Abstract
Neuroblastoma is a pediatric extracranial tumor and a major cause of death in children under age 2. Conventional therapy shows inefficacy in most cases and thus development of new therapeutic strategies is urgently needed. We explored the efficacy of combination of the small molecule Bcl-2 inhibitor HA14-1 (HA) and the isoflavonoid genistein (GST) in human malignant neuroblastoma SK-N-BE2 and SH-SY5Y cells. Combination of 10 μM HA and 250 μM GST was optimal for SK-N-BE2 cells and combination of 5 μM HA and 100 μM GST was optimal for SH-SY5Y cells for induction of apoptosis. Phase-contrast microscopy and Wright staining showed morphological features of apoptosis. Cell cycle analysis and Annexin V-FITC/PI binding assay showed that combination of HA and GST was more effective in inducing apoptosis in both cell lines than either HA or GST alone. Western blotting showed that combination of HA and GST caused upregulation of Bax and down regulation of Bcl-2 resulting in increased Bax:Bcl-2 ratio and mitochondrial release of cytochrome c, Smac, and AIF. Down regulation of survival factors such as NF-κB, N-Myc, and survivin promoted apoptosis. Activation of caspase-8, calpain, and caspase-3 occurred in course of apoptosis. Increased calpain and caspase-3 activities were confirmed in the degradation of α-spectrin to 145 kD spectrin break down product (SBDP) and 120 kD SBDP, respectively. Thus, combination of HA and GST could serve as a promising therapeutic strategy for increasing apoptosis in different human malignant neuroblastoma cells.
Keywords: apoptosis, Bcl-2, caspase, genistein, HA14-1
1. Introduction
Neuroblastoma is a pediatric extracranial tumor that exhibits complex clinical and biological heterogeneity (Brodeur et al., 2003). It is a tumor of the sympathetic nervous system and it originates mostly in adrenal gland and also in neck, chest, abdomen, and pelvis. Using aggressive multimodal therapy such as surgery, stem cell transplantation, radiation, and chemotherapy, the survival rate of children over 18 months is very low due to poor response to traditional treatment strategies (Weinstein et al., 2003). Therefore, development of novel therapeutic strategy is urgently needed for treatment of neuroblastoma in infants.
Neuroblastoma is often associated with overexpression of oncogenic survival factors and resistance to chemotherapy (Ho et al., 2002). The anti-apoptotic Bcl-2 protein prevents apoptosis and maintains cellular homeostasis (Danial, 2007). Bcl-2 mediated inhibition of chemotherapy in neuroblastoma has previously been reported (Dole et al., 1994; Ikeda et al., 1995). The molecular mechanism by which Bcl-2 performs its anti-apoptotic functions is considered to be due to blockage of mitochondrial pathway of apoptosis (Cheng et al., 2001). Thus, targeting anti-apoptotic functions of Bcl-2 could be a potential strategy for treatment of neuroblastoma. We used a small molecule Bcl-2 inhibitor named HA14-1, which fits into hydrophobic cleft of Bcl-2 protein and disrupts its anti-apoptotic functions (Manero et al., 2006). HA14-1 induces apoptosis due to inhibition of Bcl-2 interaction and binding with pro-apoptotic Bax in glioblastoma cells (Manero et al., 2006). A previous report demonstrated that HA14-1 reduced mitochondrial membrane potential and promoted activation ofcaspase-9 and caspase-3 for apoptosis in leukemia cells (Wang et al., 2006).
Recently, we reported that chemotherapeutic agents in combination are more effective than monotherapy in neuroblastoma (Das et al., 2009). Genistein (GST) is a major isoflavonoid in various soy products and it exhibits anti-cancer properties by inducing apoptosis. Anti-proliferative and anti-tumor properties of GST are attributed to negative regulation of protein tyrosine kinase activity (Akiyama et al., 1987). Further, GST has been shown to induce apoptosis in breast cancer MDA-MB-231 cells (Li et al., 1999), prostate cancer PC3 cells (Davis et al., 1998), and leukemia T cells (Spinozzi et al., 1994) by cell cycle arrest and down regulation of Bcl-2 protein. Recently, GST has been shown to induce apoptosis and cell cycle arrest at G2/M phase in neuroblastoma SK-N-MC cells (Ismail et al., 2007). We have earlier reported that GST induces apoptosis in human neuroblastoma SH-SY5Y cells by upregulating Bax and down regulating Bcl-2 and activating calpain and mitochondria mediated apoptotic pathway (Das et al., 2006).
As HA14-1 inhibits Bcl-2 (Manero et al., 2006) and GST induces apoptosis by down regulation of Bcl-2 to some extent (Spinozzi et al., 1994; Davis et al., 1998; Li et al., 1999; Das et al., 2006), use of both in combination can very effectively down regulate Bcl-2 to enhance the apoptotic process. In this investigation, we for the first time explored the effectiveness of combination of the small molecule Bcl-2 inhibitor HA14-1 and GST for increasing induction of apoptosis in human malignant neuroblastoma SK-N-BE2 and SH-SY5Y cells. Previous report showed that combination of HA14-1 with PK11195, an antagonist of mitochondrial peripheral benzodiazepine receptor, caused Bax translocation to mitochondria for cytochrome c release for induction of apoptosis (Chen et al., 2002). Our data provided the evidence that HA14-1 down regulated Bcl-2 and increased the efficacy of GST for suppressing other cell survival factors such as N-Myc and NF-κB for activating caspase cascades to induce apoptosis in two human malignant neuroblastoma cell lines.
2. Results
2.1. Changes in cell viability
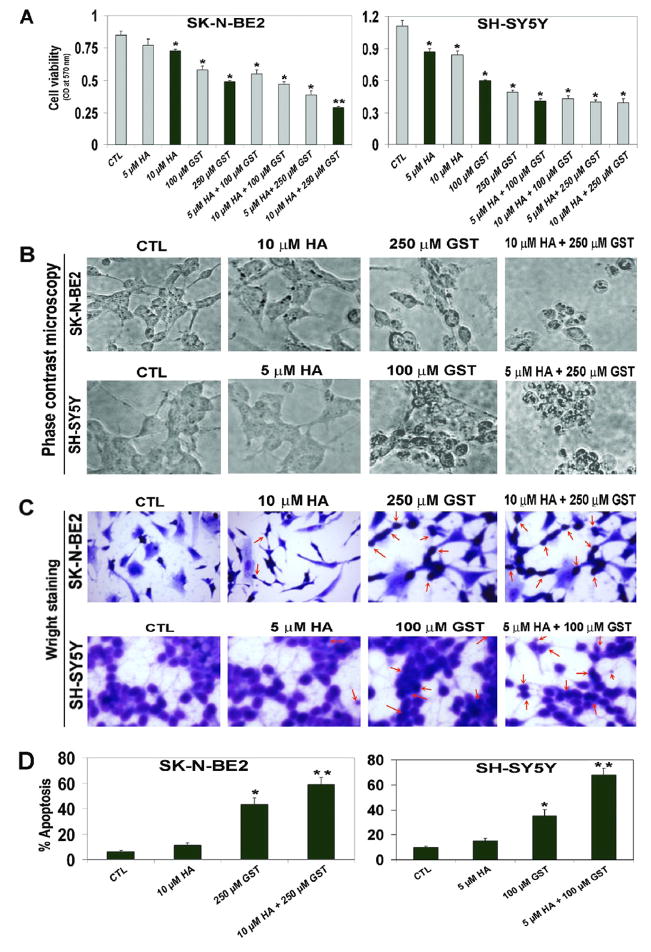
To examine the effect of HA, GST, and combination of these drugs (HA + GST) on viability of SK-N-BE2 and SH-SY5Y cells, we conducted MTT assay (Fig. 1). Results indicated that 10 μM HA or 250 μM GST as monotherapy and 10 μM HA + 250 μM GST as combination therapy could show the best efficacy for reducing cell viability in SK-N-BE2 cells (Fig. 1A). However, 5 μM HA or 100 μM GST as monotherapy and 5 μM HA + 100 μM GST as combination therapy exhibited the maximum efficacy for reducing cell viability in SH-SY5Y cells (Fig. 1A). Therefore, we selected these treatments in other experiments such as phase-contrast microscopy, Wright staining, cell cycle analysis, Annexin V-FITC/PI binding assay, and Western blotting.
Fig. 1.
Decreases in cell viability and increases in apoptosis in SK-N-BE2 and SH-SY5Y cells. Treatments (24 h): control (CTL), HA (5 and 10 μM), GST (100 and 250 μM), 5 μM HA + 100 μM GST, 5 μM HA + 250 μM GST, 10 μM HA + 100 μM GST, 10 μM HA + 250 μM GST. (A) MTT assay to assess cell viability following treatments. Based on efficacy, we decided to use 10 μM HA, 250 μM GST, and 10 μM HA + 250 μM GST in SK-N-BE2 cells; and 5 μM HA, 100 μM GST, and 5 μM HA + 100 μM GST in SH-SY5Y cells in all other experiments. (B) Phase-contrast microscopy to examine morphological changes indicating apoptosis in cells following treatments. (C) Wright staining to evaluate the morphological features of apoptosis (arrows indicate apoptotic cells). (D) Bar diagrams to show percent apoptosis based on Wright staining. Significant difference from CTL value was indicated by * p<0.05 or ** p<0.001.
2.2. Phase-contrast microscopy and Wright staining for morphological features of apoptosis
To evaluate relative efficacies of HA, GST, and HA + GST in inducing morphological features of apoptosis in SK-N-BE2 and SH-SY5Y cells, we performed phase-contrast microscopy (Fig. 1B) and Wright staining (Fig. 1C). Observations under the phase-contrast microscope showed that cells following treatment with HA, GST, and HA + GST decreased growth and committed varying degrees of apoptotic death with shrinkage and deformation of cell bodies (Fig. 1B). Following treatments, Wright staining of cells distinctly confirmed such morphological features of apoptosis (cell shrinkage, chromatin condensation, and membrane blebbing) under the light microscope (Fig. 1C), as we reported previously (Karmakar et al., 2006). Based on the Wright staining of cells from control, monotherapy, and combination therapy, we determined the percentage of apoptotic cells (Fig. 1D). Combination therapy induced more apoptosis than a monotherapy in both human malignant neuroblastoma cell lines.
2.3. Cell cycle analysis
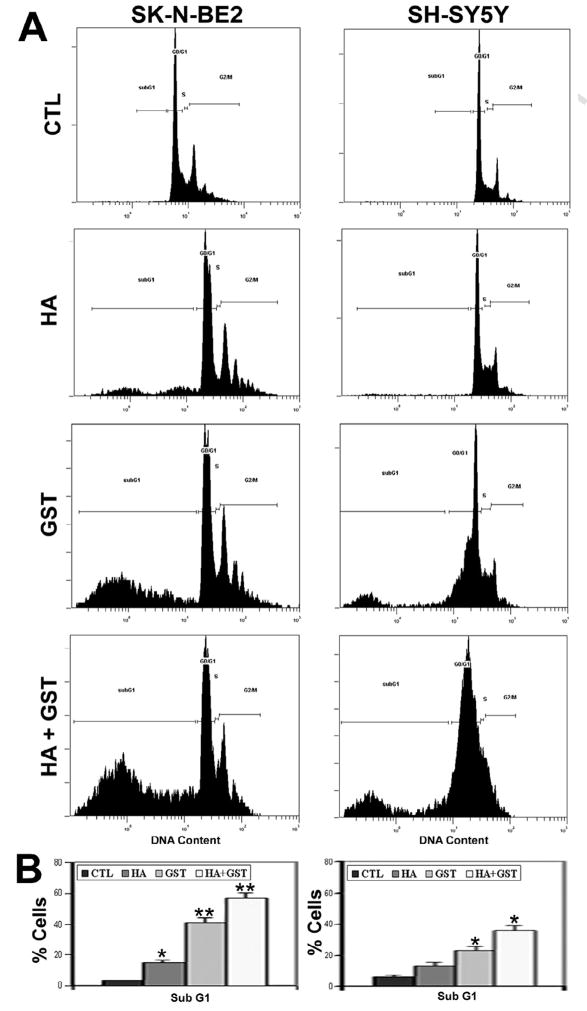
Since apoptosis often occurs as a consequence of blocking of a cell cycle phase, we used flow cytometry to examine whether the apoptotic death in SK-N-BE2 and SH-SY5Y cells following treatments with HA, GST, and HA + GST occurred due to any alteration in the cell cycle (Fig. 2). We found marked changes in cell cycle following combination therapy when we compared with control cells (Fig. 2A). Treatments with HA, GST, and HA + GST significantly increased apoptotic subG1 phase [12% (p<0.001), 38% (p<0.03), and 54% (p<0.04), respectively] in SK-N-BE2 cells (Fig. 2B). But only treatment with GST or HA + GST exhibited significant increases in apoptotic subG1 phase [17% (p<0.001) and 30% (p<0.04), respectively] in SH-SY5Y cells (Fig. 2B).
Fig. 2.
Cell cycle ananlysis by flow cytometry. Treatments (24 h): control (CTL), 10 μM HA, 250 μM GST, and 10 μM HA + 250 μM GST in SK-N-BE2 cells; and CTL, 5 μM HA, 100 μM GST, and 5 μM HA + 100 μM GST in SH-SY5Y cells. (A) Distribution of cells in different phases of cell cycle. (B) Bar diagrams to show amounts of cells in sub G1 phase. Significant difference from CTL value was indicated by * p<0.05 or ** p<0.001.
2.4. Annexin V-FITC/PI binding assay
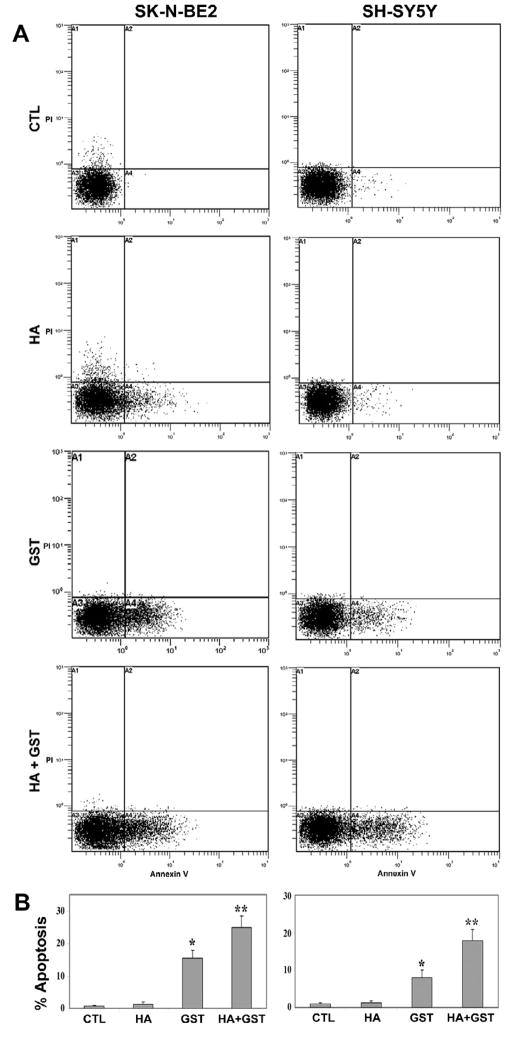
Following Annexin V-FITC/PI binding assay, we performed flow cytometry to detect the cells undergoing apoptosis (Fig. 3). An increased accumulation of cells in A4 area of the double parameter dot plots indicated apoptotic cells (Fig. 3A). Treatment with HA showed non-significant increase in apoptotic populations in both cell lines. However, treatment with GST or HA + GST caused significant increases in apoptotic populations in SK-N-BE2 cells [14.8% (p<0.01) and 24.1% (p<0.003), respectively] and also in SH-SY5Y cells [7.01% (p<0.03) and 16.9% (p<0.008), respectively], compared with corresponding control cells (Fig. 3B).
Fig. 3.
Annexin V-FITC/PI binding assay and flow cytometry for determination of apoptosis. Treatments (24 h): control (CTL), 10 μM HA, 250 μM GST, and 10 μM HA + 250 μM GST in SK-N-BE2 cells; and CTL, 5 μM HA, 100 μM GST, and 5 μM HA + 100 μM GST in SH-SY5Y cells. (A) Double parameter dot plot of FITC fluorescence (x-axis) versus PI fluorescence (y-axis) for examination of apoptotic cells. (B) Bar diagrams to show amounts of apoptotic cells in A4 quadrant. Significant difference from CTL value was indicated by * p<0.05 or ** p<0.001.
2.5. Increase in Bax:Bcl-2 ratio
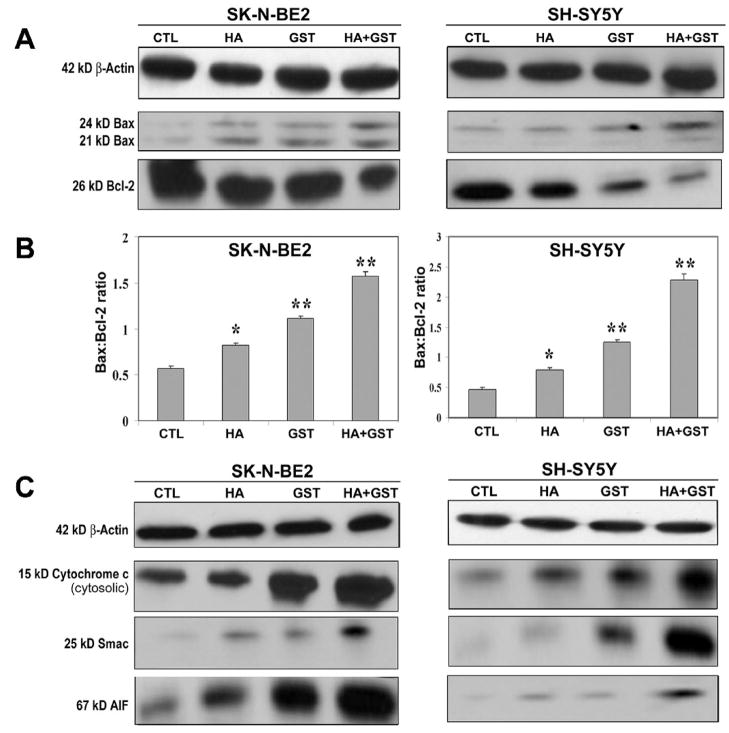
Balance in expression of pro-apoptotic Bax and anti-apoptotic Bcl-2 is a key factor for keeping the cells alive. An increase in Bax:Bcl-2 ratio due to any treatment affects mitochondrial permeability and drives the cell in the direction of apoptotic phase. We performed Western blotting to examine the relative levels of expression of Bax and Bcl-2 in the cells after the treatments (Fig. 4). To ensure the equal loading of protein samples, we monitored expression of β-actin as an internal control (Fig. 4A). We detected different levels of Bax and Bcl-2 following treatments (Fig. 4A) and determined the Bax:Bcl-2 ratio (Fig. 4B). We found significant increases in Bax:Bcl-2 ratio after treatments with HA, GST, and HA + GST in SK-N-BE2 cells [43% (p<0.002), 94% (p<0.004), and 175% (p<0.001), respectively] and also in SH-SY5Y cells [68% (p<0.002), 168% (p<0.003), and 387% (p<0.001), respectively], compared with corresponding control cells (Fig. 4B). Obviously, treatment with HA + GST caused the highest increase in Bax:Bcl-2 ratio in both cell lines, indicating an involvement of mitochondria in apoptotic process.
Fig. 4.
Western blotting for determination of Bax:Bcl-2 ratio and mitochondrial release of pro-apoptotic molecules into the cytosol in SK-N-BE2 and SH-SY5Y cells. Treatments (24 h): control (CTL), 10 μM HA, 250 μM GST, and 10 μM HA + 250 μM GST in SK-N-BE2 cells; and CTL, 5 μM HA, 100 μM GST, and 5 μM HA + 100 μM GST in SH-SY5Y cells. (A) Representative Western blots to show expression of 42 kD β-actin, 21 and 24 kD Bax, and 26 kD Bcl-2. (B) Densitometric analysis to show the Bax:Bcl-2 ratio. (C) Representative Western blots to show the levels of 42 kD β-actin, 15 kD cytochrome c, 25 kD Smac, and 67 kD AIF. Significant difference from CTL value was indicated by * p<0.05 or ** p<0.001.
2.6. Increased cytosolic levels of cytochrome c, Smac, and AIF
The increased Bax:Bcl-2 ratio could cause alteration in mitochondrial permeability to release pro-apoptotic molecules such as cytochrome c, Smac, and apoptosis-inducing factor (AIF) from mitochondria to cytosol to trigger downstream cascades of apoptosis. We performed Western blotting to examine cytosolic levels of the pro-apoptotic molecules cytochrome c, Smac, and AIF following treatments with HA, GST, and HA+GST in both SK-N-BE2 and SH-SY5Y cell lines (Fig. 4C). Again, we used uniform expression of β-actin as an internal standard in Western blotting. In both cell lines, we found some increases in cytosolic level of cytochrome c, Smac, and AIF after treatment with HA or GST alone but the most dramatic increases in cytosolic levels of these pro-apoptotic molercules only after treatment with HA + GST (Fig. 4C).
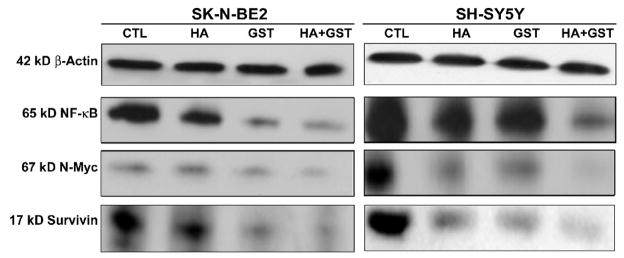
2.7. Down regulation of survival factors
We further performed Western blotting to assess the expression of survival factors such as nuclear factor-kappa B (NF-κB), N-Myc, and survivin in SK-N-BE2 and SH-SY5Y cells after treatments with HA, GST, and HA + GST (Fig. 5). Expression of β-actin was used as an internal standard in Western blotting. Treatment with HA or GST alone partially down regulated the expression of NF-κB, N-Myc, and survivin while treatment with HA + GST caused the most dramatic decrease in these survival factors in both cell lines (Fig. 5).
Fig. 5.
Western blotting for examination of levels of survival factors in SK-N-BE2 and SH-SY5Y cells. Treatments (24 h): control (CTL), 10 μM HA, 250 μM GST, and 10 μM HA + 250 μM GST in SK-N-BE2 cells; and CTL, 5 μM HA, 100 μM GST, and 5 μM HA + 100 μM GST in SH-SY5Y cells. Representative Western blots to show the levels of 42 kD β-actin, 65 kD NF-κB, 67 kD N-Myc, and 17 kD survivin in the cytosol. Down regulation of these survival factors facilitated apoptosis.
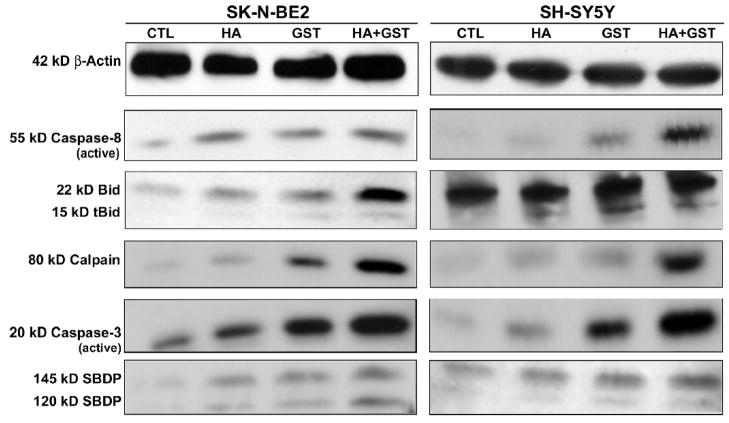
2.8. Activation caspase-8 and cleavage of Bid
Activation and proteolytic activity of caspase-8 were also examined by Western blotting (Fig. 6). Expression of β-actin was used as an internal standard in Western blotting. Treatment of SK-N-BE2 with HA or GST alone resulted in generation of active caspase-8. In case of SH-SY5Y cells, there was no apparent difference in expression of active caspase-8 between control cells and cells treated with HA, whereas cells treated with GST or HA + GST showed dramatic increases in activate caspase-8. Activation of caspase-8 induces proteolytic cleavage of Bid to tBid, which is then translocated to mitochondrial membrane for aiding mitochondrial release of pro-apoptotic factors into the cytosol. We found the highest increases in tBid in SK-N-BE2 cells as well as in SH-SY5Y cells after treatment with HA + GST (Fig. 6).
Fig. 6.
Western blotting for examination of levels of cysteine proteases and degradation of substrates during apoptosis. Treatments (24 h): control (CTL), 10 μM HA, 250 μM GST, and 10 μM HA + 250 μM GST in SK-N-BE2 cells; and CTL, 5 μM HA, 100 μM GST, and 5 μM HA + 100 μM GST in SH-SY5Y cells. Representative Western blots to show activation of 55 kD caspase-8 and 22 kD Bid cleavage to 15 kD tBid, and levels of 80 kD calpain and active 20 kD caspase-3. Increased activities of calpain and caspase-3 generated 145kD SBDP and 120 kD SBDP, respectively, in SK-N-BE2 and SH-SY5Y cells following treatment with HA + GST.
2.9. Upregulation of calpain and caspase-3
We also examined the levels of calpain, a major pro-apoptotic cysteine protease, in both neuroblastoma cell lines following treatments with HA, GST and HA + GST (Fig. 6). The treatments resulted in progressive increases in expression of 80 kD calpain in SK-N-BE2 cells as well as in SH-SY5Y cells. Caspase-3 is widely regarded as the key executioner caspase in apoptosis. In SK-N-BE2 cells, the production of active 20 kD caspase-3 was progressively increased after treatments with HA, GST, and HA + GST. Likewise, SH-SY5Y cells also exhibited increases in formation of active 20 kD caspase-3 after the treatments.
2.10. Degradation of α-spectrin indicated calpain and caspase-3 activities
We examined the calpain and caspase-3 activities in the formation of calpain-specific 145 kD α-spectrin break down product (SBDP) and caspase-3-specific 120 kD SBDP, respectively (Fig. 6). The maximum increases in 145 kD SBDP and 120 SBDP occurred in both cell lines after the treatment with HA + GST, indicating the highest increases in calpain and caspase-3 activities for induction of apoptosis in both neuroblastoma cell lines. Therefore, the treatment with HA + GST should be used for adeptly increasing apoptosis in human malignant neuroblastoma cells.
3. Discussion
Isoflavonoids present in soy products have always received extensive attention worldwide due to their anti-cancer and anti-mutagenic properties. In the current study, we demonstrated for the first time that combination of the Bcl-2 inhibitor HA14-1 (HA) and GST enhanced apoptosis in two human malignant neuroblastoma SK-N-BE2 and SH-SY5Y cell lines. The combination of these agents most effectively induced apoptosis in both cell lines by inhibiting Bcl-2 and increasing Bax:Bcl-2 ratio to release mitochondrial pro-apoptotic molecules, suppressing anti-apoptotic survival factors such as NF-κB, N-Myc, and survivin, and activating extrinsic and intrinsic caspase pathways.
Treatment with combination of HA and GST significantly reduced the cell viability and altered the morphological features of apoptosis in both human neuroblastoma SK-N-BE2 and SH-SY5Y cell lines (Fig. 1). We previously reported induction of apoptosis in SH-SY5Y cells using GST (Das et al., 2006) and also combination of retinoid and GST (Das et al., 2009). The enhancement of apoptosis following treatment with HA + GST in both neuroblastoma cell lines was further confirmed by flow cytometric analysis of cell cycle, showing robust accumulation of cells in subG1 phase (Fig. 2). Annexin V-FITC/PI binding assay further showed that the mode of cell death was apoptosis, not necrosis (Fig. 3). Previous studies reported that HA (Manero et al., 2006) and GST (Spinozzi et al., 1994; Davis et al., 1998; Li et al., 1999; Das et al., 2006; Das et al., 2009) induced apoptosis in a variety of cell lines.
The Bcl-2 family proteins consist of anti-apoptotic (e.g., Bcl-2) and pro-apoptotic (e.g., Bax) proteins and relative levels of Bacl-2 and Bax are primary regulators for cellular death by apoptosis (Reed, 1998). It is known from the previous reports that both HA (Manero et al., 2006) and GST (Spinozzi et al., 1994; Davis et al., 1998; Li et al., 1999; Das et al., 2006) can cause down regulation of Bcl-2. Our aim in this investigation was to explore whether combining both HA and GST could increase induction of apoptosis due to dramatic down regulation of Bcl-2. We examined the relative levels of Bax and Bcl-2 proteins in SK-N-BE2 and SH-SY5Y cells following treatments and our data suggested that combination of HA and GST was much more potent than HA or GST alone in both neuroblastoma cell lines to upregulate Bax and down regulate Bcl-2 (Fig. 4A) resulting in an increase in Bax:Bcl-2 ratio (Fig. 4B). The increase in Bax:Bcl-2 ratio could trigger the release of mitochondrial pro-apoptotic factors such as cytochrome c, Smac, and AIF into the cytosol for apoptosis (Green and Reed, 1998; Shimizu et al., 1999).
Our current observation of an increase in cytosolic level of cytochrome c (Fig. 4C) is in agreement with our previous study (Das et al., 2006) demonstrating that the release of cytochrome c from mitochondria to cytosol can activate caspase-9 for apoptosis. Another mitochondrial pro-apoptotic molecule called Smac (second mitochondrial activator of caspases) was found to be upregulated in both SK-N-BE2 and SH-SY5Y cells (Fig. 4C). Smac induces apoptosis by inhibiting the inhibitor-of-apoptosis proteins (IAPs) to cause indirect activation of caspases (Wilkinson et al., 2004). Similar to cytochrome c, mitochondrial Smac release is primarily regulated by Bcl-2 (Adrian et al., 2001). The increased cytosolic levels of Smac could potentially inhibit survivin, one of the IAPs, to promote the activation of caspases and thereby induce cell death (Adrian et al., 200; Wilkinson et al., 2004). We also found that combination HA and GST adeptly resulted in mitochondrial release of the pro-apoptotic molecule AIF into the cytosol (Fig. 4C). Translocation of AIF to nucleus can cause DNA fragmentation and thus promote caspase-independent apoptosis (Susin et al., 1999; Daugas et al., 2000).
We also examined anti-apoptotic survival factors, which are often overexpressed in cancers to prevent apoptosis and thereby confer resistance to therapeutic treatments. We found combination of HA and GST significantly down regulated NF-κB, N-Myc, and survivin in both SK-N-BE2 and SH-SY5Y cells (Fig. 5) to promote apoptosis. It is now well known that NF-κB is a major transcription factor that exerts anti-apoptotic effects resulting in survival of cancer cells (Beg and Baltimore, 1996). N-Myc is a member of the myc oncogene family and overexpression of N-Myc increases the malignancy in neurobalstoma (Hossain et al., 2008). Survivin, a potent member of the IAP family (Ambrosini et al., 1997), is associated with high-risk neuroblastoma in humans (Azuhata et al., 2001) and considered to be a poor prognostic marker of more aggressive type of neuroblastomas (Tajiri et al., 2001). Some reports have suggested a possible link between NF-κB and IAPs, because NF-κB promoted upregulation of IAPs and interestingly IAPs also upregulated NF-κB (Jeremias et al., 1998). On the contrary, down regulation of survivin could result in inhibition of NF-κB (Mitsiades et al., 2002) and down regulation of NF-κB (Notarbartolo et al., 2005) and NF-κB and IAPs (Karmakar et al., 2007) induced apoptosis. Recently, we reported that combination of a retinoid and GST could cause down regulation of N-Myc and IAPs to facilitate apoptosis in human neuroblastoma SH-SY5Y cells (Das et al., 2009). In this investigation, we show that combination of HA and GST caused down regulation of anti-apoptotic survival factors such as NF-κB, N-Myc, and survivin for activation of cysteine proteases for apoptosis.
In addition to activation of mitochondria-mediated intrinsic pathway of apoptosis, our results further showed that combination of HA and GST activated receptor-mediated extrinsic pathway of apoptosis through activation of caspase-8 and Bid cleavage to tBid in SK-N-BE2 and SH-SY5Y cells (Fig. 6). Our data correlated well with a previous report where GST in combination with arsenic trioxide caused activation of caspase-8 for Bid cleavage to tBid to trigger apoptosis in leukemia cells (Sánchez et al., 2008), however, this combination failed to down regulate expression of NF-κB. Our results showed that HA + GST effectively inhibited the cell survival factor NF-κB. Recently, we reported that combination of retinoid and GST caused activation caspase-8 for apoptosis in SH-SY5Y cells (Das et al., 2009). However, it is advantageous to use Bcl-2 inhibitor HA14-1 because it further facilitates the Bcl-2 down regulating property of GST, thereby increasing Bax:Bcl-2 ratio for induction of apoptosis. Another striking result from our investigation was upregulation of calpain (Fig. 6), a cysteine protease known to play an important role in apoptosis (Das et al., 2006; Karmakar et al., 2006; Karmakar et al., 2007). Increase in Bax:Bcl-2 ratio has been known to be associated with overexpression of calpain for induction of apoptosis (Ray et al., 2000; Karmakar et al., 2007). The highest activation of caspase-3, the key executioner caspase, in SK-N-BE2 and SH-SY5Y cells was detected following treatment with HA + GST (Fig. 6). A recent report suggested that HA in combination with a flavonone naringenin induced apoptosis in leukemia cells by activation of caspase-3 (Jin et al., 2009). But this study did not suggest any role of HA and naringenin in activation of calpain. Our data showed the combination of HA and GST activated calpain along with caspase-3 to promote apoptotic cell death. We further confirmed that increases in both calpain and caspase-3 activities caused cleavage of α-spectrin to generate calpain-specific 145 kD SBDP and caspase-3-specific 120 kD SBDP in course of apoptosis (Fig. 6). We previously reported that GST (Das et al., 2006) and combination of retinoid and GST (Das et al., 2009) could cause activation of calpain and caspase-3 for cleavage of α-spectrin for apoptosis in SH-SY5Y cells.
In conclusion, our current results showed activation of both the extrinsic and intrinsic proteolytic pathways and suppression of cellular survival factors for increasing apoptosis in human malignant neuroblastoma SK-N-BE2 and SH-SY5Y cells following treatment with combination of HA and GST.
4. Experimental procedures
4.1. Cells and culture conditions
We purchased the human malignant neuroblastoma SK-N-BE2 and SH-SY5Y cell lines from American Type Cell Culture Collection (Manassas, VA, USA). SK-N-BE2 cell line was established from bone marrow aspirate of a 2-year old male patient with stage 4 neuroblastoma (Biedler et al., 1978) and later characterized to harbor mutant p53 (Tweedle et al., 2001). On the other hand, SH-SY5Y cell line is a 3rd generation neuroblastoma cell line derived from SK-N-SH cell line (Biedler et al., 1973). This cell line is derived from neural crest tumors of sympathetic nervous system and harbors wild-type p53 (Schwab et al., 1985). A previous study showed that Bcl-2 was highly expressed in SH-SY5Y cell line, when compared to SK-N-BE2 cell line (Hanada et al., 1993). Hence, this striking difference between these two malignant neuroblastoma cell lines makes an attractive model to study apoptosis inhibitory properties of the Bcl-2 molecule. Cells were grown in 75-cm2 flasks (Corning Corporation, Corning, NY, USA) containing cell culture medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin in a fully humidified incubator containing 5% CO2 at 37°C. The cell culture medium was DMEM (ATCC, Manassas, VA, USA) for growing SK-N-BE2 cell line and was RPMI 1640 (Mediatech, Herndon, VA, USA) for growing SH-SY5Y cell line. Prior to drug treatment, cells were grown till 80% confluency and then starved in their respective cell culture medium containing 2% FBS for 24 h. The Bcl-2 inhibitor HA14-1 (HA) (Tocris Bioscience, Ellisville, Missouri, USA) and genistein (GST) (Sigma Chemical, St. Louis, MO, USA) were purchased. Drugs were dissolved in dimethyl sulfoxide (DMSO) to make a stock solution and aliquot were stored at −20°C until ready to use. Dose-response studies were conducted to determine the suitable doses of the drugs for induction of apoptotic death.
4.2. MTT assay for determination of cell viability
Cell viability was determined using an MTT colorimetric assay kit (Millipore, Billerica, MA, USA). The fundamental principle of this assay is to measure the activity of mitochondrial enzyme system that converts yellow MTT to purple colored formazan. Both SK-N-BE2 and SH-SY5Y cells were seeded at 3×105 cells/well in two 96-well plates separately. Different doses of HA (5 and 10 μM) and GST (100 and 250 μM) and their combination (HA + GST) were added to each plate in triplicates and plates were incubated overnight in a humidified incubator containing 5% CO2 at 37°C. Then, MTT reagent was added (100 μl/well) in each plate and incubated for 4 h at 37°C. Formazan precipitate was dissolved by pipetting each well up and down with 100 μl of isopropyl alcohol. Plates were read on a DU800 spectrophotometer (Beckman Coulter, Fullerton, CA, USA) using 570 nm as the test wavelength. Cell viability data were analyzed using Compusyn software (Combosyn, Paramus, NJ, USA) to determine a combination index (CI) for synergism in drug combination studies (Chou, 2006). Conventionally, CI > 1 indicates antagonism, CI = 1 indicates additive effect, and CI < 1 indicates synergism at the effective doses. We found a very low CI (0.11) using 10 μM HA + 250 μM GST in SK-N-BE2 cell line and also a very low CI (0.21) using 5 μM HA + 100 μM GST in SH-SY5Y cell line. Therefore, these specific doses of the drugs and their combinations were selected for their synergistic inhibitory activity on cell growth in all other experiments.
4.3. Phase-contrast microscopy and Wright staining for examination of morphological features of apoptosis
Both cell lines in culture plates were treated with HA, GST, and HA + GST for 24 h and examined under the phase-contrast microscope. Treatments induced various morphological features of apoptosis in cells on the plates. Using phase-contrast microscopy black and white photographs were taken. Further, cells from each treatment were washed with PBS and sedimented on slides by using the Eppendorf 5804R centrifuge (Brinkmann Instruments, Westbury, NY, USA) at 106xg for 5min. Cells were fixed with 95% ethanol and stained with Wright staining (Karmakar et al., 2006). Morphological featues of apoptotic cells were detected under the light microscope. Morphological features of apoptosis included reduction of cell volume, chromatin condensation, and presence of membrane-bound apoptotic bodies (Karmakar et al., 2006). Four randomly selected fields were counted for at least 800 cells. The percentage of apoptotic cells was calculated from three separate experiments.
4.4. Flow cytometry for cell cycle analysis
Both SK-N-BE2 and SH-SY5Y cells were placed in separate 6-well culture plates and starved in low FBS (2%) supplemented medium for 24 h prior to drug treatment. Cells were treated with HA and GST alone and in combination and 1 h time interval was allowed between two drugs in case of combination treatment. Following treatments, cell were incubated for 24 h and then collected by trypsinization. For flow cytometric analysis, permeabilized cells were stained with propidium iodide (PI) (Roche Molecular Biochemicals, Indianapolis, IN, USA) for DNA content. Then, 5 ml of PBS was added for the resuspension of cells, followed by fixation of cells with 70% ethanol. Cells were labeled with PI staining solution (0.05 mg/ml PI, 2 mg/ml RNase A, and 0.01% Triton X-100 in PBS) and incubated for 30 min at room temperature in darkness. Cellular DNA content was then analyzed using an Epics XL-MCL Flow Cytometer (Beckman Coulter, Fullerton, CA, USA). All experiments were performed in triplicate and analyzed for statistical significance.
4.5. Flow cytometry for determination of apoptosis
We performed Annexin V-FITC/PI staining followed by flow cytometry for quantitative determination of percentage of cells undergoing apoptosis. Cells were treated in a similar fashion as described above for cell cycle analysis. Following treatments, attached and detached cells were harvested, washed with cold PBS, resuspended in 1x binding buffer (0.1 M HEPES/NaOH, pH 7.4, 1.4 M NaCl, 25 mM CaCl2), stained with Annexin V-FITC staining kit (BD Biosciences, CA, USA) and incubated for 15 min at room temperature in darkness. Cells were then analyzed using an Epics XL-MCL Flow Cytometer (Beckman Coulter, Fullerton, CA, USA). Both PI and Annexin V-FITC negative cells were considered normal; PI negative and Annexin V-FITC positive cells were considered early apoptotic; both PI and Annexin V-FITC positive cells were considered late necrotic; PI positive and Annexin V-FITC negative cells were considered mechanically injured during the experiment. All experiments were conducted in triplicates and analyzed for statistical significance.
4.6. Protein extraction and Western blotting
Cells from control and all treatments were detached by using cell scrapper and centrifuged for 10 min at 3000 rpm in Eppendorf 5804R (Brinkmann Instruments, Westbury, NY, USA) to obtain pellets in microcentrifuge tubes and then cells in each pellet were washed twice in PBS. Cells were resuspended in ice-cold (4°C) homogenizing buffer and then protein concentration was determined using CoomassieR Plus reagent (Pierce, Rockford, IL, USA) and spectrophotometric measurement at 595 nm. Samples were then mixed with an equal volume of a diluting buffer and boiled for 5 min. Proteins in each sample were separated by (4–20%) gradient gel (Bio-Rad Laboratories, Hercules, CA, USA) using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) at 200 mV for 45 min. Following electrophoresis, gels with the resolved proteins were electroblotted to PVDF membranes (Millipore, Billerica, MA, USA) using gel electroblotting Genie apparatus (Idea Scientific, Minneapolis, MN, USA). The membranes were blocked for 1 h in 5% non-fat milk before incubation with a primary antibody. All primary antibodies were obtained commercially (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and added at appropriate dilutions to the blots for incubation overnight on a shaker at 4°C. The blots were washed three times with a washing buffer (20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.1% Tween 20) and incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody (ICN Biomedicals, Aurora, OH, USA) at a 1:2000 dilution for2 h. Blots were placed in enhanced chemiluminescence (ECL) detection system (GE Healthcare Bio-Sciences Corporation, Piscataway, NJ, USA) for 3 min and exposed to X-OMAT AR films (Eastman Kodak, Rochester, NY, USA), as we described recently (Karmakar et al., 2008). The films were scanned on an EPSON Scanner using Photoshop software (Adobe Systems, Seattle, WA, USA) and optical density (OD) of each band was determined using the NIH Image software. All experiments were performed in triplicates and samples were analyzed for statistical significance.
4.7. Statistical analysis
Results were analyzed using Minitab® 15 Statistical software (Minitab, State College, PA, USA). Data were expressed as mean ± standard error of mean (SEM) of separate experiments (n≥3) and compared by one-way analysis of variance (ANOVA) followed by Fisher’s post hoc test. Difference between control and a single or combination treatment was considered significant at * p<0.05 or ** p<0.001.
Acknowledgments
This work was supported in part by the R01 grants (NS-57811 and R01 CA-91460) from the National Institutes of Health (Bethesda, MD) to S.K.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrain C, Creagh EM, Martin SJ. Apoptosis-associated release of Smac/Diablo from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J. 2001;20:6627–6636. doi: 10.1093/emboj/20.23.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Ambrosini G, Aidida C, Altieri DC. A novel antiapoptosis gene, survivin, expressed in cancer and lymphoma. Nature Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- Azuhata T, Scott D, Takamizawa S, Wen J, Davidoff A, Fukuzawa M, Sandler A. The inhibitor of apoptosis protein survivin is associated with high-risk behavior of neuroblastoma. J Pediatr Surg. 2001;36:1785–1791. doi: 10.1053/jpsu.2001.28839. [DOI] [PubMed] [Google Scholar]
- Beg AA, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Biedler JL, Helson L, Spengler BA. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973;33:2643–2652. [PubMed] [Google Scholar]
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978;38:3751–3757. [PubMed] [Google Scholar]
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- Chen J, Freeman A, Liu J, Dai Q, Lee RM. The apoptotic effect of HA14-1, a Bcl-2-interacting small molecular compound, requires Bax translocation and is enhanced by PK11195. Mol Cancer Ther. 2002;1:961–967. [PubMed] [Google Scholar]
- Cheng EH, Wei MC, Weiler S, Flavel RA, Mak TW, Lindsten T, Korsmeyer SJ. Bcl-2, Bcl-xL sequester BH3 domain-only molecules preventing Bax- and Bak-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- Danial NN. Bcl-2 family proteins: critical checkpoints of apoptotic cell death. Clin Cancer Res. 2007;13:7254–7263. doi: 10.1158/1078-0432.CCR-07-1598. [DOI] [PubMed] [Google Scholar]
- Das A, Banik NL, Ray SK. Mechanism of apoptosis with the involvement of calpain and caspase cascades in human malignant neuroblastoma SH-SY5Y cells exposed to flavonoids. Int J Cancer. 2006;119:2575–2585. doi: 10.1002/ijc.22228. [DOI] [PubMed] [Google Scholar]
- Das A, Banik NL, Ray SK. Retinoids induce differentiation and downregulate telomerase activity and N-Myc to increase sensitivity to flavonoids for apoptosis in human malignant neuroblastoma SH-SY5Y cells. Int J Oncol. 2009;34:757–765. doi: 10.3892/ijo_00000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugas E, Nochy D, Ravagnan L, Loeffler M, Susin SA, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a ubiquitous mitochondrial oxidoreductase involved in apoptosis. FEBS Lett. 2000;476:118–123. doi: 10.1016/s0014-5793(00)01731-2. [DOI] [PubMed] [Google Scholar]
- Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer. 1998;32:123–131. doi: 10.1080/01635589809514730. [DOI] [PubMed] [Google Scholar]
- Dole M, Nuñez G, Merchant AK, Maybaum J, Rode CK, Bloch CA, Castle VP. Bcl-2 inhibits chemotherapy-induced apoptosis in neuroblastoma. Cancer Res. 1994;54:3253–3259. [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Hanada M, Krajewski S, Tanaka S, Cazals-Hatem D, Spengler BA, Ross RA, Biedler JL, Reed JC. Regulation of Bcl-2 Oncoprotein Levels with Differentiation of Human Neuroblastoma Cells1. Cancer Res. 1993;53:4978–86. [PubMed] [Google Scholar]
- Ho R, Eggert A, Hishiki T, Minturn JE, Ikegaki N, Foster P, Camoratto AM, Evans AE, Brodeur GM. Resistance to chemotherapy mediated by TrkB in neuroblastomas. Cancer Res. 2002;62:6462–6466. [PubMed] [Google Scholar]
- Hossain MS, Ozaki T, Wang H, Nakagawa A, Takenobu H, Ohira M, Kamijo T, Nakagawara A. N-Myc promotes cell proliferation through a direct transactivation of neuronal leucine-rich repeat protein-1 (NLRR1) gene in neuroblastoma. Oncogene. 2008;27:6075–6082. doi: 10.1038/onc.2008.200. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Hirato J, Akami M, Matsumaya S, Suzuku N, Takahashi A, Kuroiwa M. Bcl-2 oncoprotein expression and apoptosis in neuroblastoma. J Pediatr Surg. 1995;30:805–808. doi: 10.1016/0022-3468(95)90752-1. [DOI] [PubMed] [Google Scholar]
- Ismail IA, Kang KS, Lee HA, Kim JW, Sohn YK. Genistein-induced neuronal apoptosis and G2/M cell cycle arrest is associated with MDC1 upregulation and PLK1 down regulation. Eur J Pharmacol. 2007;575:12–20. doi: 10.1016/j.ejphar.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Jeremias I, Kupatt C, Baumann B, Herr I, Wirth T, Debatin KM. Inhibition of nuclear factor-κB activation attenuates apoptosis resistance in lymphoid cells. Blood. 1998;91:4624–4631. [PubMed] [Google Scholar]
- Jin CY, Park C, Lee JH, Chung KT, Kwon TK, Kim GY, Choi BT, Choi YH. Naringenin-induced apoptosis is attenuated by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human leukemia U937 cells. Toxicol In Vitro. 2009;23:259–265. doi: 10.1016/j.tiv.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Banik NL, Patel SJ, Ray SK. Garlic compounds induced calpain and intrinsic caspase cascade for apoptosis in human malignant neuroblastoma SH-SY5Y cells. Apoptosis. 2007;12:671–684. doi: 10.1007/s10495-006-0024-x. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Banik NL, Patel SJ, Ray SK. Combination of all-trans retinoic acid and taxol regressed glioblastoma T98G xenografts in nude mice. Apoptosis. 2007;12:2077–2087. doi: 10.1007/s10495-007-0116-2. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Weinberg MS, Banik NL, Patel SJ, Ray SK. Activation of multiple molecular mechanisms for apoptosis in human malignant glioblastoma T98G and U87MG cells treated with sulforaphane. Neuroscience. 2006;141:1265–1280. doi: 10.1016/j.neuroscience.2006.04.075. [DOI] [PubMed] [Google Scholar]
- Karmakar S, Banik NL, Ray SK. Combination of all-trans retinoic acid and paclitaxel-induced differentiation and apoptosis in human glioblastoma U87MG xenografts in nude mice. Cancer. 2008;112:596–607. doi: 10.1002/cncr.23223. [DOI] [PubMed] [Google Scholar]
- Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase-8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Upadhyay S, Bhuiyan M, Sarkar FH. Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene. 1999;18:3166–3172. doi: 10.1038/sj.onc.1202650. [DOI] [PubMed] [Google Scholar]
- Manero F, Gautier F, Gallenne T, Cauquil N, Grée D, Cartron P, Olivier Geneste O, Grée R, Vallette FM, Juin P. The small organic compound HA14-1 prevents Bcl-2 interaction with Bax to sensitize malignant glioma cells to induction of cell death. Cancer Res. 2006;66:2757–2764. doi: 10.1158/0008-5472.CAN-05-2097. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi N, Treon SP, Anderson KC. Biologic sequelae of nuclear factor-κB blockade in multiple myeloma: therapeutic applications. Blood. 2002;99:4079–4086. doi: 10.1182/blood.v99.11.4079. [DOI] [PubMed] [Google Scholar]
- Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D’Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-κB activation levels and in IAP gene expression. Cancer Lett. 2005;224:53–65. doi: 10.1016/j.canlet.2004.10.051. [DOI] [PubMed] [Google Scholar]
- Ray SK, Fidan M, Nowak MW, Wilford GG, Hogan EL, Banik NL. Oxidative stress and Ca2+ influx upregulate calpain and induce apoptosis in PC12 cells. Brain Res. 852:326–333. doi: 10.1016/s0006-8993(99)02148-4. [DOI] [PubMed] [Google Scholar]
- Reed JC, Jurgensmeier JM, Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta. 1998;1366:127–137. doi: 10.1016/s0005-2728(98)00108-x. [DOI] [PubMed] [Google Scholar]
- Sánchez Y, Amrán D, Fernández C, de Blas E, Aller P. Genistein selectively potentiates arsenic trioxide-induced apoptosis in human leukemia cells via reactive oxygen species generation and activation of reactive oxygen species-inducible protein kinases (p38-MAPK, AMPK) Int J Cancer. 2008;123:1205–1214. doi: 10.1002/ijc.23639. [DOI] [PubMed] [Google Scholar]
- Schwab M, Harold EV, Bishop JM. Human N-myc gene contributes to neoplastic transformation of mammalian cells in culture. Nature. 1985;316:160–162. doi: 10.1038/316160a0. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Spinozzi F, Pagliacci MC, Migliorati G, Moraca R, Grignani F, Riccardi C, Nicoletti I. The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells. Leuk Res. 1994;18:431–439. doi: 10.1016/0145-2126(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Tajiri T, Tanaka S, Shono K, Kinoshita Y, Fujii Y, Suita S, Ihara K, Hara T. Quick quantitative analysis of gene dosages associated with prognosis in neuroblastoma. Cancer Lett. 2001;166:89–94. doi: 10.1016/s0304-3835(01)00434-7. [DOI] [PubMed] [Google Scholar]
- Tweddle DA, Malcolm AJ, Bown N, Pearson AD, Lunec J. Evidence for the development of p53 mutations after cytotoxic therapy in a neuroblastoma cell line. Cancer Res. 2001;61:8–13. [PubMed] [Google Scholar]
- Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA. 2000;97:7124–7129. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JL, Katzenstein HM, Cohn SL. Advances in the diagnosis and treatment of neuroblastoma. Oncologist. 2003;8:278–292. doi: 10.1634/theoncologist.8-3-278. [DOI] [PubMed] [Google Scholar]
- Wilkinson JC, Wilkinson AS, Scott FL, Csomos RA, Salvesen GS, Duckett CS. Neutralization of Smac/Diablo by inhibitors of apoptosis (IAPs). A caspase-independent mechanism for apoptotic inhibition. J Biol Chem. 2004;279:51082–51090. doi: 10.1074/jbc.M408655200. [DOI] [PubMed] [Google Scholar]