Abstract
Axon regeneration after experimental spinal cord injury (SCI) can be promoted by combinatorial treatments that increase the intrinsic growth capacity of the damaged neurons and reduce environmental factors that inhibit axon growth. A prior peripheral nerve conditioning lesion is a well established means of increasing the intrinsic growth state of sensory neurons whose axons project within the dorsal columns of the spinal cord. Combining such a prior peripheral nerve conditioning lesion with the infusion of antibodies that neutralize the growth-inhibitory effects of the NG2 chondroitin sulfate proteoglycan promotes sensory axon growth through the glial scar and into the white matter of the dorsal columns. The physiological properties of these regenerated axons, particularly in the chronic SCI phase, have not been established. Here we examined the functional status of regenerated sensory afferents in the dorsal columns after SCI. Six months post-injury, we located and electrically mapped functional sensory axons that had regenerated beyond the injury site. The regenerated axons had reduced conduction velocity, decreased frequency-following ability, and increasing latency to repetitive stimuli. Many of the axons that had regenerated into the dorsal columns rostral to the injury site were chronically demyelinated. These results demonstrate that regenerated sensory axons remain in a chronic pathophysiological state and emphasize the need to restore normal conduction properties to regenerated axons after spinal cord injury.
Keywords: spinal cord injury, demyelination, pathophysiology, NG2, regeneration, myelin, conduction velocity
Transected spinal axons can undergo significant elongation if environmental growth-inhibitory factors are neutralized and intrinsic growth is stimulated. Antibody-mediated blockade of growth-inhibitory molecules (Hata et al. 2006; Tan et al. 2006), enzymatic degradation of growth-inhibitory glycosaminoglycans (GAGs) (Moon et al. 2001; Bradbury et al. 2002), and pharmacological elevation of cAMP (Qiu et al. 2002; Nikulina et al. 2004) all promote axon regeneration after spinal cord injury, as does a peripheral nerve conditioning-lesion (Richardson and Issa,1984; Neumann and Woolf, 1999). Combining some of these treatments has resulted in more favorable axon growth patterns (Lu et al. 2004; Steinmetz et al. 2005; Tan et al. 2006), longer fibers (Chau et al. 2004; Yin et al. 2006), and in some cases, limited behavioral recovery (Pearse et al. 2004; Fouad et al. 2005).
While most studies of spinal cord injury emphasize axon regrowth and functional recovery, few have directly addressed the physiological properties of the regenerated axons (Pinzon et al. 2001; Bradbury et al. 2002). Traumatized or diseased axons exhibit pathophysiological conduction properties (Nashmi and Fehlings, 2001a). Six months after compression injury to the dorsal spinal cord, axons have reduced action potential conduction velocity, are unable to follow high-frequency stimuli, and have frequency-dependent delays in conduction latency. Single Aβ fibers in the dorsal roots also display an increased latency to repetitive stimuli after nerve damage (Shin et al. 1997). Such injury-induced changes to axon conduction can lead to a loss of sensation or hypersensitivity to stimuli and pain (Hains et al. 2005; Hains and Waxman, 2006; Waxman and Hains, 2006). Whether these conduction properties return to normal when damaged axons are stimulated to regenerate is currently unknown. This is an important question since the restoration of normal function after spinal cord injury requires not only that axons grow past the site of injury but also that the regenerated axons transmit information with fidelity (Blight 2002).
In experimental models of multiple sclerosis, where axons display reduced conduction velocities and conduction failures, transplants of Schwann and olfactory ensheathing cells (Honmou et al. 1996; Ibrahim et al. 2006; Sasaki et al. 2006), treatment with pharmacological agents that facilitate conduction such as 4-aminopyridine (Sherratt et al. 1980; Bostock et al. 1981), and genetic therapies that alter ion channel expression (Hains et al. 2005) can restore normal action potential propagation. Whether these strategies are necessary for axons induced to regenerate after spinal cord injury is unknown. Therefore it is important to characterize the conduction properties of regenerated axons in order to properly design and evaluate therapeutic strategies that may help restore their normal function.
Because the functional status of sensory axons induced to regenerate have not been directly examined after spinal cord injury, particularly in the chronic SCI phase, we investigated the location and electrophysiological properties of sensory fibers that had been induced to regenerate past a dorsal column injury. The results show that at 6-8 months post-injury, sensory afferents induced to regenerate exhibit pathophysiological conduction properties including reduced compound action potential amplitude, decreased frequency-following ability, and increasing latency to repetitive stimuli. A future challenge for spinal cord injury research is to devise therapeutic approaches that can restore normal functional properties to regrowing axons.
Materials and Methods
Spinal cord injury and treatment paradigm
All experiments conformed to the University guidelines on the ethical use of animals and were approved by the Institutional Animal Care and Use Committee. Adult Sprague Dawley rats (∼150-175g) were anesthetized with a mixture of ketamine and xylazine and subjected to a peripheral nerve conditioning-lesion 1 week prior to spinal injury and antibody application. The spinal cord injury model and antibodies used here have been described previously (Tan et al. 2006). Following a dorsal laminectomy at thoracic level 8, the dura was resected to expose the spinal cord. Using microscissors, the spinal cord was transected bilaterally with the lesion extending ventrally to the depth of the central canal (∼1.5mm). Experiments were performed 6-8 months post-SCI.
A group of uninjured (sham operated: laminectomy and tracer only) animals (n=4) was used to determine the spatial resolution of the stimulus-response maps (details below), for histology, and for action potential waveform comparisons. Experiments were performed 4 weeks post-surgery.
To evaluate the possibility of surgical sparing, another group of animals (n=2) received partial spinal cord transection surgery (lesion <1mm deep). Four days prior to terminal electrophysiological experiments (details below), all 3 groups of animals were re-anaesthetized and the left sciatic nerve was exposed. 2μl of a 1.5% solution of cholera toxin B subunit (CTB, List Biological Laboratories) was injected into the nerve using a hand-held Hamilton syringe equipped with a 32 gauge needle (Bradbury et al. 2002). The solution was injected over 1 minute and the needle withdrawn over an additional 2 minutes. The wound was closed by suturing the overlying muscle and skin. All were examined for the appearance of CTB-filled axons in the gracilis nucleus which would indicate spared fibers in the dorsal columns. We did not observe CTB-labeled axons in nucleus gracilis in any of the surgically injured animals that had received regeneration-inducing treatment unlike the sham and the partially transected animals. The compound action potential waveform evoked in control spared fibers was also compared to those observed in regenerated axons.
Electrophysiological recording
Terminal electrophysiology experiments were performed on all animal groups. They were anaesthetized with ketamine (73mg/kg) and xylazine (8.8mg/kg). The jugular vein and trachea were intubated to administer supplemental anesthesia and measure end-expired CO2, respectively. Core body temperature was monitored with a rectal thermometer and maintained at 35±1°C with a water-filled thermal blanket. The animal was then suspended by spinal clamps. A laminectomy was performed at spinal cord levels between T6-L4, which exposed the spinal cord from rostral to the injury site to L5.
In all rats, evoked compound action potentials were recorded from dorsal roots L4 or L5 using two sets of bipolar platinum electrodes. The distance between electrode pairs was 0.5-1cm. A glass stimulating microelectrode (10 MΩ) filled with NaCl (2M) was attached to a stereotaxic hydraulic micromanipulator system to stimulate the dorsal columns (3.3/s, 0.05 ms stimulatus duration) with the other electrode in the ipsilateral trunk muscles. The ipsilateral dorsal columns below the spinal injury were stimulated until threshold was established. Threshold (T) was determined separately in each animal and defined as the lowest current amplitude eliciting a dorsal root response. In injured animals, this was done caudal to the injury. Mean threshold value was 0.15 ± 0.06 mA.
To determine the spatial resolution of the stimulus threshold at twice-threshold (2T) in normal animals, the stimulating electrode was moved through the dorsal column tissue at 10μm increments until no action potential could be recorded. The stimulus resolution at 2T was determined in two normal animals as 50μm since moving the stimulating electrode more than 50μm ventral or lateral out of the sensory tract abolished the imitation of an action potential.
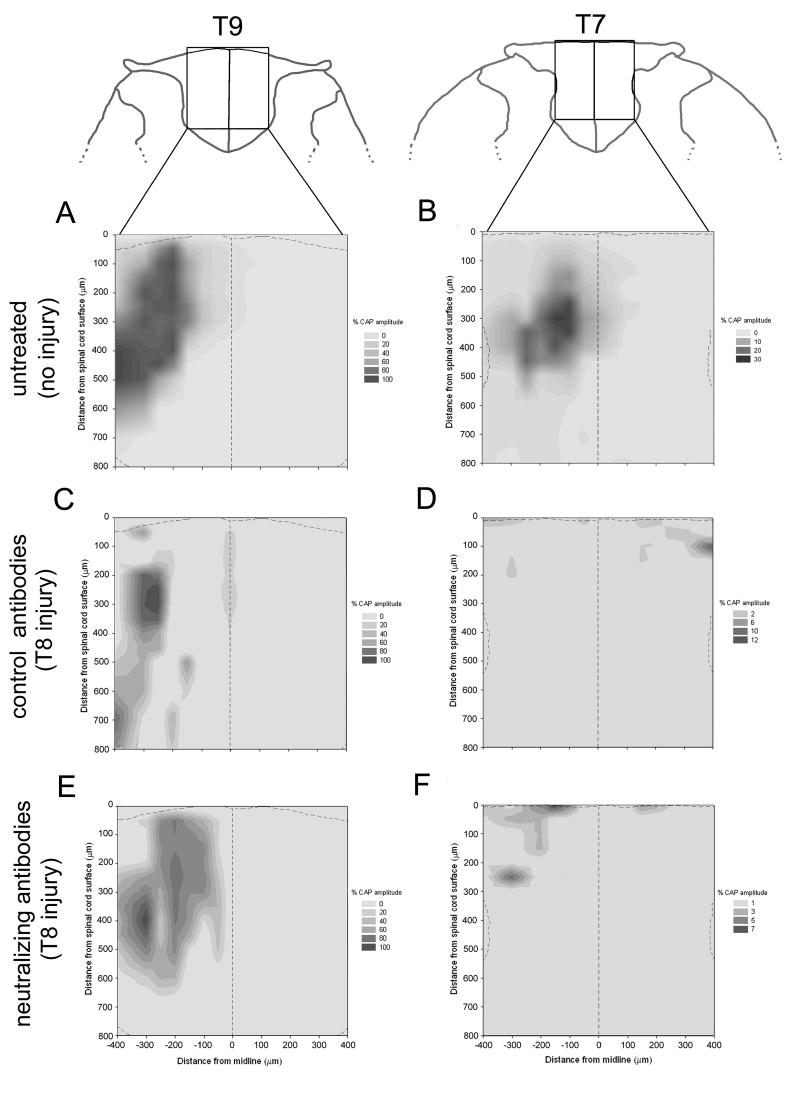
To create the spatial stimulus-response maps (figure 1A-F) a pin was inserted into the contralateral edge of the spinal cord at ∼T9 and used as a point to establish coordinates for the stimulating electrode. The midline of the spinal cord was defined by half the distance between the left and right dorsal root entry zones at the level of the pin. Surface position was defined by glass electrode contact with the spinal cord tissue surface at each medial-lateral position. The stimulation coordinates generally extended 400μm on either side of midline and to a depth of 800μm, creating a 16×16 grid made of 50μm2 squares. At each position, 30 stimulus-evoked compound action potentials were recorded. These were electronically averaged and analyzed off-line. We determined the largest compound action potential amplitude at each stimulation position at T9 (below-injury) and T7 (above-injury). These values were normalized by setting the highest CAP amplitude within each animal to 100% and plotted on the stimulus-response grid.
Figure 1.
Stimulus-response maps created from dorsal column electrical stimulation. Maps in uninjured and untreated animals show position of conducting sensory axons in the dorsal columns of the spinal cord at T9 (A) and T7 (B). Maps display axons below the injury site in animals that received a peripheral nerve conditioning-lesion and control, non-neutralizing anti-NG2 antibodies (C) or neutralizing anti-NG2 antibodies (E). Above the lesion, spatial distribution of regenerated sensory axons differs depending on treatment. In animals with conditioning-lesion and control antibodies (D), regenerated sensory axons are distributed more superficially and bilaterally. Sensory axons in animals with conditioning-lesion and neutralizing anti-NG2 antibodies (F) regenerated beyond the injury within deeper regions of the ipsilateral dorsal columns. Dashed lines on maps delineate the midline and the surface of the spinal cord. Response amplitude is expressed as % of the maximum compound action potential elicited at that site and is presented as gray-scale intensity. Drawings of coronal sections are adapted from Paxinos and Watson, 2004.
In some animals, recordings were also made from single axons (n=11) stimulated in the dorsal columns. Prior work demonstrated 2 populations of regenerating dorsal column axons; those that regenerated on the surface of the cord, and those whose regeneration through the dorsal column is dependent on neutralizing anti-NG2 antibodies treatment (Tan et al. 2006). Rostral to the injury, the stimulation electrode was placed at the coordinates (provided by results of the stimulation grid) that yielded the largest CAP from the “deep” regenerated axons. We defined axon populations in dorsal columns stimulated more than 50μm below the spinal cord surface as “deep”, and axon populations stimulated above 50μm as “superficial”. With the stimulating electrode placed in the optimal location, fascicles were teased from a dorsal rootlet until a stimulus-evoked action potential in a single axon could be recorded. To ensure single unit recordings were from the same axon stimulated above and below the injury, averaged stimulus-evoked potentials were compared and analyzed post hoc for similar amplitude and waveform.
Conduction velocity
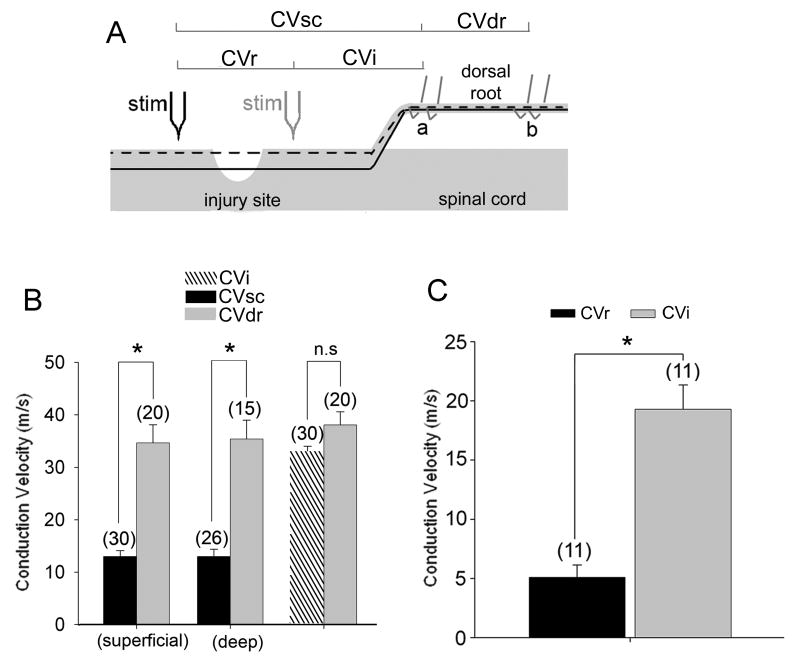
Two conduction velocities (CV) were determined for each CAP recording event: a spinal cord CV (designated CVsc) and dorsal root CV (CVdr) (figure 4A). CVsc was determined from the conduction distance between the stimulating electrode and the proximal-most recording electrode on the dorsal root. CVdr was determined from the distance between bipolar recording electrode pairs. In the case of single fiber recordings, below-injury stimulation CVi was determined similar to CVsc. The CV from an axon stimulated above the injury site incorporates the CV of both regenerated (CVr) and proximal fiber segments(CVi ). Therefore, the difference in the distance and latency of the single unit potential evoked by above and below-injury stimulation on the same axon was used to determine CVr—the CV of the regenerated segment.
Figure 4.
Regenerating axon populations stimulated above the injury exhibited lower mean conduction velocity. (A) Schematic of the electrophysiological preparation. Stim = stimulating electrode above (black) and below (faded) the injury. a and b are pairs of recording electrodes on the dorsal root. CVdr was determined from the distance and conduction time between the electrode pairs a and b. CVsc was determined from distance and time between the stimulating electrode and the proximal-most recording electrode on the dorsal root. CVsc is the average of CVr (regenerated fiber segments) and CVi (intact segment). Dashed and solid black lines represent superficial and deep axon populations, respectively. (B) Conduction velocity of the fastest component of the CAP. CVsc incorporates the CV of both the regenerated and spared segments of the axon, whereas CVdr is from the intact dorsal root. Stimulation of superficial and deep sub-populations of the dorsal columns above the lesion (CVsc) elicited volleys with much lower conduction velocity than stimulation of the dorsal root in the same experiments (CVdr) (* = p<0.001; one-way ANOVA on ranks with Dunn's test). Stimulation of the dorsal columns below the lesion (CVi) elicits volleys with conduction velocity similar to that of dorsal root. (C) Data from single units recorded in dorsal root filaments in response to stimulation of the same “deep” fiber above and below the lesion indicate that the regenerated segment had a much lower CV than the spared segment. (* = p<0.001; Student's t-test). Graphs are mean ± s.e.m and the number of axons included in analysis is in parentheses
Conduction fidelity/latency-shift
For single axon analysis, trains of twenty stimuli were delivered at 10, 20, 50, 100 and 200 Hz. Three trials were performed at each frequency on the same axon stimulated above and below the injury. The traces were scored for successful conduction by the appearance of the appropriate action potential waveform within a latency window of 2ms (to account for latency shifts with increasing frequency). Percent successful conduction was calculated as the ratio of the number of recorded single action potentials to the number of stimulations within each trial. To determine the frequency-dependent latency change to stimuli above and below the injury, the difference in latency between the 1st and the 5th action potential generated by a train of stimulations was averaged for each frequency. For statistical comparison, % successful conduction and the amount of latency shift at each stimulus position was averaged across all animals.
Tissue processing and Immunofluorescence Staining
After each experiment rats were deeply anaesthetized with ketamine and xylazine and transcardially perfused with 250ml of 0.1M phosphate buffer (PB) and heparin (0.003%) at 37°C followed by 300ml of freshly prepared ice-cold paraformaldehyde solution (4% in 0.1M PB). The spinal cord and hindbrain was removed, post-fixed for 2hr. at room temperature and cryoprotected by immersion in 30% sucrose, 0.1M PB at 4°C. A 10mm length block of spinal cord centered on the lesion was frozen in OCT (Triangle Biomedical Sciences) and 100 serial sections (20μm thick) in the sagital plane through the lesion site were cut using a Microm HM505E cryostat and thaw mounted onto gelatinized glass slides.
Immunofluorescence staining was carried out as described previously (Tan et al. 2006) using the following antibodies. Primary antibodies used were: rat anti-MBP (Serotec 1:100), goat anti-CTB antibody (List Biological Laboratories, 1:5000), and rabbit anti-GFAP (DAKO, 1:2000). The following secondary antibodies were used: FITC donkey anti-rat (Jackson ImmunoResearch Laboratories, 1:500), Alexafluor594 donkey anti-goat (1:2000), Alexafluor488 and Alexafluor488 donkey anti-rabbit (1:2000). All Alexafluor conjugated antibodies were obtained from Invitrogen, Inc. All sections were examined with a Zeiss Axioplan2 fluorescence microscope. Z-stack images were captured using a Zeiss LSM510 confocal microscope.
MBP/CTB quantitation
In five animals that had received neutralizing anti-NG2 antibodies and a peripheral nerve conditioning-lesion and subsequent CTB tracing, sagittal sections (20μm thick) from the spinal cord injury site were double-stained against myelin-basic protein (MBP) and cholera toxin-B (CTB). Thirty sagittal sections randomly sampled across a ∼2400μm width of spinal cord tissue were examined for the presence of CTB-filled axons regenerated 200-500μm rostral to the border of the injury site in the dorsal columns. The presence of CTB-labeled fibers within MBP-positive ensheathing, tube-like structures were scored.
Statistics
Compound action potentials, CVsc and CVdr, were averaged across animals and compared statistically using a one-way ANOVA on ranks with Dunn's test. The single axon CVi evoked by below-injury stimulation and the calculated regenerated segment CVr were averaged and compared by a Student's t-test. % successful conduction and latency shift were compared between above and below-injury stimulation using a Mann-Whitney Rank Sum test. All statistical tests were performed at the α-level of significance of 0.05. Data management and statistical analyses were performed using SigmaStat (version 3.0.1a; Jandel Scientific, Corte Madera, CA) and Microsoft Office Excel (2003) and graphed as mean ± s.e.m. using SigmaPlot (version 8.02a).
Results
We showed previously that transected large and medium diameter sensory axons can regenerate past the glial scar when animals are given a combinatorial treatment consisting of a prior peripheral nerve conditioning lesion and the infusion of the spinal cord lesion site with monoclonal antibodies that neutralize the growth inhibitory properties of the NG2 chondroitin sulfate proteoglycan (Tan et al. 2006). A peripheral nerve conditioning lesion alone is sufficient to promote some axonal regeneration; however, those axons grow in ectopic locations, mostly along the dorsal surface of the spinal cord. The combined treatment, on the other hand, results in axons growing through the injury site and into the dorsal columns rostral to the lesion (figures 1 and 2). Because the physiological properties of axons regenerating beyond a spinal cord injury have not been established, we analyzed the properties of regenerated axons 6-8 months after injury.
Figure 2.
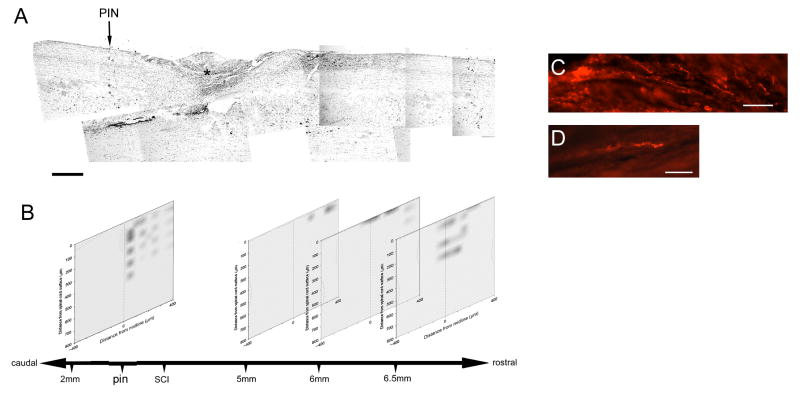
Sensory axons induced to regenerate rostral to the injury site are electrically functional. A) Nissl-stained sagittal section (18μm thick) of a bilaterally dorsal-transected spinal cord at T8. Dense cell labeling demarcates the lesion core (*). A pin (black arrow) was placed in the spinal cord tissue at an arbitrary location within T9—caudal to the injury. The rostral-caudal distances of all stimulus-response maps were calibrated to this pin. B) In this animal, four matrices created from stimulus-evoked compound action potentials and mapped with three coordinates, relative to the pin, the midline and surface of the spinal cord. The rostral-caudal distance bar is not to scale. C, and D) To assess the regeneration of sensory axons induced to grow, four-days prior to electrophysiological recordings, the transganglionic tracer, CTB, was injected into the left sciatic nerve. CTB-labeled sensory axons can be found rostral to the injury site. Scale bar A = 500μm; C, D = 50μm
Regenerated axons respond to electrical stimulation and their location can be reliably mapped
To accurately locate regenerating axons within the dorsal columns and on the surface of the spinal cord, we constructed stimulus-response (SR) maps of dorsal root axons caudal and rostral to a dorsal-over hemisection injury at spinal level T8. Activation threshold of intact axons in the dorsal columns (caudal to the injury site) was determined separately in each animal as described under Materials and Methods, and defined as the lowest current amplitude required to elicit a dorsal root response. The stimulus intensity for all experiments was set to twice threshold (2T). The spatial resolution of a 2T stimulation was determined to be 50μm (see Materials and Methods).
Stimulus response (SR) maps were constructed by measuring the largest compound action potential (CAP) amplitude at each stimulation position at T9 (below-injury) and T7 (above-injury) and projecting the stimulus location and response amplitude onto a grid representing the spinal cord in the coronal plane. As shown in figure 1, this created ‘contour’ maps of the responses above and below the injury. SR maps constructed in sham, uninjured animals exhibit the normal spatial distribution of sensory afferents within the fasiculus gracilis (LaMotte et al. 1991) (figure 1, A,B). After a combination of a peripheral nerve conditioning-lesion and the infusion of either control, non-neutralizing or neutralizing anti-NG2 antibodies, every animal had electrically-responsive axons in the white matter at spinal level T9 below the injury and ipsilateral to the dorsal root recording site. The spatial distribution of electrically responsive axons was similar after all 3 types of treatments. Responsive axons were confined to the dorsal columns ipsilateral to the recording site and the strongest responding axons were found between 300-500microns below the dorsal surface of the spinal cord (figure 1A, C, E). Above the injury at spinal level T7, however, the location of electrically-conducting axons in the dorsal columns varied according to the antibody treatment. In animals treated with control, non-neutralizing antibodies, all regenerated axons were located superficially with few deeper than 100μm below the dorsal surface (figure 1D). Axons often crossed the midline of the cord to run contralateral to the dorsal root recording site. In animals treated with the neutralizing anti-NG2 antibodies, axons regenerated within the depths of the dorsal columns, up to 300μm deep, and most, but not all, remained ipsilateral (figure 1F).
Figure 2 shows in greater detail the location of the regenerated axons and stimulation sites in a single case that was treated with the neutralizing anti-NG2 antibodies. The lesion site is clearly seen in a Nissl stained section encompassing a 7.5mm length of the spinal cord. (figure 2A, asterisk). The stimulus-response maps obtained at several levels above and below the lesion site are shown in panel B. These maps demonstrate that regenerated fibers can enter and elongate within the dorsal columns once they have grown past the injury site. Close to the lesion site, regenerated axons extend along the superficial aspects of the dorsal columns. At progressively more rostral levels, the axons lie deeper within the dorsal columns. We showed previously that after a peripheral nerve lesion and a dorsal-over hemisection lesion of the spinal cord, regenerating axons grow with relatively straight trajectories over the dorsal surface of the cord and enter the white matter rostral to the lesion site (Tan, et al., 2006). These stimulus-response maps are consistent with our previous anatomical findings. Images of individual cholera toxin B subunit labeled axons are shown in figure 2, panels C and D. The axons often had a beaded appearance which may be due to the difficulty of adequately preserving the tissue after extended electrophysiological penetrations. Alternatively, regenerated axons may undergo a protracted period of secondary degeneration and retraction. These results are consistent with our previous anatomical observations and extend them by showing that the regenerated axons are electrically excitable (Tan et al., 2006).
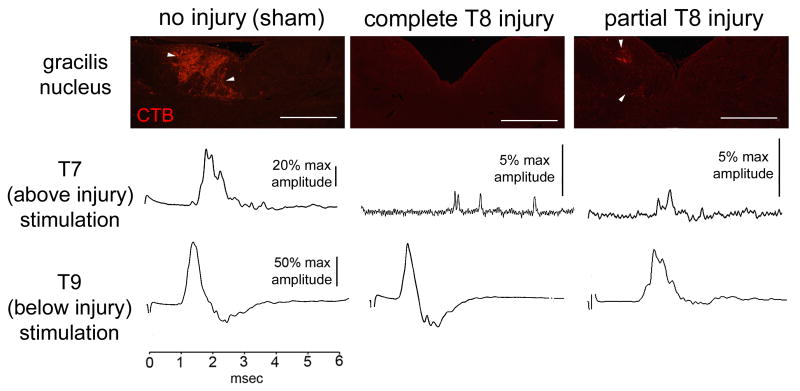
To ensure we were examining regenerated and not surgically spared fibers, additional animals were given partial lesions of the dorsal columns to intentionally spare ascending mechanosensory afferents. We compared CAP waveforms of the surgically spared axons with those of regenerated axons. In animals with regeneration-inducing treatment and a complete T8 dorsal column injury, antidromic stimulation above the injury resulted in small CAPs that were temporally dispersed (fig. 3). Larger amplitude, temporally compact CAPs were observed after above-injury stimulation in the sham-treated control animals and in those animals in which the dorsal columns were only partially transected. We confirmed the extent of the dorsal column transections by analyzing the transport of peripherally administered cholera toxin-B from the sciatic nerve into the ipsilateral gracilis nucleus. In both the sham and partially-injured animals, CTB-labeled axonal profiles were observed in the ipsilateral gracilis nucleus (Figure 3). No labeling was observed in animals with a complete T8 dorsal column injury and regeneration-inducing treatments, indicating axons were not spared. Thus, our measurements of the physiological properties were of transected axons that had regenerated into either their normal pathway or into ectopic locations.
Figure 3.
Histological and physiological representations of spared and regenerated fibers. Partial or no dorsal column injury results in presence of CTB-filled profiles in the gracilis nucleus (arrowheads). Sample raw traces from above (T7) or below (T9) injury stimulation indicate differences in temporal dispersion and amplitude of CAP in these preparations. Scale bar = 500μm.
Regenerating axon populations have reduced conduction velocity
We compared the mean conduction velocity (CV) of the fastest component of the CAP from stimulation above and below the injury. Within each axon population, 3 conduction velocities were determined: a spinal cord CV initiated by stimulation above the injury site, (designated as CVsc), a spinal cord CV initiated by stimulation below the injury site (designated as CVi) and a dorsal root CV (CVdr) (Figure 4A). For this analysis, the axons were divided into those that ran on the surface of the spinal cord and those that ran deeper than 50μm below the dorsal surface of the cord. Stimulation of both populations below the injury resulted in a spinal cord CAP CV (CVi = 32.6 ± 6.4m/s) that was not significantly different (p = 0.104) from the dorsal root CAP CV (CVdr = 38 ± 10.8m/s). These velocities are similar to that of myelinated Aβ-fibers (Feasby et al. 1981; Shin et al. 1997). In contrast, when axons were stimulated rostral to the injury site, CVsc was substantially reduced. In the case of those axons that were >50μm below the surface of the cord, the CVsc was 12.9 ± 7 m/s and CVdr was 35.2 ± 13.9m/s, (p<0.001; one-way ANOVA on ranks with Dunn's test). Superficial axon populations stimulated above the injury exhibited a similar reduction in CAP CV within the spinal cord as compared to dorsal root CAP CV (CVsc = 12.9±5.9 m/s, CVdr = 34.5 ± 15.3 m/s, p<0.001).
The CV measured after stimulation above the injury site (CVsc) includes 2 components; conduction through both the regenerated axon segment (CVr) and through the undamaged segment (CVi). To determine whether there are conduction property differences between the regenerated and intact segments of the same axon, we isolated single axons using a dorsal root teasing method. Conduction velocity was determined for the regenerated and intact portion of the axon by stimulating the same axon at two positions, one above the injury and the other below, and recording from the dorsal root at position a (fig. 4A). Single unit CV was significantly lower in regenerated axon segments (CVr = 5 ± 3.4 m/s) than in intact axon segments (CVi = 19.3 ± 6.8 m/s) (p<0.001; Student's t-test) (fig. 4C). Thus, the regenerated axonal segment has membrane conduction properties associated with slower action potential propagation.
Regenerated sensory axons have decreased frequency-following ability
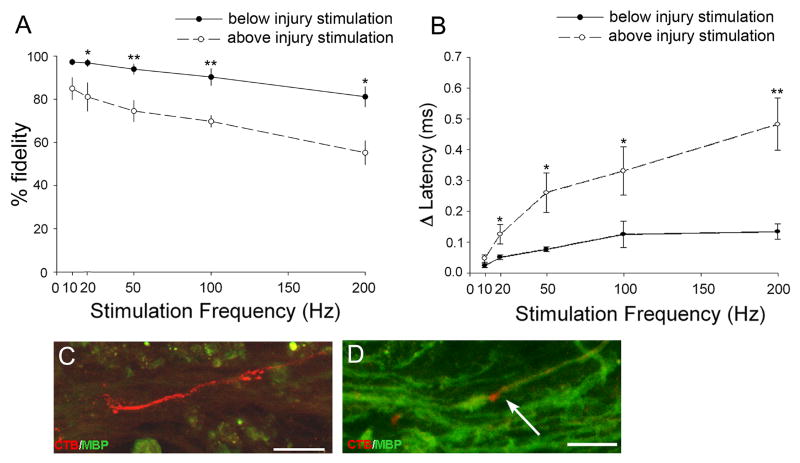
Previous studies have shown that injured and demyelinated axons (Utzschneider et al. 1994; Honmou et al. 1996) have decreased frequency following ability (Shin et al. 1997; Nashmi and Fehlings, 2001a). To determine whether regenerated axons are capable of following high frequency stimulation, we examined the conduction-fidelity of single regenerating axons stimulated above and below the injury at stimulation frequencies between 10-200Hz. While the undamaged axon segment maintained conduction fidelity close to 100%, the fidelity of conduction from the regenerated segment decreased progressively with increasing frequency (Fig. 5A). This decrease began with rates as low as 20 Hz, and dropped below 60% success at 200 Hz.
Figure 5.
Regenerated sensory axons have pathologic conduction properties. (A) Fidelity of conduction of single axons stimulated above and below the injury decreased with increasing stimulus frequency, but the decrease was greater for axons stimulated above the injury. (B) CV decreased (expressed here as latency increase) with increasing stimulation frequency. The latency delay for above-injury stimulation was greater than below-injury stimulation. (C-D) Confocal z-stack images of spinal cord tissue above (C) and below (D) the injury site immunostained against myelin-basic protein (green) and transganglionically transported cholera toxin-B (red). White arrow indicates an exposed CTB-labeled axon at a node of Ranvier. (*=p<0.01; ** = p<0.001; Mann-Whitney Rank Sum Test). Graphs are mean ± s.e.m. All scale bars = 50μm.
Conduction in regenerated axons is more sensitive to increases in stimulus frequency
With repetitive stimuli, intact sensory axons show a progressive increase in action potential conduction latency (Raymond 1979; George et al. 1984; Carley and Raymond, 1987; Shin et al. 1997). To test whether regenerated axons also exhibit increased conduction latency, we compared the latency of the 1st and the 5th response in a train of stimuli. Single fibers were stimulated both rostral and caudal to the injury. Stimulation below the injury over a range of 10-200 Hz resulted in modest increases in the action potential latency. Repetitive stimuli of the same fibers above the injury site evoked a significantly greater change in latency (Figure 5B).
The lack of myelin may account for the pathophysiological status of regenerated axons
These two properties, frequency-related conduction failure and increased conduction latency, are also features of acute and chronically demyelinated axons (Waxman 1977; Waxman and Brill, 1978; Utzschneider et al. 1994; McDonald and Ron, 1999). We used confocal microscopy to determine whether the axons that had regenerated into the white matter rostral to the injury site were myelinated. Consistent with physiological data, none of 40 cholera toxin-B filled axons regenerating in the white matter above the injury had myelin basic protein (MBP)-positive cellular sheaths (Figure 5C). Below the injury, many labeled sensory axons were found within tubular MBP-positive structures (Figure 5D). This result suggests that axons regenerated above the injury lacked myelin, which may contribute significantly to the pathophysiological properties of these fibers.
Discussion
Injured motor and sensory axons within the spinal cord can regenerate after a variety of growth-promoting treatments (Schnell and Schwab, 1993; von Meyenburg et al. 1998; Neumann and Woolf, 1999; Bradbury et al. 2002; Neumann et al. 2002; Tan et al. 2006). However, no direct physiological evaluation of the conduction properties of regenerated dorsal column axons has been performed. Here we characterized the functional status of regenerated sensory afferents during the chronic SCI phase, 6-8 months post-injury. Discrete antidromic stimulation was used to locate electrically-conducting sensory afferents above and below the injury site. This allowed us to reliably map axon location within the dorsal columns and compare the properties of the regenerated portion to those of the intact, undamaged axon segment. Because the combinatorial treatment used here allows axons to grow within the white matter rostral to the lesion site, we were able to physiologically evaluate axons in their normal locations as well as regenerated axons located ectopically (Tan, et al., 2006). Regardless of their location, regenerated sensory axons exhibited decreased conduction velocity, reduced frequency following ability, and large conduction delays with increasing rates of impulse activity. The pathological properties of regenerated sensory axons within the dorsal columns were similar to those observed in regenerated motor axons and sensory fibers regenerating (Bradbury et al. 2002; Ramer et al. 2002). Chronic spinal cord compression injuries as well as either acute or chronic demyelination also results in a similar axonal pathophysiology (Rasminsky 1984; Utzschneider et al. 1994; Fehlings and Nashmi, 1995; Honmou et al. 1996; Nashmi and Fehlings, 2001a). The thin diameter of regenerating axons, alterations in ion channel composition of the axolemma, and a lack of functional myelin may all participate in generating the pathologic properties observed here (Nashmi et al. 2000; Craner et al. 2004b; Sasaki et al. 2006).
The physiological properties reported here suggest that pathologic changes in the membrane characteristics occur within the regenerated segment of an axon while the undamaged segment remains relatively normal. An alternative explanation for this is that the regenerated axons stimulated rostral to the injury site belong to a different class of primary afferents than those stimulated below the injury site. While this may be possible for the group data shown in figure 4, the data from single fibers appear to rule this out. When we recorded from single fibers (figure 4C), the CV of the uninjured segment was well within this range for Aβ fibers (Feasby et al., 1981, Shin et al., 1997). The regenerated axon segments did not conduct slower than 2m/sec, the upper CV of C-fibers (Leem et al. 1993) and these axon segments were able to follow reliably at stimulation frequencies of up to 10 Hz, a frequency at which C-fibers show conduction changes (Koga et al. 2005). Since we stimulated regenerated fibers at spinal level T9, it is also unlikely that the fibers were displaced Ia afferents that normally terminate in Clarke's column. Lastly, the stimulus-response maps show a strong concordance with our previous anatomical reconstructions of the distribution of regenerated fibers that were based on the transganglionic transport of cholera toxin B subunit, a tracer that is taken up predominantly by medium and large diameter afferents (Tan et al., 2006). We also considered the possibility that primary afferent depolarization (i.e., dorsal root reflex, Burke and Rudomin, 1977) might have affected our physiological interpretations, but excluded this possibility because increasing frequency did not significantly reduce conduction-fidelity in the uninjured segment of single sensory axons.
The abnormal anatomical features of regenerating axons may contribute to their pathophysiologic state. After a peripheral nerve conditioning-lesion, regenerating axons often have twisted trajectories and numerous branches that could contribute to branch-point conduction failure (Feasby et al., 1981, Neumann and Woolf, 1999). However, the procedures used here to encourage axon regeneration result in axons growing past the injury site with relatively straight trajectories and few branches (Tan et al., 2006). The beaded appearance of many of these axons (figure 2) could reflect an ongoing degenerative process which may also contribute to their pathologic properties. Regenerating nerve fibers in the peripheral nervous system and in the regenerating dorsal roots have reduced axonal diameters (Feasby et al. 1981). Axons also have smaller diameters in regions of chronic demyelination (Prineas and Connell, 1978). Because CV is partially a function of axon diameter, thin regenerating axons would be expected to have a lower CV (Waxman, 1977). In our study, we were unable to record from 2 points along only the regenerated segment of the axon, so that CVr shown in figure 4C is calculated from with the different latency and displacement values of CVi and CVsc (Figure 4). Thus, it is not known how much of the reduced CV is due to slower conduction along the regenerated axon segment (CVr) or due to delays as the thinner regenerated segment transitions into the undamaged segment. Any impedance mismatch between the thinner regenerated segment and the thicker, undamaged segment or along the beaded fibers would be expected to contribute to the frequency-related conduction block.
Both astrocytes and OPCs normally contact nodes of Ranvier in the CNS, but whether these contacts affect saltatory conduction is unknown (Butt et al. 1999; Miller 1999). After SCI, there are large increases in the number of astrocytes and other glial elements and how these cells may alter axonal properties is an important question for future study.
Many of the pathophysiological properties of the regenerated axons can be explained by the loss of myelin. The regenerating axons studied here are normally myelinated but remain in a chronic demyelinated or under-myelinated state. After injuries to the spinal cord, oligodendrocytes rapidly undergo cell death (Grossman et al. 2001; McTigue et al. 2001). This period of rapid cell death is followed by the proliferation of oligodendrocyte precursor cells (OPCs) and the subsequent repopulation of the lesioned area with oligodendrocytes (Wrathall and Hudson,1998; McTigue et al. 2001). Despite oligodendrocyte numbers that may exceed those found in uninjured tissue, the injury site and adjacent areas can remain unmyelinated for up to 10 months (Totoiu and Kierstead, 2005). This lack of myelin will have profound effects on the physiology of the regenerated axons. The formation of myelin de novo is responsible for the high density clustering of sodium channels at nodes of Ranvier and the spatial restriction of potassium channels to the juxtaparanodal region (Rasband and Trimmer, 2001). As nodes of Ranvier mature, there a switch in the expression of sodium channel isoforms from Nav1.2 to Nav1.6 (Rasband et al., 2003; Rush et al. 2005). The loss of myelin in adult animals leads to the re-expression of Nav1.2 isoform and disruption of the potassium channel clusters (Black et al. 1991; Nashmi et al. 2000; Rasband et al. 2003; Craner et al. 2004a; Karimi-Abdolrezaee et al. 2004; Sinha et al. 2006). In general, the recovery from inactivation after a depolarizing stimulus is fast for Nav1.6 compared to Nav1.2, which is about three times slower (O'Leary 1998). Slower inactivation decreases the availability of channels for the next wave of stimulation, which would reduce fidelity at high frequency stimulation. Similarly, the over-expression of potassium channels and the concomitant increase in outward potassium conductance could shunt propagating action potential currents (Nashmi et al. 2000; Nashmi and Fehlings, 2001b; Karimi-Abdolrezaee et al. 2004). Such phenomena could explain the increased conduction block seen in the regenerated axons.
Given the large numbers of oligodendrocytes present in the injured spinal cord, it is puzzling that extensive remyelination does not occur. The chronically injured human spinal cord also remains in a demyelinated or under-myelinated state (Guest et al. 2005). After contusion injuries to the adult rat spinal cord, there is chronic progressive demyelination, suggesting that any myelin that is produced by either invading Schwann cells or oligodendrocytes is not stable (Totoiu and Kierstead, 2005). The thinner regenerated axons may not be able to support stable remyelination (Friede and Miyagishi, 1972). Environmental conditions within the injured spinal cord could contribute to this failure to form stable myelin. One possibility is that the dense extracellular matrix that forms at injury sites limits oligodendrocyte differentiation. A high concentration of neuregulin within the matrix, for example, might prevent the differentiation of OPCs into myelinating oligodendrocytes (Canoll et al. 1996; Sussman et al. 2005). In this regard, laminin and other integrin ligands within the matrix could also alter the development of myelinating cells (Colognato et al. 2002). Since Wallerian degeneration is protracted in the CNS, the persistence of myelin debris may also inhibit oligodendrocyte differentiation (Buss et al. 2004; Kotter et al. 2006). Together, these hostile environmental conditions, as well as the reduced electrical activity of the regenerating fibers (Demerens et al. 1996), may create a negative feedback loop wherein any myelin that does form degenerates. As is the case for multiple sclerosis, it will be important to optimize the conditions for remyelination in the damaged spinal cord so that any spared or regenerating axons can function properly.
The finding that even 8 months after injury, surviving and regenerated axons are electrically excitable suggests that treatments applied during the chronic phase of SCI may have clinical efficacy. For example, after axotomy, neurons of the red nucleus survive for up to one year. Despite being in an atrophic state, these neurons can regenerate their axons if they are provided appropriate trophic factors and a suitable terrain (Kwon et al. 2002; Tobias et al. 2003). On the other hand, transplanted neural precursor cells or stem cell-derived oligodendrocyte precursor cells can remyelinate spared axons within the damaged spinal cord and partially restore locomotor function, but only when carried out in the acute and subacute phase, not when performed in the chronic phase (Karimi-Abdolrezaee et al. 2006). This emphasizes the dynamic nature of spinal cord injuries and the need for different therapeutic strategies at different stages of the recovery process.
The observation that regenerated sensory axons remain in a chronic pathophysiologic state raises the possibility that regenerating fibers may not play a major role in the functional recovery often observed in animal models of SCI. For example, lesioned corticospinal tract axons will regenerate when the injury site is infused with chondroitinase ABC (Bradbury, et al., 2002). Stimulation of the motor cortex in these treated animals evoked reduced amplitude cord dorsum potentials that had a 4-fold longer latency than control animals, suggesting that regenerated corticospinal axons conduct as poorly as the sensory afferents under study here. Since the regenerated sensory axons did not form synapses with their targets in the brainstem, it remains unknown how synapse formation will affect the physiology of these axons. After dorsal root crush, sensory fibers have been reported to regenerate into the dorsal horn (Ramer et al. 2000; Steinmetz et al. 2005). Stimulation of the dorsal root also evokes low amplitude, long latency responses within the dorsal horn. Although these responses are indicative of the re-establishment of synaptic connections, the role of these connections in mediating the behavioral recovery observed using complex behavioral tasks is uncertain, particularly since the damaged spinal cord may have a large capacity for plasticity. Functional recovery may reflect the reorganization of existing and non-damaged connections as well as the formation of new connections due to axonal sprouting (for review, see Bradbury and McMahon, 2006).
In summary, functional recovery in the adult CNS requires that injured axons survive, elongate to proper targets, reestablish synapses, and conduct information with fidelity and proper timing (Blight 2002; Silver and Miller, 2004; Blight and Tuszynski. 2006). We have demonstrated that myelinated sensory axons induced to regenerate after SCI are electrically functional, but regardless of their anatomically correct location within the dorsal columns, are chronically unmyelinated and display pathophysiological properties. It seems important, therefore, to develop strategies to restore normal saltatory conduction after spinal cord regeneration.
Acknowledgments
Grant support by National Institute of Health (NIH), New York State Department of Health Spinal Cord Injury Research Board (NYS DOH SCIRB), and Christopher Reeve Paralysis Foundation (CRPF)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Black JA, Felts P, Smith KJ, Kocsis JD, Waxman SG. Distribution of sodium channels in chronically demyelinated spinal cord axons: immuno-ultrastructural localization and electrophysiological observations. Brain Res. 1991;544(1):59–70. doi: 10.1016/0006-8993(91)90885-y. [DOI] [PubMed] [Google Scholar]
- Blight AR. Miracles and molecules--progress in spinal cord repair. Nat Neurosci. 2002;5(Suppl):1051–4. doi: 10.1038/nn939. [DOI] [PubMed] [Google Scholar]
- Blight AR, Tuszynski MH. Clinical trials in spinal cord injury. J Neurotrauma. 2006;23(3-4):586–93. doi: 10.1089/neu.2006.23.586. [DOI] [PubMed] [Google Scholar]
- Bostock H, Sears TA, Sherratt RM. The effects of 4-aminopyridine and tetraethylammonium ions on normal and demyelinated mammalian nerve fibres. J Physiol. 1981;313:301–15. doi: 10.1113/jphysiol.1981.sp013666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7(8):644–53. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416(6881):636–40. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Burke RE, Rudimon P. Spinaol neurons and synapses. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology. The Nervous System, The Cellular Biology of Neurons Am Physiol Soc. Bethesda, MD: pp. 877–944. [Google Scholar]
- Buss A, Brook GA, Kakulas B, Martin D, Franzen R, Schoenen J, Noth J, Schmitt AB. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain. 2004;127(Pt 1):34–44. doi: 10.1093/brain/awh001. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Hornby MF, Kirvell SL, Hunter A, Levine JM, Berry M. Cells expressing the NG2 antigen contact nodes of Ranvier in adult CNS white matter. Glia. 1999;26(1):84–91. [PubMed] [Google Scholar]
- Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17(2):229–43. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Carley LR, Raymond SA. Comparison of the after-effects of impulse conduction on threshold at nodes of Ranvier along single frog sciatic axons. J Physiol. 1987;386:503–27. doi: 10.1113/jphysiol.1987.sp016548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, Chan YS, Xu XM. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. Faseb J. 2004;18(1):194–6. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, ffrench-Constant C. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4(11):833–41. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Hains BC, Lo AC, Black JA, Waxman SG. Co-localization of sodium channel Nav1.6 and the sodium-calcium exchanger at sites of axonal injury in the spinal cord in EAE. Brain. 2004;127(Pt 2):294–303. doi: 10.1093/brain/awh032. [DOI] [PubMed] [Google Scholar]
- Craner MJ, Newcombe J, Black JA, Hartle C, Cuzner ML, Waxman SG. Molecular changes in neurons in multiple sclerosis: altered axonal expression of Nav1.2 and Nav1.6 sodium channels and Na+/Ca2+ exchanger. Proc Natl Acad Sci U S A. 2004;101(21):8168–73. doi: 10.1073/pnas.0402765101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93(18):9887–92. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feasby TE, Bostock H, Sears TA. Conduction in regenerating dorsal root fibres. J Neurol Sci. 1981;49(3):439–54. doi: 10.1016/0022-510x(81)90033-2. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Nashmi R. Assessment of axonal dysfunction in an in vitro model of acute compressive injury to adult rat spinal cord axons. Brain Res. 1995;677(2):291–9. doi: 10.1016/0006-8993(95)00141-c. [DOI] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25(5):1169–78. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friede RL, Miyagishi T. Adjustment of the myelin sheath to changes in axon caliber. Anat Rec. 1972;172(1):1–14. doi: 10.1002/ar.1091720101. [DOI] [PubMed] [Google Scholar]
- George SA, Mastronarde DN, Dubin MW. Prior activity influences the velocity of impulses in frog and cat optic nerve fibers. Brain Res. 1984;304(1):121–6. doi: 10.1016/0006-8993(84)90867-9. [DOI] [PubMed] [Google Scholar]
- Grossman SD, Rosenberg LJ, Wrathall JR. Temporal-spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol. 2001;168(2):273–82. doi: 10.1006/exnr.2001.7628. [DOI] [PubMed] [Google Scholar]
- Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192(2):384–93. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- Hains BC, Saab CY, Waxman SG. Changes in electrophysiological properties and sodium channel Nav1.3 expression in thalamic neurons after spinal cord injury. Brain. 2005;128(Pt 10):2359–71. doi: 10.1093/brain/awh623. [DOI] [PubMed] [Google Scholar]
- Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;26(16):4308–17. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K, Fujitani M, Yasuda Y, Doya H, Saito T, Yamaguchi S, Mueller BK, Yamashita T. RGM inhibition promotes axonal growth and recovery after spinal cord injury. J Cell Biol. 2006;173:47–58. doi: 10.1083/jcb.200508143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honmou O, Felts PA, Waxman SG, Kocsis JD. Restoration of normal conduction properties in demyelinated spinal cord axons in the adult rat by transplantation of exogenous Schwann cells. J Neurosci. 1996;16(10):3199–208. doi: 10.1523/JNEUROSCI.16-10-03199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A, Li Y, Li D, Raisman G, El Masry WS. Olfactory ensheathing cells: ripples of an incoming tide? Lancet Neurol. 2006;5(5):453–7. doi: 10.1016/S1474-4422(06)70444-6. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Fehlings MG. Temporal and spatial patterns of Kv1.1 and Kv1.2 protein and gene expression in spinal cord white matter after acute and chronic spinal cord injury in rats: implications for axonal pathophysiology after neurotrauma. Eur J Neurosci. 2004;19(3):577–89. doi: 10.1111/j.0953-816x.2004.03164.x. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci. 2006;26(13):3377–89. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga K, Furue H, Rashid MH, Takaki A, Katafuchi T, Yoshimura M. Selective activation of primary afferent fibers evaluated by sine-wave electrical stimulation. Mol Pain. 2005;1(1):13. doi: 10.1186/1744-8069-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotter MR, Li WW, Zhao C, Franklin RJ. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26(1):328–32. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Liu J, Messerer C, Kobayashi NR, McGraw J, Oschipok L, Tetzlaff W. Survival and regeneration of rubrospinal neurons 1 year after spinal cord injury. Proc Natl Acad Sci U S A. 2002;99(5):3246–51. doi: 10.1073/pnas.052308899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte CC, Kapadia SE, Shapiro CM. Central projections of the sciatic, saphenous, median, and ulnar nerves of the rat demonstrated by transganglionic transport of choleragenoid-HRP (B-HRP) and wheat germ agglutinin-HRP (WGA-HRP) J Comp Neurol. 1991;311(4):546–62. doi: 10.1002/cne.903110409. [DOI] [PubMed] [Google Scholar]
- Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in the rat foot. J Neurophysiol. 1993;69(5):1684–99. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24(28):6402–9. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald WI, Ron MA. Multiple sclerosis: the disease and its manifestations. Philos Trans R Soc Lond B Biol Sci. 1999;354(1390):1615–22. doi: 10.1098/rstb.1999.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21(10):3392–400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH. Contact with central nervous system myelin inhibits oligodendrocyte progenitor maturation. Dev Biol. 1999;216(1):359–68. doi: 10.1006/dbio.1999.9466. [DOI] [PubMed] [Google Scholar]
- Moon LD, Asher RA, Rhodes KE, Fawcett JW. Regeneration of CNS axons back to their target following treatment of adult rat brain with chondroitinase ABC. Nat Neurosci. 2001;4(5):465–6. doi: 10.1038/87415. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Fehlings MG. Changes in axonal physiology and morphology after chronic compressive injury of the rat thoracic spinal cord. Neuroscience. 2001;104(1):235–51. doi: 10.1016/s0306-4522(01)00009-4. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Fehlings MG. Mechanisms of axonal dysfunction after spinal cord injury: with an emphasis on the role of voltage-gated potassium channels. Brain Res Brain Res Rev. 2001;38(1-2):165–91. doi: 10.1016/s0165-0173(01)00134-5. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Jones OT, Fehlings MG. Abnormal axonal physiology is associated with altered expression and distribution of Kv1.1 and Kv1.2 K+ channels after chronic spinal cord injury. Eur J Neurosci. 2000;12(2):491–506. doi: 10.1046/j.1460-9568.2000.00926.x. [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34(6):885–93. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23(1):83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci U S A. 2004;101(23):8786–90. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary ME. Characterization of the isoform-specific differences in the gating of neuronal and muscle sodium channels. Can J Physiol Pharmacol. 1998;76(10-11):1041–50. doi: 10.1139/cjpp-76-10-11-1041. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates: The New Coronal Set. Academic Press; Sydney: 2004. [Google Scholar]
- Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10(6):610–6. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- Pinzon A, Calancie B, Oudega M, Noga BR. Conduction of impulses by axons regenerated in a Schwann cell graft in the transected adult rat thoracic spinal cord. J Neurosci Res. 2001;64(5):533–41. doi: 10.1002/jnr.1105. [DOI] [PubMed] [Google Scholar]
- Prineas JW, Connell F. The fine structure of chronically active multiple sclerosis plaques. Neurology. 1978;28(9 Pt 2):68–75. doi: 10.1212/wnl.28.9_part_2.68. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, Filbin MT. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34(6):895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Bishop T, Dockery P, Mobarak MS, O'Leary D, Fraher JP, Priestley JV, McMahon SB. Neurotrophin-3-mediated regeneration and recovery of proprioception following dorsal rhizotomy. Mol Cell Neurosci. 2002;19(2):239–49. doi: 10.1006/mcne.2001.1067. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403(6767):312–6. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Kagawa T, Park EW, Ikenaka K, Trimmer JS. Dysregulation of axonal sodium channel isoforms after adult-onset chronic demyelination. J Neurosci Res. 2003;73(4):465–70. doi: 10.1002/jnr.10675. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS. Developmental clustering of ion channels at and near the node of Ranvier. Dev Biol. 2001;236(1):5–16. doi: 10.1006/dbio.2001.0326. [DOI] [PubMed] [Google Scholar]
- Rasminsky M. Pathophysiology of demyelination. Ann N Y Acad Sci. 1984;436:68–85. doi: 10.1111/j.1749-6632.1984.tb14776.x. [DOI] [PubMed] [Google Scholar]
- Raymond SA. Effects of nerve impulses on threshold of frog sciatic nerve fibres. J Physiol. 1979;290(2):273–303. doi: 10.1113/jphysiol.1979.sp012771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309(5971):791–3. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Rush AM, Dib-Hajj SD, Waxman SG. Electrophysiological properties of two axonal sodium channels, Nav1.2 and Nav1.6, expressed in mouse spinal sensory neurones. J Physiol. 2005;564(Pt 3):803–15. doi: 10.1113/jphysiol.2005.083089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Black JA, Lankford KL, Tokuno HA, Waxman SG, Kocsis JD. Molecular reconstruction of nodes of Ranvier after remyelination by transplanted olfactory ensheathing cells in the demyelinated spinal cord. J Neurosci. 2006;26(6):1803–12. doi: 10.1523/JNEUROSCI.3611-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell L, Schwab ME. Sprouting and regeneration of lesioned corticospinal tract fibres in the adult rat spinal cord. Eur J Neurosci. 1993;5(9):1156–71. doi: 10.1111/j.1460-9568.1993.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Sherratt RM, Bostock H, Sears TA. Effects of 4-aminopyridine on normal and demyelinated mammalian nerve fibres. Nature. 1980;283(5747):570–2. doi: 10.1038/283570a0. [DOI] [PubMed] [Google Scholar]
- Shin HC, Oh SJ, Jung SC, Choi YR, Won CK, Leem JW. Activity-dependent conduction latency changes in A beta fibers of neuropathic rats. Neuroreport. 1997;8(12):2813–6. doi: 10.1097/00001756-199708180-00032. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sinha K, Karimi-Abdolrezaee S, Velumian AA, Fehlings MG. Functional changes in genetically dysmyelinated spinal cord axons of shiverer mice: role of juxtaparanodal Kv1 family K+ channels. J Neurophysiol. 2006;95(3):1683–95. doi: 10.1152/jn.00899.2005. [DOI] [PubMed] [Google Scholar]
- Steinmetz MP, Horn KP, Tom VJ, Miller JH, Busch SA, Nair D, Silver DJ, Silver J. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci. 2005;25(35):8066–76. doi: 10.1523/JNEUROSCI.2111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman CR, Vartanian T, Miller RH. The ErbB4 neuregulin receptor mediates suppression of oligodendrocyte maturation. J Neurosci. 2005;25(24):5757–62. doi: 10.1523/JNEUROSCI.4748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Colletti M, Rorai AT, Skene JH, Levine JM. Antibodies against the NG2 proteoglycan promote the regeneration of sensory axons within the dorsal columns of the spinal cord. J Neurosci. 2006;26(18):4729–39. doi: 10.1523/JNEUROSCI.3900-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias CA, Shumsky JS, Shibata M, Tuszynski MH, Fischer I, Tessler A, Murray M. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp Neurol. 2003;184(1):97–113. doi: 10.1016/s0014-4886(03)00394-7. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486(4):373–83. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Utzschneider DA, Archer DR, Kocsis JD, Waxman SG, Duncan ID. Transplantation of glial cells enhances action potential conduction of amyelinated spinal cord axons in the myelin-deficient rat. Proc Natl Acad Sci U S A. 1994;91(1):53–7. doi: 10.1073/pnas.91.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Meyenburg J, Brosamle C, Metz GA, Schwab ME. Regeneration and sprouting of chronically injured corticospinal tract fibers in adult rats promoted by NT-3 and the mAb IN-1, which neutralizes myelin-associated neurite growth inhibitors. Exp Neurol. 1998;154(2):583–94. doi: 10.1006/exnr.1998.6912. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Conduction in myelinated, unmyelinated, and demyelinated fibers. Arch Neurol. 1977;34(10):585–9. doi: 10.1001/archneur.1977.00500220019003. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Brill MH. Conduction through demyelinated plaques in multiple sclerosis: computer simulations of facilitation by short internodes. J Neurol Neurosurg Psychiatry. 1978;41(5):408–16. doi: 10.1136/jnnp.41.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Hains BC. Fire and phantoms after spinal cord injury: Na(+) channels and central pain. Trends Neurosci. 2006;29(4):207–15. doi: 10.1016/j.tins.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Wrathall JR, Li W, Hudson LD. Myelin gene expression after experimental contusive spinal cord injury. J Neurosci. 1998;18(21):8780–93. doi: 10.1523/JNEUROSCI.18-21-08780.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci. 2006;9(6):843–52. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]