Abstract
Objective:
To investigate the medicative effects of medium-polar (benzene:acetone, 1:1, v/v) extract of leaves from Stevia rebaudiana (family Asteraceae) on alloxan-induced diabetic rats.
Materials and Methods:
Diabetes was induced in adult albino Wistar rats by intraperitoneal (i.p.) injection of alloxan (180 mg/kg). Medium-polar extract was administered orally at daily dose of 200 and 400 mg/kg body wt. basis for 10 days. The control group received normal saline (0.9%) for the same duration. Glibenclamide was used as positive control reference drug against Stevia extract.
Results:
Medium-polar leaf extract of S. rebaudiana (200 and 400 mg/kg) produced a delayed but significant (P < 0.01) decrease in the blood glucose level, without producing condition of hypoglycemia after treatment, together with lesser loss in the body weight as compared with standard positive control drug glibenclamide.
Conclusions:
Treatment of diabetes with sulfonylurea drugs (glibenclamide) causes hypoglycemia followed by greater reduction in body weight, which are the most worrisome effects of these drugs. Stevia extract was found to antagonize the necrotic action of alloxan and thus had a re-vitalizing effect on β-cells of pancreas.
Keywords: Alloxan-induced diabetic rats, antidiabetic activity, benzene:acetone extract, Compositae, Stevia rebaudiana
As a devastating disease, diabetes is affecting approximately 3% of the population worldwide, 90% of which suffer from type 2 diabetes. [1] The World Health Organization (WHO) estimates that more than 180 million people worldwide have diabetes and this number is likely to more than double by 2030 and an estimated 1.1 million people died from diabetes in 2005. WHO estimates that over the next 10 years (2006–2015), China will lose $ 558 billion in foregone national income due to heart disease, stroke and diabetes alone. [2] India leads the world with the largest number of diabetic subjects, earning the dubious distinction of being termed the “diabetes capital of the world”. According to the Diabetes Atlas 2006 published by the International Diabetes Federation, the number of people with diabetes in India, currently around 40.9 million, is expected to rise to 69.9 million by 2025 unless urgent preventive steps are taken. [3]
Stevia rebaudiana (Bertoni) is one of the 950 genera of the Compositae (Asteraceae). The plant was rediscovered by Dr. Moises Santiago Bertoni in 1887. The plant was used extensively by Gaurani Indians for more than 1500 years. [4] Stevia has a long history of medicinal use in Paraguay and Brazil, and while many of the therapeutic applications of Stevia are anecdotal, they must be considered in that they have spanned generations. There are now known to be more than 150 Stevia species but this is the only one with significant sweetening properties; other species do contain other biochemicals of interest. Leaves contain approximately 4–15% of steviosides, which are intensely sweet compounds (150–300 times sweeter than sugar). The leaves have been traditionally used for hundreds of years in Paraguay and Brazil to sweeten local teas, medicines and as a “sweet treat”. [5]
S. rebaudiana possesses various activities like antimicrobial, [6] antifungal, [7] hepatoprotective, [8] hypoglycemic (water extract), [9] antitumor, [6] antirotavirus, [10] anti-HIV, [11] anti-hypertension, [12,13] antiviral activity, [14] etc. Other folk applications of Stevia and stevioside (primarily in Latin America and the Orient) include the following: stimulate alertness and counter fatigue; facilitate digestion and gastrointestinal functions; regulate blood glucose levels (BGLs); nourish the liver, pancreas and spleen; help the body sustain a feeling of vitality and well-being and external application for blemishes. Some Stevia and stevioside users report a decrease in desire for sweets and fatty foods. Additionally, some users have reported that drinking Stevia tea or Stevia enhanced teas helped to reduce their desire for tobacco and alcoholic beverages. [15] Stevia and stevioside have been shown in studies to inhibit the growth and reproduction of some bacteria that are responsible for tooth decay. [15,16]
Studies on the comparative effects of leaves and stevioside on glycemia and hepatic gluconeogenesis have already been reported. [17] Hypoglycemic effect [18] of stevioside has also been studied, together with protective effects of stevioside against the toxic actions of alloxan. [19] Chen et al.[18] suggested that stevioside was able to regulate BGLs by enhancing not only insulin secretion, but also insulin utilization in insulin-deficient rats; the latter was due to decreased PEPCK gene expression in rat liver by stevioside's action of slowing down gluconeogenesis. Further studies of this agent for the treatment of diabetes appear warranted. These studies on hypoglycemic actions were centralized on stevioside, a polar molecule, which can be extracted completely either with methanol or with water, [20] whereas non-polar and medium-polar solvents like n-hexane, benzene, methylene dichloride, ethyl acetate acetate and have lesser affinity toward extraction of stevioside. Polar (methanol and water) extracts containing stevioside are well studied for hypoglycemic action, whereas low-polar and medium-polar extracts are yet to be investigated. Therefore, our group decided to study the hypoglycemic effects of medium-polar extractive of its leaves. For this, we generated medium-polar solvent by mixing benzene:acetone in 1:1, v/v ratio. Benzene, being toxic in nature, was selected because it dissolves fatty acids/esters, acetylenes and less to medium-polar plant components. Traces of benzene were removed before the use of extract on animals.
During our investigation of this sweet herb of Paraguay, we carried out extraction of its leaves with benzene:acetone, 1:1, v/v, after defatting the plant material with n-hexane. This medium-polar extract was taken for evaluation of in vivo antidiabetic effects to assess its hypoglycemic (antidiabetic) value.
Materials and Methods
Plant material
Leaves of S. rebaudiana were purchased from Sun Fruits Ltd., Pune (India), which were pulverized manually through hands and filtered with a sieve of mesh size 14. A portion (1.0 kg) of these leaves was kept for extraction. S. rebaudiana was authenticated by Dr. Gyanendra Tiwari (Taxonomist, Department of Fruit Science, K. N. K. College of Horticulture, Mandsaur) and a voucher specimen (MIP/PD/HN/Stevia/S-01) of the plant was deposited in the herbarium of Department of Pharmacognosy, Mandsaur Institute of Pharmacy, Mandsaur (MP), India.
Extract preparation
Dried leaves of S. rebaudiana plant (1.0 kg) were first defatted with several extractions of n-hexane and then these leaves were again extracted using benzene:acetone in the ratio of 1:1, v/v. The medium-polar extract of the leaves thus obtained was distilled and simultaneously dried in vacuo using rotatory evaporator (Bόchi, Switzerland). Benzene was removed completely by distillation, making an azeotropic mixture with alcohol–water. [21] A portion (20 g) of this dried extract was stored under refrigeration at 4.0 ± 2°C until used for biological activity.
Thin-layer chromatographic profiles of Stevia extract
Few chromatographic signatures (plant metabolite profiling) of medium-polar (benzene:acetone) extract of S. rebaudiana were developed using thin-layer chromatography (TLC) coupled with densitometric detection. For this, we optimized three different mobile phases for three different categories of metabolites (non-polar, medium-polar and polar) present in benzene:acetone extract.
Non-polar, medium-polar and polar constituents of the extract were separated onto TLC plates [Figure 1a–c] using hexane:diethyl ether, chloroform:methanol, and chloroform:methanol:water in ratios of 1:1, 85:15, and 60:36:4, (all v/v), respectively. Pure stevioside was used as reference / marker component, which was a kind gift from Dr. Jaroslav Pól (University of Helsinki, Finland).
Figure 1.
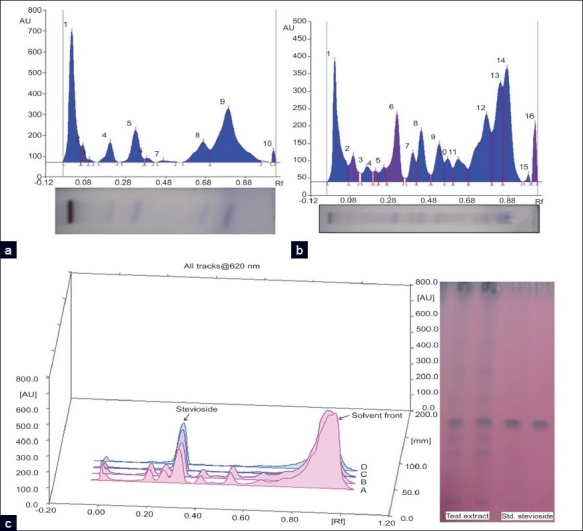
Non-polar (a), middle-polar (b) and polar (c) metabolite profiles of benzene:acetone extract [In (c), A and B = test benzene:acetone extract; C and D = standard stevioside tracks]
Extraction and determination of the extracted amount of stevioside
One gram sample of dried leaves was packed in an extraction thimble of Whatmann filter paper number 1 and extracted by hot soxhlet extraction over a water bath for 48 h. The extract thus obtained was dried in vacuo and redissolved in 10 mL methanol. Quantitative analysis was performed by spotting 5 μL of this test solution on pre-coated silica gel 60 F254 TLC plate (Item code: 1.05554.0007; Make: Merck, Darmstadt, Germany) against 5 μL of standard stevioside solution of concentration 1 mg/mL (methanol). TLC was developed in chloroform:methanol:water in a ratio of 60:36:4, v/v/v and allowed to stand in air for evaporation of the solvent completely. Dark green colored spots of well-resolved Stevia glycosides were visualized by heating derivatized plate on Camag TLC plate heater at at 110°C for 10–20 minutes. Post-chromatographic derivatization of TLC plate was performed with freshly prepared anisaldehyde–sulfuric acid reagent. [22] TLC was scanned via CAMAG TLC Scanner 3 in visible mode (tungsten lamp) at 620 nm. A calibration curve was plotted between increasing amounts of standard stevioside per spot and their peak area responses. A straight line was obtained between 1 and 10 μg/spot with a correlation coefficient (r) 0.99907 (r2 = 0.998; sdv = 3.15). The linear regression equation (y = mx + C) was found to be y = 268.2x + 52.07, where y is the peak area, m is slope of calibration curve, x is the concentration and C is the intercept. Stevioside content in benzene:acetone extract was found to be 0.45% dry leaves weight basis [Table 1].
Table 1.
Stevioside content in benzene: Acetone extract

Chemicals and reference drugs
Alloxan monohydrate was procured from Loba Chemie, Mumbai, India, and other reagents used in the experiment were of analytical grade. Chemically, alloxan is 2,4,4,6-tetra oxo hexahydropyrimidine. Glibenclamide, a reference antidiabetic drug used in this study, was purchased from a local medical store (Aventis Pharma Ltd., Goa, India) and stored as per the instructions given on the pack (i.e., below 25°C).
Animals
Adult Wistar strain albino rats (120–140 g; n = 70) of either sex were obtained from B. R. N. C. P., Mandsaur animal house, after getting permission from Institution of Animal Ethics Committee (IAEC). The rats were maintained under standard laboratory conditions at 25 ± 2°C, relative humidity 50 ± 15% and normal photoperiod (12 h dark/12 h light) for the experiment. Standard pellet diet (Hindustan Lever Ltd., Mumbai, India) and water were provided ad libitum. The experimental protocol has been approved by the IAEC and by the Regulatory body of the government (Animal Ethical Committee number 1019/C/06/CPCSEA). Blood was collected by making a small cut at terminal tail vein for measuring BGL. Estimation of blood glucose was done by using Accu-Check Advantage blood glucose system (strip method).
Acute toxicity study
Acute toxicity study of extracts was carried out in albino rats of either sex by “up and down method” (OECD Guidelines 425). Animals were treated with extract (2000 mg/kg) and observed continuously for the first 4 hours for general behavioral, neurological, autonomic profiles and mortality within 24 h. One-fifth and one-tenth of safe dose was selected as the experimental dose. [23]
Evaluation of antidiabetic activity
Induction of diabetes
Hyperglycemia was induced in 18-h fasted adult Wistar rats (n = 50) weighing 120–140 g by a single intraperitoneal (i.p.) injection of freshly prepared alloxan monohydrate (180 mg/kg) [24] dissolved in normal saline; a 20% glucose solution was also injected intraperitoneally after 4–6 h. The rats were then kept for the next 24 h on 5% glucose solution bottles in their cages to prevent hypoglycemia. [25] Fasting BGLs were estimated by commercially available glucose kit based on glucose oxidase method. [26] The elevated glucose level in plasma, determined at 48 h after injection, confirmed hyperglycemia. Rats with blood glucose more than 250 mg/dl were included in the study (n = 31). 1 unit of insulin i.p. was also given to prevent motility (due to triphasic response) after induction of diabetes. [23,27]
Experimental design
Animals were divided into five groups (five animals in each group). The first group (control/sham) received normal saline (0.9%) and the second group received alloxan monohydrate (180 mg/kg) and served as negative control. Groups from second to fifth were alloxan treated groups (diabetic animals). The third group received antidiabetic reference drug glibenclamide (10 mg/kg) as positive control. The remaining (fourth and fifth) groups received 200 mg/kg (body wt.) and 400 mg/kg (body wt.) of Stevia extract. The blood glucose concentrations of the animals were measured at the beginning of the study and the measurements were repeated on 3rd, 7th and 10th days. [28] All the animals were regularly observed for their general behavior. Changes in the body weight were also measured.
Statistical analysis
All values were expressed as mean ± standard error mean (SEM). The differences were compared using one-way analysis of variance (ANOVA) followed by Dunnet's test. P values <0.01 were considered as significant.
Results and Discussion
Alloxan, a β-cytotoxin, destroys β-cells of islets of Langerhans of pancreas, resulting in a decrease in endogenous insulin secretion and paves the way for the decreased utilization of glucose by the tissues. [29–31] In vitro studies have shown that alloxan is selectively toxic to pancreatic β-cells, leading to the induction of cell necrosis. [32,33] The cytotoxic action of alloxan is mediated by reactive oxygen species, with a simultaneous massive increase in cystolic calcium concentration, leading to a rapid destruction of β-cells. [34] Decreased utilization of glucose by the tissues results in the elevation of BGL.
Expression of elevated fasting BGL confirms induction of diabetes in alloxan-induced experimental rats. The experiment focused on exploring the competence of medium-polar (benzene:acetone, 1:1, v/v) extract from the leaves of S. rebaudiana for medication of diabetes against positive control reference drug glibenclamide. The difference in the initial and final fasting BGLs of different groups in long-term (10-day) studies exposed a significant elevation in BGL in diabetic controls as compared with that of normal control, extract treated and glibenclamide treated rats. Treatment of BGL with Stevia extract indicates the effectiveness of the extract in experimental diabetic animals.
Medium-polar extract from leaves of S. rebaudiana, when administered orally (200 and 400 mg/kg) for 10 days, produced a significant (P < 0.01) dose-dependent reduction in BGL [Table 2] as well as in the body weight [Table 3], although body weight was regained by rats treated with both glibenclamide and Stevia extract. Stevia extract exhibited a significant control of BGLs in diabetic rats, together with lowest decrease in the body weight, as compared with glibenclamide. Alternative exogenous treatments to diabetes include dosage of insulin and sulfonylurea drugs (e.g., glibenclamide), which cause hypoglycemia followed by greater reduction in body weight are the most worrisome effects. Treatment with Stevia extract did not cause hypoglycemia as well as significant decrease in body weight of diabetic rats. Stevia extract was found to revitalize β-cells of pancreas, antagonizing β-necrotic action of alloxan.
Table 2.
Effects of Stevia extract on blood glucose level (mg/dL) of diabetic rats
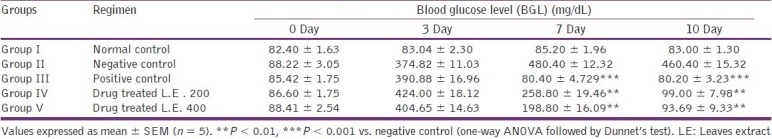
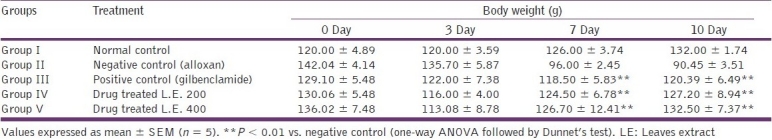
Table 3.
Effects of Stevia extract on body weight of diabetic rats
Excessive hepatic glycogenolysis and gluconeogenesis associated with decreased utilization of glucose by tissue is the fundamental mechanism underlying hyperglycemia in the diabetic state. [35] Aberration of liver glycogen synthesis or glycogenolysis in diabetes may be due to lack of or resistance to insulin, which is essential to activate glycogen synthase system. The significant increase of liver glycogen level in Stevia extract-treated groups may be due to reactivation of the glycogen synthase system by improving insulin secretion. Diabetes is associated with weight loss. [36] The reversal of weight loss in extract-treated diabetic group indicates that the restorative effect of the extract may be due to the reversal of gluconeogenesis and glycogenolysis.
Experimental results also reflect that the Stevia extract is capable of reducing the oxidative state associated with diabetes. Alloxan produces diabetes by liberating oxygen-free radicals which cause lipid peroxide-mediated pancreatic injury. [37] The extract may scavenge free radicals and facilitate reconstruction of pancreatic cells to release more insulin and ultimately produces an antidiabetic effect.
Effects on blood glucose level
Administration of benzene:acetone extract (200 and 400 mg/kg) produced a significant (P < 0.01) dose-dependant reduction in BGL of alloxan-induced diabetic rats. Alloxanized rats of group II (negative control) suffered from hyperglycemia as they did not receive any drug, whereas alloxanized rats of group III (positive control) treated with the reference antidiabetic drug glibenclamide showed significant reduction in BGL to the required standard blood glucose level on the 7th day and the levels were continuously maintained up to 10th day. Rats of group IV treated with Stevia extract (200 mg/kg) showed nearly normal BGL (99.00 ± 7.98 mg/dL) value on the 10 th day, whereas group V rats treated with Stevia extract (400 mg/kg) also showed decrease in blood glucose level to nearly normal (93.69 ± 9.33 mg/dL) value, which is very close to 0 day BGL of group V. Table 2 shows that positive control glibenclamide treated rats attained normalized BGL on the 7th day of treatment, whereas Stevia extract treated rats attained nearby normal BGL on the 10th day.
Effects on body weight
Administration of benzene:acetone extract of Stevia (200 and 400 mg/kg) produced a significant (P < 0.01) dose-dependent reduction in body weight of alloxan-induced diabetic rats. Group II (alloxan-induced negative control) rats revealed 4.46, 32.41 and 36.32% decrease in the body weight on 3rd, 7th and 10th days, respectively, with respect to 0 day control value. Group III (positive control with glibenclamide) rats revealed 5.50, 8.21 and 6.71% decrease in the body weights monitored on 3rd, 7th and 10th days of treatment, respectively. Rats in group IV treated with Stevia extract (200 mg/kg) revealed 10.81, 4.27 and 2.20% decrease in the body weight, while group V (400 mg/kg) rats revealed 16.87, 6.85 and 2.59% decrease in body weight on 3rd, 7th and 10th days, respectively, as compared with 0 day value. Least decrease in body weight was observed in group IV rats (200 mg/kg), i.e., 2.20% on the 10th day [Table 3].
Glibenclamide versus Stevia extract treatment
The effects of oral administration of medium-polar (benzene:acetone, 1:1, v/v) extract of S. rebaudiana leaves are shown in Tables 2 and 3. Experimental studies clearly reveal that the medium-polar extract from S. rebaudiana leaves (200 and 400 mg/kg) orally administered for 10 days produced a delayed but significant decrease in the blood glucose level, together with lesser loss in the body weight, as compared with standard positive control drug in the model of alloxan-induced diabetes in rats.
Effects on liver, renal and pancreatic weights
Table 4 shows the effect of medium-polar extract of S. rebaudiana on renal, pancreatic and hepatic weights of normal, diabetic and diabetic treated rats. A significant intergroup difference (P < 0.05) was observed in glibenclamide treated group and diabetic control group. The liver weight of the normal rats was greater as compared to that of the diabetic control rats and treated diabetic rats. As shown in Table 4, administration of alloxan decreased the liver mass to 1.15 ± 0.2 g/100 g body weight, which showed significant difference (P < 0.01) with respect to non-diabetic rats. The liver mass was increased in diabetic treatment groups and glibenclamide treatment groups significantly (P < 0.05) with respect to diabetic control groups. Alloxan administration also caused a decrease in the pancreatic tissue weight. Treatment with the extract caused a significant increase in pancreatic tissue weight (P < 0.05) with respect to diabetic control. S. rebaudiana extract reduced the elevated kidney weight slightly as compared to untreated diabetic rats, although this did not reach statistically significant level.
Table 4.
Effect on liver, pancreatic and kidney weights in diabetic rats

Long-term pretreatment with sulfonylurea glyburide (GB) causes elevated basal insulin secretion (BIS) and decreased glucose-stimulated insulin secretion (GSIS). These characteristics may play an important role in the development of hypoglycemia and secondary failure. Results revealed that stevioside was able to counteract the desensitizing effects of GB and may be a putative new drug candidate for the treatment of type 2 diabetes mellitus. [38] Abudula et al. in 2004 [39] showed that rebaudioside A potentially stimulates insulin secretion from isolated mouse islets in a dose-, glucose- and Ca2+ -dependent manner. According to the study of Dyrskog et al., [40] rebaudioside A failed to show beneficial effects in diabetic animals. In continuation of the previous study, Abudula et al. in 2008 [41] reported the mechanism for the insulinotropic action of rebaudioside A.
According to the study of Gardana et al.[42] on the metabolism of stevioside and rebaudioside A from S. rebaudiana extracts by human microflora, both stevioside and rebaudioside A were completely hydrolyzed to their aglycon steviol in 10 and 24 h, respectively. Interestingly, the human intestinal microflora was not able to degrade steviol, which suggests that Stevia glycosides are zero calorie sweeteners and thus can be utilizable as a dietary supplement by diabetic patients or these sweeteners can also be used for preparing cough syrups.
Stevioside is not absorbed by the human gut; only bacteria of the colon degrade stevioside to steviol. Part of this steviol is absorbed by the colon and transported to the liver by portal blood. In the liver, steviol glucuronide is formed, which is released into the blood and filtered out by the kidneys into the urine. High levels of steviol glucuronide in the urine suggest that there is no accumulation of steviol derivatives in the human body. The steviol glucuronide still present is expected to be excreted in the urine during the next 24 h. Besides steviol glucuronide, no free steviol or any other possible steviol metabolite could be detected in blood or urine. Hepatic metabolism of steviol is extremely low, if existing at all, which is in agreement with the results of Koyama et al.,[43,44] who demonstrated by their in vitro experiments that the steviol metabolism by human microsomes was 4 times lower than that by rat microsomes, and the latter one was already very low. [44]
A recent in vivo study by Melis et al., in 2009, [45] carried out on 30 male rats, toward evaluation of the renal excretion of steviol suggested that steviol at all doses (0.5, 1.0 and 3.0 mg/kg/h) used, except the lowest (0.5 mg/kg/h), induced a statistically significant increase in glucose clearance when compared to control and exhibited a dose-dependent effect. In our medium-polar extract, the amount of stevioside was 0.45% (dry leaves weight basis) as determined by high-performance thin-layer chromatography (HPTLC) method. Thus, the antidiabetic (hypoglycemic) effects of this extract may be due to the presence of stevioside, rebaudioside A and other sweet glycosides, as was also shown in polar chromatographic signature/profile [Figure 1c] of benzene:acetone extract.
Conclusions
In conclusion, the present data suggest that Stevia extract produced good antidiabetic effects together with lesser loss in body weight. Thus, purified Stevia sweeteners can also be used in the preparation of cough syrups and cold beverages for diabetes patients.
Acknowledgments
The authors would like to thank Dr. V. B. Gupta, Director, B. R. Nahata Group of Institutions and Research centre, for in vivo evaluation of antidiabetic activity. Authors are grateful to Dr. Jaroslav Pól (University of Helsinki, Finland) for providing gift sample of stevioside reference standard and to Ms. Barbora Hohnova for shipment of the same to our place. Authors are also very thankful to Mr. Deepak Jain and Mr. Ashish Bharillya for the purchase arrangement of S. rebaudiana leaves from M/s. Sun Fruits Limited, India.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Skyler JS. Diabetes mellitus: Pathogenesis and treatment strategies. J Med Chem. 2004;47:4113–7. doi: 10.1021/jm0306273. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Geneva: WHO; 2008. Diabetes, Fact sheet no. 312. [Last cited on 2010 May 23]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/index.html .
- 3.Mohan V, Sandeep R, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 4.Mowrey DB. Life with stevia: how sweet it is! [Last accessed on 2010 Oct 20]. Available from: http://healthfree.com/stevlife.html .
- 5.Midmore DJ, Rank AH. A new rural industry – Stevia – to replace imported chemical sweeteners 2002. A report for the rural industries research and development corporation Australia: RIRDC web. Publication no. W02/022, RIRDC, Project no. UCQ-16A; 2002. [Last accessed on 2010 Jan 02]. Available from: https://rirdc.infoservices.com .au/downloads/W02-022.pdf .
- 6.Satishkumar J, Sarvanan MM, Seethalakshmi I. In-vitro antimicrobial and antitumor activities of Stevia rebaudiana (Asteraceae) leaf extracts. Trop J Pharm Res. 2008;7:1143–9. [Google Scholar]
- 7.Silva PA, Oliveira DF, Prado NR, Carvalho DA, Carvalho GA. Evaluation of the antifungal activity by plant extracts against Colletotrichum gloeosporioides PENZ. Ciência e Agrotecnologia. 2008;32:420–8. [Google Scholar]
- 8.Mohan K, Robert J. Hepatoprotective effects of Stevia rebaudiana Bertoni leaf extract in CCl4-induced liver injury in albino rats. Med Arom Plant Sci Biotechnol. 2009;3:59–61. [Google Scholar]
- 9.Oviedo CA, Fronciani G, Moreno R, Maas L. Hypoglycemic action of Stevia rebaudiana. Excerpta Med. 1970;209:92–6. [Google Scholar]
- 10.Takahashi K, Matsuda M, Ohashi K, Taniquchi K, Nakaqomi O, Abe Y, et al. Analysis of anti-rotavirus activity of extract from Stevia rebaudiana. Antiviral Res. 2001;49:15–24. doi: 10.1016/s0166-3542(00)00134-0. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi K, Iwata Y, Mori S, Shigeta S. In-vitro anti-HIV activity of extract from Stevia rebaudiana. Antiviral Res. 1998;37:A59. doi: 10.1016/s0166-3542(00)00134-0. [DOI] [PubMed] [Google Scholar]
- 12.Chan P, Xu DY, Liu JC, Chen YJ, Tomlinson B, Huang WP, et al. The effect of stevioside on blood pressure and plasma catecholamines in spontaneously hypertensive rats. Life Sci. 1998;63:1679–84. doi: 10.1016/s0024-3205(98)00439-1. [DOI] [PubMed] [Google Scholar]
- 13.Lee CN, Wong KL, Liu JC, Chen YJ, Cheng JT, Chan P. Inhibitory effect of stevioside on calcium influx to produce anti-hypertension. Planta Med. 2001;67:796–9. doi: 10.1055/s-2001-18841. [DOI] [PubMed] [Google Scholar]
- 14.Kedik SA, Yartsev EI, Stanishevskaya IE. Antiviral activity of dried extract of Stevia. Pharm Chem J. 2009;43:198–9. doi: 10.1007/s11094-009-0270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevia rebaudiana Bertoni. [Last accessed on 2010 Oct 04]. Available from: http://www.emperorsherbiologist.com/stevia.php .
- 16.Sharma V. Chattopadya (assisted by) Stevia: prospects as an emerging natural sweetener. [Last accessed on 2007]. Available from: http://www.whoindia.org/LinkFiles/Food_safety_stevia.pdf .
- 17.Ferreira EB, de Assis Rocha Neves F, da Costa MA, do Prado WA, de Araújo Funari Ferri L, Bazotte RB. Comparative effects of Stevia rebaudiana leaves and stevioside on glycaemia and hepatic gluconeogenesis. Planta Med. 2006;72:691–6. doi: 10.1055/s-2006-931586. [DOI] [PubMed] [Google Scholar]
- 18.Chen TH, Chen SC, Chan P, Chu YL, Yang HY, Cheng JT. Mechanism of the hypoglycemic effect of stevioside, a glycoside of Stevia rebaudiana. Planta Med. 2005;71:108–13. doi: 10.1055/s-2005-837775. [DOI] [PubMed] [Google Scholar]
- 19.Raskovic A, Gavrilovic M, Jakovljevic V, Sabo J. Glucose concentration in the blood of intact and alloxan-treated mice after pretreatment with commercial preparations of Stevia rebaudiana (Bertoni) Eur J Drug Metab Pharmacokinet. 2004;29:87–90. doi: 10.1007/BF03190581. [DOI] [PubMed] [Google Scholar]
- 20.Pól J, Varadová Ostrá E, Karásek P, Roth M, Benešová K, Kotlaríková P, et al. Comparison of two different solvents employed for pressurised fluid extraction of stevioside from Stevia rebaudiana: methanol versus water. Anal Bioanal Chem. 2007;388:1847–57. doi: 10.1007/s00216-007-1404-y. [DOI] [PubMed] [Google Scholar]
- 21.Azeotropic mixtures. Available from; http://www.chestofbooks.com/[Last updated on 2009 Feb 21]. Canada: StasoSphere Base c2007-2009. Available from: http://www.chestofbooks.com/food/beverages/Alcohol-Properties/Azeotropic-Mixtures.html[Last cited on 2010 May 21] [Google Scholar]
- 22.Misra H, Dwivedi BK, Mehta D, Mehta BK, Jain DC. Development and validation of high-performance thin-layer chromatographic method for determination of α-mangostin in fruit pericarp of mangosteen plant (Garcinia mangostana L.) using ultraviolet – visible detection. Rec Nat Prod. 2009;3:178–86. [Google Scholar]
- 23.Nagappa AN, Thakurdesai PA, Venkat Rao N, Singh J. Antidiabetic activity of Terminalia catappa Linn.fruits. J Ethnopharmacol. 2003;88:45–50. doi: 10.1016/s0378-8741(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 24.Patel N, Raval S, Goriya H, Jhala M, Joshi B. Evaluation of Antidiabetic Activity of Coldenia procumbens in Alloxan-Induced Diabetes in Rat. J Herb Pharmacother. 2007;7:13–23. [PubMed] [Google Scholar]
- 25.Barry JA, Hassan IA, Al-Hakiem MH. Hypoglycaemic and antihyperglycaemic effects of Trigonella foenum-graecum leaf in normal and alloxan induced diabetic rats. J Ethnopharmacol. 1997;58:149–55. doi: 10.1016/s0378-8741(97)00101-3. [DOI] [PubMed] [Google Scholar]
- 26.Trinder P. Determination of blood glucose using an oxidase peroxidase system with a noncarcinogenic chromogen. J Clin Pathol. 1969;22:158–61. doi: 10.1136/jcp.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy S, Sehgal R, Padhy BM, Kumar VL. Antioxidant and protective effect of latex of Calotropis procera against alloxan-induced diabetes in rats. J Ethnopharmacol. 2005;102:470–3. doi: 10.1016/j.jep.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Aslan M, Orhan DD, Orhan N, Sezik E, Yesilada E. In-vivo antidiabetic and antioxidant potential of Helichrysum plicatum spp.in streptozotocin induced diabetic rats. J Ethanopharmacol. 2006;109:54–9. doi: 10.1016/j.jep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Lenzen S, Panten U. Alloxan: History and mechanism of action. Diabetologia. 1988;31:337–42. doi: 10.1007/BF02341500. [DOI] [PubMed] [Google Scholar]
- 30.Oberley LW. Free radicals and diabetes. Free Radic Biol Med. 1988;5:113–24. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto H, Uchigata Y, Okamoto H. Streptozotocin and Alloxan induce DNA strand breaks and poly (ADP-ribose) synthetase in pancreatic islets. Nature. 1981;294:284–6. doi: 10.1038/294284a0. [DOI] [PubMed] [Google Scholar]
- 32.Jorns A, Munday R, Tiedge M, Lenzen S. Comparative toxicity of alloxan, N-alkyl-alloxans and ninhydrin to isolated pancreatic islets in-vitro. J Endocrinol. 1997;155:283–93. doi: 10.1677/joe.0.1550283. [DOI] [PubMed] [Google Scholar]
- 33.LeDoux SP, Woodley SE, Patton NJ, Wilson GL. Mechanism of notrosourea-induced â-cells damage - alterations in DNA. Diabetes. 1986;35:866–72. doi: 10.2337/diab.35.8.866. [DOI] [PubMed] [Google Scholar]
- 34.Szkudelski T. The mechanism of alloxan and streptozotocin action in B-cells of the rat pancreas. Physiol Res. 2001;50:537–46. [PubMed] [Google Scholar]
- 35.Swanston-Flatt SK, Day C, Bailey CJ, Flatt PR. Traditional plant treatments for diabetes: Studies in normal and streptozotocin diabetic mice. Diabetologia. 1990;33:462–4. doi: 10.1007/BF00405106. [DOI] [PubMed] [Google Scholar]
- 36.Huang X, Vaag A, Hanson M, Weng J, Laurila E, Groop L. Impaired insulin stimulated expression of the glycogen synthase gene in sekeletal muscle of type 2 diabetic patient is acquired rather than inherited. J Clin Endocrinol Metab. 2000;85:1584–90. doi: 10.1210/jcem.85.4.6535. [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B, Gutterdge JM. London: Oxford Claredon Press; 1985. Free radicals in biology and medicine; pp. 24–86. [Google Scholar]
- 38.Chen J, Jeppesen PB, Nordentoft I, Hermansen K. Stevioside counteracts the glyburide-induced desensitization of the pancreatic beta-cell function in mice: studies in-vitro. Metabolism. 2006;55:1674–80. doi: 10.1016/j.metabol.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Abudula R, Jeppesen PB, Rolfsen SE, Xiao J, Hermansen K. Rebaudioside A potentially stimulates insulin secretion from isolated mouse islets: Studies on the dose-, glucose-, and calcium-dependency. Metabolism. 2004;53:1378–81. doi: 10.1016/j.metabol.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 40.Dyrskog SE, Jeppesen PB, Chen J, Christensen LP, Hermansen K. The diterpene glycoside, Rebaudioside A, does not improve glycemic control or affect blood pressure after eight weeks treatment in the Goto-Kakizaki rats. Rev Diabet Stud. 2005;2:84–91. doi: 10.1900/RDS.2005.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abudula R, Matchkov VV, Jeppesen PB, Nilsson H, Aalkjaer C, Hermansen K. Rebaudioside A directly stimulates insulin secretion from pancreatic beta cells: A glucose-dependent action via inhibition of ATP-sensitive K+-channels. Diabetes Obes Metab. 2008;10:1074–85. doi: 10.1111/j.1463-1326.2008.00864.x. [DOI] [PubMed] [Google Scholar]
- 42.Gardana C, Simonetti P, Canzi E, Zanchi R, Pietta P. Metabolism of stevioside and rebaudioside A from Stevia rebaudiana extracts by human microflora. J Agric Food Chem. 2003;51:6618–22. doi: 10.1021/jf0303619. [DOI] [PubMed] [Google Scholar]
- 43.Geuns JM. 2007. [Last assessed on 2009 Nov 18]. Steviol glycosides as food additive, Summary of new applications by eustas Maladeta, no. 20, 22300 Barbastro, Huesca, Spain: European Stevia Association. Available from: http://www.eustas.org/Steviol_glycosides_summary_application.pdf . [Google Scholar]
- 44.Koyama E, Sakai N, Ohori Y, Kitazawa K, Izawa O, Kakegawa K, et al. Absorption and metabolism of glycosidic sweeteners of stevia mixture and their aglycone, steviol, in rats and humans. Food Chem Toxicol. 2003;41:875–83. doi: 10.1016/s0278-6915(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 45.Melis MS, Rocha ST, Augusto A. Steviol effect, a glycoside of Stevia rebaudiana, on glucose clearances in rats. Braz J Biol. 2009;69:371–4. doi: 10.1590/s1519-69842009000200019. [DOI] [PubMed] [Google Scholar]


