Abstract
Herbs have been used for medicinal purposes for centuries. According to recent investigations, they may help reduce the risk of chronic diseases, cardiovascular disease, and cancer due to antioxidant properties, which in turn can be attributed to the various phytoconstituents. With this intention, evaluation of antioxidant activity was performed. Methanol extract of aerial parts of Artemisia pallens Wall was screened for its antioxidant activity due to phenolic and flavonoid contents, by employing radical scavenging assays; 2,2 –diphenyl, 1-picryl hydrazyl (DPPH) and nitric oxide. Ascorbic acid was used as a standard. Quantitative determination of phenols and flavonoids were carried out using spectrophotometric method. Total flavonoid content was determined as quercetin equivalent and total phenolic content was determined as pyrocatechol equivalent using Folin-Ciocalteu reagent. Plant produces more phenolic compounds than flavonoids. IC50 value of methanol extract for DPPH free radical scavenging activity was found to be 292.7 μg, whereas for nitric oxide it was 204.61 μg. The result obtained in the present study indicates that the aerial parts of this plant are a rich source of natural antioxidants
Keywords: Antioxidant, Artemisia pallens, DPPH, nitric oxide, flavonoids, phenols
An antioxidant is a molecule capable of slowing or preventing the oxidation of other molecules. Oxidation is the transfer of electrons from one atom to another and represents an essential component of aerobic life and our metabolism, since oxygen is the ultimate electron acceptor in the electron flow system that produces energy in the form of ATP.[1] However, chronic diseases may result due to uncoupling of electron flow (transfer of unpaired single electrons) leading to generation of free radicals.[2] Low levels of antioxidants or inhibition of the antioxidant enzymes causes oxidative stress and may lead to damage or death of cells. Antioxidants protect cells against damage caused by free radicals. They also protect the living organisms from damage caused by uncontrolled production of reactive oxygen species and the concomitant lipid peroxidation, protein damage and DNA strand breakage.[3] It has also been reported that there is an inverse relationship between the dietary intake of antioxidant rich food and the incidence of human diseases.[4]
Plants and animals maintain complex systems of multiple types of antioxidants, such as glutathione, ascorbic acid, etc. as well as enzymes like catalase, superoxide dismutase, and various peroxidases. The antioxidant effects of plants are mainly due to the presence of phenolic constituents like phenols, flavonoids, phenolic acids, phenolic diterpenes, and tannins.[5]
Phenolic compounds are ubiquitous bioactive compounds and a diverse group of secondary metabolites universally present in higher plants. Accordingly, bioactive poly phenols have attracted special attention because they can protect the human body from the oxidative stress which may cause many diseases including cancer, cardiovascular problems, and aging.[6] Flavonols and flavones are plant-derived polyphenolic compounds that are commonly consumed in the diet. Epidemiological studies indicate that high dietary intake of flavonols reduce the risk of mortality due to coronary heart disease, which has provoked interest in the mechanism of these observed cardioprotective effects.[7,8] Therefore, there is a need to identify effective natural antioxidants in this era.
Artemisia pallens Wall, an aromatic medicinal herb, is cultivated for its fragrant leaves and flowers, which are used in floral decorations, religious offerings, and for the extraction of an essential oil - Davana oil. This oil is mainly used in the flavouring of cakes, pastries, tobacco, and also some costly beverages.[9] Plants are accredited with anthelmintic, tonic, and antipyretic properties. They are also considered as good fodder.[10] Earlier, the phenolic content of microwave-assisted ethanol extract was reported by Kanimozhi P. et al.[11] In the present communication, radical scavenging activity of this medicinal herb is reported.
Materials and Methods
UV-Vis S1700 Pharmaspectrophotometer (Schimadzu) was used for the measurement of absorbance. All solvents used were of AR-grade and were obtained from Merck, Mumbai (India).
Plant material
The plant material was collected from Jejuri, Maharashtra, India. It was authenticated at Botanical Survey of India, Pune, with the authentication number BSI/WC/Tech/2008/1059.
Extraction
Air-dried and powdered plant material (10 g) was extracted with methanol (50 ml) by keeping for 24 hours at room temperature. Solvent was recovered under reduced pressure to obtain crude methanol extract 11.6 %.
Determination of total phenolics
The total phenolic contents of aerial parts of A. pallens Wall were determined using Folin-Ciocalteau reagent and Na2CO3according to the method described by Malik and Singh.[12] The concentration of phenol was determined as equivalent of phenol /g of extract by measuring absorption at 650 nm using pre-calibrated standard curve employing pyrocatechol. Experiment was carried out in triplicate and results were recorded as mean ± SEM
Determination of total flavonoids
The aluminum chloride method was used for the determination of the total flavonoid content of the sample extracts.[13] After addition of AlCl3, sodium-potassium tartarate and incubation absorbance was measured at 415 nm. The concentration of flavonoid in the test extracts was calculated from the calibration plot and expressed as mg quercetin equivalent /g of extract. Experiment was carried out in triplicate and results were recorded as mean ± SEM
2,2–Diphenyl, 1-picryl hydrazyl-free radical scavenging activity
The 2,2 –diphenyl, 1-picryl hydrazyl (DPPH)-free radical scavenging activity was determined using the method described by Sanja et al.[14] with slight modification. Methanolic DPPH solution (1.65 mM) was prepared. The initial absorbance of DPPH in methanol was measured at 516 nm and did not change throughout the period of assay. Different volume levels of test samples (50 μl to 200 μl) were diluted with methanol and volume was made up to 3 ml. One hundred and fifty microliter DPPH solution was added to each test tube. Absorbance was taken at 516 nm after 30 minutes incubation using methanol as blank. The free radical scavenging activity was calculated using the following equation.
% inhibition = (Acont–Asamp) × 100 / Acont,
where Aconl is absorbance of control and Asamp is absorbance of sample.
IC50 values calculated denote the concentration of a sample required to decrease the absorbance by 50 %.
Nitric oxide-free radical scavenging activity
The nitric oxide-free radical scavenging activity was determined using the method described by S. D. Sanja et al.[14] with slight modification. Sodium nitro prusside (1.49 g) in phosphate buffer (10 ml) was mixed with different volume levels of test sample (100 μl to 600 μl). Each dose was made of 1 ml. To this 4 ml of methanol was added and the solution was incubated at room temperature for 90 minutes. The same reaction mixture without the extract but equivalent amount of methanol served as control. After the incubation period Griess reagent was added and the absorbance was taken in UV visible spectrophotometer at 546 nm. Ascorbic acid was used as positive control. IC50 values calculated denote the concentration of a sample required to decrease the absorbance by 50 %.
Result and Discussion
Total phenolic content for A. pallens is obtained from the regression equation of calibration curve of pyrocatechol (y = 0.0275x - 0.0278, R2 = 0.9915) and expressed as pyrocatechol equivalent. Total flavonoid content for A. pallens is obtained from the regression equation of calibration curve of quercetin (y = 0.0307x - 0.0035, R2 = 0.9978) and expressed as quercetin equivalent. The phenolic and flavonoid content of A. pallens in methanol extract is recorded in Table 1.
Table 1.
Total phenolic and flavonoid content

Plant phenolics are the major group of compounds acting as primary antioxidants. Flavonoids, one of the most diverse and widespread group of natural compounds, are probably the most important natural phenols. These compounds encompass a broad spectrum of chemical and biological properties including radical scavenging properties.
The antioxidant activity of methanol extracts of A. pallens is examined by comparing it to the activity of known natural free radical scavenger ascorbic acid.[15]
DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule. The effect of antioxidants on DPPH radical scavenging is thought to be due to their hydrogen donating ability. The reduction capability of DPPH radicals was determined by the decrease in its absorbance at 516 nm induced by antioxidants. DPPH free radical scavenging activity of methanol extract of A. pallens is depicted in Figure 1. IC50 values of DPPH free radical scavenging activity for methanol extract and ascorbic acid are recorded in Table 2.
Figure 1.
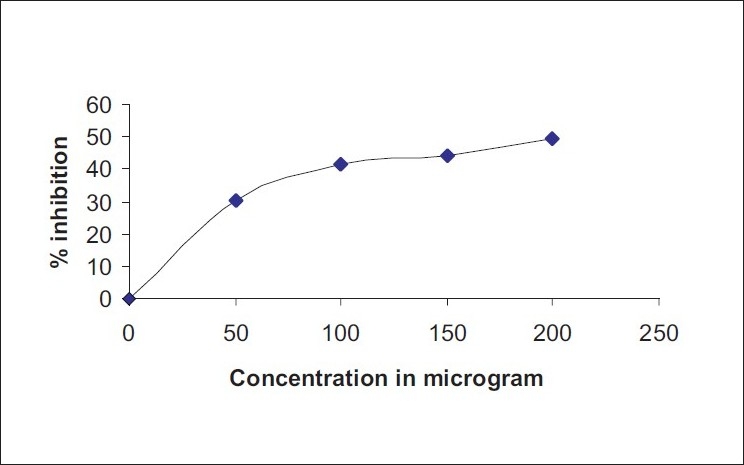
DPPH-free radical scavenging activity of Artemisia pallens methanol extract
Table 2.
DPPH radical scavenging activity

Active oxygen species and free radicals are involved in a variety of pathological events. In addition to Reactive oxygen species (ROS), nitric oxide is also implicated in inflammation, cancer, and other pathological conditions. Nitric oxide free radical scavenging activity of methanol extract of A. pallens is depicted in Figure 2. IC50 values of nitric oxide free radical scavenging activity for methanol extract and ascorbic acid are recorded in Table 3.
Figure 2.
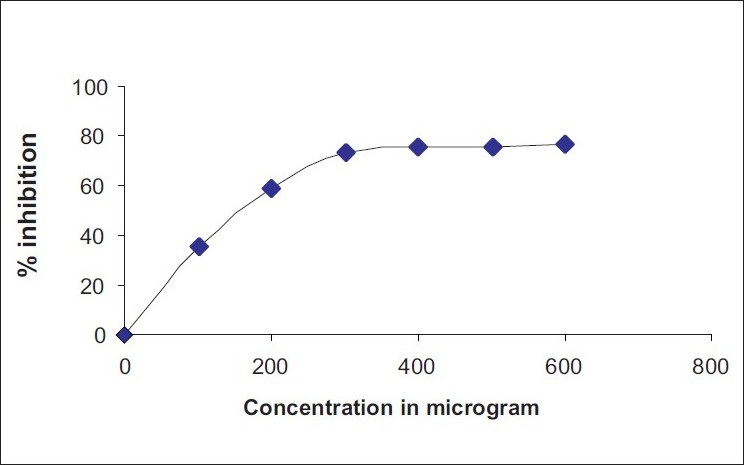
Nitric oxide-free radical scavenging activity of A. pallens methanol extract
Table 3.
Nitric oxide radical scavenging activity

It can be seen that the DPPH and nitric oxide free radical scavenging activity for the methanol extract of A. pallens is found to be significant. Presence of major phytocompounds i.e. phenolics, flavonoids, etc. may have been responsible for the observed antioxidant activity.
The plant is reported to have antimicrobial[16] as well as potent analgesic and anti-inflammatory[17] properties. This may be because of presence of bioactive molecule/s having anti-oxidant properties.
Conclusion
The results of the present study conclusively demonstrated that Artemisia pallens Wall possess high antioxidant potential. The plant is found to be rich in phenolic compounds. This would be responsible for its high antioxidant potential. Further studies are being carried out to identify the molecule/s having strong antioxidant potential.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Davies KJ. Oxidative stress: The paradox of aerobic life. [Las accessed on 2010 Sep 12];Biochem Soc Symp. 1995 61:1–31. doi: 10.1042/bss0610001. Available from: http://www.ncbi.nlm.nih.gov . [DOI] [PubMed] [Google Scholar]
- 2.Pietta PG. Flavonoids as Antioxidants (reviews) J Nat Prod. 2000;63:1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 3.Ghosal S, Tripathi VK, Chauhan S. Active constituents of Emblica officinalis: Part 1: The Chemistry and antioxidant effects of two new hydrolysable tannins, emblicanin A and B. Indian J Chem. 1996;35:941–8. [Google Scholar]
- 4.Rich-Evan CA, Sampson J, Bramely PM, Hollwa DE. Why we do except carotenoids to be antioxidants in vivo. Free Rad Res. 1997;26:381–98. doi: 10.3109/10715769709097818. [DOI] [PubMed] [Google Scholar]
- 5.Ayoola GA, Folawewo AD, Adesegun SA, Abioro OO, Adepoju-Bello AA, Coker HA. B Phytochemical and antioxidant screening of some plants of apocynaceae from South West Nigeria. African J of Plant Sci. 2008;2:124–8. [Google Scholar]
- 6.Robards K, Prernzler PD, Tucker G, Swatsitang P, Glover W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999;66:401–36. [Google Scholar]
- 7.Woodman OL, Chan EC. Vascular and anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol Physiol. 2004;31:786–90. doi: 10.1111/j.1440-1681.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 8.Alan Crozier, Jennifer Burns, Azlina A Aziz, Amanda J Stewart, Helena S Rabiasz, Gareth I Crozier A, Burns J, Aziz AA, Stewart AJ, Rabiasz HS, Jenkins GI, et al. Antioxidant flavonols from fruits, vegetables and beverages: Measurements and bioavailability. Biol Res. 2000;33:79–88. doi: 10.4067/s0716-97602000000200007. [DOI] [PubMed] [Google Scholar]
- 9.Hussain A, Virmani OP, Sharma A, Kumar A, Misra LN. Lucknow, India: Central Institute of Medicinal and Aromatic Plants; 1998. Major Essential oil Bearing Plants of India. [Google Scholar]
- 10.Ambasta S P. Editor in Chief The Useful Plants of India. India: National Institute of Science and Communication (CSIR); 2000 [Google Scholar]
- 11.Kanimozhi P, Ilango K, Chowdary A, Arun B, Ammar M Musa Adam, Nethaji A. Microwave Assisted Extraction Of Artemisia pallens For Tyrosinase Iihibitory Activity. Int J Pharm Bio Sci. 2010;1:1–6. [Google Scholar]
- 12.Malik EP, Singh MB. New Delhi: Kalyani Publishers; 1980. Plant Enzymology and Hittoenzymology.1st ed. [Google Scholar]
- 13.Mervat MM El Far, Hanan AA Taie. Antioxidant activities, total anthrocynins, phenolics and flavonoids contents of some sweet potato genotypes under stress of different concentrations of sucrose and sorbitol. Australian J Basic Applied Sc. 2009;3:3609–16. [Google Scholar]
- 14.Sanja SD, Sheth NR, Patel NK, Patel D, Patel B. Characterization And Evaluation Of Antioxident Activity Of Portulaca oleracea. Int J Pharma Pharmaceut Sci. 2009;1:74–84. [Google Scholar]
- 15.Ayoola GA, Folawewo AD, Adesegun SA, Abioro OO, Adepoju-Bello AA, Coker HA. Phytochemical and antioxidant screening of some plants of apocynaceae from South West Nigeria. African J Plant Sci. 2008;2:124–8. [Google Scholar]
- 16.Ruikar A, Kamble G, Puranik VG, Nirmala R. Deshpande Antimicrobial Screening of a Medicinal Plant – Artemisia pallens. Int J Pharm Tech Res. 2009;1:1164–6. [Google Scholar]
- 17.Praveen Ashok, Upadhaya K. Analgesic and Anti-inflammatory properties of Artemisia pallens Wall Ex.DC. Pharma Res. 2010;3:249–56. [Google Scholar]


