Abstract
Introduction
Muscle fibrosis is a prominent pathological feature that directly causes muscle dysfunction in Duchenne muscular dystrophy (DMD). The DMD mouse models, mdx mice and mdx mice with haploinsufficiency of the utrophin gene (mdx/utrn+/−), display progressive diaphragm fibrosis.
Materials and Methods
We performed unrestrained whole-body plethysmography (WBP) in mdx and mdx/utrn+/− mice, and compared them with wild-type controls.
Results
Respiratory function gauged by respiratory frequency, tidal volume, minute volume, peak inspiratory flow, and peak expiratory flow was significantly impaired in mdx mice. Consistent with more severe diaphragm fibrosis in mdx/utrn+/− mice, respiratory impairment was worse than in mdx mice at 6 months.
Discussion
WBP is useful in monitoring in vivo respiratory function of mdx and mdx/utrn+/− mice, and it may serve as an outcome measurement of therapies that target diaphragm fibrosis. Mdx/utrn+/− mice may be a better model than mdx mice for testing antifibrotic therapies, especially at a severe stage.
Keywords: Duchenne muscular dystrophy, mdx mice, diaphragm fibrosis, respiratory function, whole body non-invasive plethysmography
INTRODUCTION
DMD is the most common genetic muscle disease, affecting 1 in 3,500 live male births (1). Caused by a defective dystrophin gene on the X chromosome, DMD is characterized by progressive skeletal and cardiac muscle weakness with premature death (2). Muscle inflammation and fibrosis are prominent pathological features of DMD, which lead to muscle dysfunction and clinical weakness.
Mdx mice, which harbor a nonsense mutation in exon 23 of the dystrophin gene, have been widely used to study the pathophysiology and explore potential therapies for DMD. However, the muscular dystrophy phenotype in this mouse model is mild as compared to human DMD. Skeletal muscle inflammation starts at 3 weeks of age, peaks between 8 and 16 weeks, and then subsides spontaneously in limb muscles. Slowly progressive endomysial fibrosis only develops in the diaphragm (3–5) and causes impaired contractility (6, 7). To improve this model, mice deficient in both the dystrophin gene and its autosomal homolog utrophin gene (dko mice) were generated (8, 9). Dko mice display early onset of muscle dystrophy, severe muscle weakness, joint contracture, growth retardation, and premature death, which mimics the phenotype of human DMD (8). However, early death at around 10 weeks of age makes dko mice difficult to obtain or maintain for testing therapeutic interventions. To circumvent this difficulty, we previously characterized and quantified skeletal muscle fibrosis and inflammation in mdx/utrn+/− mice, and compared them with mdx mice (10). Diaphragm inflammation and fibrosis were worse in mdx/utrn+/− mice than in mdx mice at 3 and 6 months of age. At 6 months, diaphragm fibrosis was moderate in mdx mice but severe in mdx/utrn+/− mice. This may provide a better model for testing antifibrotic therapies (10).
There is no effective therapy for DMD at this point. Mdx mice have been widely used for testing potential therapies. In vivo functional improvement would be an important criterion to assess the effectiveness of potential therapies. Therefore, in this study we performed whole body plethysmography (WBP) in mdx and mdx/utrn+/− mice to address whether respiratory function was impaired in mdx mice and was more impaired in mdx/utrn+/− mice to correspond to more severe diaphragm fibrosis. We explored whether WBP could be used to serve as a functional measurement of diaphragm muscle dystrophy in mdx and mdx/utrn+/− mice.
MATERIALS AND METHODS
Animals
Male mdx mice (C57BL/10J) were purchased from the Jackson laboratory or obtained from crossbreeding of mdx and mdx/utrn+/− mice. Mdx/utrn+/− mice were kindly provided by Dr. Jill Rafael-Fortney (Columbus, OH) and were crossed with mdx mice (C57BL/10J) from the Jackson lab for at least 9 generations. Wild-type C57BL/10J mice were also purchased from the Jackson laboratory. All three types of mice were in the C57BL/10J background. Mice at age 3 months (BL10: n=19, mdx: n=21, and mdx/utrn+/−: n=17) and 6 months (BL10: n=26, mdx: n=24, and mdx/utrn+/−: n=20) were used for the study. We followed the Cleveland Clinic guide for the care and use of laboratory animals.
Respiratory function analysis
Respiratory function in conscious and unrestrained mice was assessed by whole body non-invasive plethysmography (WBP) (Buxco Research Systems, Sharon, CT) and Biosytems XA software. Unrestrained Plethysmograph was calibrated according to the manufacturer’s instructions (User manual for Whole Body Unrestrained Plethysmographs PLY3211). The Bias Flow Regulator was calibrated with room temperature and humidity and flow set at 1.2 liter per minute. Mice were placed in the “free moving” plethysmograph chamber and allowed to acclimate for 5 minutes. Afterwards, measurement of airway mechanics, including respiratory frequency (F), tidal volume (TV), minute volume (MV), peak inspiratory flow (PIF), and peak expiratory flow (PEF), were taken over a ten-minute interval. Values measured during the 10-minute sequence were then averaged.
Histopathological analysis
Muscle tissue was fresh-frozen in liquid nitrogen-cooled isopentane, sectioned at 8 micron (μm), stained with hematoxylin and eosin, and viewed under a bright field microscope.
Diaphragm collagen measurements
The diaphragm collagen content was measured by the Sircol collagen assay (Bioclor Ltd., Belfast, UK) following the manufacturer’s instructions. Briefly, Sirius red reagent was added to each diaphragm homogenate and mixed for 30 minutes. The collagen-dye complex was precipitated by centrifugation at 16,000 g for 5 minutes, washed with ethanol, and dissolved in 0.5 M NaOH. The samples were then transferred to a microplate reader, and the absorbance was determined at 540 nm.
Statistical analysis
Data were expressed as mean ±SD. Differences of measurements among different groups were evaluated by the Kruskal-Wallis test. The association between the diaphragm collagen content and the individual respiratory parameters was evaluated by the Pearson correlation. A p-value of <0.05 was considered statistically significant.
RESULTS
Respiratory function was impaired in mdx and mdx/utrn+/− mice at 3 months of age
At 3 months of age, mdx and mdx/utrn+/− mice displayed multifocal endomysial inflammation in respiratory muscles, including diaphragm and intercostal muscles (Fig. 1) (10), and mild endomysial fibrosis in the diaphragm (10). To address whether respiratory function was impaired in mdx and mdx/utrn+/− mice as a result of respiratory muscle pathology, we performed whole body non-invasive plethysmography on these mice at 3 months of age, and compared them with age-controlled wild-type BL10 controls. Respiratory frequency, tidal volume, minute volume, peak inspiratory flow, and peak expiratory flow were all significantly reduced in mdx and mdx/utrn+/− mice (Table 1). Tidal volume adjusted by body weight (ml/kg) was also significantly reduced in mdx (p<0.001) and mdx/utrn+/− mice (p<0.001) as compared with controls. There was no significant difference in the respiratory function parameters between mdx and mdx/utrn+/− mice at this age.
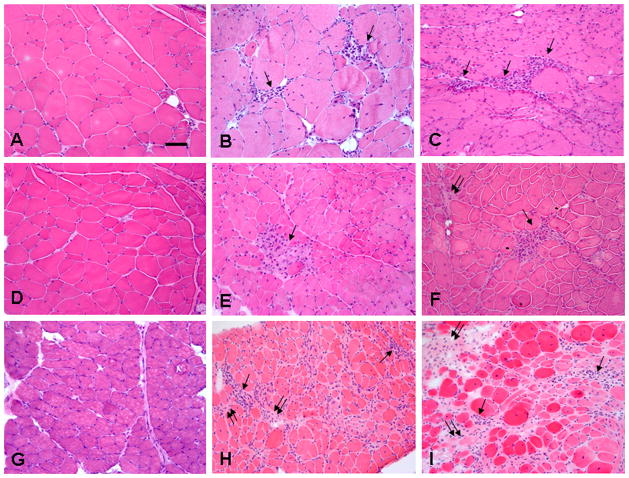
Figure 1.
Inflammation and fibrosis in respiratory muscles of mdx and mdx/utrn+/− mice.
H&E staining showed multifocal small foci of endomysial inflammatory cells (arrows) in intercostal muscles of mdx (B, E) and mdx/utrn+/− (C, F) mice at 3 (B, C) and 6 (E, F) months of age. Inflammatory cells (arrows) were more widespread in the endomysium of diaphragm muscles of mdx (H) and mdx/utrn+/− mice (I) at 6 months of age. Focal areas of increased endomysial collagen deposition were detected at 6 months of age (double arrows). They were mild in intercostal muscles of mdx/utrn+/− (F) mice, moderate in the diaphragm of mdx mice (H), and severe in the diaphragm of mdx/utrn+/− mice (I). Wild-type BL10 mice showed no inflammation or fibrosis in intercostal muscles at 3 (A) or 6 (D) months of age or diaphragm at 6 months of age (G). Bar=50μm.
Table 1.
Respiratory Parameters in BL10, Mdx, and Mdx/utrn+/− Mice at 3 Months
| BL10 | Mdx | Mdx/utrn+/− | * p1 | * p2 | * p3 | |
|---|---|---|---|---|---|---|
| F (breath/min) | 426.5±24.1 | 347.6±50.2 | 344.9±31.6 | <0.001 | <0.001 | 0.97 |
| TV (ml) | 0.37±0.05 | 0.29±0.04 | 0.28±0.05 | <0.001 | <0.001 | 0.55 |
| TV/BW (ml/kg) | 12.5±1.9 | 9.3±1.3 | 8.9±1.5 | <0.001 | <0.001 | 0.40 |
| MV (ml) | 154.4±19.4 | 100.5±20.9 | 95.5±19.4 | <0.001 | <0.001 | 0.48 |
| PIF (ml/s) | 10.7±1.6 | 7.6±1.5 | 7.3±1.1 | <0.001 | <0.001 | 0.51 |
| PEF (ml/s) | 8.1±1.1 | 6.4±1.6 | 6.4±1.2 | <0.001 | <0.001 | 0.90 |
p1: comparisons between BL10 and mdx mice;
p2: comparisons between BL10 and mdx/utrn+/− mice;
p3: comparisons between mdx and mdx/utrn+/− mice.
Respiratory function was impaired in mdx and mdx/utrn+/− mice at 6 months of age, and it was worse in mdx/utrn+/− mice
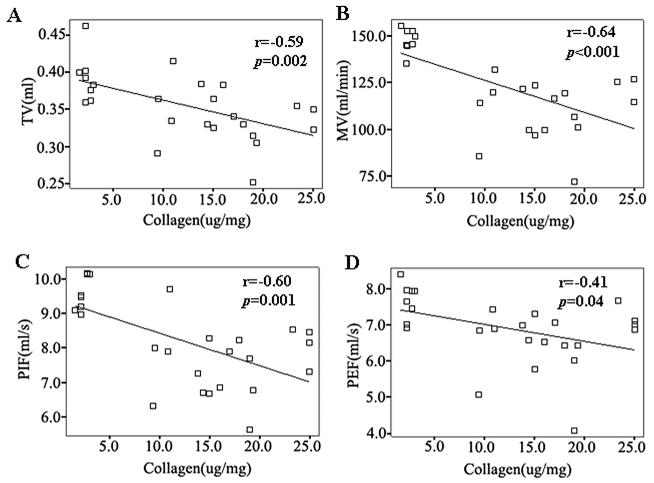
Mdx and mdx/utrn+/− mice displayed remarkable diaphragm fibrosis at 6 months of age that was worse in mdx/utrn+/− mice (10). At this age, diaphragm fibrosis was moderate in mdx mice, and it was severe in mdx/utrn+/− mice (10). Intercostal muscles also showed scattered endomysial inflammation with mild fibrosis in mdx and mdx/utrn+/− mice (Fig. 1). To address whether respiratory function was impaired in mdx and mdx/utrn+/− mice as a result of prominent diaphragm fibrosis and intercostal muscle pathology, we performed whole body non-invasive plethysmography at 6 months of age, and compared the results with age-controlled wild-type BL10 controls. Respiratory frequency, tidal volume, minute volume, peak inspiratory flow, and peak expiratory flow were all significantly reduced in mdx and mdx/utrn+/− mice (Table 2). Tidal volume adjusted by body weight (ml/kg) was also significantly reduced in mdx (p<0.001) and mdx/utrn+/− mice (p<0.001) as compared with controls. Consistent with more severe diaphragm fibrosis in mdx/utrn+/− mice than in mdx mice (10) (Fig. 1), tidal volume (p=0.001), tidal volume adjusted by body weight (p=0.002), minute volume (p=0.04), and peak inspiratory flow (p=0.04) were more severely impaired in mdx/utrn+/− mice than in mdx mice. The degree of diaphragm fibrosis gauged by the diaphragm collagen content inversely correlated with tidal volume (r=−0.59, p<0.01), minute volume (r=−0.64, p<0.001), peak inspiratory flow (r=−0.60, p<0.01), and peak expiratory flow (r=−0.41, p<0.05), but not respiratory frequency (Fig. 2).
Table 2.
Respiratory Parameters in BL10, Mdx, and Mdx/utrn+/− Mice at 6 Months
| BL10 | Mdx | Mdx/utrn+/− | * p1 | * p2 | * p3 | |
|---|---|---|---|---|---|---|
| F (breath/min) | 400.9±26.9 | 352.16±49.7 | 349.3±23.8 | <0.01 | <0.001 | 0.80 |
| TV (ml) | 0.42±0.06 | 0.37±0.05 | 0.32±0.03 | 0.002 | <0.001 | 0.001 |
| TV/BW (ml/kg) | 12.1±1.7 | 10.2±1.5 | 9.1±0.7 | <0.001 | <0.001 | 0.002 |
| MV (ml) | 166.0±23.8 | 126.8±27.5 | 111.7±15.6 | <0.001 | <0.001 | 0.04 |
| PIF (ml/s) | 11.1±1.5 | 8.9±2.0 | 7.7±0.9 | 0.001 | <0.001 | 0.04 |
| PEF (ml/s) | 9.1±1.9 | 7.8±2.0 | 6.9±1.0 | 0.03 | <0.001 | 0.10 |
p1: comparisons between BL10 and mdx mice;
p2: comparisons between BL10 and mdx/utrn+/− mice;
p3: comparisons between mdx and mdx/utrn+/− mice.
Figure 2.
Diaphragm collagen content inversely correlated with respiratory parameters.
The scatter plots show that the diaphragm collagen content inversely correlated with tidal volume (A), minute volume (B), peak inspiratory flow (C), and peak expiratory flow (D).
DISCUSSION
Skeletal muscle fibrosis is a prominent pathological feature of muscle in patients with DMD, and it directly causes muscle dysfunction and clinical weakness. Studies by our lab and others using the mdx mouse model showed that ameliorating skeletal muscle fibrosis may represent a useful therapeutic approach to slow down disease progression and improve the clinical phenotype (11, 12). The diaphragm is the major muscle in mdx mice that undergoes remarkable progressive fibrosis. It is thus useful for studying fibrogenesis associated with dystrophin deficiency and for testing potential antifibrotic therapies. Our previous study showed that diaphragm fibrosis was worse in mdx/utrn+/− mice than in mdx mice (10), which provides a better model for testing antifibrotic therapies, especially at a severe stage of fibrosis. Consistent with progressive diaphragm fibrosis, in vitro diaphragm contractility was greatly impaired in mdx mice (6, 7). However, in order to assess the effect of a potential antifibrotic therapy in the DMD mouse models, an easy and reliable in vivo function test is very much desired. Since the diaphragm is one of the respiratory muscles, which also include intercostal muscles, we assessed respiratory function by whole body non-invasive plethysmography to see if it was significantly impaired in mdx and mdx/utrn+/− mice and would correlate with diaphragm fibrosis. Our findings demonstrated that several respiratory function parameters gauged by WBP were significantly impaired in mdx and mdx/utrn+/− mice and were worse in mdx/utrn+/− mice. The diaphragm collagen content inversely correlated with the respiratory parameters, including tidal volume, minute volume, peak inspiratory flow, and peak expiratory flow. Therefore, WBP can serve as a valuable respiratory function test to assess the in vivo functional changes of the diaphragm and can be used as an outcome measurement of antifibrotic therapies.
At 3 months of age, both diaphragm and intercostal muscles show multifocal endomysial inflammation, and the diaphragm also shows mild endomysial fibrosis in mdx and mdx/utrn+/− mice (10). Impaired respiratory function at this age is likely the result of dysfunction of both types of respiratory muscles. At 6 months of age, inflammation remained in intercostal muscles of mdx and mdx/utrn+/− mice, and only mild morphological fibrosis was detected. However, diaphragm at this age shows remarkable endomysial fibrosis. It is moderate in mdx mice and severe in mdx/utrn+/− mice (10). Therefore, impaired respiratory function at this age is greatly affected by diaphragm dysfunction due to fibrosis. Consistent with more severe fibrosis in mdx/utrn+/− mice at this age, three respiratory function parameters, including tidal volume, minute volume, and peak inspiratory flow, are more severely reduced in mdx/utrn+/− mice than in mdx mice. Peak inspiratory flow mainly reflects the function of the diaphragm as it contracts and descends to create negative intrathoracic pressure for air inspiration. Peak expiratory flow mainly reflects the function of intercostal muscles. The significant reduction in PIF but not in PEF in mdx/utrn+/− mice as compared with mdx mice at 6 months of age is consistent with the finding that worsening of diaphragm fibrosis in mdx/utrn+/− mice is out of proportion to the worsening of intercostal muscle pathology in comparison with mdx mice.
Using plethysmography to compare respiratory function in mdx and BL10 mice has been previously reported by a few groups (6, 13, 14). Gosselin et al reported that tidal volume was not significantly different in wild-type and mdx mice at 7 months of age, but it was lower in mdx mice than in wild-type mice in response to hypercapnia (6). Gayraud et al reported no difference in respiratory rate, tidal volume, or minute ventilation during air breathing or hypercapnia in mdx and wild-type mice at 5 months of age, but respiratory rate and minute ventilation were significantly lower in mdx mice than in wild-type controls at 16 months of age in response to hypercapnia (13). We used more recent and better version of plethysmography than these two groups did, and our results were different. Our study showed that respiratory rate, tidal volume, and minute volume during air breathing were all significantly reduced in mdx and mdx/utrn+/− mice at both 3 and 6 months of age. Ishizaki et al (14) appeared to use a similar version of WBP from Buxco Electronics as we did. They showed that respiratory rate was reduced in mdx mice at 2 and 4 months of age, but it increased at 7 months of age as compared with wild-type controls. Our results showed reduced respiratory frequency at both 3 and 6 months of age. Their study showed no significant change in tidal volume in mdx mice as compared with wild-type mice at 2 and 4 months of age, but it was reduced at 7 months of age. Our study showed unequivocal reduction of tidal volume and minute volume (even adjusted by body weight) in mdx and mdx/utrn+/− mice at both 3 and 6 months of age, which was worse in mdx/utrn+/− mice at 6 months of age. In addition, they did not show PIF and PEF results, and our results showed that both PIF and PEF were significantly reduced in mdx and mdx/utrn+/− mice at 3 and 6 months of age, with PIF being worse in mdx/utrn+/− mice than in mdx mice at 6 months of age. The discrepancies may be in part due to differences in plethysmography calibration which is critical, and their method of calibration was not described (14). Since we noticed individual variations in respiratory parameters, we used a large number of mice (around 20 per group) for our study. Ishizaki et al (14) used only 4–8 mice per group for their study, which was probably not powerful enough to detect significant differences in respiratory parameters between mdx and control mice.
We chose to study respiratory function in mice at 3 and 6 months of age, because our previous diaphragm muscle pathology quantification and comparisons were done in mdx and mdx/utrn+/− mice at these two ages. Our previous studies showed that diaphragm fibrosis was mild in mdx and mdx/utrn+/− mice at 3 months of age, moderate in mdx mice at 6 months of age, and severe in mdx/utrn+/− mice at 6 months of age. Consistent with the pathology findings, this study showed that respiratory function was impaired in mdx and mdx/utrn+/− mice at both ages, worse in mdx/utrn+/− mice at 6 months of age. Our findings suggest that the respiratory parameters, including TV, MV, PIF and PEF, can be used to assess in vivo respiratory muscle function in DMD mouse models and can serve as a useful functional outcome measurement for antifibrotic therapies. The findings further support the notion that with more severe diaphragm fibrosis and greater impairment of respiratory function, mdx/utrn+/− mice may serve as a better model than mdx mice for testing antifibrotic therapies, especially at a severe stage of muscle fibrosis.
Acknowledgments
Work supported by K08 NS049346 (LZ) and MDA grant #91682 (LZ).
Abbreviations
- DMD
Duchenne muscular dystrophy
- WBP
whole-body plethysmography
- F
respiratory frequency
- TV
tidal volume
- MV
minute volume
- PEF
peak expiratory flow
- PIF
peak inspiratory flow
References
- 1.Emery AE. Population frequencies of inherited neuromuscular diseases--a world survey. Neuromuscul Disord. 1991;1(1):19–29. doi: 10.1016/0960-8966(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 2.Emery A. Duchenne muscular dystrophy. New York: Oxford University Press; 1993. [Google Scholar]
- 3.Dupont-Versteegden EE, McCarter RJ. Differential expression of muscular dystrophy in diaphragm versus hindlimb muscles of mdx mice. Muscle Nerve. 1992;15(10):1105–10. doi: 10.1002/mus.880151008. [DOI] [PubMed] [Google Scholar]
- 4.Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352(6335):536–9. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L, Porter JD, Cheng G, Gong B, Hatala DA, Merriam AP, et al. Temporal and spatial mRNA expression patterns of TGF-beta1, 2, 3 and TbetaRI, II, III in skeletal muscles of mdx mice. Neuromuscul Disord. 2006;16(1):32–8. doi: 10.1016/j.nmd.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Gosselin LE, Barkley JE, Spencer MJ, McCormick KM, Farkas GA. Ventilatory dysfunction in mdx mice: impact of tumor necrosis factor-alpha deletion. Muscle Nerve. 2003;28(3):336–43. doi: 10.1002/mus.10431. [DOI] [PubMed] [Google Scholar]
- 7.Lynch GS, Hinkle RT, Faulkner JA. Force and power output of diaphragm muscle strips from mdx and control mice after clenbuterol treatment. Neuromuscul Disord. 2001;11(2):192–6. doi: 10.1016/s0960-8966(00)00170-x. [DOI] [PubMed] [Google Scholar]
- 8.Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, et al. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90(4):717–27. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 9.Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90(4):729–38. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Rafael-Fortney JA, Huang P, Zhao XS, Cheng G, Zhou X, et al. Haploinsufficiency of utrophin gene worsens skeletal muscle inflammation and fibrosis in mdx mice. J Neurol Sci. 2008;264(1–2):106–11. doi: 10.1016/j.jns.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13(2):204–10. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang P, Zhao XS, Fields M, Ransohoff RM, Zhou L. Imatinib attenuates skeletal muscle dystrophy in mdx mice. FASEB J. 2009;23(8):2539–48. doi: 10.1096/fj.09-129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gayraud J, Matecki S, Hnia K, Mornet D, Prefaut C, Mercier J, et al. Ventilation during air breathing and in response to hypercapnia in 5 and 16 month-old mdx and C57 mice. J Muscle Res Cell Motil. 2007;28(1):29–37. doi: 10.1007/s10974-007-9101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishizaki M, Suga T, Kimura E, Shiota T, Kawano R, Uchida Y, et al. Mdx respiratory impairment following fibrosis of the diaphragm. Neuromuscul Disord. 2008;18(4):342–8. doi: 10.1016/j.nmd.2008.02.002. [DOI] [PubMed] [Google Scholar]