Abstract
Objectives:
The purpose of this study was to investigate the anticancer activity of anticancer drugs (5-fluorouracil and 6-thioguanine) in polymeric nanocapsules in the presence and in the absence of gold and iron oxide nanoparticles toward Hep2 cancer cells.
Materials and Methods:
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was used for quantitative measurements for the anticancer cell activity. Encapsulated drug in polyethylene terephthalate-polylactic acid copolymer (PET-co-PLA) nanocapsules in the presence and absence of gold and iron oxide nanoparticles were prepared via the W/O/W emulsification solvent-evaporation method. Morphology of the nanoparticles was characterized by transmission electron microscopy and scanning electron microscopy.
Conclusion:
The average size of the polymeric nanocapsules, gold nanoparticles, and iron oxide nanoparticles were found to be in range of 230-260, 18 -20 nm, 5-10 nm, respectively. The findings in this study inferred that incorporated drug in polymeric nanocapsules with gold nanoparticles and iron oxide nanoparticles show better anticancer activity when compared with encapsulated drug in polymeric nanocapsules.
Keywords: Emulsification, encapsulation, MTT assay, nanocapsules, nanoparticles
Gold nanoparticles which have been known for 2500 years finds extensive application, namely, optical, electronic, magnetic, catalytic, and biomedical. This fact is due to the extraordinary diversity of their modes of preparations involving ceramics, glasses, polymers, ligands, surfaces, films, oxides, biomolecules, and bioorganisms.[1] Gold nanoparticles show tremendous promise as vectors for targeted drug delivery, it is important to keep in mind that work on pharmaceutical applications based on gold colloids is still very much in its early stages.
Magnetic nanoparticles offer exciting new opportunities allowing to develop effective drug delivery systems, which feasible to produce, characterize, and specifically tailor their functional properties[2] for drug delivery applications. If the magnetic polymeric sub-micron particles are designed appropriately, they could be used to increase antitumor efficacy and to reduce systemic side-effects of anticancer drugs.
In nanomedicine the use of nanometer-sized particles and systems to detect and treat diseases at the molecular level plays an essential role in eliminating death from cancer.[3] Nanoscale devices have the potential to radically change cancer therapy by increasing the number of highly effective therapeutic agents. Nanoparticles can be in the form of nanospheres (matrix systems in which drugs are dispersed throughout the polymeric particles) and nanocapsules (drug is confined in an aqueous or oily cavity surrounded by a single polymeric membrane).[4] Nanoparticles can serve as customizable, targeted drug delivery vehicles capable of ferrying chemotherapeutic agents or therapeutic genes into malignant cells while sparing healthy cells. This fact allows the use of smaller doses of toxic substances because the drugs are delivered directly into the target tissue. It is also possible to deliver the toxin in a controlled and time-release manner.
In recent years the synthesis of potentially biodegradable copolyesters of PET has received growing interest. Copolyesters, such as poly ethylene terephthalate-poly lacticacid are biodegradable materials used for tissue engineering, bone reconstruction, and controlled drug delivery systems.[5,6] Copolyesters of PET and aliphatic copolyesters, such as PET-polylactide, PET-polyglycolide, PET-polyethyleneadipate, and PET-poly(caprolactone) have been described as environmentally degradable or hydrolyzable.
5-Fluorouracil (5-Fu) is one of the oldest chemotherapy drugs,[7] and has been used for decades. It is an active drug against many cancers.[8] 5-Fu is a clear and colorless solid, and actually it is administered by intravenous route. 6-Thioguanine (6-TG) is a guanine analog. It belongs to the family of drugs called antimetabolites. This drug is used for the treatment of acute leukemia.
Cell viability assay has been gaining much attention as an alternative to using animals. It has also been used in cancer chemotherapy to select an anticancer drug as well as its dose. The development of in vitro cytotoxicity assays has been driven by the need to rapidly evaluate the potential toxicity of large numbers of compounds, to limit animal experimentation whenever possible, and to carry out tests with small quantities.[9] The MTT assay is a cell viability assay often used to determine cytotoxicity following exposure to toxic substances. MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) is a water-soluble tetrazolium salt, which is converted to an insoluble purple formazan by cleavage of the tetrazolium ring by succinate dehydrogenase within the mitochondria. The formazan product is impermeable to the cell membranes and therefore it accumulates in healthy cells. The MTT assay was tested for its validity in various cell lines.[10]
Hence in this paper the anticancer activity of encapsulated anticancer drugs (5-Fu, 6-TG) in polymeric nanocapsules (PLA-co-PET) in the presence and absence of gold[11–13] and iron oxide nanoparticles[14–16] toward human epidermoid cell (Hep2) cancer cells was carried out for practical application. Hep2 was the cell line model selected for in vitro cytotoxicity studies.
Materials and Methods
Chloroauric acid (HAuCl4.3H2O, 98%), bis-(2-hydroxy ethyl terephthalate), polyvinyl alcohol, stannous octoate, were obtained from Aldrich. l-lactic acid, polyethylene glycol, trisodium citrate (99%), methanol, ethyl cellulose, and tin powders were obtained from SRL India. Hep2 cell line used for the anticancer assay was received from King Institute of Preventive Medicine, Guindy, Chennai – 600 032, India. Minimum essential medium (MEM) 10% and w/o fetal bovine serum (FBS), 0.45 micron filter, (3-(4,5-dimethyl thiazol-2yl)-2,5-diphenyl tetrazolium bromide) solution (MTT) powder, dimethyl sulfoxide were purchased from Sigma-Aldrich, Bangalore India. Millipore water was used for anticancer studies.
For HR-TEM studies, was employing a JEOL instrument with an accelerating voltage of 120 KV. A JEOL JSM-6360 field emission scanning electron microscope was used. For SEM measurements the surface of the nanocapsules were coated with gold by high vacuum evaporation.
Preparation of citrate-capped gold nanoparticles
Trisodium citrate (38.8 mM, 50 cm3) was added to a boiling HAuCl4 solution (1 mM, 500 cm3). As a result, the previously yellow solution of gold chloride turned to wine red color and it was obtained a characteristic absorbance at 518 nm in the UV-vis spectrum.
Preparation of oleic acid-coated iron oxide nanoparticles
FeCl2 (0.42 g) and FeCl3 (1.09 g) were dissolved in 10 mL of aqueous solution of HCl with the concentration 1 N by mechanical stirring.[17] This acid solution was added drop wise to an aqueous solution (90 mL) of 1 N KOH under nitrogen atmosphere leading to a black precipitate. The precipitate was isolated by decantation and washed several times with deionized water until the pH of the medium was 9. After washing step, 20 mL of oleic acid was added to the alkaline medium containing iron oxide nanoparticles under vigorous stirring for 1 h at room temperature. The wet precipitate was dried in an oven at 40°C during 48–72 h before use.
Preparation of polymer nanocapsules with gold nanoparticles
Nanocapsules were prepared by the solvent evaporation method.[18] PLA-co-PET copolymer was synthesized using bis(2-hydroxy ethylene terephthalate) and l-lactic acid.[19] For polymeric nanocapsules with gold nanoparticles study, fluorouracil or thioguanine drug (30 mg) was added in 5 mL of nanogold aqueous suspension with 30 min shaking under ultrasound to help the adsorption of drug on gold nanoparticles and the final solution was added to an organic polymeric solution (400 mg of copolymer + 8 mL CH2Cl2) under stirring condition. This was continued until the solvent was completely evaporated. The suspension became clear after all the nanocapsules precipitated out of the solution. These nanocapsules were collected by filtration and washed with deionized water to remove any undesirable residuals. Finally, the clean nanocapsules were dried in a vacuum oven at 40°C for 24 h to ensure the complete removal of the solvent and the residual deionized water. All the nanocapsules were stored in a desiccator at 25°C. Polymeric nanocapsules with encapsulated drug were also prepared under similar conditions.
Preparation of polymer nanocapsules with iron oxide nanoparticles
Polymer nanocapsules were prepared by the solvent evaporation technique in oil/water emulsion with copolymer as the encapsulation material. PLA-co-PET (400 mg), Fe3O4 (80 mg), and drug (30 mg) were dissolved in dichloromethane (8 mL) and vortexed during 10 min to make the organic phase. The organic phase was then poured into 50 mL of stirred aqueous solution containing 1% polyvinyl alcohol as emulsifier. The mixture was sonicated for 120 s with an ultrasonic processor (Sonics, VCX 130 PB). The formed oil/water emulsion was then stirred at room temperature overnight with a magnetic stirrer to evaporate the solvent. The particles were collected by centrifugation at 4000 rpm for 10 min and washed three times with deionized water. The nanoparticles were resuspended with 10 mL water and freeze-dried for 2 days. The polymeric nanocapsules in the absence of Fe3O4 nanoparticles were prepared using the same conditions.
Preparation of ingredients
Penicillin and streptomycin: (conc. 100 IU of penicillin and 100 μg of streptomycin)
1 × 106 units of crystalline penicillin and 1 g of streptomycin were dissolved in 100 mL of phosphate buffer saline (PBS). One milliliter of this stock solution was added to 100 mL of growth medium (with FBS and w/o FBS) to give a final concentration of 100 IU of penicillin and 100 g of streptomycin and stored at –20°C.
Fetal bovine serum
FBS (500 mL) was taken at 25°C and inactivated at 56°C in water bath for 30 min and cooled to room temperature. The solution was filtered through Seitz filter and stored at –20°C.
Preparation of TPVG solution (trypsin, PBS, Versene, glucose solution) (1000 mL)
PBS (840 mL), 2% of trypsin (50 mL), 0.2% of EDTA (100 mL), 10% of glucose (5 mL) and 100 units of penicillin (5 mL) and 100 μg of streptomycin were mixed and the pH was adjusted to 7.4 using 0.1 N HCl or 0.1 N NaOH. This solution was stored at –20°C and sterilization was started.
Minimum essential medium preparation
The MEM (10% w/v) powder was dissolved in the presterilized millipore double distilled water. It was then sterilized at 15 lbs, 121°C for 15 min and then cooled to 30°C, the ingredients were added in quantities as indicated below depending on the concentration of FBS (5% w/v and 2% w/v). Table 1 shows the composition of MEM for various concentrations of FBS.
Table 1.
Composition of minimum essential medium for various concentrations of fetal bovine serum
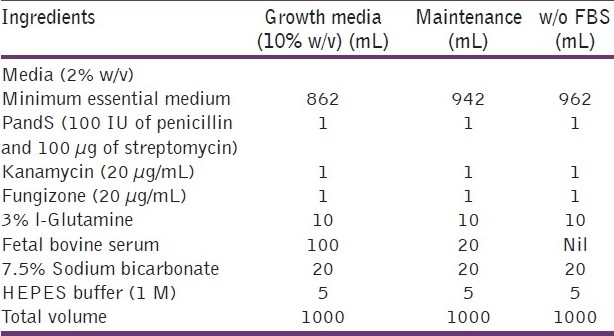
All the above ingredients were mixed well and care was taken to avoid spills. The pH of the solution was adjusted to 7.4 by using 0.1 N HCl or 0.1 N NaOH. The MEM bottles were kept for 2 days at 35°C and checked for sterility using pH drop and floating particles.
Dispersion and subculturing “seeding” of cells
The MEM and TPVG were brought at 35°C. The tissue culture flask was observed for growth, cell degeneration, pH and microbial contamination and then the flask was selected for splitting.
Procedure for subculture
Spirit was applied to the mouth of the flask. The medium was poured off and gently rinse the cells with MEM (w/o FBS) for 3 times; 5 mL of (prewarmed to 35°C) TPVG was added over the cells. After 5 min TPVG was decanted from the flask. The flask was incubated at 37°C till monolayer shows signs of dispersion.
Five milliliters of 10% MEM was added to the flask. The cell clusters were broken off by gently pipetting back and forth with pipette and cells were counted in a hemocytometer. Based on the cell count 24 wells plate was seeded with 1 × 105 cells/mL. One milliliter was added per well and incubated at 37°C in CO2 incubator.
Cell counting
0.2 mL of the cell suspension was diluted to 0.4 mL of trypan blue. This was mixed with pipette and aspirate sufficient volume to fill hemocytometer. Cells in each of the four corners of the counting chamber was counted and the cells lying on the right hand and lower lines were omitted. The mean count is multiplied by 10,000 and by the dilution factor of cell suspension to give number of cells per milliliter of cell suspension; 2.5 mL was taken from cell suspension and it was diluted with 22.5 mL of 10% MEM to get 1 lakh cells/mL concentration.
Stock drug concentration
A weighed amount of drug was dissolved in 10 mL of water giving an aqueous solution with a final concentration of 5 mg of drug. A fresh stock solution (5 mg/10 mL) was prepared and filtered through 0.45-μm filter before each assay. Working concentrations of drug ranging from 0.1 to 25 μg was prepared in MEM without FBS (from stock solution).
Cytotoxicity assay
Drug concentration required in different micrograms per milliliter was prepared from the stock solution. Hep2 cells at a concentration of 10,000 cells/0.1 mL/well (104 cells/mL/well) were seeded in 24-well micro titer plates. The plates were microscopically examined for confluent monolayer, turbidity, and toxicity. The wells were labeled for different concentration of the drug (as depicted in plate model), polymer/drug nanocapsules, polymer/drug with gold nanocapsules, polymer/drug with iron oxide nanocapsules, and cell control (cell control was carried out for each plate).
The growth medium was removed from subcultures using pipette. Care was taken so that the tip of the pipette does not touch the cell sheet. The cell monolayer was washed once with PBS and removed as described in subculture procedure. To the washed cell sheet, 1 mL of medium (w/o FBS) containing different concentrations of the drug and drug-coated nanoparticles encapsulated drug were added in tetrads in the designated wells and cell control. The same pipette was used when going from lower to higher concentration. One milliliter well of drug-free medium was added to cell control wells. The plates were incubated at 37°C in 5% CO2 environment. MTT assay was used for quantitative measurements for the anticancer cell activity. Table 2 shows the cytotoxicity assay protocol for plate model.
Table 2.
Cytotoxicity assay protocol–plate model

MTT assay
Preparation of MTT (3-(4,5-dimethyl thiazol-2yl)-2,5-diphenyl tetrazolium bromide) solution (5 mg/mL)
MTT (50 mg) and PBS (10 mL) were vortexed for 20 min and filtered with 0.45 μm filter. The bottles were wrapped in aluminum foil or paper to block light, as MTT is light sensitive and stored at 4°C. A fresh stock solution was prepared before every assay.
Procedure for MTT assay
The medium was removed from wells for MTT assay and 200 μL of 5 mg/mL of MTT (filter sterilized) solution was added to each well. The plate was incubated for 12 h at 37°C and 1 mL of dimethyl sulfoxide was added at the end of the incubation to each well. This was gently pipetted back and forth using pasteur pipette to break the cells and liberate the Formazan crystals. The suspension was then transferred into the cuvutte of spectrophotometer and optical densities (OD) were taken at 690 nm.
Results and Discussions
Morphological characterization
Figure 1(a) and (b) show the TEM images of gold nanoparticles and iron oxide nanoparticles. From the TEM measurements, the average diameters of the gold nanoparticles were found to be in the range of 18–20 nm. The monodisperse Fe3O4 nanoparticles have an average diameter of 5–9 nm and are nearly spherical in shape. Iron is dispersed throughout the polymer matrix mainly in the form of iron oxide particles.
Figure 1.

(a) TEM images of gold nanoparticles and (b) iron oxide nanoparticles
The color of PLA-co-PET/drugs nanocapsules is white, PLA-co-PET/drugs with gold nanocapsules is purple/blue probably due the presence of the gold nanoparticles. Such differences were not seen for nanocapsules prepared using iron oxide nanoparticles. From Figures 2 and 3, it was concluded that the penetrations of drug in metal nanoparticles were observed during the process of encapsulation drugs, which confirms the presence of drugs in the nanocapsules.
Figure 2.
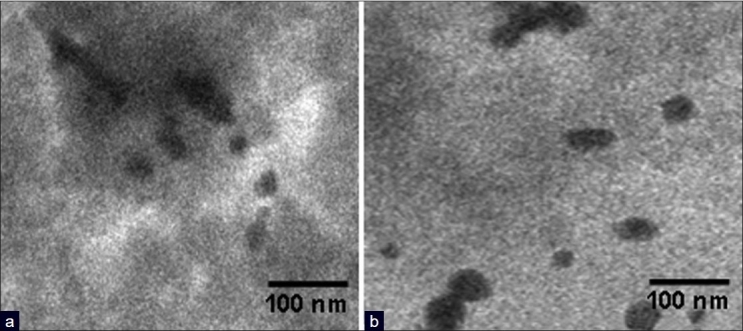
(a) TEM images of encapsulated fluorouracil in polymeric nanocapsules with gold nanoparticles and (b) TEM images of encapsulated thioguanine in polymeric nanocapsules with gold nanoparticles
Figure 3.
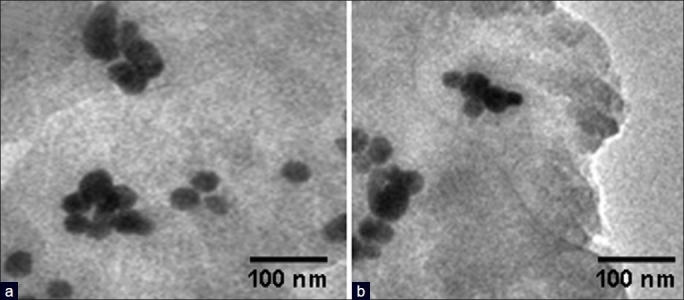
(a) TEM images of encapsulated fluorouracil in polymer nanocapsules with iron oxide nanoparticles and (b) TEM images of encapsulated thioguanine in polymer nanocapsules with iron oxide nanoparticles
The scanning electron micrographs of PLA-co-PET nanocapsules containing anticancer and antituberculosis drugs in the presence and absence of gold and iron oxide nanoparticles were shown in Figures 4 and 5. The surface of nanocapsules appeared to be smooth and it was clear that some nanospheres had been collapsed. Nanocapsules were spherical in shape and drug particles were adhered at surface as shown in SEM micrographs. The surface of polymeric nanocapsules with gold and iron oxide nanoparticles was smoother than that of nanocapsules made from PLA-co-PET/drug. The average size of the nanocapsules were found to be in the range of 230–250 nm.
Figure 4.
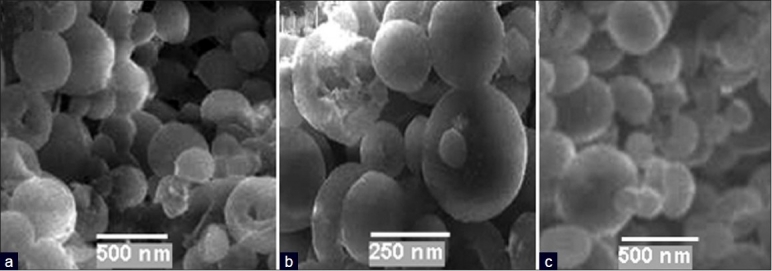
(a) SEM images of encapsulated thioguanine in polymer nanocapsules; (b) encapsulated thioguanine in polymer nanocapsules with iron oxide nanoparticles; and (c) encapsulated thioguanine in polymer nanocapsules with gold nanoparticles
Figure 5.
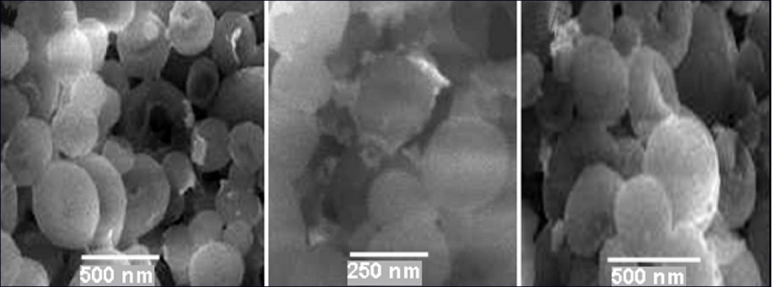
(a) SEM images of encapsulated fluorouracil in polymer nanocapsules; (b) encapsulated fluorouracil in polymer nanocapsules with iron oxide nanoparticles; and (c) encapsulated fluorouracil in polymer nanocapsules with gold nanoparticles
Anticancer studies
Hep2 cell line was used for microtissue culture assay. The cultures were prepared as described in the experimental section. Hep2 cells (concentration 10,000 cells/0.1 mL) were seeded in 24-well microtiter plates, and incubated for 48 h at 36°C in 5% CO2 environment. The growth medium is decanted and different dilutions of polymer nanocapsules (diluted in MEM without FBS) were added in tetrads. Cell control, diluent controls, and cell controls (without drugs) were included and then the plates were incubated at 36°C in 5% CO2 environment. In the first day reading was noted microscopically for cytotoxicity, however, MTT (3-(4,5-dimethyl thiazol-2yl)-2,5-diphenyl tetrazolium bromide) assay was performed to find out the percentage of viability and death cells after 48 h. MTT is cleaved by mitochondrial dehydrogenase in viable cells, yielding a measurable purple product formazan. This formazan production is proportional to the viable cell number and inversely proportional to the degree of cytotoxicity.
The cytotoxicity assay was carried out for two different anticancer drugs (6-TG and 5-FU) and their effect with gold and iron oxide nanoparticles were measured. The supernatant layer was discarded and washed once with cold PBS. 5 mg/ml of MTT was added to each well. The plate was incubated at 37°C for 4 h. One milliliter of DMSO was added to each well after the incubation and aspirated to break the cells to dissolve the formazan crystals. The color developed was measured spectrophotometrically at 690 nm. Cell control was included in each assay to compare the fall of cell viability in anticancer activity. The optical density (OD) values were directly proportional to the cellular viability. The OD values of the cell control, solvent control, and the toxic dose of anticancer drugs, encapsulated drugs in polymeric nanocapsules with gold or iron oxide nanoparticles were studied for both the anticancer therapeutic systems.
The test was repeated for 3 times at different periods for reproducibility in the present investigation. The cytotoxicity can be measured by using the following formula:
Toxicity = sample OD × 100/control OD (in terms of live cells)
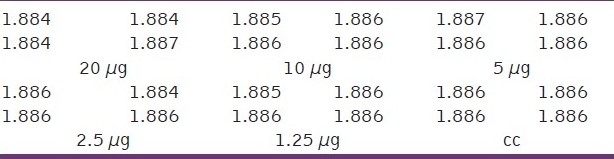
The toxicity assay of polymeric nanocapsules encapsulated with anticancer drugs in the presence of gold or iron oxide nanoparticles were carried out in Hep2 cancer cells and shown in Figures 6 and 7. The toxicity assays of encapsulated thioguanine in polymeric nanocapsules with gold or iron oxide nanoparticles and encapsulated thioguanine in polymeric nanocapsules toward the cancer cell were depicted in Tables 3–5, respectively. Encapsulated thioguanine (3.12 μg) in polymeric nanocapsules with gold and 6.25 μg of encapsulated thioguanine in polymeric nanocapsules with iron oxide gives 50% toxicity for Hep2 cancer cells (ie, 50% cancerous live cells are left behind), whereas 8 μg of pure thioguanine encapsulated in PLA-co-PET nanocapsules was required for 50% toxicity toward Hep2 cancer cells.
Figure 6.
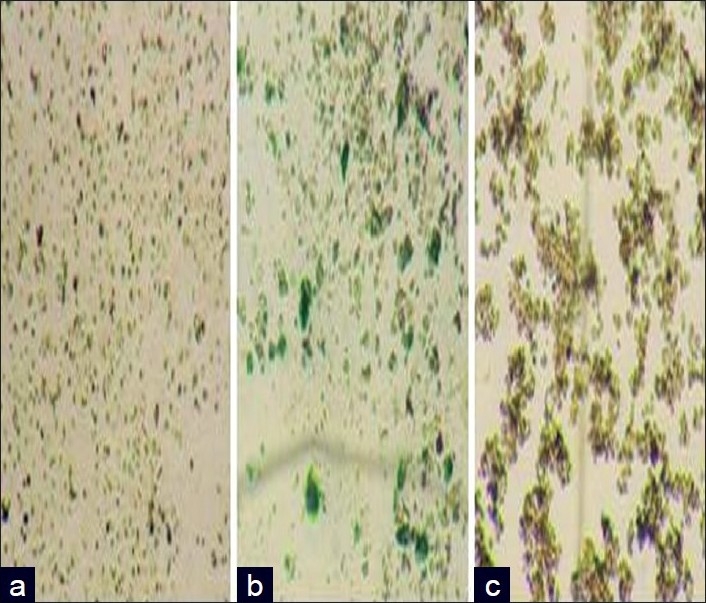
Optical images showing cytotoxicity of (a) encapsulated fluorouracil in polymer nanocapsules; (b) encapsulated fluorouracil in polymer nanocapsules with iron oxide nanoparticles; and (c) encapsulated fluorouracil in polymer nanocapsules with gold nanoparticles
Figure 7.
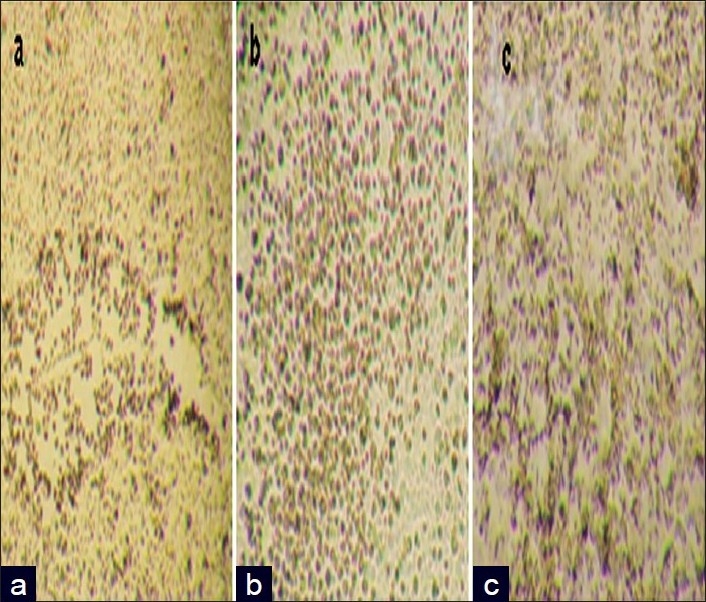
Optical images showing cytotoxicity of encapsulated thioguanine in polymer nanocapsules; (b) encapsulated thioguanine in polymer nanocapsules with iron oxide nanoparticles; and (c) encapsulated thioguanine in polymer nanocapsules with gold nanoparticles
Table 3.
Optical density (OD) measurements at different concentrations of encapsulated thioguanine in polymeric nanocapsules with gold nanoparticles (μg)
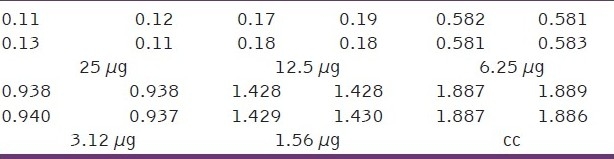
Table 5.
Optical density (OD) measurements at different concentrations of encapsulated thioguanine in polymeric nanocapsules (μg)
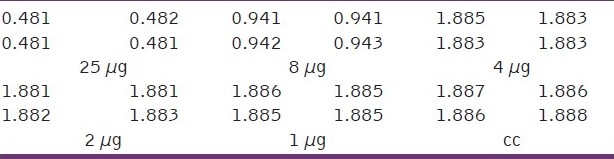
Table 4.
Optical density (OD) measurements at different concentrations of encapsulated thioguanine in polymeric nanocapsules with iron oxide nanoparticles (μg)
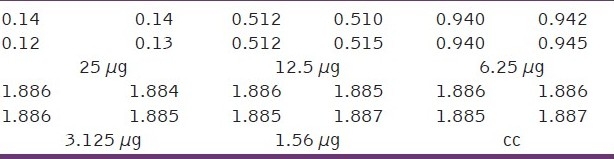
The toxicity assays of encapsulated fluorouracil in polymeric nanocapsules with gold nanoparticles, encapsulated fluorouracil in polymeric nanocapsules with iron oxide nanoparticles and encapsulated fluorouracil in polymeric nanocapsules toward the cancer cell are presented in Tables 6–8, respectively. Encapsulated fluorouracil (12.5 μg) in polymeric nanocapsules with gold nanoparticles and 15 μg of encapsulated fluorouracil in polymeric nanocapsules with iron oxide nanoparticles gives 50% toxicity for Hep2 cancer cells (ie, 50% cancerous live cells are left behind), whereas 20 μg of encapsulated fluorouracil in polymeric nanocapsules was required for 50% toxicity toward Hep2 cancer cells.
Table 6.
Optical density (OD) measurements at different concentration of encapsulated Fluorouracil in polymeric nanocapsules with gold nanoparticles (μg)
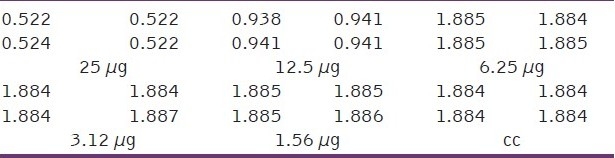
Table 8.
Optical density (OD) measurements at different concentrations of encapsulated fluorouracil in polymeric nanocapsules (μg)
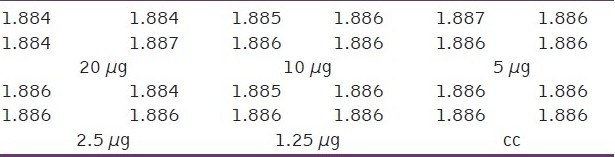
Table 7.
Optical density (OD) measurements at different concentrations of encapsulated fluorouracil in polymeric nanocapsules with iron oxide nanoparticles (μg)
From the results, it was concluded that encapsulated drugs in polymeric nanocapsules with gold and iron oxide nanoparticles show higher anticancer activities compared with encapsulated drugs in pure polymeric nanocapsules. However, encapsulated drugs in polymeric nanocapsuled with gold nanoparticles show higher anticancer activity compared with iron oxide nanoparticles. This may be due to high interaction of gold nanoparticles with anticancer drugs. This strong interaction leads to controlled and sustained release of drugs, which in turn leads to higher anticancer activity for gold therapeutic nanosystems.
Hence, high entrapment efficiency, high drug loading, controlled drug release, and smooth nanosphere formation gives higher anticancer activity for drugs encapsulated polymer nanocapsules with gold nanoparticles.
Conclusion
In summary, we have synthesized polymeric nanocapsules in presence and absence of gold or iron oxide nanoparticles. Morphological characterization of nanoparticles revealed that nanocapsules prepared using gold or iron oxide nanoparticles were found to be smooth and spherical when compared to pure polymeric nanocapsules. An effort has been taken to probe the anticancer activity of polymer nanocapsules for antileukemic drugs like 5-fluorouracil and 6-thioguanine in the presence and absence of gold or iron oxide nanoparticles in cell line studies (in vitro). From the obtained results, it was inferred that encapsulated drugs in polymeric nanocapsules with gold nanoparticles show better anticancer activity when compared with encapsulated drugs in polymeric nanocapsules with iron oxide nanoparticles and encapsulated drugs in polymeric nanocapsules, which may be due to high interaction of gold nanoparticles with anticancer drugs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Daniel MC, Astruc D. Gold Nanoparticles: Assembly, supramolecular chemistry, Quantum-size-related properties and applications towards biology, catalysis and Nanotechnology. Chem Rev. 2004;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Misra RD. Magnetic drug-targeting carrier encapsulated with thermosensitive smart polymer: Core-shell nanoparticle carrier and drug release response. Acta Biomaterialia. 2007;3:838–50. doi: 10.1016/j.actbio.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 3.Yih TC, Wei C. Nanomedicine in cancer treatment. Nanomedicine. 2005;1:191–2. doi: 10.1016/j.nano.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Reis CP, Neufeld RJ, Ribeiro AJ, Veiga F, Nanoencapsulation I. Methods for preparation of drug loaded polymeric nanoparticles. Nanomedicine. 2006;2:8–21. doi: 10.1016/j.nano.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Yuan X, Mak AF, Yao K. Comparative observation of accelerated degradation of poly(L-lactic acid) fibres in phosphate buffered saline and a dilute alkaline solution. Polymer Degrad Stabil. 2002;75:45–53. [Google Scholar]
- 6.Van Nostrum CF, Veldhuis TF, Bos GW, Hennik WE. Hydrolytic degradation of oligo(lactic acid): A kinetic and mechanistic study. Polymer. 2004;45:6779–87. [Google Scholar]
- 7.Sathish Kumar K, Selvaraj V, Alagar M. Synthesis of PET-PLA/Drug Nanoparticles for controlled drug release in cancer chemotherapy. J Nanotechnol. [cited on 2008 Apr 4 389512[about4p]]. Available from: http://wwwhindwaicom/journals/jnt/2008/389512html .
- 8.Selvaraj V, Alagar M. Analytical detection and biological assay of antileukemic drug 5-fluorouracil using gold nanoparticles as probe. Int J Pharma. 2007;337:275–81. doi: 10.1016/j.ijpharm.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Patel RM, Patel NJ. In vitro cytotoxicity screening of curcumin compounds on Hep2 cancer cell line. Int J Pharma Res. 2010;1:88–99. [Google Scholar]
- 10.Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 11.Asadishad B, Vossoughi M, Alamzadeh I. In vitro release behavior and cytotoxicity of doxorubicin-loaded gold nanoparticles in cancerous cells. Biotechnol Lett. 2010;32:649–54. doi: 10.1007/s10529-010-0208-x. [DOI] [PubMed] [Google Scholar]
- 12.Qu Y, Lü X. Aqueous synthesis of gold nanoparticles and their cytotoxicity in human dermal fibroblasts-fetal 4. Biomed Mater. 2009;4:025007. doi: 10.1088/1748-6041/4/2/025007. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoudi M, Simchi A, Milani AS, Stroeve P. Cell toxicity of superparamagnetic iron oxide nanoparticles. J Colloid Interface Sci. 2009;336:510–8. doi: 10.1016/j.jcis.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 14.Naqvi S, Samim M, Abdin MZ, Ahmed FJ, Maitra AN, Prashant CK, Dinda AK. Concentration-dependent toxicity of iron oxide nanoparticles mediated by increased oxidative stress. Int J Nanomed. 2010;5:983–9. doi: 10.2147/IJN.S13244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Hafeli UO, Pauer GJ. In vitro and in vivo toxicity of magnetic microspheres. J Magnet Magnet Mat. 1999;194:76–82. [Google Scholar]
- 16.Li J, Chen C, Wang X, Gu Z, Chen B. Novel strategy to fabricate PLA/Au nanocomposites as an efficient drug carrier for human leukemia cells in vitro. Nanoscale Res Lett. 2011;6:29. doi: 10.1007/s11671-010-9762-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okassa LN, Marchais H, Eyrolles LD, Katel H, Simon CJ, Emilie M, et al. Optimization of iron oxide nanoparticles encapsulation within poly(D,L-lactide-co-glycolide) sub-micron particles. Eur J Pharma Biopharma. 2007;67:31–8. doi: 10.1016/j.ejpb.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Olewnik E, Czerwinski W, Nowaczyk J, Sepulchre MO, Tessier M, Salhi S, et al. Synthesis and structural study of copolymers of L-Lactic acid and bis(2-hydroxyethyl terephthalate) Eur Polymer J. 2007;43:1009–19. [Google Scholar]
- 19.Beck LR, Lewis DH. A new long acting injectable microcapsule system for the administration of progesterone. Fertil Steril. 1979;31:545–51. doi: 10.1016/s0015-0282(16)44002-1. [DOI] [PubMed] [Google Scholar]


