Abstract
Introduction:
1,2,4-triazoles and its derivatives have been reported to possess anti-inflammatory, analgesic, antimicrobial, anticancer, antitumor, antitubercular, anticonvulsant, openers of Ca-activated potassium (Maxi-K) channels, antiviral properties, hypoglycemic, anxiolytic and antidepressant activity. Therefore, 1,2,4-triazole seems to be an important pharmacophore.
Materials and Methods:
The synthesis of 4-(substituted ethanoyl) amino-3-mercapto-5-(4-methoxy) phenyl-1,2,4-triazoles (6a-o) were prepared following six step starting 4-methoxy benzoic acid and using different secondary amines and were characterized with the help of FT-IR,1H,13C NMR, FAB Mass and nitrogen analysis. These synthesized compounds (6a-o) were then evaluated for anti-inflammatory activity by carrageenan induced paw edema method.Out of these synthesized compounds, some (6f, i and k) were evaluated for antinociceptive activity by Hot plate method and Tail immersion method.
Results and Discussion:
The synthesis of 4-(substituted amino)-3-mercapto-5-(4-methoxy) phenyl-1,2,4-triazoles (6a-o) was accomplished. The IR spectra exhibited characteristic bands for C-N, C=N, SH and C=O at 1350-1360, 1511-1548, 2520-2594.3 and 1650-1719 cm-1. The C-O-C asymmetric and symmetric str. was at 1250-1254 and 1027-1079.3 cm-1 respectively. In1H-NMR spectra, a singlet of CONH was found in the range of δ 9.92-10.18 ppm and another singlet of thiol group was observed in the range of δ 8.63-9.92 ppm. A singlet of Ar-OCH3 was also found between δ 3.57-3.91 ppm. In13 C- NMR spectra, C-3 and C-5 of the 1,2,4 - triazole nucleus were observed in the range of δ 147-166.9 ppm. Carbonyl carbon and methylene carbon of –NHCOCH2 N< were found between δ 166.5-177.5 and δ 47.1-62 ppm respectively. Acute toxicity study was donr following OECD-423 and cut-off dose was found to be between 1000-1500 mg/kg body weight. At the dose level of 100 mg/kg, 6f, 6i and 6k exhibited appreciable inhibition of oedema especially 6k exhibiting a percentage of oedema inhibition of 40.28%, which was comparable to that of the standard drug indomethacin (62.50% at 10mg/kg dose). Among the compounds tested, compound 6k exhibited good anti-nociceptive activity in both methods used. Pethidine (20mg/kg body weight s.c) is used as the standard drug.
Conclusion:
SAR of these synthesized compounds shows that substitution with heterocyclic moiety at C-2 of the acetamido group at position 4 of the 1,2,4-triazole produces appreciable activity as compared to substitution with aliphatic moieties since among all the synthesized compounds, the most active ones are 6f, 6i and 6k that have piperdine, 1-benzyl piperazine and morpholine group, respectively at C-2 of the acetamido group at position 4 of the 1,2,4-triazole.
Keywords: 1,2,4-Triazoles; anti-inflammatory; antinociceptive; cyclization
1,2,4-Triazole is a five-membered ring structure composed of three nitrogen atoms and two carbon atoms and is present in different medicinal agents like fluconazole, itraconazole, voriconazole, posaconazole, ravuconazole (as antifungal agents), rilmazafone (anxiolytic) trazadone, nafazodone (as antidepressant), triazolam (sedative and hypnotic), anastrozole (Estrogen synthesis inhibitor), trapidil (antihypertensive), banzel (antiepileptic), and ribavirin (antiviral).[1] 1,2,4-triazole and its derivatives have been reported to possess anti-inflammatory, analgesic,[2–5] antimicrobial,[6,7] anticancer,[8] antitumor,[9] antitubercular,[10] and anticonvulsant characteristics;[11] they are openers of Ca-activated potassium (Maxi-K) channels,[12] have antiviral properties,[13,14] and hypoglycemic,[15] anxiolytic,[16] and antidepressant activities.[17] Therefore, 1,2,4-triazole seems to be an important pharmacophore. Thus our research is focused on 1,2,4-triazole moiety.
Therefore, it was envisaged that different substitutions on the 1,2,4-triazole ring would result in compounds of interesting biological activities. In view of these findings, we have attempted to incorporate different secondary amines on the 1,2,4-triazole ring, to obtain the title compounds, to evaluate them for their anti-inflammatory and analgesic activities.
Materials and Methods
All the solvents were of LR grade and were obtained from Merck, Rankem, Fluka, and S.D. Fine Chemicals. The melting points were determined in open capillary tubes and were uncorrected. The purity of the synthesized compound was confirmed by thin layer chromatography (TLC) using silica gel G (Merck) in a developing solvent system of ethyl acetate and petroleum ether (1 : 1) and the spots were visualized with the help of iodine vapors or sulfuric acid (30% v/v).
Infrared spectra (KBr disk) were recorded on a Nicolet-6700 FT-IR spectrophotometer, using potassium bromide pellets in the range of 4000 – 400 cm-1.
1H and13C NMR spectra were scanned on a Brucker Aviance II 400 MHz spectrophotometer (Brucker, USA). Chemical shifts were expressed in parts per million (δ) units, relative to an internal standard of tetramethylsilane, using DMSO-d6/CDCl3 as solvents.
The mass spectra were recorded on Jeol Sx 102 / DA-600 mass spectrometer / Data System using the fast atom bombardment (FAB) technique. Nitrogen analysis of the newly synthesized compounds was performed using an elemental analyzer Elementar Vario EL III Carlo Erba 1108. Nitrogen analysis of all the compounds were in agreement with the calculated values.
Anti-inflammatory and analgesic activities were performed in the animal house of the Pharmaceutical Sciences Department, Dr. H.S. Gour University, Sagar (M.P.), and the experimental protocol was approved by the University Animal Ethical Committee of Dr. H. S. Gour University, Sagar (M.P.)-India. (379/01/ab/CPCSEA).
Chemistry
The synthesis of 4-(substituted ethanoyl) amino-3-mercapto-5-(4-methoxy) phenyl-1,2,4-triazoles (6a-o) was prepared by following stepwise procedures, as mentioned in Figure 1.
Figure 1.
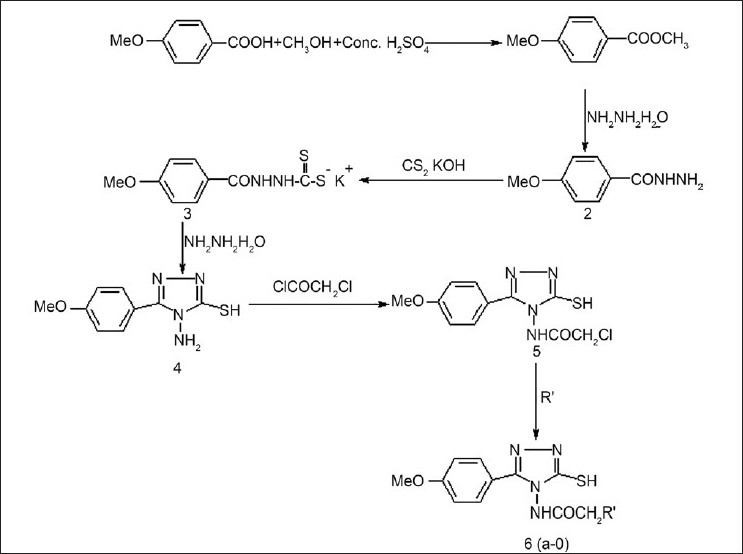
Scheme for the synthesis of compounds (6a-o)
Synthesis of methyl ester of 4-methoxy-benzoic acid (1)
The required amount of 4-methyl benzoic acid (0.1 mol) was placed in methanol (100 mL) in a round bottom flask and concentrated sulfuric acid (5.7 mL) was added to it. The mixture was refluxed for four to six hours. Excess methanol was then distilled off. After cooling, the contents were transferred to a separating funnel containing distilled water (100 mL). The synthesized ester was repeatedly extracted several times with carbon tetrachloride (30 mL). The combined organic layers were washed with a solution of sodium-bi-carbonate (20%) to remove any unreacted acid. After washing with distilled water the organic layer was dried over anhydrous MgSO4. Carbon-tetrachloride was then distilled off, under reduced pressure, to get the ester, which was recrystallized from absolute alcohol.
Synthesis of acid hydrazide (2)
Hydrazine hydrate (99%) (5.7 mL, 0.15 mol) was placed in a flat bottom flask and a solution of methyl ester ( 1 , 0.1 mol, 16.6 g) in ethanol was added dropwise, with gentle stirring. After complete addition, the mixture was transferred into a round bottomed flask and refluxed for four to six hours. Ethanol was distilled off under reduced pressure. The precipitate of acid hydrazide was filtered off and recrystallized from ethanol.
Synthesis of potassium 3-aroyl dithiocarbazate (3)
A mixture of aroyl hydrazide ( 2 , 0.1 mol, 16.6 g) and potassium hydroxide (0.15 mol, 8.4 g) in absolute ethanol (100 mL) was treated with carbon disulfide (0.15 mol, 9.06 mL). This mixture was diluted with absolute ethanol (75 mL) and stirred for 12 – 16 hours at room temperature. The solvent was distilled off under reduced pressure. The salt prepared, as described earlier in the text, was obtained in nearly quantitative yield and was employed without further purification.
Synthesis of 4-amino-3-mercapto-5-(4-methoxy) phenyl-1,2,4-triazole (4)
A suspension of potassium salt ( 3 ) (0.1 mol, 28 g), hydrazine hydrate 99% (0.2 mol, 9.72 mL), and water (6 mL), in absolute alcohol was refluxed for two to three hours. The color of the reaction mixture changed to green, with evolution of hydrogen sulfide gas, and a homogenous solution resulted. Cold distilled water (100 mL) was added and the solution was acidified with concentrated hydrochloric acid. The precipitated solid was filtered, washed with two portions of cold water, each of 30 mL and recrystallized from aqueous ethanol (50%).
Synthesis of 4-chloroacyl amino-3-mercapto-5-(4-methoxy) phenyl 1,2,4-triazole (5)
A solution of compound 4-amino-3-mercapto-5-(4-methoxy) phenyl-1,2,4-triazole ( 4 , 0.1 mol, 22.2 g) in dioxane (50 mL) was placed in a two necked round bottom flask fitted with a reflux condenser and a separating funnel. A solution of chloroacetyl chloride (0.11 mol, 8.75 mL) in dioxane (25 mL) was placed in a separating funnel and added portion-wise to the vessel. After complete addition, the contents of the flasks were refluxed for an hour. After cooling, the contents were poured on crushed ice. The precipitated product was filtered and washed several times with ice / cold distilled water.
Synthesis of 4-(Substituted ethanoyl)amino-3-mercapto-5-(4-methoxy)phenyl-1,2,4-triazoles (6a-o)
Compound (5), (0.025 mol, 29.8 g) and the respective amine (0.030 mol) along with triethylamine (0.030 mol, 4.18 mL) in benzene (75 mL) were placed in a round bottomed flask. The contents were refluxed for three to four hours. The precipitated triethylamine hydrochloride was separated out. The organic layer was washed several times with distilled water to remove the last traces of hydrochloride. Benzene was distilled off under vacuum and the crude product was separated. Purification of the compounds was done by repeated crystallization of the appropriate solvents.
Acute toxicity study
This involves the estimation of the median lethal dose (LD50), which is the dose that will kill 50% of the animal population within 24 hours post treatment with the test substance. All animal experiments were conducted under the conditions of the Animal Scientific Procedures and as per the OECD-423 Guidelines.[18] The experimental protocol was approved by the University Animal Ethical Committee of Dr. H. S. Gour University, Sagar (M.P.), India. (379/01/ab/CPCSEA). Groups of Swiss albino mice consisted of three animals and were maintained in colony cages at 25 ± 2°C, relative humidity of 50 – 60%, under a 12 hour light and dark cycle; they were fed the standard animal feed and water ad libitum.
When there is no information on a substance to be tested, for animal welfare reasons, it is recommended to use the starting dose of 300 mg/kg body weight. The animals were given the lowest dose of 300 mg/kg of the compounds at the first instance. Then the animals were observed for three days. They were treated orally with different doses of tested compounds (200, 400, 600, 800, 1000, 2000, 2500 mg/kg-1). The animals were then observed for 24 hours for any behavioral effects such as nervousness, excitement, dullness, in-coordination or even death.
Anti-inflammatory activity
The anti-inflammatory activity was evaluated by the carrageenan-induced, paw edema method.[19] Albino rats of Wistar strains, weighing 100 – 200 g, of either sex, were divided into seventeen groups of six animals each. The animals were maintained under normal environmental conditions. They were fed the standard feed, with water ad libitum. To each group of six animals, with the exception of the control group, the tested compounds (100 mg/kg of body weight) were administered orally (p.o.). The control group received an equivalent amount of CMC. To one group the standard drug Indomethacin (10 mg/kg) was administered. After one hour, carrageenan (0.1 mL, 1% w/v solution in sterile saline) was injected into the subplantar tissue of the left paw of all the animals. The right paw served as the reference non-inflamed paw for comparison. The initial paw volume was measured using a plethysmograph within 30 seconds of the injection. After three hours, the final paw volume of each animal was measured. The percentage of reduction in the paw volume was calculated by subtracting the difference between the right and left hind paw volumes in the treated group from the difference in the control group and dividing it by the difference in the control group. The anti-inflammatory activity of the tested compounds and the standard reference drug was determined by using the formula, % anti-inflammatory activity = (1-Vt/Vc) × 100, where Vt represented the mean increase in paw volume of rats treated with test compounds and Vc represented the mean increase in paw volume in the control group of rats.
Antinociceptive activity
Out of all the synthesized compounds evaluated for anti-inflammatory activity, some of the compounds that exhibited anti-inflammatory activity higher than 37% (6f, 6i, and 6k) were further tested for their antinociceptive activity at the same dose used for anti-inflammatory activity.
Hot plate method
Some of the newly synthesized compounds (6f, 6i, and 6k) were tested for antinociceptive activity by using the Eddy and Leimbach method.[20] Swiss albino mice were divided into five groups of six mice each. One group served as the control and was administered 1% w/v CMC, i.p., another group was used as a standard and received pethidine solution 20 mg/kg body weight, s.c., while the other groups received 100 mz/kg body weight of tested compounds i.p., suspended in 1% CMC. The reaction time in seconds was noted for all the groups on Eddy's hot plates, 15, 30, 60, and 120 minutes after the administration of the drug.
Tail immersion method
The synthesized compounds were also tested for antinociceptive activity by the tail immersion method.[21] The animals were divided in groups as per the Hot plate method. After intraperitoneal injection of the test substance, each mouse was placed in house made mouse holder and the tail was dipped in a water bath at 55 ± 0.5°C. The actual flick response of the mice, that is, time taken in seconds to withdraw it from the hot water source was noted and compared with that of the standard drug. The experiments were carried out after 15, 30, 60, and 120 minutes of drug administration.
Statistical analysis
All the statistical analyses were carried out using the software GraphPad InStat 3.0 using ANOVA, followed by the Dunnet's multiplet comparison test, and the results were expressed in Mean ± SEM.
Results and Discussion
In the present investigation, we have synthesized a series of novel 4-(substituted amino)-3-mercapto-5-(4-methoxy)-1,2,4-triazoles using 4-methoxy benzoic acid as the starting material. This acid is converted into the respective ester. Then the ester is converted into hydrazide, hydrazine into the potassium salt, and then into the 1,2,4-triazole. This 1,2,4-triazole is acylated with chloroacetyl chloride and then this acyl derivative is reacted with different secondary amines to get the tested compounds. [Figure 1]. The compounds are evaluated in vivo sfor their anti-inflammatory activity by the carrageenan-induced paw edema method, and some of the compounds (6f, 6i, and 6k) are evaluated in vivo for the antinociceptive activity by the hot plate and tail immersion methods.
Chemistry
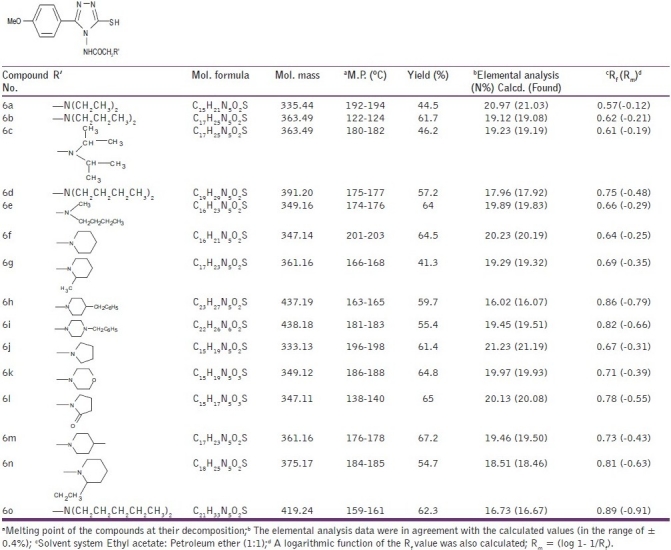
The synthesis of 4-(substituted amino)-3-mercapto-5-(4-methoxy) phenyl-1,2,4-triazoles (6a-o) was accomplished as presented in Scheme-1. It involves the preparation of 4-amino-3-mercapto-5-(4-methoxy) phenyl-1,2,4-triazole and then its reaction with chloroacetyl chloride in dioxane, to give acyl derivative. This acyl triazole (5) was reacted with different secondary amines to give the title compounds (6a-o). The synthesized compounds were characterized by elemental analysis, IR,1H NMR,13C NMR, and mass spectra. The IR spectra exhibited characteristic bands for C-N, C = N, SH, and C = O, at 1350 – 1360, 1511 – 1548, 2520 – 2594.3, and 1650 – 1719 cm-1. The C-O-C asymmetric and symmetric str. was at 1250 – 1254 and 1027 – 1079.3 cm-1 , respectively. In1H-NMR spectra, a singlet of CONH was found in the range of δ 9.92 – 10.18 ppm and another singlet of the thiol group was observed in the range of δ 8.63 – 9.92 ppm. A singlet of Ar-OCH3 was also found between δ 3.57 – 3.91 ppm. In13C- NMR spectra, C-3 and C-5 of the 1,2,4 - triazole nucleus were observed in the range of δ 147 – 166.9 ppm. Carbonyl carbon and methylene carbon of -NHCOCH2 N < were found between δ 166.5 and 177.5 and δ 47.1 and 62 ppm, respectively. A solvent peak of DMSO-d6 was observed at 44 ppm. Analytical and spectral data were in good agreement with the composition of the synthesized compounds and the data are given in Table 1.
Table 1.
Spectral characterization of the synthesized compounds (6a-o)

The physicochemical properties of the titled compounds are presented in Table 2.
Table 2.
Physicochemical properties of the synthesized compounds (6a-o)
Acute toxicity study
As per the OECD Guidelines-423 (Acute toxic classic method) LD50 was calculated for all the synthesized compounds and the cut-off dose was found to be between 1000 and 1500 mg/kg body weight.
Anti-inflammatory activity
The pharmacological evaluation of the tested compounds (6a-o) was carried out as per the protocol specified. The anti-inflammatory activity of the synthesized compounds was carried out using the carrageenan-induced rat paw edema method. The anti-inflammatory activity data for the compounds is given in Table 3.
Table 3.
Anti-inflammatory data of the title compounds (6a-o)
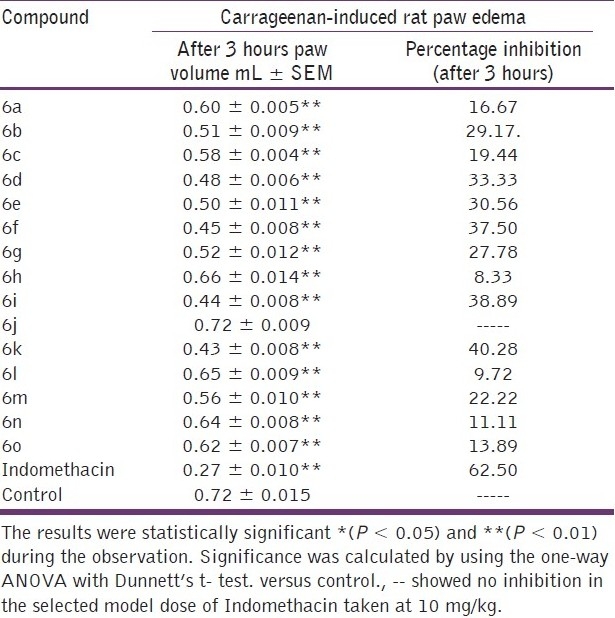
At the dose level of 100 mg/kg, 6f, 6i, and 6k exhibited appreciable inhibition of edema, especially 6k, which exhibited a percentage of edema inhibition of 40.28%, which was comparable to that of the standard drug indomethacin (62.50% at 10 mg/kg dose). Among the compounds tested, compounds 6b, 6d, 6g, and 6m exhibited mild anti-inflammatory activity.
Antinociceptive activity
Out of all the synthesized compounds evaluated for anti-inflammatory activity, some of the compounds that exhibited anti-inflammatory activity higher than 37% (6f , 6i, and 6k ) were further tested for their antinociceptive activity at the same dose used for the anti-inflammatory activity. The hot plate method [Table 4] and tail immersion method [Table 5] were used for evaluation of the synthesized compounds.
Table 4.
Evaluation of antinociceptive activity of title compounds (6f, I, and k) by the hot plate method
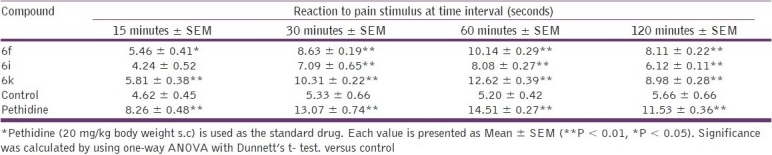
Table 5.
Evaluation of the antinociceptive activity of the title compounds (6f, i, and k) by the tail immersion method
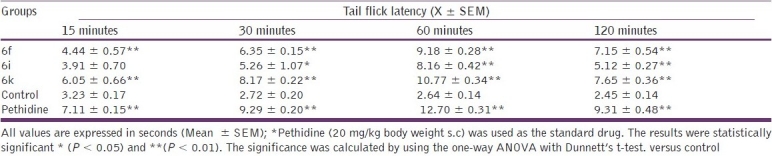
Among the compounds tested, compound 6k exhibited good antinociceptive activity in both the methods used. Pethidine (20 mg/kg body weight s.c) was used as the standard drug.
Conclusion
In the present study, synthesis of a new series of 4-(substituted amino)-3-mercapto-5-(4-methoxy) phenyl-1,2,4-triazoles (6a-o) has been described. Out of these compounds 6f, 6i, and 6k were found to be the most active synthesized compounds, with significant anti-inflammatory activity. Out of these compounds the most potent active derivative was 6k, with promising antinociceptive activity. The Structure Activity Relationship (SAR) of these synthesized compounds showed that substitution with a heterocyclic moiety at C-2 of the acetamido group, at position 4 of the 1,2,4-triazole, produced appreciable activity as compared to substitution with aliphatic moieties, as among all the synthesized compounds, the most active ones were 6f, 6i, and 6k, which had the piperdine, 1-benzyl piperazine, and morpholine groups, respectively, at the C-2 of the acetamido group, at position 4 of the 1,2,4-triazole. The other compounds 6b, 6d, and 6e that had di-propyl amino, di-butyl amino, and N-butyl methyl amino groups at C-2 of the acetamido group, produced moderate anti-inflammatory activity. Another structural feature of all the synthesized compounds was that these also had a free thiol group at C-3 of the 1,2,4-triazole. Out of all these synthesized compounds, the most promising compound was 6k, which showed not only appreciable anti-inflammatory activity, but also antinociceptive activity.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Kharb R, Sharma PC, Yar MS. Pharmacological significance of triazole scaffold. J Enzyme Inhib Med Chem. 2010;26:1–21. doi: 10.3109/14756360903524304. [DOI] [PubMed] [Google Scholar]
- 2.Navidpour L, Shafaroodi H, Abdi K, Amini M, Ghahremani MH, Dehpour AR, et al. Design, synthesis, and biological evaluation of substituted 3-alkylthio-4,5-diaryl-4H-1,2,4-triazoles as selective COX-2 inhibitors. Bioorg Med Chem. 2006;14:2507–717. doi: 10.1016/j.bmc.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 3.Palaska E, Sahin G, Kelicen P, Durlu NT, Altinok G. Synthesis and anti-inflammatory activity of 1-acylthiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4- thiadiazoles and 1,2,4-triazole-3-thiones. IL Farmaco. 2002;57:101–7. doi: 10.1016/s0014-827x(01)01176-4. [DOI] [PubMed] [Google Scholar]
- 4.Amir M, Kumar H, Javed SA. Condensed bridgehead nitrogen heterocyclic system: Synthesis and pharmacological activities of 1,2,4-triazolo-[3,4-b]-1,3,4-thiadiazole derivatives of ibuprofen and biphenyl-4-yloxy acetic acid. Eur J Med Chem. 2008;43:2056–66. doi: 10.1016/j.ejmech.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Turan-Zitouni G, Sιvacι M, Kιlιc F, Erol K. Synthesis of some triazolyl-antipyrine derivatives and investigation of analgesic activity. Eur J Med Chem. 2001;36:685–9. doi: 10.1016/s0223-5234(01)01252-1. [DOI] [PubMed] [Google Scholar]
- 6.Turan-Zitouni G, Kaplancιklι ZA, Yιldιz MT, Chevallet P, Kaya D. Synthesis and antimicrobial activity of 4-phenyl/cyclohexyl-5-(1-phenoxyethyl)-3-[N-(2-thiazolyl)acetamido]thio-4H-1,2,4-triazole derivatives. Eur J Med Chem. 2005;40:607–13. doi: 10.1016/j.ejmech.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Ezabadi IR, Camoutsis C, Zoumpoulakis P, Geronikaki A, Sokovic M, GlamocČilijad J, et al. Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg Med Chem. 2008;16:1150–61. doi: 10.1016/j.bmc.2007.10.082. [DOI] [PubMed] [Google Scholar]
- 8.Holla BS, Veerendra B, Shivananda MK, Poojary B. Synthesis characterization and anticancer activity studies on some Mannich bases derived from1,2,4-triazoles. Eur J Med Chem. 2003;38:759–67. doi: 10.1016/s0223-5234(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 9.Demirbaş N, Ugurluoglu R, Demirbaş A. Synthesis of 3-alkyl(Aryl)-4-alkylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-ones and 3-alkyl-4- alkylamino-4,5-dihydro-1H-1,2,4-triazol-5-ones as antitumor agents. Bioorg Med Chem. 2002;10:3717–23. doi: 10.1016/s0968-0896(02)00420-0. [DOI] [PubMed] [Google Scholar]
- 10.Shiradkar M, Kumar GV, Dasari V, Tatikonda S, Akula KC, Shah R. Clubbed triazoles: A novel approach to antitubercular drugs. Eur J Med Chem. 2007;42:807–16. doi: 10.1016/j.ejmech.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Küçükgüzel I, Küçükgüzel ŞG, Rollas S, Sanιs GÖ, Özdemir O, Bayrak I, et al. Synthesis of some 3-(Arylalkylthio)-4-alkyl/aryl-5-(4-aminophenyl)-4H-1,2,4-triazole derivatives and their anticonvulsant activity. IL Farmaco. 2004;59:893–901. doi: 10.1016/j.farmac.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Romine JL, Martin SW, Gribkoff VK, Boissard CG, Dworetzky SI, Natale J, et al. 4,5-Diphenyltriazol-3-ones: Openers of large-conductance Ca 2+ activated potassium (Maxi-K) channels. J Med Chem. 2002;45:2942–52. doi: 10.1021/jm010569q. [DOI] [PubMed] [Google Scholar]
- 13.Kritsanida M, Mouroutsou A, Marakos P, Pouli N, Papakonstantinou-Garoufalias S, Pannecouque C, et al. Synthesis and antiviral activity evaluation of some new 6-substituted 3-(1-adamantyl)-1,2,4- triazolo[3,4-b][1,3,4]thiadiazoles. IL Farmaco. 2002;57:253–7. doi: 10.1016/s0014-827x(01)01189-2. [DOI] [PubMed] [Google Scholar]
- 14.Witkowski JT, Robins RK, Sidwell RW, Simon LN. Design, synthesis and broad spectrum antiviral activity of 1-beta-D ribofuranosyl-1,2,4-triazole-3-carboxamide and related nucleosides. J Med Chem. 1972;15:1150–4. doi: 10.1021/jm00281a014. [DOI] [PubMed] [Google Scholar]
- 15.Deliwala CV, Mhasalkar MY, Shah MH, Nikam ST, Anantanarayanan KG. Further studies in substituted 4H-1,2,4-triazoles for possible hypoglycemic activity. J Med Chem. 1971;14:260–2. doi: 10.1021/jm00285a029. [DOI] [PubMed] [Google Scholar]
- 16.Gall M, Lahti RA, Rudzik AD, Duchamp DJ, Chidester C, Scahill T. Novel anxiolytic agents derived from alpha-amino-alpha-phenyl-o-tolyl-4H-triazoles and imidazoles. J Med Chem. 1978;21:542–8. doi: 10.1021/jm00204a008. [DOI] [PubMed] [Google Scholar]
- 17.Kane JM, Dudley MW, Sorensen SM, Miller FP. 2,4-Dihydro-3-H-1,2,4-triazol-3-ones as potential antidepressant agents. J Med Chem. 1988;31:1253–8. doi: 10.1021/jm00401a031. [DOI] [PubMed] [Google Scholar]
- 18.Ecobichon DJ. The Basis of Toxicology Testing. New York: CRC Press; 1997. pp. 43–86. [Google Scholar]
- 19.Winter CA, Risley EA, Nuss GW. Carrageenan-induced oedema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 20.Eddy NB, Leimbach D. Synthetic analgesics: II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther. 1953;107:385–93. [PubMed] [Google Scholar]
- 21.Ben-Bassat J, Peretz E, Sulman FG. Analgesimetry and ranking of analgesic drugs by the receptacle method. Arch Int Pharmacodyn. 1959;122:434–47. [PubMed] [Google Scholar]


