Abstract
Objective
Sugar consumption affects insulin release and, in hypertension, may stimulate cardiac signaling mechanisms that accelerate left ventricular hypertrophy and the development of heart failure. We investigated the effects of high-fructose or sucrose diets on ventricular function and mortality in hypertensive Dahl salt-sensitive rats.
Methods
Rats were fed chows that were either high starch (70% starch, 10% fat by energy), high fat (20% carbohydrates, 60% fat), high fructose (61% fructose, 9% starch, 10% fat), or high sucrose (61% sucrose, 9% starch, 10% fat). Hypertension was induced by adding 6% salt to the chow (n = 8–11/group).
Results
After 8 weeks of treatment, systolic blood pressure and left ventricular mass were similarly increased in all rats that were fed high-salt diets. Hypertension caused a switch in mRNA myosin heavy chain isoform from α to β, and this effect was greater in the high-salt sucrose and fructose groups than in starch and fat groups. The cardiac mRNA for atrial natriuretic factor was also increased in all high-salt groups compared to respective controls, with the increase being significantly greater in the hypertensive sucrose fed group. Mortality was greater in the sucrose group (44%) compared to all the other hypertensive groups (12–18%), as was cardiomyocyte apoptosis. Left ventricular ejection fraction was lower in the high-salt sucrose group, which was due to an increase in end-systolic volume, and not increased end-diastolic volume.
Conclusion
Diets high in sugar accelerated cardiac systolic dysfunction and mortality in hypertension compared to either a low-carbohydrate/high-fat or high-starch diet.
Keywords: cardiac, fat, glucose, heart failure, insulin, nutrition
Introduction
Hypertension is a major risk factor for left ventricular hypertrophy (LVH), heart failure, and early mortality [1,2]. There is a paucity of information regarding the role of macronutrient intake for the development of heart failure and mortality in hypertension [3]. Recent studies on hypertensive rats suggest that a low-carbohydrate/high-fat diet attenuates development of LVH and heart failure [4,5], which is particularly relevant in light of the well documented increase in the intake of sugar in developed countries and the positive association with metabolic and cardiovascular diseases [6–8]. At present, the optimum dietary intake of fat and carbohydrate, particularly sugar, is not known for hypertensive patients.
Recent epidemiological studies found no reduction in cardiovascular disease associated with consumption of a low-fat/high-carbohydrate diet, and suggest there may be reduced risk with consumption of a very low-carbohydrate/high-fat diet [9,10]. Furthermore, consuming a diet with high sugar content (i.e. a higher ‘glycemic load’) was strongly associated with an increased risk of coronary heart disease [9]. The effects of sugar consumption on the development of LVH or cardiac failure in patients with hypertension have not been reported. We recently observed that feeding a high-carbohydrate diet, comprised mainly of fructose, to hypertensive rats impaired LV function and increased mortality up to 85% over a 13 weeks period compared to either a high-starch or a low-carbohydrate/high-fat diet [11]. Due to a low survival in this study, it was not possible to determine the influence of a high-sugar diet on key cardiovascular parameters or cardiac markers of molecular stress and hypertrophy [11]. A high-sugar diet may trigger greater insulin release, which activates cardiac protein synthesis and may increase LVH in response to hypertension [12], as suggested from studies in transgenic mice where activation of the insulin signaling pathways resulted in LVH and cardiac dysfunction [13,14].
The objective of the present investigation was to determine the effects of high-sugar diets on cardiac function, gene expression, LV remodeling, and mortality in hypertension. Studies with dietary interventions were performed with 8 weeks of hypertension in the well established Dahl salt-sensitive rat model of salt-induced hypertension [15]. This model results in a compensatory LVH during the initial 8–10 weeks of hypertension, followed by progressive deterioration of LV function, and development of heart failure [16]. Diets high in fructose or sucrose were compared to a high-starch or a low-carbohydrate/high-fat chow. LV function was assessed by echocardiography and cardiomyocyte apoptosis was measured histologically. Indices of cardiac remodeling were assessed, including LV end-diastolic diameter, levels of the mRNA that encode atrial natriuretic factor (ANF) and myosin heavy chain (MHC) isoforms (MHC-α and MHC-β), myocyte cross-sectional area, and interstitial fibrosis.
Methods
Animal care
The present study was conducted according to the guidelines for the care and use of laboratory animals (NIH publication No. 85-23) and was approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University. We performed the study using the Dahl salt-sensitive rat (Harlan, Indianapolis, Indiana, USA), which predictably develops concentric LVH after 6 weeks of salt-induced hypertension (6% NaCl by mass milled into rat chow) [11,15]. This model of salt-induced hypertension is devoid of any inflammatory response that may be attributed to a surgically-induced pressure-overload model. Male Dahl salt-sensitive rats were obtained at 9–11 weeks of age, and were housed in controlled conditions (23 ± 1°C) with a 12 h reverse light–dark cycle (06 : 00–18 : 00). All rats had free access to food and water.
Experimental design
Rats were initially fed standard lab chow (14% fat, 60% carbohydrates, 26% protein by energy, and 0.26% sodium; LabDiet, St Louis, Missouri, USA). After a baseline systolic blood pressure assessment rats were assigned to one of four dietary groups (high starch (STC), high fat (FAT), high fructose (FRU), and high sucrose (SUC)). After a week on low-salt chow, half the rats from each of the groups were placed on a high-salt background (+S; 6% NaCl by mass; n = 8 to 11/group). Rats were maintained on these diets for the duration of the 8 weeks study, and food consumption and rat weights were monitored weekly. At 6 weeks, another systolic blood pressure measurement was made. Echocardiography was performed at 7 weeks, and, at weeks, rats were sacrificed under isoflurane anesthesia, and aortic blood was drawn and tissue was rapidly harvested, weighed, and frozen for biochemical analysis. All physiological and biochemical measurements were performed with the investigator blinded to treatment.
Diets
All chows were manufactured by Research Diets, Inc. (New Brunswick, New Jersey, USA). The high-starch diet (STC) was composed of 70% carbohydrate (82% cornstarch, 18% maltodextrin), 10% fat (lard base – 37% saturated, 45% monounsaturated, 11% polyunsaturated), and 20% protein by energy. The high-fat (FAT) diet was composed of 60%oftotalenergyfrom fat comprised mainly of long chain saturated fatty acid from cocoa butter as previously described [17], 20% carbohydrates (65% maltodextrin, 35% sucrose), and 20% protein. The high-fructose (FRU) diet was composed of 70% carbohydrates (87% fructose, 13% cornstarch), 20% protein, and 10% fat (lard base). The high-sucrose (SUC) diet was composed of 70% carbohydrates (87% sucrose, 13% cornstarch), 20% protein, and 10% fat (lard base). After rats were assigned to one of these four low-salt diets for 1 week, half the rats from each of these diets (n = 8–11) were placed on a high-salt background for the duration of the study (8 weeks). The salt content in these diets was 0.29% and 6.0% by mass in the low and high-salt chows, respectively.
Blood pressure measurements
Systolic arterial blood pressure was measured at baseline prior to treatment and at 6 weeks of treatment using the tail cuff method as previously described [18].
Echocardiography
Echocardiographic measurement of LV function and dimensions were made at 7 weeks of treatment as previously described [4,5,19]. Briefly, LV function and dimensions were measured at 7 weeks of treatment using an ACUSON Sequoia C256 system (Siemens Medical Solutions, Malvern, Pennsylvania, USA) with a 15-MHz linear array transducer. Rats were anesthetized with 1.2–2.0% isoflurane by mask. After shaving their chest, they were situated in the supine position on a warming pad, and electrocardiogram limb electrodes were placed. With investigator blinded to treatment, two-dimensional guided M-mode and Doppler echocardiographic studies of aortic flows were performed from parasternal and foreshortened apical windows. Mitral inflow was not evaluated. Data were analyzed offline using software resident on the echocardiography system. LV end-diastolic and end-systolic diameters were determined from the short-axis view at the midpapillary level. Fractional shortening, myocardial performance index, aortic velocity–time integral, relative wall thickness, and velocity of circumferential shortening were calculated as previously described [4,5,19]. Absolute wall thickness was taken as the average of the anterior and posterior walls at end diastole.
Metabolic measurements
All terminal studies were performed on fed rats between 3 and 6 h from the initiation of the dark phase (06 : 00) of the daily reverse light–dark cycle. At 8 weeks of treatment, rats were weighed and anesthetized with 1.5–2% isoflurane. Blood samples were drawn from the inferior vena cava and processed for both plasma and serum. The heart was removed, and the atria and right ventricle were quickly separated from the LV. The LV was removed and weighed with a portion quickly embedded in a histological matrix (Tissue-Tek O.C.T., Bayer Corp., Pittsburgh, Pennsylvania, USA) and stored on dry ice. The remaining LV was freeze clamped and stored at −80°C. Plasma glucose, insulin, insulin-like growth factor-1, fatty acids, triglycerides, and serum adiponectin were assayed using commercially available kits. The activity of the mitochondrial fatty acid oxidation enzyme medium chain acyl-coenzyme A (CoA) dehydrogenase and the citric acid cycle enzyme citrate synthase were measured in heart homogenates as previously described [20].
mRNA measurements
Rat heart tissue was disrupted and homogenized by shaking in with 5-mm stainless-steel bead for 3 min at rate 30/s (Mixermill 300; Qiagen, Valencia, California, USA), followed by RNA isolation (RNeasy Mini Kit, Qiagen). On column DNase digestion was performed using RNase-free DNase set (Qiagen). RNA samples were eluted in 50 μl of nuclease-free water and stored at −80°C. RNA (1 μg) was mixed with 2 μl Oligo(dT)16 (Applied Biosystems, Foster City, California, USA) and 0.5 μl random primers (Invitrogen, Carlsbad, California, USA), and brought to a total volume of 15 μl. Sample/primer mix was heated to 70°C for 10 min, then placed immediately on the ice for 2 min, followed by the addition of the reverse transcriptase (RT) reaction mix containing 5× buffer (5 μl) and 0.1 mol/l dithiothreitol (DTT) (2.5 μl) (Superscript II RT, Invitrogen), 10 mmol/l deoxyribonucleotide triphosphate (dNTP) (1.25 μl; Invitrogen), and RNase inhibitor (0.25 μl; Applied Biosystems). Reaction mix was incubated for 2 min at 42°C, 1 μl reverse transcriptase added, and incubation continued at 42°C for 1 h. Reaction mix was then incubated at 70°C for 10 min, and placed on ice for 2 min. The resulting cDNA samples were stored at −20°C. Quantitative RT-PCR was performed using an ABI 7900 and the following protocol: 2 min at 50°C, 10 min at 95°C, 40 cycles at 95°C for 15 s, and 1 min at 60°C. Each reaction was 25 μl, consisting of 1.0 μl cDNA sample, 1.25 μl TaqMan Gene Expression Assay (Applied Biosystems), 12.5 μl 2× TaqMan PCR master mix, and 10.25 μl nuclease-free water. PCR was performed for each of the following genes, using TaqMan Gene Expression Assays (Applied Biosystems): ANF, MHC-α, MHC-β, medium chain acyl-CoA dehydrogenase, citrate synthase, carnitine palmitoyltransferase-β, pyruvate dehydrogenase kinase-4, uncoupling protein-3, peroxosome proliferator activated receptor-α (PPARα), and cyclophilin a. mRNA values for these genes were normalized to cyclophilin a mRNA, and expressed relative to the mean of the STC low-salt group.
Western immunoblot analysis
As dietary sugar might alter insulin signaling pathways that affect cardiac hypertrophy and function [12], cardiac tissue was analyzed for changes in the serine/threonine kinases AMP activated protein kinase (AMPK) and Akt. In addition, forkhead transcription factor 3a (FoxO3a), a member of the forkhead family of transcription factors involved in protein degradation, was also measured. Frozen powdered LV samples were homogenized in ice-cold buffer containing sufficient protease and phosphatase inhibitors (Sigma-Aldrich, St Louis, Missouri, USA) as previously described [21]. Crude homogenate was subjected to 20 800 g centrifuge at 4°C for 10 min. The supernatant was collected and its protein concentration determined using modified Lowry’s method (DC protein assay reagents, Bio-Rad, Hercules, California, USA). Seventy-five micrograms protein of each sample was mixed with 10 μl Laemmli buffer, boiled and loaded to 10% SDS-polyacrylamide gel for electrophoresis, before being transferred to nitrocellulose membranes (Pierce Biotechnology, Rockford, Illinois, USA) as previously described [22]. Membranes were blocked for 1 h at room temperature in LI-COR blocking buffer (LI-COR Biosciences, Lincoln, Nebraska, USA) and incubated overnight at 4°C with primary antibodies. Primary antibodies, including AMPK α2, phospho-AMPK α (Thr-172), Akt, phospho-Akt (Ser-473), Foxo3a, phosphor-Foxo3a (Thr-32), were all diluted at 1 : 1000 ratio in 5% BSA following instruction from manufacturer (Cell Signaling Technology, Danvers, Massachusetts, USA). Membranes were incubated with infrared fluorescence-labeled secondary antibodies (LI-COR Biosciences) for 1 h at room temperature, and then subjected to densitometry scan in Odyssey system (LI-COR Biosciences). Phosphorylated proteins were detected first, before the membranes were stripped (Pierce Restore stripping buffer) and reprobed for unphosphorylated proteins.
Histological measurements
Cardiomyocyte apoptosis in the LV free wall was measured by nuclear DNA fragmentation (nDNAf) in myosin positive cells [23,24]. Briefly, nDNAf was assessed using ApopTag in-situ fluorescein apoptosis detection kit (Oncor, Gaithersburg, Maryland, USA). The DeadEnd Fluorometric TUNEL (TdT-mediated dUTP Nick-End Labeling) System measured the fragmented DNA of apoptotic cells by incorporating fluorescein-12-dUTP at 3′-OH DNA ends using the terminal deoxynucleotidyl transferase (rTdT). Cardiomyocyte cross-sectional area, interstitial fibrosis, and capillarization were assessed as previously described [5,25].
Protein synthesis
As hypertension and diet may affect cardiac protein synthesis, we measured LV protein synthesis using the 2H2O labeling method as previously described [26–28]. Briefly, an 8-ml bolus of 0.9% NaCl in 2H2O was injected intraperitoneally 5 h prior to terminal surgery to allow for adequate isotope distribution into body water and free alanine and the incorporation of 2H-labeled alanine into proteins. Gas chromatography–mass spectrometry was used to determine the enrichment of 2H in body water and protein-bound alanine for calculations of LV protein fractional synthetic rate (FSR) [26–28] and body fat percentage [29].
Statistical analysis
Two-way analysis of variance (ANOVA) with post-hoc Bonferroni t-tests was used to assess the effects of diet and salt. Differences in mortality were assessed by a Kaplan–Meier survival curve with chi-square analysis (Graph Pad Prizm 4.0; Hearne Scientific Software, Chicago, Illinois, USA). Data are presented as mean ± SEM. P <0.05 was accepted as statistically significant.
Results
Blood pressure
There were no differences in arterial systolic blood pressure among any of the treatment groups at baseline (Table 1). After 6 weeks of treatment, systolic blood pressure increased significantly in the FAT, FRU, and SUC group compared to baseline values. The addition of salt caused pronounced hypertension in all diets (STC + S, FAT + S, FRU + S, SUC + S), but there were no differences in systolic blood pressure among any of the high-salt diets. There were no differences in heart rate between any of the groups at baseline or at 6 weeks of treatment (data not shown).
Table 1.
Body and heart masses, blood pressure, and histological data
| Parameter | STC | STC + S | FAT | FAT + S | FRU | FRU + S | SUC | SUC + S |
|---|---|---|---|---|---|---|---|---|
| Baseline body mass (g) | 381 ± 5 | 378 ± 9 | 370 ± 10 | 375 ± 8 | 360 ± 9 | 371 ± 18 | 384 ± 9 | 381 ± 4 |
| Final body mass (g) | 473 ± 8 | 427 ± 24* | 457 ± 18 | 440 ± 12 | 420 ± 9 | 373 ± 23*,† | 468 ± 13 | 409 ± 15* |
| Body fat (%) | 20.4 ± 2.1 | 19.9 ± 2.9 | 27.0 ± 1.6|| | 20.0 ± 1.5* | 27.9 ± 2.1|| | 17.1 ± 2.3* | 28.8 ± 1.2|| | 21.4 ± 2.3* |
| Arterial Systolic Blood Pressure (mmHg) | ||||||||
| Baseline | 127 ± 0.6 | 127 ± 1.2 | 128 ± 0.5 | 126 ± 0.9 | 127 ± 0.9 | 127 ± 0.7 | 127 ± 0.4 | 126 ± 0.6 |
| 6 weeks | 126 ± 0.7 | 222 ± 0.9* | 174 ± 1.1† | 222 ± 0.9* | 189 ± 0.9† | 225 ± 1.1* | 205 ± 1.3† | 225 ± 1.8* |
| Body fat (%) | 20.4 ± 2.1 | 19.9 ± 2.9 | 27.0 ± 1.6|| | 20.0 ± 1.5* | 27.9 ± 2.1|| | 17.1 ± 2.3* | 28.8 ± 1.2|| | 21.4 ± 2.3* |
| LV mass (g) | 1.01 ± 0.02 | 1.26 ± 0.06* | 0.91 ± 0.03 | 1.24 ± 0.04* | 0.82 ± 0.02 | 1.17 ± 0.06* | 1.07 ± 0.02‡ | 1.32 ± 0.05* |
| LV mass/body mass (mg/g) | 2.14 ± 0.04 | 3.00 ± 0.19* | 2.00 ± 0.04 | 2.82 ± 0.06* | 1.96 ± 0.04 | 3.17 ± 0.09*,† | 2.29 ± 0.09§ | 3.23 ± 0.16*,† |
| LV mass/tibial length (mg/cm) | 251 ± 7 | 307 ± 13* | 225 ± 9 | 304 ± 10* | 200 ± 5 | 290 ± 12* | 261 ± 7‡ | 320 ± 16* |
| RV mass/tibial length (mg/cm) | 66 ± 3 | 70 ± 5 | 65 ± 5 | 76 ± 5 | 61 ± 2 | 67 ± 3 | 67 ± 4 | 71 ± 7 |
| Myocyte cross-sectional area (μm2) | 467 ± 15 | 546 ± 26 | 472 ± 37 | 533 ± 33 | 457 ± 25 | 539 ± 31 | 467 ± 47 | 551 ± 41 |
| Interstitial fibrosis (%) | 10.6 ± 0.7 | ND | ND | ND | 10.6 ± 0.7 | 10.5 ± 0.8 | 10.4 ± 0.8 | 9.7 ± 0.6 |
| LV protein fractional synthesis rate (% newly made protein/hr) | 1.05 ± 0.09 | 1.09 ± 0.10 | 0.82 ± 0.06 | 1.11 ± 0.08* | 1.03 ± 0.08 | 1.02 ± 0.08 | 0.96 ± 0.11 | 1.14 ± 0.08 |
Values are means ± SEM. FAT, fat; FRU, fructose; LV, left ventricular; RV, right ventricular; S, salt; STC, starch; SUC, sucrose. ND, analysis was not done for these groups.
P <0.05 vs. same diet with low salt.
P <0.05 vs. FAT + S.
P <0.05 vs. FAT, FRU.
P <0.05 vs. FRU.
P <0.05 vs. STC.
Survival data
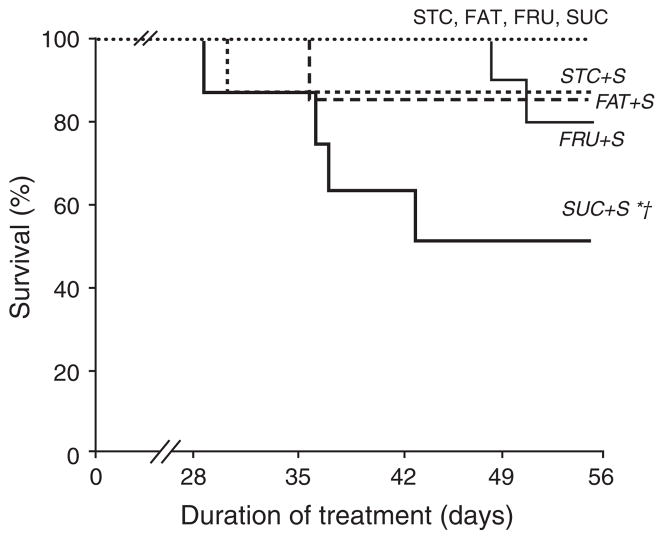
All rats that were fed low-salt diets (STC, FAT, FRU, and SUC) survived for the 8 weeks duration of the study (Fig. 1). Mortality was observed in the STC + S, FAT + S, and FRU + S groups (12%, 12%, and 18%, respectively), and was significantly greater in the SUC + S group (44%) compared to all other high-salt groups. There was no evidence of decompensated heart failure (edema, pulmonary congestion, lethargy) or stroke in any of the animals.
Fig. 1.
Survival of rats over the 8 weeks duration of the study. *P <0.05 compared to the same dietary group with low salt. † P <0.05 compared to all other diets with high salt. Values are means ± SEM.
Body and heart mass, and protein synthesis
There was no difference in body mass at baseline, but final body mass was higher in the STC, FRU, and SUC groups compared to the same diet with high salt (Table 1). Left ventricular (LV) mass was increased in the all high-salt groups compared to the same diet on low salt, but right ventricular (RV) mass/tibia length was unchanged (Table 1). There was no difference in FSR of endogenous protein among the low-salt dietary groups (Table 1), but there was an increase in FSR in the FAT + S group compared to FAT. There was no effect of dietary salt on FSR in the other diet groups. Percentage body fat was increased in the FAT, FRU, and SUC groups compared to the STC group; however, there were no differences among the high-salt groups (Table 1). The FAT + S, FRU + S, and SUC + S had low-percentage body fat than the same diets with low salt.
Cardiac dimensions and performance
LV wall thickness was increased by the addition of salt in all diets, whereas relative wall thickness was increased only in the FAT + S and FRU + S groups (Table 2). LV end-diastolic diameter was unaffected by hypertension or diet, suggesting that these animals were in the compensatory phase of LVH in the well described progression to heart failure that occurs in this model [15,16] (Table 2). End-systolic diameter was elevated in the FRU + S and SUC + S groups compared to the same diet with low salt. Area of fractional shortening and velocity of circumferential shortening were decreased in the FRU + S and SUC + S groups compared to the same diets with low salt (Table 2). Velocity of circumferential and fractional shortening was also decreased in the SUC + S group compared to STC + S and FAT + S groups.
Table 2.
Echocardiography measurements after 7 weeks of treatment
| STC | STC + S | FAT | FAT + S | FRU | FRU + S | SUC | SUC + S | |
|---|---|---|---|---|---|---|---|---|
| End-diastolic diameter (mm) | 8.4 ± 0.2 | 8.6 ± 0.03 | 8.1 ± 0.1 | 8.5 ± 0.3 | 8.1 ± 0.2 | 8.1 ± 0.3 | 8.3 ± 0.1 | 8.4 ± 0.3 |
| End-systolic diameter (mm) | 4.8 ± 0.2 | 4.9 ± 0.3 | 4.5 ± 0.1 | 4.7 ± 0.3 | 4.4 ± 0.2 | 5.0 ± 0.3* | 4.5 ± 0.1 | 5.5 ± 0.2* |
| Fractional shortening (%) | 55 ± 1 | 53 ± 2 | 57 ± 1 | 53 ± 3 | 59 ± 1 | 46 ± 3* | 57 ± 1 | 42 ± 5*,† |
| Velocity of circumferential shortening (per s) | 5.99 ± 0.18 | 5.61 ± 0.16 | 6.55 ± 0.26 | 6.43 ± 0.42 | 6.14 ± 0.24 | 5.10 ± 0.19* | 6.18 ± 0.29 | 4.62 ± 0.49*,† |
| Absolute wall thickness (mm) | 2.0 ± 0.1 | 2.3 ± 0.2* | 2.0 ± 0.1 | 2.4 ± 0.1* | 1.8 ± 0.1 | 2.4 ± 0.1* | 1.9 ± 0.05 | 2.4± 0.1* |
| Relative wall thickness (mm) | 0.48 ± 0.02 | 0.54 ± 0.04 | 0.47 ± 0.02 | 0.57 ± 0.04* | 0.46 ± 0.02 | 0.58 ± 0.03* | 0.47 ± 0.02 | 0.54 ± 0.03 |
| Myocardial performance index | 0.42 ± 0.02 | 0.47 ± 0.04 | 0.43 ± 0.03 | 0.45 ± 0.05 | 0.40 ± 0.01 | 0.46 ± 0.04 | 0.38 ± 0.02 | 0.44 ± 0.02 |
| Aortic velocity–time integral (cm) | 2.46 ± 0.14 | 1.86 ± 0.09* | 2.61 ± 0.14 | 2.51 ± 0.16‡ | 2.54 ± 0.17 | 2.29 ± 0.18 | 2.60 ± 0.19 | 2.32 ± 0.21 |
Values are means ± SEM. FAT, fat; FRU, fructose; S, salt; STC, starch; SUC, sucrose.
P <0.05 vs. same diet with low salt.
P <0.05 compared to STC + S and FAT + S groups.
P <0.05 vs. STC + S.
Atrial natriuretic factor and myosin heavy chain expression
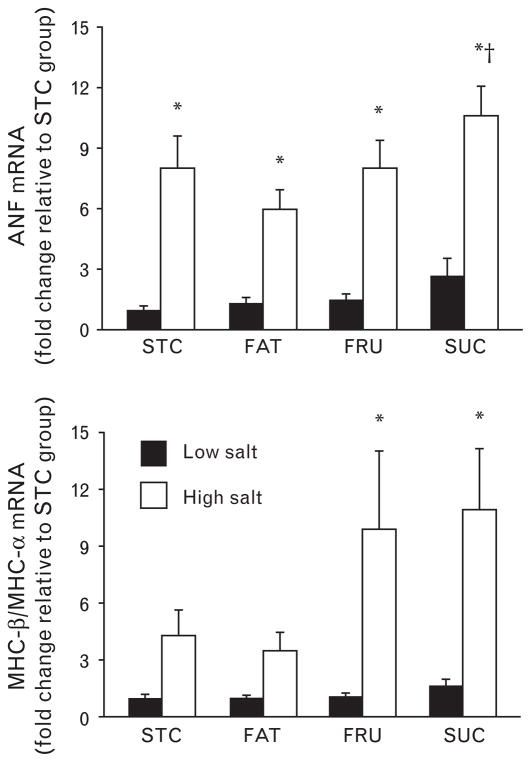
ANF mRNA was increased in all high-salt groups compared to the same diets on low salt, and there was a further increase in the SUC + S compared to the FAT + S group (Fig. 2). Hypertension caused a switch in the isoforms of MHC from α to β in the FRU and SUC groups, and this effect was greater in the SUC + S and the FRU + S groups than in the STC + S and FAT + S groups (Fig. 2). There was a decrease MHC-α mRNA in all high-salt diets compared to the same diets on low salt and there was an increase in MHC-β in the FAT + S, FRU + S, and SUC + S groups compared to same diets with low salt (Table 3).
Fig. 2.
mRNA levels for ANF (top) and the ratio of mRNA for MHC-β/MHC-α (bottom). Filled bars represent low-salt diets and open bars represent high-salt diets. *P <0.05 compared to the same dietary group with low salt. † P <0.05 compared to FAT + S group. ‡P <0.05 compared to STC + S and FAT + S groups. Values are means ± SEM.
Table 3.
Cardiac mRNA expressed as a fraction of the low-salt STC group
| Mass | STC | STC + S | FAT | FAT + S | FRU | FRU + S | SUC | SUC + S |
|---|---|---|---|---|---|---|---|---|
| MHCα | 1.00 ± 0.05 | 0.53 ± 0.09* | 0.95 ± 0.04 | 0.54 ± 0.07* | 0.88 ± 0.05 | 0.30 ± 0.05* | 0.72 ± 0.09§ | 0.27 ± 0.08* |
| MHCβ | 1.00 ± 0.22 | 1.43 ± 0.15 | 0.76 ± 0.12 | 1.42 ± 0.12* | 0.80 ± 0.18 | 1.74 ± 0.21* | 0.95 ± 0.15 | 1.97 ± 0.24* |
| Citrate synthase | 1.00 ± 0.05 | 0.96 ± 0.08 | 1.09 ± 0.03 | 0.95 ± 0.07 | 0.99 ± 0.07 | 0.70 ± 0.05* | 0.79 ± 0.13† | 0.79 ± 0.07 |
| PPARα | 1.00 ± 0.02 | 0.74 ± 0.05* | 0.92 ± 0.03 | 0.87 ± 0.04 | 0.95 ± 0.05 | 0.66 ± 0.03*,‡ | 0.85 ± 0.05§ | 0.76 ± 0.06 |
| Carnitine palmitoyl transferase-1β | 1.00 ± 0.07 | 0.84 ± 0.04 | 1.00 ± 0.03 | 1.00 ± 0.06 | 0.86 ± 0.06 | 0.73 ± 0.06 | 0.77 ± 0.06 | 0.75 ± 0.08 |
| Uncoupling protein 3 | 1.00 ± 0.10† | 0.68 ± 0.12‡ | 2.01 ± 0.20 | 1.31 ± 0.16* | 0.95 ± 0.10† | 0.43 ± 0.08*,‡ | 0.84 ± 0.18† | 0.29 ± 0.06*,‡ |
| Pyruvate dehydrogenase kinase 4 | 1.00 ± 0.17† | 1.38 ± 0.20 | 2.37 ± 0.34 | 1.63 ± 0.28* | 0.85 ± 0.14† | 0.85 ± 0.17 | 1.03 ± 0.15† | 0.66 ± 0.15 |
| Medium chain acyl-CoA dehydrogenase | 1.00 ± 0.03 | 0.69 ± 0.05*,‡ | 1.16 ± 0.06 | 0.97 ± 0.05 | 0.99 ± 0.07 | 0.65 ± 0.09*,‡ | 0.82 ± 0.08† | 0.56 ± 0.12*,‡ |
Values are means ± SEM. FAT, fat; FRU, fructose; MHC, myosin heavy chain; PPARα, peroxosome proliferator activated receptor-α; S, salt; STC, starch; SUC, sucrose. Samples were standardized to cyclophilin A mRNA.
P <0.05 vs. same diet with low salt.
P <0.05 vs. FAT.
P <0.05 vs. FAT + S.
P <0.05 vs. STC.
Histology
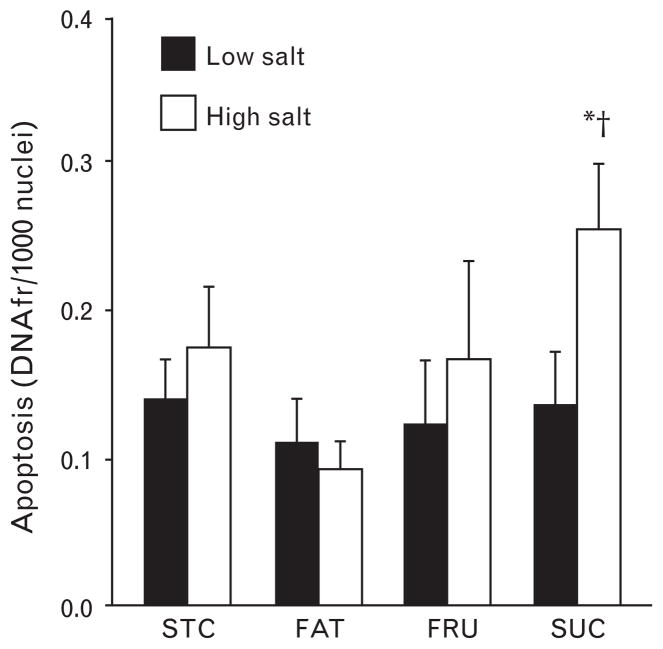
Analysis of nuclear DNA fragmentation indicates that there was significantly greater apoptosis in the SUC + S groups compared to the SUC, STC + S, and FAT + S groups (Fig. 3). There was a trend for an increase in cardoimyocyte cross-sectional area in the groups fed high-salt diets (~16% increase), but this difference did not reach statistical significance in any of the dietary treatments (Table 1). In addition, capillary density was similar among groups (data not shown), as was interstitial fibrosis (Table 1). The lack of a significant increase in cardiomyocyte cross-sectional area despite a clear increase in LV mass is likely attributable to the greater coefficient of variation in the measurement of myocyte cross-sectional area compared to gravimetrically measured LV mass (Table 1).
Fig. 3.
Levels of apoptosis as indicated by the presence of nuclear DNA fragmentation (DNAfr). Filled bars represent low-salt diets and open bars represent high-salt diets. *P <0.05 compared to the same dietary group with low salt. † P <0.05 compared to STC + S and FAT + S groups. Values are means ± SEM.
Metabolic parameters
Plasma glucose concentration was similar among all dietary treatments with low salt (Table 4), and the addition of salt decreased plasma glucose in the FRU + S S and SUC + S groups. Plasma insulin was also similar among all dietary treatments with low salt, but was lower in the FAT + S compared to the FAT group (Table 4). Insulin-like growth factor-1, a peptide hormone released by the liver that has similar effects to insulin, was lower in the FAT group compared to all other low-salt groups, and was lower in the STC + S, FRU + S, and SUC + S compared to the same diets with low salt (Table 4). Plasma-free fatty acids in the FAT group were greater compared to STC and FRU groups, and they were lesser in the FAT + S and SUC + S groups compared to the same diets on low salt (Table 4).
Table 4.
Metabolite and hormone concentrations in plasma or serum in fed animals after 8 weeks of treatment
| Metabolite | STC | STC + S | FAT | FAT + S | FRU | FRU + S | SUC | SUC + S |
|---|---|---|---|---|---|---|---|---|
| Plasma glucose (mmol/l) | 8.49 ± 0.70 | 8.77 ± 0.34 | 8.82 ± 0.78 | 8.64 ± 0.64 | 9.92 ± 0.80 | 7.48 ± 0.31* | 9.61 ± 0.43 | 7.35 ± 0.57* |
| Plasma-free fatty acids (mmol/l) | 0.39 ± 0.03†,‡ | 0.38 ± 0.04 | 0.82 ± 0.1 | 0.44 ± 0.05* | 0.55 ± 0.05† | 0.42 ± 0.04 | 0.70 ± 0.04 | 0.35 ± 0.09* |
| Plasma triglycerides (mg/ml) | 1.39 ± 0.24 | 1.23 ± 0.14 | 1.15 ± 0.13 | 0.95 ± 0.08 | 2.02 ± 0.39† | 1.24 ± 0.19* | 1.50 ± 0.26 | 1.24 ± 0.18 |
| Plasma insulin (pmol/l) | 80 ± 16 | 35 ± 7 | 150 ± 47 | 53 ± 15* | 83 ± 22 | 51 ± 9 | 89 ± 33 | 36 ± 9 |
| Plasma IGF-1 (ng/ml) | 939 ± 50† | 780 ± 47* | 736 ± 52 | 759 ± 60 | 955 ± 46† | 724 ± 60* | 936 ± 44† | 754 ± 64* |
| Serum adiponectin (μg/ml) | 9.0 ± 0.5§ | 9.1 ± 0.5|| | 8.1 ± 0.4 | 8.7 ± 0.5 | 6.7 ± 0.3 | 7.4 ± 0.4 | 9.6 ± 0.6§ | 9.4 ± 0.7|| |
Values are means ± SEM. FAT, fat; FRU, fructose; S, salt; STC, starch; SUC, sucrose; IGF-1, insulin-like growth factor-1.
P <0.05 vs. same diet with low salt.
P <0.05 vs. FAT.
P <0.05 vs. SUC.
P <0.05 vs. FRU.
P <0.05 vs. FRU + S.
In general, the mRNA levels of PPARα-regulated genes were decreased by high-salt chow (Table 3). High salt intake decreased cardiac PPARα mRNA levels in the STC + S and FRU + S groups (Table 3), but not in FAT + S and SUC + S groups. The high-fat diet increased expression of the PPARα-regulated target genes UCP3 and PDK-4 compared to all other low-salt diets. mRNA for medium chain acyl-CoA dehydrogenase was lower in the STC + S, FRU + S, and SUC + S groups compared to the same diets with low salt (Table 3). However, the FAT + S group did not exhibit the decrease in medium chain acyl-CoA dehydrogenase mRNA that was observed in all other high-salt groups. The diet-induced changes in medium chain acyl-CoA dehydrogenase mRNA were not mirrored by changes in enzymatic activity or the ratio of medium chain acyl-CoA dehydrogenase/citrate synthase activity, as this ration was lower in all high-salt groups regardless of diet (Table 5).
Table 5.
Activity of medium chain acyl-CoA dehydrogenase and citrate synthase in the heart
| Enzyme activities | STC | STC + S | FAT | FAT + S | FRU | FRU + S | SUC | SUC + S |
|---|---|---|---|---|---|---|---|---|
| MCAD activity (μmol/min/g) | 14.5 ± 0.9 | 11.4 ± 0.7*,† | 12.1 ± 0.4 | 8.2 ± 0.6* | 14.2 ± 0.50 | 10.1 ± 0.7* | 14.2 ± 0.5 | 10.4 ± 0.8* |
| CS activity (μmol/min/g) | 153 ± 6 | 137 ± 6* | 150 ± 2 | 140 ± 4 | 156 ± 3 | 133 ± 6* | 150 ± 5 | 131 ± 10* |
| MCAD/CS activity | 0.094 ± 0.003 | 0.083 ± 0.002* | 0.081 ± 0.002‡ | 0.059 ± 0.007*,§ | 0.091 ± 0.002 | 0.075 ± 0.003* | 0.094 ± 0.003 | 0.079 ± 0.002* |
Values are means ± SEM. CS, citrate synthase; FAT, fat; FRU, fructose; MCAD, medium chain acyl-CoA dehydrogenase; S, salt; STC, starch; SUC, sucrose.
P <0.05 vs. same diet with low salt.
P <0.05 vs. FAT + S.
P <0.05 vs. all other low-salt diets.
P <0.05 vs. all other high-salt diets.
Western immunoblots
There were no differences in total Akt, phospho-Akt, or the ratio of phospho-Akt/total Akt between the STC and FRU or SUC groups with or without salt (Table 6). Both total Akt and phospho-Akt were increased in the SUC group compared to FAT, however, the ratio of phospho-Akt/total Akt was not different. There were no differences in phospho-AMPK among treatment groups; however, total AMPK expression was increased in STC + S and FRU + S compared to respective low-salt control (P <0.05) (Table 6). In addition, total AMPK and the ratio of phospho-AMPK/total AMPK were greater in the STC group than in the FAT group. There were no differences in FoxO3a total protein or phosphorylated protein (Table 6).
Table 6.
Western blot densitometry data (arbitrary units)
| Protein | STC | STC + S | FAT | FAT + S | FRU | FRU + S | SUC | SUC + S |
|---|---|---|---|---|---|---|---|---|
| Phospho-AMPK | 4.34 ± 0.36 | 5.13 ± 0.31 | 4.17 ± 0.20 | 4.57 ± 0.30 | 4.43 ± 0.19 | 4.41 ± 0.17 | 4.44 ± 0.17 | 4.44 ± 0.12 |
| Total AMPK | 14.5 ± 0.63 | 18.2 ± 1.00*,‡ | 12.9 ± 0.98† | 14.5 ± 0.7 | 14.0 ± 1.4† | 17.4 ± 0.9* | 17.6 ± 0.83 | 18.1 ± 0.9 |
| Phospho-AMPK/total AMPK | 0.300 ± 0.025 | 0.281 ± 0.008 | 0.330 ± 0.016† | 0.315 ± 0.011 | 0.334 ± 0.035† | 0.257 ± 0.014* | 0.254 ± 0.012 | 0.246 ± 0.012 |
| Phospho-Akt | 1.49 ± 0.02 | 1.52 ± 0.05 | 1.45 ± 0.02† | 1.46 ± 0.02 | 1.51 ± 0.03 | 1.45 ± 0.03§ | 1.57 ± 0.04 | 1.58 ± 0.04 |
| Total Akt | 8.50 ± 0.52 | 10.05 ± 0.74 | 7.45 ± 0.54† | 8.73 ± 0.86 | 7.96 ± 0.72 | 9.25 ± 0.50 | 9.95 ± 0.50 | 10.99 ± 1.19 |
| Phospho-Akt/total Akt | 0.180 ± 0.011 | 0.154 ± 0.008 | 0.200 ± 0.012 | 0.176 ± 0.018 | 0.197 ± 0.016 | 0.160 ± 0.009* | 0.160 ± 0.007 | 0.148 ± 0.011 |
| Phospho-FoxO3a | 6.00 ± 0.21 | 6.62 ± 0.22 | 6.06 ± 0.23 | 6.16 ± 0.33 | 6.34 ± 0.22 | 5.93 ± 0.25 | 6.10 ± 0.21 | 5.77 ± 0.10 |
| Total FoxO3a | 4.68 ± 0.04 | 4.83 ± 0.07 | 4.63 ± 0.06 | 4.71 ± 0.10 | 4.75 ± 0.07 | 4.65 ± 0.06 | 4.69 ± 0.06 | 4.61 ± 0.05 |
| Phospho-FoxO3a/total FoxO3a | 1.28 ± 0.04 | 1.37 ± 0.03 | 1.31 ± 0.04 | 1.31 ± 0.05 | 1.33 ± 0.03 | 1.27 ± 0.04 | 1.300 ± 0.04 | 1.25 ± 0.02 |
Values are means ± SEM. AMPK, AMP activated protein kinase.
P <0.05 vs. same diet with low salt.
P <0.05 vs. SUC.
P <0.05 vs. FAT + S.
P <0.05 vs. SUC + S.
Discussion
Despite a similar severity of hypertension, a high-sucrose diet increased LV dysfunction causing a greater MHC isoform switch and upregulation of ANF, accelerated apoptosis, and increased mortality compared to either a low-carbohydrate/high-fat or high-starch diet. Moreover, although, in our previous study, similar effects were observed with a high-fructose chow [11], in the present investigation, the high-sucrose diet triggered an earlier onset of mortality and greater apoptosis than observed with all other diets. Taken together, the results of the present investigation are consistent with the concept that macronutrient composition, especially the contribution of sugars, can have a profound effect on the development of cardiac dysfunction and mortality in hypertension [5,11].
Left ventricular function was impaired in the SUC + S group compared to all other treatment groups, as reflected by an increase in end-systolic volume and a lower ejection fraction (Table 2). On the contrary, there was no indication of LV chamber dilation, as reflected in the maintenance a normal end-diastolic diameter (Table 2). Hypertension also induced expression of MHC-β and ANF, with a greater increase in ANF in the SUC + S compared to all other groups (Fig. 2), suggesting that LV dysfunction with sucrose feeding is due at least in part to changes in gene expression. Rat cardiomyocytes express both MHCα and MHCβ, with MHCα being associated with faster velocity of shortening than MHCβ [30]. This suggests that the greater isoform switch with high-sugar diets contributes to worse LV systolic function in these animals.
The mechanisms for greater mortality with a high-sucrose diet in hypertension are unclear. Previous studies demonstrated that enhanced stimulation of the insulin-Akt signaling pathway caused hypertrophy and dysfunction in transgenic animals and isolated cardiomyocytes [13,31–33]; however, in the present investigation, rats fed high-sugar diets did not have greater LVH (Table 1), elevated insulin levels (Table 4), or phosphorylation of Akt [33,34] (Table 6). This may be attributed to salt-induced alterations in eating behavior, consistent with the trend for decreased weight gain in the high-salt groups compared to the rats fed low-salt diets (Table 1). On the contrary, we recently observed a similar increase in LV dysfunction and mortality with a high-sugar diet compared to a high-starch diet in mice subjected to pressure overload induced by transverse aortic constriction [35]. Plasma insulin levels were increased on the high-sugar diet; however; Akt phosphorylation was unchanged in all experimental groups. An alternative explanation for accelerated cardiac pathology and mortality with fructose and sucrose diets is the generation of reactive oxygen species, perhaps linked to flux through the oxidative pentose phosphate pathway, as suggested recently in advanced heart failure in dogs and patients [36,37]. In addition, deterioration in renal function may also play a role, as suggested by a recent study showing that a high-fructose diet can cause kidney hypertrophy, glomerular hypertension, cortical vasoconstriction, and a decreased glomerular filtration rate in rats [38].
The Dahl salt-sensitive rat develops worsening heart failure and mortality from 12–20 weeks of salt-induced hypertension when fed a standard laboratory chow, which corresponds with a progressive increase in cardiomyocyte apoptosis [15]. The present results extend these findings by demonstrating that there is an early increase in cardiomyocyte apoptosis with high sucrose consumption compared to a high-starch or low-carbohydrate/high-fat diet in hypertensive Dahl salt-sensitive rats. This observation suggests the possibility that sucrose consumption triggers proapoptotic mechanisms that lead to LV dysfunction and early mortality. However, it is important to note that the increase in apoptosis is relatively modest in the sucrose fed hypertensive animals (~90%; Fig. 3), which is far less than the 15-fold increase in more advanced heart failure (~14 weeks of hypertension) in this model) [15]. Thus, the modest increase in apoptosis observed with sucrose feeding in the present study, while statistically significant, is unlikely to be responsible for the LV dysfunction and mortality observed in this treatment group.
Protein synthesis rate, as measured with the 2H2O method [26,27], showed no effect of hypertension on protein synthesis despite an increase in LV mass (Table 1). To our knowledge, this is the first study to assess protein synthesis in the heart with 2H2O method. The values that we obtained (~1.0%/h) are similar to the values previously reported in conscious rats using the [2,6-3H]phenylalanine incorporation method [39]. From the increase in LV mass and the fractional synthesis rate, the relative imbalance between protein synthesis and breakdown that is required to result in LVH can be estimated. Assuming that there was 30% increase in LV mass with hypertension that occurred linearly over 60 days, and 25% of cardiac protein turns over daily [39] (Table 1), the net accumulation of protein in the heart each day would be 0.5% of cardiac protein that reflects only 2% of the rate of protein synthesis (0.5%/25%). These data suggest that only a minor imbalance in protein synthesis and breakdown are required to trigger cardiac hypertrophy.
The animals fed the low-salt FAT, FRU and SUC diets had hypertension after 6 weeks, but did not develop LVH or LV dysfunction. The mechanism for this response is unclear, but this observation suggests that the LVH and cardiac pathology in this model is at least partially dependent on high salt intake. Previous studies documented that diets high in simple sugar can result in moderate levels of hypertension due to increased sympathetic activity [40]. We have previously observed that high-fat diets that have no effect on Wistar rats result in modest hypertension in the Dahl salt-sensitive rat [5,11,17], suggesting that blood pressure in the Dahl strain is uniquely sensitive to dietary fat intake. The lack of LVH and contractile dysfunction in the low-salt FAT, FRU, and SUC fed groups suggests that there is a blood pressure threshold that has to be achieved to before hypertrophic mechanisms are activated.
In summary, the present study demonstrates that consuming a high-sucrose diet during hypertension causes early LV dysfunction and mortality compared to high-starch or low-carbohydrate/high-fat diet. While current American Heart Association (AHA) dietary guidelines for the general population recommend a high-carbohydrate/low-fat diet [3] and suggest minimizing the intake of sugar, there is little experimental evidence showing that the risk for cardiovascular disease is affected by consumption of simple sugar, particularly in the setting of hypertension [7]. Our current findings demonstrate a profound consequence attributed to sugar consumption during hypertension. These results, taken together with our previous findings in the same model [4,5,11], suggest that an optimal dietary prescription for preventing heart failure in hypertension should include minimizing sugar intake. There is little direct evidence supporting this recommendation in patients; however, recent epidemiological studies show an increased risk for coronary heart disease as the dietary ‘glycemic load’ increases [9]. Future studies should assess the effect of sugar consumption on the development of LVH and heart failure in hypertensive patients.
Acknowledgments
We would like to thank Dr Margaret Chandler for her insight and advice; Janean Johnson for conducting the blood pressure measurements; Tracy McElfresh, Hazel Huang and Cody Rutledge for technical support; and Danielle Gilge for assistance with the GCMS analysis. This study was supported by National Institutes of Health grants HL-074237, RoadMap 1R33DK070291-01, and Mt. Sinai Healthcare Foundation (Cleveland, Ohio, USA). Naveen Sharma was supported by a predoctoral fellowship from the American Heart Association, Ohio Valley affiliate.
Abbreviations
- AMPK
AMP activated protein kinase
- ANF
atrial natriuretic factor
- FSR
fractional synthetic rate
- FAT
high fat
- FRU
high fructose
- SUC
high sucrose
- LVH
left ventricular hypertrophy
- MHC
myosin heavy chain
- nDNAf
nuclear DNA fragmentation
- PPARα
peroxosome proliferator activated receptor-α
- STC
high starch
Footnotes
There are no conflicts of interest.
References
- 1.Gradman AH, Alfayoumi F. From left ventricular hypertrophy to congestive heart failure: management of hypertensive heart disease. Prog Cardiovasc Dis. 2006;48:326–341. doi: 10.1016/j.pcad.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–173. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 4.Okere IC, Chess DJ, McElfresh TA, Johnson J, Rennison J, Ernsberger P, et al. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive Dahl salt-sensitive rat. Clin Exp Pharmacol Physiol. 2005;32:825–831. doi: 10.1111/j.1440-1681.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- 5.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, et al. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 6.Fried SK, Rao SP. Sugars, hypertriglyceridemia, and cardiovascular disease. Am J Clin Nutr. 2003;78:873S–880S. doi: 10.1093/ajcn/78.4.873S. [DOI] [PubMed] [Google Scholar]
- 7.Griel AE, Ruder EH, Kris-Etherton PM. The changing roles of dietary carbohydrates: from simple to complex. Arterioscler Thromb Vasc Biol. 2006;26:1958–1965. doi: 10.1161/01.ATV.0000233384.97125.bd. [DOI] [PubMed] [Google Scholar]
- 8.Popkin BM, Nielsen SJ. The sweetening of the World’s diet. Obesity Res. 2003;11:1325–1332. doi: 10.1038/oby.2003.179. [DOI] [PubMed] [Google Scholar]
- 9.Halton TL, Willett WC, Liu S, Manson JE, Albert CM, Rexrode K, et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N Engl J Med. 2006;355:1991–2002. doi: 10.1056/NEJMoa055317. [DOI] [PubMed] [Google Scholar]
- 10.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, et al. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 11.Sharma N, Okere IC, Duda MK, Johnson J, Yuan CL, Chandler MP, et al. High fructose diet increases mortality in hypertensive rats compared to a complex carbohydrate or high fat diet. Am J Hypertens. 2007;20:403–409. doi: 10.1016/j.amjhyper.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Sharma N, Okere IC, Duda MK, Chess DJ, O’Shea KM, Stanley WC. Potential impact of carbohydrate and fat intake on pathological left ventricular hypertrophy. Cardiovasc Res. 2007;73:257–268. doi: 10.1016/j.cardiores.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109:629–639. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, et al. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation. 2003;107:1664–1670. doi: 10.1161/01.CIR.0000057979.36322.88. [DOI] [PubMed] [Google Scholar]
- 15.Kang PM, Yue P, Liu Z, Tarnavski O, Bodyak N, Izumo S. Alterations in apoptosis regulatory factors during hypertrophy and heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H72–H80. doi: 10.1152/ajpheart.00556.2003. [DOI] [PubMed] [Google Scholar]
- 16.Klotz S, Hay I, Zhang G, Maurer M, Wang J, Burkhoff D. Development of heart failure in chronic hypertensive Dahl rats: focus on heart failure with preserved ejection fraction. Hypertension. 2006;47:901–911. doi: 10.1161/01.HYP.0000215579.81408.8e. [DOI] [PubMed] [Google Scholar]
- 17.Okere IC, Chandler MP, McElfresh TA, Rennison JH, Sharov V, Sabbah HN, et al. Differential effects of saturated and unsaturated fatty acid diets on cardiomyocyte apoptosis, adipose distribution, and serum leptin. Am J Physiol Heart Circ Physiol. 2006;291:H38–H44. doi: 10.1152/ajpheart.01295.2005. [DOI] [PubMed] [Google Scholar]
- 18.Ernsberger P, Nelson DO. Refeeding hypertension in dietary obesity. Am J Physiol Regul Integr Comp Physiol. 1988;254:R47–R55. doi: 10.1152/ajpregu.1988.254.1.R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morgan EE, Faulx MD, McElfresh TA, Kung TA, Zawaneh MS, Stanley WC, et al. Validation of echocardiographic methods for assessing left ventricular dysfunction in rats with myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H2049–H2053. doi: 10.1152/ajpheart.00393.2004. [DOI] [PubMed] [Google Scholar]
- 20.Panchal AR, Stanley WC, Kerner J, Sabbah HN. Beta-receptor blockade decreases carnitine palmitoyl transferase I activity in dogs with heart failure. J Card Fail. 1998;4:121–126. doi: 10.1016/s1071-9164(98)90252-4. [DOI] [PubMed] [Google Scholar]
- 21.Lei B, Matsuo K, Labinskyy V, Sharma N, Chandler MP, Ahn A, et al. Exogenous nitric oxide reduces glucose transporters translocation and lactate production in ischemic myocardium in vivo. Proc Natl Acad Sci USA. 2005;102:6966–6971. doi: 10.1073/pnas.0500768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem. 2003;278:39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 23.Goussev A, Sharov VG, Shimoyama H, Tanimura M, Lesch M, Goldstein S, et al. Effects of ACE inhibition on cardiomyocyte apoptosis in dogs with heart failure. Am J Physiol. 1998;275 (2 Pt 2):H626–H631. doi: 10.1152/ajpheart.1998.275.2.H626. [DOI] [PubMed] [Google Scholar]
- 24.Sabbah HN, Sharov VG, Gupta RC, Todor A, Singh V, Goldstein S. Chronic therapy with metoprolol attenuates cardiomyocyte apoptosis in dogs with heart failure. J Am Coll Cardiol. 2000;36:1698–1705. doi: 10.1016/s0735-1097(00)00913-x. [DOI] [PubMed] [Google Scholar]
- 25.Sabbah HN, Stanley WC, Sharov VG, Mishima T, Tanimura M, Benedict CR, et al. Effects of dopamine beta-hydroxylase inhibition with nepicastat on the progression of left ventricular dysfunction and remodeling in dogs with chronic heart failure. Circulation. 2000;102:1990–1995. doi: 10.1161/01.cir.102.16.1990. [DOI] [PubMed] [Google Scholar]
- 26.Dufner D, Previs SF. Measuring in vivo metabolism using heavy water. Curr Opin Clin Nutr Metab Care. 2003;6:511–517. doi: 10.1097/00075197-200309000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Dufner DA, Bederman IR, Brunengraber DZ, Rachdaoui N, Ismail-Beigi F, Siegfried BA, et al. Using 2H2O to study the influence of feeding on protein synthesis: effect of isotope equilibration in vivo vs. in cell culture. Am J Physiol Endocrinol Metab. 2005;288:E1277–E1283. doi: 10.1152/ajpendo.00580.2004. [DOI] [PubMed] [Google Scholar]
- 28.Previs SF, Fatica R, Chandramouli V, Alexander JC, Brunengraber H, Landau BR. Quantifying rates of protein synthesis in humans by use of 2H2O: application to patients with end-stage renal disease. Am J Physiol Endocrinol Metab. 2004;286:E665–E672. doi: 10.1152/ajpendo.00271.2003. [DOI] [PubMed] [Google Scholar]
- 29.McCabe BJ, Bederman IR, Croniger C, Millward C, Norment C, Previs SF. Reproducibility of gas chromatography-mass spectrometry measurements of 2H labeling of water: application for measuring body composition in mice. Anal Biochem. 2006;350:171–176. doi: 10.1016/j.ab.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Pauletto P, Vescovo G, Scannapieco G, Angelini A, Pessina AC, Dalla LL, et al. Changes in rat ventricular isomyosins with regression of cardiac hypertrophy. Hypertension. 1986;8:1143–1148. doi: 10.1161/01.hyp.8.12.1143. [DOI] [PubMed] [Google Scholar]
- 31.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 32.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 33.Shiojima I, Yefremashvili M, Luo Z, Kureishi Y, Takahashi A, Tao J, et al. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J Biol Chem. 2002;277:37670–37677. doi: 10.1074/jbc.M204572200. [DOI] [PubMed] [Google Scholar]
- 34.Daly M. Sugars, insulin sensitivity, and the postprandial state. Am J Clin Nutr. 2003;78:865S–8872S. doi: 10.1093/ajcn/78.4.865S. [DOI] [PubMed] [Google Scholar]
- 35.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Deleterious effects of sugar and protective effects of starch on cardiac remodeling, contractile dysfunction, and mortality in response to pressure overload. Am J Physiol Heart Circ Physiol. 2007;293:H1853–H1860. doi: 10.1152/ajpheart.00544.2007. [DOI] [PubMed] [Google Scholar]
- 36.Gupte RS, Vijay V, Marks B, Levine RJ, Sabbah HN, Wolin MS, et al. Upregulation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase activity increases oxidative stress in failing human heart. J Card Fail. 2007;13:497–506. doi: 10.1016/j.cardfail.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, et al. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol. 2006;41:340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Lozada LG, Tapia E, Jimenez A, Bautista P, Cristobal M, Nepomuceno T, et al. Fructose-induced metabolic syndrome is associated with glomerular hypertension and renal microvascular damage in rats. Am J Physiol Renal Physiol. 2007;292:F423–F429. doi: 10.1152/ajprenal.00124.2006. [DOI] [PubMed] [Google Scholar]
- 39.Samuels SE, Thompson JR, Christopherson RJ. Skeletal and cardiac muscle protein turnover during short-term cold exposure and rewarming in young rats. Am J Physiol. 1996;270 (6 Pt 2):R1231–R1239. doi: 10.1152/ajpregu.1996.270.6.R1231. [DOI] [PubMed] [Google Scholar]
- 40.Hwang IS, Ho H, Hoffman BB, Reaven GM. Fructose-induced insulin resistance and hypertension in rats. Hypertension. 1987;10:512–516. doi: 10.1161/01.hyp.10.5.512. [DOI] [PubMed] [Google Scholar]