Abstract
Background:
Ophthalmic drug delivery systems are the challenging subject for the researchers because of delicate nature of ocular membrane and preventive barriers leading to less than 1 % of Bioavailability. Reasons for reduced bioavailability are due to rapid pre corneal elimination, tear turnover, lacrimal drainage, blinking and degradation by enzymes. Less bioavailability causes short duration of action and increased frequency of administration.
Materials and Methods:
Timolol maleate was used as model drug. Dynamic drug release studies were used to study the polymeric hydrogels and ophthalmic inserts. Rheological studies were carried out by Brookfield Viscometer LVDV- II+.
Result and Discussion:
Viscosity value lies in the range of 4.08 to 31.8 cps. Drug release data was fitted to various kinetic equations such as First order plots, Higuchi plots, Peppa's exponential plots. The results shows fairly linear curve and the slope value of the Peppa's equation is less than 0.5 and hence follows the fickian diffusion.
Conclusion:
The developed hydrogels and inserts were therapeutically effacious, stable, non irritant and provide a sustained release of drug over 8 hours time period.
Keywords: Polymeric hydrogel, ophthalmic inserts, timolol maleate, ophthalmic deliver
Although it is commonly believed that the innovation of new drug delivery systems is of paramount importance for improving the health and quality of life of patients, there is also a keen recognition regarding upward-spiraling costs of innovation, drug discovery, and drug development against a backdrop of dwindling successes in research and development efforts.
Within the last few decades, in response to the advent of potent and versatile therapeutic agents, the diversity of conventional ophthalmic formulations has gradually evolved, extending well beyond simple solutions, and now includes a variety of types of drug administration.[1]
In the ophthalmic drug-delivery systems, the physiological constraints imposed by the protective mechanism of the eye lead to the low absorption of drugs resulting in the short duration of action.[2] The bioavailability of traditional ocular drug delivery systems such as eye drops is very poor because eye is protected by a series of complex defense mechanisms that make it difficult to achieve an effective drug concentration within the target area of the eye.
Barriers in effective ophthalmic drug delivery
The physiological barriers imposed by the protective mechanism of the eye lead to the low absorption of drugs and results in a short duration of therapeutic action. A high frequency of the eye drops instillation is associated with patient's noncompliance.[3] After the instillation of eye drop into the eye cavity, the effective tear drainage and blinking action of eye results in a 10 times reduction in the drug concentration within 4 to 20 minutes.[4] Due to the tear drainage, most of the administered dose passes through nasolacrimal duct into the gastrointestinal tract leading to the side effects. Rapid elimination of the eye drops often results in a short duration of the therapeutic effect. The normal volume of tear in the eye is 7 μl; although a nonblinking eye can accommodate a maximum of 30 μl of the fluid, blinking eye can hold only 10 μl, both normally and externally added solution are rapidly drained from eye. The usual single drop size of an instilled drug solution is up to 50 μl and thus most of the drug instilled as eye drop is lost.
Novel innovations and optimizations
Ophthalmic therapy can be improved by altering corneal residence time of drug. Several new drug delivery systems[5] are widely used, such as ocular inserts,[6] collagen shields,[7] etc. These systems are able to prolong the contact time of vehicle on the ocular surface and also slow down drug elimination. However, these systems are having some disadvantage such as poor compliance, uncomfort, especially by the elderly people, and many patients sometimes lose it without noticing it.
Many approaches have been developed to solve the problem in recent decades, of which colloidal drug delivery system has been paid much attention. For example, sol to gel phase transition on ocular surface such as temperature-dependent concept (pluronics),[8,9] pH-triggered systems (including cellulose acetate hydrogen phthalate latex,[10,11] Carbopols),[12] Ion-activated systems including Gelrite,[13] Gellan,[14] and Carbopol/pluronics,[15] liposome and emulsion are reported as effective ocular drug carries.[16–19] But they still have many disadvantages like expensive excipients and poor stability.
Nanostructured lipid carrier (NLC) was developed in early 1990s; now, it is a good alternative carrier of traditional colloidal-controlled release drug delivery system.[20] NLC is composed of a solid lipid matrix with certain content of liquid lipid; it has many advantages such as good biocompatibility due to the use of physiological and biodegradable lipids of low systemic toxicity, possibility of production on large industrial scale, and reduction of drug leakage during storage. Another approach is to increase the transcorneal passage of drugs by incorporating permeation enhancers into formulations.[21]
This problem can be overcome by using hydrogel systems and ophthalmic inserts. Hydrogel is a network of polymer chains that are water-insoluble, sometimes found as a colloidal gel in which water is the dispersion medium. Hydrogels are superabsorbent (they can contain more than 99% water) natural or synthetic polymers. Hydrogels possess also a degree of flexibility very similar to natural tissue, due to their significant water content.[22] Hydrogel systems can be formulated in liquid phase suitable to be administrated by instillation into the eye cavity and which upon exposure to the stimuli such as pH, temperature, ion activation etc., changes to the gel phase and thus improves the residence time and corneal bioavailability of the drug.
The objective of the present work was to develop a novel drug delivery system with improved bioavailability, with reduction in frequency of administration by the help of hydrogels and ophthalmic insert of timolol maleate.
Materials and Methods
Materials
Timolol maleate is provided by the FDC pvt. ltd, Mumbai.
Carbopols 934p was provided by the Noveon polymers, Arihant trading Co. Mumbai. Viscolizers, i.e, Hydroxyl propyl methyl cellulose was made available by S.d fine chem. ltd, Biosar. Triethanolamine, ethyl cellulose, glycerine, polyvinylpyrrolidone, sodium hydroxide flakes; sodium chloride was provided by S.d fine chem. pvt. ltd. Mumbai. All the reagents were of the analytical grade.
Preparation of hydrogels
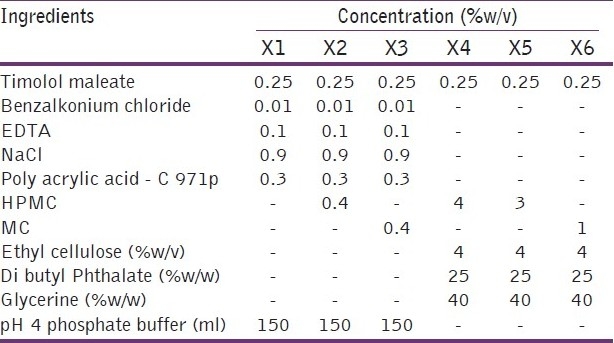
Hydrogels were prepared by using timolol maleate (antiglaucoma agent) along with some formulation additives such as preservatives, tonicity contributors, chelating agents, thickening agents, solvents, or buffers to attain the desirable qualities of the ophthalmic dosage forms. The ingredients included in the hydrogels such as the benzalkonium chloride (preservative), ethylenediaminetetraacetic acid (chelating agent), sodium chloride[23] (tonicity contributors), and viscolizer, i.e, hydroxypropyl methyl cellulose.
Weighed quantities [Table 1] of timolol maleate, benzalkonium chloride, EDTA, and NaCl were dissolved in the pH 4 phosphate buffer under aseptic conditions. Furthermore, polyacrylic acid was slowly added with continuous stirring at the speed of 1 500 to 2 000 rpm to minimize the formation of the undispersible lumps, then viscolizers was added with a slow stirring to avoid the foam formation. Stirring was continued until a clear dispersion was formed. The prepared hydrogels were evaluated for the viscosity study in order to identify the composition suitable for the use.
Table 1.
Composition details of hydrogels and ophthalmic inserts
Preparation of ophthalmic inserts
Accurately weighed quantity of HPMC was soaked in one-third volume of distilled water for 24 hours. Specified quantity of plasticizer was then dissolved in remaining amount of water. Both the solution was mixed together with the help of magnetic stirrer. 5 ml of resultant mixture was poured into each fabricated plastic ring of a specific area (25.53 cm2) placed on a mercury substrate.
Drying was carried out at 80°C for 24 hours in hot air oven. After the drying, resultant polymeric patches are cut into a diameter of 6 mm for different evaluation studies.
Formulation of rate-controlling membrane:- The ethyl cellulose was dissolved in one-third quantity of chloroform, dibutyl phthalate as a plasticizers in remaining chloroform, then mixed both the solutions, thoroughly to get a uniform dispersion. This solution was poured on mercury substrate and dried at room temperature for 24 hours.
After drying, 7-mm patches in diameter were cut out with the help of borer. Drug containing 6-mm patches were sandwiched between two pieces of 7-mm diameter of rate-controlling membrane and pasted with the help of chloroform.
Finally, ophthalmic inserts containing drug reservoir were entrapped between two layers of rate-controlling membrane. Each insert was containing a drug equivalent to the three drops of eye drop. Dose calculation was done on the basis of total area of patch having drug.
Evaluation of the hydrogels and ophthalmic inserts
Rheological studies for hydrogel
Viscosity determination of the prepared hydrogels were determined using Brookfields viscometer LVDV II+. The viscosity of the hydrogel was measured at different rpm (10, 20, 30, 50. 60, 100). The correct viscosity of the hydrogel was noted at particular spindle at which it shows maximum percent torque value [Table 2].
Table 2.
Viscosity details of the hydrogels at pH 4.0 and pH 7.4

The results demonstrate that at acidic pH 4, hydrogel were low viscous and at pH 7.4 (pH of eye cavity), it changes into a highly viscous preparation. The literature also suggests that the viscosity value in the range of 15 to 50 cps significantly improves the contact time of the formulation on the corneal surface and higher viscosity values offer no significant advantage and have a tendency to leave a noticeable residue on the lid margin.[24]
Evaluation of ophthalmic inserts
Thickness[25]
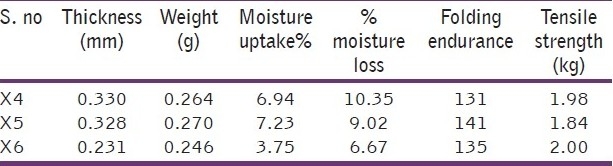
The thickness of the occusert was measured using screw gauge with a least count of 0.01 mm at different spots of the patches. The thickness was measured at five different spots of the patch and average was taken. Thickness was found to be in the range of 0.231 to 0.330 mm [Table 3].
Table 3.
Evaluation parameter of ophthalmic inserts
Weight measurement[25]
Five films were taken from each batch and their individual weights were determined by using electronic balance. Weight of ophthalmic insert was found to be in the range of 0.246 to 0.270 g [Table 3].
Moisture uptake[26]
The percentage moisture uptake test was carried out to check physical stability or integrity of occuserts. Occuserts were weighed and placed in a dessicator containing 100 ml of saturated solution of aluminium chloride and 79.5% humidity was maintained. After three days, the occuserts were taken out and reweighed, the percentage moisture uptake was calculated by using formula. Values were found to be in the range of 3.75 to 7.23 [Table 3].
![]()
Moisture loss[27]
The percentage moisture loss was carried out to check integrity of the film at dry condition. Occuserts were weighed and kept in a dessicator containing anhydrous calcium chloride. After 3 days, the occuserts were taken out and reweighed, the percentage moisture loss was calculated using the formula and values were found in the range of 6.67 to 10.35 [Table 3].
![]()
Folding endurance[28,29]
The flexibility of occuserts can be measured quantitatively in terms of what is known as folding endurance. Folding endurance of the patches was determined by repeatedly folding a small strip of the patch (approximately 2 x 2 cm) at the same place till it broke. The number of times patch could be folded at the same place, without breaking, gives the value of folding endurance. Ophthalmic inserts show the folding endurance in the range of 131 to 141 [Table 3].
Tensile strength
The tensile strength of occusert refers to tension or force required to tear off the patch apart into two places. This was determined with an instrument assembled in the laboratory. For this, both the ends of the patches were enclosed between two pairs of acrylic slides with the help of clamps. One pair of acrylic slides enclosed with the upper end of the patch was fixed to a metal stand; elongation can be conveniently observed with the travelling microscope. To the other pair of acrylic slides, a pan was suspended with the help of a wire loop. Strips of 6 cm in length and 1 cm in width were cut using a razor blade and stainless steel guide. This procedure was preferred to die cutting in order to avoid notching of the specimen. To small marking 4-cm apart and 1-m of each end of specimen that were made on sample with ink samples were than observed under microscope to detect flaws. Tensile strength was found in the range of 1.84 to 2.0 kg [Table 3].
Evaluation of hydrogel and ophthalmic inserts drug polymer interaction studies
Drug polymer interaction studies were carried out by infrared spectral analysis. Infrared spectra of timolol maleate pure drug were scanned by using Perkin elenmeyer 1600 FTIR, by a thin film method. The drug timolol maleate in its infrared spectrum exhibits a strong peak at 3 445 cm-1, indicating the presence of -OH group. The absorption due to the -NH group present molecule is supported by exhibition of a shoulder to the main peak around 2 100 cm-1 . The drug contain more than one C=N absorption present in the thiadiazole moiety of the heterocyclic ring system. When pure drug was formulated with the Carbopol 934p and viscolizers, the spectrum obtained for this formulated product exhibit a broad absorption peak from 3 050 to 3 500 cm-1, indicating the participation of the alkali hydroxyl in forming gel preparation. The increased viscosity leads to a broadening of peak [Figure 1]. The spectral data suggest that the intactness of the thiadiazole ring structure of the timolol maleate indicated by the absence of the additional peaks which confirm the opening of the thiadiazole ring is not taking place. Hence, the drug timolol maleate was not reacting with the polymers used in the formulation.
Figure 1.
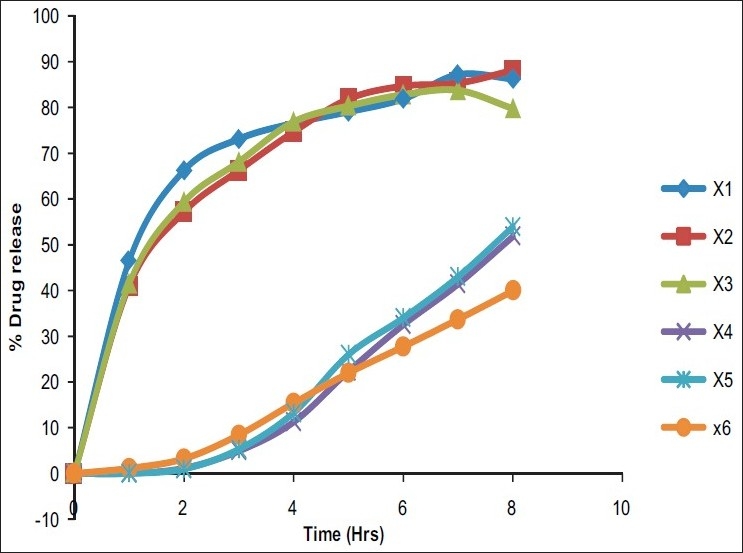
Comparative % drug release study
In vitro release study of timolol maleate
In vitro release rate of the timolol maleate from the sol-gel system for the corneal drug availability was determined by the diffusion process. 1 ml of the formulation was kept in the donor compartment over a cellophane membrane which was rinsed and soaked for 24 hours in the diffusion medium. The donor compartment was immersed in the receptor compartment containing 50 ml of the phosphate buffer of pH 7.4, the beaker containing diffusion medium (receptor compartment) was maintained at 370 C with the constant stirring at 22 rpm using the magnetic stirrer. 1 ml aliquots were withdrawn from the diffusion medium every hour for the 8 hours and same quantity of fresh, prewarmed diffusion medium was replaced for the amount withdrawn[30] [Table 3]. The samples withdrawn were analyzed spectrophotometrically at 294 nm for the timolol maleate using Shimazdu Double-beam UV-Visible spectrophotometer.[31] Drug release data [Tables 4 and 5] [Figure 1] were fitted to various kinetic equations such as Flux plots [Figure 2], First order plots, Higuchi plots and Peppa's exponential plots. The results show fairly linear curve and the slope value of the Peppa's equation is less than 0.5 and hence follows the Fickian diffusion.
Table 4.
Hydrogel and ophthalmic insert % drug release comparison
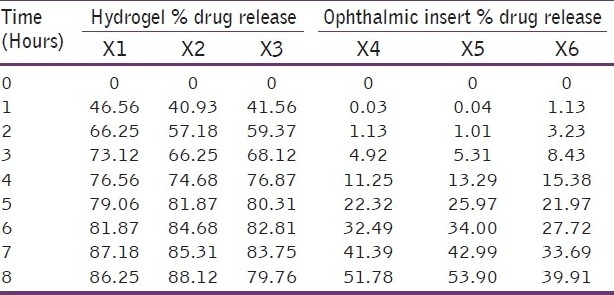
Table 5.
Comparison of hydrogel flux and ophthalmic insert flux
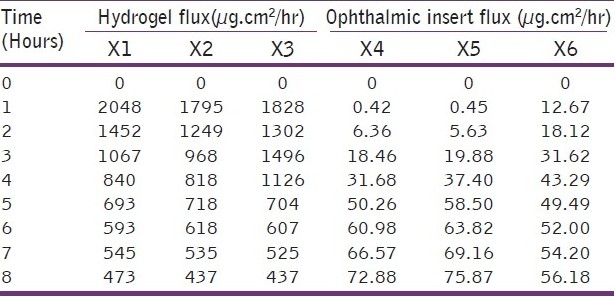
Figure 2.
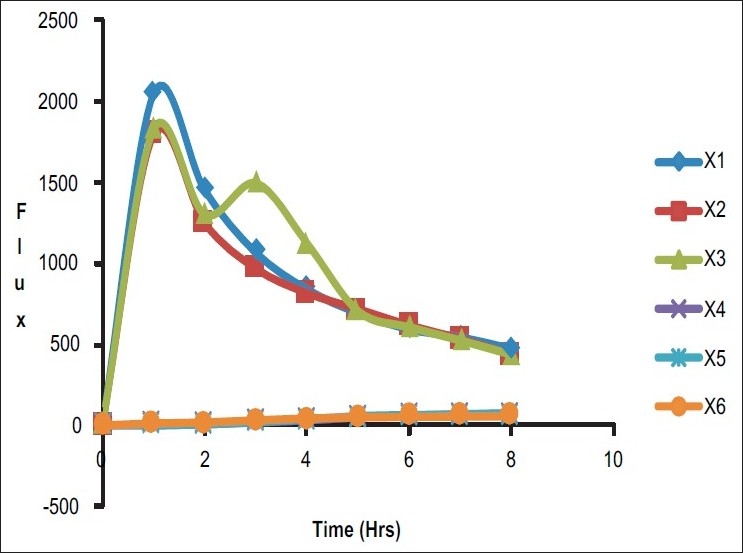
Comparative flux study
Sterility testing
The sterility testing of the ophthalmic drug delivery systems were performed for the aerobic bacteria, anaerobic bacteria, and fungi by using alternative thioglycolate medium and soybean casein digest medium. The positive control (growth promotion) and negative control (sterility) test were also carried out.[32] Bacillus subtilis was used as a test organism in case of aerobic bacteria test. Bacteroides vulgatus was used as a test organism in case of anaerobic bacteria test and Candida albicans in fungi test.
Incubation was carried in all cases and growth was checked. The overall results of the sterility test showed that ophthalmic formulation prepared passes the sterility test as there was no evidence of the growth found in the negative control test tubes. Thus, the hydrogels and inserts were found sterile in nature.
Results
Viscosity of the prepared hydrogels lies in the optimum range, i.e, 25 cps at the pH 4 and up to 50 cps at the pH 7.4. I.R studies show that there was no interaction of the thiadiazole ring, which shows that the drug and polymer was not reacting together. The hydrogel formed provide a sustained drug release up to 90% over an 8-hour period. All the formulation passes the test for the sterility.
In case of ophthalmic inserts, weight was observed and result lies in the range of 0.246 to 0.270 g. Moisture uptake values were found to be in the range of 3.75 to 7.23. Moisture loss values were found in the range of 6.67 to 10.35. Ophthalmic inserts show the folding endurance in the range of 131 to 141. Tensile strength was found in the range of 1.84 to 2.0 kg.
Drug release data were fitted to various kinetic equations such as First order plots, Higuchi plots, and Peppa's exponential plots. The results show fairly linear curve and the slope value of the Peppa's equation is less than 0.5 and hence follows the Fickian diffusion.
Conclusion
In the present research work, timolol maleate was successfully formulated in hydrogels and inserts. The hydrogels were liquid at pH 4 buffer and undergo rapid gelation after change in the pH 7.4. The hydrogels were evaluated for the several parameters like viscosity, drug-polymer interaction, in vitro drug release, sterility testing, and in vivo study. The hydrogels are safe, provide increased precorneal residence time, and are therapeutically efficacious. Similarly, ophthalmic inserts were also evaluated for % moisture loss, % moisture uptake, thickness, and tensile strength, and it was found that prolonged and sustained drug release was observed. Finally, in conclusion to our studies, we suggest that hydrogels are considered as a good choice of ophthalmic drug delivery because there is less chance of irritation during insertion and expulsion from eye cavity. Hydrogels are able to deliver increased flux, whereas insert provide very less flux.
Acknowledgments
Authors are thankful to the Luqman College of Pharmacy and HKE's college of Pharmacy, Gulbarga, Karnataka, for providing the help during this work. Authors are also thankful to Sardar Bhagwan Singh Post graduate Institute of Biomedical Sciences and Research, Balawala, Dehradun.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Babizhayev MA. Current ocular drug delivery challenges for N- acetylcarnosine: Novel Patented Routes and Modes of delivery, Design for enhancement of therapeutic activity and drug delivery relationship. Recent Pat Drug Deliv Formul. 2009;3:229–65. doi: 10.2174/187221109789105621. [DOI] [PubMed] [Google Scholar]
- 2.Singh V, Bushetti SS, Appala R, Shareef A, Imam SS, Singh M. Stimuli Sensitive hydrogels: A Novel Ophthalmic Drug Delivery System. Indian J Ophthalmol. 2010;58:477–81. doi: 10.4103/0301-4738.71677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh V, Bushetti SS, Appala R, Ahmad R, Singh M. Invitro and Invivo evaluation of stimuli sensitive hydrogel for ophthalmic drug delivery. Indian J Pharm EducRes. 2010;44:380–5. [Google Scholar]
- 4.Maurice DM. Kinetics of topical applied drugs. In: SaettoneMS, BucciP, Speiser P, editors. Ophthalmic drug delivery, Biopharmaceutical, technological and clinical aspects. Fidia research series. Vol. 11. Padova: Liviana press; 1987. pp. 19–26. [Google Scholar]
- 5.Ding Recent development in ophthalmicdrug delivery. Pharm Sci Technol Today. 1998;1:328–35. [Google Scholar]
- 6.Mishra DN, Gilhotra RM. Design and characterization of bioadhesive in-situ gelling ocular inserts ofgatifloxin sesquihydrate. DARU. 2008;16:328–35. [Google Scholar]
- 7.Hill JM, O‘Callaghan RJ, Hobden JA, Kaufman E. Controlled collagen shield for oculardelivery. In: MitraAK, editor. Ophthalmic drug deliverysystems. New York: Marcel Dekker; 1993. pp. 261–75. [Google Scholar]
- 8.Desai SD, Blanchard J. In vitroevaluation of pluronic F 127 based controlled release oculardeliverysystemfor the pilocarpine. J Pharm Sci. 1998;87:226–30. doi: 10.1021/js970090e. [DOI] [PubMed] [Google Scholar]
- 9.El-Kamel AH. Invitro and in vivo evaluationof pluronicF 127 basedoculardeliverysystembasedon the oculardelivery systemfor timolol maleate. Int J Pharm. 2002;241:47–55. doi: 10.1016/s0378-5173(02)00234-x. [DOI] [PubMed] [Google Scholar]
- 10.GurnyR Preliminary study of prolonged actingdrugdelivery systemfor the treatment of glaucoma. PharmActa Helv. 1981;56:130–2. [PubMed] [Google Scholar]
- 11.Gurny R, Boye T, Ibrahim H. Oculartherapy with nanoparticle systemfor controlleddrug delivery. JControl Rel. 1985;2:353. [Google Scholar]
- 12.Srividya B, CardozaRM, Amin PD. Sustained ophthalmicdelivery of the oflaxin froma phtriggered insitu gelling systems. JControl Rel. 2001;73:205–11. [Google Scholar]
- 13.Rosier A, Manuel C, Groove J, Plazonet B. Gelrite, a novelion activatedin situgelling polymers for ophthalmic vehicles, effect on bioavailability of timolol maleate. IntJ Pharm. 1989;57:163–8. [Google Scholar]
- 14.Sanzgiri YD, Maschi S, Crescenzi V, Callingaro L, Top EM, StellaVJ Gellanbasedsystemsforophthalmicsustained delivery of methyl prednisonolone. JControl Release. 1993;26:195–201. [Google Scholar]
- 15.Lin HR, Sung KC. Carbopol /pluronicphase change solution for the ophthalmicdrug delivery. J Control Release. 2000;69:379–88. doi: 10.1016/s0168-3659(00)00329-1. [DOI] [PubMed] [Google Scholar]
- 16.Assil KK, Frucht-Perry J, Ziegler E, Schanzlin DJ, Schneiderman T, Weinreb RN. Tobramycin liposomes, single subconjunctival therapy of pseudomonal keratitis. Invest Ophthalmol Vis Sci. 1991;32:3216–20. [PubMed] [Google Scholar]
- 17.Meisner D, Mezei M. Liposome ocular drug delivery system. Adv Drug Deliv Rev. 1995;16:75–93. [Google Scholar]
- 18.Nagarsenker MS, Londhe VY, Nadkarni GD. Preparation and evaluation of liposomal formulations of Tropicamide for ocular delivery. Int J Pharm. 1999;190:63–71. doi: 10.1016/s0378-5173(99)00265-3. [DOI] [PubMed] [Google Scholar]
- 19.Law SL, Huang KJ, Chiang CH. Acyclovir containing liposomes for potential ocular delivery.Corneal penetration and absorption. J Control Release. 2000;63:135–40. doi: 10.1016/s0168-3659(99)00192-3. [DOI] [PubMed] [Google Scholar]
- 20.Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery-a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–77. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 21.Diane DS, Tang L, Joseph BR, Robert JW, Harun T. Effects of four penetration enhancers on corneal permeability of drugs in vitro. J Pharm Sci. 1994;83:85–90. doi: 10.1002/jps.2600830120. [DOI] [PubMed] [Google Scholar]
- 22.Bushetti SS, Singh V, Appala RS, Javed A, Veermaram Stimuli Sensitivehydrogels: A review. Indian J Pharm Educ Res. 2009;43:10–9. [Google Scholar]
- 23.Wade A, Weller PJ. Sodiumchloride “Handbook of pharmaceuticalexcipients. 2nd ed. London: Pharmaceutical Press; 1994. pp. 439–42. [Google Scholar]
- 24.Gennaro AR. Remington pharmaceutical sciences. 18th ed. Pennsylvania: Mack Publishing Co; 1990. Ophthalmic preparation; p. 1581. [Google Scholar]
- 25.Balamurugan K, Pandit JK, Choudary PK, Balasubramaniam Systemic absorption of propranalol hydrochloride from Buccoadhesive Films. Indian J Pharm Sci. 2001;63:473–80. [Google Scholar]
- 26.Koteshwar KB, Udupa N, Vasantha K. Design and Evaluation of Captopril transdermal preparation. Indian Drugs. 1992;29:680–5. [Google Scholar]
- 27.Sankar V. Design and Evaluation of Nifedipien transdermal patches. Indian J Pharm Sci. 2003;65:510–5. [Google Scholar]
- 28.Khanna R, Agrawal SP, Ahuja A. Preparation and Evaluation of Mucoadhesive buccal films of Clotrimazole for oral candida infections. Indian J Pharm Sci. 1997;59:299–305. [Google Scholar]
- 29.Manvi FV. Formulation of a transdermal Drug Delivery System of Ketotifen Fumarate. Indian J Pharm Sci. 2003;65:239–43. [Google Scholar]
- 30.Ali A, Sharma SN. Faricationofthrough flow apparatus for theinvitro determination of drugsfrom ophthalmicpreparation. Indian drugs. 1991;29:157–60. [Google Scholar]
- 31.Mazzo DJ, Loper AE. Timolol maleate -analytical profile of the drug substances. Vol. 16. London: Academic Press; 1987. pp. 641–92. [Google Scholar]
- 32.Singh V, Bushettii SS, Appala R, Ahmad R, Singh M. Glaucoma: A treatment by hydrogel. Pharm Sci Monitar. 2010;2:285–94. [Google Scholar]


