Abstract
Neuroblastoma is the most common extracranial solid tumor in infants and young children. Current treatments are not always effective and new therapies are needed. We examined efficacy of combination of the small molecule Bcl-2 inhibitor HA14-1 (HA) and the dietary isoflavonoid apigenin (APG) in human malignant neuroblastoma cells. Dose-response studies indicated that treatment with HA and APG for 24 h synergistically reduced cell viability in human malignant neuroblastoma SK-N-DZ, SH-SY5Y, and IMR32 cells. For further studies, we selected SK-N-DZ cells that showed the highest sensitivity following treatment with 2.5 μM HA, 100 μM APG, or combination (2.5 μM HA + 100 μM APG). Wright staining showed increase in morphological features of apoptosis. Cell cycle distribution and Annexin V assay showed that combination therapy caused more apoptosis than either treatment alone. Western blotting revealed that combination therapy down regulated angiogenic factors and also induced extrinsic pathway of apoptosis with activation of caspase-8 for Bid cleavage to tBid. Alterations in Bax and Bcl-2 levels resulted in an increase in Bax:Bcl-2 ratio to activate intrinsic pathway of apoptosis with mitochondrial release of cytochrome c into the cytosol and activation of proteases. Increases in calpain and caspase-3 activities generated 145 kD spectrin break down product (SBDP) and 120 kD SBDP, respectively. Results showed that combination of HA and APG could be used for down regulation of angiogenic factors and activation of extrinsic and intrinsic pathways of apoptosis in malignant neuroblastoma cells.
Keywords: apigenin, apoptosis, Bcl-2 inhibitor, caspase, neuroblastoma
Introduction
Neuroblastoma is the most common extracranial solid tumor in childhood, accounting for approximately 15% of the total pediatric oncology deaths [1]. Neuroblastoma begins from any neural crest element of the sympathetic nervous system and mostly occurs in one of the adrenal glands but it can also occur in neck, chest, abdomen, and pelvis [1].
Bcl-2 is a potent suppressor of apoptosis [2] and Bcl-2 mediated resistance to chemotherapy is a major problem in neuroblastoma [3, 4]. The small molecule HA14-1 (henceforth called HA) is a cell-permeable, non-peptide apoptosis-inducer that binds to and inactivates Bcl-2 [5]. This small molecule induces translocation of Bax from cytosol to mitochondria and cells deficient in Bax exhibit resistance to HA, suggesting that Bax is essential for HA mediated apoptosis [5]. HA sensitizes the Bcl-2 overexpressing cancer cells to apoptosis in a number of malignancies such as colon [6], leukemia [7], prostate [8], and breast [9] cancers.
Phytochemicals including flavonoids and isoflavonoids are abundantly found in nature and have been highly regarded for their anti-tumor effects on multiple human malignancies [10]. Dietary isoflavonoids including apigenin (henceforth called APG) may play a role in modulating a number of key elements in cellular signal transduction pathways in cancers [11]. APG showed dose-dependent and time-dependent cytotoxicity, G2/M arrest, and activation of caspase-9 and caspase-3 in esophageal squamous cell carcinoma KYSE-510 cells [12] and activation of caspase-7, caspase-10, caspase-9, and caspase-3 in HepG2 cells [13] for apoptosis. APG activates caspases in breast cancer MDA-MB-453 cells [14] and causes G2/M arrest in breast cancer SK-BR-3 cells due to upregulation of p53, p21, and Bax for apoptosis [15]. Further, APG down regulated NF-κB and increased Bax:Bcl-2 ratio in prostate cancer LNCap cells [16] and it also down regulated Bcl-2 and VEGF in prostate cancer PC-3 cells [17] for apoptosis. The cytotoxicity of APG is partly due to decrease in anti-apoptotic Bcl-xL and Bcl-2 levels and increase in pro-apoptotic Bax level for caspase activation in prostate cancer 22Rv1 cells [18], indicating that down regulation of anti-apoptotic Bcl-2 proteins potentiates APG effects for apoptosis.
In this study, we showed synergistic efficacy of combination of HA and APG in three malignant neuroblastoma cell lines. Our results indicated that combination therapy was more efficacious than monotherapy to induce apoptosis through activation of both extrinsic and intrinsic pathways in SK-N-DZ cells.
Materials and methods
Cell Culture and drugs
Human malignant (neuroblastic or N-type) neuroblastoma SK-N-DZ (amplified N-Myc and wild-type p53), SH-SY5Y (single copy N-Myc and wild-type p53), and IMR32 (amplified N-Myc and wild-type p53) cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and grown separately in 75-cm2 flasks containing 10 ml of DMEM (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT) and 1% penicillin and streptomycin (GIBCO-Invitrogen, Grand Island, NY) at 37°C in a fully-humidified incubator containing 5% CO2. Prior to drug treatments, cells were starved in DMEM containing only 1% FBS for 24 h. HA (Tocris Bioscience, Ellisville, MO) and APG (Sigma-Aldrich) were purchased and dissolved in dimethyl sulfoxide (DMSO) to make stock solutions and aliquots were stored at −20°C until ready to use.
MTT assay for dos- response and cell viability
Dose-response studies were conducted by MTT assay using 1×104 cells in 100 μl medium per well in a 96-well plate and growing at 37°C in an incubator with 5% CO2. Drugs were added at various concentrations (alone or in combinations and 1 h time interval was given in between two drugs in case of combination treatment) to the wells and incubated for 24 h. MTT solution (10 μl of 5 mg MTT/ml) was added to all wells and incubated for 4 h at 37°C. Then, 100 μl of DMSO was added to each well to dissolve the formazan (MTT metabolic product) and optical density (OD) was read at 570 nm. Cell viability data were analyzed using Compusyn software (ComboSyn, Paramus, NJ) to generate a combination index (CI). Conventionally, CI >1 indicates antagonism, CI = 1 indicates additive effect, and CI < 1 indicates synergism at effective dose (ED).
Wright staining for analysis of morphological features of apoptosis
For detection of morphological features of apoptosis, cells from each treatment were washed with PBS and were sedimented by using centrifuge Eppendorf 5804R (Brinkmann Instruments, Westbury, NY, USA) at 106 g for 5 min. Cells were fixed with 95% ethanol before Wright staining. Morphology of apoptotic cells were examined under the optical microscopy.
Cell cycle analysis
Following treatment, cells were collected by trypsinization and centrifugation, then washed with PBS, and fixed with 70% ethanol. Cells were labeled with propidium iodide (PI) solution (0.05 mg/ml PI, 2 mg/ml RNase A, 0.01% Triton X-100 in PBS) and incubated for 30 min at room temperature in darkness. Cell cycle (DNA content) was analyzed using an Epics XL-MCL Flow Cytometer (Beckman Coulter, Fullerton, CA).
Determination of apoptosis by flow cytometry
Following treatments, attached and detached cells were harvested, washed with cold PBS, resuspended in 1× binding buffer (0.1 M HEPES/NaOH, pH 7.4, 1.4 M NaCl, 25 mM CaCl2), stained with Annexin V-FITC/PI staining kit (BD Biosciences, San Jose, CA) and incubated for 15 min at room temperature in darkness. Cells were analyzed using an Epics XL-MCL Flow Cytometer (Beckman Coulter).
Protein extraction and Western blot analysis
Western blotting was used for analyzing specific proteins. Briefly, total protein samples from cells were denatured, and loaded onto the SDS-polyacrylamide gradient (4-20%) gels (Bio-Rad, Hercules, CA), resolved by electrophoresis, and electroblotted to membranes. The blots were incubated with a primary IgG antibody followed by incubation with an alkaline horseradish peroxidase (HRP)-conjugated secondary IgG antibody. Specific protein bands on the blots were detected by HRP/H2O2 catalyzed oxidation of luminol in alkaline condition using enhanced chemiluminescence (ECL) system (GE Healthcare Bio-Sciences, Piscataway, NJ) followed by autoradiography. Autoradiograms were scanned on a UMAX PowerLook Scanner (UMAX, Fremont, CA) using Photoshop software (Adobe Systems, Seattle, WA), and OD of each band was determined using NIH Image software.
Statistical analysis
Results were analyzed using Minitab® 15 Statistical Software (Minitab Inc., State College, PA). Results were expressed as mean ± standard error of mean (SEM) of separate experiments (n≥3) and compared by one-way analysis of variance (ANOVA) followed by Fisher's post hoc test. Difference between two treatments was considered significant at * p<0.05 or ** p<0.001.
Results
Effect of treatments on cell viability
Various doses of HA and APG were used as monotherapy and combination therapy to examine treatment efficacy in decreasing cell viability in SK-N-DZ, SH-SY5Y, and IMR32 cells (Fig. 1). Combination of HA and APG dose-dependently decreased the cell viability (Fig. 1A). Synergistic effect of HA + APG was confirmed in SK-N-DZ (CI = 0.133 at ED50), SH-SY5Y (CI = 0.05 at ED50), and IMR32 (CI = 0.37 at ED50) cells (Fig. 1A). Notably, SK-N-DZ cell line exhibited maximum efficacy in reducing cell viability following treatment with 2.5 μM HA, 100 μM APG, or 2.5 μM HA + 100 μM APG. We selected above doses of the drugs and SK-N-DZ cell line for further experiments.
Fig. 1.
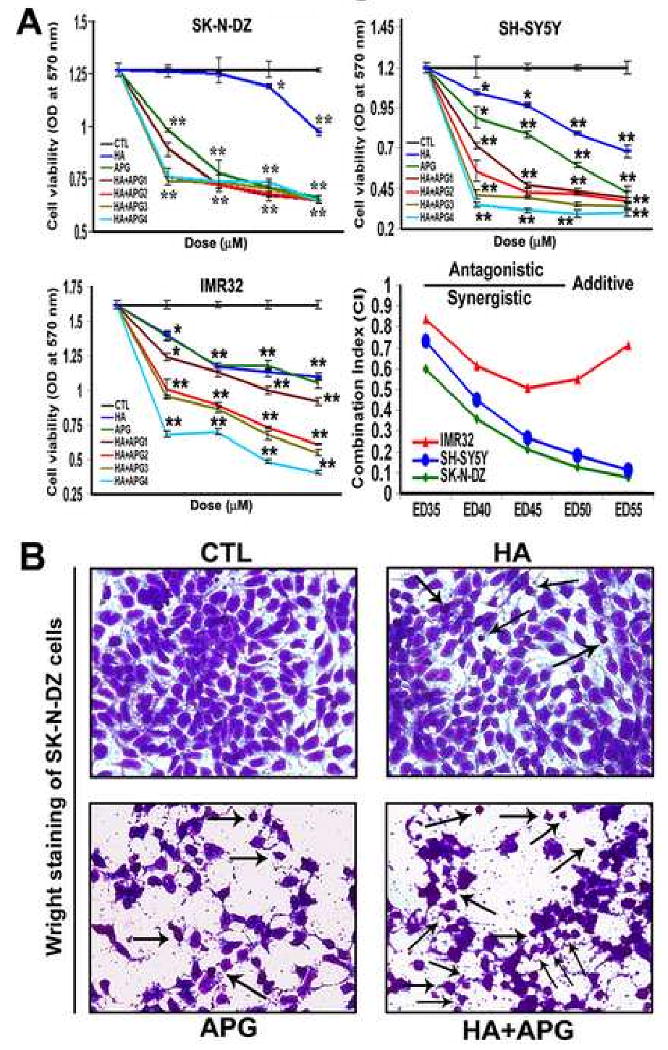
Decrease in cell viability and increase in morphological features of apoptosis in human malignant neuroblastoma cells. (A) Determination of dose-response and combination index (CI) in SK-N-DZ, SH-SY5Y, and IMR32 cells. Treatments (24 h): control (CTL), HA (2.5, 5, 10 and 20 μM), APG (50, 100, 200, 400 μM), HA + APG1 (2.5 + 50, 2.5 + 100, 2.5 + 200, and 2.5 + 400 μM), HA + APG2 (5 + 50, 5 + 100, 5 + 200, and 5 + 400 μM), HA + APG3 (10 + 50, 10 + 100, 10 + 200, and 10 + 400 μM), and HA + APG4 (20 + 50, 20 + 100, 20 + 200, and 20 + 400 μM). Combination of HA and APG showed synergism in reducing cell viability in all neuroblastoma cell lines. Finally, SK-N-DZ cell line and CTL, 2.5 μM HA, 100 μM APG, and 2.5 μM HA + 100 μM APG treatment groups were selected for further studies. (B) Wright staining for morphological features of apoptosis (arrows) in SK-N-DZ cells. Treatments (24 h): CTL, 2.5 μM HA, 100 μM APG, and 2.5 μM HA + 100 μM APG.
Wright staining for morphological features of apoptosis
Wright staining was used to evaluate morphological features of apoptosis in SK-N-DZ cells following treatments with HA, APG, and HA + APG (Fig. 1B). Wright-stained cells under the light microscope showing morphological features such as cell shrinkage, chromatin condensation, and membrane blebbing indicated apoptotic death. Treatment with 2.5 μM HA + 100 μM APG maximally induced morphological features of apoptosis in SK-N-DZ cells (Fig. 1B).
Cell cycle analysis
We performed flow cytometry following treatments with HA, APG, and HA + APG in SK-N-DZ cells to observe whether the apoptotic death occurred due to any alteration in the cell cycle (Fig. 2). We found a marked change in cell cycle distribution when compared with control cells. Flow cytometric analysis of cell cycle demonstrated that HA + APG accumulated more subG1 apoptotic population than monotherapy in SK-N-DZ cells (Fig. 2A).
Fig. 2.
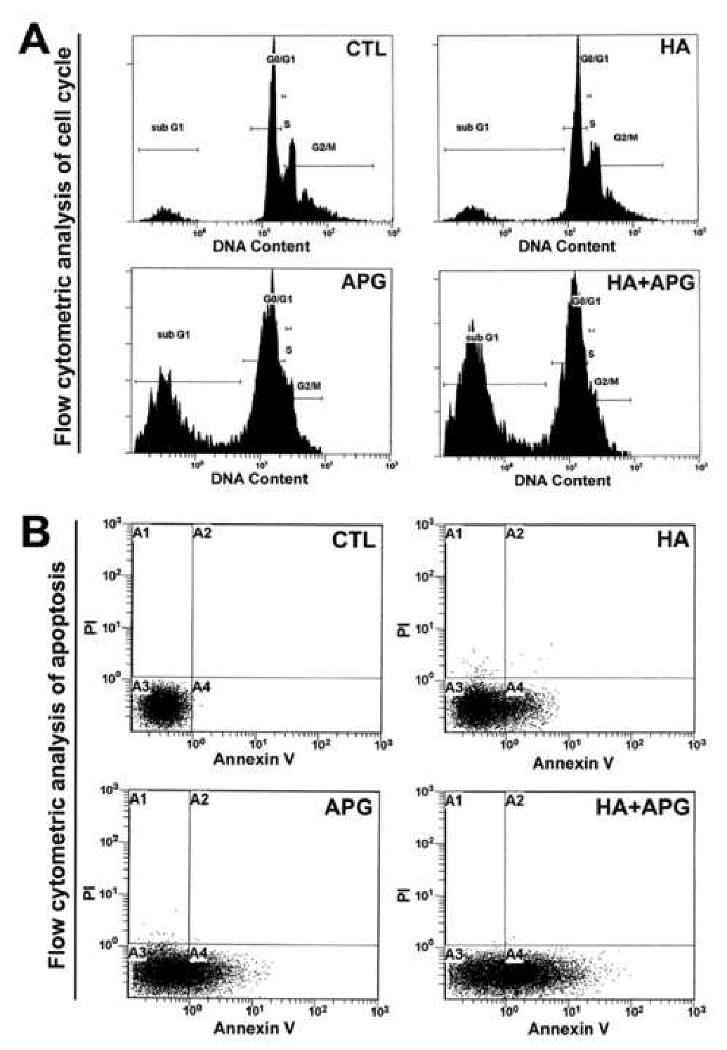
Flow cytometric analyses of cell cycle and apoptosis. Treatments (24 h): CTL, 2.5 μM HA, 100 μM APG, and 2.5 μM HA + 100 μM APG. (A) Cell cycle analysis. (B) Flow cytometric analysis for apoptosis.
Annexin V-FITC/PI analysis
Annexin V-FITC/PI double labeling technique was used to distinguish necrotic (A2) and apoptotic (A4) death in SK-N-DZ cells after the treatments (Fig. 2B). Combination therapy was more effective in inducing apoptotic death than monotherapy (Fig. 2B). Annexin V-FITC/PI assay confirmed that mode of cell death was exclusively apoptosis due to accumulation of only Annexin V-positive/PI-negative (early apoptotic) population maximally after combination therapy (Fig. 2B).
Combination therapy inhibited expression of angiogenic factors
Vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) are highly overexpressed in neuroblastoma to promote angiogenesis and tumor progression, respectively. We employed Western blotting to elucidate the anti-angiogenic and anti-tumor effects of HA + APG in SK-N-DZ cells (Fig. 3). Expression of β-actin was used as a loading control (Fig. 3A). We found strong suppression of expression of both VEGF and EGFR in SK-N-DZ cells following combination therapy (Fig. 3A).
Fig. 3.
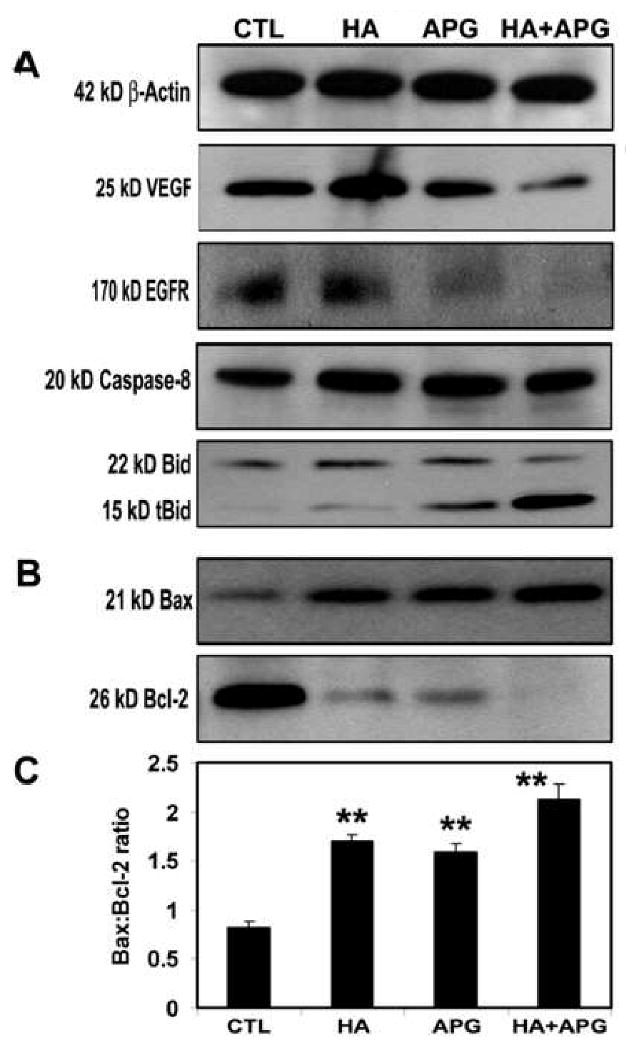
Western blotting for examining angiogenic factors and molecules in extrinsic pathway of apoptosis. Treatments (24 h): CTL, 2.5 μM HA, 100 μM APG, and 2.5 μM HA + 100 μM APG. (A) Representative Western blots to show levels of 42 kD β-actin, 25 kD VEGF, 170 kD EGFR, 20 kD active caspase-8, and 22 kD Bid cleavage to 5 kD tBid. (B) Representative Western blots to show upregulation of 21 kD Bax, and down regulation of 26 kD Bcl-2 to trigger the intrinsic pathway of apoptosis. (C) Densitometric analysis to show Bax:Bcl-2 ratio.
Combination therapy induced activation of caspase-8 and cleavage of Bid to tBid
We examined the changes in apoptosis related proteins in SK-N-DZ cells after treatments (Fig. 3A). We found activation of caspase-8 in generation of 20 kD active caspase-8 fragment, which cleaved 22 kD Bid to 15 kD tBid (Fig. 3A) for mitochondrial translocation to trigger apoptosis.
Combination therapy induced apoptosis with an increase in Bax:Bcl-2 ratio
We examined the levels of expression of Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic) proteins (Fig. 3B) to determine the changes in Bax:Bcl-2 ratio (Fig. 3C). Cells treated with HA + APG showed an increase in Bax expression and a decrease in Bcl-2 expression (Fig. 3B). We performed densitometric analysis to determine the Bax:Bcl-2 ratio (Fig. 3C). The Bax:Bcl-2 ratio was significantly increased after treatment with HA + APG, indicating involvement of mitochondrial events as well for apoptosis.
Combination therapy caused release of cytochrome c from mitochondria and activation of caspase-3
We examined the cytosolic cytochrome c and caspase-3 levels (Fig. 4). Treatment with HA + APG caused an increase in cytosolic cytochrome c and activation of caspase-3 (Fig. 4). Caspase-3 causes cleavage of various cellular substrates and induces morphological and biochemical features of apoptosis including cell shrinkage, DNA fragmentation, chromatin condensation, membrane blebbing.
Fig. 4.
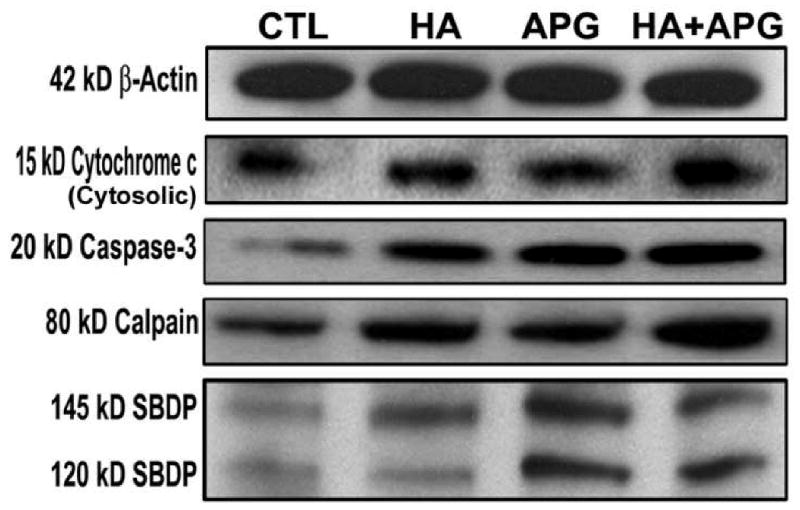
Western blotting for examining molecules in intrinsic pathway of apoptosis. Treatments (24 h): CTL, 2.5 μM HA, 100 μM APG, and 2.5 μM HA + 100 μM APG. (A) Representative Western blots to show levels of 42 kD β-actin, 15 kD cytochrome c (cytosolic), 20 kD active caspase-3, 80 kD calpain, 145 kD SBDP, and 120 kD SBDP.
Combination therapy activated calpain and increased calpain and caspase-3 activities for α-spectrin degradation
We also determined activation of calpain, a major pro-apoptotic cysteine protease, in neuroblastoma SK-N-DZ cells following treatments with HA, APG, and HA + APG (Fig. 4). Calpain and caspase-3 activities are known to be activated simultaneously in course of apoptosis. So, we assessed calpain and caspase-3 activities in the formation of calpain-specific 145 kD spectrin breakdown product (SBDP) and caspase-3-specific 120 kD SBDP, respectively, in SK-N-DZ cells (Fig. 4). We found increases in 145 kD SBDP and 120 kD SBDP following combination therapy indicating increases in activities of calpain and caspase-3, respectively, in SK-N-DZ cells.
Discussion
In this study, we demonstrated for the first time that combination of HA and APG worked synergistically to reduce cell viability in human malignant neuroblastoma SK-N-DZ, SH-SY5Y, and IMR32 cells. Morphological and biochemical studies indicated that combination of HA and APG caused more apoptosis than either treatment alone in SK-N-DZ cells. Previous studies reported induction of dose-dependent cytotoxicity and apoptosis by HA in leukemia [2] and glioblastoma [19] and by APG in HepG2 [13], prostate cancer 22Rv1 [18], and esophageal squamous cell carcinoma KYSE-510 [12] cells. Combination of APG with gemcitabine in pancreatic cancer [20] and retinoic acid with genistein in neuroblastoma [21] showed better efficacy than single drug in inhibiting cell viability and inducing apoptosis.
The massive accumulation of cells in subG1 phase and Annexin V-FITC/PI binding assay showed the mode of cell death was apoptosis and not necrosis. Earlier reports demonstrated that APG caused G2/M arrest for apoptosis in breast cancer SK-BR-3 cells [15]. Combination of APG with gemcitabine induced apoptosis due to cell cycle arrest in pancreatic cancer cells [20]. A previous study also used Annexin V-FITC/PI assay to report that APG induced apoptosis in prostate carcinoma PC-3 cells [17].
We found that combination of HA and APG inhibited expression of VEGF and EGFR. As a potent inducer of angiogenesis, VEGF is associated with cancer cell proliferation, migration, and vascular permeability and it is overexpressed in neuroblastoma [22]. Elevated EGFR is involved in cancer development and progression [23]. VEGF mediated upregulation of Bcl-2 is associated with decreased apoptosis in neuroblastoma cells [24]. APG suppresses EGFR in thyroid cancer cells [25] and inhibits VEGF in prostate carcinoma PC-3 cells [17].
Combination therapy worked via extrinsic pathway through Bid cleavage to tBid in SK-N-DZ cells for apoptosis. This finding is correlated with previous reports where APG activated caspase-8 in breast cancer [14] and prostate cancer [26] cells for apoptosis. Caspase-8-dependent cleavage of Bid to tBid provides a link between extrinsic and intrinsic pathways of apoptosis.
Levels of expression of Bcl-2 family proteins regulate the commitment of cells to apoptosis [27]. Levels of expression of the pro-apoptotic Bax and the anti-apoptotic Bcl-2 proteins were altered resulting in significant increase in Bax:Bcl-2 ratio after combination therapy. This observation is in agreement with the earlier studies demonstrating that HA prevents Bcl-2 interaction with Bax to cause apoptosis in glioblastoma cells [19] and APG increases Bax and decreases Bcl-2 expression leading to an increase in Bax:Bcl-2 ratio in prostate [16, 18] and neuroblastoma [28] cells. Increased Bax:Bcl-2 ratio could trigger the mitochondrial release of pro-apoptotic factors into the cytosol [29, 30] for apoptosis via intrinsic pathway.
The Bcl-2 family proteins regulate the release of cytochrome c from mitochondria [30]. Mitochondrial release of cytochrome c into the cytosol is a pre-condition for activation of caspases for apoptosis through intrinsic pathway [30, 31]. Western blotting showed that combination therapy caused activation of caspase-3. This finding is correlated with previous studies showing that APG induces apoptosis in human prostate cancer [18] and neuroblastoma [28] cells due to activation of caspase-3 and cleavage of specific substrate [32].
The Ca2+-dependent cysteine protease calpain plays an important role in apoptosis [33]. We also found an increase in calpain expression following combination therapy. Co-operation between calpain and caspase-3 has previously been demonstrated in apoptosis [34]. Increased calpain and caspase-3 activities can cause cleavage of 270 kD α-spectrin at specific sites to generate 145 kD SBDP and 120 kD SBDP [33], respectively. We found increases in calpain and caspase-3 activities in SK-N-DZ cells for apoptosis.
In conclusion, our results showed that combination of HA and APG worked synergistically in reducing cell viability in malignant neuroblastoma cells and suppressed expression of angiogenic factors and activated both the extrinsic and intrinsic pathways for increasing apoptosis in SK-N-DZ cells.
Acknowledgments
This work was supported in part by the R01 grants (NS-57811 and CA-91460) from the National Institutes of Health (Bethesda, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 2.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dole M, Nuñez G, Merchant AK, Maybaum J, Rode CK, Bloch CA, Castle VP. Bcl-2 inhibits chemotherapy-induced apoptosis in neuroblastoma. Cancer Res. 1994;54:3253–3259. [PubMed] [Google Scholar]
- 4.Ikeda H, Hirato J, Akami M, Matsuyama S, Suzuki N, Takahashi A, Kuroiwa M. Bcl-2 oncoprotein expression and apoptosis in neuroblastoma. J Pediatr Surg. 1995;6:805–808. doi: 10.1016/0022-3468(95)90752-1. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Freeman A, Liu J, Dai Q, Lee RM. The apoptotic effect of HA14-1, a Bcl-2-interacting small molecular compound, requires Bax translocation and is enhanced by PK11195. Mol Cancer Ther. 2002;1:961–967. [PubMed] [Google Scholar]
- 6.Sinicrope FA, Penington RC, Tang XM. Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human colon cancer cells. Clin Cancer Res. 2004;10:8284–8292. doi: 10.1158/1078-0432.CCR-04-1289. [DOI] [PubMed] [Google Scholar]
- 7.Reiners JJ, Jr, Kessel D. Susceptibility of myelomonocytic leukemia U937 cells to the induction of apoptosis by the non-peptidic Bcl-2 ligand HA14-1 is cell cycle phase-dependent. Cancer Lett. 2005;221:153–163. doi: 10.1016/j.canlet.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An J, Chervin AS, Nie A, Ducoff HS, Huang Z. Overcoming the radioresistance of prostate cancer cells with a novel Bcl-2 inhibitor. Oncogene. 2007;26:652–661. doi: 10.1038/sj.onc.1209830. [DOI] [PubMed] [Google Scholar]
- 9.Witters LM, Witkoski A, Planas-Silva MD, Berger M, Viallet J, Lipton A. Synergistic inhibition of breast cancer cell lines with a dual inhibitor of EGFR-HER-2/neu and a Bcl-2 inhibitor. Oncol Rep. 2007;17:465–469. [PubMed] [Google Scholar]
- 10.Chen D, Chen MS, Cui QC, Yang H, Dou QP. Structure-proteasome-inhibitory activity relationships of dietary flavonoids in human cancer cells. Front Biosci. 2007;12:1935–1945. doi: 10.2741/2199. [DOI] [PubMed] [Google Scholar]
- 11.Ramos S. Cancer chemoprevention and chemotherapy: dietary polyphenols and signaling pathways. Mol Nutr Food Res. 2008;52:507–526. doi: 10.1002/mnfr.200700326. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Zhao XH, Wang ZJ. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol In Vitro. 2009 doi: 10.1016/j.tiv.2009.04.007. In press. [DOI] [PubMed] [Google Scholar]
- 13.Khan TH, Sultana S. Apigenin induces apoptosis in Hep G2 cells: possible role of TNF-alpha and IFN-γ. Toxicology. 2006;217:206–212. doi: 10.1016/j.tox.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 14.Choi EJ, Kim GH. Apigenin Induces Apoptosis through a Mitochondria/Caspase-Pathway in Human Breast Cancer MDA-MB-453 Cells. J Clin Biochem Nutr. 2009;44:260–265. doi: 10.3164/jcbn.08-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi EJ, Kim GH. Apigenin causes G2/M arrest associated with the modulation of p21Cip1 and Cdc2 and activates p53-dependent apoptosis pathway in human breast cancer SK-BR-3 cells. J Nutr Biochem. 2009;20:285–290. doi: 10.1016/j.jnutbio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- 17.Shukla S, Gupta S. Suppression of constitutive and tumor necrosis factor alpha-induced nuclear factor NF-κB activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: correlation with down-regulation of NF-κB-responsive genes. Clin Cancer Res. 2004;10:3169–3178. doi: 10.1158/1078-0432.ccr-03-0586. [DOI] [PubMed] [Google Scholar]
- 18.Shukla S, Gupta S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic Biol Med. 2008;44:1833–1845. doi: 10.1016/j.freeradbiomed.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manero F, Gautier F, Gallenne T, Cauquil N, Grée D, Cartron PF, Geneste O, Grée R, Vallette FM, Juin P. The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 2006;66:2757–2764. doi: 10.1158/0008-5472.CAN-05-2097. [DOI] [PubMed] [Google Scholar]
- 20.Strouch MJ, Milam BM, Melstrom LG, McGill JJ, Salabat MR, Ujiki MB, Ding XZ, Bentrem DJ. The flavonoid apigenin potentiates the growth inhibitory effects of gemcitabine and abrogates gemcitabine resistance in human pancreatic cancer cells. Pancreas. 2009;38:409–415. doi: 10.1097/MPA.0b013e318193a074. [DOI] [PubMed] [Google Scholar]
- 21.Das A, Banik NL, Ray SK. Retinoids induce differentiation and downregulate telomerase activity and N-Myc to increase sensitivity to flavonoids for apoptosis in human malignant neuroblastoma SH-SY5Y cells. Int J Oncol. 2009;34:757–765. doi: 10.3892/ijo_00000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaghloul N, Hernandez SL, Bae JO, Huang J, Fisher JC, Lee A, Kadenhe-Chiweshe A, Kandel JJ, Yamashiro DJ. Vascular endothelial growth factor blockade rapidly elicits alternative proangiogenic pathways in neuroblastoma. Int J Oncol. 2009;34:401–407. [PMC free article] [PubMed] [Google Scholar]
- 23.Hegymegi-Barakonyi B, Eros D, Szántai-Kis C, Breza N, Bánhegyi P, Szabó GV, Várkondi E, Peták I, Orfi L, Kéri G. Tyrosine kinase inhibitors - Small molecular weight compounds inhibiting EGFR. Curr Opin Mol Ther. 2009;11:308–321. [PubMed] [Google Scholar]
- 24.Beierle EA, Strande LF, Chen MK. VEGF upregulates Bcl-2 expression and is associated with decreased apoptosis in neuroblastoma cells. J Pediatr Surg. 2002;37:467–471. doi: 10.1053/jpsu.2002.30868. [DOI] [PubMed] [Google Scholar]
- 25.Yin F, Giuliano AE, Van Herle AJ. Signal pathways involved in apigenin inhibition of growth and induction of apoptosis of human anaplastic thyroid cancer cells (ARO) Anticancer Res. 1999;19:4297–4303. [PubMed] [Google Scholar]
- 26.Morrissey C, O'Neill A, Spengler B, Christoffel V, Fitzpatrick JM, Watson RW. Apigenin drives the production of reactive oxygen species and initiates a mitochondrial mediated cell death pathway in prostate epithelial cells. Prostate. 2005;63:131–142. doi: 10.1002/pros.20167. [DOI] [PubMed] [Google Scholar]
- 27.Danial NN. BCL-2 family proteins: critical checkpoints of apoptotic cell death. Clin Cancer Res. 2007;13:7254–7263. doi: 10.1158/1078-0432.CCR-07-1598. [DOI] [PubMed] [Google Scholar]
- 28.Das A, Banik NL, Ray SK. Mechanism of apoptosis with the involvement of calpain and caspase cascades in human malignant neuroblastoma SH-SY5Y cells exposed to flavonoids. Int J Cancer. 2006;119:2575–2785. doi: 10.1002/ijc.22228. [DOI] [PubMed] [Google Scholar]
- 29.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. Erratum in: Nature 407 (2000) 767. [DOI] [PubMed] [Google Scholar]
- 31.Kim R. Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer. 2005;103:1551–1560. doi: 10.1002/cncr.20947. [DOI] [PubMed] [Google Scholar]
- 32.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 33.Karmakar S, Weinberg MS, Banik NL, Patel SJ, Ray SK. Activation of multiple molecular mechanisms for apoptosis in human malignant glioblastoma T98G and U87MG cells treated with sulforaphane. Neuroscience. 2006;141:1265–1280. doi: 10.1016/j.neuroscience.2006.04.075. [DOI] [PubMed] [Google Scholar]
- 34.Neumar RW, Xu YA, Gada H, Guttmann RP, Siman R. Cross-talk between calpain and caspase proteolytic systems during neuronal apoptosis. J Biol Chem. 2003;278:14162–14167. doi: 10.1074/jbc.M212255200. [DOI] [PubMed] [Google Scholar]


