Abstract
Background:
Peptic ulcer is a global health problem of the gastrointestinal tract characterized by mucosal damage secondary to pepsin and gastric acid secretion which occurs due to due to an imbalance between offensive and defensive factors.
Objective:
The present study was carried out with methanolic extract of the seed coat of Tamarindus indica Linn. to evaluate its antiulcer potential on ibuprofen, alcohol and pyloric ligation induced gastric lesions.
Materials and Methods:
Doses of 100 mg/kg & 200 mg/kg of methanolic extract wre administered orally to rats of different groups. Ranitidine at a dose of 50 mg/kg was used as a standard drug for these gastric ulcer models. The gastric content was collected and the volume was measured. The ulceration index was determined by examining the inner lining of each stomach. Furthermore, the effect was assessed by free acidity, pepsin activity, total carbohydrate (TC), protein content (PK).
Result:
The result showed that the methanolic extract of seed coats of Tamarindus indica significantly reduce the total volume of gastric juice, free and total acidity of gastric secretion (P < 0.01) in pylorus ligation induced ulcer model as is comparable with the standard drug ranitidine. There was also a significant reduction in ulcer index (P < 0.01) as compared to control group.
Conclusion:
The methanolic extracts of seed coat of Tamarindus indica can be used as a new source of antiulcer agent in animals.
Keywords: Peptic ulcer, ranitidine, Tamarindus indica, ulcer index
A peptic ulcer, also known as ulcus pepticum, or peptic ulcer disease (PUD), is an ulcer (defined as mucosal erosions equal to or greater than 0.5 cm) of an area of the gastrointestinal tract that is usually acidic and thus extremely painful. It is a sore in the lining of the stomach or duodenum. If peptic ulcers are found in the stomach, they are called gastric ulcers. If they are found in the duodenum, they are called duodenal ulcers. Peptic ulcer disease is a problem of the gastrointestinal tract characterized by mucosal damage secondary to pepsin and gastric acid secretion. It usually occurs in the stomach and proximal duodenum; less commonly, it occurs in the lower esophagus, the distal duodenum or in the jejunum. It is one of the major gastrointestinal disorders that occurs due to an imbalance between offensive and defensive factors along with weakness of the mucosal barrier.[1] The major offensive factors are acid, non-steroidal anti-inflammatory drugs (NSAIDs), pepsin, Helicobacter pylori (H-pylori), and bile salts, and defensive factors involve bicarbonate secretion and prostaglandins. Approximately 4500 people in the United Kingdom (UK) and 15,000 in United States (US) die each year from complications of PUD.[2] Its prevalence in India is quite high. Several field studies from different parts of our country suggest its occurrence in 4 to 10 per thousand populations. Tamilnadu, Andhra Pradesh, and Jammu and Kashmir are considered to be very high risk areas. The estimated lifetime prevalence of PUD (gastric or duodenal ulceration) is 12% for men and 9% for women.
Tamarindus indica Linn. (Leguminosae) is a tree-type plant, which is indigenous to tropical Africa, North and South America, Florida, Brazil, and is also cultivated in subtropical China, India, Pakistan, Indochina, Philippines, Java, and Spain.[1,2] Tamarind seeds are reported to contain phenolic compounds,[3] polymeric tannins,[4] and Fatty acids;[5] the leaves contain triterpenes,[6] Flavones, and flavonols;[7] the pericarp contains (+)-catechin, procyanidin B2, (-) epicatechin, procyanidin trimer, procyanidin tetramer, procyanidin pentamer, procyanidin hexamer, taxifolin, apigenin, eriodictyol, luteolin, and naringenin;[4] and the fruits contain L-ascorbic acid, tocopherol, and carotenes. The seeds are useful for stomach algia, diarrhea, dysentery, burning sensation, giddiness, vertigo, inflammation, abscess, hemorrhoids, vaginopathy, and diabetes.[1,2] However, the seed coats have so far not been scientifically evaluated for their antiulcer activity. T0 herefore ,0 the present study is undertaken to evaluate the antiulcer potential of the seed coat of Tamarindus indica Linn. Furthermore, the effect of the plant extract on the biochemical parameters (Free acidity activity, pepsin activity, total carbohydrate, protein content, etc.) of gastric juice in pylorus-ligated rats has also been noted.
Materials and Methods
Collection of plants
The dried seeds of Tamarindus indica were purchased from the local market of Hisar, Haryana, India. The crude drugs were authenticated by Dr. H. B. Singh, National Institute of Science Communication and Information Resources (NISCAIR), New Delhi.
Preparation of the extract
The dried seeds of Tamarindus indica were heated in a hot air oven at 140°C, for 45 minutes, cooled, and cracked, to separate their outside brown layer. Only the brown–red seed coat was collected and ground into fine powder. Then they were defatted with petroleum ether. After defatting with petroleum ether, the seed coat powder was extracted with methanol for 48 hours and filtered through Whatman No. 4 filter paper. The residues were re-extracted with an additional 100 ml of methanol. The solvent of the combined extract was evaporated under reduced pressure (34 – 36 kPa) using a rotary vacuum evaporator at 40°C and the contents were air dried.
Animals
Male Albino Wistar rats weighing 100 – 150 g were obtained from the Disease Free Animal House, Chaudhary Charan Singh Haryana Agricultural University, Hisar (Haryana, India). The animals were kept in environmentally controlled rooms (25 ± 2°C, 12-hour light and dark cycle) with free access to food and water. Food given to animals consisted of wheat flour kneaded with water and mixed with a small amount of vegetable oil. The experimental protocol was approved by the Institutional Animals Ethics Committee (IAEC) and care of laboratory animals was taken as per the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India (Reg. No.0436). All the animals were deprived of food for 24 hours before subjecting them to ulcerogens and were randomly allocated to different experimental groups.
Drugs and chemicals
Ranitidine (Martin Brown, India), Ibuprofen (Martin Brown, India), Thiobarbituric acid (Spectrochem), Reduced glutathione (Hi-media), 5,5′-dithionitrobenzoic acid (Hi-media), Bovine serum albumin (Himedia), Trichloroacetic acid (Qualigens fine chemicals), Potassium chloride (Qualigens Fine Chemicals), Sucrose (Qualigens Fine Chemicals), Ethylenediamine tetraacetic acid (S. D. Fine Chem), Sodium hydroxide (Spectrochem), Petroleum ether (40 – 60°C), Ethanol, Phenolphthalein (S. D. Fine Chem), Phenol reagent (Ciocalteau-Folins reagent; S. D. Fine Chem), Phenol (Qualigens Fine Chemicals), Sulfuric acid (Qualigens Fine Chemicals), Glucose (Qualigens Fine Chemicals), Anhydrous sodium acetate (Qualigens Fine Chemicals), Alkaline reagent (S. D. Fine Chem), Phenol red indicator, Pyridine (Spectrochem), n-Butanol (Spectrochem), Tris buffer (Spectrochem).
Experimental design
The following groups were made for the antiulcer study. Six animals were taken for each group:
Group 1: Vehicle (Distilled water; DW)
Group 2: DW + Ibuprofen (control)
Group 3: DW + Pylorus ligation (control)
Group 4: DW + Alcohol (control)
Group 5: DW + Alcohol + Ranitidine (50 mg/kg)
Group 6: DW + Ibuprofen + Ranitidine (50 mg/kg)
Group 7: DW + Pylorus ligation + Ranitidine (50 mg/kg)
Group 8: DW + Ibuprofen + Methanolic fractions (100 mg/kg)
Group 9: DW + Ibuprofen + Methanolic fractions (200 mg/kg)
Group 10: DW + Alcohol + Methanolic fractions (100 mg/kg)
Group 11: DW + Alcohol + Methanolic fractions (200 mg/kg)
Group 12: DW + Pylorus ligation + Methanolic fractions (100 mg/kg)
Group 13: DW + Pylorus ligation + Methanolic fractions (200 mg/kg)
All animals were deprived of food for 24 hours, but not of water, before subjecting them to ulcerogens.
Ibuprofen-induced ulcer model
A non-steroidal anti-inflammatory drug, ibuprofen, was given in two doses of 300 mg/kg p.o. at 15-hour intervals, to 24-hour fasted animals.[8] The plant extract was given 30 minutes before each dose of ibuprofen, by oral administration. Animals were sacrificed six hours after the second dose of ibuprofen. The stomach was removed, cut along the greater curvature, washed with normal saline (0.9%), and the ulcer index was scored.
Alcohol-induced ulcer model
Gastric ulcers were induced by administration of absolute alcohol at a dose of 1 ml / 200 g of body weight, orally, after 45 minutes of oral administration fractions to all groups of animals.[9] Animals were sacrificed one hour after the administration of ethanol. The stomach was removed, cut along the greater curvature, washed with normal saline (0.9%), and the ulcer index was scored.[10]
Pylorus ligation-induced ulcer model
This is the oldest animal model of gastric ulcers developed by Shay in 1945. Wistar rats weighing 100 – 150 g were fasted for 24-hours prior to pyloric ligation. Under light ether anesthesia the abdomen was opened by a small midline incision below the Xiphoid process. The pyloric position of the stomach was lifted out slightly and ligated, avoiding traction to the pylorus or damage to its blood supply. The stomach was replaced carefully and the abdominal wall was closed by interrupted sutures. The animals were deprived of both food and water during the postoperative period and were sacrificed at the end of six hours, after the operation. The stomachs were dissected out, the contents were drained into tubes and were subjected for analysis of biochemical parameters like free acidity activity, pepsin activity, total carbohydrate, and protein content.[8] The stomachs were then cut along the greater curvature and the inner surfaces were examined for ulcerations, and the ulcer indices were calculated.
Drug administration
The test drug (Tamarindus indica Linn. in a dose of 100 mg/kg and 200 mg/kg, and the standard drug (50 mg/kg) were administered by oral route for 15 days in all the three different ulcer models.
Measurement of the ulcer index
Ulcer scoring was done by viewing ulcers with a magnifying glass. The following arbitrary scoring system was used to grade the incidence and severity of the lesions.[11]
Shedding of epithelium = 10
Petechial and frank hemorrhages = 20
One or two ulcers = 30
More than two ulcers = 40
Perforated ulcers = 50
Ulcer index was calculated from scoring as follows:
UI = Us + Up × 10 - 1; where,
Us = Mean severity of ulcer score
Up = percentage of animals with ulcer incidence
Percentage protection index was calculated as
C - T/C × 100 (where, C = ulcer index in the control group; T = ulcer index in the treated group)
Biochemical parameters investigated in gastric juice
Estimation of dissolved mucosubstances is done by determining the total carbohydrates and protein in 95% ethanol precipitate of gastric juice. The total carbohydrate and the total carbohydrate : protein (TC : PR) ratio has been accepted as a reliable index of mucus secretion and mucosal resistance.[12] From gastric juices, the following parameters were determined, that is, free acidity, pepsin activity,[13] total carbohydrates,[14] and protein content.[15]
Estimation of free acidity
Centrifuge the gastric content at 1000 × g for 10 minutes. Note the volume of gastric juice. Pipette out 1 ml of the supernatant liquid and dilute it to 10 ml with distilled water. Then total acidity of gastric juice was estimated by titration with 0.01 N sodium hydroxide using phenolphthalein as an indicator. The result was expressed as the free acid output, which was expressed as mEq / liter of body weight.[13]
Estimation of pepsin activity
Centrifuged gastric juice of 0.1 ml (5000 × g for 10 minutes) was added to 1 ml of bovine albumin (0.5% w/v in 0.01 N HCl, pH 2) and incubated for 20 minutes at 37° C. A duplicate background control tube (gastric juice blank) in which 1 ml albumin was replaced with 1 ml of 0.01 N HCl was run simultaneously. The hydrolysis was stopped by adding 2 ml of 10% trichloroacetic acid. All tubes were heated in boiling water for 5 minutes and cooled. After denaturation of the protein by heating in a boiling water bath for 5 minutes, the precipitate was removed by centrifugation (9000 × g for 10 minutes). A total of 1 ml of the supernatant was mixed with 0.4 ml of 2.5 N NaOH and 0.1 ml of the Folin-Ciocalteu reagent and the volume was adjusted to 10 ml with distilled water. The absorbance was measured at 700 nm. The peptic activity was calculated in terms of micrograms of tyrosine liberated per milliliter of gastric juice.[13]
Estimation of total carbohydrate
One milliliter of 5% phenol was pipetted out into test tubes containing 0.15 m gastric juice and a blank containing 0.15 ml of distilled water and mixed thoroughly. Five milliliters of 96% H2SO4 was added and mixed slowly. After 10 minutes, the test tubes were shaken and placed in water kept at 20°C for 20 minutes. The optical density of the developed yellow orange chromophore was read in a UV spectrophotometer at 482 nm. Several concentrations of glucose standard solution were run to prepare a standard curve. Total carbohydrates were expressed in terms of micrograms per milliliter liberated in gastric juice. Mucoadhesive activity was expressed as the ratio of total carbohydrates and protein content.[14]
Estimation of protein content
Estimation of protein was carried out as described by Lowry in 1951.[15] One milliliter of gastric juice and 9 ml of 95% alcohol was mixed, shaken, and then the mixture was centrifuged at 3000 × g for 15 minutes to obtain the precipitation. This precipitate was dissolved in 1 ml of 0.1 N NaOH. Next 0.9 ml of distilled water was added to 0.1 ml of the above-mentioned solution. Out of this solution, 0.4 ml was taken in another test tube. Four milliliters of alkaline reagent was added to this test tube and kept for 10 minutes. Then 0.4 ml of phenol reagent was added to this test tube and kept for 10 minutes for color development. The readings were taken against the blank prepared with distilled water. The protein content was obtained by calculating with the use of standard curve prepared with bovine albumin. The concentrations of proteins were expressed in terms of micrograms per milliliter of gastric juice ± SEM.
Statistical analysis
All the results were expressed as mean ± standard error of mean (SEM). The data of all the groups were analyzed using one-way ANOVA followed by Dunnett's t-test using the software Instat 3.0. In all the tests, the criterion for statistical significance was P < 0.05.
Results
Ibuprofen-induced ulcer model
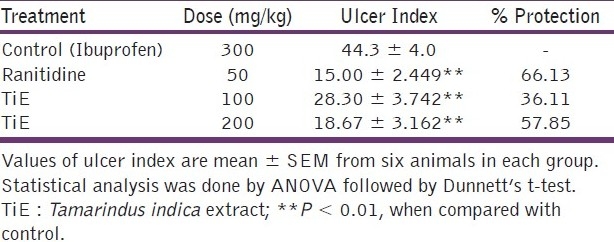
In the ibuprofen-induced ulcer model the ulcer index and percentage protection of ulcer index [Table 1]. The extract of the plant shows significant inhibition of the ulcer index (57.85%) in a dose of 200 mg/kg (P < 0.01) when compared with the control animals. The standard drug, Ranitidine also shows significant effect in the reduction of the ulcer index (66.13%) in a dose of 50 mg/kg when compared with the control groups (P < 0.01).
Table 1.
Effect of Tamarindus indica extract (TiE) on Ibuprofen-induced ulcers
Alcohol-induced ulcer model
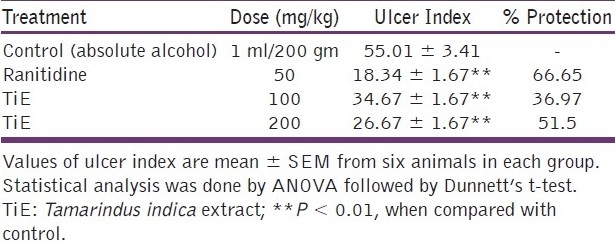
Table 2 shows the ulcer index and percentage protection of the ulcer in the alcohol-induced ulcer model. The dose-dependent effect was observed with the methanolic extract of Tamarindus indica. The extract of the plant shows significant protection of ulcer 51.5% in a dose of 200 mg/kg (P < 0.01) and 36.97% protection in a dose of 100 mg/kg when compared with the control animals. The standard drug, Ranitidine, also shows significant effect in the protection of ulcer (66.65%) in a dose of 50 mg/kg when compared with the control groups (P < 0.01).
Table 2.
Effect of Tamarindus indica extract (TiE) in alcoholinduced ulcers
Pylorus ligation-induced ulcer model
The ulcer index and percentage protection of ulcer in the pylorus-induced ulcer model [Table 3]. The dose-dependent effect was observed in the methanolic extract of Tamarindus indica. The extract of the plant showed significant protection of ulcer 36.88% in a dose of 200 mg/kg (P < 0.01) and 18.5% protection in a dose of 100 mg/kg when compared with the control animals. The standard drug, Ranitidine, also shows significant effect in the protection of ulcers (62.95%) in a dose of 50 mg/kg, when compared to the control groups (P < 0.01). The free acidity and pepsin activity were also significantly decreased in a dose of 200 mg/kg of the extract as shown in Table 3 when compared with the control animals. Total carbohydrate / protein content ratios (TC / PR) were significantly increased when compared with the control groups.
Table 3.
Effect of Tamarindus indica extract (TiE) in PL-induced ulcers
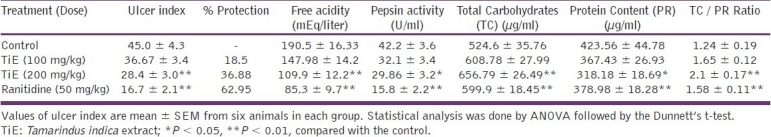
The result indicates that the methanolic fraction of the seed coat by Tamarindus indica has a potent dose-dependent ulcer protective effect against three used animal models. A significant decrease in the ulcer index (P < 0.01) was observed with the treatment of methanolic fractions of the seed coat of Tamarindus indica Linn. at a dose of 200 mg/kg. The plant fraction also significantly increased the glycoprotein content of the mucosal cells seen from the increase in the TC : PC ratio of the gastric mucosa.
Discussion
Peptic ulcers are due to imbalances between offensive (NSAIDs, alcohol, acid, and pepsin) and defensive (bicarbonate, mucosal blood supply) factors.[16] Acid and pepsin are relatively less important causative agents, but defects in the defensive mechanism of the gastric mucosa are the first step toward ulcer formation.[17] The mucosal barrier is normally impermeable, to back the diffusion of hydrogen ions, but it is weakened by either decreased mucus secretion or by disturbance in the turnover of epithelial cells, caused by stress, the Adrenocorticosteroid hormone, cortisone, aspirin, phenylbutazone, ingestion of chillies, tobacco, smoking, and so on.[18,19] The aim of this study is to evaluate the anti-ulcer effect of the methanolic fraction of the seed coat of Tamarindus indica Linn. in several experimental ulcer models, namely, alcohol, ibuprofen, and pylorus ligation. The plant fraction showed dose-dependent, ulcer-protective effects against all three animal models. In the alcohol-induced model, ulcers are caused due to perturbations of superficial epithelial cells, notably the mucosal mast cells, leading to the release of vasoactive mediators including histamine[20] and reactive oxygen species (ROS),[21] resulting in the damage of rat gastric mucosa. Mucosal blood flow has been considered to be an important factor in the damage caused by alcohol and is modulated by prostaglandin.[22] The effectiveness of the plant fraction in protecting against mucosal damage caused by alcohol is an indication of its effect on prostaglandins synthesis and on the free radical scavenging activity.[23] It has been also accepted that alcohol-induced ulcers are not only inhibited by anti-secretory agents such as ranitidine, but also by agents that enhance mucosal defensive factors.[24,25] Thus, it could be assumed that the existence of the cytoprotective effect of compound(s) is present in the methanolic fraction of the seed coat of Tamarindus indica Linn.
Nonsteroidal anti-inflammatory drugs (NASAIDs) such as ibuprofen have been reported to induce gastric ulceration due to inhibition of the biosynthesis of cytoprotective prostaglandins, for example, PGE's and PGI2 (by inhibition of the cyclooxygenase pathway of arachidonic acid metabolism), resulting in overproduction of leukotrienes and other products of the 5-lipoxygenase pathway.[26] Ibuprofen causes a direct irritation effect by increasing H+ ion transport and free radical formation. It also decreases mucus content, surface-active phospholipid bicarbonate secretion, and mucosal proliferation.[27] The possible protective effect of the plant fraction in ibuprofen-induced ulcers may be due to increased mucus secretion.
Pylorus-ligation-induced gastric ulcers occur because of an increase in acid-pepsin accumulation due to pyloric obstruction and subsequent mucosal digestion. A copious amount of mucus is secreted during superficial damage and provides favorable microenvironment in repair. Mucin is a viscous glycoprotein producing, relatively resistant, acid barrier.[28] It makes up a major part of the mucus, an important pre-epithelial factor that acts as a first line of defense against ulcerogens.[29] Increase in the mucin is due to a significant increase in the individual mucopolysacharide-like sialic acid and total hexoses, leading to a significant increase in total carbohydrates. The plant fraction also significantly increases the glycoprotein content of mucosal cells as seen from the increase in the TC : PC ratio of the gastric mucosa. The second dose, 200 mg/kg, significantly decreases the free acidity and causes a significant increase in the mucus content. The ineffectiveness of the lower may be attributed to the low effectiveness of the lower doses, in protecting the mucosa from ulcerogens, as compared to the higher doses. A significant increase in mucus content and decrease in free acidity is also observed with treatment of the seed coat of Tamarindus indica Linn. at a dose of 200 mg/kg. Previous studies have reported that the seed coat of Tamarindus indica Linn. contains polyphenolic compounds, which mainly include pocyanidins, epicatechin, and polymeric tannins.[4] These substances have been seen to possess cytoprotective properties[30] and have been associated with antiulcer activity in other plants.[31] The anti-ulcer potential of these tannins or polyphenolic compounds may be due to their antioxidants and free radical scavenging activities. Tannins may also prevent ulcer development due to protein precipitating and vasoconstricting effects.[32] Their astringent action can help in precipitating microproteins on the ulcer site, thereby forming an impervious layer over the lining that hinders gut secretions and protects the underlying mucosa from toxins and other irritants.[33] Based on our studies, the efficacy of plant extracts in animal ulcer models is attributed to its cytoprotective effect. This cytoprotective effect may be due to the free radical scavenging activity or increase in mucus secretion. However, their antisecretory effect cannot be ruled out. Moreover, a further study has to be conducted, to exploit the possible mechanism of action of the plant extract used in our study.
Conclusion
Results of our studies indicate that the methanolic fraction of Tamarindus indica Linn. produces a significant, dose-dependent cytoprotective effect in ibuprofen, alcohol, and pylorus-ligation-induced ulcer models (P < 0.01). Thus, the methanolic extracts from the seed coat of the plant can be used as a new source of antiulcer drug in animals.
Relevance
Nowadays there is greater use of NSAIDs as analgesics and anti-inflammatories in our daily life, due to which ulcers may form. Various other factors, such as alcohol, which is consumed by many people, also causes ulcers. Thus, in these situations we require drugs that have lesser side effects and higher potency. In the light of these facts the present study is highly relevant and necessary, to point out the utility of tamarind seeds as an antiulcer agent.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Alkofahi A, Atta AH. Pharmacological screening of the anti-ulcerogenic effects of some Jordanian medicinal plants in rats. J Ethnopharmacol. 1999;67:341–34. doi: 10.1016/s0378-8741(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 2.Valle DL. Peptic ulcer disease and related disorders. In: Brawn WC, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. 16th. New York: McGraw Hill; 2005. pp. 1746–62. [Google Scholar]
- 3.Tsuda T, Watanabe M, Ohshima K, Yamamoto A, Kawakishi S, Osawa T. Antioxidative components isolated from the seed of Tamarind (Tamarindus indica L.) J Agri Food Chem. 1994;42:2671–4. [Google Scholar]
- 4.Sudjaroen Y, Haubner R, Wurtele G, Hull EW, Erben G, Spiegelhalder B, et al. Isolation and structure elucidation of phenolic antioxidants from Tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem Toxicol. 2005;43:1673–82. doi: 10.1016/j.fct.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Andriamanantena RW, Artaud J, Gaydou EM, Iatrides MC, Chevalier JL. Fatty acid and sterol composition of Malagasy tamarind kernel oils. J Am Oil Chem Soc. 1983;60:318–21. [Google Scholar]
- 6.Imam S, Azhar I, Hasan MM, Ali MS, Ahmed SW. Two triterpenes lupanone and lupeol isolated and identified from Tamarindus indica Linn. Pak J Pharm Sci. 2007;20:125–7. [PubMed] [Google Scholar]
- 7.Bhatia VK, Gupta SR, Seshadri TR. C-glycosides of Tamarind leaves. Phytochemistry. 1996;5:77–181. [Google Scholar]
- 8.Parmar NS, Desai JK. A review of current methodology for the evaluation of gastric and duodenal antiulcer agents. Indian J Pharmacol. 1993;25:120. [Google Scholar]
- 9.Suleyman H, Demirezer LO, Kuruuzum-Uz A. Effects of Rumex patientia root fractions on indomethacin and ethanol induced gastric damage in rats. Pharmazie. 2004;59:147–9. [PubMed] [Google Scholar]
- 10.Shay H, Komarov SA, Fels SS, Meranze D, Gruenstein M, Siplet H. A simple method for the uniform production of gastric ulceration. Gastroenterol. 1945;5:43–61. [Google Scholar]
- 11.Srivastava SK, Nath C, Gupta MB, Vrat S, Sinha JN, Dhawan KN, et al. Protection against gastric ulcers by Verapamil. Pharmacol Res. 1991;23:81–6. doi: 10.1016/s1043-6618(05)80109-4. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal AK, Mitra PK, Goel RK. A modified method to estimate dissolved mucosubstances in gastric juices. Ind J Exp Biol. 1983;21:78–80. [PubMed] [Google Scholar]
- 13.Prino G, Paglialunga S, Nardi G, Lietti A. Inhibition of experimentally-induced gastric ulcers in the rat by a new sulfated glycopeptide. Eur J Pharmacol. 1971;15:119–26. doi: 10.1016/0014-2999(71)90086-0. [DOI] [PubMed] [Google Scholar]
- 14.Nair BR. Trivendrum (Kerala): University of Kerala; 1976. Investigations on the neuron of south Indian scorpion hetrometrus scaber (dissertation) [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin-phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 16.Goel RK, Bhattacharya SK. Gastroduodenal mucosal defense and mucosal protective agents. Indian J Exp Biol. 1991;29:701–14. [PubMed] [Google Scholar]
- 17.Baron TH, Langman MT, Wastell C. Stomach and duodenum.In: Bouchier IA, editor. In: Bouchier IA, editor. Recent Advances in Gastroenterology. London: Churchill Livingston; 1980. p. 23. [Google Scholar]
- 18.Croft PN. Cell turnover and loss and the gastric mucosal barrier. Am J Dig Dis. 1977;22:383. doi: 10.1007/BF01072198. [DOI] [PubMed] [Google Scholar]
- 19.Menguy R, Masters YF. Effects of aspirin on gastric mucus secretion. Surg Gynecol Obstet. 1965;120:92–8. [PubMed] [Google Scholar]
- 20.Miller TA, Henagan JM. Indomethacin decreases resistance of gastric barrier to disruption by alcohol. Dig Dis Sci. 1984;29:141–9. doi: 10.1007/BF01317055. [DOI] [PubMed] [Google Scholar]
- 21.Mizui T, Sato H, Hirose F, Doteuchi M. Effect of antiperoxidative drugs on gastric damage induced by ethanol in rats. Life Sci. 1987;41:755–63. doi: 10.1016/0024-3205(87)90456-5. [DOI] [PubMed] [Google Scholar]
- 22.Hollander D, Tarnawski A, Gergely H, Zipser RD. Sucralfate protection of the gastric mucosa against ethanol-induced injury: A prostaglandin-mediated process? Scand J Gastroentero. 1984;19:97–102. [PubMed] [Google Scholar]
- 23.Soll AH. Pathogenesis of peptic ulcer and implications for therapy. N Engl J Med. 1990;322:909–16. doi: 10.1056/NEJM199003293221307. [DOI] [PubMed] [Google Scholar]
- 24.Hunt HR, Cederberg C, Dent J, Halter F, Howden C, Marks SI, et al. Optimizing acid suppression for treatment of acid-related diseases. Dig Dis Sci. 1995;40:24s–49. doi: 10.1007/BF02214870. [DOI] [PubMed] [Google Scholar]
- 25.Wolfe MM, Sachs G. Acid suppression: Optimizing therapy for gastroduodenal ulcer healing, gastroesophageal reflux disease, and stress-related erosive syndrome. Gastroenterol. 2000;118:s9–31. doi: 10.1016/s0016-5085(00)70004-7. [DOI] [PubMed] [Google Scholar]
- 26.Rainsford KD, Brune K. Role of the parietal cell in gastric damage induced by aspirin and related drug implications for same therapy. Med J Aust. 1976;1:881–3. [PubMed] [Google Scholar]
- 27.Scheiman JM, Dubois RN, Giardiello FM. Vol. 25. Philadelphia: Saunders; 1996. NSAIDs, Eicosonoids, and the Gastroenteric Tract; pp. 279–89. [Google Scholar]
- 28.Flemstrong G, Garner A. Gastroduodenal HCO3 transport: Characteristics and proposed role in acidity regulation and mucosal protection. Am J Physiol. 1982;242:G153–93. doi: 10.1152/ajpgi.1982.242.3.G183. [DOI] [PubMed] [Google Scholar]
- 29.Zalewsky CA, Moody FG. Mechanisms of mucus release in exposed canine gastric mucosa. Gastroenterol. 1979;77:719–29. [PubMed] [Google Scholar]
- 30.Gonzales E, Iglesias I, Carretero E, Villar A. Gastric cytoprotection of Bolivian medicinal plants. J Ethanopharmacol. 2000;70:329–33. doi: 10.1016/s0378-8741(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 31.Konig M, Scholz E, Hartmann R, Lehmann W, Rimpler H. Ellagitannins and complex tannins from Quercus petraea bark. J Nat Prod. 1994;57:1411–5. doi: 10.1021/np50112a010. [DOI] [PubMed] [Google Scholar]
- 32.Aguwa CN, Nwako SO. Preliminary studies of the root fractions of Nauclea latifolia Smith, for anti-ulcer properties. J Pharm Sci Nigerian. 1988;4:16–23. [Google Scholar]
- 33.Nwafor PA, Effraim KD, Jacks TW. Gastroprotective effects of aqueous fractions of Khaya senegalensis bark on indomethacin-induced ulceration in rats. West Afr J Pharmacol Drug Res. 1996;12:46–50. [Google Scholar]


