite of ginseng saponin, induces apoptosis via caspase-8-dependent pathway in HL-60 human leukemia cells. BMC Cancer. 2009;9:449. doi: 10.1186/1471-2407-9-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Kim TW, Singh SV. Ginsenoside Rh2-mediated G1 phase cell cycle arrest in human breast cancer cells is caused by p15 Ink4B and p27 Kip1-dependent Inhibition of cyclin-dependent kinases. Pharm Res. 2009;26:2280–2288. doi: 10.1007/s11095-009-9944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- Court WE. Ginseng: the history of an insignificant plant. Pharm Hist (Lond) 2000;30:38–44. [PubMed] [Google Scholar]
- Cui JF, Bjorkhem I, Eneroth P. Gas chromatographic-mass spectrometric determination of 20(S)-protopanaxadiol and 20(S)-protopanaxatriol for study on human urinary excretion of ginsenosides after ingestion of ginseng preparations. J Chromatogr B. 1997;689:349–355. doi: 10.1016/s0378-4347(96)00304-0. [DOI] [PubMed] [Google Scholar]
- Dascalu A, Sievenpiper JL, Jenkins AL, Stavro MP, Leiter LA, Arnason T, Vuksan V. Five batches representative of Ontario-grown American ginseng root produce comparable reductions of postprandial glycemia in healthy individuals. Can J Physiol Pharmacol. 2007;85:856–864. doi: 10.1139/Y07-030. [DOI] [PubMed] [Google Scholar]
- Dey L, Xie JT, Wang A, Wu J, Maleckar SA, Yuan CS. Anti-hyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003;10:600–605. doi: 10.1078/094471103322331908. [DOI] [PubMed] [Google Scholar]
- Du XW, Wills RBH, Stuart DL. Changes in neutral and malonyl ginsenosides in American ginseng (Panax quinquefolium) during drying, storage and ethanolic extraction. Food Chem. 2004;86:155–159. [Google Scholar]
- Fuzzati N. Analysis methods of ginsenosides. J Chromatogr B. 2004;812:119–133. doi: 10.1016/j.jchromb.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Giustarini D, Dalle-Donne I, Tsikas D, Rossi R. Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers. Crit Rev Cl Lab Sci. 2009;46:241–281. doi: 10.3109/10408360903142326. [DOI] [PubMed] [Google Scholar]
- Ha YW, Ahn KS, Lee JC, Kim SH, Chung BC, Choi MH. Validated quantification for selective cellular uptake of ginsenosides on MCF-7 human breast cancer cells by liquid chromatography-mass spectrometry. Anal Bioanal Chem. 2010;396:3017–3025. doi: 10.1007/s00216-010-3515-0. [DOI] [PubMed] [Google Scholar]
- Han KL, Jung MH, Sohn JH, Hwang JK. Ginsenoside 20(S)-protopanaxatriol (PPT) activates peroxisome proliferator-activated receptor gamma (PPAR gamma) in 3T3-L1 adipocytes. Biol Pharm Bull. 2006;29:110–113. doi: 10.1248/bpb.29.110. [DOI] [PubMed] [Google Scholar]
- Hasegawa H. Proof of the mysterious efficacy of ginseng: Basic and clinical trials: Metabolic activation of ginsenoside: Deglycosylation by intestinal bacteria and esterification with fatty acid. J Pharmacol Sci. 2004;95:153–157. doi: 10.1254/jphs.fmj04001x4. [DOI] [PubMed] [Google Scholar]
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Heistad DD, Wakisaka Y, Miller J, Chu Y, Pena-Silva R. Novel aspects of oxidative stress in cardiovascular diseases. Circ J. 2009;73:201–207. doi: 10.1253/circj.cj-08-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CC, Ho MC, Lin LC, Su B, Hsu MC. American ginseng supplementation attenuates creatine kinase level induced by submaximal exercise in human beings. World J Gastroenterol. 2005;11:5327–5331. doi: 10.3748/wjg.v11.i34.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WH, Zhang QW, Wang CZ, Yuan CS, Li SP. Isolation and identification of two new polyynes from a North American ethnic medicinal plant-Oplopanax horridus (Smith) Miq. Molecules. 2010;15:1089–1096. doi: 10.3390/molecules15021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa T, Li JQ, Nagarkatti P, Nagarkatti M, Hofseth LJ, Windust A, Cui TX. American ginseng preferentially suppresses STAT/iNOS signaling in activated macrophages. J Ethnopharmacol. 2009;125:145–150. doi: 10.1016/j.jep.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SM, Lee JH, Kim JH, Lee BH, Yoon IS, Kim DH, Rhim H, Kim Y, Nah SY. Stereospecificity of ginsenoside Rg3 action on ion channels. Mol Cells. 2004;18:383–389. [PubMed] [Google Scholar]
- Jia JM, Wang ZQ, Wu LJ. Two new acetylated ginsenosides from the roots of Panax quinquefolium. Chinese Chem Lett. 2008;19:1099–1102. [Google Scholar]
- Jia L, Zhao YQ. Current Evaluation of the Millennium Phytomedicine-Ginseng (I): Etymology, Pharmacognosy, Phytochemistry, Market and Regulations. Curr Med Chem. 2009;16:2475–2484. doi: 10.2174/092986709788682146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HP, Qiu YK, Cheng DR, Kang TG, Dou DQ. Structure elucidation and complete NMR spectral assignments of two new dammarane-type tetraglycosides from Panax quinquefolium. Magn Reson Chem. 2008;46:786–790. doi: 10.1002/mrc.2247. [DOI] [PubMed] [Google Scholar]
- Jin Y, Hofseth AB, Cui XL, Windust AJ, Poudyal D, Chumanevich AA, Matesic LE, Singh NP, Nagarkatti M, Nagarkatti PS, Hofseth LJ. American ginseng suppresses colitis through p53-mediated apoptosis of inflammatory cells. Cancer Prev Res. 2010;3:339–347. doi: 10.1158/1940-6207.CAPR-09-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Kotakadi VS, Ying L, Hofseth AB, Cui XL, Wood PA, Windust A, Matesic LE, Pena EA, Chiuzan C, Singh NP, Nagarkatti M, Nagarkatti PS, Wargovich MJ, Hofseth LJ. American ginseng suppresses inflammation and DNA damage associated with mouse colitis. Carcinogenesis. 2008;29:2351–2359. doi: 10.1093/carcin/bgn211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo SS, Won TJ, Lee DI. Reciprocal activity of Ginsenosides in the production of proinflarnmatory repertoire, and their potential roles in neuroprotection in vitro. Planta Med. 2005;71:476–481. doi: 10.1055/s-2005-864145. [DOI] [PubMed] [Google Scholar]
- Kang DI, Lee JY, Yang JY, Jeong SM, Lee JH, Nah SY, Kim Y. Evidence that the tertiary structure of 20(S)-ginsenoside Rg(3) with tight hydrophobic packing near the chiral center is important for Na+ channel regulation. Biochem Bioph Res Co. 2005;333:1194–1201. doi: 10.1016/j.bbrc.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Kang KS, Kim HY, Yamabe N, Yokozawa T. Stereospecificity in hydroxyl radical scavenging activities of four ginsenosides produced by heat processing. Bioorg Med Chem Lett. 2006;16:5028–5031. doi: 10.1016/j.bmcl.2006.07.071. [DOI] [PubMed] [Google Scholar]
- Kang KS, Yokozawa T, Yamabe N, Kim HY, Park JH. ESR study on the structure and hydroxyl radical-scavenging activity relationships of ginsenosides isolated from Panax ginseng C. A. MEYER. Biol Pharm Bull. 2007;30:917–921. doi: 10.1248/bpb.30.917. [DOI] [PubMed] [Google Scholar]
- Kim HY, Kang KS, Yamabe N, Nagai R, Yokozawa T. Protective effect of heat-processed American ginseng against diabetic renal damage in rats. J Agr Food Chem. 2007a;55:8491–8497. doi: 10.1021/jf071770y. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hong YH, Lee JH, Kim DH, Nam G, Jeong SM, Lee BH, Lee SM, Nah SY. A role for the carbohydrate portion of ginsenoside Rg(3) in Na+ channel inhibition. Mol Cells. 2005;19:137–142. [PubMed] [Google Scholar]
- Kim SW, Kwon H, Chi DW, Shim JH, Park JD, Lee YH, Pyo S, Rhee DK. Reversal of P-glycoprotein-mediated multidrug resistance by ginsenoside Rg(3) Biochem Pharmacol. 2003;65:75–82. doi: 10.1016/s0006-2952(02)01446-6. [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim DH, Han SJ, Hyun JW, Kim HS. Repression of matrix metalloproteinase gene expression by ginsenoside Rh2 in human astroglioma cells. Biochem Pharmacol. 2007b;74:1642–1651. doi: 10.1016/j.bcp.2007.08.015. [DOI] [PubMed] [Google Scholar]
- King ML, Murphy LL. Role of cyclin inhibitor protein p21 in the inhibition of HCT116 human colon cancer cell proliferation by American ginseng (Panax quinquefolius) and its constituents. Phytomedicine. 2010;17:261–268. doi: 10.1016/j.phymed.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- Lau AJ, Seo BH, Woo SO, Koh HL. High-performance liquid chromatographic method with quantitative comparisons of whole chromatograms of raw and steamed Panax notoginseng. J Chromatogr A. 2004;1057:141–149. doi: 10.1016/j.chroma.2004.09.069. [DOI] [PubMed] [Google Scholar]
- Lee LS, Wise SD, Chan C, Parsons TL, Flexner C, Lietman PS. Possible differential induction of phase 2 enzyme and antioxidant pathways by American ginseng, Panax quinquefolius. J Clin Pharmacol. 2008a;48:599–609. doi: 10.1177/0091270008314252. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Ko WG, Kim JH, Sung JH, Lee SJ, Moon CK, Lee BH. Induction of apoptosis by a novel intestinal metabolite of ginseng saponin via cytochrome c-mediated activation of caspase-3 protease. Biochem Pharmacol. 2000;60:677–685. doi: 10.1016/s0006-2952(00)00362-2. [DOI] [PubMed] [Google Scholar]
- Lee TK, O’Brien KF, Wang WD, Johnke RM, Sheng C, Benhabib SM, Wang T, Allison RR. Radioprotective effect of American ginseng on human lymphocytes at 90 minutes postirradiation: A study of 40 cases. J Altern Complement Med. 2010;16:561–567. doi: 10.1089/acm.2009.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Wang WD, O’Brien KF, Johnke RM, Wang T, Allison RR, Diaz AL. Effect of North American ginseng on cs-137-induced micronuclei in human lymphocytes: A comparison with WR-1065. Phytother Res. 2008b;22:1614–1622. doi: 10.1002/ptr.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WK, Kao ST, Liu IM, Cheng JT. Increase of insulin secretion by ginsenoside Rh2 to lower plasma glucose in wistar rats. Clin Exp Pharmacol Physiol. 2006;33:27–32. doi: 10.1111/j.1440-1681.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Kim HY, Kang KS, Lee JG, Yokozawa T, Park JH. The chemical and hydroxyl radical scavenging activity changes of ginsenoside-Rb-1 by heat processing. Bioorg Med Chem Lett. 2008c;18:4515–4520. doi: 10.1016/j.bmcl.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Li BH, Wang CZ, He TC, Yuan CS, Du W. Antioxidants potentiate American ginseng-induced killing of colorectal cancer cells. Cancer Lett. 2010a;289:62–70. doi: 10.1016/j.canlet.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GY, Zeng YM, Meng H, Li X, Wang JH. A new triterpenoid saponin from the leaves and stems of Panax quinquefolium L. Chinese Chem Lett. 2009a;20:1207–1210. doi: 10.1080/10286020802682734. [DOI] [PubMed] [Google Scholar]
- Li HF, Ye M, Guo HZ, Tian Y, Zhang J, Zhou JP, Hu YC, Guo DA. Biotransformation of 20(S)-protopanaxadiol by Mucor spinosus. Phytochemistry. 2009b;70:1416–1420. doi: 10.1016/j.phytochem.2009.07.041. [DOI] [PubMed] [Google Scholar]
- Li JQ, Ichikawa T, Jin Y, Hofseth LJ, Nagarkatti P, Nagarkatti M, Windust A, Cui TX. An essential role of Nrf2 in American ginseng-mediated anti-oxidative actions in cardiomyocytes. J Ethnopharmacol. 2010b;130:222–230. doi: 10.1016/j.jep.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Liu Y, Zhang JW, Ai CZ, Xiang N, Liu HX, Yang L. Anti-androgen-independent prostate cancer effects of ginsenoside metabolites In Vitro: Mechanism and possible structure-activity relationship investigation. Arch Pharm Res. 2009c;32:49–57. doi: 10.1007/s12272-009-1117-1. [DOI] [PubMed] [Google Scholar]
- Li XL, Sun S, Du GJ, Qi LW, Williams S, Wang CZ, Yuan CS. Effects of Oplopanax horridus on human colorectal cancer cells. Anticancer Res. 2010c;30:295–302. [PMC free article] [PubMed] [Google Scholar]
- Li XL, Wang CZ, Sun S, Mehendale SR, Du W, He TC, Yuan CS. American ginseng berry enhances chemopreventive effect of 5-FU on human colorectal cancer cells. Oncol Rep. 2009d;22:943–952. [PubMed] [Google Scholar]
- Liao B, Newmark H, Zhou R. Neuroprotective effects of ginseng total saponin and ginsenosides Rb1 and Rg1 on spinal cord neurons in vitro. Exp Neurol. 2002;173:224–234. doi: 10.1006/exnr.2001.7841. [DOI] [PubMed] [Google Scholar]
- Lin E, Wang Y, Mehendale S, Wang CZ, Xie JT, Aung H, Yuan CS. Antioxidant protection by American ginseng in pancreatic beta-cells. Faseb J. 2005;19:A97–A97. doi: 10.1142/S0192415X08006399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E, Wang Y, Mehendale S, Sun S, Wang CZ, Xie JT, Aung HH, Yuan CS. Antioxidant protection by American ginseng in pancreatic beta-cells. Am J Chinese Med. 2008;36:981–988. doi: 10.1142/S0192415X08006399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Shiono J, Shimizu K, Yu HS, Zhang CZ, Jin FX, Kondo R. 20(R)-Ginsenoside Rh2, not 20(S), is a selective osteoclastgenesis inhibitor without any cytotoxicity. Bioorg Med Chem Lett. 2009;19:3320–3323. doi: 10.1016/j.bmcl.2009.04.054. [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Zhao M, Zhang Y, Xue JF, Chen NH. Ginsenoside Rg1 promotes glutamate release via a calcium/calmodulin-dependent protein kinase II-dependent signaling pathway. Brain Res. 2010;1333:1–8. doi: 10.1016/j.brainres.2010.03.096. [DOI] [PubMed] [Google Scholar]
- Liu ZQ, Luo XY, Liu GZ, Chen YP, Wang ZC, Sun YX. In vitro study of the relationship between the structure of ginsenoside and its antioxidative or prooxidative activity in free radical induced hemolysis of human erythrocytes. J Agr Food Chem. 2003;51:2555–2558. doi: 10.1021/jf026228i. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Gravenstein S, Cole SK, Davidson E, O’Neill D, Petitjean S, Rumble B, Shan JJ. A placebo-controlled trial of a proprietary extract of North American ginseng (CVT-E002) to prevent acute respiratory illness in institutionalized older adults. J Am Geriatr Soc. 2004;52:13–19. doi: 10.1111/j.1532-5415.2004.52004.x. [DOI] [PubMed] [Google Scholar]
- Mehendale SR, Wang CZ, Shao ZH, Li CQ, Xie JT, Aung HH, Yuan CS. Chronic pretreatment with American ginseng berry and its polyphenolic constituents attenuate oxidant stress in cardiomyocytes. Eur J Pharmacol. 2006;553:209–214. doi: 10.1016/j.ejphar.2006.09.051. [DOI] [PubMed] [Google Scholar]
- Musende AG, Eberding A, Wood C, Adomat H, Fazli L, Hurtado-Coll A, Jia W, Bally MB, Guns ET. Pre-clinical evaluation of Rh2 in PC-3 human xenograft model for prostate cancer in vivo: formulation, pharmacokinetics, biodistribution and efficacy. Cancer Chemother Pharmacol. 2009;64:1085–1095. doi: 10.1007/s00280-009-0965-1. [DOI] [PubMed] [Google Scholar]
- Nah SY, Kim DH, Rhim H. Ginsenosides: Are any of them candidates for drugs acting on the central nervous system? Cns Drug Rev. 2007;13:381–404. doi: 10.1111/j.1527-3458.2007.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Sugimoto S, Matsuda H, Yoshikawa M. Medicinal flowers. XVII. New dammarane-type triterpene glycosides from flower buds of American ginseng, Panax quinquefolium L. Chem Pharm Bull. 2007;55:1342–1348. doi: 10.1248/cpb.55.1342. [DOI] [PubMed] [Google Scholar]
- Park S, Ahn IS, Kwon DY, Ko BS, Jun WK. Ginsenosides Rb1 and Rg1 suppress triglyceride accumulation in 3T3-L1 adipocytes and enhance beta-cell insulin secretion and viability in Min6 cells via PKA-dependent pathways. Biosci Biotechnol Biochem. 2008;72:2815–2823. doi: 10.1271/bbb.80205. [DOI] [PubMed] [Google Scholar]
- Pawar AA, Tripathi DN, Ramarao P, Jena G. Protective effects of American ginseng (Panax quinquefolium) against mitomycin C induced micronuclei in mice. Phytother Res. 2007;21:1221–1227. doi: 10.1002/ptr.2245. [DOI] [PubMed] [Google Scholar]
- Peralta EA, Murphy LL, Minnis J, Louis S, Dunnington GL. American ginseng inhibits induced COX-2 and NFKB activation in breast cancer cells. J Surg Res. 2009;157:261–267. doi: 10.1016/j.jss.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Popovich DG, Kitts DD. Structure-function relationship exists for ginsenosides in reducing cell proliferation and inducing apoptosis in the human leukemia (THP-1) cell line. Arch Biochem Biophys. 2002;406:1–8. doi: 10.1016/s0003-9861(02)00398-3. [DOI] [PubMed] [Google Scholar]
- Predy GN, Goel V, Lovlin RE, Basu TK. Immune modulating effects of daily supplementation of COLD-fX (a proprietary extract of north American ginseng) in healthy adults. J Clin Biochem Nutr. 2006;39:160–165. [Google Scholar]
- Prior RL, Cao G. Analysis of botanicals and dietary supplements for antioxidant capacity: a review. J AOAC Int. 2000;83:950–956. [PubMed] [Google Scholar]
- Qi LW, Liu EH, Chu C, Peng YB, Cai HX, Li P. Anti-Diabetic Agents from natural products-An update from 2004 to 2009. Curr Top Med Chem. 2010a;10:434–457. doi: 10.2174/156802610790980620. [DOI] [PubMed] [Google Scholar]
- Qi LW, Wang CZ, Yuan CS. American ginseng: Potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010b;80:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Qiu YK, Dou DQ, Cai LP, Jiang HP, Kang TG, Yang BY, Kuang HX, Li MZC. Dammarane-type saponins from Panax quinquefolium and their inhibition activity on human breast cancer MCF-7 cells. Fitoterapia. 2009;80:219–222. doi: 10.1016/j.fitote.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Qu CL, Bai YP, Jin XQ, Wang YT, Zhang K, You JY, Zhang HQ. Study on ginsenosides in different parts and ages of Panax quinquefolius L. Food Chem. 2009;115:340–346. [Google Scholar]
- Radad K, Gille G, Liu LL, Rausch WD. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 2006;100:175–186. doi: 10.1254/jphs.crj05010x. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Moldzio R, Saito H, Ishige K, Rausch WD. Ginsenosides Rb1 and Rg1 effects on survival and neurite growth of MPP+-affected mesencephalic dopaminergic cells. J Neural Transm. 2004a;111:37–45. doi: 10.1007/s00702-003-0063-1. [DOI] [PubMed] [Google Scholar]
- Radad K, Gille G, Moldzio R, Saito H, Rausch WD. Ginsenosides Rb-1 and Rg(1) effects on mesencephalic dopaminergic cells stressed with glutamate. Brain Res. 2004;1021:41–53. doi: 10.1016/j.brainres.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Rausch WD, Liu S, Gille G, Radad K. Neuroprotective effects of ginsenosides. Acta Neurobiol Exp. 2006;66:369–375. doi: 10.55782/ane-2006-1625. [DOI] [PubMed] [Google Scholar]
- Ren GX, Chen F. Degradation of ginsenosides in American ginseng (Panax quinquefolium) extracts during microwave and conventional heating. J Agr Food Chem. 1999;47:1501–1505. doi: 10.1021/jf980678m. [DOI] [PubMed] [Google Scholar]
- Schlag EM, McIntosh MS. Ginsenoside content and variation among and within American ginseng (Panax quinquefolius L.) populations. Phytochemistry. 2006;67:1510–1519. doi: 10.1016/j.phytochem.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Scholey A, Ossoukhova A, Owen L, Ibarra A, Pipingas A, He K, Roller M, Stough C. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: an acute, randomised, double-blind, placebo-controlled, crossover study. Psychopharmacology. 2010;212:345–356. doi: 10.1007/s00213-010-1964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, Yeung HW, Wong RNS, Sasisekharan R, Fan TPD. Modulating angiogenesis - The yin and the yang in ginseng. Circulation. 2004;110:1219–1225. doi: 10.1161/01.CIR.0000140676.88412.CF. [DOI] [PubMed] [Google Scholar]
- Shang W, Yang Y, Jiang B, Jin H, Zhou L, Liu S, Chen M. Ginsenoside Rb1 promotes adipogenesis in 3T3-L1 cells by enhancing PPARgamma2 and C/EBPalpha gene expression. Life Sci. 2007;80:618–625. doi: 10.1016/j.lfs.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Shang W, Yang Y, Zhou L, Jiang B, Jin H, Chen M. Ginsenoside Rb1 stimulates glucose uptake through insulin-like signaling pathway in 3T3-L1 adipocytes. J Endocrinol. 2008;198:561–569. doi: 10.1677/JOE-08-0104. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Xie JT, Vanden Hoek TL, Mehendale S, Aung H, Li CQ, Qin Y, Schumacker PT, Becker LB, Yuan CS. Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochim Biophys Acta. 2004;1670:165–171. doi: 10.1016/j.bbagen.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Shin YW, Bae EA, Han MJ, Kim DH. Metabolism of ginsenoside Rg5, a main constituent isolated from red ginseng, by human intestinal microflora and their antiallergic effect. J Microbiol Biotechn. 2006a;16:1791–1798. [Google Scholar]
- Shin YW, Bae EA, Kim DH. Inhibitory effect of ginsenoside Rg5 and its metabolite ginsenoside Rh3 in an oxazolone-induced mouse chronic dermatitis model. Arch Pharm Res. 2006b;29:685–690. doi: 10.1007/BF02968253. [DOI] [PubMed] [Google Scholar]
- Sievenpiper JL, Arnason JT, Leiter LA, Vuksan V. Decreasing, null and increasing effects of eight popular types of ginseng on acute postprandial glycemic indices in healthy humans: the role of ginsenosides. J Am Coll Nutr. 2004a;23:248–258. doi: 10.1080/07315724.2004.10719368. [DOI] [PubMed] [Google Scholar]
- Sievenpiper JL, Arnason JT, Vidgen E, Leiter LA, Vuksan V. A systematic quantitative analysis of the literature of the high variability in ginseng (Panax spp. ) Diabetes Care. 2004b;27:839–840. doi: 10.2337/diacare.27.3.839-a. [DOI] [PubMed] [Google Scholar]
- Stavro PM, Woo M, Heim TF, Leiter LA, Vuksan V. North American ginseng exerts a neutral effect on blood pressure in individuals with hypertension. Hypertension. 2005;46:406–411. doi: 10.1161/01.HYP.0000173424.77483.1e. [DOI] [PubMed] [Google Scholar]
- Stavro PM, Woo M, Leiter LA, Heim TF, Sievenpiper JL, Vuksan V. Long-term intake of North American ginseng has no effect on 24-hour blood pressure and renal function. Hypertension. 2006;47:791–796. doi: 10.1161/01.HYP.0000205150.43169.2c. [DOI] [PubMed] [Google Scholar]
- Sun S, Qi LW, Du GJ, Mehendale SR, Wang CZ, Yuan CS. Red notoginseng: higher ginsenoside content and stronger anticancer potential than Asian and American ginseng. Food Chem. 2011;125:1299–1305. doi: 10.1016/j.foodchem.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawab MA, Bahr U, Karas M, Wurglics M, Schubert-Zsilavecz M. Degradation of ginsenosides in humans after oral administration. Drug Metab Disp. 2003;31:1065–1071. doi: 10.1124/dmd.31.8.1065. [DOI] [PubMed] [Google Scholar]
- Tian J, Fu F. Neuroprotective effects of 20(S)-ginsenoside Rg3 on dopamine levels in the 6-hydroxydopamine lesioned mouse brain. Movement Disord. 2006;21:S76–S76. [Google Scholar]
- Tian Y, Guo H, Han J, Guo D. Microbial transformation of 20(S)-protopanaxatriol by Mucor spinosus. J Nat Prod. 2005;68:678–680. doi: 10.1021/np049688+. [DOI] [PubMed] [Google Scholar]
- Van Kampen J, Robertson H, Hagg T, Drobitch R. Neuroprotective actions of the ginseng extract G115 in two rodent models of Parkinson’s disease. Exp Neurol. 2003;184:521–529. doi: 10.1016/j.expneurol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Vohra S, Johnston BC, Laycock KL, Midodzi WK, Dhunnoo I, Harris E, Baydala L. Safety and tolerability of north American ginseng extract in the treatment of pediatric upper respiratory tract infection: A phase II randomized, controlled trial of 2 dosing schedules. Pediatrics. 2008;122:E402–E410. doi: 10.1542/peds.2007-2186. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Sievenpiper JL. Herbal remedies in the management of diabetes: Lessons learned from the study of ginseng. Nutr Metab Cardiovasc Dise. 2005;15:149–160. doi: 10.1016/j.numecd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000a;160:1009–1013. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Sievenpiper JL, Wong J, Xu Z, Beljan-Zdravkovic U, Arnason JT, Assinewe V, Stavro MP, Jenkins AL, Leiter LA, Francis T. American ginseng (Panax quinquefolius L.) attenuates postprandial glycemia in a time-dependent but not dose-dependent manner in healthy individuals. Am J Clin Nutr. 2001a;73:753–758. doi: 10.1093/ajcn/73.4.753. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Sievenpiper JL, Xu Z, Wong EYY, Jenkins AL, Beljan-Zdravkovic U, Leiter LA, Josse RG, Stavro MP. Konjac-mannan and American ginsing: Emerging alternative therapies for type 2 diabetes mellitus. J Am Coll Nutr. 2001b;20:370S–380S. doi: 10.1080/07315724.2001.10719170. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Stavro MP, Sievenpiper JL, Beljan-Zdravkovic U, Leiter LA, Josse RG, Xu Z. Similar postprandial glycemic reductions with escalation of dose and administration time of American ginseng in type 2 diabetes. Diabetes Care. 2000b;23:1221–1226. doi: 10.2337/diacare.23.9.1221. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Stavro MP, Sievenpiper JL, Koo VYY, Wong E, Beljan-Zdravkovic U, Francis T, Jenkins AL, Leiter LA, Josse RG, Xu Z. American ginseng improves glycemia in individuals with normal glucose tolerance: Effect of dose and time escalation. Journal of the American College of Nutrition. 2000c;19:738–744. doi: 10.1080/07315724.2000.10718073. [DOI] [PubMed] [Google Scholar]
- Wang AB, Wang CZ, Wu JA, Osinski J, Yuan CS. Determination of major ginsenosides in Panax quinquefolius (American ginseng) using, high-performance liquid chromatography. Phytochem Anal. 2005;16:272–277. doi: 10.1002/pca.838. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Aung HH, Ni M, Wu JA, Tong RB, Wicks S, He TC, Yuan CS. Red American ginseng: Ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007a;73:669–674. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Li XL, Wang QF, Mehendale SR, Fishbein AB, Han AH, Sun S, Yuan CS. The mitochondrial pathway is involved in American ginseng-induced apoptosis of SW-480 colon cancer cells. Oncol Rep. 2009a;21:577–584. doi: 10.3892/or_00000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YZ, Chen J, Chu SF, Wang YS, Wang XY, Chen NH, Zhang JT. Improvement of memory in mice and increase of hippocampal excitability in rats by ginsenoside Rg1’s metabolites ginsenoside Rh1 and protopanaxatriol. J Pharmacol Sci. 2009b;109:504–510. doi: 10.1254/jphs.08060fp. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: Active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007b;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Yuan CS. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Zhang B, Song WX, Wang AB, Ni M, Luo XJ, Aung HH, Xie JT, Tong R, He TC, Yuan CS. Steamed American ginseng berry: Ginsenoside analyses and anticancer activities. J Agr Food Chem. 2006;54:9936–9942. doi: 10.1021/jf062467k. [DOI] [PubMed] [Google Scholar]
- Wang JH, Li W, Sha Y, Tezuka Y, Kadota S, Li X. Triterpenoid saponins from leaves and stems of Panax quinquefolium L. J Asian Nat Prod Res. 2001;3:123–130. doi: 10.1080/10286020108041379. [DOI] [PubMed] [Google Scholar]
- Wang KC, Wang PH, Lee SS. Microbial transformation of protopanaxadiol and protopanaxatriol derivatives with Mycobacterium sp (NRRL B-3805) J Nat Prod. 1997;60:1236–1241. [Google Scholar]
- Wang W, Zhao YQ, Rayburn ER, Hill DL, Wang H, Zhang RW. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemoth Pharm. 2007c;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- Xie JT, McHendale S, Yuan CS. Ginseng and diabetes. Am J Chin Med. 2005a;33:397–404. doi: 10.1142/S0192415X05003004. [DOI] [PubMed] [Google Scholar]
- Xie JT, Mehendale SR, Li X, Quigg R, Wang X, Wang CZ, Wu JA, Aung HH, PAR, Bell GI, Yuan CS. Anti-diabetic effect of ginsenoside Re in ob/ob mice. Biochim Biophys Acta. 2005b;1740:319–325. doi: 10.1016/j.bbadis.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Xie JT, Mehendale SR, Wang A, Han AH, Wu JA, Osinski J, Yuan CS. American ginseng leaf: ginsenoside analysis and hypoglycemic activity. Pharmacol Res. 2004a;49:113–117. doi: 10.1016/j.phrs.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Xie JT, Shao ZH, Vanden Hoek TL, Chang WT, Li J, Mehendale S, Wang CZ, Hsu CW, Becker LB, Yin JJ, Yuan CS. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol. 2006;532:201–207. doi: 10.1016/j.ejphar.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Xie JT, Wang CZ, Zhang B, Mehendale SR, Li XL, Sun S, Han AH, Du W, He TC, Yuan CS. In vitro and in vivo anticancer effects of American ginseng berry: Exploring representative compounds. Biol Pharm Bull. 2009;32:1552–1558. doi: 10.1248/bpb.32.1552. [DOI] [PubMed] [Google Scholar]
- Xie JT, Wu JA, Mehendale S, Aung HH, Yuan CS. Anti-hyperglycemic effect of the polysaccharides fraction from American ginseng berry extract in ob/ob mice. Phytomedicine. 2004b;11:182–187. doi: 10.1078/0944-7113-00325. [DOI] [PubMed] [Google Scholar]
- Xie JT, Zhou YP, Dey L, Attele AS, Wu JA, Gu M, Polonsky KS, Yuan CS. Ginseng berry reduces blood glucose and body weight in db/db mice. Phytomedicine. 2002;9:254–258. doi: 10.1078/0944-7113-00106. [DOI] [PubMed] [Google Scholar]
- Xu BB, Liu CQ, Gao X, Zhang WQ, Wang SW, Cao YL. Possible mechanisms of the protection of ginsenoside Re against MPTP-induced apoptosis in substantia nigra neurons of Parkinson’s disease mouse model. J Asian Nat Prod Res. 2005;7:215–224. doi: 10.1080/10286020410001690172. [DOI] [PubMed] [Google Scholar]
- Xu L, Chen WF, Wong MS. Ginsenoside Rg1 protects dopaminergic neurons in a rat model of Parkinson’s disease through the IGF-I receptor signalling pathway. Br J Pharmacol. 2009;158:738–748. doi: 10.1111/j.1476-5381.2009.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue JF, Liu ZJ, Hu JF, Chen H, Zhang JT, Chen NH. Ginsenoside Rb1 promotes neurotransmitter release by modulating phosphorylation of synapsins through a cAMP-dependent protein kinase pathway. Brain Res. 2006;1106:91–98. doi: 10.1016/j.brainres.2006.05.106. [DOI] [PubMed] [Google Scholar]
- Ye RD, Li NL, Han JL, Kong XW, Cao R, Rao ZR, Zhao G. Neuroprotective effects of ginsenoside Rd against oxygen-glucose deprivation in cultured hippocampal neurons. Neurosci Res. 2009;64:306–310. doi: 10.1016/j.neures.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Yokozawa T, Yasui T, Oura H. Stimulation of rna-polymerase activity by ginsenoside-Rb2 in diabetic rats. Phytother Res. 1993;7:240–243. [Google Scholar]
- Yoshikawa M, Murakami T, Yashiro K, Yamahara J, Matsuda H, Saijoh R, Tanaka O. Bioactive saponins and glycosides. XI. Structures of new dammarane-type triterpene oligoglycosides, quinquenosides I, II, III, IV, and V, from American ginseng, the roots of Panax quinquefolium L. Chem Pharm Bull. 1998;46:647–654. doi: 10.1248/cpb.46.647. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Han EJ, Sung JH, Chung SH. Anti-diabetic effects of compound K versus metformin versus compound K-metformin combination therapy in diabetic db/db mice. Biol Pharm Bull. 2007;30:2196–2200. doi: 10.1248/bpb.30.2196. [DOI] [PubMed] [Google Scholar]
- Yu Y, Zhou O, Hang Y, Bu XX, Jia W. Antiestrogenic effect of 20S-protopanaxadiol and its synergy with tamoxifen on breast cancer cells. Cancer. 2007;109:2374–2382. doi: 10.1002/cncr.22659. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Dey L. Multiple effects of American ginseng in clinical medicine. Am J Chin Med. 2001;29:567–569. doi: 10.1142/S0192415X01000599. [DOI] [PubMed] [Google Scholar]
- Yuan CS, Wei G, Dey L, Karrison T, Nahlik L, Maleckar S, Kasza K, Ang-Lee M, Moss J. Brief communication: American ginseng reduces warfarin’s effect in healthy patients - A randomized, controlled trial. Ann Intern Med. 2004;141:23–27. doi: 10.7326/0003-4819-141-1-200407060-00011. [DOI] [PubMed] [Google Scholar]
- Yuan QL, Yang CX, Xu P, Gao XQ, Deng L, Chen P, Sun ZL, Chen QY. Neuroprotective effects of ginsenoside Rb1 on transient cerebral ischemia in rats. Brain Res. 2007;1167:1–12. doi: 10.1016/j.brainres.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Yue PYK, Wong DYL, Wu PK, Leung PY, Mak NK, Yeung HW, Liu L, Cai Z, Jiang ZH, Fan TPD, Wong RNS. The angiosuppressive effects of 20(R)- ginsenoside Rg(3) Biochem Pharmacol. 2006;72:437–445. doi: 10.1016/j.bcp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Yun TK. Panax ginseng--a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- Zhang GZ, Liu AL, Zhou YB, San X, Jin TW, Jin Y. Panax ginseng ginsenoside-Rg(2) protects memory impairment via anti-apoptosis in a rat model with vascular dementia. J Ethnopharmacol. 2008;115:441–448. doi: 10.1016/j.jep.2007.10.026. [DOI] [PubMed] [Google Scholar]
- Zhao HF, Li Q, Pei XR, Zhang ZF, Yang RY, Wang JB, Li Y. Long-term ginsenoside administration prevents memory impairment in aged C57BL/6J mice by up-regulating the synaptic plasticity-related proteins in hippocampus. Behav Brain Res. 2009a;201:311–317. doi: 10.1016/j.bbr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Zhao HF, Li Q, Zhang ZF, Pei XR, Wang JB, Li Y. Long-term ginsenoside consumption prevents memory loss in aged SAMP8 mice by decreasing oxidative stress and up-regulating the plasticity-related proteins in hippocampus. Brain Res. 2009b;1256:111–122. doi: 10.1016/j.brainres.2008.12.031. [DOI] [PubMed] [Google Scholar]
- Zhao WJ, Li PY. Ginsenoside Re improved memory impairment in aged rats and mice. Neurobiol Aging. 2004;25:S582–S583. [Google Scholar]