Abstract
Depression is currently the fourth leading cause of disease or disability worldwide. Antidepressant is approved for the treatment of major depression (including paediatric depression), obsessive-compulsive disorder (in both adult and paediatric populations), bulimia nervosa, panic disorder and premenstrual dysphoric disorder. Antidepressant is a psychiatric medication used to alleviate mood disorders, such as major depression and dysthymia and anxiety disorders such as social anxiety disorder. Many drugs produce an antidepressant effect, but restrictions on their use have caused controversy and off-label prescription a risk, despite claims of superior efficacy. Our current understanding of its pathogenesis is limited and existing treatments are inadequate, providing relief to only a subset of people suffering from depression. Reviews of literature suggest that heterocyclic moieties and their derivatives has proven success in treating depression.
Keywords: Antidepressant, depression, heterocyclic
Depression is a chronic, recurring and potentially life-threatening illness that affects up to 20% of the population across the globe.[1] The etiology of the disease is suboptimal concentrations of the monoamine neurotransmitters serotonin (5-HT) and norepinephrine (NE) in the central nervous system (CNS). It is also due to consequence of dysfunctional endocrine, immune and neurotransmitter systems.[2]
Depression is a chronic condition.[3] Primary clinical manifestations of depression are significant depression of mood and impairment of function. Some features of depressive disorders overlap those of the anxiety disorders, including panic-agoraphobia syndrome, severe phobias, generalized anxiety disorder, social anxiety disorder, posttraumatic stress disorder, and obsessive-compulsive disorder.[4]
Recent advancement in antidepressant therapeutics include the monoamine oxidase inhibitors (MAOIs, e.g. Nardil®) tricyclic antidepressants (TCAs, e.g. Elavil). They increases the synaptic concentration of either two (5-HT and NE) or all three (5-HT, NE and dopamine (DA)) neurotransmitters. The combined effect of Serotonin selective reuptake inhibitor (SSRI) (Prozac®) and serotonin reuptake transporter (SERT) inhibitor increases synaptic concentration of 5-HT and its duration of action.
Recent studies suggest that combination therapy using bupropion, a Dopamine transporter (DAT) inhibitor, and an SSRI or Serotonin–norepinephrine reuptake inhibitor (SNRI) offers improved efficacy in the treatment of depression compared with monotherapy alone.[2] For example, DOV-21947 (by DOV pharmaceutical, Phase II), SEP-225289 (by Sepracor, Phase II), and NS-2359 (by NeuroSearch, Phase II).[5] A new SNRI class of antidepressants (Cymbalta®) are also approved to treat depression along with anxiety (approximately 60% of patients).[2]
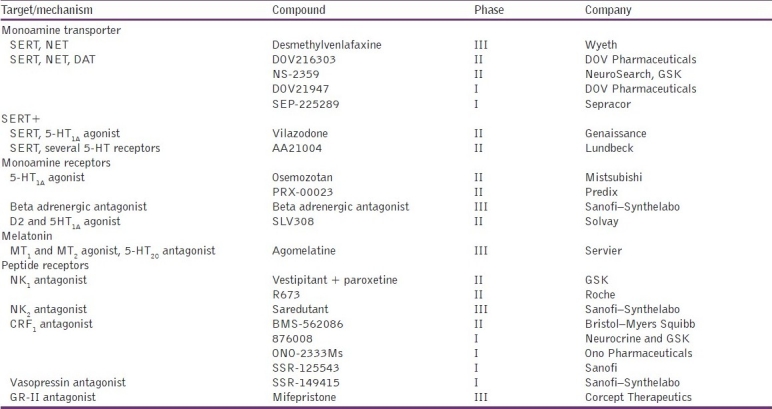
The major problems of existing antidepressant drugs include delayed clinical benefit, serious side-effects, and a response in less than 50% of patients.[6] Neither the pathophysiology of depression nor the mechanism of action of various antidepressant agents is fully understood.[7] Consequently, there is still a great need for faster-acting, safer, and more effective treatments for depression. In an effort to expand beyond classical monoamine-based strategies, recent medication development activities have focused on neurotrophic factors, glutamatergic systems, and the hypothalamic–pituitary axis (HPA) as well as a number of other less well-characterized novel targets [Table 1].
Table 1.
Survey of the pharmacological mechanisms of new accepted antidepressants in clinical development[8]
The present review focuses on potent heterocyclic nuclei and derived compounds showing antidepressant activity.[6]
Indole
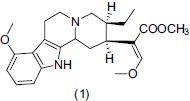
Indole is a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. Numerous pharmacological active compounds have an indole nucleus such as Tryptophan (essential amino acid), serotonin (neurotransmitter), melatonin (hormone). Indole nucleus shows potent antidepressant activity, various derivatives of indole and 5-HT serve as a template for the development of new chemical entities that act on 5-HT1A receptor and the SERT.[9] Mattson et al.,[10] synthesized some potent serotonin reuptake inhibitors based on indole nucleus and they reported that compound (12) reveals potent hSERT binding (Ki) 0.18 nM) in vitro. Indole nucleus is present in many important classes of therapeutic agents.[11] Indole alkaloidal analogues and indoles condensed with different heterocycles[12] showed different biological activities. Idayu et al.,[13] investigated the antidepressant effect of Mitragynine (1) isolated from Mitragyna speciosa Korth. Mitragynin showed potent antidepressant activity at dose of 10 mg/kg and 30 mg/kg i.p. and reduced the immobility time of mice in both Forced swimming test (FST) and Tail suspension test (TST) without any significant effect on locomotor activity in open field test (OFT).
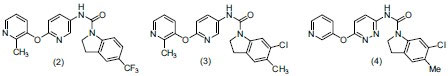
Bispyridyl ethers have indoline nucleus and have selected or potent activity towards CNS disorder. (SB-242084) and (SB-243213) (Phase 1 clinical trials) are bispyridyl ethers having potent 5-HT2C receptor inverse agonistic activity and selective activity for both the 5-HT2A and 5-HT2B receptors. Bromidge et al.,[14] synthesized a series of bisaryl ethers and evaluated their antidepressant activity. Compounds (2), (3) and (4) possess potent activity in the rat hypolocomotion assay.
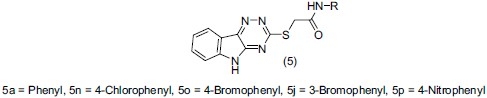
Shelke et al.,[15] synthesized a series of 2-(5H-[1, 2, 4] triazino [5, 6-b]indol-3-ylthio)-N-(substituted phenyl) acetamides. Selected compounds were screened for potential antidepressant activity on animal model by tail suspension test (TST) in mice. On the basis of quantitative structure activity relationship (QSAR) study they proved that compounds like (5a), (5n), (5o), (5j) and (5p) have activity compared to standard antidepressant drugs moclobemide, imipramine and fluoxetine.
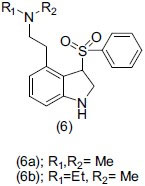
Heffernam et al.,[16] synthesized a series of 4-amino ethyl -3- (phenyl sulfonyl)1-H indole(6) and screened the compound for dual-acting norepinephrine reuptake inhibitor and 5-HT2A reuptake antagonist. Compound (6a) and (6b) were potent inhibitors of norepinephrine reuptake and possessed modest h5-HT2A antagonist activity. When the amino-ethyl chain shifted from the fourth to fifth and seventh position, it behaved as a potent antagonist.
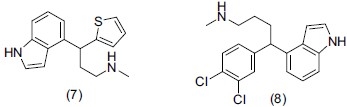
Manning et al.,[17] synthesized a series of enantiomerically pure 1-naphthyl and 4-indolyl arylalkylamines and evaluated them for binding affinities to the monoamine transporters. They designed (S)-4-(3, 4-dichlorophenyl)-4-(1H-indol-4-yl)-N-methylbutan-1-amine as a potent inhibitor for the dopamine and serotonin transporters. They showed that compounds (7) and (8) have strong binding affinity to DAT and SERT (3.83 and 0.815 nM respectively) and a 1000-fold selectivity compared to the Norepinephrine transporter (NET) binding affinity.
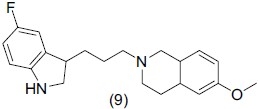
Serotonergic neurotransmission is known to modulate various physiological and behavioral functions in the CNS. It is related to ′integrating′ emotion, cognition, motor function, pain, circadian rhythm and neuroendocrine functions. Ben-Daniel et al.,[9] synthesized a new class of indole derivatives, some potent compounds have low nanomolar affinity for the SERT and high selectivity for 5-HT1A receptor. Six indolylpropylamine derivatives were synthesized and evaluated for SERT inhibitory activity. The most potent compound (9), 2-[3-(5-fluoro-1H-indol-3-yl)-propyl]-6-methoxy-1, 2, 3, 4-tetrahydro isoquinoline showed promising antidepressant effect and was also evaluated in vivo using positron emission tomography (PET) in animal models.
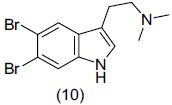
Brominated indole alkaloids form a common class of metabolites obtained from sponges of the order Verongida. Kochanowska et al.,[18] reported the isolation, structure determination, and activity of metabolites obtained from three Florida sponges, namely Verongula rigida (order Verongida, family Aplysinidae), Smenospongia aurea, and S. cerebriformis (order Dictyoceratida, family Thorectidae). They isolated some compounds among which compound (10) 5, 6-dibromo-N,N-dimethyltryptamine exhibited significant antidepressant activity on rodent FST model.
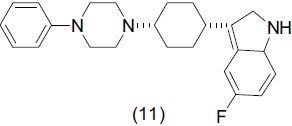
Selective serotonin reuptake inhibitors (SSRIs) have great success in treating depression and have fewer and less severe side-effects than first-generation drugs. Zhou et al.,[19] reported a series of analogues of arylpiperazine-4-yl-cyclohexylindole (11) evaluated as 5-HT transporter inhibitor and 5-HT1A receptor antagonist.
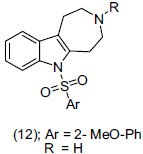
Liu et al.,[20] reported the tryptamine derivatives for antidepressant activity. They suggested the regiospecific synthesis of a series of 1-sulfonyl azepinoindoles bearing a sulfonyl group at indole N1 position, which showed potent 5-HT6 activity. They concluded that rigidified structures of azepino indole derivatives bind effectively to the 5-HT6 receptor in a constrained conformational mode. According to the structure activity relationship (SAR) studies, alkyl group is unfavorable to the peptide backbone of the receptor. Selected compounds were evaluated for 5-HT6 functional activity. Compound (12) showed promising antidepressant activity with (IC50 = 162 nM).
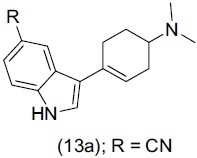
5-Hydroxytryptamine (5-HT, serotonin) is an important neurotransmitter that plays a significant role in a variety of physiological functions in the CNS and peripheral tissues. Deskus et al.,[21] synthesized a series of indole tetrahydropyridine and indole cyclohexenylamines and studied their binding affinities at the human serotonin transporter (SERT). They found out that the nitrile substituent at the C5 position of the indole ring gave potent antidepressant activity through selectivity over SERT. On the basis of modeling studies they conclude that Compound (13a) and especially R-enantiomer reveal selectivity for serotonin transporter IC50 (nM) over dopamine and norepinephrine transporters.
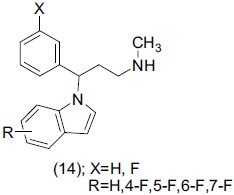
Mahaney et al.,[22] designed a variety of 3-(1H-indole-1yl)-3-aryl propan-1amines (14) and evaluated them for dual-acting norepinephrine and serotonin reuptake inhibitors. The antidepressant effect of compound (14) was found to be promising.
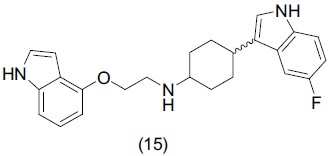
Evrard et al.,[23] synthesized some derivatives of serotonin reuptake inhibitor 4-(5-fluoro-1H indol-3-yl)cyclohexylamine and evaluated them for antidepressant activity. Compounds containing 1-(4-indolyl)piperazine and 2-(1H-indol-4-yloxy)ethylamine showed promising activity. They identify 4-(5-fluoro-1H-indol-3-yl) cyclohexylamine as a more optimized template for the design of dual-acting SSRI/5-HT1A antagonists. Compound (15) possessed potent and balanced affinities for the two molecular targets and exhibited potent antidepressant activity.
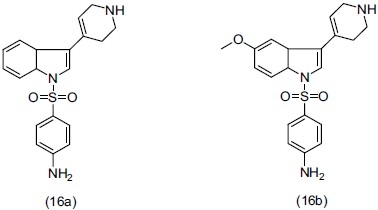
The serotonin (5-HT) receptors have been divided into seven subclasses (5-HT1-7). Numerous antidepressants and antipsychotic drugs have high affinity for the 5-HT6 receptor. Cole et al.,[24] synthesized a series of N1-arylsulfonyl-3-(1, 2, 3, 6-tetrahydropyridin-4-yl)indole derivatives. Some synthesized compounds were shown to have high affinity for the 5-HT6 receptor. Two analogs, 4-[3-(1, 2, 3, 6-tetrahydropyridin-4-yl)-1H-indole-1-sulfonyl]- phenyl amine (16a) and 4-[3-(1, 2, 3, 6-tetrahydropyridin-4-yl)-5-methoxy-1H-indole-1 sulfonyl]-phenylamine (16b), have 0.4 and 3.0nM affinity, and antagonized the production of adenylate cyclase at sub-nanomolar concentrations.
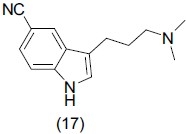
Simple homotryptamines are potential SSRIs. Schmitz et al.,[25] synthesized a series of N,N dimethylhomotryptamines and binding affinities of the synthesized compounds were determined by using HEK-293 cells that stably expressed human serotonin transporters (HEK-hSERT cells). They also revealed that compounds possessing an electron withdrawing substituent at the C5 position of the indole nucleus are potent SSRIs. From the synthesized compounds 5-cyano homotryptamine (17) was the most potent compound with (IC50 = 2 nM).
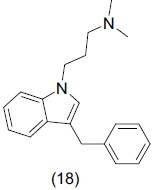
Tryptamine and isotryptamine are compounds having indole nucleus. Kolanos et al.,[26] studied the binding affinity of indolic nitrogen for h5-HT6 serotonin receptors. They concluded that indolic nitrogen atom is essential for the binding of N1-benzyltryptamines at h5-HT6 serotonin receptors.
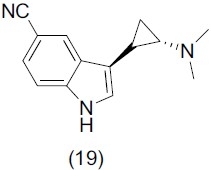
The serotonergic (5-HT) system is a key target for antidepressant drugs; human serotonin transporter (hSERT) is one of the major regulators of synaptic 5-HT levels. Mattson et al.,[10] synthesized a series of indole cyclopropylmethylamines and some selected synthesized compounds were evaluated for antidepressant activity. On the basis of structure activity relationship, Nitrile substituents at the 5 and 7 positions of the indole ring gave high affinity for hSERT, while N-1 Substitution in indole ring with methyl or ethyl group decreases in 10- to 30-fold affinity for hSERT. Compound (+)-(19) demonstrated potent hSERT binding (Ki) 0.18 nM) in vitro and was more than 1000-fold less potent at hDAT, hNET, 5-HT1A, and 5-HT6. The maximal response of this compound was similar to that of fluoxetine.
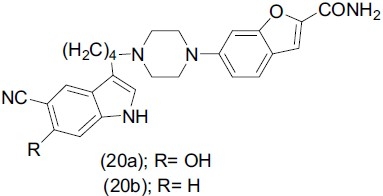
Heinrich et al.,[27] synthesized derivatives of 5-cyano-6-hydroxy-1H-indole, which is a major metabolite of the potential antidepressant drug Vilazodone. Compounds (20a) and (20b), evaluated for antidepressant activity showed an innovative dual mechanism at the 5-HT1A receptor.
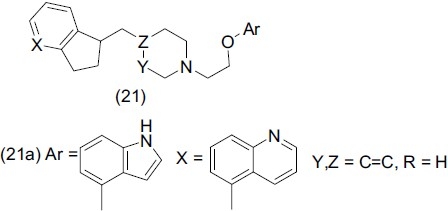
Mewshow et al.,[28] designed and synthesized a novel series of indole derivatives (21) and evaluated them for dual 5-HT transporter reuptake and 5-HT1A antagonist activity. Compound (21a) showed much higher 5-HT1A and 5-HT transporter affinity.
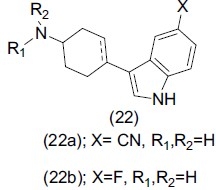
Meagher et al.,[29] reported a set of indonyl cylohexylamine (22). The synthesized compounds were evaluated for potent and selective serotonin reuptake inhibition. Substitution at the indole fifth position with cyano (22a) and fluoro (22b) led to an improvement in 5-HT transporter affinity.
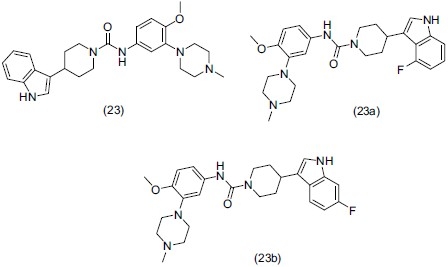
Serotonin (5-HT) is a biogenic amine neurotransmitter; antidepressant effect of the selective 5-HT reuptake inhibitors (SSRIs) is due to enhancement of postsynaptic 5-HT levels. Matzen et al.,[30] prepared a series of unsymmetrical ureas and evaluated them as 5-HT reuptake inhibitors with 5-HT1B/1D antagonistic activities. For the designing of the compounds, they coupled the various indole derivatives (inhibit 5-HT reuptake), with three different aniline moieties (5-HT1B/1D ligands). Compound no. (23) (4-(5-fluoro-1H-indol-3-yl)piperidine-1-carboxylic acid [4-methoxy-3-(4-methylpiperazin-1-yl) phenyl] amide), (23a), and (23b) showed the most potent antidepressant activity through 5-HT reuptake inhibition and 5-HT1B/1D antagonism in vitro using rabbit saphenous vein model and 5-HT release from guinea pig cortical slices.
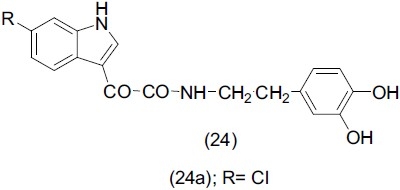
Primofiore et al.,[31] synthesized a range of N-(indole-3-ylgloxyl) (24) dopamine derivatives. All products were evaluated for their antidepressant activity. The N-(5-chloro indole-3-ylgloxylyl) dopamine (24a) proved to be of particular interest and exhibited good activity.
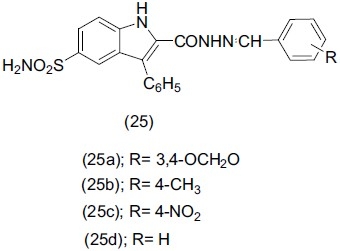
Ergence et al.,[32] synthesized a series of arylidenehydrazides (25) by reacting 3- phenyl-5-sulfonamidoindol-2- carboxylic acid hydrzide with various aldehydes and evaluated them for antidepressant activity by Porsolt's forced swim method by using tranylcypromine as standard drug. Compound 3-phenyl -5-sulfonamidomindol-2-corboxylic acid, 3, 4- methylene dioxybenzylidene hydrazide (25a), 3-phenyl-5-sulfonamidoindole-2-carboxylic acid 4-methylbenzylidene hydrazide (25b), 3-phenyl-5-sulfonamidoindole-2-carboxylic acid 4-nitrobenzylidene hydrazide (25c), 3-phenyl-5-sulfonamidoindole-2-corboxylic acid benzylidene hydrazide (25d), showed antidepressant activity at 100 mg/kg.
Thiadiazole
The thiadiazole system is a cyclic analogue of thiosemicarbazide having the toxophoric N=C=S linkage.[33] Thiadiazole is derived from thiophene by replacing two -CH= group by pyridine-type nitrogen (-N=) and is found in four isomeric forms depending on the relative positions of nitrogen atoms. A number of synthetic products have been framed on the basis of thiadiazole nucleus which show broad range of biological activities, for example, the thiadiazole SCH-202676 is a promising allosteric modulator of G-protein coupled receptors-4, KC12291 showed cardioprotective action, and small heterocyclic thiadiazolidinones (TDZD) are first non-ATP competitive glycogen synthase kinase 3b inhibitors.[34] Thiadiazole derivative compounds displayed potent antidepressant and anxiolytic activity. Clerici et al.,[35] synthesized some potent compounds based on 2-amino-5-sulfanyl-1, 3, 4-thiadiazole and evaluated them for CNS activity. Compound (26) exhibited marked antidepressant activity.[36]
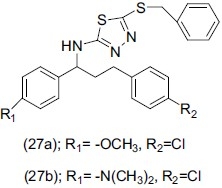
Yusuf et al.,[37] synthesized a series of new imine derivatives of 5-amino-1,2,3-thiadiazole 2-thiol. Antidepressant activity was tested against imipramine as reference drug using FST in mice. Compound (27a) 5-{[1-(4-chlorophenyl)-3-(4-methoxyphenyl)prop-2-ene-1ylidine]-amino]-5-benzyl thio-1,3,4-thiadiazole, and compound (27b) 3-{[1-(chloropheny)-3-(4-dimethyl-amino phenyl)-prop-2ene1-ylidine]amino)-5-benzylthio-1,3,4-thiadiazole showed significant antidepressant activity. Compound (27a) and (27b) decreased immobility time by 77.99% and 76.26% compared to the standard imipramine (82%).
Pathanayk et al.,[38] synthesized novel 2-amino-5-sulfanyl1, 3, 4 thaidiazole analogues (28). Potentially active analogues (28a), (28b) and (28c) characterized by spectral analysis and evaluated for antidepressant activity exhibited promising antidepressant activity in pharmacological models and in also comparison with reference antidepressant drugs.
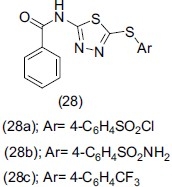
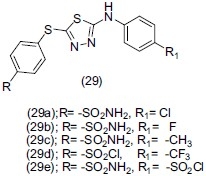
Sharma et al.,[39] worked on a compound 2-amino-5-sulfanyl 1, 3, 4-thiadiazole(29) and synthesized a new series of compounds (29a), (29b), (29c), (29d), (29e) .The newly synthesized compounds were characterized by analytical and spectral methods. Compounds (29d), (29e), (29f), (29g), (29h) exhibited significant antidepressant activity in comparison to the reference drugs.
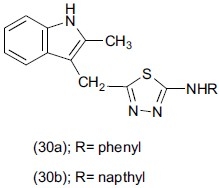
Varvarsou et al.,[40] synthesized a series of N-5-(2 methyl-1H-3-indolyl) melhyl)-1, 3, 4-thiadiazol-2-yl-N-arylamine. Compounds (30a and 30b) were evaluated for antidepressant activity by forced swim test using imipramine as standard drug.
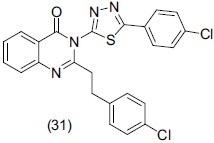
Jatav et al.,[41] synthesized a series of 3-[5-substituted phenyl-1, 3, 4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Out of 18 synthesized compounds, compound no (31) exhibited potent antidepressant activity through forced swim test method.
Piperidine
Piperidine ring is an omnipresent molecular skeleton. A large number of piperidine-containing compounds are biologically and medicinally important. Biological properties of piperidines are highly dependent on the type and location of substituents on the heterocyclic ring.[42] Various N-heterocyclic piperidine compounds have been mentioned in clinical and preclinical studies.[43] Piperidine nucleus occurs in active pharmacological substances, for example Eliprodil (SL-82.0715) (N-Methyl-D-aspartic acid (NMDA) antagonist).[44]
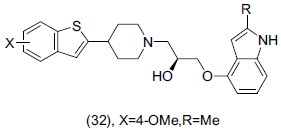
Takeuchi et al.,[45] synthesized a series of 1-(1H-indol-4- yloxy)-3-(4-benzo[b]thiophen-2-ylpiperidinyl) propan-2-ols and evaluated them for antidepressant activity. They identified some fused bicyclic aryl-substituted piperidines as an essential pharmacophore for 5-HT reuptake inhibition and also having potent dual 5-HT1A receptor antagonism and serotonin reuptake inhibition. On the basis of potency and activity compound (32) showed combined 5-HT1A/SSRI activity in a single molecule with no negative feedback effect.
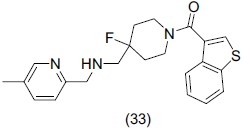
Bollinger et al.,[46] synthesized 1, 4-disubstituted aromatic piperidines and piperazines (1,4-DAPs) and showed the effect of some synthesized compounds on endogenous neurotransmitters including dopamine, serotonin, and (nor)epinephrine. Compound (33) reported to be a potent agonist for 5HT1A receptor and showed promising antidepressant effect having Ki value of 2.7 nM and a maximal efficacy of 124%.
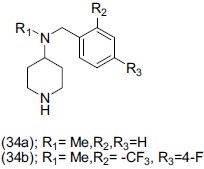
Boot et al.,[47] reported a set of N-alkyl-N-arymethylpiperidin-4-amines (34a) and derivatives were demonstrated to be inhibitors of both serotonin and norepinephrin reuptake. 4- Fluro-2-trifluoromethyl substitution (34b) pattern was evaluated for optimization towards dual SERT and NET inhibition.
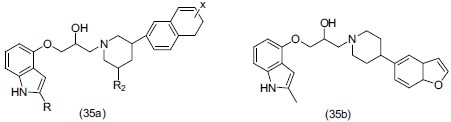
Rocco et al.,[48] designed a series of 1-(1H indole-4-yloxy)-3-(4-aryl pipridinyl)propan-2-ols (35a). Compound (35b) was found to be a potent dual 5-HT1A receptor antagonist and serotonin reuptake inhibitor.
Steckler et al.,[49] designed a set of derivatives of 4-phenyl-4-[1H-imidazole-2-yl]-piperidine (36). Compound (36a) acted as a selective non-peptide delta opoid agonist with antidepressant and anxiolytic activity.
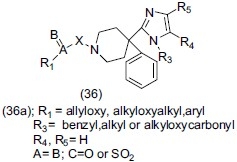
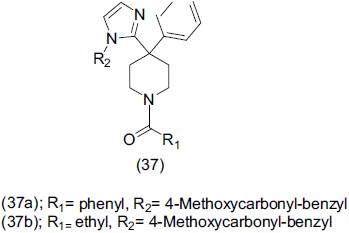
Trabanco et al.,[50] synthesized 4-phenyl-4-(1H-imidazol-2-yl) Piperidine derivatives (37) and evaluated their antidepressant-like effect by mouse tail suspension test. Compound (37a), (37b) stand as the most potent compounds prepared within this series.
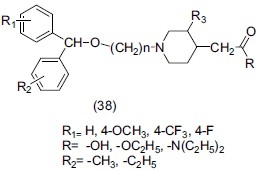
Ahmed et al.,[51] synthesized several benzhydroxylalkylpiperidine derivatives (38) and evaluated them by pharmacological tests for antagonism of reserpine and apomorphine-induced hypothermia. Compounds were evaluated by tail suspension test in mice. Preferred compounds were also studied pharmacologically by binding study to serotonin (5HT), norepinephrine (Ne) and dopamine (DA) reuptake site and showed antidepressant activity.
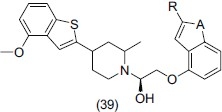
Takeuchi et al.,[52] synthesized a series of 1-aryloxy-3-piperidinylpropan-2-ols having potent dual 5-HT1A receptor antagonism and serotonin reuptake inhibition. They also proposed some beneficial modification of potential metabolic sites of 1-(1H-indol-4-yloxy)-3-(4-benzo[b]thiophen-2-ylpiperidinyl) propan-2-ols, which is essential for improved in vitro binding affinities and functional antagonism over 5HT1A receptor and 5-HT reuptake sites. The most potent compound identified was (39).
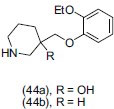
Takeuchi et al.,[53] synthesized a series of 1-(1H-indol-4-yloxy)-3-(4-benzo[b]thiophen-2-ylpiperidinyl) propan-2-ols. Some potent derivatives showed 5-HT1A/SSRIs activity at low nanomolar and subnanomolar concentrations. They found that incorporation of the α-methyl group in the piperidine ring with its specific stereochemistry enhanced binding affinity at the 5-HT reuptake site and also in vitro 5-HT1A antagonist functional activity. Compound (40) and the exo-isomer showed potent antidepressant activity.
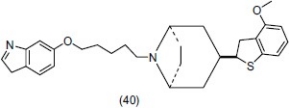
Ong et al.,[54] synthesized a series of l-arylspiro[indoline-3,4′-piperidines] and some potent compounds were evaluated for potential antidepressant activity in the reference of tetrabenazine (TBZ) ptosis prevention and potentiation of 5-hydroxytryptophan (5-HTP)-induced head twitching in pargyline-pretreated rats. Compound (41) showed potent in vitro antidepressant activity.
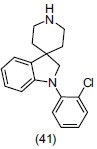
Many drugs showed antidepressant effect by interacting with σ receptors present in brain regions. Wang et al.,[55] studied two novel σ receptor agonists UMB23 (1-(3-phenylpropyl) piperidine oxalate) (42), UMB82 (2-(3,4-dichlorophenyl)-N-ethyl-N-(2-piperidin-1-ylethyl)ethanamine oxalate) (43) and evaluated them for antidepressant-like activity in mice through two standard methods, first radio ligand binding studies and second the forced swim test. Compound UMB23 precisely displayed a high degree of selectivity, showing Nano molar affinities for each of the σ receptor subtypes. They also reveal that σ receptor antagonist BD1047 attenuated the antidepressant-like effects of UMB23 and UMB82.
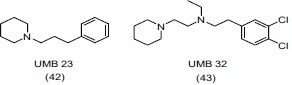
Balsamo et al.,[56] synthesized a series of 3-[1-(2-ethoxyphenoxy)methyl] piperidine derivatives. They screened some potential antidepressant compounds through reserpine interaction test in mice and evaluated them for reuptake inhibition of biogenic amines in pig brain synaptosomal fractions. On the basis of in vivo and in vitro tests they revealed that compound (44a) and (44b) have antidepressant activity comparable to that of reference antidepressant drug viloxazine.
He et al.,[57] synthesized a series of 4-(4-Chlorophenyl) piperidine derivatives having a thioacetamide side chain and evaluated them for potent antidepressant activity through dopamine transporter/norepinephrine transporter (DAT/NET) selectivity. They concluded that compounds (-)-trans-(45a), (+)-cis-(45b), and (-)-cis(45c) show fairly good potency, the Ki value of (+)-cis-(45b) at the NET is 5.5 nM and showed best selectivity for the NET.
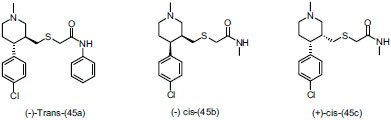
Orjales et al.,[58] prepared a series of [aryl(aryloxy)methyl] piperidine derivatives and selected synthesized compounds were evaluated for antidepressant activity. These compounds had high affinity for serotonin (5-HT) transporter (SERT), 5-HT1A and 5-HT2A receptors and also Norepinephrine (NE) transporters and linked directly or through a methylene chain to different substituted and unsubstituted nuclei (isoquinoline, piperazine, piperidine, tetrahydropyran, or cyclopentane) for potentiation of antidepressant effect. They reported that Compound (-)-(46) (coded as F-98214-TA) possess a dual binding character and very high affinity value for SERT and NET (Ki)1.9 and 13.5 nM, respectively from rat brain using radioligand binding assay.
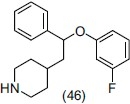
Vacher et al.,[59] prepared a series of aryl-{[4-(6-R-pyridin-2-ylmethyl)-amino]-methyl}- piperidin-1-yl-methanone as 5-HT1A receptor agonists. They reported that the fluorine atom at the C4 position of the piperidine ring enhances the oral bioavailability and 5-HT1A agonist activity in rats. Some potent derivatives (47), (48), and (49) have higher affinity and selectivity to 5-HT1A receptors. For comparing the conformation of the pharmacophore and agonistic activity they synthesized a series of 3-chloro-4-fluorophenyl-(4- fluoro-4{[(5-(H or CH3)-6-R-pyridin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl-methanone derivatives. They concluded that compounds (50), (51), and (52) have potent antidepressant activity measured through forced swimming test (FST) in rats.
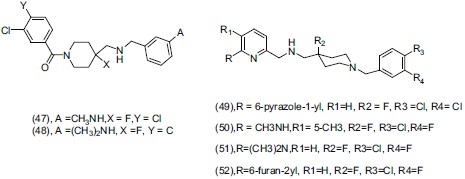
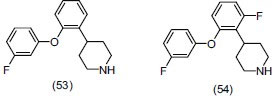
Gray et al.,[60] synthesized a series of aryl piperazine and piperidine ethers and selected compounds were evaluated for antidepressant activity through both norepinephrine reuptake inhibitors (NRI) and 5-HT1A partial agonist effect. They concluded that compounds (53) and (54) are the most promising and potent for antidepressant activity. Compounds (54) in 10 mg/kg subcutaneous injection, penetrates into the brain and binds to both target receptors in Sprauge-Dawley rats.
Piperazine
Piperazine is a six-membered heterocyclic ring having two opposing nitrogen atoms. the piperazine nucleus has potent antidepressant activity. Various derivatives of piperazine show potent serotonin antagonist/reuptake inhibitors, YM-992, LY367265, Nefazodone and Aripiprazole are the typical examples.[61] WAY-100635 is a 5-HT1A receptor antagonist.[62] 1-(2-Pyrimidinyl)-piperazine is a major active metabolite of tandospirone, gepirone, ipsapirone, and buspirone which also have antidepressant/anxiolytic 5-HT1A agonists′ activity.[63] The pharmaceutical importance of this nucleus is due to extensive occurrence in current marketed drug.[64] Various antidepressant drugs have a piperazine nucleus such as Amoxapine, Befuraline, Binospirone, Alnespirone, Buspirone, Flesinoxan, Gepirone, Ipsapirone, Nefazodone, Piberaline, and Tandospirone. Arylpiperazine moiety is used as a template for designing CNS-active agents. It constitutes the main pharmacophoric fragment, for serotonergic, dopaminergic, and adrenergic receptors.[65]
Fray et al.,[66] synthesized a new class of dual serotonin noradrenalin reuptake inhibitor N-(1,2-diphenylethyl) piperazine (55). Two compounds possessed comparable in vitro profile to the dual reuptake inhibitor duloxetine. N-substituted piperazine (55a) potently inhibited both [3H]-5HT and [3H]-NA reuptake in HEK 293 cell transfected with human amine transporter with 100-fold selectivity over the DA transporter.
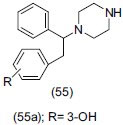
Kang et al.,[67] synthesized aryl piperzine containing pyrrole-3-carboxamide derivatives (56). These derivatives were evaluated for binding to 5HT2A, 5-HT2C receptor and 5-HT transporter. Compound (56a) showed good efficacy. Antidepressant activity of interesting compounds was evaluated by FST on mice using fluoxetine as reference drug. Compound (56b) proved to be the most potent.
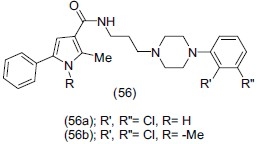
Kling et al.,[68] synthesized 2- methoxy arylpiperazine (57) series based on the N-4-aryl-piperazinyl-N-ethyl-5, 6, 7, 8-tetrahydropyrido [4,3,4.5] thieno (2,3-d) pyrimidine-4(3H)-one core. Isoquinoline analogue (57a) displayed high affinity and an antagonistic mode of action for the 5-HT1A and the 5-HT1B receptors. Compound (57a) was shown to increase [3H] 5-HT release from rat brain cortical slices.
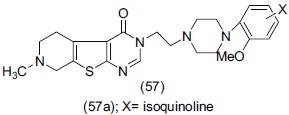
Torrado et al.,[69] synthesized a series of compounds combining the naphthylpiperazine (58) and thienopyran scaffolds and evaluated for 5-HT reuptake inhibitor with 5-HT1D antagonist activity. The designed compound (59) has been based on the ′overlapping type strategy where two pharmacophores (58) and thinopyran are linked in a single molecule, the resultant compound (59) has a dual pharmacological profile and has the potential to deliver a more efficient treatment for depression. Analogue (59a) chloro substituted at C3 position of the theopyran showed the most promising profile.
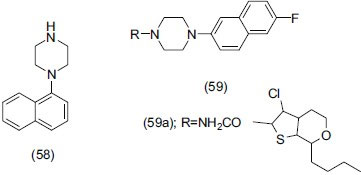
Zajdel et al.,[70] synthesized compound bearing (3-arylamino) pyrrolidine-2,5-dione (60) and N-arylprolinamide (61) moieties showing high affinity for 5-HT1A, high to low for 5-HT2A and low for D2 receptor. Compounds (60a) and (60b) were found to be 5-HT1A partial agonist and compound (60c) was a mixed 5-HT1A antagonist.
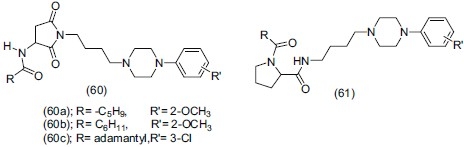
Paluchowska et al.,[71] reported some potent flexible and rigid imides with 1-(m-trifluorophenyl)piperazine fragment showing very high affinity (Ki = 0.3-34 nM) for 5-HT1A receptors. They screened some potent compounds having antidepressant-like activity in the swim tests in mice. Compounds (62a), (62b) and (62c) showed potent antidepressant effect, greater than that induced by imipramine as a reference antidepressant.
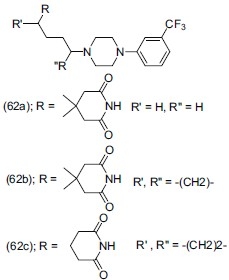
5-HT7 receptor belongs to G protein-coupled receptor (GPCRs) and most recently discovered, found in the thalamus, hypothalamus, brainstem, and hippocampus in the brain, and blood vessels in the periphery. SB-269970 a 5-HT7 receptor antagonist having piperazine nucleus, exhibited the maximum affinity and the greatest selectivity. Yoon et al.,[72] synthesized a series of 4-methoxy-N-[3-(4-substituted phenyl-piperazine-1-yl) propyl] benzene sulfonamides and N-[3-(4-substituted phenyl-piperazine-1-yl) propyl] naphthyl sulphonamides and evaluated them for 5-HT7 receptor antagonistic activity. The synthesized compound (63) showed a noble activity on 5-HT7 receptors and good selectivity on 5-HT1a, 5-HT2a, 5-HT2c, and 5-HT6 receptors.
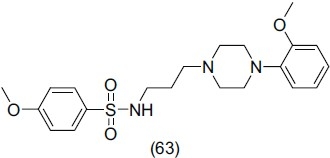
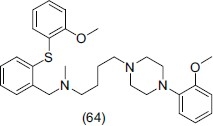
Wu et al.,[73] synthesized and reported a novel series of arylpiperazine derivatives of diphenylsulfide and evaluated them for dual 5- HT1A/SSRI activities. They concluded that 2-methoxyphenyl-piperazine moiety is essential for 5-HT1A affinity. Compound (64) of this series showed best antidepressant activity.
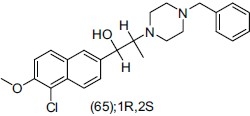
Weng et al.,[74] synthesized four optical isomers of SIPI5056 (2-(4-benzylpiperazin-1-yl) 1-(5-chloro-6-methoxynaphthalen-2-yl) propan-1-ol) and evaluated them for antidepressant activities and acute toxicities as novel reuptake inhibitors of monoamine transmitters. They studied two optical isomers (1S, 2R) and (1R, 2S) which have difference in activity and safety. The ED50 values of four isomers were measured through single oral dosing mouse tail suspension test (TST) and mouse forced swimming test (FST). They concluded that ED50 value of (1R, 2S) isomer was 12.6 mg/kg and (1S, 2R) isomer was 16.5 mg/kg. Isomer (1R,2S)-SIPI5056 is most potent for antidepressant activity.
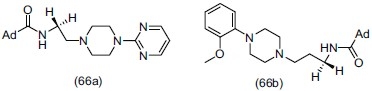
Abou-Gharbia et al.,[75] synthesized novel adamantyl aryl- and heteroarylpiperazine derivatives and evaluated them for serotonin receptor activities. They reported that the compound (66a) (WY-50,324, SEB-324, adatanserin), adamantyl-1-carboxylic acid 2-[4-(2-pyrimidinyl)- 1-piperazinyl]ethylamide, and (66b), adamantyl-1-carboxylic acid 2-[4-(2-methoxyphenyl)- 1-piperazinyl]ethylamide, revealed high affinity for 5-HT1A (Ki) (1nM for both) and also moderate affinity for 5-HT2 receptors (Ki) (73 and 75 nM, respectively). Partial agonistic activity for 5-HT1A was measured in vivo in rat serotonin syndrome and 5-HT2 antagonist activity in quipazine- and DOI-induced head shakes paradigms. On the basis of animal model results, they confirmed that compound (66a) showed combined anxiolytic and antidepressant activity.
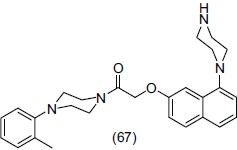
Jorand-Lebrun et al.,[76] reported a series of arylpiperazide derivatives of 1- naphthylpiperazine (1-NP) and evaluate them for 5-HT1B antagonists as potent antidepressant compounds. They observed that arylpiperazide at 7 positions of 1-NP increases the selectivity for 5-HT1B/1D receptor. From the synthesized compounds 2-[[8-(4-methylpiperazin-1-yl)naphthalen-2-yl]oxy]-1-(4-o-tolylpiperazin-1-yl) ethanone (67) was identified as a potent 5-HT1B antagonist and has a capability to antagonize the inhibition of 5-HT release induced by 5-CT (5-carbamoyltryptamine) in guinea pig hypothalamus slices and also antagonizes the hypothermia induced by selective 5-HT1B/1D agonist in vivo in the guinea pig following oral administration (ED50 = 0.13 mg/kg).
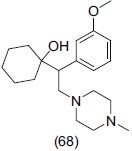
Mahaney et al.,[77] discovered a series of piperazine-containing analogues for norepinephrine reuptake inhibitors (NRIs) in animal models. On the basis of substitution pattern and animal testing profile, compound (68) (S)-(-)-(WAY-256805) was found to be a potent norepinephrine reuptake inhibitor (IC50 = 82 nM and Ki = 50 nM) and showed excellent selectivity over both the serotonin and dopamine transporters. Its antidepressant-like effects were also measured in the mouse tail suspension model.
Quinoline
Quinoline is a stable high-boiling liquid with a sweetish odor and is alkaloidal in nature, having a fused benzene and pyridine ring. OPC-14523 (OPC; [1-[3-[4-(3-Chlorophenyl)-1-piperazinyl] propyl]-5-methoxy-3,4-dihydro-2-quinolinone monomethanesulfonate)] is a novel compound with high affinity for σ and 5-HT1A receptors as well as for the 5-HT transporter.[78] Kynuramine has antidepressant activity, bearing quinoline nucleus.[79] Protoberbines and 8-Oxoberbines are known for antileukemic, antitumor and anticancer activity.[80] 2′-quinidinone and 3-hydroxy-quinidine, an active metabolite of quinoline ring is also used in cardiac drug therapy.[81] Oshiro et al., synthesized prototypic tricyclic antidepressant, 10,11-dihydro- N,N-dimethyl-5H-dibenz[b,f]azepine-5-propanamine (imipramine) which showed antidepressant activity by inhibiting the presynaptic reuptake of monoamines, especially 4-(2-amino-1-hydroxyethyl)-1,2- benzenediol (norepinephrine) and 5-hydroxytryptamine (serotonine, 5-HT).[82]
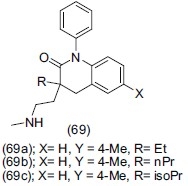
Beadle et al.,[83] developed a novel series of 1-aryl-3,4-dihydro-1H-quinolin-2-one (69). Analogues of compound (69a), (69b), (69c) were reported as potent and selective norepinephrine reuptake inhibitors.
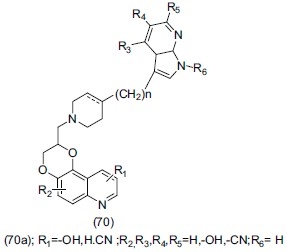
Tran et al.,[84] developed an antidepressant compound 2,3-dihydro 1,4-dioxino (2,3-f) quinoline (70). Compound (70a) is a derivative of azahetrocyclomethyl. Compound (70a) was evaluated as an antidepressant agent.
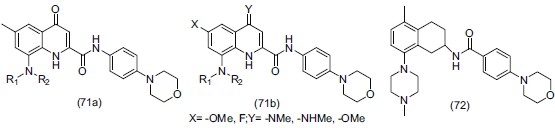
Horchler et al.,[85] synthesized 6-methoxy-8-amino-4oxo-1,4-dihydro-quinolin-2-carboxylic acid(-4-morpholin-4yl-phenyl)amides (71a) and 4-amino-6-methoxy-8-(-4-methyl-piperazin-1-yl)-quinoline-2-carboxylic and (4-morpholin-4yl-phenyl)amides (71b). Compound AR-A000002 (72), a selective 5- HT1B antagonist has been shown to have potential as both an antidepressive and an anxiolytic agent.
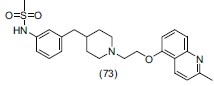
Ward et al.,[86] prepared some novel derivatives of (SB-649915) 6-((1-(2-(2-methylquinolin-5-yloxy)ethyl)piperidin-4-yl)methyl)-2H-benzo[b][1,4]oxazin-3(4H)-one as potent, selective 5-HT1 receptor antagonists. They reported that molecules with pKi > 8 against the 5-HT1A, 5-HT1B, and 5-HT1D receptors have prominently low intrinsic activity and good selectivity towards serotonin transporter (SERT) and are also free of the side-effects associated with the SSRIs. On the basis of SAR, they modified the structure of (SB-649915) and formed (SB-714786) (8-((4-(2-(2-methylquinolin-5-yloxy) ethyl) piperazin-1-yl) methyl) quinolone) which is a selective 5-HT1D receptor antagonist. Compound no. (73) showed potent antidepressant profile acting as a 5-HT1B antagonist in vivo.
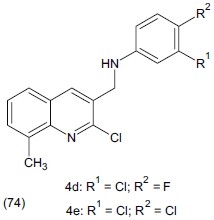
Kumar et al.,[87] prepared a new series of N-[(2-chloro-8-methylquinolin-3-yl)methyl]-(substituted)-aniline / butylamine / cyclohexylamine / benzylamine derivatives. The antidepressant activity of the synthesized compounds was evaluated by FST in rats and their neurotoxicity was evaluated by the rotarod test. Test compounds and clomipramine were administered intraperitoneally at a dose of 100 mg/kg and 20 mg/kg respectively. Preliminary antidepressant screening of compounds revealed that compound (74) significantly (P<0.01) reduces the duration of immobility time.
Pyrazoline
Pyrazoline is characterized by a five-membered heterocyclic ring structure. Pyrazolines represent an important class of heterocycles due to their highly pronounced biological and pharmacological activities.[88]
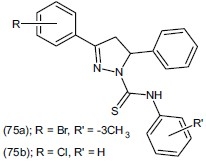
Siddiqui et al.,[89] designed and synthesized a set of derivatives of N,3-(substituted diphenyl)-5- phenyl-1H-pyrazoline-2-carbothioamide(75). Two compounds (75a) and (75b) significantly reduced the duration of the immobility time at a 25 mg/kg dose, when compared to control.
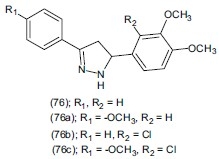
Palaska et al.,[90] synthesized a series of ten new 3, 5-diphenyl-2-pyrazoline derivatives (76). The antidepressant activities of these compounds were evaluated by the Porsolt's behavioral despair test on Swiss-Webster mice. Compounds (76a), (76b) and (76c) reduced 41.94-48.62% immobility time at 100 mg/kg dose level. It was found that 4-methoxy derivative (76a) and 4-chloro derivative (76b) on the phenyl ring at 3 of the pyrazoline ring increased the antidepressant activity.
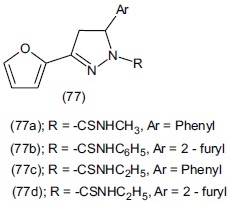
Ozdemir et al.,[91] synthesized 12 1-phenyl,1-thiocarbomoyl and 1-N substituted thiocarbomoyl(-3-(2-furyl)-5-phenyl (77a) or (2-furyl)-2-pyrazoline (77b) derivatives. Compounds (77c) and (77d) reduce 33.80-31.42% duration of immobility times at 10 mg/kg dose level. The antidepressant activities of the compounds were investigated by Porsolt's behavioral despair (forced swim) test on albino mice.
Can et al.,[92] synthesized a series of some 1, 3, 5-trisubstituted-2-pyrazoline derivatives (78) and evaluated for antidepressant activity in modified forced swim test. Compound pyrazoline–benzoxazole derivative (78a) and pyrazoline-benzimidazole derivative (78b) showed significant antidepressant activity.
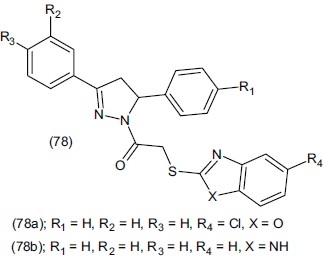
Gok et al.,[93] synthesized 1-[(N,N-disubstituted thiocorbamoylthio)acetyl]-3-(2-thienyl)-5 aryl-2-pyrazolines (79) and evaluated for antidepressant activity by FST. Compounds (79a), (79b), and (79c) significantly shortened immobility time compared to control. Compound (79a) was found to be more effective in FST than clomipramine and tranylcypromine used as reference drugs.
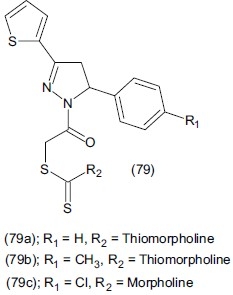
Antidepressants and anticonvulsants are the most beneficial drugs for the treatment of CNS disorders. The pyrazole structure possesses a broad spectrum of biological activities. Abdel-Aziz et al.,[94] synthesized some novel pyrazole derivatives and evaluated them for antidepressant activity. They prepared a series of diacylhydrazines and 5-amino-1-substiuted pyrazole-3, 3, 4-tricarbonitriles by reaction of substituted carboxylic acid hydrazides with ethenetetracarbonitril in presence of dimethyl formamide. Some potent compounds which are derivative of pyrazole (80a) and (80b) show great antidepressant activity compared to imipramine at 10 mg/kg dose level.
Pyrrolidine
Numerous substances have this nucleus, for example PF-877423 has established potent in vitro activity against both human and mouse 11b-HSD1 enzymes.[95] Spiro[9, 10- dihydro-anthracene]-9,30-pyrrolidine (SpAMDA) have high affinity as 5-HT2A antagonists,[96] Sulfonamide hydroxamate CGS-27023A as potent MMP inhibitors,[97] R121919 as CRF-1 receptor antagonists, used in treatment of depression.[98] AHR-1118 is a pyrrolidine derivative with clinically established antidepressant efficacy.[99] (+/-)-(1′R∗,3R∗)-3-phenyl-1-[(1′,2′,3′,4′-tetrahydro-5′,6′-methylene-dioxy-1′-naphthalenyl)methyl]pyrrolidine methane sulfonate (ABT-200) antagonized the uptake of [3H]-norepinephrine into synaptosomes of rat hypothalamus (IC50 = 841 nM).[100] Rolipram is a PDE4-inhibitor and is being studied as a possible alternative to current antidepressant treatment.[101]
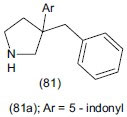
Bannwart et al.,[102] designed a series of 3,3-disubstituted pyrrolidine derivatives (81). Synthesized compounds were evaluated as monomaine triple reuptake inhibitors. It showed in vivo antidepressant-like effect in the mouse tail suspension assay with a minimum effective dose of 30 mg/kg; i.p. The first compound (81a) revels potent inhibitor activity.
Lucas et al.,[2] synthesized two new series of monoamine triple reuptake inhibitors (TRIS) through scaffold homologation of recently reported series of 3, 3-disubstituted pyrrolidine (82) TRIS. The region isomeric 2 and 3 ketopyrrolidine (82a) has a high level of potency against all three monoamine transporters as well as has good human in vitro stability.
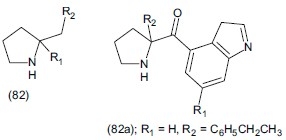
Zelle et al.,[103] synthesized a novel compound ABT-200(+), (11R∗-3R∗)-3-phenyl-1[(11,21,31,41- tetrahydro-51,61-methylene-dioxy-11-napthalanyl) methyl] pyrrolidine (83) which was the first example of a new structural class of potent α- antagonists, which possesses the additional property of nor-epinephrine reuptake inhibitor. The compound was evaluated for antidepressant activity and expected to have utility in the treatment of depression.
Imidazole
Imidazole is a five-membered heterocyclic system with three carbons and two nitrogen atoms, at Position 1 is pyrrole type and at Position 2 is pyridine type. Imidazole exists in three partially reduced forms, 2-imidazoline, 3-imidazoline and 4-imidazoline. Imidazole analogues show potent antidepressant activity. Hadizadeh et al.,[104] synthesized some moclobemide derivatives and evaluated them for antidepressant activity. Phenyl ring of moclobemide was replaced by substituted imidazoles, and N-[(4-morpholinyl)ethyl)]-1-benzyl-2-(alkkylthio)-1H-imidazole-5-carboxamides (7a-c) were formed. Antidepressant activity was evaluated by FST in mice. Some synthesized compounds were found to be more potent than moclobemide.
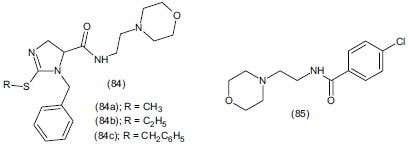
Hadizadeh et al.,[104] synthesized N-[(4-morpholinyl)ethyl)]-1-benzyl-2-(alkylthio)-1H-imidazole-5-carboxamide (84) and evaluated it as antidepressant agent using FST in mice. The investigation demonstrated that analogues (84a), (84b) and (84c) are more potent than moclobemide (85) in FST model. Replacement of electron-deficient 4-chlorophenyl in moclobemide (85) with substituted electron-deficient ring imidazole on analogues (84a), (84b), (84c) increases antidepressant potency and also toxicity.
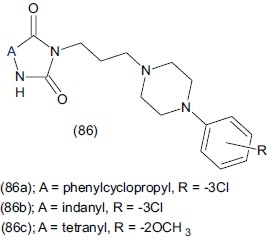
Czopek et al,.[105] synthesized 5-cycloalkyl-5-phenyl and 5-spiroimidazoline-2, 4-dione derivatives with an arylpiperazinyl moiety (86). Compounds showed high affinity for 5-HT1A receptors as well as for 5-HT2A receptors. Compounds (86a), (86b) and (86c) showed antagonistic, partial agonistic and agonistic activity respectively towards 5-HT1A receptors. Compound (86c) (1-{3-(4-(-2-methoxyphenyl)piperazine-1-yl)propyl]-31,41,-dihydro-21H-spiro(imidazoline-4,11-napthalene1-2,5-dione) show agonistic activity towards pre and post synaptic 5-HT2A receptor and evaluated them for antidepressant like effect that is more pronounced in Imipramine on forced swim test in mice without affecting locomotor activity.
Zagorska et al.,[106] synthesized a series of N-8 arylperazinyl-propyl derivatives of 1,3-dimethyl-(1H,8H)-imidazole[2, 1-f]purine-2,4-dione (87). Derivatives were evaluated in vitro and found to be potent 5-HT1A receptor ligands. Compound 8[-3(N4-phenyl) piperazine-N1-yl-propyl]-1,3- dimethyl-(1H,8H)-imidazo[2, 1-f] purine-2,4 dione (87a) exerts anxiolytic-like activity in the four-plate test in mice. Compounds (87a) and 8-[3-(N4-21-metoxyphenyl)- piperazine-N-1yl-propyl]-1,3-dimethyl-(1H,8H)imidazole-[2, 1-f]-purin-2,4-dione (87b) behave like antidepressants in the FST in mice by using imipramine as standard.
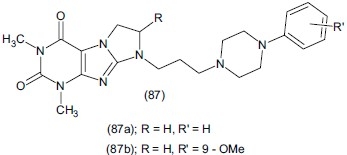
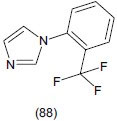
Ulak et al.,[107] studied the antidepressant effect and synergistic interaction between 1-(2-trifluoromethylphenyl)-imidazole (TRIM) (88), a novel neuronal nitric oxide synthase (nNOS) inhibitor and conventional antidepressants of different classes and evaluated them in FST in rats. TRIM decreased the immobility time at 50 mg/kg doses.
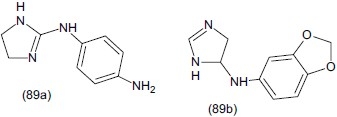
Rodriguez et al.,[108] studied some potential (bis) guanidine and (bis) 2-aminoimidazoline derivatives. The antidepressant activity of some selected compounds showing pKi value larger than 7 was determined in functional [35S]GTPçS binding assays in human brain tissue. In vivo activity was determined through microdialysis experiments in rats. They also revealed that the 2-aminoimidazoline derivatives are more potent than the guanidine derivative and have higher affinity. They reported that compounds (89a) and (89b) are most potent for antidepressant activity.
Pyrimidine
Pyrimidine and its derivatives attracted great interest due to the wide variety of important pharmacological activities with good pharmacokinetic properties.
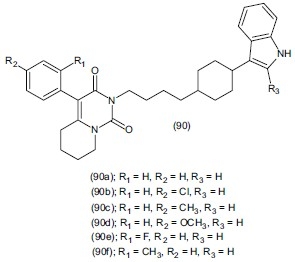
Herold et al.,[109] synthesized some analogues of 4-aryl-5, 6, 7, 8-tetrahydropyrido[1,2-c]pyrimidine with 3-(1, 2, 3, 6-tetrahydro-pyridin-4-yl)- 1H-indole or 2-methyl-3-(1, 2, 3, 6-tetrahydro-pyridin-4-yl)-1H-indole. On the basis of structure activity relationship they concluded that compounds with 3-(1, 2, 3, 6- tetrahydropyridin-4-yl)-1H-indole group have higher affinity to the 5-HT1A receptors than the analogous 2-methyl derivatives. Some synthesized compounds (90a), (90b), (90c), (90d), (90e) and (90g) are selective agonists, while (90i) has an antagonistic effect of 5-HT1A on presynaptic receptors in the inducible hypothermia test in mice having Ki value from 28,3 to 642 nM and 42,4 nM - 1,8μ M respectively for both 5-HT1A and SERT.
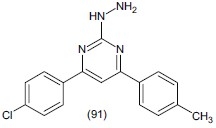
Rodrigues et al.,[110] synthesized 4-(4-chlorophenyl)-6-(411-methylphenyl)-2-hydrazin pyrimidine (91) evaluate.
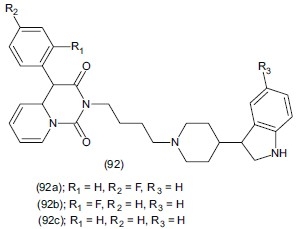
Herold et al.,[111] synthesized a series of new derivatives of 4-aryl-pyrido[1,2-c] pyrimidine (92) containing the 3-(4-piperidyl)-1H indole residue or its methoxy derivatives. Presence of -F or -CH3 group at ortho or para in aryl ring and the presence of 3-(4-piperidyl)-1H-indole residue promoted low Ki value for 5-HT1A auto receptors and post-synaptic 5-HT1A receptors. In contrast, the presence of a 5- methoxy -3-(4- piperidyl)-1H-indole residue as well as -Cl or -OCH3 substituents at the para position markedly reduced the receptor affinity. Compounds (92a), (92b), (92c) showed agonistic effect on post-synaptic 5-HT1A receptor in the FST in mice. Compound (92a) had an effect at 10 mg/kg dose, while compounds (92b), (92c) required higher dose, 20 mg/kg, to be effective.
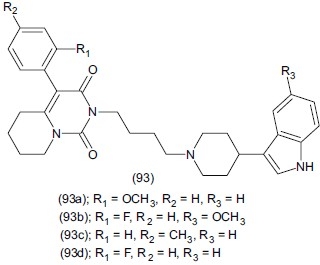
Herold et al.,[112] synthesized derivatives of 4-aryl-5, 6, 7, 8-tetrahydro-pyrido[1,2,-c] pyrimidine (93). These compounds contain the 3-(4 piperidyl)-1-H indole residue or its methoxy or 2- methyl derivatives. SAR analysis showed the presence of the 3-(4-piperidyl)-1H-indole group or its 5- methoxy derivative as well as a para substitution with -CH3(93a) or -F (93b) in the aryl ring of 4-aryl-5, 6, 7, 8 tetrahydro-pyrido[1,2-c] pyrimidine results in an affinity for both the 5-HR1A receptor and SERT. The agonistic effects of compounds (93c), (93d), (93e) were demonstrated on post-synaptic 5-HT1A receptors.
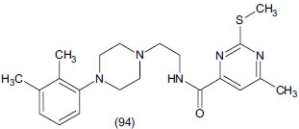
Kim et al.,[113] synthesized a series of arylpiperazine-containing pyrimidine 4-carboxamide derivatives. Some synthesized compounds were evaluated for binding affinity to serotonin receptors and transporters. They reported in vitro and SAR studies and suggested that compound (94) (IC50μM >10) is the most potent in this series.
Triazole
Triazole nucleus has planer five-membered heterocyclic system containing three nitrogen atoms, one pyrrole and two pyridine type.[114] Two structural isomeric forms of triazole are 1,2,3 and 1,2,4.[115] On the basis of the pharmacological profiles of triazoles, their antidepressant properties seem to be best documented. Nefazodone has triazole nucleus and possesses antidepressant activity. It inhibits the binding of [3H] ketanserin to cortical serotonin2 (5-HT2) binding sites, it antagonizes the 5-HT2 and also inhibits cortical serotonin uptake.[116] Some potent marketed drugs having triazole nucleus are Ribavirin (antiviral), Alprazolam (anxiolytic), Fluconazole, Itraconazole (antifungal) and Rizatriptan (antimigrane). The ambient nucleophilic centers present in triazoles are used for synthesis of various N-bridged heterocycles.[117]
Kaplancikli et al.,[118] synthesized some triazole-pyrazoline derivatives and investigated their potential antidepressant activity. Antidepressant-like activity of the test compounds (100 mg/Kg)1-[{4-amino-3-[2-(4-hydroxyphenyl)ethyl]-4H-1, 2, 4-triazole-5yl)thioacetyl]-3-(2-thieyl)-5-aryl-2-pyrazoline (95) were screened using both modified forced swimming and tail suspension tests. The test compounds in the series, unsubstituted derivatives (95a) and chlorine-substituted (95b) were more effective than the test compounds (95c), (95d) as compared to reference drug fluoxetine.
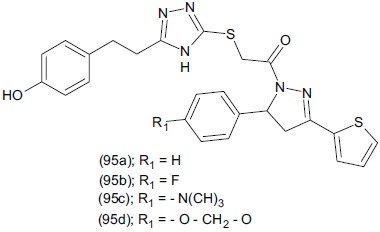
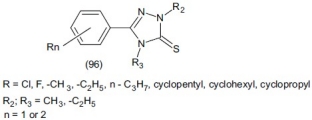
Kane et al.,[119] synthesized 5-aryl-2,4-dialkyl-3H-1, 2, 4-triazole-3-thiones (96) and evaluated their pharmacological properties.
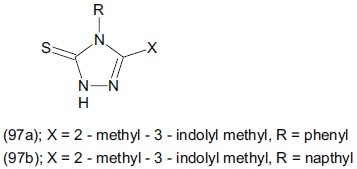
Varvaresou et al.,[120] synthesized a derivatives of 3-{(2-methyl-1H-3-indolyl) methyl)-4-aryl-4,5-dihydro-1H-1, 2, 4-triazole-5-thione (97). The antidepressant profile of compounds (97a), (97b) was studied on mice with respect to that of analogue 3-(1H-indolylmethyl)-4-aryl-4,5dihydro-1H-1, 2, 4-triazole-5-thione. Compound (97a) was evaluated as a potent antidepressant.
Current perspective
This review demonstrated that all mentioned heterocyclic moieties and their derivatives have potent antidepressant activity. Additionally, these modifications can be utilized to develop more potentially active agents in order to improve the mental health of the patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Srinath R, Pinkal DV, Saravanan J, Pravin SJ, Prashant R, Aravind S. Synthesis and evaluation of Antidepressant like Activity of Some Novel Thieno-1,2,3-triazine 4-ones. Int J Res Pharm Sci. 2010;1:143–50. [Google Scholar]
- 2.Lucas MC, Weikert RJ, Carter DS, Cai HY, Greenhouse R, Lyer PS, et al. Design, synthesis and biological evaluation of new monoamine reuptake inhibitors with potential therapeutic utility in depression and pain. Bioorg Med Chem Lett. 2010;20:5559–66. doi: 10.1016/j.bmcl.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Iversen L, Glennon RA. Antidepressants. In: Abraham DJ, editor. Burger's Medicinal Chemistry and Drug Discovery. 6th. Vol. 6. New York: John wiley and sons publication; 1998. pp. 483–524. [Google Scholar]
- 4.Gilman A, Goodman L. Drug therapy of depression and anxiety disorders. In: Brunton L, Parker K, Blumenthal D, Buxton D, editors. Goodman and Gilman's Manual of Pharmacology and Therapeutics. 7th. McGraw-Hill: New York; 2008. pp. 278–98. [Google Scholar]
- 5.Wenga Z, Li J. Synthesis and antidepressant activity of optical isomers of 2-(4-benzylpiperazin-1-yl)-1-(5-chloro-6-methoxynaphthalen-2-yl)propan-1-ol (SIPI5056) Bioorg Med Chem Lett. 2010;20:1256–9. doi: 10.1016/j.bmcl.2009.11.108. [DOI] [PubMed] [Google Scholar]
- 6.Fishback JA, Robson MJ, Xu YT, Matsumoto RR. Sigma receptors: Potential targets for a new class of antidepressant drug. Pharmacol Ther. 2010;127:271–82. doi: 10.1016/j.pharmthera.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboukhatwa MA, Undieh AS. Antidepressant stimulation of CDP-diacylglycerol synthesis does not require monoamine reuptake inhibition. BMC Neurosci. 2010;11:10. doi: 10.1186/1471-2202-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez C. Allosteric modulation of monoamine transporters-new drug targets in depression. Drug Discov Today Ther Strateg. 2006;3:483–8. [Google Scholar]
- 9.Ben-Daniel R, Deuther-Conrad W, Scheunemann M, Steinbach J, Brust P, Mishani E. Carbon-11 labeled indolylpropylamine analog as a new potential PET agent for imaging of the serotonin transporter. Bioorg Med Chem. 2008;16:6364–70. doi: 10.1016/j.bmc.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Mattson RJ, Catt JD, Denhart DJ, Deskus JA, Ditta JL, Higgins MA, et al. Conformationally restricted homotryptamines 2 Indole cyclopropylmethylamines as selective serotonin reuptake inhibitors. J Med Chem. 2005;48:6023–34. doi: 10.1021/jm0503291. [DOI] [PubMed] [Google Scholar]
- 11.Biradar JS, Sasidhar BS, Parveen R. Synthesis, antioxidant and DNA cleavage activities of novel indole derivatives. Eur J Med Chem. 2010;45:4074–8. doi: 10.1016/j.ejmech.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 12.Csomós P, Fodor L, Bernáth G, Csámpai A, Sohár P. Synthesis of 4-thiaharmalan analogue 4-aryl-1,3-thiazino[5,6-b]indole derivatives by prevention of rearrangements to position two of the indole moiety. Tetrahedron. 2008;64:8646–51. [Google Scholar]
- 13.Farah Idayua N, Taufik Hidayata M, Moklasa MA, Sharida F, Nurul Raudzaha AR, Shamima AR, et al. Antidepressant-like effect of mitragynine isolated from Mitragyna speciosa Korth in mice model of depression. Phytomedicine. 2010 doi: 10.1016/j.phymed.2010.08.011. [In press] [DOI] [PubMed] [Google Scholar]
- 14.Bromidge SM, Dabbs S, Davies S, Duckworth DM, Forbes IT, Jones GE, et al. 1-[2-[(Heteroaryloxy) heteroaryl]carbamoyl] indolines: Novel and selective 5-HT2C receptor inverse agonists with potential as antidepressant/anxiolytic agents. Bioorg Med Chem Lett. 2000;10:1863–6. doi: 10.1016/s0960-894x(00)00364-4. [DOI] [PubMed] [Google Scholar]
- 15.Shelke SM, Bhosale SH. Synthesis, antidepressant evaluation and QSAR studies of novel 2-(5H-[1,2,4] triazino [5,6-b] indol-3-ylthio)-N-(substituted phenyl)acetamides. Bioorg Med Chem Lett. 2010;20:4661–4. doi: 10.1016/j.bmcl.2010.05.100. [DOI] [PubMed] [Google Scholar]
- 16.Haffernan GD, Coghlan RD, Manas ES, McDevit RE, Li Y, Mahaney PE, et al. Dual acting norepinephrine reuptake inhibitors and 5-HT2A receptor antagonistsn: Identifation, synthesis and activity of novel 4-aminoethyl-3-(phenylsulfonyl)-1H-indoles. Bioorg Med Chem. 2009;17:7802–15. doi: 10.1016/j.bmc.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Manning JR, Sexton T, Childers SR, Davies HM. 1-Naphthyl and 4-indolyl arylalkylamines as selective monoamine reuptake inhibitors. Bioorg Med Chem Lett. 2009;19:58–61. doi: 10.1016/j.bmcl.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Kochanowska AJ, Rao KV, Childress S, El-Alfy A, Matsumoto RR, Kelly M, et al. Secondary metabolites from three florida sponges with antidepressant activity. J Nat Prod. 2008;71:186–9. doi: 10.1021/np070371u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou D, Zhou P, Evrard DA, Meagher K, Webb M, Harrison BL, et al. Studies towards the discovery of the next generation of antidepressant.Part 6: Dual 5HT1A receptor and serotonine transporter affinity within a class of arylpiperazinyl-cyclohexyl indole derivateves. Bioorg Med Chem. 2008;16:6707–23. doi: 10.1016/j.bmc.2008.05.075. [DOI] [PubMed] [Google Scholar]
- 20.Liu KG, Lo JR, Comery TA, Zhang GM, Zhang JY, Kowal DM, et al. A regiospecific synthesis of a series of 1-sulfonyl azepinoindoles as potent 5-HT6 ligands. Bioorg Med Chem Lett. 2008;18:3929–31. doi: 10.1016/j.bmcl.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 21.Deskus JA, Epperson JR, Sloan CP, Cipollina JA, Dextraze P, Qian-Cutrone J, et al. Conformationally restricted homotryptamines 3. Indole tetrahydropyridines and cyclohexenylamines as selective serotonin reuptake inhibitors. Bioorg Med Chem Lett. 2007;17:3099–104. doi: 10.1016/j.bmcl.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 22.Mahaney PE, Vu AT, McComas CC, Zhang P, Nogle LM, Watts WL, et al. Synthesis and activity of a new class of dual acting norepinephrine and serotonine reuptake inhihibitor: (3-(1H-indole-1-yl)-3-arylpropan-1-amines. Bioorg Med Chem. 2006;14:8455–66. doi: 10.1016/j.bmc.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 23.Evrard DA, Zhou P, Yi SY, Zhou D, Smith DL, Sullivan KM, et al. Studies towards the next generation of antidepressants. Part 4: Derivatives of 4-(5-fluoro-1H-indol-3-yl)cyclohexylamine with affinity for the serotonin transporter and the 5-HT1A receptor. Bioorg Med Chem Lett. 2005;15:911–4. doi: 10.1016/j.bmcl.2004.12.064. [DOI] [PubMed] [Google Scholar]
- 24.Cole DC, Ellingboe JW, Lennox WJ, Mazandarani H, Smith DL, Stock JR, et al. N1 arylsulfonyl-3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-indole derivatives are potent and selective 5-HT6 receptor antagonists. Bioorg Med Chem Lett. 2005;15:379–83. doi: 10.1016/j.bmcl.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz WD, Denhart DJ, Brenner AB, Ditta JL, Mattson RJ, Mattson GK, et al. Homotryptamines as potent and selective serotonin reuptake inhibitors (SSRIs) Bioorg Med Chem Lett. 2005;15:1619–21. doi: 10.1016/j.bmcl.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 26.Kolanos R, Siripurapu U, Pullagurla M, Riaz M, Setola V, Roth BL, et al. Binding of isotryptamines and indenes at h5-HT6 serotonin receptors. Bioorg Med Chem Lett. 2005;15:1987–98. doi: 10.1016/j.bmcl.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 27.Heinrich T, Böttcher H. A new synthesis of indole 5- carboxylic acids and 6- hydroxyl- indole-5-carboxylic acids in the preparation of an o-hydroxyated of vilazodone. Bioorg Med Chem Lett. 2004;14:2681–4. doi: 10.1016/j.bmcl.2004.01.110. [DOI] [PubMed] [Google Scholar]
- 28.Mewshaw RE, Meagher KL, Zhou P, Zhou D, Shi X, Scerni R, et al. Studies towards the discovery of the next generation of antidepressant.Part 2: Incorporating a 5HT1A antagonist component into a class of serotonin reuptake inhibitors. Bioorg Med Chem Lett. 2002;12:307–10. doi: 10.1016/s0960-894x(01)00746-6. [DOI] [PubMed] [Google Scholar]
- 29.Meagher KL, Mewshaw RE, Evrard DA, Zhou P, Smith DL, Scerni R, et al. Studies towards the next generation of antidepressants. Part 1: Indolylcyclohexylamines as potent serotonin reuptake inhibitors. Bioorg Med Chem Lett. 2001;11:1885–988. doi: 10.1016/s0960-894x(01)00334-1. [DOI] [PubMed] [Google Scholar]
- 30.Matzen L, van Amsterdam C, Rautenberg W, Greiner HE, Harting J, Seyfried CA, et al. 5-HT Reuptake inhibitors with 5-HT1B/1D antagonistic activity: A new approach toward efficient antidepressants. J Med Chem. 2000;43:1149–57. doi: 10.1021/jm9811054. [DOI] [PubMed] [Google Scholar]
- 31.Primofiore G, Marini AM, Settimo FD, Franzone JS, Mason U, Reboani MC, et al. N-(Indole-3-ylglyoxylyl) dopamine derivatives: Preparation and antidepressant activity. Eur J Med Chem. 1998;23:397–401. [Google Scholar]
- 32.Ergenc N, Gunay NS, Demirdamar R. Synthesis and antidepressant evaluation of new 3- phenyl-5-sulfonamidoindole derivatives. Eur J Med Chem. 1998;33:143–8. [Google Scholar]
- 33.Sah P, Bidawat P, Seth M, Gharu CP. Synthesis of formazans from Mannich base of 5-(4-chlorophenyl amino)-2-mercapto-1,3,4-thiadiazole as antimicrobial agents. Arabian J Chem. 2010 [In press] [Google Scholar]
- 34.Castro A, Castaño T, Encinas A, Porcaland W, Gil C. Advances in the synthesis and recent therapeutic applications of 1,2,4-thiadiazole heterocycles. Bioorg Med Chem. 2006;14:1644–52. doi: 10.1016/j.bmc.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Clerici F, Pocar D, Guido M, Loche A, Perlini V, Brufani M. Synthesis of 2-amino-5-sulfanyl-1,3,4-thiadiazole derivatives and evaluation of their antidepressant and anxiolytic activity. J Med Chem. 2001;15:931–6. doi: 10.1021/jm001027w. [DOI] [PubMed] [Google Scholar]
- 36.Clerici F, Pocar D, Guido M, Loche A, Perlini V, Brufani M. Synthesis of 2-Amino-5-sulfanyl-1,3,4-thiadiazole derivatives and evaluation of their antidepressant and anxiolytic activity. J Med Chem. 2001;44:931–6. doi: 10.1021/jm001027w. [DOI] [PubMed] [Google Scholar]
- 37.Yusuf M, Khan RA, Ahmed B. Syntheses and anti-depressant activity of 5-amino-1,3,4- thiadiazole-2-thiol imines and thiobenzyl derivatives. Bioorg Med Chem. 2008;16:8029–34. doi: 10.1016/j.bmc.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 38.Pattanayak P, Sharma R, Sahoo PK. Synthesis and evaluation of 2-amino-5-sulfanyl-1,3,4-thiadiazole as antidepressant, anxiolytic, and anticonvulsant agents. Med Chem Res. 2009;18:351–61. [Google Scholar]
- 39.Sharma R, Mishra GP, Sainey J, Chaturvedi SC. Synthesis and biological evaluation of 2-amino-5-sulfanyl-1,3,4-thiadiazole derivatives as antidepressant, anxiolytics and anticonvulsants agents. Med Chem Res. 2010 [In press] [Google Scholar]
- 40.Varvaresou A, Siatra-Papastaikoudi T, Dalla Tsotinis A, Tsantili-Kakoulidou A, Vamvakides A. Synthesis, lipophilicity and biological evaluation of indole-containing derivatives of 1,3,4- thiadiazole and 1,2,4-triazole. Farmaco. 1998;53:320–6. doi: 10.1016/s0014-827x(98)00024-x. [DOI] [PubMed] [Google Scholar]
- 41.Jatav V, Mishra P, Kashaw S, Stables JP. CNS depressant and anticonvulsant activities of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur J Med Chem. 2008;43:1945–54. doi: 10.1016/j.ejmech.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Takeuchi K, Kohn TJ, Honigschmidt NA, Rocco VP, Spinazze PG, Koch DJ, et al. Advances toward new antidepressants beyond SSRIs: 1-aryloxy-3-piperidinylpropan-2-ols with dual 5-HT1A receptor antagonism/SSRI activities.Part 1. Bioorg Med Chem Lett. 2003;13:1903–5. doi: 10.1016/s0960-894x(03)00303-2. [DOI] [PubMed] [Google Scholar]
- 43.Källström S, Leino R. Synthesis of pharmaceutically active compounds containing a disubstituted piperidine framework. Bioorg Med Chem. 2008;16:601–35. doi: 10.1016/j.bmc.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 44.Layer RT, Popik P, Olds T, Skolnick P. Antidepressant-like actions of the polyamine site NMDA antagonist, eliprodil (SL-82.0715) Pharmacol Biochem Behav. 1995;52:621–7. doi: 10.1016/0091-3057(95)00155-p. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi K, Kohn TJ, Honigschmidt NA, Rocco VP, Spinazze PG, Hemrick-Luecke SK, et al. Advances toward new antidepressants beyond SSRIs: 1-Aryloxy-3-piperidinylpropan-2-ols with dual 5-HT1A receptor antagonism/SSRI activities.Part 5. Bioorg Med Chem Lett. 2006;16:2347–51. doi: 10.1016/j.bmcl.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Bollinger S, Hübner H, Heinemann FW, Meyer K, Gmeiner P. Novel pyridylmethylamines as highly selective 5-HT1A superagonists. J Med Chem. 2010;53:7167–79. doi: 10.1021/jm100835q. [DOI] [PubMed] [Google Scholar]
- 47.Boot JR, Boulet SL, Clark BP, Cases-Thomas MJ, Delhaye L, Diker K, et al. N-alkyl-N-arylmethylpiperidin-4-amines: Novel dual inhihibitors of serotonin and norepinephrine reuptake. Bioorg Med Chem Lett. 2006;16:2714–8. doi: 10.1016/j.bmcl.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 48.Rocco VP, Spinazze PG, Kohn TJ, Honigschmidt NA, Nelson DL, Wainsscott DB, et al. Advances toward new antidepressants beyond SSRIs: 1-aryloxy-3-piperidinylpropane-2-ols with dual 5HT 1A receptor antagonism/SSRI activities.Part 4. Bioorg Med Chem Lett. 2004;14:2653–6. doi: 10.1016/j.bmcl.2004.02.088. [DOI] [PubMed] [Google Scholar]
- 49.Steckler TH, Janssens FE, Leenaerts JE, Fernandez-Gadea FJ, Gomez-Sanchez A, Meert TF. Substituted 4-phenyl-4-(1H-Imidazole-2-yl)-Piperidine derivatives as selective non-peptide delta opiod agonist with antidepressant and anxiolytic activity. US Patents. 2006;31:513. [Google Scholar]
- 50.Trabanco AA, Aerts N, Alvarez RM, Andrés JI, Boeckx I, Fernández J, et al. 4-phenyl-4-(1H-imidazol-2-yl)-piperidine derivatives as non-peptides selective opiod agonist with potential anxiolytic/ antidepressant properties. Part2. Bioorg Med Chem Lett. 2007;17:3860–3. doi: 10.1016/j.bmcl.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Ahmad YE, Maillet P, Laurent E, Talab A, Teste JF, Cedat MJ, et al. New N-(benzhydroxyalkyl)-4-(carboxy/carbamoylmethyl)piperidine derivatives with antidepressant activity. Eur J Med Chem. 1997;32:205–18. [Google Scholar]
- 52.Takeuchi K, Kohn TJ, Honigschmidt NA, Rocco VP, Spinazze PG, Atkinson ST, et al. Advances toward new antidepressants beyond SSRIs: 1-aryloxy-3-piperidinylpropan-2-ols with dual 5-HT1A receptor antagonism/SSRI Activities. Part 3. Bioorg Med Chem Lett. 2003;13:3939–42. doi: 10.1016/j.bmcl.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 53.Takeuchi K, Kohn TJ, Honigschmidt NA, Rocco VP, Spinazze PG, Koch DJ, et al. Advances toward new antidepressants beyond SSRIs: 1-aryloxy-3-piperidinylpropan-2-ols with dual 5-HT1A receptor antagonism/SSRI activities.Part 2. Bioorg Med Chem Lett. 2003;13:2393–7. doi: 10.1016/s0960-894x(03)00392-5. [DOI] [PubMed] [Google Scholar]
- 54.Ong HH, Profitt JA, Fortunato J, Glamkowski EJ, Ellis DB, Geyer HM, et al. Novel tetracyclic spiropiperidines. 3. 1-Arylspiro[indoline-3,4′-piperidine]s as potential antidepressants. J Med Chem. 1983;26:981–6. doi: 10.1021/jm00361a009. [DOI] [PubMed] [Google Scholar]
- 55.Wang J, Mack AL, Coop A, Matsumoto RR. Novel sigma (σ) receptor agonists produce Antidepressant like effects in mice. Eur Neuropsychopharmacol. 2007;17:708–16. doi: 10.1016/j.euroneuro.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balsamo A, Giorgi I, Lapucci A, Lucacchini A, Macchia B, Macchia F, et al. 3-[(2-Ethoxyphenoxy)methyl]piperidine derivatives.Synthesis and antidepressant activity. J Med Chem. 1987;30:222–5. doi: 10.1021/jm00384a040. [DOI] [PubMed] [Google Scholar]
- 57.He R, Kurome T, Giberson KM, Johnson KM, Kozikowski AP. Further structure-activity relationship studies of piperidine-based monoamine transporter inhibitors: Effects of piperidine ring stereochemistry on potency. Identification of norepinephrine transporter selective ligands and broad-spectrum transporter inhibitors. J Med Chem. 2005;48:7970–9. doi: 10.1021/jm050694s. [DOI] [PubMed] [Google Scholar]
- 58.Orjales A, Mosquera R, Toledo A, Pumar M, García N, Cortizo L, et al. Syntheses and binding studies of new [(aryl)(aryloxy)methyl]piperidine derivatives and related compounds as potential antidepressant drugs with high affinity for serotonin (5-HT) and norepinephrine (NE) transporters. J Med Chem. 2003;46:5512–32. doi: 10.1021/jm0309349. [DOI] [PubMed] [Google Scholar]
- 59.Vacher B, Bonnaud B, Funes P, Jubault N, Koek W, Assie MB, et al. Novel derivatives of 2-pyridinemethylamine as selective, potent, and orally active agonists at 5-HT1A receptors. J Med Chem. 1999;42:1648–60. doi: 10.1021/jm9806906. [DOI] [PubMed] [Google Scholar]
- 60.Gray DL, Xu W, Campbell BM, Dounay AB, Barta N, Boroski S, et al. Discovery and pharmacological characterization of aryl piperazine and piperidine ethers as dual acting norepinephrine reuptake inhibitors and 5-HT1A partial agonists. Bioorg Med Chem Lett. 2009;19:6604–7. doi: 10.1016/j.bmcl.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Kim JY, Kim D, Kang SY, Park W, Kim HJ, Jung ME, et al. Arylpiperazine-containing pyrimidine-4-carboxamide derivatives targeting serotonin 5-HT2A, 5-HT2C, and the serotonin transporter as a potential antidepressant. Bioorg Med Chem Lett. 2010;20:6439–42. doi: 10.1016/j.bmcl.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 62.Hovels N, Sager TN, Mørk A. Combination of escitalopram and a 5-HT1A receptor antagonist selectively decreases the extracellular levels of dopamine in the nucleus accumbens relative to striatum through 5-HT2C receptor stimulation; suggestive of antipsychotic potential. Pharmacol Biochem Behav. 2011;97:479–85. doi: 10.1016/j.pbb.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Raghavan N, Zhang D, Zhu M, Zeng J, Christopher L. CYP2D6 catalyzes 5-hydroxylation of 1-(2-pyrimidinyl)-piperazine, an active metabolite of several psychoactive drugs, in human liver microsomes. Drug Metab Dispos. 2005;33:203–8. doi: 10.1124/dmd.104.001198. [DOI] [PubMed] [Google Scholar]
- 64.Bishnoi A, Srivastava K, Singh S, Tripathi CM. Synthesis of some novel piperazine salts and their antimicrobial property against Escherichia coli and Bacillus subtilis. Der Pharma Chemica. 2010;2:446–52. [Google Scholar]
- 65.Bojarski AJ, Paluchowska MH, Duszynska B, Bugno R, K³odzinska A, Tatarczynska E, et al. Structure–intrinsic activity relationship studies in the group of 1-imido/amido substituted 4-(4-arylpiperazin-1-yl)cyclohexane derivatives; new, potent 5-HT1A receptor agents with anxiolytic- like activity. Bioorg Med Chem. 2006;14:1391–402. doi: 10.1016/j.bmc.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 66.Jonathan Fray M, Bish G, Brown AD, Fish PV, Stobie A, Wakenhut F, et al. N-(1,2-Diphenylethyl)piperazine: A new class of dual serotonin/noradrenaline reuptake inhibitor. Bioorg Med Chem Lett. 2006;16:4345–8. doi: 10.1016/j.bmcl.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 67.Kang SY, Park EJ, Park WK, Kim HJ, Jeong D, Jung ME, et al. Arylpiperizine-containing pyrrole 3-carboxamide derivatives targeting serotonin 5-HT2A, 5-HT2C, and the serotonin transporter as a potential antidepressant. Bioorg Med Chem Lett. 2010;20:1705–11. doi: 10.1016/j.bmcl.2010.01.093. [DOI] [PubMed] [Google Scholar]
- 68.Kling A, Lange UE, Mack H, Bakkerr MH, Drescher KU, Hornburger W, et al. Synthesis and SAR of highly potent dual 5-HT1A and 5-HT1B antagonists as potential antidepressant drug. Bioorg Med Chem Lett. 2005;15:5567–73. doi: 10.1016/j.bmcl.2005.04.077. [DOI] [PubMed] [Google Scholar]
- 69.Torrado A, Lamas C, Agejas J, Jimenaz A, Diaz N, Gilmore J, et al. Novel selective and potent 5-HT reuptake inhibitor with 5-HT1D antagonist activity: Chemistry and pharmacological evaluation of a series of thienopyran derivatives. Bioorg Med Chem. 2004;12:5277–99. doi: 10.1016/j.bmc.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 70.Zajdel P, Subra G, Bojarski AJ, Duszynska B, Tatarczynska E, Nikiforuk A, et al. Novel class of arylpiperazine containing N-acylated amino acids: Their Synthesis, 5-HT1A, 5HT2A receptor affinity, and in vivo pharmacological evaluation. Bioorg Med Chem. 2007;15:2907–19. doi: 10.1016/j.bmc.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 71.Paluchowska MH, Bugno R, Duszyn´ska B, Tatarczyn´ska E, Nikiforuk A, Lendac T, et al. The influence of modifications in imide fragment structure on 5-HT1A and 5-HT7 receptor affinity and in vivo pharmacological properties of some new 1-(m-trifluoromethylphenyl)piperazines. Bioorg Med Chem. 2007;15:7116–25. doi: 10.1016/j.bmc.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 72.Yoon J, Yoo EA, Kim J, Pae AN, Rhim H, Park W, et al. Preparation of piperazine derivatives as 5-HT 7 receptor antagonists. Bioorg Med Chem. 2008;16:5405–12. doi: 10.1016/j.bmc.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 73.Wu XD, Liu DZ, Li AJ, Zhou XQ. Arylpiperazine derivatives of diphenylsulfide: Synthesis and evaluation for dual 5-HT1A/SSRI activities. Chin Chem Lett. 2008;19:291–4. [Google Scholar]
- 74.Weng Z, Li J. Synthesis and antidepressant activity of optical isomers of 2-(4-benzylpiperazin-1-yl)-1-(5-chloro-6-methoxynaphthalen-2-yl)propan-1-ol(SIPI5056) Bioorg Med Chem Lett. 2010;20:1256–9. doi: 10.1016/j.bmcl.2009.11.108. [DOI] [PubMed] [Google Scholar]
- 75.Abou-Gharbia MA, Childers WE, Jr, Fletcher H, McGaughey G, Patel U, Webb MB, et al. Synthesis and SAR of adatanserin: Novel adamantyl aryl- and heteroarylpiperazines with dual serotonin 5-HT1A and 5-HT2 activity as potential anxiolytic and antidepressant agents. J Med Chem. 1999;42:5077–94. doi: 10.1021/jm9806704. [DOI] [PubMed] [Google Scholar]
- 76.Jorand-Lebrun C, Pauwels PJ, Palmier C, Moret C, Chopin P, Perez M, et al. 5-HT1B receptor antagonist properties of novel arylpiperazide derivatives of 1-naphthylpiperazine. J Med Chem. 1997;40:3974–8. doi: 10.1021/jm9703552. [DOI] [PubMed] [Google Scholar]
- 77.Mahaney PE, Gavrin LK, Trybulski EJ, Stack GP, Vu AT, Cohn ST, et al. Structure-activity relationships of the cycloalkanol ethylamine scaffold: Discovery of selective norepinephrine reuptake inhibitors. J Med Chem. 2008;51:4038–49. doi: 10.1021/jm8002262. [DOI] [PubMed] [Google Scholar]
- 78.Bermack JE, Haddjeri N, Debonnel G. Effects of the potential antidepressant OPC-14523 [1-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-5-methoxy-3,4-dihydro-2-quinolinone monomethanesulfonate] a combined σ and 5-HT1A ligand: Modulation of neuronal activity in the dorsal raphe nucleus. J Pharmacol Exp Ther. 2004;310:578–83. doi: 10.1124/jpet.104.066472. [DOI] [PubMed] [Google Scholar]
- 79.Naoi M, Nagatsu T. Quinoline and quninaldine as naturally occurring inhibitors specific for type A monoamine oxidase. Life Sci. 1987;40:1075–82. doi: 10.1016/0024-3205(87)90570-4. [DOI] [PubMed] [Google Scholar]
- 80.Kategaonkar AH, Shinde PV, Kategaonkar AH, Pasale SK, Shingate BB, Shingare MS. Synthesis and biological evaluation of new 2-chloro-3-((4-phenyl-1H-1,2,3-triazol-1-yl)methyl)quinoline derivatives via click chemistry approach. Eur J Med Chem. 2010;45:3142–6. doi: 10.1016/j.ejmech.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 81.Guentert TW, Daly JJ, Riegelman S. Isolation, characterisation and synthesis of a new quinidine metabolite. Eur J Drug Metab Pharmacokinet. 1982;7:31–38. doi: 10.1007/BF03189540. [DOI] [PubMed] [Google Scholar]
- 82.Oshiro Y, Sakurai Y, Sato S, Kurahashi N, Tanaka T, Kikuchi T, et al. 3,4-Dihydro-2(1H)-quinolinone as a novel antidepressant drug: Synthesis and pharmacology of 1-[3-[4-(3-Chlorophenyl)-1-piperazinyl]propyl]-3,4- dihydro-5-methoxy-2(1H)-quinolinone and its derivatives. J Med Chem. 2000;43:177–89. doi: 10.1021/jm980333v. [DOI] [PubMed] [Google Scholar]
- 83.Beadle CD, Boot J, Camp NP, Dezutter N, Findlay J, Hayhurst LM, et al. 1-Aryl-3,4-dihydro-1H-quinolin-2-one derivatives, novel and selective norepinephrine reuptake inhibitors. Bioorg Med Chem Lett. 2005;15:4432–7. doi: 10.1016/j.bmcl.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 84.Tran M, Stack GP. Antidepressant Azaheterocyclymethyl derivatives of 2, 3- dihydro-1,4- dioxino(2,3-F)quinoline. US Patent. 2002/0165245 A1. [Google Scholar]
- 85.Horchler CL, McCauley JP, Jr, Hall JE, Snyder DH, Moore WC, Hudzik TJ, et al. Synthesis of novel quinolone and quinoline-2-carboxylic acid (4-morpholin-4-yl-phenyl)amides: A late- stage diversification approach to potent 5HT1B antagonists. Bioorg Med Chem. 2007;15:939–50. doi: 10.1016/j.bmc.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 86.Ward SE, Eddershaw PJ, Scott CM, Gordon LJ, Lovell PJ, Moore SH, et al. Discovery of potent, orally bioavailable, selective 5-HT1A/B/D receptor antagonists. J Med Chem. 2008;51:2887–90. doi: 10.1021/jm8001444. [DOI] [PubMed] [Google Scholar]
- 87.Kumar S, Bawa S, Drabu S, Gupta H, Machwal L, Kumar R. Synthesis, antidepressant and antifungal evaluation of novel 2-chloro-8-methylquinoline amine derivatives. Eur J Med Chem. 2011;46:670–5. doi: 10.1016/j.ejmech.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 88.Barsoum FF, Girgis AS. Facile synthesis of bis(4,5-dihydro-1H-pyrazole-1-carboxamides) and their thio-analogues of potential PGE2 inhibitory properties. Eur J Med Chem. 2009;44:2172–7. doi: 10.1016/j.ejmech.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 89.Siddiqui N, Alam P, Ahsan W. Design, synthesis, and in-vivo pharmacological screening of N,3-(substituted diphenyl)-5-phenyl-1H-pyrazoline-1-carbothioamides derivatives. Arch Pharm (Weinheim) 2009;342:173–81. doi: 10.1002/ardp.200800130. [DOI] [PubMed] [Google Scholar]
- 90.Palaska E, Aytemir M, Uzbay IT, Erol D. Synthesis of antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur J Med Chem. 2001;36:539–43. doi: 10.1016/s0223-5234(01)01243-0. [DOI] [PubMed] [Google Scholar]
- 91.Ozdemir Z, Kandilci HB, Gümüşel B, Caliş U, Bilgin AA. Synthesis of studies on antidepressant and anticonvusant activities of some 3-(2-furyl)-pyrazoline derivatives. Eur J Med Chem. 2007;42:373–9. doi: 10.1016/j.ejmech.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 92.Can OZ, Ozkay UD, Kaplancikli ZA, Oztürk Y. Effect of some 1.3,4- trisubtituted-2-pyrazoline derivatives on depression and anxiety parameters of mice. Arch Pharm Res. 2009;32:1293–9. doi: 10.1007/s12272-009-1915-5. [DOI] [PubMed] [Google Scholar]
- 93.Gok S, Demet MM, Ozdemir A, Turan-Zitouni G. Evaluation of antidepressant- like effect of 2-pyrazoline derivatives. Med Chem Res. 2010;19:94–101. [Google Scholar]
- 94.Abdel-Aziz M, Abuo-Rahma G, Hassan AA. Synthesis of novel pyrazole derivatives and evaluation of their antidepressantand anticonvulsant activities. Eur J Med Chem. 2009;44:3480–7. doi: 10.1016/j.ejmech.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 95.Cheng H, Hoffman J, Le P, Nair SK, Cripps S, Matthews J, et al. The development and SAR of pyrrolidine carboxamide 11β-HSD1 inhibitors. Bioorg Med Chem Lett. 2010;20:2897–902. doi: 10.1016/j.bmcl.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 96.Peddi S, Roth BL, Glennon RA, Westkaempe RB. Structural determinants for high 5-HT2A receptor affinity of spiro[9,10-dihydroanthracene]-9,3¢-pyrrolidine (SpAMDA) Bioorg Med Chem Lett. 2004;14:2279–83. doi: 10.1016/j.bmcl.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 97.Cheng XC, Wang Q, Fang H, Tang W, Xu WF. Synthesis of new sulfonyl pyrrolidine derivatives as matrix metalloproteinase inhibitors. Bioorg Med Chem. 2008;16:7932–8. doi: 10.1016/j.bmc.2008.07.073. [DOI] [PubMed] [Google Scholar]
- 98.Yoon T, De Lombaert S, Brodbeck R, Gulianello M, Chandrasekhar J, Horvath RF, et al. The design, synthesis and structure-activity relationships of 1-aryl-4-aminoalkyl isoquinolines: A novel series of CRF-1 receptor antagonists. Bioorg Med Chem Lett. 2008;18:891–6. doi: 10.1016/j.bmcl.2007.12.050. [DOI] [PubMed] [Google Scholar]
- 99.DeMet EM, Vosmer G, Halaris AE. Noncompetitive amine uptake inhibition by the new antidepressant pridefine. J Neurochem. 1981;36:917–23. doi: 10.1111/j.1471-4159.1981.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 100.Hancock AA, Buckner SA, Giardina WJ, Brune ME, Lee JY, Morse PA, et al. Preclinical pharmacological actions of (+/-)-(1′R∗,3R∗)-3-phenyl-1-[1′,2′,3′,4′-tetrahydro-5′,6′-methylene-dioxy-1′-naphthalenyl) methyl] pyrrolidine methanesulfonate (ABT-200), a potential antidepressant agent that antagonizes alpha-2 adrenergic receptors and inhibits the neuronal uptake of norepinephrine. J Pharmacol Exp Ther. 1995;272:1160–9. [PubMed] [Google Scholar]
- 101.Bobon D, Breulet M, Gerard-Vandenhove MA, Guiot-Goffioul F, Plomteux G, Sastre-y-Hernández M, et al. “Is phosphodiesterase inhibition a new mechanism of antidepressant action.A double blind double-dummy study between rolipram and desipramine in hospitalized major and/or endogenous depressives”. Eur Arch Psychiatry Neurol Sci. 1988;238:2–6. doi: 10.1007/BF00381071. [DOI] [PubMed] [Google Scholar]
- 102.Banwart LM, Carter DS, Cai HY, Choy JC, Greenhouse R, Jaime-Figueroa S, et al. Novel 3,3-disubstituted pyrrolidines as selective triple serotonin/norepinephrine/dopamine reuptake inhibitors. Bioorg Med Chem Lett. 2008;18:6062–6. doi: 10.1016/j.bmcl.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 103.Zelle RE, Hancock AA, Buckner SA, Basha FZ, Tieje K, Debernardis JF, et al. Synthesis and pharmacological characterization of ABT-200:A putative novel antidepressant combining potent α-2 antagonism with moderate NE uptake inhibition. Bioorg Med Chem Lett. 1994;4:1319–22. [Google Scholar]
- 104.Hadizadeh F, Hosseinzadeh H, Motamed-Shariaty VS, Seifi M, Kazemi S. Synthesis and antidepressant activity of N-substituted imidazole-5-caboxamide in forced swimming test model. Iran J Pharm Res. 2008;7:29–33. [Google Scholar]
- 105.Czopek A, Byrtus H, Kolaczkowski M, Pawlowski M, Dybala M, Nowak G, et al. Synthesis and pharmacological evaluation of new 5-(cyclo)alkyl-5-phenyl and 5-spiroimidazolidine-2,4-dione derivatives.Novel 5-HT1A receptor agonist with potential antidepressant and anxiolytic activity. Eur J Med Chem. 2010;45:1295–303. doi: 10.1016/j.ejmech.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 106.Zagorska A, Jurczy S, Pawlowski M, Dybala M, Nowak G, Tatarczynska E, et al. Synthesis and preliminary pharmacological evaluation of imidazo(2,1-f)purine-2,4-dione derivatives. Eur J Med Chem. 2009;44:4288–96. doi: 10.1016/j.ejmech.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 107.Ulak G, Mutlu O, Akar FY, Komsuoðlu FI, Tanyeri P, Erden BF. Neuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole augment the effects of antidepressants acting via serotonergic system in the forced swimming test in rats. Pharmacol Biochem Behav. 2008;90:563–8. doi: 10.1016/j.pbb.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 108.Rodriguez F, Rozas I, Ortega JE, Erdozain AM, Meana JJ, Callado LF. Guanidine and 2-aminoimidazoline aromatic derivatives as α2-adrenoceptor antagonists. 2. Exploring alkyl linkers for new antidepressants. J Med Chem. 2008;51:3304–12. doi: 10.1021/jm800026x. [DOI] [PubMed] [Google Scholar]
- 109.Herold F, Chodkowski A, Izbicki Q, Turio J, Dawidowski M, Kleps J, et al. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1A activity. Part 3. Eur J Med Chem. 2011;46:142–9. doi: 10.1016/j.ejmech.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 110.Rodrigues ALS, Rosa JM, Gadotti VM, Goulart EC, Santos MM, Silva AV, et al. Antidepressant-like antinociceptive-like action of 4-(4′-cholorophenyl)-6-(4“-methylphenyl)-2-hydrazinepyrimidine Mannich base in mice. Pharmacol Biochem Behav. 2005;82:156–62. doi: 10.1016/j.pbb.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Herhold F, Chodkowski A, Izbicki L, Krol M, Kleps J, Turio J, et al. Novel 4-aryl-pyrido[1,2-C]pyrimidines with dual SSRI and 5-HT1A activity. Part 1. Eur J Med Chem. 2009;44:1710–7. doi: 10.1016/j.ejmech.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 112.Herhold F, Izbicki L, Chodkowski A, Dawidowski M, Krol M, Kleps J, et al. Novel 4-aryl-pyrido[1,2-c]pyrimidines with dual SSRI and 5-HT1A activity: Part2. Eur J Med Chem. 2009;44:4702–15. doi: 10.1016/j.ejmech.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 113.Kim JY, Kim D, Kang SY, Park WK, Kim HJ, Jung ME, et al. Arylpiperazine-containing pyrimidine 4-carboxamide derivatives targeting serotonin 5-HT(2A), 5-HT(2C), and the serotonin transporter as a potential antidepressant. Bioorg Med Chem Lett. 2010;20:6439–42. doi: 10.1016/j.bmcl.2010.09.081. [DOI] [PubMed] [Google Scholar]
- 114.Gupta RR, Kumar M, Gupta V. Berlin, Heidelberg, New York: Springer-Verlag; 1999. Heterocyclic Chemistry: Five Membered Heterocycles; pp. 492–3. [Google Scholar]
- 115.Finar IL. 5th. Vol. 2. India: Pearson education; 2004. Organic Chrmistry: Stereochemistry and the Chemistry of Natural Product; pp. 621–622. [Google Scholar]
- 116.Eison AS, Eison MS, Torrente JR, Wright RN, Yocca FD. Nefazodone: preclinical pharmacology of a new antidepressant. Psychopharmacol Bull. 1990;26:311–5. [PubMed] [Google Scholar]
- 117.El Shehry MF, Abu-Hashem AA, El-Telbani EM. Synthesis of 3-((2,4-dichloro phenoxy)methyl)-1,2,4-triazolo(thiadiazoles and thiadiazines) as anti-inflammatory and molluscicidal agents. Eur J Med Chem. 2010;45:1906–11. doi: 10.1016/j.ejmech.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 118.Kapilncki ZA, Ozdemir A, Turan-Zitouni G, Altintop MD, Can OD. New Pyrazoline derivatives and their antidepressant activity. Eur J Med Chem. 2010;45:4383–7. doi: 10.1016/j.ejmech.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 119.Kane JM, Dudley MW, Sorensen SN, Miller FP. 2,4-Dihydro-3-H-1,2,4-triazole-3-thiones as potential antidepressant agents. J Med Chem. 1988;31:1253–8. doi: 10.1021/jm00401a031. [DOI] [PubMed] [Google Scholar]
- 120.Varvaresou A, Siatra-Papastaikoudi T, Dalla Tsotinis A, Tsantili-Kakoulidou A, Vamvakides A. Synthesis, lipophilicity and biological evaluation of indole-containing derivatives of 1,3,4-thiadiazole and 1,2,4-triazole. Farmaco. 1998;53:320–6. doi: 10.1016/s0014-827x(98)00024-x. [DOI] [PubMed] [Google Scholar]


