Abstract
Permanent loss of cardiomyocytes and scar tissue formation after myocardial infarction (MI) results in an irreversible damage to the cardiac function. Cardiac repair (replacement, restoration, and regeneration) is, therefore, essential to restore function of the heart following MI. Existing therapies lower early mortality rates, prevent additional damage to the heart muscle, and reduce the risk of further heart attacks. However, there is need for treatment to improve the infarcted area by replacing the damaged cells after MI. Thus, the cardiac tissue regeneration with the application of stem cells may be an effective therapeutic option. Recently, interest is more inclined toward myocardial regeneration with the application of stem cells. However, the potential benefits and the ability to improve cardiac function with the stem cell-based therapy need to be further addressed. In this review, we focus on the clinical applications of stem cells in the cardiac repair.
Keywords: Cardiomyocytes, myocardial infarction, stem cells, stem cell transplantation
Myocardial infarction (heart attack; abbreviated as "MI") remains a major clinical problem and the leading causes of mortality in the world. In the United States alone, approximately 1 million people suffer MI each year. In the UK, the annual incidence of MI (using 2006 CHD mortality data) was estimated to be about 146 000 of all aged individuals (men: ∼87 000 and women: ∼59 000), and the estimated prevalence in those aged >35 years is more than 1.4 million (men: ∼970 000 and women: ∼439 000).[1] MI can be defined by pathology as myocardial cell death due to prolonged ischemia.[2] The most common cause of MI is coronary atherosclerotic plaque rupture or erosion, resulting in the exposure of thrombogenic contents to the blood. This leads to thrombus formation and consequently MI. Several risk factors are associated with MI as listed below.
The risk factors include
non-modifiable risk factors: age, gender, low-birth weight, race/ethnicity, genetic factors;
well-documented and modifiable risk factors: Hypertension, exposure to cigarette smoke, diabetes, atrial fibrillation and certain other cardiac conditions, dyslipidemia, carotid artery stenosis, sickle cell disease, postmenopausal hormone therapy, poor diet, less physical activity, obesity, and body fat distribution; and
less well-documented or potentially modifiable risk factors: Metabolic syndrome, alcohol abuse, drug abuse, oral contraceptive use, sleep-disordered breathing, migraine headache, hyperhomocysteinemia, elevated lipoprotein(a), elevated lipoprotein-associated phospholipase, hypercoagulability, inflammation, infection.[3]
Cardiac troponins are more specific markers of myocardial necrosis. The measurement of cardiac troponins is the new gold standard diagnostic indicator of myocardial injury. These molecules are valuable and sensitive cardiac biochemical markers for the heart damage compared with total creatine kinase or its isoform, creatine kinase-MB.[4] "The Universal Definition of MI requires detection of increasing or decreasing cardiac biomarkers (preferably cardiac troponin) with ≥1 value >99th percentile, together with clinical symptoms, new ischemic electrocardiographic changes, or typical imaging findings indicative of myocardial necrosis as diagnostic criteria for acute myocardial infarction".[5]
The treatment of MI involves reperfusion therapy to reduce early mortality rates.[6] The drug therapy includes treatment with the combination of the following (Grade A): angiotensin-converting enzyme inhibitor, aspirin (an antiplatelet drug), beta-blocker, and statin.[7] Existing therapies lower the early mortality rates, prevent additional damage to the heart muscle, and reduce the risk of further heart attacks. However, there is need for a treatment to improve the clinical conditions by replacing the damaged heart cells. Thus, the cardiac tissue regeneration with the application of stem cells may be an effective therapeutic option.
Stem cells (both adult and embryonic stem cells [ESCs]) have the ability to self-replicate and transform into an array of specialized cells. Stem cells (unspecialized) are becoming the most important tool in "regenerative medicine."[8] These cells have the potential to differentiate into cardiomyocytes. It would, therefore, be useful to find out if the differentiated cells can restore and improve cardiac function with efficacy and a good safety profile.
In the present review, we summarize the recent progress in this field of science and focus on the potential clinical applications of stem cells in the cardiac repair.
Types of Stem Cells
Both embryonic and adult stem cells are sources of undifferentiated cells, with the ability to repair and regenerate tissue. Some of the stem cell types that can be used to improve infarcted heart are as follows: embryonic, cardiac, cardiomyocyte progenitor cells, hematopoietic cells, bone marrow mesenchymal stem cells, skeletal myoblast, fetal and umbilical cord blood cells.[9–13] Several routes can be used to deliver such cells to the human myocardium or to the coronary circulation. These routes include intravenous infusion, direct injection into the ventricular wall, or transepicardial/transendocardial infusions. However, questions relating to dose, timing, and route of administration need to be addressed.
Embryonic stem cells
ESCs hold promise for myocardial cellular therapy, but the clinical use of these cells raises ethical issues and political controversies.[14] The ethical problems specific to ESC research, however, can be avoided by reprogramming somatic cells to produce induced pluripotent stem cells. Takahashi and Yamanaka[15] demonstrated the induction of pluripotent stem cells from mouse embryonic or adult fibroblasts by introducing four factors (Oct3/4, Sox2, c-Myc, and Klf4). The induced pluripotent stem cells showed the morphology and growth properties of ESC and expressed ESC marker genes. More recently, Zhang et al.[16] have shown that human-induced pluripotent stem cells can differentiate into functional cardiomyocytes and these cells may be suitable for therapeutic application.
Kehat et al.[17] have succeeded in growing the heart cells from human ESCs. The results showed that human ESCs have the ability to differentiate into myocytes with the structural and functional properties of cardiomyocytes. The preclinical studies have shown that ESC transplant results in improvement in cardiac structure and function.[18–20] Caspi et al.[21] evaluated the ability of human ESCs and their cardiomyocyte derivatives to engraft and improve myocardial performance in the rat chronic infarction model. The results of this study showed the formation of stable cardiomyocyte grafts, attenuation of the remodeling process, and functional benefit, highlighting their potential for myocardial cell therapy strategies.
Adult stem cells
Currently, adult stem cells are the only stem cell types that can be used for transplant to treat diseases like MI. These unspecialized cells are found in within a tissue or organ and have the ability to develop into specialized cells. The purpose of these cells is to primarily maintain and repair the tissue in which they are found. The adult human heart contains small populations of indigenous committed cardiac stem cells or multipotent cardiac progenitor cells. These stem cells are intrinsically programmed to generate cardiac tissue.[22] The markers used to identify cardiac stem cells and their biologic functions in human are SSEA-1, Oct-3/4, Isl-1, c-Kit (CD117, SCFR), Sca-1 (Ly 6), MDR-1 (Abcb1, Pgp), Abcg2 (MXR1, BCRP), CD133 (prominin), CD90 (Thy-1), CD105 (endoglin), CD34, CD31(PECAM-1), and CD45 (LCA).[23] To date, at least five apparently different cardiac stem and/or progenitor cell types have been described.
In addition, the adult bone marrow stem cells consist of hematopoietic stem cells, endothelial progenitor cells, and mesenchymal stem cells. Orlic et al.[24] first suggested that bone marrow cells have the ability to regenerate infarcted myocardium. Mangi et al.[25] genetically engineered rat mesenchymal stem cells using ex vivo retroviral transduction and these stem cells repaired infarcted myocardium. Recent study showed that granulocyte colony-stimulating factor promoted bone marrow cells to migrate into the infarcted heart and differentiate into cardiomyocytes; however, proportional contribution of cells from bone marrow was small when compared with non-bone marrow in the infarction model.[26] Zhang et al.[27] identified a single non-hematopoietic mesenchymal stem cell subpopulation isolated from human bone marrow, clonally purified, and compared the effects with unpurified mesenchymal stem cell, mononuclear cells, or peripheral blood mononuclear cells on myocardial repair after induction of MI in rats. They observed that transplantation with single clonally purified mesenchymal stem cells was more beneficial to the cardiac repair when compared with other stem cells. Recent in vivo studies indicate that engrafted bone marrow cells survive and grow within the spared myocardium after infarction by forming junctional complexes with resident myocytes. These cells generate de novo myocardium composed of integrated cardiomyocytes and coronary vessels.[28] Previously, we tried to find out if bone marrow-derived stem cells from patients can differentiate into cardiomyocyte precursors when cocultured with atrial tissue condition medium, and we observed that these cells resembled smooth muscles probably cardiomyocytes.[29] Further studies need to be conducted to confirm the results. More recently, a randomized, double-blind, placebo-controlled, dose-escalation study (reperfused MI patients [n = 53])[30] showed that intravenous allogeneic human mesenchymal stem cells are safe in patients after MI.
Studies have also shown that adipose tissue-derived cells have the ability to give rise to functional cardiomyocyte-like cells.[31] Adipose tissue contains a population of adult multipotent mesenchymal stem cells and endothelial progenitor cells with extensive proliferative capacity. These cells have the potential to differentiate into several lineages including cardiomyocytes, suggesting their potential as cell source for repairing the damaged tissues. More recently, Bai et al.[32] showed that both freshly isolated human adipose tissue-derived cells and cultured human adipose tissue-derived stem cells engrafted into infarcted myocardium survived and improved myocardial function. The results suggest that both cells are promising alternative source for myocardial repair post-MI. To explore the clinical application of human stem cells, Kim et al.[33] transplanted human unrestricted somatic stem cells into porcine hearts post-MI. Four weeks after transplantation, the engrafted cells were detected in the infarct region, resulting in improved regional and global function of the porcine heart, suggesting positive potential for their implantation for cellular cardiomyoplasty.
Umbilical cord blood is a source of human hematopoietic precursor cells. These cells have a potential for cell-based therapy to treat acute MI by contributing to the repair processes post-MI, thus providing a useful therapeutic option.[34] Wu et al.[35] investigated the therapeutic potential of human umbilical cord-derived stem cells in a rat MI model. They showed that transplanted cells provide benefit in cardiac function recovery after acute MI. More recently, Schlechta et al.[36] have shown that umbilical cord blood-derived hematopoietic precursor cells can be reproducibly expanded ex vivo and retain their potential to improve cardiac function post-MI.
Comparison of Stem Cells
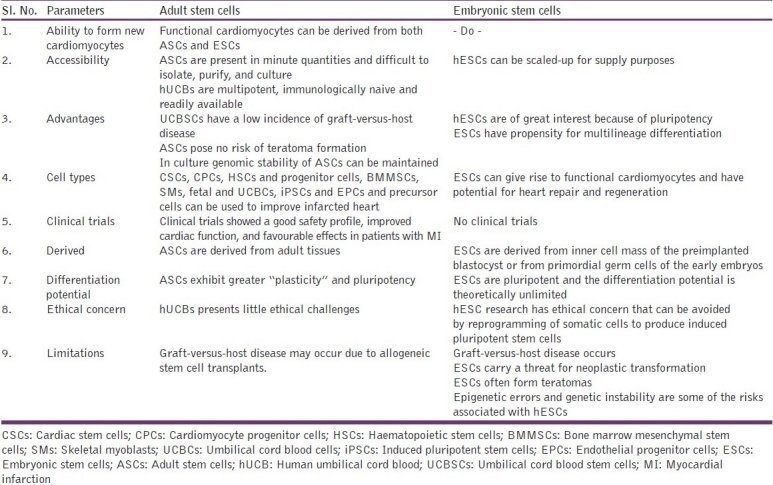
Both adult and ESCs can be used to improve an infarcted heart. The best cell type as a source for transplantation into the ischemic heart remains a controversy because of lack of well-defined cell markers. Studies need to address the source and the best types of stem cells for clinical applications [Table 1].
Table 1.
Types of stem cells and their application
Stem Cell-based Therapy
Currently, various therapeutic options are available to heal the damaged heart. Gene therapy is an interesting option, but the use of viral vectors for gene delivery is a concern due to safety issues. Another more promising addition to the existing option is the cell replacement therapy. Both in vitro and in vivo studies performed in animal models have demonstrated that the transplanted cells undergo differentiation in an attempt to repair damaged myocardium.[37,38] Previous studies in the animal models have suggested that ESC transplantation post-MI is beneficial. In rats, ESCs differentiated into cardiomyocytes and improved cardiac function.[39] Singla et al.[40] have concluded that transplanted mouse ESCs have the ability to regenerate infarcted myocardium and improve cardiac structure and function. Kolossov et al.[41] have examined the fate and functional impact of bone marrow cells and ESC-derived cardiomyocytes after transplantation into the infarcted mouse heart. The results have shown that ESC-based therapy is a promising approach for the treatment of impaired myocardial function; this therapy also provides better results when compared with the bone marrow-derived cells. Mangi et al.[42] have genetically engineered rat mesenchymal stem cells that have the ability to repair infarcted myocardium to nearly normal cardiac performance. A new therapeutic strategy for cardiac tissue regeneration was discovered when Miyahara et al.[43] cultured adipose tissue-derived mesenchymal stem cells and transplanted the monolayered cells onto the scarred myocardium that improved cardiac function in rats.
The results from the clinical studies have demonstrated a good safety profile with improved cardiac function, and favorable effects in patients with MI.[44–49] The randomized controlled clinical trial (60 patients; 6-month follow-up)[45] has shown that intracoronary transfer of autologous bone-marrow cells promoted improvement of left-ventricular systolic function in MI patients. The trial (59 patients with acute MI) has demonstrated that intracoronary infusion of progenitor cells (circulating progenitor or bone marrow-derived progenitor cells) is safe and feasible in patients after acute MI successfully revascularized by stent implantation.[46] In a prospective, nonrandomized, open-label study with five heart transplant candidates with severe ischemic heart failure, the autologous bone-marrow mononuclear cell injections were performed safely and resulted in improved exercise capacity (6-month follow-up evaluation).[47] In the cell-randomized clinical trial (27 patients), granulocyte colony-stimulating factor therapy with intracoronary infusion of peripheral blood stem cells have shown improved cardiac function, and promoted angiogenesis in MI patients.[48] Bartunek et al.[49] in Phase I/II study have shown that intracoronary administration of enriched bone marrow CD133+ cells in patients with acute MI (19/35 patients) although feasible is associated with increased incidence of coronary events.
Autologous cell transplantation uses stem cells to regenerate myocardium. This process involves implantation of bone marrow stem cells into the infarcted area of heart in an effort to restore the viability of the tissue. Stamm et al. have injected autologous AC133+ bone marrow cells into the infarct area of patients with MI and restored tissue viability.[50] A clinical trial[51] in patients with old MI and ischemic coronary artery disease (n = 12) has shown that treatment with skeletal myoblast in conjunction with coronary artery bypass is safe and feasible. A randomized controlled trial[52] (69 patients with acute MI) has shown that transplantation of bone marrow mesenchymal stem cells might improve cardiac function and is safe and feasible with no deaths or malignant arrhythmias. In another clinical trial, Katritsis et al.[53] have shown that intracoronary transplantation of mesenchymal stem cells and endothelial progenitors is feasible, safe, and may also contribute to regional regeneration of myocardial tissue early or late following MI. Klein et al.[54] in a Phase I clinical study have shown that CD133+ -enriched stem cell transplantation improved cardiac function in all patients. Li et al.[55] have shown that autologous peripheral blood stem cell transplantation by intracoronary infusion in patients with acute MI is safe. A Phase I/IIa double-blind, randomized, placebo-controlled, dose-escalating trial[56] of intramyocardial injection of autologous CD34+ cells in patients with intractable angina (n = 24) provided evidence for feasibility, safety, and bioactivity. Furthermore, a larger Phase IIb study is underway to evaluate this therapy. In the Doppler substudy of the randomized, double-blind, placebo-controlled Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction trial,[57] microvascular function of the infarct-related artery was restored after intracoronary transplantation of bone marrow progenitor cells in patients with reperfused acute MI. In an open-labeled prospective clinical trial, Choi et al.[58] have shown that intracoronary transplantation of autologous peripheral blood stem cells (mobilized by granulocyte colony-stimulating factor) was safe in patients who underwent percutaneous coronary intervention and improved myocardial function, but could not significantly improve left ventricular function compared with standard reperfusion treatment. Balogh et al.[59] transplanted autologous bone marrow-derived CD34 + stem cells in patients with MI, and these cells improved left ventricular function and viability after MI. A Phase I clinical study[60] has shown CD133+ -enriched stem cell transplantation into ischemic myocardium without coronary artery bypass grafting that was feasible, safe, and improved cardiac function in all patients. More recently, in a prospective, randomized study (60 patients), Gyöngyösi et al.[61] have compared the safety and feasibility of early and late delivery of bone marrow mononuclear stem cells after MI with combined delivery approach. They have shown that this approach induces a moderate but significant improvement in myocardial infarct size and left ventricular function. Recently, Martin-Rendon et al.[62] have evaluated evidence from randomized control trials on the effectiveness of adult bone marrow-derived stem cells to treat acute MI (13 RCTs; 811 participants). The results of this systematic review have suggested that there is little evidence to assess the clinical effects of this treatment. Mohyeddin-Bonab et al. [63] have investigated the efficacy of autologous bone marrow-derived mesenchymal stem cells in improving the heart function in patients (n = 8) with old MI. They have shown that this transplantation is safe, feasible, and that the cells improved the cardiac function without serious adverse effects. Meluzín et al.[64] have shown that the autologous mononuclear bone marrow cell transplantation (60 patients) provided sustained improvement in global left ventricular systolic function in patients with acute MI. More recently, in a Phase I clinical trial, Krause et al.[65] have shown that (12-month follow-up) left ventricular electromechanical mapping and percutaneous intramyocardial cell injection in patients with acute MI was a safe procedure.
Discussion
The paradigm that the adult heart is a postmitotic organ was established in the 1970s. Later, this concept was changed because of the growing body of evidence challenging this traditional view. In adults, myocardium can regenerate from the cardiac stem cells that enter the cell cycle, contributing to changes in the myocardial mass.[66,67] A low level of cardiomyocyte renewal may occur throughout life, which may be in response to cardiac stem cell activity or migration of stem cells from distant tissues. Such repair following MI is grossly inadequate to compensate for the severe loss of functional cardiomyocytes. Existing therapies lower the early mortality rates, prevent additional damage to the heart muscle, and reduce the risk of further heart attacks. However, there is need for a treatment to improve the clinical conditions by replacing the damaged heart cells. Thus, the cardiac tissue regeneration with the application of stem cells may be an effective therapeutic option.
The regeneration of cardiomyocytes (heart muscle cells) to improve postinfarcted heart with the application of stem cells has been broadly studied. Recently, interest has been concentrated on myocardial regeneration with the application of stem cells. Studies have shown that both embryonic and adult stem cells have the ability to repair and regenerate tissue, and in turn, these cell types can be used in the cardiomyocyte regeneration. However, there are major hurdles for the therapeutic use of adult stem cells which include harvesting and expansion of cells in vitro, advanced methods to obtain desired cell types, optimization of route of administration of stem cells, and specific induction of heart tissue regeneration.
ESCs hold promise for myocardial cellular therapy, but the clinical uses of these cells raise ethical issues and political controversies. The ethical issues specific to ESC research can be avoided by reprogramming somatic cells to produce induced pluripotent stem cells. In vitro human ESCs give rise to cardiomyocytes; however, the regenerative capacity of undifferentiated human ESCs after in vivo engraftment needs to be established. The major hurdle for the clinical application of ESCs is the formation of teratoma by undifferentiated cells, as they may not be directed to form new myocardium after transplantation.
Although ESCs have potential to repair the damaged tissue, the use of ESCs carries a threat for neoplastic transformation due to inherent risk for unguided differentiation. The malignant tumorigenic potential of ESCs needs to be defined, and the risk of teratoma formation has to be eliminated before ESC-based therapy is considered. Guided cardiopoiesis uses the definitive engagement of stem cells to generate cardiac tissue avoiding teratomas. Cardiopoietic programming offers a tumor-resistant approach for regeneration. Behfar et al.[68] demonstrated that use of cardiopoietic cells eliminates reliance on host heart signaling for differentiation. These cells delivered into infarcted hearts generated cardiomyocytes integrating with host myocardium for tumor-free repair. Other challenges include the need for preparations of high cardiac purity, improved delivery methods, and overcome immune rejection and graft cell death.
Research has been focused on understanding how stem cells target injured tissue.[69] A review by Smart and Riley[70] focuses on existing insights into the trafficking of stem cells in the context of cardiac regenerative therapy. In addition, the mechanisms of action of stem cell transplantation are not fully understood. The transplanted stem cells into injured tissue express paracrine signaling factors (cytokines, chemokines, and growth factors) involved in the process of stem cell-driven repair. Future studies need to address the mechanistic basis for stem cell-mediated paracrine enhancement.
In conclusion, stem cell transplantation appears to be a safe and effective option for treating the postinfarcted heart. To date, there are several preclinical studies that have demonstrated the potential of stem cell-based therapy in the treatment of MI. These clinical studies have demonstrated a good safety profile, improved cardiac function, and favorable effects in patients with MI. The results obtained from animal studies are promising, and the data obtained from the human clinical trials are even more encouraging.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Allender S, Peto V, Scarborough P, Kaur A, Rayner M. Coronary heart disease statistics London: British Heart Foundation; 2008. Available from: http://www.heartstats.org.
- 2.Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction.Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–88. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, et al. American Heart Association/American Stroke Association Stroke Council; Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; Quality of Care and Outcomes Research Interdisciplinary Working Group; American Academy of Neurology.Primary prevention of ischemic stroke: A guideline from the American Heart Association/American Stroke Association Stroke Council: Co-sponsored by the Atherosclerotic Peripheral Vascular Disease Interdisciplinary Working Group; Cardiovascular Nursing Council; Clinical Cardiology Council; Nutrition, Physical Activity, and Metabolism Council; and the Quality of Care and Outcomes Research Interdisciplinary Working Group: The American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37:1583–633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 4.Senter S, Francis GS. A new, precise definition of acute myocardial infarction. Cleve Clin J Med. 2009;76:159–66. doi: 10.3949/ccjm.75a.08092. [DOI] [PubMed] [Google Scholar]
- 5.Eggers KM, Lind L, Venge P, Lindahl B. Will the universal definition of myocardial infarction criteria result in an overdiagnosis of myocardial infarction? Am J Cardiol. 2009;103:588–91. doi: 10.1016/j.amjcard.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 6.van Domburg RT, Sonnenschein K, Nieuwlaat R, Kamp O, Storm CJ, Bax JJ, et al. Sustained benefit 20 years after reperfusion therapy in acute myocardial infarction. J Am Coll Cardiol. 2005;46:15–20. doi: 10.1016/j.jacc.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 7.Cooper A, Skinner J, Nherera L, Feder G, Ritchie G, Kathoria M, et al. London: National Collaborating Centre for Primary Care and Royal College of General Practitioners; 2007. Clinical Guidelines and Evidence Review for Post Myocardial Infarction: Secondary prevention in primary and secondary care for patients following a myocardial infarction. [PubMed] [Google Scholar]
- 8.Krishna KA, Rao KR. India: I.K. International Publishing; 2010. Regenerative medicine: Stem cells and their applications. pp. 1–208. [Google Scholar]
- 9.Gallo P, Peschle C, Condorelli G. Sources of cardiomyocytes for stem cell therapy: An update. Pediatr Res. 2006;59:79R–83. doi: 10.1203/01.pdr.0000203551.63437.9b. [DOI] [PubMed] [Google Scholar]
- 10.van Laake LW, Hassink R, Doevendans PA, Mummery C. Heart repair and stem cells. J Physiol. 2006;577:467–78. doi: 10.1113/jphysiol.2006.115816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Laake LW, Van Hoof D, Mummery CL. Cardiomyocytes derived from stem cells. Ann Med. 2005;37:499–512. doi: 10.1080/07853890500327843. [DOI] [PubMed] [Google Scholar]
- 12.Smits AM, van Vliet P, Hassink RJ, Goumans MJ, Doevendans PA. The role of stem cells in cardiac regeneration. J Cell Mol Med. 2005;9:25–36. doi: 10.1111/j.1582-4934.2005.tb00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gersh BJ, Simari RD, Behfar A, Terzic CM, Terzic A. Cardiac cell repair therapy: A clinical perspective. Mayo Clin Proc. 2009;84:876–92. doi: 10.4065/84.10.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204–13. doi: 10.1210/er.2008-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–14. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodgson DM, Behfar A, Zingman LV, Kane GC, Perez-Terzic C, Alekseev AE, et al. Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H471–9. doi: 10.1152/ajpheart.01247.2003. [DOI] [PubMed] [Google Scholar]
- 19.Ménard C, Hagège AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: A preclinical study. Lancet. 2005;366:1005–12. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- 20.Yamada S, Nelson TJ, Crespo-Diaz RJ, Perez-Terzic C, Liu XK, Miki T, et al. Embryonic stem cell therapy of heart failure in genetic cardiomyopathy. Stem Cells. 2008;26:2644–53. doi: 10.1634/stemcells.2008-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–93. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 22.Barile L, Chimenti I, Gaetani R, Forte E, Miraldi F, Frati G, et al. Cardiac stem cells: Isolation, expansion and experimental use for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2007;4:S9–14. doi: 10.1038/ncpcardio0738. [DOI] [PubMed] [Google Scholar]
- 23.Smith RR, Barile L, Messina E, Marbán E. Stem cells in the heart: What's the buzz all about.-Part 1: Preclinical considerations? Heart Rhythm. 2008;5:749–57. doi: 10.1016/j.hrthm.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 25.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 26.Fukuhara S, Tomita S, Nakatani T, Yutani C, Kitamura S. Endogenous bone-marrow-derived stem cells contribute only a small proportion of regenerated myocardium in the acute infarction model. J Heart Lung Transplant. 2005;24:67–72. doi: 10.1016/j.healun.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Ge J, Sun A, Xu D, Qian J, Lin J, et al. Comparison of various kinds of bone marrow stem cells for the repair of infarcted myocardium: Single clonally purified non-hematopoietic mesenchymal stem cells serve as a superior source. J Cell Biochem. 2006;99:1132–47. doi: 10.1002/jcb.20949. [DOI] [PubMed] [Google Scholar]
- 28.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–8. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishna KA, Balaji CS, Ameena A, Soma G, Rao SG. Guntur, India: National Seminar on Modern Biology; 2003. Effect of atrial tissue condition medium on bone marrow derived mesenchymal stem cells. [Google Scholar]
- 30.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Planat-Bénard V, Menard C, André M, Puceat M, Perez A, Garcia-Verdugo JM, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–9. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 32.Bai X, Yan Y, Song YH, Seidensticker M, Rabinovich B, Metzele R, et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 2010;31:489–501. doi: 10.1093/eurheartj/ehp568. [DOI] [PubMed] [Google Scholar]
- 33.Kim BO, Tian H, Prasongsukarn K, Wu J, Angoulvant D, Wnendt S, et al. Cell transplantation improves ventricular function after a myocardial infarction: A preclinical study of human unrestricted somatic stem cells in a porcine model. Circulation. 2005;112:I96–104. doi: 10.1161/01.CIRCULATIONAHA.105.524678. [DOI] [PubMed] [Google Scholar]
- 34.Ma N, Stamm C, Kaminski A, Li W, Kleine HD, Müller-Hilke B, et al. Human cord blood cells induce angiogenesis following myocardial infarction in NOD/scid-mice. Cardiovasc Res. 2005;66:45–54. doi: 10.1016/j.cardiores.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Wu KH, Zhou B, Yu CT, Cui B, Lu SH, Han ZC, et al. Therapeutic potential of human umbilical cord derived stem cells in a rat myocardial infarction model. Ann Thorac Surg. 2007;83:1491–8. doi: 10.1016/j.athoracsur.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 36.Schlechta B, Wiedemann D, Kittinger C, Jandrositz A, Bonaros NE, Huber JC, et al. Ex-vivo expanded umbilical cord blood stem cells retain capacity for myocardial regeneration. Circ J. 2010;74:188–94. doi: 10.1253/circj.cj-09-0409. [DOI] [PubMed] [Google Scholar]
- 37.Tomita S, Li RK, Weisel RD. Autologous transplantation of bone marrow cells improves damaged heart function. Circulation. 1999;100:247–56. doi: 10.1161/01.cir.100.suppl_2.ii-247. [DOI] [PubMed] [Google Scholar]
- 38.Li RK, Mickle DA, Weisel RD, Zhang J, Mohabeer MK. In vivo survival and function of transplanted rat cardiomyocytes. Circ Res. 1996;78:283–8. doi: 10.1161/01.res.78.2.283. [DOI] [PubMed] [Google Scholar]
- 39.Min JY, Yang Y, Sullivan MF, Ke Q, Converso KL, Chen Y, et al. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg. 2003;125:361–9. doi: 10.1067/mtc.2003.101. [DOI] [PubMed] [Google Scholar]
- 40.Singla DK, Hacker TA, Ma L, Douglas PS, Sullivan R, Lyons GE, et al. Transplantation of embryonic stem cells into the infarcted mouse heart: formation of multiple cell types. J Mol Cell Cardiol. 2006;40:195–200. doi: 10.1016/j.yjmcc.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203:2315–27. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 43.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–65. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 44.Strauer BE, Brehm M, Zeus T, Gattermann N, Hernandez A, Sorg RV, et al. Intracoronary, human autologous stem cell transplantation for myocardial regeneration following myocardial infarction. Dtsch Med Wochenschr. 2001;126:932–8. doi: 10.1055/s-2001-16579-2. [DOI] [PubMed] [Google Scholar]
- 45.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 46.Schächinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: Final one-year results of the TOPCARE-AMI Trial. J Am Coll Cardiol. 2004;44:1690–9. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Silva GV, Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, et al. Catheter-based transendocardial delivery of autologous bone-marrow derived mononuclear cells in patients listed for heart transplantation. Tex Heart Inst J. 2004;31:214–9. [PMC free article] [PubMed] [Google Scholar]
- 48.Kang HJ, Kim HS, Zhang SY, Park KW, Cho HJ, Koo BK, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction: The MAGIC cell randomised clinical trial. Lancet. 2004;363:751–6. doi: 10.1016/S0140-6736(04)15689-4. [DOI] [PubMed] [Google Scholar]
- 49.Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, De Bondt P, et al. Intracoronary injection of CD133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: Feasibility and safety. Circulation. 2005;112:I178–83. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 50.Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet. 2003;361:45–6. doi: 10.1016/S0140-6736(03)12110-1. [DOI] [PubMed] [Google Scholar]
- 51.Herreros J, Prósper F, Perez A, Gavira JJ, Garcia-Velloso MJ, Barba J, et al. Autologous intramyocardial injection of cultured skeletal muscle-derived stem cells in patients with non-acute myocardial infarction. Eur Heart J. 2003;24:2012–20. doi: 10.1016/j.ehj.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 52.Chen SL, Fang WW, Qian J, Ye F, Liu YH, Shan SJ, et al. Improvement of cardiac function after transplantation of autologous bone marrow mesenchymal stem cells in patients with acute myocardial infarction. Chin Med J (Engl) 2004;117:1443–8. [PubMed] [Google Scholar]
- 53.Katritsis DG, Sotiropoulou PA, Karvouni E, Karabinos I, Korovesis S, Perez SA, et al. Transcoronary transplantation of autologous mesenchymal stem cells and endothelial progenitors into infarcted human myocardium. Catheter Cardiovasc Interv. 2005;65:321–9. doi: 10.1002/ccd.20406. [DOI] [PubMed] [Google Scholar]
- 54.Klein HM, Ghodsizad A, Marktanner R, Poll L, Voelkel T, Mohammad Hasani MR, et al. Intramyocardial implantation of CD133+ stem cells improved cardiac function without bypass surgery. Heart Surg Forum. 2007;10:E66–9. doi: 10.1532/HSF98.20061054. [DOI] [PubMed] [Google Scholar]
- 55.Li ZQ, Zhang M, Jing YZ, Zhang WW, Liu Y, Cui LJ, et al. The clinical study of autologous peripheral blood stem cell transplantation by intracoronary infusion in patients with acute myocardial infarction (AMI) Int J Cardiol. 2007;115:52–6. doi: 10.1016/j.ijcard.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Losordo DW, Schatz RA, White CJ, Udelson JE, Veereshwarayya V, Durgin M, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina: A phase I/IIa double-blind, randomized controlled trial. Circulation. 2007;115:3165–72. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 57.Erbs S, Linke A, Schächinger V, Assmus B, Thiele H, Diederich KW, et al. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: The Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation. 2007;116:366–74. doi: 10.1161/CIRCULATIONAHA.106.671545. [DOI] [PubMed] [Google Scholar]
- 58.Choi JH, Choi J, Lee WS, Rhee I, Lee SC, Gwon HC, et al. Lack of additional benefit of intracoronary transplantation of autologous peripheral blood stem cell in patients with acute myocardial infarction. Circ J. 2007;71:486–94. doi: 10.1253/circj.71.486. [DOI] [PubMed] [Google Scholar]
- 59.Balogh L, Czuriga I, Hunyadi J, Galuska L, Kristóf E, Edes I. Effects of autologous bone marrow derived CD34+ stem cells on the left ventricular function following myocardial infarction. Orv Hetil. 2007;148:243–9. doi: 10.1556/OH.2007.27994. [DOI] [PubMed] [Google Scholar]
- 60.Klein HM, Ghodsizad A, Marktanner R, Poll L, Voelkel T, Mohammad Hasani MR, et al. Intramyocardial implantation of CD133+ stem cells improved cardiac function without bypass surgery. Heart Surg Forum. 2007;10:E66–9. doi: 10.1532/HSF98.20061054. [DOI] [PubMed] [Google Scholar]
- 61.Gyöngyösi M, Lang I, Dettke M, Beran G, Graf S, Sochor H, et al. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: The MYSTAR prospective, randomized study. Nat Clin Pract Cardiovasc Med. 2009;6:70–81. doi: 10.1038/ncpcardio1388. [DOI] [PubMed] [Google Scholar]
- 62.Martin-Rendon E, Brunskill S, Dorée C, Hyde C, Watt S, Mathur A, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2008;4:CD006536. doi: 10.1002/14651858.CD006536.pub2. [DOI] [PubMed] [Google Scholar]
- 63.Mohyeddin-Bonab M, Mohamad-Hassani MR, Alimoghaddam K, Sanatkar M, Gasemi M, Mirkhani H, et al. Autologous in vitro expanded mesenchymal stem cell therapy for human old myocardial infarction. Arch Iran Med. 2007;10:467–73. [PubMed] [Google Scholar]
- 64.Meluzín J, Janousek S, Mayer J, Groch L, Hornácek I, Hlinomaz O, et al. Three-, 6-, and 12-month results of autologous transplantation of mononuclear bone marrow cells in patients with acute myocardial infarction. Int J Cardiol. 2008;128:185–92. doi: 10.1016/j.ijcard.2007.04.098. [DOI] [PubMed] [Google Scholar]
- 65.Krause K, Jaquet K, Schneider C, Haupt S, Lioznov MV, Otte KM, et al. Percutaneous intramyocardial stem cell injection in patients with acute myocardial infarction: First-in-man study. Heart. 2009;95:1145–52. doi: 10.1136/hrt.2008.155077. [DOI] [PubMed] [Google Scholar]
- 66.Beltrami AP, Urbanek K, Kajstura J, Yan SM, Finato N, Bussani R, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med. 2001;344:1750–7. doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 67.Ellison GM, Torella D, Karakikes I, Nadal-Ginard B. Myocyte death and renewal: Modern concepts of cardiac cellular homeostasis. Nat Clin Pract Cardiovasc Med. 2007;4:S52–9. doi: 10.1038/ncpcardio0773. [DOI] [PubMed] [Google Scholar]
- 68.Behfar A, Perez-Terzic C, Faustino RS, Arrell DK, Hodgson DM, Yamada S, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med. 2007;204:405–20. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Penn MS, Zhang M, Deglurkar I, Topol EJ. Role of stem cell homing in myocardial regeneration. Int J Cardiol. 2004;95:S23–5. doi: 10.1016/s0167-5273(04)90007-1. [DOI] [PubMed] [Google Scholar]
- 70.Smart N, Riley PR. The stem cell movement. Circ Res. 2008;102:1155–68. doi: 10.1161/CIRCRESAHA.108.175158. [DOI] [PubMed] [Google Scholar]


