Abstract
Supplementation with eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) from fish oil may prevent development of heart failure through alterations in cardiac phospholipids that favorably impact inflammation and energy metabolism. A high-fat diet may block these effects in chronically stressed myocardium. Pathological left ventricle (LV) hypertrophy was generated by subjecting rats to pressure overload by constriction of the abdominal aorta. Animals were fed: (1) standard diet (10% of energy from fat), (2) standard diet with EPA+DHA (2.3% of energy intake as EPA+DHA), (3) high fat (60% fat); or (4) high fat with EPA+DHA. Pressure overload increased LV mass by ≈40% in both standard and high-fat diets without fish oil. Supplementation with fish oil increased their incorporation into cardiac phospholipids, and decreased the proinflammatory fatty acid arachidonic acid and urine thromboxane B2 with both the standard and high-fat diet. Linoleic acid and tetralinoloyl cardiolipin (an essential mitochondrial phospholipid) were decreased with pressure overload on standard diet, which was prevented by fish oil. Animals fed high-fat diet had decreased linoleic acid and tetralinoloyl cardiolipin regardless of fish oil supplemention. Fish oil limited LV hypertrophy on the standard diet, and prevented upregulation of fetal genes associated with heart failure (myosin heavy chain-β and atrial natriuetic factor). These beneficial effects of fish oil were absent in animals on the high-fat diet. In conclusion, whereas treatment with EPA+DHA prevented tetralinoloyl cardiolipin depletion, LV hypertrophy, and abnormal genes expression with pressure overload, these effects were absent with a high-fat diet.
Keywords: Omega-3 fatty acids, cardiac hypertrophy, heart failure, cardiolipin, phospolipids
Initial observations of Greenland Eskimos and subsequent epidemiological studies identified an association between increased consumption of marine oil and reduced cardiovascular mortality.1–4 Prospective randomized trials showed dietary supplementation with ω-3 polyunsaturated fatty acids (ω-3 PUFA) from fish oil (specifically eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) can reduce mortality postmyocardial infarction.5,6 Epidemiological data also suggested that increased consumption of fish oil high in EPA and DHA correlated with a lower incidence of congestive heart failure.7 The recent Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico-Heart Failure (GISSI-HF) study showed the addition of even a low dose of ω-3 PUFA to a modern heart failure (HF) regimen decreased mortality in patients with symptoms.8
Multiple mechanisms may potentially account for the beneficial effects of ω-3 PUFA from fish oil in HF, including a reduction in inflammation,9 improved cardiac energetics,10 and prevention of adverse left ventricle (LV) remodeling.11,12 EPA and DHA displace ω-6 PUFA in cellular membranes, competitively removing arachidonic acid, the precursor of inflammatory mediators.12,13 Dietary supplementation with fish oil high in EPA and DHA increases cardiolipin (CL),14,15 a mitochondrial tetra-acyl phospholipid that is essential for optimal mitochondrial function.16 There is emerging evidence CL plays a critical role in cardiac function and pathology in HF and is affected by dietary intake of PUFA and saturated fatty acids. Linoleic acid (18:2n-6) is the main fatty acyl moiety in cardiac CL, with tetralinoleoyl CL (L4CL) being the primary form of CL in the heart.16–19 Diets deficient in linoleic acid are associated with decreased L4CL18,19 and decreased L4CL in the heart may worsen cardiac function by adversely altering energy metabolism and promoting apoptosis.16 There is L4CL depletion with aging, LV hypertrophy (LVH), and HF, which is associated with impaired mitochondrial function.16,18,19 The effects of dietary supplementation with fish oil on L4CL have not been reported; however previous studies suggest that fish oil might prevent depletion of L4CL in LVH,14,15 which could subsequently prevent development of HF.20
While dietary supplementation with fish oil may improve cardiac energy metabolism and decrease inflammation, a high-fat diet might block the incorporation of EPA and DHA into membrane phospholipids and prevent their protective effect on the heart. From a clinical perspective, dietary fat intake has not been considered in studies with fish oil supplementation, however high fat consumption could interfere with the positive effects of EPA and DHA supplementation. Therefore, we assessed the effects of fish oil (EPA+DHA at 2.3% of energy intake) in rats subjected to arterial pressure overload induced hypertrophy and fed either a standard low-fat diet or a high-fat diet (10% or 60% of energy from fat). We hypothesized that dietary supplementation of fish oil would increase membrane incorporation of EPA and DHA and promote favorable effects on inflammation, cardiolipin profile, and cardiac remodeling in the face of increased myocardial afterload. Furthermore, we hypothesized that a high-fat diet would block the cardioprotective effects of fish oil.
Methods
Experimental Design
The investigators were blinded from information identifying treatment groups. The animal protocol was conducted according to the guidelines for the care and use of laboratory animals (NIH publication No. 85-23) and was approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee. Animals were maintained on a reverse light-dark cycle. All measurements and procedures were performed in the fed state between 3 to 6 hours of initiation of the dark phase.
Five-week old male Wistar rats (Harlan Labs, Indianapolis, Ind) underwent sham surgery or abdominal aortic banding and were then randomized to different diets: (1) standard low-fat (n=9 sham and 10 banded), (2) standard low-fat diet supplemented with fish oil (n=9 sham and 11 banded), (3) high-fat diet (n=9 sham and 10 banded), or (4) high-fat diet with fish oil (n=9 sham and 9 banded). Measurement of systolic arterial pressure was performed at 3 weeks of treatment using the tail cuff method as previously described.12 Echocardiography was performed 7 weeks after surgery. At 8 weeks, the animals were anesthetized with 1.5%–2.0% isoflurane. Serum was obtain from clotted samples and plasma from tubes containing EDTA. Organs were immediately harvested, weighed, freeze clamped, and stored at –80°C for later analysis. Adipose tissue was also dissected, weighed, and frozen. Adipose tissue mass and organ mass were indexed by tibia length.
Diets
The diets were manufactured by Research Diet Inc. The normal low-fat diets derived 70% of their energy (kcal) from carbohydrates (75% corn starch, 15% maltodextrin, and 10% sucrose), 20% from protein, and 10% from fat. The diets high in fat derived 60% of their energy from cocoa butter (96%) and soybean oil (4%). The fatty acid composition of the cocoa butter was 28% palmitate, 65% stearate, 5% oleic acid, and 2% linoleic acid. In diets supplemented with fish oil, the ω-3 PUFA (21% EPA and 48% DHA by mass) constituted 3% of the total energy (2.3% as EPA+DHA) and replaced the caloric equivalent of the cocoa butter derived energy (Table S1 in Data Supplement, please see http://hyper.ahajournals.org). This dose was selected based on our recent dose–response study that showed that 2.3% gave the optimal response in prevention of LV pathology under conditions of pressure overload.12
Abdominal Aortic Banding
The rats were anesthetized with 2.0 to 2.5% isoflurane delivered by face mask. The suprarenal abdominal aorta was visualized with a midline incision. A 3-0 silk suture was tied around the aorta and a 21-gauge needle. The needle was removed immediately after the suture was secured. The residual aortic lumen diameter approximated that of the needle. Sham surgery rats were subjected to the same procedure but without banding.
Echocardiography
Please see Data Supplement at http://hyper.ahajournals.org.
Membrane Lipid Composition
The fatty acid composition of phospholipids isolated from LV tissues by solid phase extraction21,22 as assessed by gas-liquid chromatography with a flame ionization detector according to a modification of the transesterification method.23,24 Cardiolipin composition was assessed on extracts from whole cardiac tissue homogenates by electrospray ionization mass spectrometry as previously described.18,19
Metabolic Variables
The plasma was analyzed for free fatty acids, triglycerides, and glucose using enzymatic spectrophotometric methods. Adiponectin, leptin, and insulin were measured by ELISA. The urine thromboxane B2 was measured by ELISA. mRNA encoding atrial natriuretic factor (ANF) and myosin heavy chain (MHC) α and β were measured by real-time RT-PCR as previously described.11 Activities of the mitochondrial marker enzymes citrate synthase, medium chain acyl CoA dehydrogenase (MCAD), aconitase, and isocitrate dehydrogenase were measured spectophotometrically.
Statistical Analysis
The values for continuous variables are presented as means±SEM. Comparisons between groups were performed with a univariate 3-way analysis of variance. Analysis for individual subgroup comparison was performed as individual t tests. A probability value <0.05 was considered statistically significant.
Results
Body and Heart Mass
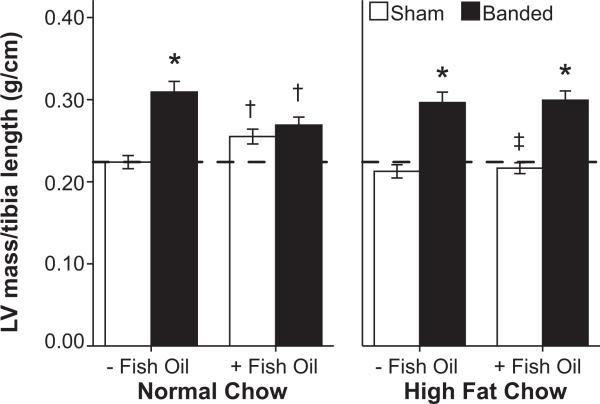
Initial body mass was matched among groups and was similar at the termination of treatment among normal low-fat diet and high-fat diet groups (Table 1). There was no difference in tail blood pressure among dietary treatment groups, and abdominal banding resulted in a consistent decrease of 4 to 8 mm Hg in blood pressure compared to sham (P<0.001). LV mass increased in both the normal low-fat diet (40% increase in LV mass/tibia length compared to respective sham group) and high-fat diet (42% increase) groups (Figure 1). Dietary supplementation with fish oil limited LVH in animals on the normal low-fat diet (4% increase in LV mass/tibia length compared to respective sham), but not in those receiving the high-fat diet (36% increase; Table 1, Figure 1).
Table 1.
Blood Pressure, Body Mass, and LV Mass
| Normal Low Fat |
Normal Low Fat+Fish Oil |
High Fat |
High Fat+Fish Oil |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Sham | Banded | Sham | Banded | Sham | Banded | Sham | Banded | Significant P Values |
| Tail systolic blood pressure, mm Hg | 125±3 | 118±3 | 128±2 | 120±1* | 125±1 | 121±2 | 129±2 | 121±2* | PS<0.001 |
| Heart rate, bpm | 510±11 | 488±13 | 471 ±11† | 469±6 | 489±14 | 500±13 | 479±16 | 502±14‡ | PD<0.024 |
| Terminal body mass, g | 461±13 | 438±15 | 487±10 | 452±10* | 452±13 | 468±17 | 475±13 | 478±15 | PSD=0.049 |
| Tibia length, mm | 44.3±0.4 | 43.5±0.4 | 43.5±0.5 | 43.3±0.4 | 43.9±0.5 | 43.6±0.4 | 43.7±0.3 | 43.6±0.3 | |
| LV mass/tibia length, g/cm | 0.22±0.01 | 0.31 ±0.01* | 0.26±0.01† | 0.27±0.01† | 0.21±0.01 | 0.30±0.01* | 0.22±0.01‡ | 0.30±0.01* | PS<0.001; PSD=0.024; PSF=0.015; PSDF=0.018 |
Data are the mean±SEM.
P≤0.05 vs respective sham
P≤0.05 vs same diet without fish oil
P≤0.05 vs respective low fat diet.
PS, banded animals vs sham animals; PD, low-fat diet vs high-fat diet; PF, fish oil vs no fish oil.
PSD, interaction between surgery and diet; PSF, interaction between surgery and fish oil; PDF, interaction between diet and fish oil; PSDF, interaction between surgery, diet, and fish oil.
Figure 1.
Left ventricular (LV) mass indexed by tibia length (n=9 to 11 per group). *P≤0.05 vs respective sham, †P≤0.05 vs same diet without fish oil, ‡P≤0.05 vs respective normal low-fat diet. The hatched line marks the mean LV mass for sham animals receiving standard chow without fish oil.
Diet composition or supplementation of fish oil did not alter adipose, liver, or kidney mass. Abdominal aortic banding was associated with a modest but significant increase in kidney mass and decreased perirenal adipose mass (Table S2).
Echocardiographic Results
Echocardiography revealed decreased LV ejection fraction (P=0.046) and increased LV end systolic volume (P=0.041) in animals with aortic banding. There was no measured diet or fish oil effect on echocardiographic measurements of LV size or function (Table S3).
MHC Isoforms and ANF Expression
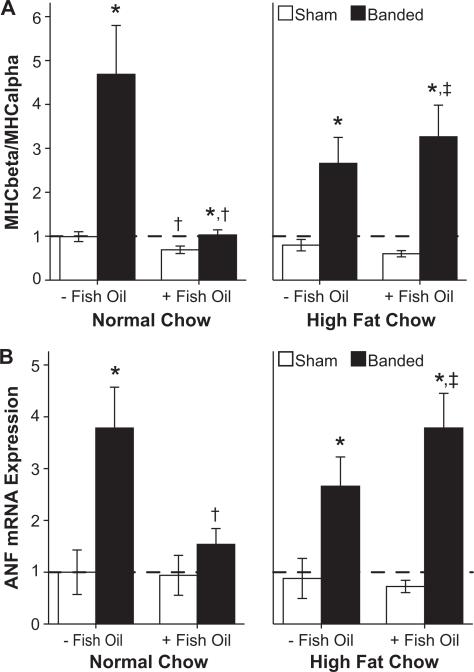
The MHC composition of the heart changes with development of HF, with increased RNA transcription of MHCβ in relation to MHCα.25 ANF is synthesized and secreted from myocytes in response to increased afterload. Banding produced an increase in MHCβ, MHCβ/MHCα ratio, and ANF (P<0.001). In banded animals, fish oil supplementation preserved expression of MHCα (P=0.024), and blunted the upregulation in the expression of MHCβ (P=0.004). Fish oil also blunted the increase in the MHCβ/MHCα ratio (P=0.009) (Figure 2a) and the mRNA for ANF (P=0.022; Figure 2b) only in animals receiving diets low in fat. There was a significant interaction between fish oil and diet: MHCβ (P=0.003), MHCβ/MHCα (P=0.005), and ANF (P=0.024). Thus the attenuation in the classic pressure overload-induced alterations in cardiac gene expression were prevented by fish oil supplementation only in animals on the normal low-fat diet.
Figure 2.
Gene expression of (A) myosin heavy chain (MHC) isoforms and (B) atrial natriuretic factor (ANF; n=9 to 11 per group). *P≤0.05 vs respective sham, †P≤0.05 vs same diet without fish oil, ‡P≤0.05 vs respective normal low-fat diet. The hatched line marks the mean level for sham animals receiving standard chow without fish oil.
Cardiac Phospholipid Fatty Acid Composition
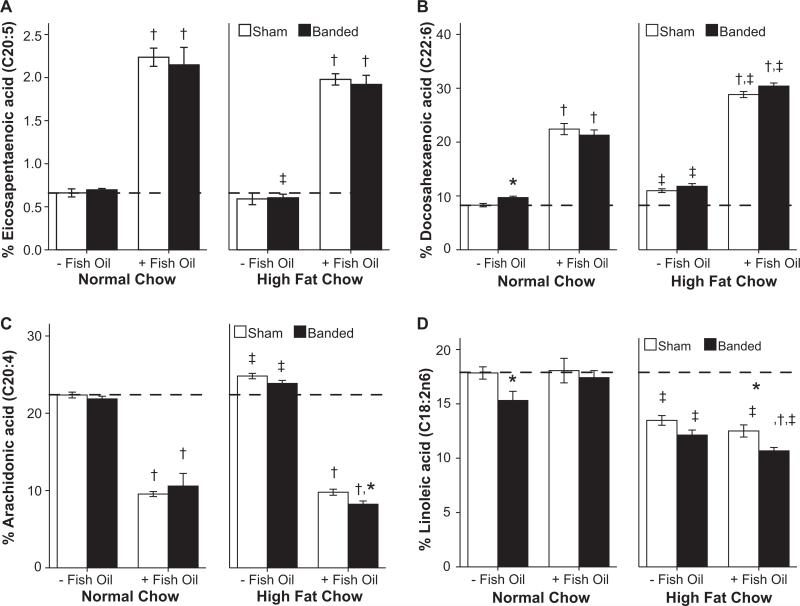
EPA and DHA incorporation into cardiac phospholipids was increased with fish oil supplementation in both the normal low-fat and high-fat diets (both P<0.001; Figure 3a and 3b). Levels of arachidonic acid in cardiac phospholipids decreased in all fish oil groups (P<0.001; Figure 3c). Animals consuming the high-fat diet without fish oil had increased DHA composition (P<0.001), but decreased EPA composition (P=0.03). Linoleic acid membrane composition decreased with high-fat diet consumption (Figure 3d).
Figure 3.
Percentage membrane composition of (A) eicosapentaenoic acid, (B) docosahexaenoic acid, (C) arachidonic acid, and (D) linoleic acid (n=9 to 11 per group). *P≤0.05 vs respective sham, †P≤0.05 vs same diet without fish oil, ‡P≤0.05 vs respective normal low-fat diet. The hatched line marks the mean level for sham animals receiving standard chow without fish oil.
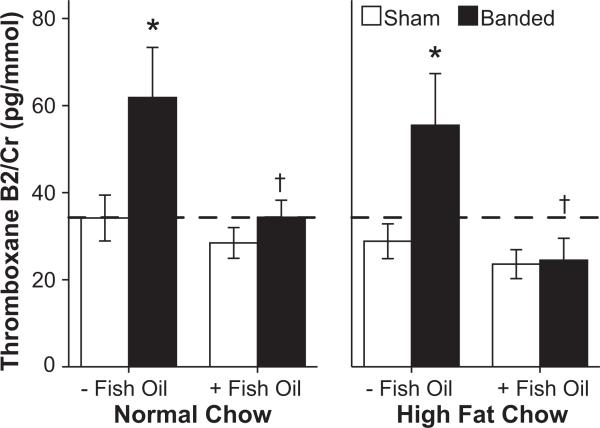
Arachidonic acid in membrane phospholipids is the precursor to thromboxane, which increases in patients with heart failure. The urinary excretion of thromboxane B2, the stable breakdown product of thromboxane A2, was increased with aortic banding (P=0.004). Supplementation with fish oil prevented this increase (P<0.001; Figure 4), consistent with lower levels of arachidonic acid in cardiac phospholipids (Figure 3d). Other fatty acid membrane compositions are presented in Table S4.
Figure 4.
Urinary thromboxane concentration adjusted for baseline creatinine (n=9 to 11 per group). *P≤0.05 vs respective sham, †P≤0.05 vs same diet without fish oil. The hatched line marks the mean level for sham animals receiving standard chow without fish oil.
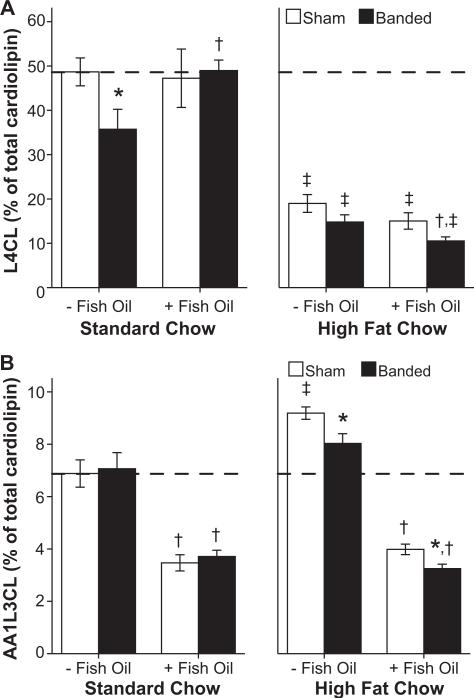
L4CL levels in cardiac tissue decreased in rats subjected to pressure overload fed the normal low-fat diet, consistent with previous studies, and supplementation with fish oil prevented this decline (Figure 5a, left panel).18 Treatment with the high-fat diet caused a dramatic decrease in L4CL in both sham and banded rats regardless of fish oil treatment (Figure 5a). Cardiolipin containing 1 arachidonic acid and 3 linoleic acid chains (AA1L3CL) was not affected by aortic banding, but was decreased by ≈50% with fish oil treatment in both the normal low-fat and high-fat diet groups (P<0.001; Figure 5b).
Figure 5.
Percentage membrane composition of (A) tetra cardiolipin (L4CL) and (B) cardiolipin containing 1 arachidonic acid and 3 linoleic acid chains (AA1L3CL; n=9 to 11 per group). *P≤0.05 vs respective sham, †P≤0.05 vs same diet without fish oil, ‡P≤0.05 vs respective normal low-fat diet. The hatched line marks the mean level for sham animals receiving standard chow without fish oil.
Hormones and Metabolic Parameters
Supplementation with fish oil reduced circulating plasma insulin concentrations in animals receiving the low-fat diets; however, they had no effect on insulin concentrations in animal consuming diets high in fat. Diet composition alone had no effect on insulin levels (Table 2). Plasma glucose was similar in all groups (Table S5).
Table 2.
Metabolites and Hormones
| Normal Low Fat |
Normal Low Fat+Fish Oil |
High Fat |
High Fat+Fish Oil |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Sham | Banded | Sham | Banded | Sham | Banded | Sham | Banded | Significant P Values |
| Serum FFA, mM/L | 0.49±0.07 | 0.52±0.08 | 0.53±0.07 | 0.49±0.04 | 0.66±0.03‡ | 0.60±0.08 | 0.70±0.03‡ | 0.59±0.05 | PD=0.003 |
| Plasma insulin, ng/ml | 3.27±0.51 | 3.78±0.46 | 1.47±0.23† | 1.55±0.31† | 2.63±0.51 | 3.27±0.51 | 3.00±0.53‡ | 3.62±0.48‡ | PF=0.012; PDF<0.001 |
| Plasma triglycerides, mg/ml | 1.05±0.11 | 1.07±0.2 | 0.50±0.06† | 0.51 ±0.1† | 0.94±0.11 | 1.11±0.2 | 0.59±0.06† | 0.66±0.07 | PF<0.001 |
| Serum leptin, ng/ml | 2.38±0.28 | 2.15±0.30 | 1.24±0.09† | 0.77±0.10*† | 1.09±0.13‡ | 0.80±0.13‡ | 2.64±0.32†‡ | 2.05±0.28†‡ | PS=0.016; PDF<0.001 |
| Plasma Adiponectin, ug/mL | 10.82±0.82 | 6.86±1.00* | 20.73±2.60† | 19.66±2.90† | 9.44±1.16 | 6.86±1.11 | 22.94±2.96† | 17.71±2.65† | PS=0.015; PF<0.001 |
Data are the mean±SEM.
P≤0.05 vs respective sham
P≤0.05 vs same diet without fish oil
P≤0.05 vs respective low-fat diet.
PS, banded animals vs sham animals; PD, low-fat diet vs high-fat diet; PF, fish oil vs no fish oil.
PSD, interaction between surgery and diet; PSF, interaction between surgery and fish oil; PDF, interaction between diet and fish oil; PSDF, interaction between surgery, diet, and fish oil.
Plasma triglyceride concentrations decreased with supplementation of fish oil (Table 2). Free fatty acid levels increased with high-fat diet, but were unaffected by fish oil (Table 2).
Circulating leptin concentrations decreased with abdominal aortic banding and diets high in fat. Fish oil increased leptin in animals receiving diets high in fat while decreasing leptin levels in animals receiving the normal low-fat diet (Table 2). Adiponectin concentration decreased with abdominal aortic banding in the absence of fish oil, and it increased with fish oil in both the normal low-fat diet and the high-fat diet groups (Table 2).
Cardiac hypertrophy and HF are characterized by altered energy metabolism with downregulation of gene expression for enzymes of fatty acid oxidation and mitochondrial function. A high-fat diet may preserve or increase mitochondrial enzyme activity.26 Abdominal aortic banding produced a significant decrease in citrate synthase (P<0.001) and MCAD (P=0.001) activity. High-fat diet increased aconitase activity (P=0.033). Fish oil did not affect citrate synthase, aconitase, MCAD, or isocitrate dehydrogenase activity (Table S5).
Discussion
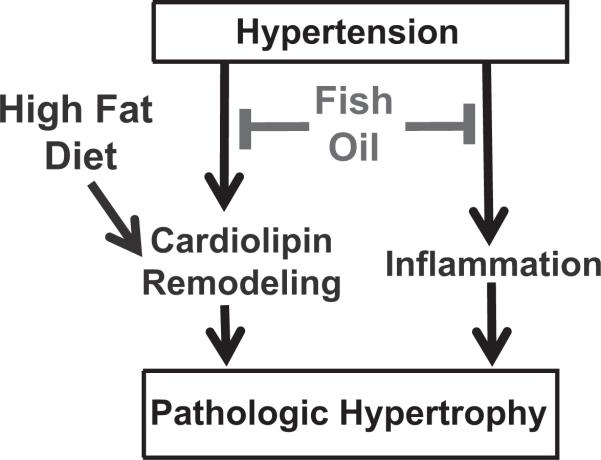
The primary findings of this study are (1) supplementation with fish oil prevented LVH and the decline in L4CL levels in cardiac tissue in response to pressure overload in animals fed the normal low-fat/high carbohydrate diet, (2) this beneficial effect of fish oil was not observed with a high-fat diet composed of saturated fat, and (3) the high–saturated fat diet decreased linoleic acid and L4CL in cardiac membranes. Thus the beneficial effects of fish oil on cardiac remodeling appear to be nullified in rats consuming diets high in fat, possibly because of decreased linoleic acid availability and adverse cardiolipin remodeling (Figure 6). This suggests that to reach maximum benefit from fish oil supplementation, patients at risk for HF should not consume a high–saturated fat diet.
Figure 6.
Simplified schematic summary of the results of the present study. Treatment with fish oil exerted antiinflammatory effects and prevented tetralinoloyl cardiolipin depletion, which corresponded with prevention of LV hypertrophy and expression of fetal genes. A high-fat diet exacerbated cardiolipin remodeling and prevented the beneficial effects of fish oil on prevention of pathological LV hypertrophy.
EPA and DHA were readily incorporated into cardiac membranes regardless of diet. Additionally, downstream effects of fish oil including reduced triglyceride concentrations, increased adiponectin levels, and decreased urine thromboxane levels were observed irrespective of diet. These observations suggest high fat intake did not abolish key biochemical effects of fish oil.
Cardiac membranes contained less linoleic acid in animals receiving high-fat diets. Furthermore, these animals had marked reductions in L4CL, the dominant cardiolipin species comprising 4 linoleic acid fatty acyl side chains. L4CL is a critical component of the inner mitochondrial membrane and plays important roles in mitochondrial respiratory function.16 Alterations in cardiolipin composition could impair mitochondrial function and accelerate development of HF. In fact, Barth Syndrome, an X-linked myopathy associated with abnormal cardiolipin synthesis, is associated with dilated cardiomyopathy. Patients with Barth syndrome exhibit decreased L4CL content in myocyte mitochondrial membranes and develop progressive cardiac dysfunction during infancy.27,28 Decreased membrane cardiolipin composition has also been observed in patients with late-stage dilated cardiomyopathy.29 In a pressure overloaded rat model, Chicco and Sparagna noted a progressive loss of L4CL species and acyl chain remodeling (alternative CL species formation) in the myocyte mitochondria. This finding was associated with decreased activity in the electron transport enzyme cytochrome oxidase and accelerated LVH and remodeling.18,19 In our study, fish oil supplementation preserved L4CL composition at levels equal to sham animals. However, adverse cardiolipin remodeling (decrease in L4CL) was associated with the high-fat diet and possibly nullified the beneficial effects of fish oil despite robust incorporation of EPA and DHA into membrane phospholipids. This could be attributable to impaired mitochondrial function as a result of depletion of L4CL.
Fish oil elicited classic changes in circulating adiponectin and triglyceride levels in both the normal and high-fat diets. Because there was no evidence that fish oil was beneficial with the high-fat diet, this suggests that changes in adiponectin and triglyceride are not major mediators of the cardioprotective effects of fish oil. We previously found a strong negative correlation between plasma adiponectin concentration and LV end systolic and diastolic volumes in a more prolonged model of pressure overload in the rat, suggesting a causal role for adiponectin in the prevention of LV remodeling.12 Future studies should use the adiponectin–/– mouse model to address the role of adiponectin in mediating the protective effect of fish oil in chronic pressure overload.
The results of the present investigation suggest several possibilities for further investigation. Future studies should elucidate the consequence of these changes in CL content and composition on the function of cardiac mitochondria. It is also important to note that the present study used a relatively short duration of pressure overload with early stage HF, and these results may not be applicable to more severe models of heart failure. Further, future studies should evaluate the effects of fish oil and dietary fat intake on the progression of LV dysfunction in previously established heart failure. Lastly, one should keep in mind that the present findings may be limited to pathological cardiac hypertrophy induced by pressure overload, and may not be found with other forms of heart failure (eg, heart failure resulting from volume overload, tachycardiac, ischemic heart disease, or primary cardiomyopathies).
Perspectives
There is emerging evidence from pharmacological studies demonstrating that high intake of fish oil can prevent the development and progression of HF.8 Previous clinical studies did not consider the role of dietary fat intake with fish oil supplementation, however high-fat consumption could interfere with the positive effects of fish oil supplementation. The novel findings of the present investigation are that a high–saturated fat diet negatively modulates the effects of fish oil on LVH and pathological gene expression. This suggests that to maximize the benefit from fish oil supplementation, patients at risk for HF should not consume a high–saturated fat diet.
Supplementary Material
DATA SUPPLEMENT THE CARDIOPROTECTIVE EFFECTS OF FISH OIL DURING PRESSURE OVERLOAD ARE BLOCKED BY HIGH FAT INTAKE: ROLE OF CARDIAC PHOSPHOLIPID REMODELING
Keyur B. Shah1, Monika K. Duda1,2, Karen M. O'Shea 1,3, Genevieve C. Sparagna4, David J. Chess1, Ramzi J. Khairallah1, Isabelle Frayne-Robillard5, Wenhong Xu1, Robert C. Murphy6. Christine Des Rosiers5, William C. Stanley1,3
1 Division of Cardiology, Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland
2 Department of Clinical Physiology, Medical Center of Postgraduate Education, Warsaw, Poland
3Department of Nutrition, Case Western Reserve University; Cleveland, Ohio
4 Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO
5 Department of Nutrition and Montreal Heart Institute, Université de Montréal, Montreal, Canada
6 Department of Pharmacology, University of Colorado Denver and Health Sciences Center, Aurora, CO
Correspondence to: William C. Stanley, Ph.D. Professor Division of Cardiology, Department of Medicine University of Maryland-Baltimore 20 Penn Street, HSF2, Room S022 Baltimore, MD 21201 Phone: 410-706-3585 Fax: 410-706-3586 wstanley@medicine.umaryland.edu
Methods
Echocardiography
Cardiac structure and function were evaluated with Vevo 770 High-Resolution Imaging Systems (Visual Sonics) with a 30-MHz linear array transducer (model 716). The rats were anesthetized with 1.5-2.0% isoflurane delivered by face mask, the chest was shaved, and the animal was secured to electrocardiogram limb electrodes on a warming pad. Two-dimensional and M-mode images were obtained from the parasternal long and short axis views. Pulse-wave Doppler of the transmitral flow was acquired in the foreshortened apical view. The data were analyzed offline on the software resident on the device.
Table S1.
Table S2. Blood pressure, body mass, and tissue masses
Table S3. Echocardiographic data.
Table S4. Cardiac phospholipid fatty acid composition.
Table S5. Metabolic Parameters and Activities for Enzymes
Acknowledgments
The authors thank Dr Willem J. Kop for advice on the statistical analysis and Anselm Tintinu for assistance in the laboratory.
Sources of Funding
This work was supported by NIH grants HL074237 and HL091307 (to W.C.S.).
Footnotes
Disclosures
None.
References
- 1.Hu FB, Bronner L, Willett WC, Stampfer MJ, Rexrode KM, Albert CM, Hunter D, Manson JE. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–1821. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003;107:1372–1377. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- 3.Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, Inaba Y, Tanabe N, Tamakoshi A. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the J Am Coll Cardiol (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Sinclair HM. Deficiency of essential fatty acids and atherosclerosis, etcetera. Lancet. 1956;270:381–383. [PubMed] [Google Scholar]
- 5.Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 6.Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989;2:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 8.Gissi-Hf I. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 9.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 10.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–2308. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- 11.Duda MK, O'Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hoit BD, Kop WJ, Stanley WC. Dietary supplementation with omega-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc Res. 2007;76:303–310. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duda MK, O'Shea KM, Tintinu A, Xu W, Khairallah RJ, Barrows BR, Chess DJ, Azimzadeh AM, Harris WS, Sharov VG, Sabbah HN, Stanley WC. Fish oil, but not flaxseed oil, decreases inflammation and prevents pressure overload-induced cardiac dysfunction. Cardiovasc Res. 2009;81:319–327. doi: 10.1093/cvr/cvn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metcalf RG, James MJ, Gibson RA, Edwards JR, Stubberfield J, Stuklis R, Roberts-Thomson K, Young GD, Cleland LG. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am J Clin Nutr. 2007;85:1222–1228. doi: 10.1093/ajcn/85.5.1222. [DOI] [PubMed] [Google Scholar]
- 14.McMillin JB, Bick RJ, Benedict CR. Influence of dietary fish oil on mitochondrial function and response to ischemia. Am J Physiol. 1992;263:H1479–H1485. doi: 10.1152/ajpheart.1992.263.5.H1479. [DOI] [PubMed] [Google Scholar]
- 15.Pepe S, Tsuchiya N, Lakatta EG, Hansford RG. PUFA and aging modulate cardiac mitochondrial membrane lipid composition and Ca2+ activation of PDH. Am J Physiol. 1999;276:H149–H158. doi: 10.1152/ajpheart.1999.276.1.H149. [DOI] [PubMed] [Google Scholar]
- 16.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 17.Schlame M, Ren M, Xu Y, Greenberg ML, Haller I. Molecular symmetry in mitochondrial cardiolipins. Chem Phys Lipids. 2005;138:38–49. doi: 10.1016/j.chemphyslip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, Moore RL. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Sparagna GC, Johnson CA, McCune SA, Moore RL, Murphy RC. Quantitation of cardiolipin molecular species in spontaneously hypertensive heart failure rats using electrospray ionization mass spectrometry. J Lipid Res. 2005;46:1196–1204. doi: 10.1194/jlr.M500031-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Chess DJ, Lei B, Hoit BD, Azimzadeh AM, Stanley WC. Effects of a high saturated fat diet on cardiac hypertrophy and dysfunction in response to pressure overload. J Card Fail. 2008;14:82–88. doi: 10.1016/j.cardfail.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khairallah RJ, Khairallah M, Gelinas R, Bouchard B, Young ME, Allen BG, Lopaschuk GD, Deschepper CF, Des Rosiers C. Cyclic GMP signaling in cardiomyocytes modulates fatty acid trafficking and prevents triglyceride accumulation. J Mol Cell Cardiol. 2008;45:230–239. doi: 10.1016/j.yjmcc.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruiz J, Antequera T, Andres AI, Petron M, Muriel E. Improvement of a solid phase extraction method for analysis of lipid fractions in muscle foods. Analytica Chimica Acta. 2004;520:201–205. [Google Scholar]
- 23.Lepage G, Roy CC. Direct transesterification of all classes of lipids in a one-step reaction. J Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 24.Harris WS, Sands SA, Windsor SL, Ali HA, Stevens TL, Magalski A, Porter CB, Borkon AM. Omega-3 fatty acids in cardiac biopsies from heart transplantation patients: correlation with erythrocytes and response to supplementation. Circulation. 2004;110:1645–1649. doi: 10.1161/01.CIR.0000142292.10048.B2. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchimochi H, Sugi M, Kuro-o M, Ueda S, Takaku F, Furuta S, Shirai T, Yazaki Y. Isozymic changes in myosin of human atrial myocardium induced by overload. Immunohistochemical study using monoclonal antibodies. J Clin Invest. 1984;74:662–665. doi: 10.1172/JCI111466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 27.Hauff KD, Hatch GM. Cardiolipin metabolism and Barth Syndrome. Prog Lipid Res. 2006;45:91–101. doi: 10.1016/j.plipres.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Vreken P, Valianpour F, Nijtmans LG, Grivell LA, Plecko B, Wanders RJ, Barth PG. Defective remodeling of cardiolipin and phosphatidylglycerol in Barth syndrome. Biochem Biophys Res Commun. 2000;279:378–382. doi: 10.1006/bbrc.2000.3952. [DOI] [PubMed] [Google Scholar]
- 29.Heerdt PM, Schlame M, Jehle R, Barbone A, Burkhoff D, Blanck TJJ. Disease-specific remodeling of cardiac mitochondria after a left ventricular assist device. Ann Thorac Surg. 2002;73:1216–1221. doi: 10.1016/s0003-4975(01)03621-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
DATA SUPPLEMENT THE CARDIOPROTECTIVE EFFECTS OF FISH OIL DURING PRESSURE OVERLOAD ARE BLOCKED BY HIGH FAT INTAKE: ROLE OF CARDIAC PHOSPHOLIPID REMODELING
Keyur B. Shah1, Monika K. Duda1,2, Karen M. O'Shea 1,3, Genevieve C. Sparagna4, David J. Chess1, Ramzi J. Khairallah1, Isabelle Frayne-Robillard5, Wenhong Xu1, Robert C. Murphy6. Christine Des Rosiers5, William C. Stanley1,3
1 Division of Cardiology, Department of Medicine, University of Maryland School of Medicine, Baltimore, Maryland
2 Department of Clinical Physiology, Medical Center of Postgraduate Education, Warsaw, Poland
3Department of Nutrition, Case Western Reserve University; Cleveland, Ohio
4 Department of Integrative Physiology, University of Colorado Boulder, Boulder, CO
5 Department of Nutrition and Montreal Heart Institute, Université de Montréal, Montreal, Canada
6 Department of Pharmacology, University of Colorado Denver and Health Sciences Center, Aurora, CO
Correspondence to: William C. Stanley, Ph.D. Professor Division of Cardiology, Department of Medicine University of Maryland-Baltimore 20 Penn Street, HSF2, Room S022 Baltimore, MD 21201 Phone: 410-706-3585 Fax: 410-706-3586 wstanley@medicine.umaryland.edu
Methods
Echocardiography
Cardiac structure and function were evaluated with Vevo 770 High-Resolution Imaging Systems (Visual Sonics) with a 30-MHz linear array transducer (model 716). The rats were anesthetized with 1.5-2.0% isoflurane delivered by face mask, the chest was shaved, and the animal was secured to electrocardiogram limb electrodes on a warming pad. Two-dimensional and M-mode images were obtained from the parasternal long and short axis views. Pulse-wave Doppler of the transmitral flow was acquired in the foreshortened apical view. The data were analyzed offline on the software resident on the device.
Table S1.
Table S2. Blood pressure, body mass, and tissue masses
Table S3. Echocardiographic data.
Table S4. Cardiac phospholipid fatty acid composition.
Table S5. Metabolic Parameters and Activities for Enzymes